Abstract
Objectives
To examine health-related quality of life (HRQoL) among sibling pediatric hematopoietic stem cell donors from predonation through 1 year postdonation, to compare donor-reported HRQoL scores with proxy-reports by parents/guardians and those of healthy norms, and to identify predonation factors (including donor age) potentially associated with postdonation HRQoL, to better understand the physical and psychosocial effects of pediatric hematopoietic stem cell donation.
Study design
A random sample of 105 pediatric donors from US centers and a parent/guardian were interviewed by telephone predonation and 4 weeks and 1 year postdonation. The interview included sociodemo-graphic, psychosocial, and HRQoL items. A sample of healthy controls matched to donors by age, gender, and race/ethnicity was generated.
Results
Key findings included (1) approximately 20% of donors at each time point had very poor HRQoL; (2) child self-reported HRQoL was significantly lower than parent proxy-reported HRQoL at all 3 time points and significantly lower than that of norms at predonation and 4 weeks postdonation; and (3) younger children were at particular risk of poor HRQoL.
Conclusions
Additional research to identify the specific sources of poorer HRQoL among at-risk donors (eg, the donation experience vs having a chronically ill sibling) and the reasons that parents may be overestimating HRQoL in their donor children is critical and should lead to interventions and policy changes that ensure positive experiences for these minor donors.
During the past 50 years, allogeneic hematopoietic stem cell (HSC) transplantation has become a preferred treatment for multiple blood and immune-related disorders.1 Allogeneic HSC donation involves removing stem cells from a healthy donor, in this case a sibling child, through either a surgical bone marrow collection or a peripheral blood stem cell (PBSC) procedure, and infusion of these cells into the ill sibling recipient.2 In 2013, there were 1578 US pediatric HSC transplants, and the number of pediatric HSC transplants has been increasing yearly.3 Although the use of minors as HSC donors is considered medically safe4 and legally accepted given that no alternative approach of comparable effectiveness exists, policy statements by the American Academy of Pediatrics5 and published reviews of the literature cite a lack of understanding of the physical and psychosocial effects of pediatric HSC donation and call for investigations of such effects.6-8
Published reviews identified only a handful of studies of health-related quality of life (HRQoL) in sibling pediatric HSC donation.6,7 Authors of these reviews and other published investigations conclude that there is a critical need to better understand the donation-related experiences of this group.8-11 The few published findings suggest that pediatric donors may experience psychosocial issues around the time of and following donation including higher anxiety and lower self-esteem than nondonors,12 moderate levels of posttraumatic stress, depression, behavioral problems, identity problems, guilt, and resentment.7,12,13 Young donors may also fear the medical aspects and pain involved in donation and experience anxiety and ambivalence about donation.14,15 Following donation, 25%-35% of donors and their families have expressed a need for more predonation information about the donation process.8,16 Although there is evidence of the potential HRQoL risks associated with pediatric HSC donation, the investigations providing this evidence have limitations including descriptive cross-sectional designs, small, nonrepresentative samples, varying time of posttransplant data collection, and lack of child self-reported HRQOL.6,7
The current investigation of sibling pediatric donors was part of a larger study focused on the medical safety and HRQoL of related HSC donation. In addition to large samples of related and unrelated adult HSC donors, the parent study included a smaller sample of sibling pediatric donors. The goals of the pediatric HRQoL substudy were to (1) longitudinally examine HRQoL among sibling pediatric HSC donors from predonation through 1 year postdonation and to compare donor child self-reported scores with parent/guardian proxy-reported scores and normative sample HRQoL scores; (2) examine the potential association of donor age with HRQoL; and (3) determine which predonation factors were most strongly associated with donor child HRQoL at 4 weeks and 1 year postdonation.
Methods
This investigation was approved by the Institutional Review Boards at the University of Pittsburgh, the National Marrow Donor Program, and participating transplant centers.All parents signed informed consent and children gave assent before completing interviews.
Donors and Their Parents/Guardians
This investigation included sibling pediatric HSC donors ages 5-18 years from 24 transplant centers enrolled in the parent Multi-Institutional Study of HSC Donor Safety and Quality Life investigation (ClinicalTrials.gov: NCT00948636) who donated bone marrow or PBSC in the US between April 2010 and May 2013 and 1 of their parents/guardians.
Potential participants were required to meet the standard requirements for donation, be first-time donors, and assent/consent to participate in both the parent Multi-Institutional Study of HSC Donor Safety and Quality Life and the donor HRQoL substudy. Potential participants were excluded if they did not speak English, were unable to complete a telephone interview because of cognitive or linguistic difficulties, or had no access to a telephone as determined by the transplant centers interacting with them.
Individual transplant centers consented participants for the study and passed contact information of enrolled donors to University of Pittsburgh staff. Parent-child pairs who consented entered the random selection pool for the HRQoL substudy with a target sample goal of 100. Interviewers from the University of Pittsburgh contacted participants by telephone within 4 weeks prior to bone marrow donation, or 4 weeks prior to initiation of granulocyte colony stimulating factor administration, to complete a baseline interview. All donors were interviewed again at 4 weeks and 1 year after donation. The interviews required approximately 20 minutes to complete.
Healthy Normative Sample
A normative sample of 537 healthy children matched to the donor sample by age, sex, and race/ethnicity was generated from existing data and provided for this work by the developer of the Pediatric Quality of Life Inventory (PedsQL). Norm-based guidelines for the PedsQL are also available as derived from existing sample of >9500 healthy children assessed during the PedsQL validation phases.17-19
Study Measures
Three categories of participant characteristics were assessed by HRQoL (primary outcome), sociodemographic, and psychosocial characteristics. Measures were previously validated scales/items with established measurement properties either created for, or used in, other donation-related settings. Recipient status at 1 year following donation was collected directly from transplant center records.
HRQoL
HRQoL was assessed with the PedsQL 4.0 Generic Core Scales comprising 23 items assessing functioning in the past month across 4 dimensions: physical (8 items), emotional (5 items), social (5 items), and school (5 items).17-19 Age appropriate-validated versions of the PedsQL were administered. The parent version of the PedsQL asks the same questions as the child version. Parents and children completed the interviews independent of one another. Following standard procedures, responses were transformed to a 0-100 score with a higher score indicating better HRQoL. Responses from the emotional, social, and school functioning scales comprised the psychosocial health summary score. All items combine to produce a total HRQoL score. In addition to a total score, an “at risk” cut-off score of ≤69.71 has been suggested by the PedsQL developers.19 Children scoring at or below this cut-off have HRQoL similar to that of chronically ill children.18,19
Sociodemographic Characteristics
Donor, recipient, and parent/family sociodemographic characteristics were gathered from parents/guardians and included (1) donor age, sex, race/ethnicity, and relationship to the recipient, (2) recipient age and sex, and (3) parent/guardian relationship to the donor, age, education level, relationship status, number of children, and family income. For most analyses, donor age was converted to a categorical variable corresponding to the developmental age categories defined by the PedsQL.17 These age categories are 5-7 years, 8-12 years, and 13-18 years, and age appropriate but statistically comparable versions of the PedsQL are administered to these groups. Donors were assigned to an age group based on their age at the predonation assessment and remained in that group for all analyses.
Psychosocial Characteristics
Included psychosocial characteristics have been associated with donation-related decisions/beliefs/behaviors in the context of adult HSC donation. Family cohesion (parent-report) was measured using 8 items from the expressiveness subscale of the family environment scale. These items assessed feelings of family unity (1 = false, 2 = true) and were averaged to form a scale ranging from 1 to 2 with a higher score indicating greater family cohesion.20 Importance/influence of religion (parent-report) was assessed with 2 standard items gauging the importance and influence of religious beliefs (1 = not at all important/have no religion, 9 = extremely important/religious faith is the center of life). Items were averaged to create a scale ranging from 1 to 9 with a higher score indicating greater importance/influence of religious beliefs.21-24 Finally, we included an open-ended question asking parents whether they believed that their child's age mattered in how he/she experienced the donation process, and if so, how.
Statistical Analyses
Data were cleaned and exported from the CATI system to SPSS Statistics for Windows v 22.0, Released 2013 (IBM Corporation, Armonk, New York) for analysis. Self-reported donor HRQoL and parent proxy-reports across time were described using means and SDs. Potential differences in child donor, parent, and normative sample HRQoL at each time point were examined using independent t tests for comparisons with the normative sample and paired t tests for donor-parent comparisons. Repeated measures general linear modeling was used to examine child and parent HRQoL scores across the 3 data collection time points. Linear mixed models were used to examine predonation predictors of child donor self-reported HRQoL at 4 weeks and 1 year postdonation. Prior to multivariable analyses, we examined predictor (predonation) variables for collinearity and eliminated a variable from any pair of variables that were correlated at ≥0.50. We ran the model both including and excluding predonation HRQoL as a predictor of postdonation HRQoL.
Results
One hundred fifty-eight donors and their parents/guardians agreed to participate in the parent study and also consented for the HRQoL substudy. Donors were randomly selected for the HRQoL study from the eligible pool of potential participants. Based on statistical power and study duration considerations, our goal was to sample approximately 100 donor/parent participants. We ended up randomly selecting 111 potential participants and approaching them for participation during the study time period; 105 (95%) completed the predonation interview and 6 families were unreachable at predonation. Table I lists interview completion rates by time point. Ninety-eight (93%) donors donated via the surgical bone marrow donation procedure. Five of these donations were granulocyte colony stimulating factor-primed, and 7 (7%) donated PBSCs. A total panel of 94 (86%) completed all 3 interviews. The pattern of results from the panel with complete data did not differ from that of the full sample; results presented here are based on all available data from all time points.
Table I.
Response rates and total PedsQL scores for children, parents, and norms at 3 time points
| Study variables | Predonation PedsQL | 4 wk postdonation PedsQL | 1 y postdonation PedsQL |
|---|---|---|---|
| Child N (%) | 105 (95%) | 102 (94%) | 94 (86%) |
| Parent N (%) | 105 (95%) | 102 (94%) | 94 (86%) |
| Child total PedsQL score Mean* (SD) | 81.66ab (13.34) | 81.41ab (14.57) | 83.52a (15.45) |
| Parent total PedsQL score Mean (SD) | 87.66a (8.29) | 85.68a (11.91) | 90.72a (9.20) |
| Normative total score | 85.11b (11.64) | 85.11b (11.64) | 85.11 (11.64) |
Mean values in a given column with the same superscript letter are significantly different from each other (P ≤ .006).
Predonation demographic and psychosocial characteristics are presented in Table II (available at www.jpeds.com). Mean donor age was 11.10 years (age range 5-17 years), a slight minority was females (41%), and there was substantial ethnic diversity (52% White, 23% Black, 18% Hispanic, 6% Asian/Pacific Islander). The most common donor-recipient relationship was recipient as younger brother to donor (38%),and the mean recipient age was 10.78 years; 38% of recipients were females. Respondent parents/guardians tended to be donors’ mothers (74%), mean parent/guardian age was 40.75 years, 36% had at least a Bachelor's Degree, and most were married (87%). Donor families had a mean of 3.6 children and were relatively evenly distributed across the 3 income categories listed in Table II.
Table II.
Predonation demographic and psychosocial characteristics
| Characteristics | Predonation n = 105 |
|---|---|
| Donors | |
| Age (mean, SD) | 11.10 (3.41) |
| Age group (%) | |
| 5-7 | 18 |
| 8-12 | 43 |
| 13-17 | 39 |
| Sex (% female) | 41 |
| Ethnicity (%) | |
| White | 52 |
| Black | 23 |
| Hispanic | 18 |
| Asian/Pacific Islander | 6 |
| Native American | 1 |
| Relationship to recipient (%) | |
| Recipient is older brother | 19 |
| Recipient is younger brother | 38 |
| Recipient is older sister | 20 |
| Recipient s younger sister | 18 |
| Recipient is twin | 4 |
| Recipient is parent | 1 |
| Recipients | |
| Age (mean, SD) | 10.78 (6.07) |
| Sex (% female) | 38 |
| Parent/guardian | |
| Respondent is mother (%) | 74 |
| Age (mean, SD) | 40.75 (7.36) |
| Education (% ≥ Bachelors) | 36 |
| Relationship status (% married) | 87 |
| Number of children | 3.60 (1.47) |
| Income (%) | |
| < $35 000/y | 35 |
| $35 000-$75 000/y | 29 |
| > $75 000/y | 35 |
| Psychosocial | |
| Importance/influence of religion (mean, SD) | 7.69 (1.89) |
| Family environment (mean, SD) | 1.87 (0.14) |
| Child's age makes a difference (% yes) | 68 |
Parents/guardians generally indicated that religion was an important part of their lives; mean of 7.69 on a 1-9 scale and reported a high degree of family unity and mean of 1.87 on a 1-2 scale. Sixty-eight percent of parents/guardians indicated that their child's age made a difference in how the child experienced the donation process.
HRQoL Comparisons
Table I presents donor self-reported, parent-reported, and healthy controls’ total PedsQL mean scores at predonation and 4 weeks and 1 year postdonation. We first examined HRQoL scores longitudinally across the 3 time points. Among donors, there was a significant change in PedsQL scores across time (F[2,92] = 3.27, P = .04). This overall difference was the result of a statistically significant improvement in scores from 4 weeks postdonation to 1 year postdonation; other time point comparisons did not differ significantly. A similar pattern was observed for parent/guardian proxy report scores, an overall statistically significant difference across time (F[2,91] = 15.34, P < .001) with statistically significant declines from pre- to 4 weeks postdonation and then statistically significantly higher scores at 1 year postdonation compared with the 2 previous time points. We then compared child, parent/guardian proxy, and normative scores cross-sectionally at each of the 3 time points using paired t tests for child-parent comparisons and independent t tests for comparisons with norms (Table I). Child self-reported scores were significantly lower than parent-reported scores for their child at all 3 time points (all P ≤ .006) and significantly lower than child-reported normative scores from the matched sample at pre- and 4 weeks postdonation (both P ≤ .005).
Finally, we examined the percentage of donors below the PedsQL cut-off score of ≤69.71. Approximately 20% of pediatric donors at each time point (19%, 17%, and 21%, respectively) scored at/below this cut-off. Donors did not tend to be consistently below the cut-off; 16% were below cut-off at 1 time point only, 13% were below at 2 time points only, and 5% were below cut-off at all 3 time points.
HRQoL and Donor Age
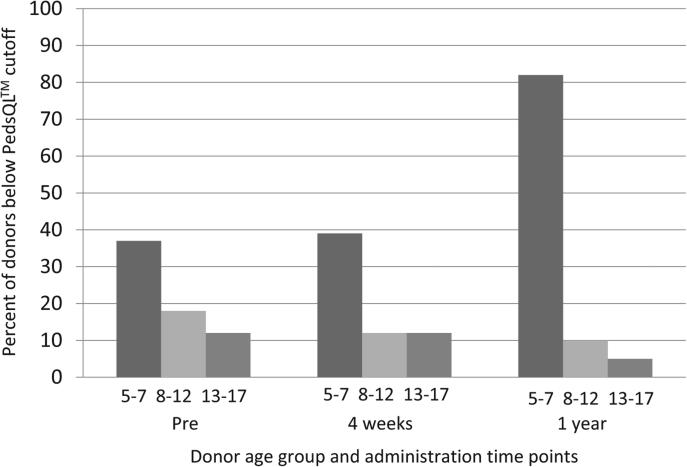
Because we anticipated that pediatric donors of different ages might experience the donation differently, we examined the percentage of children falling below the PedsQL cut-off by each developmental age group (5-7 years, n = 19; 8-12 years, n = 45; 13-18 years, n = 41) at each of the 3 time points (Figure). It was clear that although there were at-risk children in all age categories, the youngest children were most likely to be at risk of poor HRQoL. Predonation, 37% of the youngest age group of donors was below the cut-off vs 18% and 12% of the 8- to 12- and 13- to 18-year-old age groups, respectively. Four weeks postdonation, from youngest to oldest, 39%, 12%, and 12% were below the cut-off, and at 1 year postdonation, from youngest to oldest, 82%, 10%, and 5% were below the cut-off.
Figure.
Percent below PedsQL cut-off by age group (in years).
Table III presents the mean values for each of the PedsQL subscales and the total score for each age group at each of the 3 data collection time points. An ANOVA test of mean differences for each subscale at each time point indicated that at predonation, the mean values for the 3 age groups differed significantly for all but school function. At 4 weeks postdonation, means values differed for all but physical and emotional function. At 1 year postdonation, mean values for all subscales differed. Mean differences, when they existed, always involved the youngest donor group having lower mean scores than one or both of the older groups.
Table III.
PedsQL subscale scores by age group at 3 time points
| Predonation PedsQL |
4 wk postdonation PedsQL |
1 y postdonation PedsQL |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study variables | N | Mean§ | SD | F | N | Mean | SD | F | N | Mean | SD | F |
| Physical function | ||||||||||||
| 5-7 | 19 | 78.28a | 17.8 | 4.19* | 18 | 73.95 | 22.3 | 2.21 | 17 | 65.07ab | 24.5 | 23.90*** |
| 8-11 | 45 | 86.28 | 15.1 | 43 | 83.66 | 14.8 | 39 | 88.86b | 11.0 | |||
| 12-18 | 41 | 89.93a | 11.9 | 41 | 81.70 | 15.4 | 38 | 92.10a | 9.3 | |||
| Emotional function | ||||||||||||
| 5-7 | 19 | 68.94a | 25.3 | 4.42* | 18 | 74.44 | 24.7 | 1.37 | 17 | 63.52ab | 17.2 | 12.26*** |
| 8-11 | 45 | 74.55 | 19.0 | 43 | 83.25 | 22.7 | 39 | 86.53b | 16.2 | |||
| 12-18 | 41 | 82.92a | 12.5 | 41 | 83.41 | 15.9 | 38 | 84.60a | 16.9 | |||
| Social function | ||||||||||||
| 5-7 | 19 | 68.94ab | 20.2 | 11.87*** | 18 | 67.77ab | 19.5 | 12.74*** | 17 | 61.76ab | 21.8 | 27.57*** |
| 8-11 | 45 | 85.11b | 13.4 | 43 | 86.86b | 17.3 | 39 | 89.10b | 14.7 | |||
| 12-18 | 41 | 88.78a | 13.5 | 41 | 89.87a | 12.1 | 38 | 92.63a | 10.2 | |||
| School function† | ||||||||||||
| 5-7 | 15 | 68.66 | 16.8 | 1.75 | 15 | 64.00b | 18.8 | 8.52*** | 16 | 64.37ab | 20.9 | 4.43* |
| 8-11 | 36 | 78.05 | 16.9 | 37 | 84.86b | 13.8 | 32 | 80.62b | 17.9 | |||
| 12-18 | 31 | 73.22 | 17.2 | 30 | 76.66 | 18.6 | 26 | 79.03a | 17.9 | |||
| Psychosocial function | ||||||||||||
| 5-7 | 19 | 68.94ab | 18.9 | 6.52** | 18 | 68.42ab | 18.8 | 7.27*** | 17 | 63.29ab | 17.0 | 18.52*** |
| 8-11 | 45 | 79.58b | 12.9 | 43 | 84.26b | 17.2 | 39 | 86.04b | 14.1 | |||
| 12-18 | 41 | 82.88a | 12.3 | 41 | 84.22a | 13.0 | 38 | 86.64a | 12.6 | |||
| Total score‡ | ||||||||||||
| 5-7 | 19 | 72.33ab | 16.1 | 7.21** | 18 | 70.45ab | 18.0 | 6.96*** | 17 | 63.88ab | 17.0 | 25.91*** |
| 8-11 | 45 | 82.06b | 11.8 | 43 | 84.15b | 13.9 | 39 | 87.06b | 12.0 | |||
| 12-18 | 41 | 85.42a | 11.1 | 41 | 83.34a | 11.2 | 38 | 88.66a | 10.3 | |||
F, ANOVA test of mean differences for each subscale at each time point.
P ≤ .05
P ≤ .01
P ≤ .001.
School function was collected only for participants currently attending school.
Total score is an average of all items and was calculated for all participants even if the school function score was missing.
Mean values in a given column within each PedsQL subscale with the same superscript letter are significantly different from each other (P ≤ .05).
Because of the elevated risk of poor HRQoL for the youngest donors 5-7 years of age, we examined parents’ responses for this group of donors to the open-ended question asking whether they believed their child's age made a difference in how the child experienced the donation process. A theme that emerged across the qualitative parental data was the perception that the donation process had minimal impact on the child donor and that the younger age of these donors was a protective factor in the donation experience. In fact, nearly 70% of parents of this younger age group who responded that their child's age made a difference (10 of 15) indicated that they believed their child's age made donation easier. This contrasts with the poor HRQoL reported by these children.
Predonation Predictors of Postdonation HRQoL
The goal of the multivariable analysis was to determine which predonation variables were associated with donor HRQoL at 4 weeks and 1 year postdonation. Prior to this analysis, we evaluated predonation variables that were potential predictors of postdonation HRQoL for any large intercorrelations (≥0.50). Recipient age and donor-recipient age relationship were highly correlated (r = 0.64; P < .01) and family income and respondent education were highly correlated (r = 0.54; P < .01). Donor-recipient relationship and family income were, there fore, excluded from multivariable analyses. As indicated in Table IV, only predonation HRQoL scores significantly predicted HRQoL at 4 weeks postdonation, and both predonation HRQoL and younger donor age predicted HRQoL at 1 year postdonation (all significant at P < .001). We also evaluated the models excluding predonation HRQoL as a predictor. In these models, younger donor age continued to be significantly as-sociated with poorer postdonation HRQoL, and predonation parental marital status (not married) was identified as a significant predictor for poorer HRQoL both at 4 weeks postdonation (Wald χ2 = 6.27, P = .01) and at 1 year postdonation (Wald χ2 = 4.41, P = .04).
Table IV.
Predonation predictors of HRQoL at 4 weeks and 1 year postdonation
| 4 wk postdonation PedsQL child-report total score |
1 y postdonation PedsQL child-report total score |
|||||||
|---|---|---|---|---|---|---|---|---|
| Study variables | B | SE of B | Wald χ2 | P | B | SE of B | Wald χ2 | P |
| Donor age group | ||||||||
| 5-7 | −5.79 | 3.76 | 2.37 | .12 | −19.67 | 3.77 | 27.41 | <.001 |
| 8-11 | 3.30 | 2.62 | 1.59 | .21 | −0.10 | 2.58 | 0.00 | .97 |
| 12-18 | — | — | — | — | — | — | — | — |
| Donor sex (male) | −2.19 | 2.25 | 0.96 | .33 | 1.77 | 2.30 | 0.59 | .44 |
| Donor race (minority) | −0.40 | 2.40 | 0.03 | .87 | 0.54 | 2.36 | 0.05 | .82 |
| Recipient age | 0.15 | 0.22 | 0.47 | .49 | −0.06 | 0.21 | 0.09 | .76 |
| Recipient sex (male) | −1.07 | 2.49 | 0.06 | .67 | 1.02 | 2.56 | 0.16 | .69 |
| Parent sex (male) | −2.54 | 2.76 | 0.84 | .36 | 0.89 | 2.85 | 0.10 | .75 |
| Parent age | 0.14 | 0.20 | 0.48 | .49 | 0.06 | 0.20 | 0.09 | .77 |
| Parent education (<Bachelors) | 0.60 | 2.43 | 0.06 | .80 | −2.27 | 2.37 | 0.92 | .34 |
| Parent relationship status (not married) | −6.41 | 3.53 | 3.29 | .07 | −5.05 | 3.53 | 2.04 | .15 |
| Parent importance of religion | −0.02 | 0.65 | 0.00 | .97 | 0.28 | 0.63 | 0.19 | .66 |
| Family environment | 3.79 | 8.23 | 0.21 | .65 | −4.38 | 7.96 | 0.30 | .58 |
| PedsQL total score (predonation) | 0.64 | 0.09 | 52.54 | <.001 | 0.44 | 0.09 | 24.63 | <.001 |
B, beta coefficient.
Only 7 recipients died during the study period, and there were no statistically significant donor HRQoL differences between those whose siblings died vs those who survived, but the power to detect such differences was low given the small number of deaths.
Discussion
Despite existing evidence that some pediatric HSC donors experience the donation as physically and psychologically stressful and calls for investigations into pediatric donor HRQoL, there has been very limited research focused on HRQoL in pediatric HSC donors. The findings presented here are from one of the first large-scale, longitudinal, multicenter investigations of pediatric HSC donor HRQoL.
A key finding was that donor children had poorer HRQoL than did norms at pre- and shortly postdonation and then return to normative levels by 1 year postdonation. It should be noted that the total PedsQL differences between donors and norms were smaller than the minimal clinically important difference of 4.4 points (3.45 predonation and 3.70 postdonation) as established by the PedsQL developers.19 However, approximately 20% of donors at all 3 time points exhibited clinically important (below at-risk cut-off point) HRQoL deficits. There are several possible explanations for low HRQoL scores among a subset of donors, and these potential explanations are difficult to disentangle based on findings from this study alone. First, the lower donor HRQoL scores near the time of donation could suggest that something specific to the donation experience is affecting HRQoL, or alternatively, be a result of having a sibling who is critically ill and at a particularly important medical intervention point.
Evidence about whether siblings of chronically ill children have poorer HRQoL, in general than their counterparts is mixed, but some studies have reported HRQoL deficits among siblings of chronically ill children.25 Alternately, the lower HRQoL scores could be a result of family dynamics more generally (eg, the donor's sense of exclusion because of parental focus on the ill child, or parents’ emotional and coping status throughout the process).
A second key finding is that parents seem to overestimate their child's HRQoL. Again, there are several possible explanations for the divergent parent-child reports. First, parents may be motivated to believe that the donor child is doing well because they are being exposed to the medical risks of donation with no direct self-benefits. Second, it is possible that the donor child appears healthy in contrast to their ill sibling. Third, it is possible that the reporting parent may be located remotely with the ill child and geographically separated from the donor child. This could lead to an inability to accurately report on the HRQoL of the donor. Finally, it is possible that parental focus on the ill child causes them to fail to notice signs, symptoms, and behaviors that would indicate poor HRQoL in the donor child.12,13 Recent review articles suggest that child-parent HRQoL discrepancies (1) are inconsistent and occur in both directions; (2) may occur because children and parents base HRQoL judgements on different information; (3) may themselves have clinical implications; and (4) provide a strong justification for assessing both child and parent HRQoL reports.26,27
A third important finding is that younger donors seem to be most at-risk of poor HRQoL.
Younger donors scored lower than their older counterparts on virtually every HRQoL domain at all 3 time points; the exceptions were school function at predonation and physical and emotional function at 4 weeks postdonation. In addition, younger donor age was a predictor of poorer HRQoL at 1 year postdonation. It is possible that the younger children may not have developed the necessary coping skills to deal with the significant stress of having a seriously ill sibling, and the resultant effects on the family. Alternatively, having a very ill sibling may be more distressing to younger children given that their lives may be more disrupted because of their greater dependence on their parents.
This investigation has some limitations. First, the lack of follow-up beyond 1 year prevents us from knowing the longer-term relationship between donor-reported, parent-reported, and normative HRQoL. Second, our streamlined approach to data collection meant that we did not ask about several factors (eg, quality of the donor-recipient relationship, parental emotional status, parent respondent geographic proximity to the donor) that would have allowed us to provide fuller explanations for our findings. Third, it might have been ideal to include a comparison group of nondonor siblings, but we anticipated difficulty identifying families with >3 children (patient, donor, nondonor sibling) in which both the donor and the nondonor sibling were in appropriate age ranges for the PedsQL. The inclusion of nondonor siblings will be critical for future research to disentangle the effects of being a pediatric donor from those of being the sibling of a child who is critically ill. Finally, although the donor sample was generated from multiple sites and included a high degree of donor and family diversity, expanding the number of children studied in this manner would strengthen our ability to ensure that the findings are generalizable to the larger population of pediatric HSC donors.
Despite these limitations, this investigation represents a significant advance in our understanding of the HRQoL of pediatric donors and raises concerns about their safety and well-being during donation. Future investigations should focus on determining the reasons for HRQoL deficits among pediatric donors and for the differences between parent and child HRQoL scores. Gathering quantitative and qualitative data from the entire family during and after the donation process, follow-up with families beyond the 1-year time point, and making explicit HRQoL comparisons between sibling donors and nondonors would help address these issues. Regardless of the explanation for low HRQoL scores among some donors in the current study (ie, whether they are a product of the donation experience specifically or having an ill sibling or family functioning more generally), it is critical to take steps to minimize HRQoL risks among these children who have agreed to a medical procedure with no direct self-benefit. A first step might be to ensure that all centers have a psychosocial clinician who meets regularly with the child to identify HRQoL concerns and provide psychosocial support. Minimization of such risks will require research to identify the specific sources of poorer HRQoL in this context, followed by interventions and policy changes to ensure positive experiences for these donors.
Acknowledgments
We thank the following site principal investigators at participating transplant centers: Aly Abdel-Mageed, MD, Helen DeVos Children's Hospital at Spectrum Health, Grand Rapids, MO; Edward D. Ball, MD, University of California San Diego Medical Center, San Diego, CA; Brian Bolwell, MD, Cleveland Clinic Foundation, Cleveland, OH; Michael Boyer, MD, University of Utah Blood and Marrow Program, Salt Lake City, UT; Nancy Bunin, MD, Children's Hospital of Philadelphia, Philadelphia, PA; Alexandra Cheerva, MD, MS, Kosair Children's Hospital, Louisville, KY; Jignesh Dalal, MD, Children's Mercy Hospital, Kansas City, MO; David Delgado, MD, Riley Children's Hospital at IU Health, Indianapolis, IN; Christopher Dvorak, MD, Benioff Children's Hospital, University of California San Francisco, San Francisco, CA; Steven C. Goldstein, MD, University of Michigan Medical Center, Ann Arbor, MI; Theresa Hahn, PhD, Roswell Park Cancer Institute, Buffalo, NY; Ann E. Haight, MD, Children's Healthcare of Atlanta, Emory University School of Medicine, Atlanta, GA; Brandon Hayes-Lattin, MD, Knight Cancer Institute, Oregon Health and Science University, Portland, OR; David Jacobsohn, MD, ScM, Children's National Health System, Washington DC; Kimberly A. Kasow, DO, University of North Carolina, Chapel Hill, NC; Michael Linenberger, MD, Fred Hutchinson Cancer Research Center, Seattle, WA; Shahram Mori, MD, PhD, Florida Hospital Cancer Institute, Florida Center for Cellular Therapy, Orlando, FL; Aleksandra Petrovic, MD, All Children's Hospital, St. Petersburg, FL; Vinod K. Prasad, MD, FRCP (London), Duke University Medical Center, Durham, NC; Scott Rowley, MD, Hackensack University Medical Center, Hackensack, NJ; Indira Sahdev, MD, Cohen Children's Medical Center of New York, New York, NY; Jeffrey Schriber, MD, City of Hope Samaritan, Phoenix, AZ; Paul Shaughnessy, MD, Texas Transplant Institute, San Antonio, TX; Shalini Shenoy, MD, St. Louis Children's Hospital, Washington University School of Medicine, St. Louis, WA; Margarida Silverman, MD, University of Iowa Hospitals & Clinics, Iowa City, IA; and William Tse, MD, James Graham Brown Cancer Center, Louisville, KY.
Supported by the National Heart, Lung, and Blood Institute (R01 HL085707). J.V. holds the copyright and the trademark for the Pediatric Quality of Life Inventory and receives financial compensation from the Mapi Research Trust, which is a nonprofit research institute that charges distribution fees to for-profit companies that use the Pediatric Quality of Life Inventory.
Glossary
- HRQoL
Health-related quality of life
- HSC
Hematopoietic stem cell
- PBSC
Peripheral blood stem cell
- PedsQL
Pediatric Quality of Life Inventory
Footnotes
The other authors declare no conflicts of interest.
References
- 1.Copelan EA. Medical progress: hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–26. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 2.Center for International Blood and Marrow Transplant Research Statistical center of the Center for International Blood and Marrow Transplant Research. 2015.
- 3.Pulsipher MA, Nagler A, Iannone R, Nelson RM. Weighing the risks of G-CSF administration, leukopheresis, and standard marrow harvest: ethical and safety considerations for normal pediatric hematopoietic cell donors. Pediatr Blood Cancer. 2006;46:422–33. doi: 10.1002/pbc.20708. [DOI] [PubMed] [Google Scholar]
- 4.Pulsipher MA, Levine JE, Hayashi RJ, Chan KW, Anderson P, Duerst R, et al. Safety and efficacy of allogeneic PBSC collection in normal pediatric donors: the pediatric blood and marrow transplant consortium experience (PBMTC) 1996-2003. Bone Marrow Transplant. 2005;35:361–7. doi: 10.1038/sj.bmt.1704743. [DOI] [PubMed] [Google Scholar]
- 5.Committee on Bioethics Children as hematopoietic stem cell donors. Pediatrics. 2010;125:392–404. doi: 10.1542/peds.2009-3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauk K, D'Auria J, Andrews A, Presler CM. The pediatric sibling donor experience in hematopoietic stem cell transplant: an integrative review of the literature. J Pediatr Nurs. 2013;28:235–42. doi: 10.1016/j.pedn.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Wiener LS, Steffen-Smith E, Fry T, Wayne AS. Hematopoietic stem cell donation in children: a review of the sibling donor experience. J Psychosoc Oncol. 2007;25:45–66. doi: 10.1300/J077v25n01_03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiener LS, Steffen-Smith E, Battles HB, Wayne A, Love CP, Fry T. Sibling stem cell donor experiences at a single institution. Psychooncology. 2008;17:304–7. doi: 10.1002/pon.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Walraven SM, Straathof LM, Switzer GE, Lankester A, Korthof ET, Brand A, et al. Immediate and long-term somatic effects and health-related quality of life of BM donation during early childhood. A single center report in 210 pediatric donors. Bone Marrow Transplant. 2013;48:40–5. doi: 10.1038/bmt.2012.102. [DOI] [PubMed] [Google Scholar]
- 10.van Walraven SM, Ball LM, Koopman HM, Switzer GE, Ropes-de Jong CM, de Jong A, et al. Managing the dual role – experiences and coping strategies of parents donating haploidentiacal G-CSF mobilized peripheral blood stem cells to their children. Psychooncology. 2012;21:168–75. doi: 10.1002/pon.1885. [DOI] [PubMed] [Google Scholar]
- 11.Weisz V, Robbennolt JK. Risks and benefits of pediatric bone marrow donation: a critical need for research. Behav Sci Law. 1996;14:375–91. doi: 10.1002/(SICI)1099-0798(199623)14:4<375::AID-BSL247>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 12.Packman W, Crittenden MR, Rieger Fischer JB, Schaffer E, Bongar B, Cowan MJ. Siblings’ perceptions of the bone marrow transplantation process. J Psychosoc Oncol. 1997;15:81–105. [Google Scholar]
- 13.Packman W, Crittenden MR, Schaeffer E, Bongar B, Fischer J, Cowan MJ. Psychosocial consequences of bone marrow transplantation in donor and nondonor siblings. J Dev Behav Pediatr. 1997;18:244–53. [PubMed] [Google Scholar]
- 14.Kinrade LC. Preparation of sibling donor for bone marrow transplant harvest procedure. Cancer Nurs. 1987;10:77–81. [PubMed] [Google Scholar]
- 15.MacLeod KD, Whitsett SF, Mash EJ, Pelletier W. Pediatric sibling donors of successful and unsuccessful hematopoietic stem cell transplants (HCST): a qualitative study of their psychosocial experience. J Pediatr Psychol. 2003;28:223–31. doi: 10.1093/jpepsy/jsg010. [DOI] [PubMed] [Google Scholar]
- 16.Pentz RD, Alderfer MA, Pelletier W, Stegenga K, Haight AE, Hendershot KA, et al. Unmet needs of siblings of pediatric stem cell transplant recipients. Pediatrics. 2014;133:1156–62. doi: 10.1542/peds.2013-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varni JW, Seid M, Kurtin PS. PedsQL™4.0: reliability and validity of the pediatric quality of life inventory™version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39:800–12. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Varni JW, Limbers CA, Burwinkle TM. Impaired health-related quality of life in children and adolescents with chronic conditions: a comparative analysis of 10 disease clusters and 33 disease categories/severities utilizing the PedsQL™ 4.0 Generic Core Scales. Health Qual Life Outcomes. 2007;15:1–15. doi: 10.1186/1477-7525-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL™ 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr. 2003;3:329–41. doi: 10.1367/1539-4409(2003)003<0329:tpaapp>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 20.Moos R, Moos B. Family environment scale manual: development, applications, research. third ed. Consulting Psychologist Press; Palo Alto (CA): 1999. [Google Scholar]
- 21.Switzer GE, Simmons RG, Dew MA. Helping unrelated strangers: physical and psychological reactions to the bone marrow donation process among anonymous donors. J Appl Soc Psychol. 1996;26:469–90. [Google Scholar]
- 22.Switzer GE, Dew MA, Goycoolea JM, Myaskovsky L, Abress L, Confer DL. Attrition of potential bone marrow donors at two key decision points leading to donation. Transplantation. 2004;77:1529–34. doi: 10.1097/01.tp.0000122219.35928.d6. [DOI] [PubMed] [Google Scholar]
- 23.Switzer GE, Myaskovsky L, Goycoolea JM, Dew MA, Confer DL, King R. Factors associated with ambivalence about bone marrow donation among newly recruited unrelated potential donors. Transplantation. 2003;75:1517–23. doi: 10.1097/01.TP.0000060251.40758.98. [DOI] [PubMed] [Google Scholar]
- 24.Switzer GE, Dew MA, Harrington DJ, Crowley-Matoka M, Myaskovsky L, Abress L, et al. Ethnic differences in donation-related characteristics among potential hematopoietic stem cell donors. Transplantation. 2005;80:890–6. doi: 10.1097/01.tp.0000173648.60978.30. [DOI] [PubMed] [Google Scholar]
- 25.Limbers CA, Skipper S. Health-related quality of life measurement in siblings of children with physical chronic illness: a systematic review. Fam Syst Health. 2014;32:8–415. doi: 10.1037/fsh0000077. [DOI] [PubMed] [Google Scholar]
- 26.Eisner C, Varni JW. Health-related quality of life and symptom reporting: similarities and differences between children and their parents. Eur J Pediatr. 2013;172:1299–304. doi: 10.1007/s00431-013-2049-9. [DOI] [PubMed] [Google Scholar]
- 27.Upton P, Lawford J, Eiser C. Parent-child agreement across child health-related quality of life instruments: a review of the literature. Qual Life Res. 2008;17:895–913. doi: 10.1007/s11136-008-9350-5. [DOI] [PubMed] [Google Scholar]