Abstract
Rationale
G protein-coupled receptor kinase 2 (GRK2) is an important molecule upregulated after myocardial injury and during heart failure. Myocyte-specific GRK2 loss before and after myocardial ischemic injury improves cardiac function and remodeling. The cardiac fibroblast plays an important role in the repair and remodeling events following cardiac ischemia; the importance of GRK2 in these events has not been investigated.
Objective
The aim of this study is to elucidate the in vivo implications of deleting GRK2 in the cardiac fibroblast after ischemia/reperfusion (I/R) injury.
Methods and Results
We demonstrate, using Tamoxifen inducible, fibroblast-specific GRK2 knockout mice, that GRK2 loss confers a protective advantage over control mice after myocardial I/R injury. Fibroblast GRK2 knockout mice presented with decreased infarct size and preserved cardiac function 24 hours post-I/R as demonstrated by increased ejection fraction (58.1±1.8% vs. 48.7±1.2% in controls, p<0.0005). GRK2 fibroblast knockout mice also had decreased fibrosis and fibrotic gene expression. Importantly, these protective effects correlated with decreased infiltration of neutrophils to the ischemia site and decreased levels of TNFα expression and secretion in GRK2 fibroblast knockout mice.
Conclusions
These novel data showing the benefits of inhibiting GRK2 in the cardiac fibroblast adds to previously published data showing the advantage of GRK2 ablation and reinforces the therapeutic potential of GRK2 inhibition in the heart after myocardial ischemia.
Keywords: Myocardial ischemia/reperfusion injury, G protein-coupled receptor kinase 2, cardiac fibroblast, fibrosis, inflammation
INTRODUCTION
Cardiac fibroblasts represent the largest population of interstitial cells in myocardium.1 They are responsible for secreting and maintaining the extracellular matrix forming a three-dimensional scaffold supporting the surrounding myocytes.2 While not contractile, fibroblasts are directly connected to myocytes through cell junctions and may act as bridges to connect myocytes electrically isolated by connective tissue.2 Unlike myocytes, cardiac fibroblasts are particularly resistant to hypoxia and so play multiple roles in the cascade of events following ischemic injury.3 After ischemic injury, cardiac fibroblasts transform into a specialized cell type called a myofibroblast marked by increased amounts of α-smooth muscle actin and characterized as being hyper-secretory. Myofibroblasts have a critical role in cardiac remodeling by forming a collagen-rich scar that allows the infarcted area to maintain structural integrity after cardiomyocyte death.4 Additionally, in response to injury, fibroblasts secrete cytokines and chemokines that have important roles in the immediate inflammatory stage.5 Fibroblasts are responsive to a variety of stimuli for these above functions including agents that activate membrane-bound G protein-coupled receptors (GPCRs). Thus, regulation of these receptors is important in the overall process of myofibroblast activation after injury.6
Ischemia/reperfusion (I/R) injury activates a ‘sterile’ inflammatory response where neutrophils are recruited to the ischemic area by necrotic myocytes and reactive oxygen species (ROS).7 Excessive neutrophil infiltration in the infarcted site is thought to be detrimental to myocyte survival as neutrophils also secrete ROS, further exacerbating cell and tissue destruction.8 Understanding inflammation and remodeling after I/R injury is important for developing novel therapies; multiple studies have demonstrated that inhibiting neutrophil extravasation reduces tissue damage and decreases infarct size.9-11 We are interested in the cardiac signals and cellular communication between myocytes and fibroblasts that may regulate or alter the tissue damage after I/R injury.
While the regulation of GPCRs in myocytes during ischemic injury has been extensively studied, fibroblast GPCR signaling during I/R injury and the focus of this study, GPCR kinase 2 (GRK2), has not been well characterized. The primary role of GRK2 is to phosphorylate agonist-activated GPCRs and target them for β-arrestin-mediated internalization; in the heart, GRK2 is up-regulated in the myocyte after injury and its enhanced activity targets the β-adrenergic receptor (βAR) to modulate contractility.12-13 Decades of research have uncovered the detrimental effects of increased GRK2 activity in heart disease and provided sweeping evidence that loss of myocyte GRK2 expression or its inhibition either before or after cardiac insult is beneficial for myocardial contractility and cardiac remodeling.14-16 More recent studies have also expanded on the versatility of GRK2 by delineating non-canonical roles in eNOS regulation17,18 , mitochondrial function19,20 and insulin signaling.21
Currently, little is known concerning the function of GRK2 in the cardiac fibroblast. In this study we sought to examine whether the loss of fibroblast GRK2 would be advantageous or detrimental to cardiac function following acute ischemic injury in vivo. Using inducible and conditional GRK2 knockout (KO) mice we subjected fibroblast-specific GRK2 KO mice to myocardial I/R injury and investigated the effects related to heart function, fibrosis and inflammation. Our results distinctly show that, in an acute situation, loss of fibroblast GRK2 is beneficial for cardiac function and remodeling and that this may be related to differences in inflammatory cell recruitment in the early hours following reperfusion. We also performed in vitro experiments in freshly isolated neonatal rat and adult mouse cardiac fibroblasts to gain insight into how the fibroblast may be influencing myocyte survival and cardiac inflammation in vivo.
METHODS
Experimental animals
To obtain inducible, fibroblast-specific GRK2 KO mice, Collagen1α2-CreER(T) mice22 (Jackson Labs Stock #029235) were crossed with GRK2fl/fl mice23 (Jackson Labs Stock # 012458). Pups were backcrossed to generate Collagen1α2-CreER(T)/GRK2fl/fl mice (referred to as GRK2 fKO). GRK2fl/fl mice were used as wild-type (WT) littermate controls. Tamoxifen (Sigma T5648) was injected intraperitoneally (IP) daily for 10 days at 40mg/kg/day. All animal studies were conducted with the approval of the Animal Care and Use Committee at Temple University.
Isolation of adult mouse cardiac fibroblasts and myocytes
Hearts were removed from >2-3 month old mice and processed as previously described.24
Isolation of bone marrow cells and lysate preparation
Femurs were removed from >2-3 month old mice. Contents were flushed using Hank's Buffered Salt Solution (Corning 21-023-CV), a syringe and a 27-gauge needle. Bone marrow cells were centrifuged and washed with 1X PBS. Cells were resuspended in RIPA buffer supplemented with protease inhibitors, sonicated and centrifuged to remove cell debris.
Isolation of vascular smooth muscle and lysate preparation
Thoracic aortas were isolated from >2-3 month old mice. After washing in 1X PBS, aortas were responded in RIPA buffer supplemented with protease inhibitors, sonicated and centrifuged to remove cell debris.
Isolation of neonatal rat cardiac fibroblasts
Neonatal rat cardiac fibroblasts are isolated as a byproduct of neonatal rat cardiac myocyte isolation, performed as previously described17.
Immunoblotting
GRK2 and GAPDH antibodies were from Santa Cruz. Phospho-AKT and total AKT antibodies were from Cell Signaling.
In vivo model of ischemia/reperfusion injury
Ischemia/reperfusion injury was performed as described previously.25 Infarct size was measured as previously described.26
Transthoracic echocardiographic analysis
Transthoracic two-dimensional echocardiography was performed blinded as described.25
Terminal hemodynamic analysis of cardiac function
Hemodynamic analysis was conducted blinded as described previously.25
Measurement of myocardial apoptosis
Myocardial apoptosis was assessed by TUNEL staining.18
Assessment of myocardial fibrosis
Collagen levels were measured using the Masson's Trichrome staining kit (Sigma HT15) and the Hydroxyproline assay kit (Sigma MAK008) without modifications.
Measurement of cAMP levels
Neonatal rat cardiac fibroblasts were treated with 10μM isoproterenol for 15 minutes. cAMP levels were measured using the cyclic AMP XP Assay Kit (Cell Signaling 4339) without modifications.
Immunofluorescence
Hearts were harvested 72 hours post reperfusion, fixed overnight in 4% paraformaldehyde, embedded in paraffin and cut on the long axis into 6um sections. After deparaffinization, rehydration and antigen retrieval using Vector Antigen Unmasking Solution (Vector Labs H3300), sections were blocked in 10% FBS/1X PBS. Primary antibody (α-SMA, Sigma A5228) was incubated with sections overnight at 4°C. Secondary antibody (Life Technologies A21463) was applied for 1 hour at room temperature. Sections were treated with DAPI mounting media and coverslipped. Fluorescence intensity was measured using ImageJ. For staining of neonatal rat cardiac fibroblasts, cells were treated with methanol for 15 minutes at −20°, washed and blocked for 1 hour at room temperature in 5% BSA/PBS. P65 antibody (Cell Signaling 6956) was incubated overnight at 4°C and secondary antibody (Life Technologies) was applied for 1 hour at room temperature. Sections were treated with DAPI mounting media and coverslipped. Cells were counted using Image J over at least five fields per group. All immunofluorescence images were taken with a Nikon Ti microscope.
Myeloperoxidase (MPO) staining
Mice were euthanized after 6 and 24 hours of reperfusion and hearts were fixed in 4% paraformaldehyde. The hearts were embedded in paraffin and cut into 5 μm thick sections. Immunohistochemistry was carried out on sections, including antigen retrieval with Vector Antigen Unmasking Solution. MPO primary antibody and secondary antibody were sourced from Santa Cruz (sc16129, sc2354, respectively). Images of the infarct area of the LV wall were taken from a least two randomly chosen fields on a Nikon DS-Ri1 and quantified in a blind manner using ImageJ.
Cytokine microarray
Adult mouse cardiac fibroblasts were isolated as described above. Cells were subjected to in vitro ischemia buffer27 and supernatant was harvested for use with the Proteome Profiler Mouse Cytokine Array Panel A (R&D ARY006) with one modification: IRDye 800CW Streptavidin (Rockland S000-31) was used.
Conditioned media experiment
Neonatal cardiac fibroblasts were treated with LacZ or shGRK2 containing adenovirus. After 24 hours fibroblasts were serum starved overnight, treated for 30 minutes with in vitro ischemia buffer and then reperfused in 0.1% DMEM for 3 hours. Supernatant was collected and placed on neonatal cardiac myocytes for 10 minutes. Fibroblasts were collected to ensure GRK2 loss and myocytes were collected for whole cell lysate.
In vitro ischemia reperfusion buffer
This buffer simulates the extracellular milieu of an ischemic environment because it is glucose deficient, hyperkalemic and acidic/lactate rich. It is composed of 137mM NaCl, 3.8mM KCl, 0.49mM MgCl2, 0.9mM CaCl2, 4.0mM Hepes supplemented with 10mM 2-deoxyglucose, 20mM sodium lactate, 1mM sodiumdithionite and 12mM KCL, pH 6.5.
Statistical analysis
Data are expressed as mean±SE. Statistical significance was determined by unpaired t test on experiments comparing two groups or ANOVA and Tukey test for experiments involving four comparisons using vassarstats.net. P values <0.05 were considered significant.
RESULTS
Decreased levels and activity of GRK2 in fibroblasts are beneficial to post-I/R myocardium
Eight-week old adult male Col1α2CreER/GRK2 flox (GRK2 fKO) mice and their control littermates (WT) were injected intraperitoneally with tamoxifen (40mg/kg/day) for 10 days (Online Figure I). After a two week washout period, freshly isolated fibroblasts from GRK2 fKO mice demonstrated approximately 60% protein loss compared to fibroblasts from WT mice (Figure 1a). GRK2 knockdown was specific for fibroblasts, as protein levels were maintained in myocytes, bone marrow cells and vascular smooth muscle cells following tamoxifen treatment in both groups (Figure 1a). This finding correlates with previously published reports using this model for other gene products.24,28 We can infer negligible interference from knockdown in circulating fibrocytes. While this cell type has fibroblast qualities, fibrocytes are monocyte-derived and GRK2 levels are maintained in the bone marrow cells. Left ventricular (LV) infarct size subsequent to 30 minutes of left anterior descending coronary artery occlusion and 24 hours of reperfusion was measured in GRK2 fKO mice and significant differences were found compared to control mice (Figure 1). In WT littermates, LV infarct size was 34.17±3.98% of the area at risk (Figure 1b,c). However, fibroblast-specific ablation of GRK2 caused a decreased infarct size of 20.67±3.03%. The LV area at risk was similar in both groups, indicating the same amount of ischemic trauma (Figure 1d). These results establish that loss of fibroblast GRK2 during acute ischemic injury confers a protective advantage to reperfused myocardial tissue.
Figure 1. GRK2 levels and post I/R left ventricular (LV) infarct size in GRK2 fKO mice.
A) Western blots and GRK2 protein quantification of freshly isolated cardiac fibroblast, myocyte, bone marrow and vascular smooth muscle cell lysate in Tamoxifen-treated WT and GRK2 fKO mice. *P<0.05 vs. WT. N=3-4. B) Representative photographs of post-I/R Evan's Blue/triphenyl tetrazolium chloride-stained sections from WT and GRK2 fKO hearts. C) LV infarct size expressed as a percentage of the area at risk. D) LV area at risk expressed as a percentage of the total LV. *P<0.05 vs. WT. N=6.
Echocardiography was performed on WT and GRK2 fKO mice after 30 minutes of ischemia and 24 hours of reperfusion (Figure 2a,b). Control mice presented with decreased LV ejection fraction of 48±2.1% compared to 62±2.5% at baseline (Figure 2a). WT mice also exhibited loss of fractional shortening (24±1.2% vs. 33±1.7% in WT baseline group)(Figure 2b). However, GRK2 fKO mice displayed partial but significantly restored cardiac function with an ejection fraction of 59±1.7% and fractional shortening of 31±1.1%. Both groups displayed similar baseline ejection fractions, demonstrating equal cardiac function prior to surgery. To correlate echocardiography data and to measure intact cardiac pressures, terminal hemodynamics was done 24 hours post-I/R injury and mice were infused with increasing doses of isoproterenol to assess their inotropic reserve (Figure 2c). WT mice presented with compromised contractility, demonstrated by decreased +dP/dTmax and −dP/dTmin compared to GRK2 fKO mice, which maintained contractility levels close to baseline. In agreement with the systolic function data, we also noted decreased cardiac output (CO) and stroke volume (SV) in the WT mice compared to GRK2 fKO mice after 24 hours (Table 1). We also assessed cardiac function at 1 and 4 weeks post I/R to determine the chronic effects of fibroblast GRK2 deletion. The WT I/R group maintained a significantly reduced ejection fraction at 1 week and 4 weeks post I/R (51.9 ± 2.3 and 53.3 ± 0.7 respectively) while the GRK2 fKO mice presented with rescued cardiac function at both time points (62.1 ± 1.2 and 60.3 ± 1.7)(Table 1). There were no survival differences between the I/R groups after 4 weeks of reperfusion (Table 1).
Figure 2. Cardiac function post I/R in WT and GRK2 fKO mice.
Cardiac function shown by A) Ejection fraction (%) and B) Fractional shortening (%) from echocardiography on WT and GRK2 fKO mice at baseline and 24 hours after I/R. *P<0.05 and #P<0.01 vs. WT post I/R, **P<0.01 vs. WT baseline. N=8. C) In vivo contractility demonstrated by + dP/dTmax and − dP/dTmin measured with terminal hemodynamics. *P<0.05 and **P<0.01 vs. WT Sham. N=8
Table 1.
Functional Measurements Obtained from Echocardiography and Survival Rate on WT and GRK2 fKO mice subjected to Sham and I/R surgery.
| Animal | Heart Rate (beats/min) |
LVEDD (mm) |
Stroke Volume (μl) |
Cardiac Output (ml/min) |
Ejection Fraction (%) |
Survival Rate (%) |
|
|---|---|---|---|---|---|---|---|
| Time post I/R | 24 hours | 24 hours | 24 hours | 24 hours | 1 week | 4 weeks | 4 weeks |
| WT Sham | 504 ± 14.1 | 4.03 ± 0.18 | 45.9 ± 2.6 | 23.2 ± 1.5 | 63.8 ± 2.2 | 60.8 ± 1.7 | 100% |
| GRK2 fKO Sham | 535± 22.8 | 3.97 ± 0.14 | 46.0 ± 3.3 | 25.1 ± 2.1 | 60.4 ± 0.9 | 59.2 ± 2.0 | 100% |
| WT I/R | 470 ± 12.3 | 4.07 ± 0.14 | 35.3 ± 1.7& | 16.6 ± 0.7& | 51.9 ± 2.3& | 53.3 ± 0.7* | 80% |
| GRK2 fKO I/R | 489 ± 9.9 | 4.01 ± 0.12 | 42.2 ± 1.6** | 20.7 ± 1.1** | 62.1 ± 1.2# | 60.3 ± 1.7# | 80% |
p<0.05 vs, WT Sham
p<0.05 vs, WT I/R
p<0.01 vs. WT Sham
p<0.01 vs. WT I/R. N=4-7 for Sham groups, N=6-9 for I/R groups.
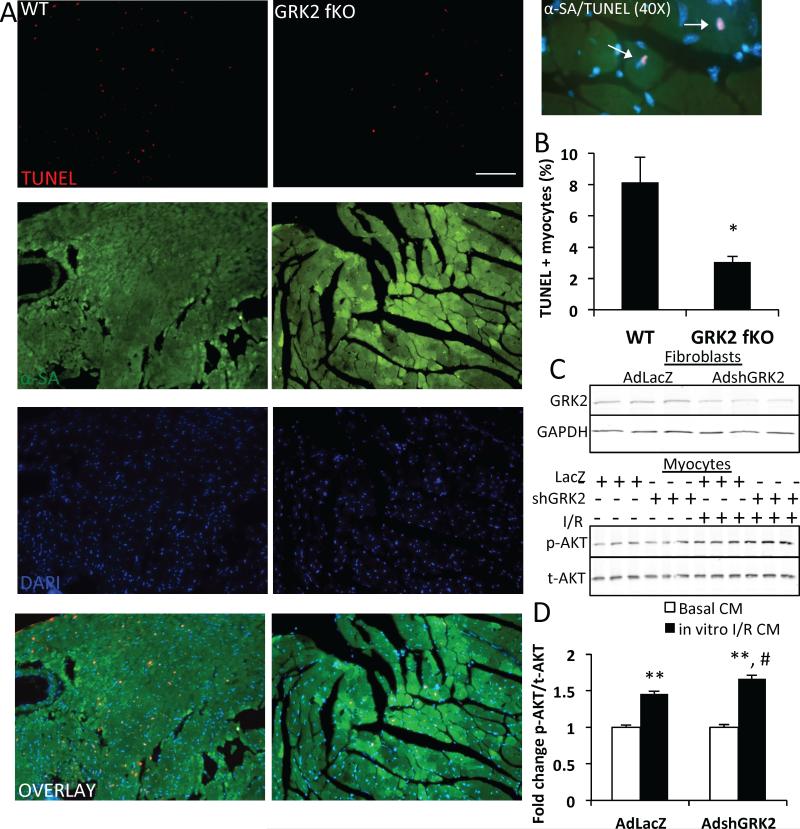
Because myocardial apoptosis is a contributing factor to I/R injury and the loss of viable myocardium, we evaluated TUNEL positivity in myocardial sections of post-I/R mice (Figure 3). WT and GRK2 fKO mice were subjected to 30 minutes of myocardial ischemia and 6 hours of reperfusion. TUNEL staining, along with α-sarcomeric actin staining for cardiomyocytes, was performed on heart sections and apoptosis levels were measured in the ischemic border zone of the infarcted area (Figure 3a). Quantification of these findings revealed that WT mice exhibited significantly more apoptotic cardiomyocytes with nearly three times the TUNEL positive myocytes of GRK2 fKO littermates (Figure 3b).
Figure 3. Apoptosis and survival signaling after fibroblast GRK2 ablation.
A) Representative photographs of TUNEL and α-sarcomeric actin (αSA) stained sections from the border zone of 6 hour post-I/R WT and GRK2 fKO hearts, including 40X image showing TUNEL and αSA+ cardiomyocytes (white arrows). Scale bar, 100 μm. α-sarcomeric actin (green), cardiomyocytes; TUNEL (red), apoptotic nuclei; DAPI (blue), total nuclei. B) TUNEL+ cardiomyocyte quantification represented as the percentage of TUNEL and αSA + cells in WT and GRK2 fKO. *P<0.05 vs. WT. N=4. C) Western blot demonstrating GRK2 knockdown in neonatal rat cardiac fibroblasts using an shRNA-containing adenovirus for GRK2 (top blot) and AKT levels in neonatal rat cardiac myocytes following conditioned media treatment (CM) from LacZ and shGRK2 adenovirus-treated fibroblasts at basal and following in vitro I/R (bottom blot). D) Quantified phosphorylated AKT normalized to total AKT in myocytes. **P<0.01 vs. Basal CM, #P<0.05 vs. AdLacZ in vitro I/R CM. N=3 separate experiments.
We also performed conditioned media experiments using both isolated myocytes and fibroblasts from neonatal rats (Figure 3c, d). Fibroblasts were treated with adenovirus containing an shRNA for GRK2 or a LacZ control adenovirus (Figure 3c top panel), exposed to an in vitro Kreb's buffer and reperfused with DMEM for 3 hours. The supernatant was collected, placed on myocytes and AKT signaling was measured (Figure 3C bottom panel). Conditioned media from LacZ adenovirus and in vitro I/R-treated fibroblasts increased phosphorylated AKT in myocytes compared to media from fibroblasts treated only with LacZ adenovirus. Knockdown of GRK2 in cardiac fibroblasts potentiated this increase in phosphorylated AKT in myocytes after treatment with in vitro I/R fibroblast conditioned media, indicating that in vitro loss of GRK2 in fibroblasts is able to modify the fibroblast secretome to enhance survival signaling in myocytes (Figure 3d).
GRK2 ablation in fibroblasts inhibits I/R induced myocardial fibrosis and fibrotic gene upregulation
Because collagen deposition by cardiac fibroblasts is linked to myocardial apoptosis, we wanted to investigate whether the decrease in apoptotic myocytes led to diminished collagen deposition. Masson's Trichrome staining was performed on heart sections after 30 minutes of ischemia and 72 hours of reperfusion. Fibrosis was measured in the infarcted area of the LV. As shown in Figure 4A, WT mice had a robust amount of reparative fibrosis (16.1±1.4% of the total area quantified) following I/R as compared to Sham treated animals. Correlating with decreased myocardial cell death, the GRK2 fKO group also presented with diminished collagen deposition (4.9±1.8% of the total area quantified). Independently, we also used the hydroxyproline assay to measure collagen levels in equal weights of heart tissue taken from the LV 72 hours after reperfusion (Figure 4b). WT hearts exhibited an increase in hydroxyproline levels (0.2049±0.0148 ug/mg tissue) compared to sham animals (0.1347±0.0013 and 0.1285±0.0073 ug/mg tissue). Like the Masson's Trichrome results, myocardial tissue from the GRK2 fKO group had decreased hydroxyproline levels (0.1407±0.0037 ug/ml tissue) compared to the WT post-I/R. We also measured mRNA levels of Collagen I and Collagen III in myocardium taken from the LV and found that while both collagen types were significantly upregulated (3 fold and 27 fold increase, respectively) in the WT mice, the GRK2 fKO mouse heart tissue contained significantly decreased levels of both Collagen I and Collagen III (1.25 fold and 11 fold increase, respectively)(Figure 4c). Attenuated levels of fibrosis were maintained in the GRK2 fibroblast knockout mouse (10.02±0.79%) after 4 weeks of reperfusion compared to wild type littermates (17.2±1.5%)(Figure 4d). Importantly, decreased fibrosis levels did not cause cardiac rupture and benefits to systolic function were still detectable in the GRK2 fKO mouse one week and four weeks after I/R injury (Table 1).
Figure 4. Fibrosis and fibrotic gene expression in GRK2 fKO mice.
A) Representative Masson's Trichrome images and quantification of the infarct area 72 hours post-I/R. Quantification expressed as a percentage of fibrosis from the total area. Scale bar, 50μm ***P<0.001 vs. WT. N=4-5. B) Quantitative analysis of collagen with hydroxyproline assay. **P<0.01 vs. Sham, #P<0.01 vs. WT I/R. N=3-7. C) mRNA levels of Collagen I and III measured by RTPCR normalized to 18S; fold change vs. WT Sham. *P<0.05 vs. Sham, #P<0.05 vs. WT I/R. N=3 Sham, 6 I/R. D) Representative Masson's Trichrome images and quantification of the infarct area 4 weeks post I/R. Quantification expressed as a percentage of fibrosis from the total area. Scale bar, 50μm **P<0.01 vs WT. N=5.
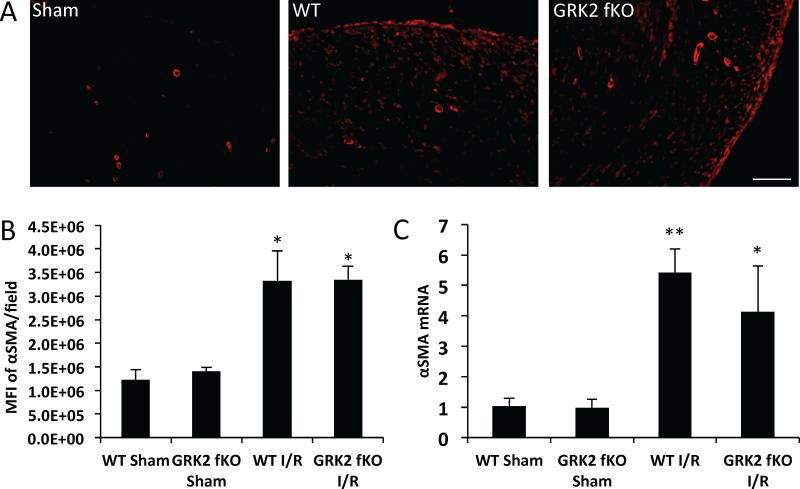
Following myocardial injury, fibroblasts undergo phenotypic changes that result in the transformation to the α-smooth muscle actin expressing myofibroblast; this change is also important for the increase in collagen production necessary for wound healing. In order to investigate if the decrease in collagen production seen in the GRK2 fKO mice is caused by the inability of fibroblasts to transform into myofibroblasts, we performed immunohistochemistry and RTPCR. After 72 hours reperfusion, mouse hearts were sectioned, probed for α-smooth muscle actin and the level of fluorescence intensity was measured using ImageJ software (Figure 5a). Interestingly, we found that both WT and GRK2 fKO mice expressed equal amounts of α-smooth muscle actin protein with both groups demonstrating a 2.64 fold increase (Figure 5b). We also found similar increases in α-SMA mRNA expression in both the WT and GRK2 fKO mice (Figure 5c).
Figure 5. Myofibroblast transformation and α-smooth much actin (αSMA) mRNA levels in WT and GRK2 fKO hearts following I/R.
A) Representative immunofluorescence images of sections stained with α-SMA. Scale bar, 100 μm. B) Quantification of the mean fluorescence intensity (MFI) of α-SMA. *p<0.05 vs. Sham. N=3-4. C) mRNA levels of α-SMA measured by RTPCR normalized to 18S; fold change vs. WT Sham. *p<0.05 vs. Sham, **p<0.01 vs. Sham, N=3 Sham, 11-12 I/R.
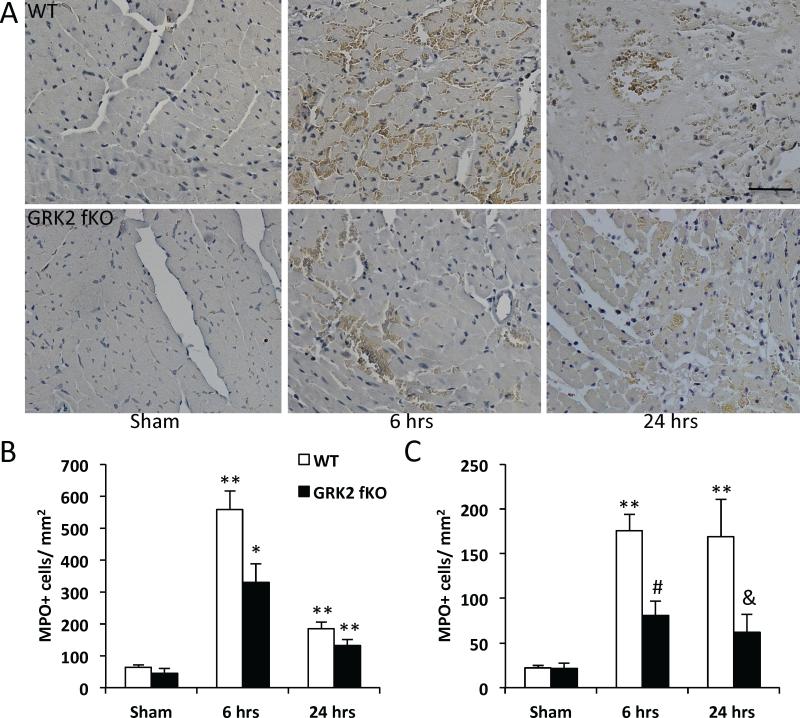
The first 48 hours following myocardial injury are marked by inflammation and diapedesis of immune cells; of these cells, neutrophils are the first to migrate to the infarcted area and have been implicated in negative repercussions for cardiomyocyte survival.29 Therefore, we assessed whether the decreases in infarct size in the GRK2 fKO mouse could be correlated to a reduction in neutrophil extravasation. To answer this question we performed immunohistochemistry for myeloperoxidase (MPO), an enzyme abundantly expressed by neutrophils. After 6 and 24 hours of reperfusion, hearts were stained for MPO; MPO-positive cells were quantified (Figure 6 and Online Figure II). After 6 hours, WT mice exhibited a robust accumulation of MPO-positive neutrophils in both the border zone and infarct area (557±59 and 176±17, respectively compared to sham groups) (Figure 6b, c). Similar results were seen at 24 hours post reperfusion for the border zone and infarct area (183±22 and 169±42, respectively compared to sham groups). Conversely, the GRK2 fKO hearts displayed attenuated neutrophil accumulation at 6 hours post I/R (329±56 in the border zone and 80±16 in the infarct area). The GRK2 fKO group also had decreased neutrophil counts at 24 hours post I/R in the border and infarct zones (132±18 and 61±20, respectively). The same experiment performed at 6 hours in the myocyte-specific KO αMHC-Cre/GRK2flox mouse line showed no differences between groups indicating that this is a fibroblast-specific event (Online Figure IIIa,b).
Figure 6. Neutrophil invasion following I/R in WT and GRK2 fKO mice.
A) Representative images of the border zone of myeloperoxidase (MPO) staining on hearts 6 and 24 hours post I/R. Scale bar, 50 μm. B) Quantification of MPO+ cells/field in the border and C) infarct area. *P<0.05 vs. Sham, **P<0.01 vs. Sham, #P<0.05 vs. WT 6 hrs, &P<0.01 vs. WT 24 hrs. N=3-4 for Sham, N=10-12 for I/R.
We first explored the possibility that expression levels of the neutrophil-recruiting CXC chemokines were affected by GRK2 ablation. Surprisingly, we found no changes in mRNA levels of CXCL1, CXCL2 or CXCL5 (Online Figure IV). Because inflammation is a complex process characterized by the activation of multiple cytokines, we turned to the Mouse Cytokine Antibody Array to glean further insight into what cytokines may be altered in the GRK2 fKO mouse after I/R injury. Adult cardiac fibroblasts were isolated from WT and GRK2 fKO mice, treated with in vitro Kreb's buffer and reperfused with DMEM. After 6 hours, the DMEM was collected and used in the Cytokine Antibody Array kit (Figure 7, Online Figure V). We noted that Tumor Necrosis Factor α (TNFα), a potent activator of inflammation, was downregulated by 30% in the media obtained from post-ischemic GRK2 fKO purified adult mouse fibroblasts (Figure 7a). Using RTPCR, we investigated mRNA levels of TNFα in myocardial tissue taken from 6 hour post ischemic hearts and found that while the WT hearts had an 3.7 fold increase in TNFα mRNA, the GRK2 fKO hearts presented with reduced TNFα with only a 1.3 fold increase (Figure 7b). There were other changes in the microarray, including decreases in IL6 and IL1β, both important for inflammation after myocardial ischemia/reperfusion injury. However, we were not able to resolve differences in mRNA expression levels as with TNFα (data not shown). There were also decreases in IL4 and IL5, however, the role of these cytokines in ischemia/reperfusion injury is less defined and so we focused on TNFα. Next, we purified GRK2 flox +/+ adult mouse cardiac fibroblasts treated with Cre adenovirus to knockdown GRK2 levels. In agreement with our in vivo RTPCR data, we found that levels of secreted TNFα were also decreased after in vitro I/R, compared to control fibroblasts treated with LacZ adenovirus (Figure 7c,d). NFκB is known to regulate TNFα transcription; therefore we were curious to see if GRK2 was affecting the ability of NFκB to enter the nucleus. We treated neonatal rat cardiac fibroblasts with shGRK2 adenovirus to knockdown GRK2 expression, subjected the cells to in vitro I/R and used immunofluorescence to track the movement of the p65 subunit of NFκB. Only in post-ischemic cells treated with control LacZ adenovirus was NFκB able to move into the nucleus. Nuclear translocation was prevented in post-ischemic cells lacking GRK2 (Figure 7E). Importantly, increased levels of cAMP, a second messenger that is indirectly affected by GRK2 activity, have been shown to prevent TNFα expression. To ascertain if this is a potential mechanism, we infected neonatal rat cardiac fibroblasts with shGRK2 adenovirus, treated with isoproterenol and measured fold changes in cAMP versus the basal control. Fibroblasts lacking GRK2 had an enhanced fold change compared to the isoproterenol treated fibroblasts infected with LacZ adenovirus (Figure 7F).
Figure 7. TNFα levels in post ischemic WT and GRK2 fibroblast knockout groups.
A) Post-ischemic conditioned media from WT and GRK2 fKO purified adult mouse cardiac fibroblasts against TNF on the Mouse Cytokine Micro Array. B) mRNA levels of TNFα measured by RTPCR in whole hearts from Sham and 6 hours post I/R WT and GRK2 fKO mice, normalized to 18S; fold change vs. WT Sham. *P<0.05 vs. Sham, #P<0.05 vs. WT I/R. N=6-7. C) Western blot demonstrating GRK2 knockdown using Cre Adenovirus in purified GRK2 flox+/+ adult mouse cardiac fibroblasts. D) Soluble TNFα protein measured by ELISA in post-ischemic purified adult mouse cardiac fibroblasts. *P<0.05. N=4-6. E) Representative images of neonatal rat cardiac fibroblasts stained for p65 at baseline and after 30 minutes of in vitro ischemia/30 minutes of reperfusion and quantification of p65+/DAPI+ nuclei. Scale bar, 100 μm. **p<0.01 vs. AdLacZ Basal, *p<0.05 vs. AdLacZ I/R. N=3 separate experiments. F) Fold change of cAMP levels normalized to respective basal controls in neonatal rat cardiac fibroblasts after isoproterenol (Iso) treatment. **p<0.01 vs Basal. N=3 separate experiments.
DISCUSSION
Fibroblasts comprise a large proportion of the non-myocyte cells in the heart, which varies from species to species but constitutes approximately 26% of the total cells in the murine heart.30 Initially thought to be a relatively inert stromal cell, recent publications have expanded on favorable and adverse fibroblast contributions after myocardial I/R injury. For example, following ischemic insult, cardiac fibroblasts undergo mesenchymal to endothelial transition, contributing to beneficial neovascularization of the injured myocardium.28 Additionally, inflammasome activation in the cardiac fibroblast has been shown to be critical for the detrimental inflammatory response seen following I/R injury.31 To our knowledge, this is the first study to investigate the in vivo effects of GRK2 ablation in the fibroblast following myocardial ischemic injury. This mouse model is beneficial because the Cre is driven by a fibroblast-specific regulatory sequence, which prevents off target effects to other collagen-expressing cell types, like vascular smooth muscle cells. Our mouse model is not cardiac specific and will incur the loss of GRK2 in all fibroblasts. However, this study will focus on myocardial specific effects of GRK2 ablation in cardiac fibroblasts following ischemic injury. We have shown here, using an inducible and cell-selective transgenic mouse model, that the absence of fibroblast GRK2 in the post-ischemic heart confers a protective advantage for cardiomyocyte survival and cardiac function. In the setting of I/R, these cardio-protective effects can be attributed to decreased neutrophil infiltration and reduced TNFα production in the infarcted tissue resulting in smaller infarct size and enhanced contractility due to more viable myocardium.
Neutrophils are the most abundant type of white blood cell and are a part of the innate immune system; they are the first immune cells to respond to injury or infection. Multiple studies have demonstrated that neutrophils are the main source of ROS in the reperfused heart, lending a negative connotation to the presence of this cell type that is typically thought of as a beneficial member of the inflammatory environment.32,33 Tumor Necrosis Factor α (TNFα) is a pleiotropic and potent activator of inflammation and is involved in neutrophil chemotaxis to the injury area.34 Cardiac fibroblasts secrete TNFα in response to different types of injury, like mechanical strain and hypoxia.2 While latent TNFα is tethered to the external plasma membrane for immediate availability via proteolytic cleavage, nascent production of TNFα is also achieved via activation of the NFκB transcription factor.35 In macrophages, GRK2 has been shown to intersect with NFκB signaling through phosphorylation of non-traditional sites of the inhibitor of NFκB, IκB, inducing degradation and subsequent release and nuclear localization of NFκB.36 This is notable in light of our data showing a reduction in TNFα mRNA following in vivo I/R in our GRK2 fKO mice. We also see in neonatal rat cardiac fibroblasts that post-ischemic nuclear localization of NFκB is prevented when GRK2 is ablated which speaks to another non-classical role for GRK2 in an injury setting.
β2-ARs have been documented to be anti-inflammatory. In the setting of murine acute lung injury, β2-AR agonists reduced infiltrating neutrophils and TNFα levels in the supernatant of LPS-treated isolated macrophages.37 Additionally, neutrophil infiltration in rat venule endothelial cells after substance P treatment was inhibited using the β2-AR agonist, formoterol.38 Cardiac fibroblasts in mice and humans are predominantly populated with the β2-AR subtype39, 40 and a major target of GRK2. Loss of GRK2 may maintain or even enhance β2-AR mediated anti-inflammatory signaling in the setting of ischemic injury, which could have translational significance in cardiac patients.
Additionally, there is evidence that cAMP inhibits TNFα production through the β2-AR.41 After LPS treatment, the selective cAMP-PDE IV inhibitor rolipram inhibited TNFα production.42 Conversely, treating murine macrophages with agents that elevate intracellular cAMP suppressed LPS-induced inflammatory gene expression, including TNFα.43 Mechanistically, elevated cAMP leads to activated PKA, which phosphorylates and facilitates the degradation of p105, the precursor of p50.44 These data hint at another potential mechanism for how decreased GRK2 can result in attenuated TNFα levels; by maintaining cAMP levels formed by preserved or enhanced β2-AR signaling. In line with this reasoning, we see that loss of GRK2 in the cardiac fibroblasts resulted in potentiated levels of cAMP compared to WT fibroblasts after isoproterenol treatment.
Our results suggesting pro-inflammatory actions of GRK2 conflict with other reports concluding GRK2 is anti-inflammatory. Myeloid cell-specific GRK2 knockdown increased tissue injury after LPS injection by enhancing NFκB1p105-ERK signaling45. Lymphocytes with reduced GRK2 levels show increased chemotaxis to CCL3, CCL4 and CCL546. However, while GRK2 hemizygous mice afflicted with experimental autoimmune encephalomyelitis (EAE) have a quicker disease onset than wild type EAE counterparts, GRK2 +/- mice do not suffer from relapses like wild type mice. The lack of relapses was correlated with attenuated inflammatory CNS infiltrates47. In addition, high levels of GRK2 directly correlate with the severity of Alzheimer's disease in human patients48. These conflicting reports on the inflammatory role of GRK2 indicate there is no definite answer and conclusions may be dependent on cell type and disease model.
Cardiac fibroblasts secrete collagen to maintain structural integrity after I/R-induced cardiomyocyte death. Initially, this is a critical reparative process but eventually excessive collagen deposition leads to myocardial stiffening and impedes contractility. Our present study demonstrates that GRK2 loss in fibroblasts decreases fibrosis after I/R. cAMP, in addition to its anti-inflammatory capabilities, also possesses anti-fibrotic qualities. Increased intracellular cAMP can attenuate the pro-fibrotic phenotype of in vitro cardiac myofibroblasts.49 However, it is likely in our case that decreased fibrosis is a reaction to limiting myocyte death in GRK2 fKO hearts after I/R. Regardless of the underlying cause, limiting myocardial apoptosis and fibrosis is a desirable outcome caused by decreased levels of fibroblast GRK2, noted previously in myocyte-specific GRK2 KO mice.50 Our conditioned media experiments suggest that fibroblasts secrete factors that influence cardiomyocyte survival after in vitro I/R and that GRK2 knockdown augments this effect. Interestingly, conditioned media from fibroblasts has been shown to improve in vitro cardiomyocyte viability as measured by mitochondrial dehydrogenase activity and troponin I levels. In this case, augmented cardiomyocyte survival was linked to TIMP1 activity; inhibiting TIMP1 abolished the effect.51 Fibroblast growth factor-2, a protein secreted by cardiac fibroblasts can improve contractility and tissue preservation in isolated perfused rat hearts; these cardioprotective qualities were attributed to increased AKT signaling.52 This is interesting in light of our findings that conditioned media from ischemic neonatal rat cardiac fibroblasts increased AKT signaling in neonatal rat myocytes. Cardiac fibroblasts are also the main source of IL33, the functional ligand of the ST2 receptor expressed basally on cardiomyocytes.53 IL33 is upregulated by Angiotensin II, the known activator of the ATII receptor, a GPCR desensitized by GRK2.53 IL33 is cardioprotective; this molecule prevented cardiomyocyte apoptosis, decreased infarct size and improved systolic function when administered to mice following ischemia/reperfusion injury.54 Loss of GRK2 in the cardiac fibroblast could augment the production of Angiotensin II induced IL33 to increase cardiomyocyte survival and preserve inotropy. Reduction in TNFα levels could also be playing a significant role in decreased myocyte apoptosis in the GRK2 fKO mice as cardiac-restricted overexpression of TNFα in mice has been shown to cause myocyte apoptosis and cardiac remodeling through the increased activation of cell death pathways.55
Another interesting aspect of our findings is that despite the reduced levels of fibrosis and fibrotic gene expression seen in the GRK2 fKO group, myofibroblast activation and α-SMA expression appears to be similar in both the WT and fKO mice. The events governing myofibroblast transformation and fibrotic genes are multi-factorial, with regards to GRK2, the cAMP axis is one signaling pathway important for regulating these events. Adenylate cyclase activation following GPCR activation leads to the formation of cAMP. cAMP can modulate responses through two signaling platforms, PKA and Epac. Swaney et al demonstrated that both cAMP and Epac activation by exogenous agents can blunt collagen synthesis, α-SMA expression and myofibroblast transformation in rat cardiac fibroblasts, indicating parallel roles.56 However, cAMP and Epac have opposing effects on fibroblast migration.57 These contrasts are most likely due to differences in protocols (ex vivo versus in vivo), species (rat versus mouse) and injury model (I/R versus exogenous fibrotic agents) but dysregulation of this dual signaling axis following I/R could explain these differences.
From a therapeutic standpoint, our results are significant because they reinforce the idea that inhibition of GRK2 in both myocytes and fibroblasts is beneficial for maintaining inotropic reserve. There is a vast body of literature demonstrating the protective effects of GRK2 ablation in the cardiac myocyte after ischemia, but limited data showing the benefits of GRK2 deletion in the cardiac fibroblast. The goal of this study was to investigate the acute and chronic effects of GRK2 ablation in the cardiac fibroblast; we have seen that fibroblast GRK2 knockdown prevents myocyte death and excessive inflammation and confers long-term benefits to cardiac function. A detailed investigation into the role of GRK2 during post-ischemic inflammation in the heart is important for future analysis. However, our current findings add depth to the various beneficial roles previously attributed to loss of GRK2, through classical or non-classical pathways.
Supplementary Material
Novelty and Significance.
What Is Known?
G protein-coupled receptor kinase 2 (GRK2) is the major GRK in the heart, and it is upregulated after cardiac injury and during heart failure.
The classical role of GRK2 in the heart is to phosphorylate and desensitize β-adrenergic receptors controlling myocyte contractility; non-classical roles for GRK2 have also been delineated in metabolism and insulin signaling.
Myocyte-specific inhibition of GRK2 before or after myocardial infarction can rescue heart function and prevent adverse remodeling.
What New Information Does This Article Contribute?
Inhibition of GRK2 via genetic ablation in the cardiac fibroblast, a major cell type in the heart, preserves cardiac contractility after ischemic injury in a mouse model.
Excessive fibrosis, increased myocyte death and inflammatory cell infiltration are also prevented.
The above beneficial effects are linked to decreased TNFα production and secretion caused by the attenuated ability of NF-κB to translocate to the nucleus in the cardiac fibroblast.
The beneficial effects of GRK2 inhibition in the myocyte after cardiac injury are well documented; however, the role of GRK2 in the cardiac fibroblast after ischemic injury has yet to be investigated. In this study, we show that in a mouse model, fibroblast-specific GRK2 deletion decreased infarct size and partially rescued the drop in contractility. Myocyte apoptosis and neutrophil extravasation were also reduced. Furthermore, we found that cardiac fibroblasts lacking GRK2 express and secrete diminished levels of TNFα, a critical modulator of inflammation. This correlates with the inability of NF-κB, a major regulator of TNFα transcription, to translocate to the nucleus after ischemia reperfusion injury when GRK2 is not present in the cardiac fibroblast. These data lend further credence to the idea that inhibition of GRK2 in the heart could be a potential therapy to treat ischemia heart disease.
ACKNOWLEDGEMENTS
We thank Z. Qu and J. Ibetti for technical support, J. Rabinowitz (AdLacZ, AdCre) and C. Murga (AdshGRK2) for adenoviruses and B. de Crombrugghe for the Col1α2CreER mouse.
SOURCES OF FUNDING
W.J.K is the W.W. Smith Chair in Cardiovascular Medicine at the Lewis Katz School of Medicine at Temple University. This project was supported by National Institute of Health (NIH) grant P01 HL091799. W.J.K. is also supported by NIH grants R37 HL061690, R01 HL085503, P01 HL075443 (Project 2) and P01 HL108806 (Project 3).
Nonstandard Abbreviations and Acronyms
- AKT
Protein kinase B
- βAR
β-Adrenergic Receptor
- Epac
exchange factor directly activated by cAMP
- GPCR
G protein-coupled receptor
- GRK2
G protein-coupled receptor kinase 2
- I/R
Ischemia/Reperfusion
- IκB
Inhibitor of κB
- KO
Knockout
- LPS
Lipopolysaccharide
- LV
Left Ventricle
- MPO
Myeloperoxidase
- NFκB
nuclear factor kappa-light-chain-enhancer of activated B cells
- PKA
Protein kinase A
- ROS
Reactive oxygen species
- TNFα
Tumor Necrosis Factor α
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Chen W, Frangogiannis NG. Fibroblasts in post-infarction inflammation and cardiac repair. Biochimica et Biophysica Acta. 2013;1833:945–953. doi: 10.1016/j.bbamcr.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Souders CA, Bowers SLK, Baudino TA. Cardiac fibroblast: the renaissance cell. Circulation Research. 2009;105:1164–1176. doi: 10.1161/CIRCRESAHA.109.209809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li P-F, Dietz R, von Harsdorf R. Superoxide induces apoptosis in cardiomyocytes, but proliferation and expression of transforming growth factor-β1 in cardiac fibroblasts. FEBS Letters. 1999;448:206–210. doi: 10.1016/s0014-5793(99)00370-1. [DOI] [PubMed] [Google Scholar]
- 4.Porter KE, Turner NA. Cardiac fibroblasts: At the heart of myocardial remodeling. Pharmacology & Therapeutics. 2009;123:255–278. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodeling. Nature Reviews Cardiology. 2014;11:255–265. doi: 10.1038/nrcardio.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacLean J, Pasumarthi KBS. Signaling mechanisms regulating fibroblast activation, phenoconversion and fibrosis in the heart. Indian Journal of Biochemistry & Biophysics. 2014;51:476–482. [PubMed] [Google Scholar]
- 7.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. Journal of Clinical Investigation. 2013;123:92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen PR. Role of neutrophils in myocardial ischemia and reperfusion. Circulation. 1995;91:1872–1885. doi: 10.1161/01.cir.91.6.1872. [DOI] [PubMed] [Google Scholar]
- 9.Romson JL, Hook BG, Kunkel SL, Abrams GD, Schork MA, Lucchesi BR. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation. 1983;67:1016–1023. doi: 10.1161/01.cir.67.5.1016. [DOI] [PubMed] [Google Scholar]
- 10.Hatori N, Roberts RL, Tadokoro H, Ryden L, Satomura K, Fishbein MC, Stiehm ER, Corday E, Drury JK. Differences in infarct size with lidocaine as compared with bretylium tosylate in acute myocardial ischemia and reperfusion in pigs. Journal of Cardiovascular Pharmacology. 1991;18:581–588. doi: 10.1097/00005344-199110000-00015. [DOI] [PubMed] [Google Scholar]
- 11.De Lorgeril M, Basmadjian A, Lavallée M, Clément R, Millette D, Rousseau G, Latour J-G. Influence of leukopenia on collateral flow, reperfusion flow, reflow ventricular fibrillation, and infarct size in dogs. American Heart Journal. 1989;117:523–532. doi: 10.1016/0002-8703(89)90724-2. [DOI] [PubMed] [Google Scholar]
- 12.Koch WJ, Rockman HA, Samama P, Hamilton RA, Bond RA, Milano CA, Lefkowitz RJ. Cardiac function in mice overexpressing the beta-adrenergic receptor kinase or a beta ARK inhibitor. Science. 1995;268:1350–1353. doi: 10.1126/science.7761854. [DOI] [PubMed] [Google Scholar]
- 13.Sato PY, Chuprun JK, Schwartz M, Koch WJ. The evolving impact of G protein-coupled receptor kinases in cardiac health and disease. Physiological Reviews. 2015;95:377–404. doi: 10.1152/physrev.00015.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raake PW, Vinge LE, Gao E, Boucher M, Rengo G, Chen X, DeGeorge BR, Jr, Matkovich S, Houser SR, Most P, Eckhart AD, Dorn GW II, Koch WJ. G protein coupled receptor kinase 2 ablation in cardiac myocytes before or after myocardial infarction prevents heart failure. Circulation Research. 2008;103:413–422. doi: 10.1161/CIRCRESAHA.107.168336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rengo G, Lymperopoulos A, Zincarelli C, Donniacuo M, Soltys S, Rabinowitz JE, Koch WJ. Myocardial adeno-associated virus serotype 6-betaARKct gene therapy improves cardiac function and normalizes the neurohormonal axis in chronic heart failure. Circulation. 2009;119:89–98. doi: 10.1161/CIRCULATIONAHA.108.803999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schumacher SM, Gao E, Zhu W, Chen X, Chuprun JK, Feldman AM, Tesmer JJG, Koch WJ. Paroxetine-mediated GRK2 inhibition reverses cardiac dysfunction and remodeling after myocardial infarction. Science Translational Medicine. 2015;7:1–12. doi: 10.1126/scitranslmed.aaa0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brinks H, Boucher M, Gao E, Chuprun JK, Pesant S, Raake PW, Huang ZM, Wang X, Qiu G, Gumpert A, Harris DM, Eckhart AD, Most P, Koch WJ. Level of G protein-coupled receptor kinase-2 determines myocardial ischemia/reperfusion injury via pro- and anti-apoptotic mechanisms. Circulation Research. 2010;107:1140–1149. doi: 10.1161/CIRCRESAHA.110.221010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang ZM, Gao E, Fonseca F, Hayashi H, Shang X, Hoffman NE, Chuprun JK, Tian X, Tilley DG, Madesh M, Lefer DJ, Stamler JS, Koch WJ. Convergence of G protein-coupled receptor and nitric oxide pathways determines the outcome to cardiac ischemia injury. Science Signaling. 2013;6:1–8. doi: 10.1126/scisignal.2004225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen M, Sato PY, Chuprun JK, Peroutka RJ, Otis NJ, Ibetti J, Pan S, Sheu S-S, Gao E, Koch WJ. Prodeath signaling of G protein-coupled receptor kinase 2 in cardiac myocytes after ischemia stress occurs via extracellular signal-regulated kinase-dependent heart shock protein 90-mediated mitrochondrial targeting. Circulation Research. 2013;112:1121–1134. doi: 10.1161/CIRCRESAHA.112.300754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato PY, Chuprun JK, Ibetti J, Cannavo A, Drosatos K, Elrod JW, Koch WJ. GRK2 compromises cardiomyocyte mitochondrial function by diminishing fatty acid-mediated oxygen consumption and increasing superoxide levels. Journal of Molecular and Cellular Cardiology. 2015;89:360–364. doi: 10.1016/j.yjmcc.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ciccarelli M, Chuprun JK, Rengo G, Gao E, Wei Z, Peroutka RJ, Gold J, Gumpert A, Chen M, Otis NJ, Dorn GW II, Trimarco B, Iaccarino G, Koch WJ. GRK2 activity impairs cardiac glucose uptake and promotes insulin resistance following myocardial ischemia. Circulation. 2011;123:1953–1962. doi: 10.1161/CIRCULATIONAHA.110.988642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng B, Zhang Z, Black CM, de Crombrugghe B, Denton CP. Ligand-dependent genetic recombination in fibroblasts. American Journal of Pathology. 2002;160:1609–1617. doi: 10.1016/S0002-9440(10)61108-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matkovich SJ, Diwan A, Klanke DA, Hammer DJ, Marreez Y, Odley AM, Brunskill EW, Koch WJ, Schwartz RJ, Dorn GW II. Cardiac-specific ablation of G-protein receptor kinase 2 redefines its roles in heart development and beta-adrenergic signaling. 2006;29:996–1003. doi: 10.1161/01.RES.0000247932.71270.2c. [DOI] [PubMed] [Google Scholar]
- 24.Lal H, Ahmad F, Zhou J, Yu J, Vagnozzi RJ, Guo Y, Yu D, Tsai EJ, Woodgett J, Gao E, Force T. Cardiac fibroblast GSK-3β regulates ventricular remodeling and dysfunction in ischemic heart. Circulation. 2014;130:419–430. doi: 10.1161/CIRCULATIONAHA.113.008364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao E, Lei YH, Shang X, Huang ZM, Zuo L, Boucher M, Fan Q, Chuprun JK, Ma XL, Koch WJ. A novel and efficient model of coronary artery ligation and myocardial infarction in the mouse. Circulation Research. 2010;107:1445–1453. doi: 10.1161/CIRCRESAHA.110.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vivaldi MT, Kloner RA, Schoen FJ. Triphenyltetrazolium staining of irreversible ischemic injury following coronary artery occlusion in rats. American Journal of Pathology. 1985;121:522–530. [PMC free article] [PubMed] [Google Scholar]
- 27.Punn A, Mockridge JW, Farooqui S, Marber MS, Heads RJ. Sustained activation of p42/p44 mitogen-activated protein kinase during recovery from simulated ischaemia mediates adaptive cytoprotection in cardiomyocytes. Biochemistry Journal. 2000;350:891–899. [PMC free article] [PubMed] [Google Scholar]
- 28.Ubil E, Duan J, Pillai ICL, Rosa-Garrido M, Wu Y, Bargiacchi F, Lu Y, Stanbouly S, Huang J, Rojas M, Vondriska TM, Stefani E, Deb A. Mesenchymal-endothelial transition contributes to cardiac neovascularization. Nature. 2014;514:585–590. doi: 10.1038/nature13839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. Journal of Molecular and Cellular Cardiology. 2010;48:504–511. doi: 10.1016/j.yjmcc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banerjee I, Fuseler JW, Price RL, Borg TK, Baudino TA. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. American Journal of Physiology: Heart and Circulatory Physiology. 2007;293:H1883–H1891. doi: 10.1152/ajpheart.00514.2007. [DOI] [PubMed] [Google Scholar]
- 31.Kawaguchi M, Takahashi M, Hata T, Kashima Y, Usui F, Morimoto H, Izawa A, Takahashi Y, Masumoto J, Koyama J, Hongo M, Noda T, Nakayama J, Sagara J, Taniguchi S, Ikeda U. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation. 2011;123:594–604. doi: 10.1161/CIRCULATIONAHA.110.982777. [DOI] [PubMed] [Google Scholar]
- 32.Duilio C, Ambrosio G, Kuppusamy P, Dipaula A, Becker LC, Zweier JL. Neutrophils are primary source of O2 radicals during reperfusion after prolonged myocardial ischemia. American Journal of Physiology: Heart and Circulatory Physiology. 2001;280:H2649–H2657. doi: 10.1152/ajpheart.2001.280.6.H2649. [DOI] [PubMed] [Google Scholar]
- 33.Mitsos SE, Askew TE, Fantone JC, Kunkel SL, Abrams GD, Schork A, Lucchesi BR. Protective effects of N-2-mercaptopropionyl glycine against myocardial reperfusion injury after neutrophil depletion in the dog: evidence for the role of intracellular-derived free radicals. Circulation. 1986;73:1077–1086. doi: 10.1161/01.cir.73.5.1077. [DOI] [PubMed] [Google Scholar]
- 34.Feldman AM, Combes A, Wagner D, Kadakomi T, Kubota T, Li YY, McTiernan C. The Role of Tumor Necrosis Factor in the Pathophysiology of Heart Failure. Journal of the American College of Cardiology. 2000;35:537–544. doi: 10.1016/s0735-1097(99)00600-2. [DOI] [PubMed] [Google Scholar]
- 35.Shakov AN, Collart MA, Vassalli P, Nedospasov SA, Jongeneel CV. Kappa B-type enhancers are involved in lippolysaccharide-mediated transcriptional activation of the tumor necrosis factor alpha gene in primary macrophages. Journal of Experimental Medicine. 1990;171:35–47. doi: 10.1084/jem.171.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patial S, Luo J, Porter KJ, Benovic JL, Parameswaran N. G-protein coupled receptor kinases mediate TNFα-induced NFκB signaling via direct interaction with and phosphorylation of IκBα. Biochemistry Journal. 2009;425:169–178. doi: 10.1042/BJ20090908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bosmann M, Grailer JJ, Zhu K, Matthay MA, Sarma JV, Zetoune FS, Ward PA. Anti-inflammatory effects of β2adrenergic receptor agonists in experimental acute lunch injury. FASEB Journal. 2012;26:2137–2144. doi: 10.1096/fj.11-201640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bowden JJ, Sulakvelidze I, McDonald DM. Inhibition of neutrophil and eosinophil adhesion to venules of rat trachea by β2adrenergic agonist formoterol. Journal of Applied Physiology. 1994;77:397–405. doi: 10.1152/jappl.1994.77.1.397. [DOI] [PubMed] [Google Scholar]
- 39.Meszaros JG, Gonzalez AM, Endo-Mochizuki Y, Villegas S, Villarreal F, Brunton LL. Identification of G protein-coupled signaling pathway in cardiac fibroblasts: cross talk between Gq and Gs. American Journal of Physiology Cell Physiology. 2000;278:C154–C162. doi: 10.1152/ajpcell.2000.278.1.C154. [DOI] [PubMed] [Google Scholar]
- 40.Turner NA, Porter KE, Smith WHT, White HL, Ball SG, Balmforth AJ. Chronic β2-adrenergic receptor stimulation increases proliferation of human cardiac fibroblasts via an autocrine mechanism. Cardiovascular Research. 2003;57:784–792. doi: 10.1016/s0008-6363(02)00729-0. [DOI] [PubMed] [Google Scholar]
- 41.Zidek Z. Adenosine-cyclic AMP pathways and cytokine expression. European Cytokine Network. 1999;10:319–328. [PubMed] [Google Scholar]
- 42.Prabhakar U, Lipshutz D, Bartus JO, Slivjak MJ, Smith EF, 3rd, Lee JC, Esser KM. Characterization of cAMP-dependent inhibition of LPS-induced TNF alpha production by rolipram, a specific phosphodiesterase IV (PDE IV) inhibitor. International Journal of Immunopharmacology. 1994;16:805–816. doi: 10.1016/0192-0561(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 43.Tannenbaum CS, Hamilton TA. Lipopolysaccharide-induced gene expression in murine peritoneal macrophages is selectively suppressed by agents that elevate intracellular cAMP. The Journal of Immunology. 1989;142:1274–1280. [PubMed] [Google Scholar]
- 44.Wall EA, Zavzavadjian JR, Chang MS, Randhawa B, Zhu X, Hsueh RC, Lui J, Driver A, Bao XR, Sternweis PC, Simin MI, Fraser IDC. Suppression of LPS-induced TNF-α production in macrophages by cAMP is mediated by PKA-AKAP95-p105. Science Signaling. 2009;2:ra28. doi: 10.1126/scisignal.2000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patial S, Saini Y, Parvataneni S, Appledorn DM, Dorn GW, II, LaPres JJ, Amalfitano A, Senagore P, Parameswaran N. Myeloid-specific GPCR kinase-2 negatively regulates NFκB1p105-ERK pathway and limits endotoxemic shock in mice. Journal of Cellular Physiology. 2011;226:627–637. doi: 10.1002/jcp.22384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vroon A, Heijnen CJ, Lombardi MS, Cobelens PM, Mayor F, Jr, Caron MG, Kavelaars A. Reduced GRK2 level in T cells potentiates chemotaxis and signaling in response to CCL4. Journal of Leukocyte Biology. 2004;75:901–909. doi: 10.1189/jlb.0403136. [DOI] [PubMed] [Google Scholar]
- 47.Vroon A, Kavelaars A, Limmroth V, Lombardi MS, Goebel MU, Van Dam A-M, Caron MG, Schedlowski M, Heijnen CJ. G protein-coupled receptor kinase 2 in multiple sclerosis and experimental autoimmune encephalomyelitis. Journal of Immunology. 2005;174:4400–4406. doi: 10.4049/jimmunol.174.7.4400. [DOI] [PubMed] [Google Scholar]
- 48.Leosco D, Fortunato F, Rengo G, Iaccarino G, Sanzari E, Golino L, Zincarelli C, Canonico V, Marchese M, Koch WJ, Rengo F. Lymphocyte G-protein-couple receptor kinase-2 is upregulated in patients with Alzheimer's disease. Neuroscience Letters. 2007;415:279–282. doi: 10.1016/j.neulet.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 49.Lu D, Aroonsakool N, Yokoyama U, Patel HH, Insel PA. Increase in cellular cyclic AMP concentrations reverses the profibrogenic phenotype of cardiac myofibroblasts: a novel therapeutic approach for cardiac fibrosis. Molecular Pharmacology. 2013;84:787–793. doi: 10.1124/mol.113.087742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fan Q, Chen M, Zuo L, Shang X, Huang ZM, Ciccarelli M, Raake P, Brinks H, Chuprun JK, Dorn GW II, Koch WJ, Gao E. Myocardial ablation of G protein-coupled receptor kinase 2 (GRK2) decreases ischemia/reperfusion injury through an anti-intrinsic apoptotic pathway. Plos ONE. 2013;8:e66234. doi: 10.1371/journal.pone.0066234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abrial M, Da Silva CC, Pillot B, Augeul L, Ivanes F, Teixeira G, Cartier R, Angoulvant D, Ovize M, Ferrera R. Cardiac fibroblasts protect cardiomyocytes against lethal ischemia-reperfusion injury. Journal of Molecular and Cellular Cardiology. 2014;68:56–65. doi: 10.1016/j.yjmcc.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 52.Jiang Z-S, Wen G-B, Tang Z-H, Srisakuldee W, Randrich RR, Kardami E. High molecular weight FGF-2 promotes postconditioning-like cardioprotection linked to activation of the protein kinase C isoforms and p70 S6 kinase. Canadian Journal of Physiology and Pharmacology. 2009;87:798–804. doi: 10.1139/Y09-049. [DOI] [PubMed] [Google Scholar]
- 53.Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie ANJ, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. The Journal of Clinical Investigation. 2007;117:1538–1549. doi: 10.1172/JCI30634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seki K, Sanada S, Kudinova AY, Steinhauser ML, Handa V, Gannon J, Lee RT. Interleukin-33 prevents apoptosis and improves survival after experimental myocardial infarction through ST2 signaling. Circulation Heart Failure. 2009;2:684–691. doi: 10.1161/CIRCHEARTFAILURE.109.873240. [DOI] [PubMed] [Google Scholar]
- 55.Haudek SB, Taffet GE, Schneider MD, Mann DL. TNF provokes cardiomyocyte apoptosis and cardiac remodeling through activation of multiple cell death pathways. Journal of Clinical Investigation. 2007;117:2692–2701. doi: 10.1172/JCI29134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Swaney JS, Roth DM, Olson ER, Naugle JE, Meszaros JG, Insel PA. Inhibition of cardiac myofibroblast formation and collagen synthesis by activation and overexpression of adenylyl cyclase. Proceedings of the National Academy of Sciences. 2005;102:437–442. doi: 10.1073/pnas.0408704102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yokoyama U, Patel HH, Lai NC, Aroonsakool N, Roth DM, Insel PA. The cyclic AMP effector Epac integrates pro- and anti-fibrotic signals. Proceedings of the National Academy of Sciences. 2008;105:6386–6391. doi: 10.1073/pnas.0801490105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.