Abstract
Background
Links between food allergens and eosinophilic esophagitis have been established, but the interplay between EoE and IgE-associated immediate hypersensitivity to foods remains unclear.
Objective
We sought to determine the prevalence of IgE-associated food allergy at time of diagnosis of EoE in children and to determine if differences existed in presentation and disease compared to subjects with EoE alone.
Methods
EoE patients were stratified based on diagnosis of IgE-associated immediate hypersensitivity (EoE+IH versus EoE-IH). Clinical, histologic, pathologic, and endoscopic differences were investigated using a retrospective database.
Results
We found that 29% of the 198 EoE patients in our cohort had EoE+IH. These subjects presented at a younger age than those without IH (6.05 years vs 8.09 years, p=0.013) and were more likely to have comorbid allergic disease. Surprisingly, the EoE+IH group presented with significantly different clinical symptoms, with increased dysphagia, gagging, cough, and poor appetite compared to their counterparts in the EoE-IH group. Male gender, allergic rhinitis, the presence of dysphagia, and younger age were independently associated with having EoE+IH. Specific IgE levels to common EoE-associated foods were higher in EoE+IH, regardless of eliciting immediate hypersensitivity symptoms. In contrast, IgE levels for specific foods triggering EoE were relatively lower in both groups than IgE levels for immediate reactions.
Conclusions & Clinical Relevance
Immediate hypersensitivity is common in children with EoE, and identifies a population of EoE patients with distinct clinical characteristics. Our study describes a subtype of EoE in which IgE-mediated food allergy may impact the presentation of pediatric EoE.
Keywords: eosinophilic esophagitis, food allergy, IgE, food sensitization
Introduction
Eosinophilic esophagitis (EoE) is a chronic allergic inflammatory disorder, characterized by eosinophil-predominant inflammation in the esophagus with notable increasing prevalence in both the pediatric and adult populations [1-3]. This trend reflects the pattern of many atopic diseases including eczema, allergic rhinitis, and IgE-mediated food allergy [4], but the cross talk between EoE and co-morbid atopic diseases, particularly IgE-mediated food allergy, remains unclear. A recent comprehensive review concluded that EoE was most likely not IgE-mediated, but this conclusion was largely based on evidence that included the inability of allergy testing to predict trigger foods and the failure of IgE-targeted therapies [5]; however, there have been few studies that have directly addressed this interplay.
Several studies seem to support a causal link between food allergen exposures and esophageal eosinophilia [6, 7]. An early study showed that children with EoE achieved remission of their clinical symptoms and esophageal inflammation when placed on an exclusively amino-acid based diet [8]. This idea has been reinforced by studies that removed specific food antigens and demonstrated subsequent improvement in the symptoms and pathology of EoE [9-11]. Recently, there have been reports of patients with IgE-mediated food allergy undergoing oral immunotherapy and then developing clinical symptoms of esophageal dysfunction and esophageal eosinophilia consistent with EoE [12-15]. Together, these studies help support the conclusions that food antigens are a major contributor to the pathogenesis of EoE and that links between IgE-mediated food allergy and EoE are likely.
Despite these associations, the utility of allergy testing for determining the trigger foods of EoE has been the subject of extensive debate. Current practices suggest that specific IgE quantification, skin prick testing and/or atopy patch testing be considered as part of a patient's evaluation. However, the results of studies that have evaluated the utility of these techniques have been highly variable with a wide range of responsiveness reported in both pediatric and adult populations [9, 16-18].
The prevalence of IgE-associated immediate hypersensitivity (i.e. IgE-mediated food allergy) within the EoE population is unclear. While some reports have used the term “food allergy” to describe any adverse reaction to food that leads to eosinophilic disease in the esophagus [19, 20], the studies in which food allergy is used precisely to describe IgE-mediated immediate hypersensitivity have limitations. Sugnanam et al, reported that 24% of their EoE cohort had anaphylaxis, but the study was limited to 45 patients [21]. In contrast, Spergel et al evaluated a much larger number of patients, but their study was limited by patient self-reported history of immediate reactions [16] rather than physician-diagnosed assessment.
In this study, we evaluated a large retrospective cohort of pediatric EoE patients to determine the clinical, endoscopic, histologic, and laboratory differences amongst patients who had EoE with evidence of IgE-mediated food allergy, which we will refer to as IgE-associated immediate hypersensitivity reactions (EoE+IH), compared to those who had EoE with no immediate hypersensitivity to foods (EoE-IH). Our findings suggest that IgE-associated immediate hypersensitivity is common within our pediatric EoE cohort and that these subjects exhibit a strikingly different clinical presentation. In addition, subjects with EoE+IH have significantly higher specific IgE to a number of foods compared to those who have EoE-IH, regardless of whether that food had been demonstrated to trigger clinical symptoms of immediate hypersensitivity or EoE. Furthermore, we demonstrate that for both cohorts IgE values to foods that had been identified as being EoE-trigger foods were generally low, particularly when compared to specific IgE values for immediate hypersensitivity food-allergic triggers. Consequently, our data support the conclusions that IgE is most likely not necessary for EoE disease progression but does define a subgroup of EoE patients with IgE-associated reactions to foods and in whom the presentation of EoE is dramatically altered. We therefore propose that EoE+IH may reflect a unique endotype of EoE.
Methods
Cohort Selection
A retrospective chart review was conducted using an existing electronic database of patients who underwent evaluation at the Eosinophilic Gastrointestinal Diseases Clinic at the Ann & Robert H. Lurie Children's Hospital of Chicago (previously Children's Memorial Hospital) between 2004 and 2014. Included patients were under the age of 18. The majority of patients were seen by an allergist-immunologist in addition to a gastroenterologist and underwent skin prick testing (SPT) to a panel of aeroallergens and various foods based on diet, clinical history, and potential treatment options. The majority of patients also underwent serologic assessment of food-specific IgE values.
Inclusion Criteria
Patients were included in this study if pathology and endoscopy reports were available in the electronic medical record to assess for EoE and if sufficient clinical information was available to identify the presence or absence of IgE-associated immediate hypersensitivity reactions. Subjects were excluded from the study if there was insufficient information in the medical record to assess for IgE-associated reactions, if the diagnosis of EoE could not be confirmed, or if other gastrointestinal diseases including eosinophilic gastroenteritis and PPI-responsive EoE (PPIREE) were present. The first 300 charts randomly selected from the electronic database were reviewed for inclusion in this study.
Clinical Definitions
The confirmation of the diagnosis of EoE was based on the presence of clinical symptoms of esophageal dysfunction and esophageal biopsies with ≥15 eosinophils per high-powered field after other causes of esophageal eosinophilia (particularly reflux) were excluded. Patients who underwent endoscopy and were found to have GERD, celiac disease, or a functional etiology were classified as non-EoE controls and were excluded from the analyses. IgE-associated reactions were defined either by an oral food challenge or by the presence of both a history of immediate clinical reactivity to a food (e.g. cutaneous, respiratory, cardiovascular, and/or gastrointestinal symptoms) within 2 hours of food ingestion and the detection of specific IgE by skin prick test and/or serum IgE testing. An EoE-trigger food was defined by elimination of the food from the diet leading to symptomatic and histologic remission of EoE followed by disease recrudescence upon reintroduction of the food into the diet. Food-specific IgE levels and SPT information were obtained by chart review from allergy testing performed during the EoE evaluation (i.e. the initial visit to the multidisciplinary EGID clinic). For each food, only children with a specific IgE level greater than the 95% PPV, as outlined by Sampson and Ho, were considered to have positive IgE findings [22]. All charts were reviewed by an allergy-immunology physician.
For all subjects in the study, demographic and clinical data-including the assessment of IgE-associated immediate hypersensitivity-were obtained by chart review. Information from pathology reports from the diagnostic endoscopy, as well as endoscopy reports of the gross visual findings, were collected. Symptoms of EoE were assessed by physician-guided questions at the time of the diagnostic endoscopy or by chart review by a GI physician within 2 weeks of the endoscopy prior to treatment changes. This study was approved by the institutional review board of the Ann & Robert H. Lurie Children's Hospital of Chicago.
Statistical Analysis
For all statistical analyses, patients were separated and compared based on the presence or absence of IgE-associated immediate hypersensitivity reactions. Continuous variables were compared with two-tailed paired t-tests. Categorical variables were compared using chi-squared analyses. Binary logistic regression modeling was used to ascertain the effects of statistically significant variables in predicting the presence or absence of EoE+IH while controlling for the other variables in the equation. For all associations, a p value of <0.05 was considered statistically significant. Statistical analysis was performed using Microsoft Excel (Microsoft Corporation, Redmond, WA), GraphPad Prism (GraphPad Software, San Diego, CA), and IBM SPSS for Windows (IBM Corporation, Armonk, NY).
Results
Clinical Characteristics
Since the overlap between eosinophilic esophagitis and IgE-associated food allergy has not been examined in great detail, we first determined the prevalence of IgE-associated immediate hypersensitivity reactions in our cohort of pediatric EoE subjects. Of 300 charts that were reviewed, 244 met inclusion criteria for this study. Of these, 57 (29%) had EoE with IgE-mediated food allergy (EoE+IH) while 141 (71%) had EoE with no evidence of IgE-mediated immediate hypersensitivity reactions (EoE-IH) (Table 1). The remaining 46 subjects were excluded as not possessing sufficient information to meet diagnostic inclusion criteria or due to exclusion criteria for coincident conditions including eosinophilic gastroenteritis. As is typical for EoE and food allergy, both subgroups were predominantly male and white, and there were no statistically significant differences among the racial backgrounds. Surprisingly, given the reports that children who outgrow IgE-associated food allergy develop EoE in later years [23], the EoE+IH group presented at a significantly younger age (6.05 years) than the EoE-IH cohort (8.09 years).
Table 1. Demographics and baseline atopic characteristics for study subjects.
| Total w EoE | EoE+IH | EoE-IH | P value | |
|---|---|---|---|---|
| Number, (%) | 198 | 57 (29) | 141 (71) | NA |
| Male, no (%) | 148 (74.7) | 47 (82.5) | 101 (71.6) | 0.11 |
| Average age in years, (range) | 7.7 (0.7-18.2) | 6.05 (0.8-17.6) | 8.09 (0.7-17.9) | 0.013* |
| Race | ||||
| White, no (%) | 169 (85.4) | 47 (82.5) | 122 (86.5) | 0.466 |
| African American, no (%) | 13 (6.6) | 5 (8.8) | 8 (5.7) | 0.428 |
| Asian, no (%) | 17 (8.6) | 6 (10.5) | 11 (7.8) | 0.539 |
| Other, no (%) | 8 (4.0) | 3 (5.3) | 5 (3.5) | 0.624 |
| Hispanic ethnicity, no (%) | 21 (10.6) | 5 (8.8) | 16 (11.3) | 0.297 |
| Comorbid Allergic Disease, no (%) | 155 (78.3) | 57 (100) | 98 (69.5) | <0.001* |
| Asthma, no (%) | 77 (38.9) | 31 (54.4) | 46 (32.6) | 0.211 |
| Allergic Rhinitis, no (%) | 108 (54.5) | 43 (75.4) | 65 (46.1) | 0.061 |
| Allergic Conjunctivitis, no (%) | 20 (10.1) | 9 (15.8) | 11 (7.8) | 0.884 |
| Atopic Dermatitis, no (%) | 74 (37.4) | 37 (64.9) | 37 (26.2) | 0.008* |
Denotes p value between EoE+IH and EoE-IH cohorts based on X2 test.
We next investigated the clinical manifestations of the IgE-associated immediate hypersensitivity reactions within this cohort. As is typically seen in IgE-associated food allergy, the majority of EoE+IH subjects presented with urticaria alone (33%) or with a combination of urticaria and vomiting (33%), but systemic and respiratory responses were also seen in some patients (Supplemental Table 1). Importantly, the food allergy-triggered clinical reactions seen in the EoE+IH cohort were typical of IgE-associated hypersensitivity reactions, occurring within minutes of ingestion.
It is widely reported that EoE subjects are highly atopic [16], and indeed both of our cohorts of EoE+IH and EoE-IH had a high likelihood of allergic disease. EoE+IH subjects were significantly more likely to have comorbid allergic disease (Table 1). We next investigated whether there were differences in the frequencies of specific atopic diseases between our cohorts of EoE+IH and EoE-IH. As shown in Table 1, EoE+IH patients were significantly more likely to have atopic dermatitis compared to the EoE-IH group (64.9% compared to 26.2%), consistent with this being an important factor associated with IgE-mediated food allergy [24]. We also found that 75% of the EoE+IH subjects had comorbid allergic rhinitis compared to just 46% of the EoE-IH subjects. There were no statistically significant differences in the presence of other comorbid conditions such as GERD, seizures, or diabetes mellitus type I (data not shown). Taken together, these data suggest that EoE+IH subjects exhibit a general predisposition for having more comorbid allergic diseases compared to their EoE-IH counterparts.
Focusing on the EoE presentations of the EoE+IH and EoE-IH groups, we further investigated the clinical symptoms at the time of EoE diagnosis (Table 2). Surprisingly, we found highly significant differences between the two groups. While 26% of the EoE+IH subjects presented with dysphagia, only 16% of the EoE-IH subjects reported this symptom. Similarly, the EoE+IH cohort was significantly more likely than the EoE-IH cohort to present with poor appetite, cough, and gagging. In sharp contrast, heartburn was significantly more common in the EoE-IH subjects compared to those with EoE+IH. In summary, the EoE+IH group presented with different clinical symptoms, suggesting that the presence of IgE-associated immediate hypersensitivity to foods may identify patients who exhibit a profoundly altered form of the clinical manifestations of EoE disease.
Table 2. Differences in Clinical Symptoms at time of Diagnostic EGD.
| EoE+IH, n=57 | EoE-IH, n=141 | ||||||
|---|---|---|---|---|---|---|---|
| Symptom | Yes | No | % Yes | Yes | No | % Yes | X2 |
| Abdominal Pain | 15 | 25 | 26% | 45 | 50 | 32% | 0.292 |
| Chest Pain | 6 | 30 | 10.5% | 12 | 76 | 0.1% | 0.664 |
| Extra chewing | 5 | 2 | 8.8% | 10 | 19 | 7.1% | 0.075 |
| Poor appetite | 10 | 28 | 17.5% | 10 | 78 | 7.1% | 0.035* |
| Cough | 6 | 2 | 10.5% | 3 | 10 | 2.2% | 0.019* |
| Vomiting | 21 | 21 | 37% | 41 | 55 | 29% | 0.428 |
| Regurgitation | 6 | 30 | 10.5% | 16 | 70 | 11.3% | 0.799 |
| Nausea | 4 | 31 | 7% | 5 | 81 | 3.5% | 0.286 |
| Heartburn | 1 | 33 | 1.8% | 16 | 72 | 11.3% | 0.029* |
| Odynophagia | 4 | 31 | 7% | 4 | 84 | 2.8% | 0.162 |
| Gagging | 14 | 25 | 24.6% | 11 | 79 | 7.8% | 0.002* |
| Failure to thrive | 11 | 25 | 19.3% | 23 | 66 | 16.3% | 0.592 |
| Food impaction | 9 | 27 | 15.6% | 30 | 62 | 21.3% | 0.400 |
| Feeding aversion | 11 | 28 | 19.3% | 21 | 67 | 14.9% | 0.603 |
| Early satiety | 5 | 29 | 8.8% | 9 | 78 | 6.4% | 0.500 |
| Dysphagia | 15 | 19 | 26% | 22 | 69 | 15.6% | 0.029* |
| Ave. No. Symptoms | 3.05 | 2.61 | 0.308 | ||||
To further explore these results, we developed a logistic regression model to determine the relative significance of each variable in predicting the presence of EoE+IH or EoE-IH (Table 3). The logistic regression analysis revealed that younger age, male gender, a history of allergic rhinitis, and presence of dysphagia had the biggest independent impact in predicting EoE+IH. The model was statistically significant (p<0.001 by the Omnibus test of coefficients), explained 56.7% of the variance (Nagelkerke R2), and correctly predicted classification of EoE+IH in 83.5% of scenarios. Male study subjects were 18.9 times more likely to exhibit EoE+IH than females at diagnosis. A history of allergic rhinitis and symptoms of dysphagia were 30.9 and 25.1 times more likely in EoE+IH subjects at diagnosis. Increasing age was associated with a decreased likelihood of having EoE+IH, and model predictors were significant even after controlling for age.
Table 3. Multivariate Logistic Regression Model of Factors Predicting the Presence of EoE+IH.
| Beta Coefficient | Odds Ratio | P value | 95% Confidence Interval | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Age at Diagnosis | -0.364 | 0.695 | 0.001 | 0.556 | 0.870 |
| Male Gender | 2.938 | 18.89 | 0.016 | 1.720 | 207.400 |
| History of Allergic Rhinitis | 3.432 | 30.93 | 0.002 | 3.610 | 265.031 |
| Symptom of Dysphagia | 3.224 | 25.12 | 0.001 | 3.550 | 177.716 |
Pathologic Findings
Since the clinical symptoms upon diagnosis of EoE were different between the groups, we next investigated whether the local esophageal disease had appreciable differences between the two cohorts. Endoscopy reports from the time of the diagnostic scopes were used to evaluate EREFS (edema, rings, exudates, furrows, or strictures) scores for subjects on whom scores were available. There were no appreciable differences in the presence of edema, rings, exudates, furrows, or strictures on their diagnostic endoscopies among the EoE+IH and EoE-IH groups (data not shown).
Next we reviewed the histology of the biopsy samples to investigate pathologic differences between the groups. We compared the overall peak number of esophageal eosinophils between the EoE+IH and EoE-IH groups as well as the average number of eosinophils in the distal, mid, and proximal biopsy samples, but no significant differences in eosinophil frequency were observed (Supplemental Table 2).
Atopic sensitization
Since the endoscopic and histologic findings suggested that the local esophageal disease was not significantly altered whether IgE-associated immediate hypersensitivity to foods was present or not, we next evaluated the utility of skin prick testing (SPT) and serum specific IgE testing results to differentiate these cohorts. There were no statistically significant differences between the groups with regard to SPT results for aeroallergens (data not shown). In contrast, the EoE+IH group was significantly more likely to demonstrate positive SPT responses to egg (p=0.049), milk (p=0.0056), soy (p=0.0036), peanut (p<0.0001), brazil nut (p=0.004), cashew (p=0.0018), hazelnut (p=0.0029), pecan (p=0.014), walnut (p=0.0084), and pork (p=0.025).
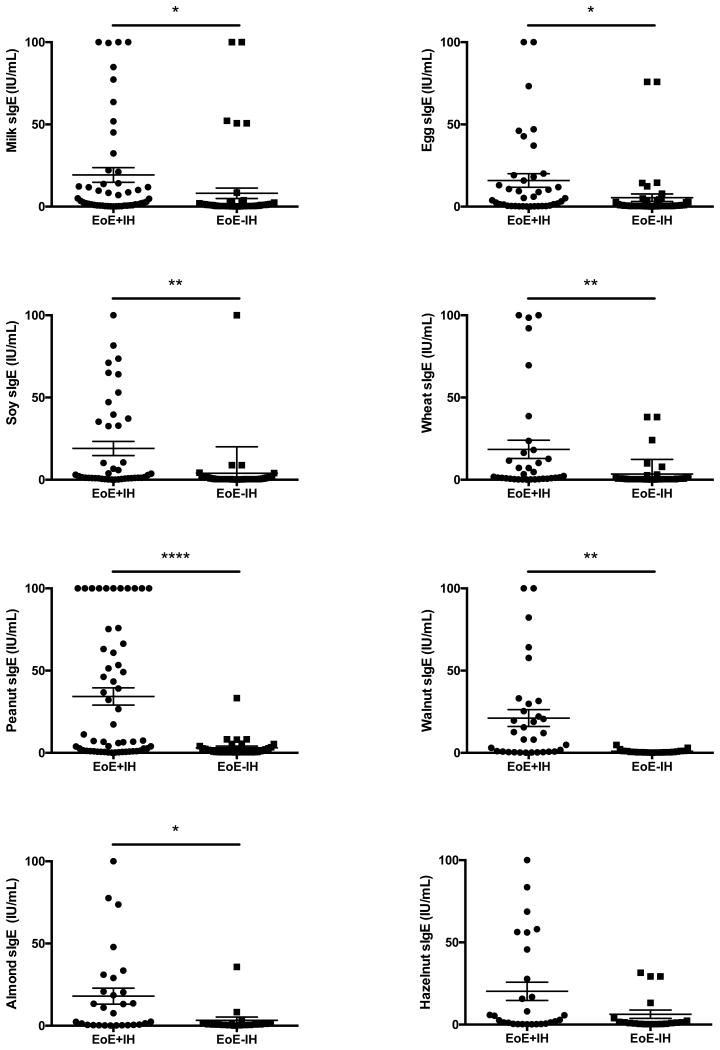
We next evaluated the levels of food-specific IgE values between the EoE subgroups. Specific IgE antibody values of 0.35 IU/mL or greater were considered positive, and only positive IgE values were then compared between the groups. Surprisingly, while there were no significant differences in the overall frequency of positive responses, the EoE+IH cohort showed higher levels of IgE towards milk, egg, soy, wheat, peanut, almond, and walnut for many subjects (Figure 1) and this was statistically significant for all these foods except hazelnut.
Figure 1. Specific IgE values for food allergens are higher in patients with immediate hypersensitivity.
For each food indicated, the reported levels of specific IgE for EoE patients with reported positive levels (>0.35IU/ml) were assessed based on the presence (EoE+IH) or absence (EoE-IH) of IgE-associated immediate hypersensitivity. *=p<0.05, **=p<0.01, ****=p<0.001 by Students t-test.
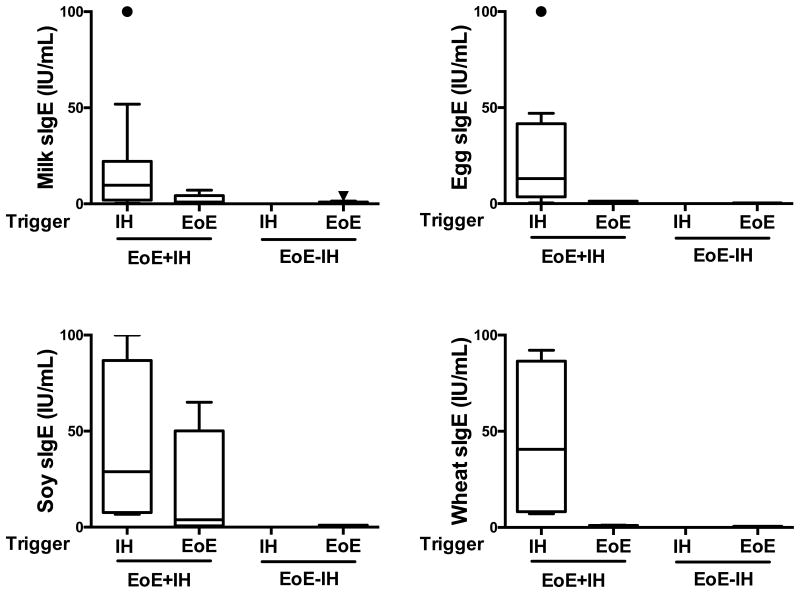
In order to determine whether these observed differences were solely due to the presence of IgE-mediated food allergy, we next examined the specific IgE values in relation to whether the specific foods were a trigger of an IgE-associated hypersensitivity reaction (determined by food challenge or clinical history, as detailed in the methods section) or a trigger of EoE (determined by food elimination and reintroduction, as detailed in the methods section). As shown in Figure 2, the quantities of specific IgE antibodies for the foods that triggered EoE were generally low (average values of 0.77 IU/mL, 0.41, and 0.62 for milk, egg, and wheat, respectively), in agreement with previous studies [25]. In contrast, specific IgE antibodies towards these same foods when they were identified as triggers of an IgE-associated food allergy reaction were significantly higher (14.59, 21.94, and 22.79, for milk, egg, and wheat, respectively). Interestingly, the specific IgE values towards soy were more wide-ranging, with an average IgE of 17.77 when soy was identified as an EoE trigger and 29.99 when identified as a food-allergy trigger. Collectively, these findings clearly demonstrate that high levels of specific IgE are associated with immediate hypersensitivity triggers to foods while the levels for EoE trigger foods are relatively lower.
Figure 2. Specific IgE levels for triggers of immediate hypersensitivity are higher than those for EoE food triggers.
IgE-mediated hypersensitivity trigger foods, determined by food challenge or clinical history, or EoE trigger foods, determined by food elimination and reintroduction, were assessed for levels of specific IgE in EoE+IH and EoE-IH groups.
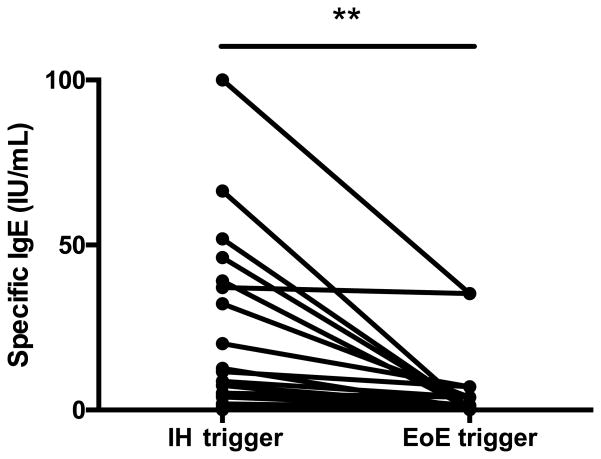
To further investigate this novel finding and further determine whether these results could be explained by food allergy alone, we identified a subset of patients in whom specific IgE values were obtained for both EoE triggers and immediate hypersensitivity triggers (EoE triggers have not yet been identified for every patient enrolled in this study). As shown in Figure 3, IgE values for identified immediate hypersensitivity triggers were consistently higher than the IgE values for foods identified as an EoE trigger. One patient who did not show this trend had an IgE value for the immediate hypersensitivity trigger (egg) and the EoE trigger (soy) that were similar.
Figure 3. IgE values for IH triggers are higher than EoE triggers.
Patients in whom both immediate hypersensitivity food triggers and EoE triggers had been determined were assessed for levels of IgE to either food. Each line represents an individual patient and the IgE levels reflect the foods specific to that individual.
We next investigated whether the quantities of food specific IgE were elevated as a characteristic of the EoE+IH cohort in general or solely because they were related to immediate hypersensitivity triggers in individual food-allergic patients. As shown in Table 4, we compared the mean IgE value of each food to the number of subjects who were sensitized to that food and in the case of the EoE+IH group to the number of subjects who were confirmed allergic to that particular food. Surprisingly, the specific IgE values detected in the EoE+IH subjects were higher than in the EoE-IH group for several foods regardless of whether the food tested was a confirmed specific immediate hypersensitivity trigger.
Table 4. Mean IgE for selected foods.
| Food | EoE+IH | Total # tested | Number (%) with specific IH | Number (%) with specific EoE trigger | EoE-IH | Total # tested | Number (%) with specific EoE trigger | P value |
|---|---|---|---|---|---|---|---|---|
| Egg | 15.89 ± 4.13 | 39 | 11 (33.33) | 1 (2.56) | 5.42 ± 2.30 | 46 | 3 (6.52) | 0.0238* |
| Milk | 19.28 ± 4.47 | 49 | 11 (22.45) | 6 (12.24) | 8.10 ± 3.18 | 50 | 13 (26) | 0.0435* |
| Soy | 19.02 ± 4.31 | 42 | 6 (14.28) | 5 (11.90) | 3.93 ± 2.62 | 38 | 1 (2.63) | 0.0046** |
| Wheat | 18.49 ± 5.55 | 34 | 4 (11.76) | 4 (11.76) | 3.45 ± 1.39 | 41 | 1 (2.44) | 0.0057** |
| Peanut | 34.31 ± 5.25 | 56 | 17 (30.36) | 1 (1.79) | 3.08 ± 1.01 | 34 | 3 (8.82) | <0.0001**** |
| Almond | 18.02 ± 4.86 | 29 | 6 (20.69) | 0 (0) | 3.31 ± 1.96 | 18 | 0 (0) | 0.0258* |
| Cashew | 22.60 ± 5.49 | 29 | 6 (20.69) | 1 (3.44) | 7.80 ± 5.52 | 18 | 0 (0) | 0.0786 |
| Hazelnut | 20.26 ± 5.57 | 28 | 4 (14.29) | 0 (0) | 6.33 ± 2.51 | 19 | 0 (0) | 0.0555 |
| Walnut | 21.15 ± 5.11 | 32 | 7 (21.88) | 0 (0) | 0.94 ± 0.25 | 21 | 0 (0) | 0.0024** |
As the presence of atopic dermatitis can associate with sensitization without clinical immediate hypersensitivity disease, we next investigated whether atopic dermatitis accounted for an increased proportion of positive IgE values. We found that atopic dermatitis alone did not have a statistically significant effect on the total IgE values or on specific IgE levels to milk, egg, soy, wheat, peanut, almond, cashew, hazelnut, pecan, or walnut (Supplemental Table 3). Furthermore, the presence of atopic dermatitis was not a statistically significant factor in the logistic regression model (Table 3).
Since the utility of allergy testing to predict trigger foods in EoE patients remains unclear, we examined whether there were differences in the ability of SPT to predict food reintroduction for identified trigger foods within the six-food elimination diet (SFED) amongst the EoE+IH and EoE-IH groups. Using SPT results for egg, milk, wheat, soy, fish, shellfish, peanuts, and tree nuts, Table 5 shows that the PPV and the sensitivity in the EoE+IH are relatively low, likely due to the prevalence of positive tests being higher amongst EoE+IH when compared to EoE-IH. Consequently, separating the EoE+IH from the overall EoE population increased the predictive values of the SPT in the EoE-IH group, as demonstrated by the higher PPV and sensitivity in the EoE-IH group alone. Additionally, the negative predictive value improved in the EoE-IH group compared to the EoE+IH group. Finally, since cow's milk is the most common food to elicit inflammation in EoE, yet has the least reliable predictive value in testing in EoE patients, many providers consider empiric milk elimination in addition to six food elimination diet (SFED). We therefore investigated the role of SPT in predicting non-cow's milk EoE food triggers. When milk was eliminated from the calculation, the NPV and accuracy of SPT improved, most notably in the EoE-IH cohort.
Table 5. Predictive Values of SPT in each subgroup.
| EoE+IH | |||||||
|---|---|---|---|---|---|---|---|
| n | PPV | NPV | Specificity | Sensitivity | DOR | Accuracy | |
| SFED | 55 | 28.6 | 76.3 | 30.8 | 74.4 | 1.3 | 63.5 |
| SFED-Dairy | 46 | 25 | 85.3 | 37.5 | 76.3 | 1.9 | 69.6 |
| EoE-FA | |||||||
| n | PPV | NPV | Specificity | Sensitivity | DOR | Accuracy | |
| SFED | 127 | 57.1 | 78.8 | 25 | 93.7 | 4.9 | 76.4 |
| SFED-Dairy | 108 | 40 | 88.8 | 26.7 | 93.5 | 5.3 | 84.3 |
All numbers are percentages except for DOR. DOR=diagnostic odds ratio. SFED=six food elimination diet.
Discussion
EoE is a chronic, antigen-mediated disease with long-term morbidity whose pathogenesis is unclear. While multiple studies have demonstrated increased allergen sensitization in EoE patients and the therapeutic utility of food allergen avoidance, the potential contribution of IgE-associated food allergy to EoE has been the subject of ongoing debate.
Our study demonstrates that IgE-associated immediate hypersensitivity is common in children with EoE. This is important for two reasons. First, it confirms that providers should be suspicious for the potential co-existence of immediate reactivity in any patient who is being evaluated for possible EoE. Second, the finding evokes questions that may help uncover mechanisms underlying EoE pathogenesis. Unlike other studies [16, 21], our study contained a relatively large cohort of patients, and the diagnosis of immediate hypersensitivity was made according to strict criteria. Although selection bias is a potential limitation of our study given its retrospective nature, the charts included were randomly selected from our existing EGID database.
Our results demonstrate that high specific IgE is not necessary for EoE disease development, in agreement with a recent review of circumstantial evidence that concluded EoE was not an IgE-mediated disease [5]. However, our data does define a subtype of EoE in which the presence of IgE-mediated food allergy dramatically alters the presentation of EoE. These findings suggest that for this particular subtype, IgE-mediated food allergy exerts some mechanistic influences that leads to an altered presentation of EoE that is not present in EoE subjects without IgE-associated immediate hypersensitivity reactions. While our work does not identify how mechanistically IgE-associated immediate hypersensitivity is associated with unique clinical symptoms such as dysphagia, it seems likely that immune sensitization to foods may alter the microenvironment of the esophagus. Prospective studies to investigate the potential underlying mechanisms are currently underway. =
The mechanistic interplay and crossovers between IgE-associated food allergy and EoE have been difficult to dissect, and current conclusions are largely based on circumstantial evidence such as the failure of omalizumab in trials for EoE treatment [26, 27]. Also, evidence of children outgrowing IgE-associated food allergy or undergoing oral immunotherapy and progressing towards EoE [13, 15, 23] has implied a progression from IgE-mediated triggering to an alternative process, likely IgG4-mediated [28]. Our findings show that the age of our EoE+IH group was significantly younger than EoE-IH, seemingly counter to this concept of progression from immediate hypersensitivity to EoE. Alternatively, it could be that our two subsets represent unique phenotypes of EoE and that disease progression differs between them. Since our study was built from information gathered at the EoE diagnosis appointment, the EoE+IH group had existing IgE-associated immediate hypersensitivity at the time of their EoE evaluation, although whether this represents disease progression or is unrelated is unclear. Our assessment of specific IgE levels within the EoE+IH group clearly demonstrated that the trigger foods for EoE and immediate hypersensitivity were largely unrelated and that the elevated specific IgE levels related to their immediate hypersensitivity reactions and less so to their EoE trigger, perhaps suggesting that the occurrence of both diseases is unrelated. However, in support of an interplay between the two diseases, the clear differences in symptoms we observed in our EoE+IH cohort suggests that patients with IgE-associated immediate hypersensitivity to foods exhibit an altered presentation of pediatric EoE. Additional studies will be needed to further explore the underlying pathophysiology.
Our ability to answer the question of disease progression versus separate phenotypes is limited by the retrospective nature of the study and being unable to address whether EoE+IH subjects were seen by a specialist at a younger age and then referred to the EGID clinic as a result. Additionally, we were unable to ascertain whether study subjects were actively avoiding potential EoE trigger foods at the time of diagnosis. The lack of antigen exposure in an elimination diet may theoretically be an important factor in determining IgE levels. However, it seems reasonable to assume that there would be no differences in the avoidance patterns of the two EoE cohorts (perhaps with the exclusion of the triggers of immediate hypersensitivity food reactions).
Currently, specific IgE testing has been problematic in its diagnostic and therapeutic value in EoE [29] and has led to considerable discussion of its value for predicting recurrence of esophageal eosinophilia upon food reintroduction after elimination diet [30]. We consistently observed that the IgE levels for EoE food triggers were low in comparison to levels seen in immediate hypersensitivity. It has been proposed that the serum might not reflect the local generation of IgE within the esophageal tissue as part of a more localized immune response [31] or that IgE is generated to minor components of foods and is thereby difficult to detect [32]. Others have suggested that detection of IgE levels might be blunted or blocked by other immunoglobulins such as IgG4 [28]. In our case, low IgE levels were a hallmark of EoE-inducing foods and separation of EoE+IH from EoE-IH allowed this conclusion to be significantly clearer. Indeed, the high food-specific IgE we observed was a hallmark of underlying immediate hypersensitivity. Interestingly, we did observe a few patients in the EoE-IH group who had elevated specific IgE levels, e.g. one patient had elevated peanut specific IgE. Prospective assessments are underway to help determine if such patients go on to present with IgE-associated, immediate reactions. Interestingly, our SPT analyses between the two cohorts showed that separating the EoE+IH cohort improved the sensitivity and specificity of the SPT for predicting outcomes of the SFED in the remaining group of patients who do not have immediate hypersensitivity. Whether the variability in predictive value of SPT results in EoE seen in previous studies was influenced by the presence or absence of underlying food allergy is unknown.
Interestingly, our cohorts did not demonstrate any significant differences in either endoscopic or histologic findings. While this may suggest that the esophageal disease is not different in these two groups, it could also be that these readouts reflect common end points of a chronic disease and that immediate hypersensitivity alters alternative aspects of EoE pathology. Additional studies are necessary to determine if there are differences in the deeper layers of tissue such as the esophageal muscle layers and submucosa and/or if cytokine and cellular infiltrates differ in these populations. Similarly, we did not evaluate the response to therapy--as determined by eosinophil counts in esophageal biopsy--in these cohorts, but this is another measure by which their esophageal disease may be different and which could have important management implications.
One limitation of our study is that our center does not perform patch testing to assess the T cell immune response in these EoE subtypes. Given that our findings have implications for disease pathogenesis, it would be interesting to investigate whether there are differences in food-specific delayed hypersensitivity responses and in T cell responses between these two cohorts. However, patch testing is not standardized for foods, is subjective in its interpretation, and can cause a considerable irritation reaction particularly in patients with atopic dermatitis.
In conclusion, our study describes a clear separation in the presentation of EoE if patients also exhibit IgE-associated immediate hypersensitivity. Notably, the EoE+IH subjects presented at a younger age, had higher frequencies of comorbid allergic disease, and had elevated IgE levels to various foods while IgE values for foods identified as EoE triggers were relatively low in both EoE+IH and EoE-IH cohorts. Taken together, our findings suggest that there is an endotype of EoE that is characterized by the presence of IgE-associated immediate hypersensitivity and within which the phenotypic presentation of EoE is altered.
Supplementary Material
Acknowledgments
The authors would like particularly thank all the patients and their families who consented to their information being included in this study. We acknowledge the contribution of Karen Rychlik, MS, for statistical expertise and advice. This study was supported in part by funding from CURED Foundation (to PB and JW), The Buckeye Foundation (to AK) and an R01 grant from the National Institutes of Health (AI076456 to PB).
Footnotes
Conflict of Interest: The authors have no conflict of interests related to the findings of this study.
References
- 1.Cherian S, Smith NM, Forbes DA. Rapidly increasing prevalence of eosinophilic oesophagitis in Western Australia. Arch Dis Child. 2006;91:1000–4. doi: 10.1136/adc.2006.100974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liacouras CA, Spergel JM, Ruchelli E, Verma R, Mascarenhas M, Semeao E, Flick J, Kelly J, Brown-Whitehorn T, Mamula P, Markowitz JE. Eosinophilic esophagitis: a 10-year experience in 381 children. Clin Gastroenterol Hepatol. 2005;3:1198–206. doi: 10.1016/s1542-3565(05)00885-2. [DOI] [PubMed] [Google Scholar]
- 3.van Rhijn BD, Verheij J, Smout AJ, Bredenoord AJ. Rapidly increasing incidence of eosinophilic esophagitis in a large cohort. Neurogastroenterol Motil. 2013;25:47–52 e5. doi: 10.1111/nmo.12009. [DOI] [PubMed] [Google Scholar]
- 4.Jackson KD, Howie LD, Akinbami LJ. Trends in allergic conditions among children: United States, 1997-2011. NCHS data brief. 2013:1–8. [PubMed] [Google Scholar]
- 5.Simon D, Cianferoni A, Spergel JM, Aceves S, Holbreich M, Venter C, Rothenberg ME, Terreehorst I, Muraro A, Lucendo AJ, Schoepfer A, Straumann A, Simon HU. Eosinophilic esophagitis is characterized by a non-IgE-mediated food hypersensitivity. Allergy. 2016 doi: 10.1111/all.12846. [DOI] [PubMed] [Google Scholar]
- 6.Simon D, Marti H, Heer P, Simon HU, Braathen LR, Straumann A. Eosinophilic esophagitis is frequently associated with IgE-mediated allergic airway diseases. The Journal of allergy and clinical immunology. 2005;115:1090–2. doi: 10.1016/j.jaci.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 7.Jyonouchi S, Brown-Whitehorn TA, Spergel JM. Association of eosinophilic gastrointestinal disorders with other atopic disorders. Immunology and allergy clinics of North America. 2009;29:85–97. doi: 10.1016/j.iac.2008.09.008. x. [DOI] [PubMed] [Google Scholar]
- 8.Kelly KJ, Lazenby AJ, Rowe PC, Yardley JH, Perman JA, Sampson HA. Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid-based formula. Gastroenterology. 1995;109:1503–12. doi: 10.1016/0016-5085(95)90637-1. [DOI] [PubMed] [Google Scholar]
- 9.Gonsalves N, Yang GY, Doerfler B, Ritz S, Ditto AM, Hirano I. Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterology. 2012;142:1451–9 e1. doi: 10.1053/j.gastro.2012.03.001. quiz e14-5. [DOI] [PubMed] [Google Scholar]
- 10.Kagalwalla AF, Sentongo TA, Ritz S, Hess T, Nelson SP, Emerick KM, Melin-Aldana H, Li BU. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2006;4:1097–102. doi: 10.1016/j.cgh.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 11.Markowitz JE, Liacouras CA. Ten years of eosinophilic oesophagitis: small steps or giant leaps? Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2006;38:251–3. doi: 10.1016/j.dld.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Hofmann AM, Scurlock AM, Jones SM, Palmer KP, Lokhnygina Y, Steele PH, Kamilaris J, Burks AW. Safety of a peanut oral immunotherapy protocol in children with peanut allergy. The Journal of allergy and clinical immunology. 2009;124:286–91. doi: 10.1016/j.jaci.2009.03.045. 91 e1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lucendo AJ, Arias A, Tenias JM. Relation between eosinophilic esophagitis and oral immunotherapy for food allergy: a systematic review with meta-analysis. Ann Allergy Asthma Immunol. 2014;113:624–9. doi: 10.1016/j.anai.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez-Garcia S, Rodriguez Del Rio P, Escudero C, Martinez-Gomez MJ, Ibanez MD. Possible eosinophilic esophagitis induced by milk oral immunotherapy. The Journal of allergy and clinical immunology. 2012;129:1155–7. doi: 10.1016/j.jaci.2011.11.042. [DOI] [PubMed] [Google Scholar]
- 15.Ridolo E, De Angelis GL, Dall'aglio P. Eosinophilic esophagitis after specific oral tolerance induction for egg protein. Ann Allergy Asthma Immunol. 2011;106:73–4. doi: 10.1016/j.anai.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Spergel JM, Brown-Whitehorn TF, Cianferoni A, Shuker M, Wang ML, Verma R, Liacouras CA. Identification of causative foods in children with eosinophilic esophagitis treated with an elimination diet. The Journal of allergy and clinical immunology. 2012;130:461–7.e5. doi: 10.1016/j.jaci.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 17.Henderson CJ, Abonia JP, King EC, Putnam PE, Collins MH, Franciosi JP, Rothenberg ME. Comparative dietary therapy effectiveness in remission of pediatric eosinophilic esophagitis. The Journal of allergy and clinical immunology. 2012;129:1570–8. doi: 10.1016/j.jaci.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henderson CJ, Collins MH, Abonia JP, Putnam PE, Rothenberg ME. Reply: To PMID 22541246. The Journal of allergy and clinical immunology. 2013;131:243–4. doi: 10.1016/j.jaci.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Spergel JM. Eosinophilic esophagitis in adults and children: evidence for a food allergy component in many patients. Current opinion in allergy and clinical immunology. 2007;7:274–8. doi: 10.1097/ACI.0b013e32813aee4a. [DOI] [PubMed] [Google Scholar]
- 20.Brown-Whitehorn TF, Spergel JM. The link between allergies and eosinophilic esophagitis: implications for management strategies. Expert review of clinical immunology. 2010;6:101–9. doi: 10.1586/eci.09.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugnanam KK, Collins JT, Smith PK, Connor F, Lewindon P, Cleghorn G, Withers G. Dichotomy of food and inhalant allergen sensitization in eosinophilic esophagitis. Allergy. 2007;62:1257–60. doi: 10.1111/j.1398-9995.2007.01454.x. [DOI] [PubMed] [Google Scholar]
- 22.Sampson HA, Ho DG. Relationship between food-specific IgE concentrations and the risk of positive food challenges in children and adolescents. The Journal of allergy and clinical immunology. 1997;100:444–51. doi: 10.1016/s0091-6749(97)70133-7. [DOI] [PubMed] [Google Scholar]
- 23.Maggadottir SM, Hill DA, Ruymann K, Brown-Whitehorn TF, Cianferoni A, Shuker M, Wang ML, Chikwava K, Verma R, Liacouras CA, Spergel JM. Resolution of acute IgE-mediated allergy with development of eosinophilic esophagitis triggered by the same food. J Allergy Clin Immunol. 2014;133:1487–9. doi: 10.1016/j.jaci.2014.02.004. 89 e1. [DOI] [PubMed] [Google Scholar]
- 24.Brough HA, Liu AH, Sicherer S, Makinson K, Douiri A, Brown SJ, Stephens AC, Irwin McLean WH, Turcanu V, Wood RA, Jones SM, Burks W, Dawson P, Stablein D, Sampson H, Lack G. Atopic dermatitis increases the effect of exposure to peanut antigen in dust on peanut sensitization and likely peanut allergy. The Journal of allergy and clinical immunology. 2015;135:164–70. doi: 10.1016/j.jaci.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erwin EA, James HR, Gutekunst HM, Russo JM, Kelleher KJ, Platts-Mills TA. Serum IgE measurement and detection of food allergy in pediatric patients with eosinophilic esophagitis. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2010;104:496–502. doi: 10.1016/j.anai.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loizou D, Enav B, Komlodi-Pasztor E, Hider P, Kim-Chang J, Noonan L, Taber T, Kaushal S, Limgala R, Brown M, Gupta R, Balba N, Goker-Alpan O, Khojah A, Alpan O. A pilot study of omalizumab in eosinophilic esophagitis. PLoS One. 2015;10:e0113483. doi: 10.1371/journal.pone.0113483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rocha R, Vitor AB, Trindade E, Lima R, Tavares M, Lopes J, Dias JA. Omalizumab in the treatment of eosinophilic esophagitis and food allergy. Eur J Pediatr. 2011;170:1471–4. doi: 10.1007/s00431-011-1540-4. [DOI] [PubMed] [Google Scholar]
- 28.Clayton F, Fang JC, Gleich GJ, Lucendo AJ, Olalla JM, Vinson LA, Lowichik A, Chen X, Emerson L, Cox K, O'Gorman MA, Peterson KA. Eosinophilic esophagitis in adults is associated with IgG4 and not mediated by IgE. Gastroenterology. 2014;147:602–9. doi: 10.1053/j.gastro.2014.05.036. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Sanchez J, Gomez Torrijos E, Lopez Viedma B, de la Santa Belda E, Martin Davila F, Garcia Rodriguez C, Feo Brito F, Olmedo Camacho J, Reales Figueroa P, Molina-Infante J. Efficacy of IgE-targeted vs empiric six-food elimination diets for adult eosinophilic oesophagitis. Allergy. 2014;69:936–42. doi: 10.1111/all.12420. [DOI] [PubMed] [Google Scholar]
- 30.Lucendo AJ, Arias A, Tenias JM, Rodriguez-Sanchez J, Gomez-Torrijos E, Feo-Brito F, Molina-Infante J. Serum IgE-targeted elimination diets for treating eosinophilic esophagitis: things are not what they seem. Allergy. 2014;69:1567–8. doi: 10.1111/all.12471. [DOI] [PubMed] [Google Scholar]
- 31.Vicario M, Blanchard C, Stringer KF, Collins MH, Mingler MK, Ahrens A, Putnam PE, Abonia JP, Santos J, Rothenberg ME. Local B cells and IgE production in the oesophageal mucosa in eosinophilic oesophagitis. Gut. 2010;59:12–20. doi: 10.1136/gut.2009.178020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Erwin EA, Tripathi A, Ogbogu PU, Commins SP, Slack MA, Cho CB, Hamilton RG, Workman LJ, Platts-Mills TA. IgE Antibody Detection and Component Analysis in Patients with Eosinophilic Esophagitis. The journal of allergy and clinical immunology In practice. 2015;3:896–904.e3. doi: 10.1016/j.jaip.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.