Abstract
Cinnamon (Cinnamomum sp.) has been suggested to help patients with type 2 diabetes mellitus (T2DM) achieve better glycemic control although conclusions from meta-analyses are mixed. To evaluate whether the use of cinnamon dietary supplements by adults with T2DM had clinically meaningful effects on glycemic control, as measured by changes in fasting plasma glucose (FPG) or hemoglobin A1c (HbA1c), a comprehensive PubMed literature search was performed. Eleven RCTs were identified meeting our inclusion criteria that enrolled 694 adults with T2DM receiving hypoglycemic medications or not. In 10 of the studies participants continued to take their hypoglycemic medications during the cinnamon intervention period. Studies ranged from 4 to 16 weeks in duration; seven studies were double-blinded. Cinnamon doses ranged from 120 to 6000 mg/d. The species of cinnamon used varied; 7 used C. cassia/C. aromaticum; 1 used C. zeylanicum, and 3 did not disclose it. Because of the heterogenity of the studies, a metaanalysis was not conducted. All 11 of the studies reported some reductions in FPG during the cinnamon intervention, and of the studies measuring HbA1c very modest decreases were also apparent with cinnamon, while changes in the placebo groups were minimal. However, only four studies achieved the American Diabetes Association treatment goals (FPG <7.2 mmol/L or 130 mg/dL and/or HbAlc <7.0). We conclude that cinnamon supplements added to standard hypoglycemic medications and other lifestyle therapies had modest effects on FPG and HbA1c. Until larger and more rigorous studies are available, dietitians and other healthcare professionals should recommend that patients continue to follow existing recommendations of authoritative bodies for diet, lifestyle changes, and hypoglycemic drugs.
Keywords: cinnamon, fasting plasma glucose, hemoglobin A1c, type 2 diabetes mellitus
Introduction
In 2012, the estimated prevalence of diabetes in adults in the US was 9.3%, with a prevalence of 25.9% in Americans over the age of 65.1 Diabetes increases risks for kidney and cardiovascular disease and is the leading preventable cause of blindness in the US and so strategies to decrease its incidence and complications are important. For individuals with type 2 diabetes (T2DM), cardiovascular risk factor management includes dyslipidemia, hypertension, smoking, albuminuria, and a family history of premature coronary disease and emphasizes control of blood glucose with diet, hypoglycemic drugs, and comprehensive cardiovascular risk factor reduction.2,3
Fasting plasma glucose (FPG) and hemoglobin A1c (HbA1c) are two commonly used measures for the diagnosis of diabetes. In 2014, the American Diabetes Association defined a HbA1c of ≥6.5%, or FPG of 126 mg/dL or 7.0 mmol/L as indicative of diabetes. HbA1c reflects glucose levels over a longer period (approximately 3 months or 90 days, the life span of the red blood cell) rather than a single point in time, and it is subject to less individual variability than FPG. HbA1c also has a strong predictive value since it is associated with decreased microvascular complications.2
Cinnamon bark oil has been used for centuries in Western and traditional Eastern medicine such as Indian Ayurvedic and Unani systems to treat many conditions.4, 5 US Physicians in the 19th century used cinnamon for a variety of disorders.6 Today, The German Commission E recognizes the use of two cinnamon species (C. verum and C. aromaticum) to treat loss of appetite, dyspeptic complaints, bloating and flatulence.4
Cinnamon is also a widely used culinary spice.7 Four of the 250 species in the genus Cinnamomum are used as spice “cinnamon”.8 C. verum Ceylon or Sri Lankan (also known as C. zeylanicum) is often referred to as “true “cinnamon. The three species related to C. cassia, which are more popular, include C. aromaticum (also known as Chinese), C. loureirii (Saigon or Vietnamese) cinnamon and C. burmanni (Indonesian). In 2013, imports of C. cassia to the US totaled over 25,000 tons, predominately from Indonesia,9 and increased by 15% in 2014.10 Cinnamon is sold in many forms, including harvested sticks of bark (or quills), pulverized bark powder, and extracts derived from the powder. The form in which cinnamon is administered is important because extracts (aqueous and/or organic solvent extraction) and powders made from pulverized bark contain different phytochemicals and may also differ in bioavailability.11 The composition of cinnamon also varies across species. Volatile oils constitute 1-4% of the products, and include 60-80% cinnamaldehyde; up to 10% eugenol; 5- 10% trans-cinnamic acid; 5-10% phenolic compounds and 4-10% of other compounds including condensed tannins, catechins, and proanthocyanidins, monoterpenes, sesquiterpenes, and traces of courmarin.4, 12 The bioactive compounds responsible for the putative effects are still under investigation, making it difficult to standardize preparations. Marker compounds, used to separate and further delineate the species, include coumarin, cinnamyl alcohol, cinnamaldehyde, cinnamic acid and eugenol. C. loureirii has a high percentage of trans-cinnamaldehyde; C. aromaticium contains high levels of methylcinnamate compared with C. loureirii, C. burmanni and C. verum. C. burmanni contains high levels of coumarin in comparison with the other three types of cinnamon.13 The spice purchased in food stores typically contains a combination of different forms of cinnamon in the bark or powdered form, with C cassia usually predominating.
Recently it has been suggested that cinnamon has beneficial effects on blood glucose control and other ailments.14 Some in vitro and in vivo studies suggested that a compound or compounds in the aqueous extract of cinnamon activated the insulin receptor by multiple mechanisms that included increased auto-phosphorylation of the insulin receptor, increased glucose transporter-4 (GLUT-4) receptor synthesis and activation, inhibition of pancreatic and intestinal amylase and glucosidase, and increased glycogen synthesis in the liver thus improving insulin sensitivity, glycemic control, and lipid levels.15-19 Interest in cinnamon's possibly beneficial properties in diabetes management increased after the discovery of a bioactive insulin potentiating agent initially identified as hydroxychalcone derived from cinnamon20, 21 and the publication of small clinical studies in subjects with T2DM claiming it reduced fasting blood glucose,21-24 although other studies were negative.25-27
Patients with diabetes who have been prescribed hypoglycemic agents, along with diet and lifestyle modifications, often ask dietitians and other health care professionals about the benefits of using cinnamon to control their blood glucose levels. Therefore, the goal of this review was to determine whether cinnamon used as a dietary supplement would be a clinically useful complementary therapy for T2DM. Clinically meaningful conclusions from the existing meta-analyses evaluating cinnamon for glycemic control were difficult to interpret;28-32 three28, 29, 31 out of five recent meta-analyses reported statistically significant decreases in FPG but only one28 out of four meta-analyses reported decreases in HbA1c. Moreover the studies reviewed were extremely heterogeneous in populations studied, preparations, doses, and experimental design, and the disparate conclusions they reached even when evaluating the same research studies.28-32
Therefore, we undertook a comprehensive and thorough review of the original studies in the literature that met our inclusion criteria to determine whether the use of cinnamon by adults with T2DM met the American Diabetic Association (ADA)2 clinical treatment guidelines for glycemic control, as measured by changes in FPG or HbA1c.
Studies Included in this Review
A literature search was performed to identify all randomized clinical trials (RCTs) using PubMed, AGRIS, Embase, Web of Science, Science Direct and CINAHL from 01/01/1994 through 12/31/2015 using the following search terms: cinnamon, blood glucose, blood sugar, glucose metabolism disorders, metabolic syndrome, diabetes, clinical trial, RCTs, systematic review, meta-analysis, humans, and English. Original research papers were included if they met the following criteria: trials with adults (>18 yr.) with T2DM defined as self-reported, clinically diagnosed, or biochemically determined (FPG or HbA1c or both); with or without simultaneous treatment with hypoglycemic agents; with stable chronic disease; and participant in a placebo or comparator- controlled RCTs testing the dietary supplement for glycemic control.
Diabetes was defined using two commonly used clinical test criteria described in the introduction, either an elevated fasting plasma glucose (FPG) ≥126 mg/dL/7.0 mmol/L or a HbA1c of ≥6.5%.2 Trials and studies were excluded if they included children; study arms of all patients with type 1 diabetes; patients with unstable chronic disease and/or acute conditions (e.g., severe heart failure, hemodialysis, acute myocardial infarction) or with HIV; and studies of combination therapies without a separate cinnamon supplement arm. Also excluded were observational, non-randomized, and unblinded studies that did not report pre and post intervention results with respect to either FPG or HbA1c. Eleven studies were identified in patients with T2DM who met the inclusion criteria. These 11 RCTs that measured the effects of cinnamon supplements as single preparations on FPG and HbA1c in patients with T2DM were then reviewed
For this analysis, a clinically relevant outcome was deemed to be a treatment success (meeting recommended ADA treatment goals) if the supplement intervention resulted in a decrease in FPG to ≤130 mg/dl/7.2 mmol/L or a decrease to ≤7.0% for HbA1c2. A secondary criterion, substantial decline in HbA1c (a decrease of ≥ 0.5%), was also evaluated based on the expected reduction achieved by successful drug therapy, which is often cited in the literature as an indicator of treatment success.2 A similar criterion for a substantial decline in FPG was not available, and so none was used.
Evaluation of Individual RCTs
This review includes 11 RCTs21, 23, 25-27, 33-38, three of which, Mirfeiz and colleagues 36, Vafa and colleagues 37, and Sharma and colleagues 38 had not been included in the meta-analyses noted above. A total of 694 subjects (both placebo and cinnamon treatment) were enrolled across 11 individual RCTs with 15 treatment arms. All studies except one enrolled both male and female participants with T2DM. Ten studies enrolled subjects who were taking hypoglycemic medications and continued this treatment during the intervention; one study did not. Body mass index was reported in 73% of the studies and ranged from 24.4 to 30.7 kg/m2. Most of the studies were small, and only 73% were double-blinded. Three studies used a placebo that was not described. Few of the studies were carried out in major research institutions and 73% of the studies were conducted in low or middle income countries outside of Western Europe and the US. The study by Khan and colleagues21 in Pakistan was the only dose ranging study employing 3 dose arms. Most of the studies were short, from 4 to 16 weeks duration. Dropout rates varied from 3 to 20% and were described in 82% studies. Most studies (73%) analyzed their data using an intention-to-treat analysis which includes all individuals who started the intervention.
The species of cinnamon reported in the studies reviewed varied and included the following: C. cassia and/or C. aromaticum (64%), three studies did not disclose the species of cinnamon used and simply stated as “cinnamon” and one study used a formulation of C. zeylanium (Ceylon) cinnamon. Most provided either the full name of the manufacturer of the cinnamon product or a clinical trial identification number. In contrast, documentation of purity testing was rare [Table 1]. Most studies (73%) lacked authentication describing how the investigator ensured the integrity of the product being tested. Seven studies used C. cassia in a powdered capsule; three studies used an unspecified cinnamon extract (1 in tablet form and 2 in capsule form); and one used a non-specified C. zeylanicum in a capsule formulation. Doses of the product were stated to be 120 mg/d to 6000 mg/d, but the information on dose did not provide the amount of the presumed active compounds in the product. Only four studies obtained dietary intake records during the study, so it was impossible to determine precisely what the total dietary exposure of cinnamon was since some diets may have contained small amounts of it. Of greater concern was that it was not possible to rule out other changes that might also have affected FPG and HbA1c, such as changes in adherence to hypoglycemic medications, diet, physical activity and exercise regimens during the intervention period.
Table 1.
Characteristics of Cinnamon Intervention Products Used in the Clinical Trials Reviewed
| First Author/Date/Location | Product Formulation | Placebo Formulation | Cinnamon Dose | Manufacturer/Supplement Source |
|---|---|---|---|---|
| Akilen 2010 UK33 | 100 % cinnamon bark, C. cassia, Ref HBL14020NB. | Starch (with 80% amylose and 20% amylopectin) | 2 g/d (4, 500 mg capsule) for 12 weeks; 1 capsule at breakfast, 2 at lunch, 1 at dinner. | Holland and Barrett Ltd (London, UK) |
| Blevins 2007 USA25 | C. cassia | Wheat flour | 1 g/d (500 mg capsule) twice daily at breakfast and dinner for 3 months | Not specified |
| Khan 2003 Pakistan21 | C. cassia certified by State R&D, NWFP Forest Dept. Peshawar, Pakistan | Wheat flour placebo taken with meals. | 1 g/d (2, 500 mg capsules), 3 g/d (6, 500 mg capsules), or 6 g/d (12, 500 mg capsules) for 40 d; no supplement 41-60d. | Mehran Traders Pharmaceutical Suppliers, Peshawar, Pakistan |
| Khan 2010 Pakistan34 | Not specified, purchased at local market and encapsulated | Maize flour 500 mg at each meal | 1.5 g/d (500 mg capsule) at each meal for 30 days | Prepared by researcher |
| Lu 2012 China35 | 60 mg extract = 2.4 g of cinnamon; bark of Chinese Cinn aromaticum. Each 60 mg tab cinn extract isolated from 2.4 g cinn [Shanghai Yitian Bio Scientific Co.; appr for use G20110080 by State FDA | Not specified | 4.8 g/d (2, 60 mg tablet extract) or 14.4 g (6, 60 mg tablet extract) prior to breakfast for 3 months | Developed by Shanghai Yitian Bio-Scientific Co, Ltd (Shanghai, China), and produced by Shanghai Jinsijia Health-Care Food Co, Ltd (Shanghai, China), |
| Mang 2006 Germany23 | 112 mg of the aqueous cinnamon extract (C. cassia) = 1g of cinnamon [TC112, Finzelberg, Andernach, Ger] | Microcrystalline cellulose only, 3 times a day, total 3 gm/d | 3 g/d (3, 1 g capsule) for 4 months | Prepared by: Finzelberg (Andernach, Germany) Donated by Truw Arzneimittel Vertriebs GmbH (Diabetruw®, Gürersloh, Germany) |
| Mirfeizi 2015 Iran36 | Cinnamon (not specified) and Caucasian whortleberry (Vaccinium arctostaphylos L.) | starch (1000 mg/d) | 1 g/d (500 mg capsule) after morning and evening meal or Caucasian whortleberry (1000 mg/d) | Three herbs were sent to the Herbarium at the College of Pharmacy, Tehran University of Medical Sciences, for identification, qualitative and qualitative analysis, purification, and formulation as powders before being sent to a pharmacy in Karaj to be made up into 500-mg cinnamon, Caucasian whortleberry, and starch capsules. |
| Sharma 2012 India 38 | Not specified; possibly C. tamala (Indian cassia) | Not specified | 3 g/d (1000 mg capsule) at breakfast, lunch and dinner or 6 g/ds (2000 mg capsule) at breakfast, lunch and dinner, immediately after meals for 3 months | Prepared by pharmacy of the National Institute of Ayurveda (NIA) Jaipur, India |
| Suppapitiporn 2006 Thailand26 | Cinnamon powder C. cassia | Not specified | 1.5 g/d (500 mg capsule × 3) with meals for 3 months | Not specified |
| Vafa 2012 Iran37 | C. zeylanicum (Ceylon) | matching wheat flour capsule | 3 g/d (500 mg/capsule) for 8 wks. 2 capsules with breakfast, lunch, and dinner | Not specified |
| Vanschoonbeek 2006 Netherlands27 | C. cassia (Verstegen) | 1500 mg wheat flour, (Verstegen) 1 cap at each main meal | 1.5 g (500 mg capsule) with meals for 6 weeks | Not specified |
Studies Achieving Diabetes Treatment Goals
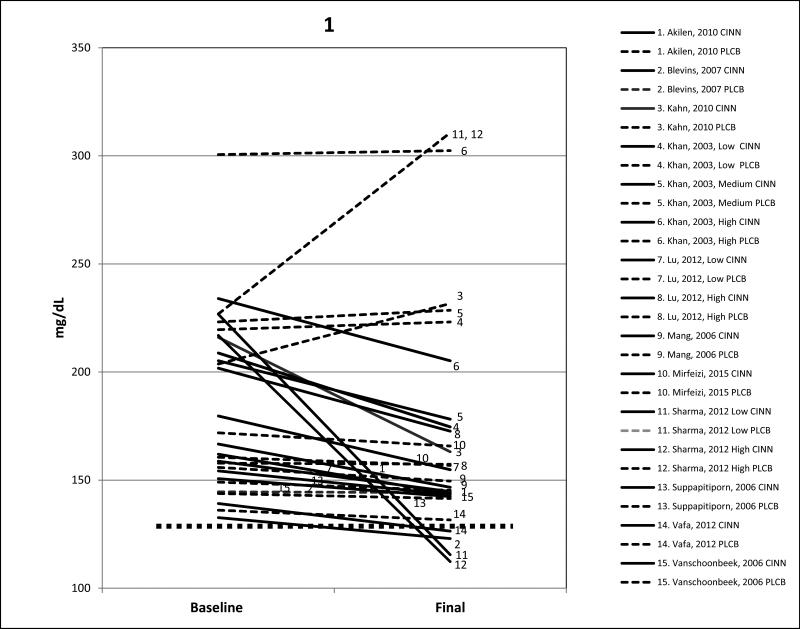
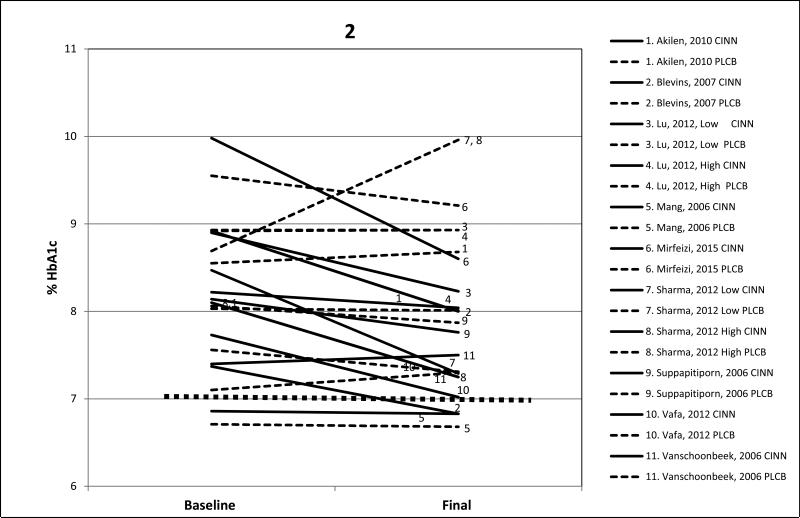
Figures 1 and 2 display pre-post cinnamon and placebo intervention results for FPG and HbA1c. Only four of the 11 studies achieved treatment goals. In the study by Blevins and colleagues,25 FPG and HbA1c decreased sufficiently to reach at least the clinical treatment goals of ≤130 mg/dL/7.2 mmol/L and ≤7.0% respectively; however, baseline levels in that study were only slightly evaluated above the treatment goals to begin with; 133mg/dL/7.4 mmol/L and 7.0% respectively. In the study by Mang and colleagues,23 baseline HbA1c levels were already at the clinical treatment goal of ≤7.0% and they changed little by study end, so treatment effects were not apparent. In the study by Vafa and colleagues,37 FPG (127 mg/dL/7.02 mmol/L) and HbA1c (6.9%) had decreased sufficiently to fall below treatment goals, but at baseline both FPG and HbAlc were only modestly elevated 144 mg/dL/8 mmol/L and 8.0% respectively. In the largest study (n=150) by Sharma and colleagues,38 treatment goals were met for FPG with both the 3000 mg and 6000 mg doses.
Figure 1.
Mean changes in FPG baseline to post cinnamon supplementation for 11 studies (15 arms) and placebo. Solid line is cinnamon treatment, dashed line is placebo control, and dotted line represents FPG treatment goal of ≤130 mg/dL/7.2 mmol/L. CINN = cinnamon, PLCB = placebo. Conversion factor to mmol/L multiply by .05551.
Figure 2.
Mean changes in HbA1c baseline to post cinnamon supplementation for all 9 studies (11 arms) and placebo arms. Solid line is cinnamon treatment, dashed line is placebo control, and dotted line represents HbA1c treatment goal of ≤7.0%. CINN = cinnamon; PLCB = placebo.
Studies with Clinically Significant Lowering of FPG or HbA1c
All of the 11 studies (15 treatment arms) showed varying degrees of reductions in mean FPG during the cinnamon intervention. The study by Khan and colleagues21 with three treatment arms (low dose 1000 mg/d, medium dose 3000 mg/d, and high dose 6000 mg/d) achieved statistically significant lowering (12-16%; P<0.05) of FPG in all three arms. However, those randomized to the placebo group study arms had higher baseline FPGs than those in the cinnamon groups. Lu and colleagues,35 who also studied a low (120 mg/d) and high dose (360 mg/d) found statistically significant reductions (11-14%; P<0.01) at both cinnamon doses. Sharma and colleagues38 found statistically significant declines in both FPG and HbA1c in the intervention group after 3 months of cinnamon; 49% (p<0.001) and 14% (p<0.005) respectively but significant increases in both the FPG (37%) and HbA1c (15%) in the placebo group were also apparent.
In the nine studies (11 treatment arms) that measured mean HbA1c, the percent reductions were not as marked as those for FPG. Both the studies by Lu35 and Sharma and colleagues38 reported a drop ≥0.5% in HbA1c values at both low and high doses. Overall, the addition of cinnamon was associated with a mean lowering of 21% in FPG and a more modest lowering of 6% in HbA1c, and similar indices for the placebos showed very small changes from baseline to final values.
Discussion
Although FPG and HbA1c decreased significantly with cinnamon in several studies, only 4 studies reached the ADA treatment goals of FPG <130 mg/dL/7.2 mmol/L or HbA1c <7.0%. The United Kingdom Perspective Diabetes Study39 and the Diabetes Control and Complications Trial40 showed that a decrease of 1.0% in HbA1c cut the risk of microvascular complications by 28%. Unfortunately, in the studies reviewed here, the mean reduction in HbA1c was only 0.49%, in all studies combined, most of which involved concurrent hypoglycemic drug therapy. In general, the quality of the studies reviewed was poor, and there was a uniform lack of striking beneficial effects.
A number of limitations of the studies we reviewed called into question the efficacy of the cinnamon intervention. First, the data were lacking in the description of patient groups, such as information on their weight status or adherence to hypoglycemic medications, diet, or physical activity changes over the course of the study. Another limitation was that the forms in which cinnamon was administered differed; extracts (aqueous and/or organic solvent extraction) and powders (pulverized bark material) each possibly provided different bioactive compounds with varying levels of bioavailability and possibly potency.11 Comparisons across studies were also difficult because of the multitude of cinnamon products, species, forms, doses and bioactive ingredients that may have been present. No two studies used the same intervention product at a similar dose, and levels of the presumed bioactives were not provided, making dose-dependent effects difficult to discern. The background diet at baseline or during intervention was rarely reported; only 6 of the 11 RCTs reported any form of dietary intake assessment during the study period (dietary monitoring or 3-day dietary intake records). Less than half of the studies documented adherence to the supplement by pill counts. Most of the studies did not comment on whether side effects or adverse events occurred during the study. The lowering of FPG was most apparent in those patients that had the highest mean baseline values to begin with and who may have altered their adherence to other treatments during the interventional period. It may have been this phenomenon, rather than a significant dose-dependent effect of the cinnamon supplement that seemed to be operating to improve glucose control.28 Also, when baseline levels differed between control and treatment groups, adjustments were not made.
Our results agree with Kirkham and colleagues41 that statistically significant lowering of blood glucose levels may not be clinically significant.
We conclude that these data suggest that cinnamon, when added to conventional hypoglycemic medication and other lifestyle therapies, may have only modest effects on FPG and HbA1c, and that it does not achieve levels in them that indicate the success of treatment, although it may provide some additional lowering. Our findings agree with the recommendations of professional and authoritative bodies. The National Institutes of Health (NIH) National Center for Complementary and Integrative Health (NCCIH) does not recognize the use of cinnamon for either the treatment or prevention of diabetes or other disease.42 Neither the American Diabetes Association (Grade C evidence level for use of cinnamon for treatment of diabetes)2 nor the Canadian Diabetes Association (Grade D evidence level for individuals with diabetes) endorse its use.43 The Canadian Diabetes Association has concluded that it is premature to recommend cinnamon for widespread use but that it merits consideration for further research.43 Nevertheless, in spite of these authoritative recommendations, some patients with diabetes consume cinnamon containing dietary supplements, perhaps in the hopes that they will complement standard medical therapies for diabetes management.44
This review highlights many of the common problems with studies of botanical dietary supplements and of meta-analyses that do not adhere to PRISMA guidelines45.
Dietary supplements are not approved by the Food and Drug Administration to treat disease although over 800 supplement products in the US marketplace contain cinnamon. Many claim to assist in blood sugar regulation.46 Cinnamon bark is regulated as a food additive and as a dietary supplement ingredient in the US, and C. cassia, burmanni and loureirii are listed as Generally Recognized as Safe (GRAS) for use in culinary applications. The FDA Code of Federal Regulations under GRAS Sec. 182.10 Spices and other natural seasonings and flavorings47 states that “the quantity of a substance added to food does not exceed the amount reasonably required to accomplish its intended physical, nutritional, or other technical effect in food.” GRAS levels are substantially lower than doses of 2-4 gm cinnamon bark that have been suggested in the German Commission E Monograph for preventive or therapeutic purposes.48 Very few cinnamon supplements have been tested for their clinical efficacy or safety at the doses recommended. Currently, 15 studies of cinnamon interventions for therapeutic purposes are listed in clinicaltrials.gov, including 10 focused on studying hypoglycemic effects on blood glucose and HbA1c, or other outcomes such as gastric emptying or post-prandial blood glucose that employ varying doses of cinnamon extracts ranging from 1-9 gm/d.
Very little safety data has been collected from clinical trials to evaluate cinnamon's adverse effects. The most common are irritation or contact allergies.49 C. cassia (Chinese) cinnamon has been safely used in clinical trials lasting up to 4 months and appears to be well-tolerated in spite of the fact that some forms of it are high in coumarin, a benzo[a]pyrene, a known hepatotoxin and nephrotoxin in both rodents and humans when fed at high doses.50, 51 Bark from C. verum (Ceylon cinnamon) has lower levels of coumarin than C. cassia but it is expensive and rarely used in foods and supplements.51 Caution is indicated in using high doses that have not been assessed for safety. Although the American Herbal Products Association Botanical Safety Handbook lists cinnamon as safety class 2b (not for use in pregnancy except under the supervision of a qualified healthcare practitioner), no clinical trials on potential drug or supplement interactions are available.52
Double-blind placebo controlled RCTs are regarded as the gold standard for causal inference. Of the 11 studies reviewed, 8 were double-blind, all were placebo controlled, and 8 were analyzed using the stronger intention-to-treat analysis including all patients in the data analysis rather than per protocol analysis. However, the majority of the studies lacked a description of product authentication describing how the investigator ensured the integrity of the product raising questions regarding the quality of the intervention and the reported results. While clinically significant lowering of FPG and HbA1c occurred in a small subset of the clinical studies, sample sizes were small and some trials were conducted among subjects who often had either exceedingly high or only modestly elevated levels of FPG, and who were being treated at the same time with hypoglycemic agents.
Larger high quality RCTs are needed in well-defined population groups utilizing well-defined interventions in order to determine the efficacy if any, of using cinnamon in patients with diabetes. Whether the long-term use of cinnamon can reduce the risk of diabetes progression is yet to be determined. The data from this narrative review suggest that additional studies, such as dose-titration or dose ranging studies of sufficient sample size with a well-defined cinnamon intervention in T2DM might be an appropriate next research step.
The strengths of our review include its limited focus to studies on patients with T2DM, and inclusion of several studies published after the meta-analyses28-32 were completed. It highlighted the heterogeneity of the products used, the lack of details about them, including the identification and quantification of the presumed bioactives or marker compounds, standardization of the product, and verification of product ingredients. It also emphasizes the need for caution in accepting meta-analyses that do not adhere to PRISMA guidelines at face value. A limitation of our review is that a full systematic review or meta-analysis as outlined by PRISMA guidelines was not conducted; hence neither study bias nor assignment of a study grade was given to the RCTs we examined.
We conclude that the evidence to date does not suggest that the addition of a cinnamon supplement achieve hypoglycemic treatment goals nor cause a reliable and clinically significant drop in FPG or HbA1c in patients with T2DM. Dietitians and other health professionals should recommend that patients with diabetes follow existing recommendations of authoritative bodies for diet and lifestyle changes, along with their hypoglycemic drugs if they are prescribed.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease Control and Prevention [December 12, 2015];2014 National Diabetes Statistics Report. http://www.cdc.gov/diabetes/data/statistics/2014statisticsreport.html. Last updated May 15, 2015.
- 2.American Diabetes Association Standards of medical care in diabetes--2016. Diabetes Care. 2016;39(Suppl 1):S6–59. doi: 10.2337/dc16-er09. [DOI] [PubMed] [Google Scholar]
- 3.Chamberlain JJ, Rhinehart AS, Shaefer CF, Jr., Neuman A. Diagnosis and Management of Diabetes: Synopsis of the 2016 American Diabetes Association Standards of Medical Care in Diabetes. Ann Intern Med. 2016;164(8):542–52. doi: 10.7326/M15-3016. [DOI] [PubMed] [Google Scholar]
- 4.American Botanical Council . Herbal Medicine: Expanded Commission E Monographs. Integrative Medicine Communications; Newton, MA: 2000. [Google Scholar]
- 5.Ayurveda Pharmacopoeia Committee . The Ayurvedic Pharmacopoeia of India, Part I, Volume VI. Department of Ayurveda, Yoga & Naturopathy, Unani, Siddha and Homoeopathy (AYUSH); New Delhi: 1989. Darusita Taila. pp. 111–112. [Google Scholar]
- 6.Felter HW, Lloyd JU. King's American Dispensatory. Eclectic Institute; Sandy, OR: 1993. [Google Scholar]
- 7.Abascal K, Yarnell E. The medicinal uses of cinnamon. Integrative Med. 2010;9(1):28–32. [Google Scholar]
- 8.Jayaprakasha GK, Rao LJ. Chemistry, biogenesis, and biological activities of Cinnamomum zeylanicum. Crit Rev Food Sci Nutr. 2011;51(6):547–562. doi: 10.1080/10408391003699550. [DOI] [PubMed] [Google Scholar]
- 9.International Trade Centre (ITC) [April 11, 2016];Update of USA Spice & Herb Imports. http://www.intracen.org/uploadedFiles/intracenorg/Content/Exporters/Market_Data_and_Information/Market_information/Market_Insider/Spices/USA%20June%20%202014%20stat%20update%20docx.pdf. Published 2014.
- 10.Nutrition Business Journal. Market Data & Overview. 2015:26. [Google Scholar]
- 11.Cheng DM, Kuhn P, Poulev A, Rojo LE, Lila MA, Raskin I. In vivo and in vitro antidiabetic effects of aqueous cinnamon extract and cinnamon polyphenol-enhanced food matrix. Food Chem. 2012;135(4):2994–3002. doi: 10.1016/j.foodchem.2012.06.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engels G, Brinckmann J. [April 11 2016];Cinnamon. http://cms.herbalgram.org/herbalgram/issue95/hg95-herbpro-cinnamon.html?ts=1460496353&signature=2ab407a93ce945f19834fdd6f8777747. Published 2012.
- 13.Avula B, Smillie TJ, Wang YH, Zweigenbaum J, Khan IA. Authentication of true cinnamon (Cinnamon verum) utilising direct analysis in real time (DART)-QToF-MS. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2015;32(1):1–8. doi: 10.1080/19440049.2014.981763. [DOI] [PubMed] [Google Scholar]
- 14.Gruenwald J, Freder J, Armbruester N. Cinnamon and health. Crit Rev Food Sci Nutr. 2010;50(9):822–834. doi: 10.1080/10408390902773052. [DOI] [PubMed] [Google Scholar]
- 15.Medagama AB. The glycaemic outcomes of Cinnamon, a review of the experimental evidence and clinical trials. Nutr J. Oct 16. 2015;14:108. doi: 10.1186/s12937-015-0098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beejmohun V, Peytavy-Izard M, Mignon C, et al. Acute effect of Ceylon cinnamon extract on postprandial glycemia: alpha-amylase inhibition, starch tolerance test in rats, and randomized crossover clinical trial in healthy volunteers. BMC Complement Altern Med. Sep 23. 2014;14:351. doi: 10.1186/1472-6882-14-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qin B, Dawson HD, Schoene NW, Polansky MM, Anderson RA. Cinnamon polyphenols regulate multiple metabolic pathways involved in insulin signaling and intestinal lipoprotein metabolism of small intestinal enterocytes. Nutrition. 2012;28(11-12):1172–1179. doi: 10.1016/j.nut.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 18.Cao H, Polansky MM, Anderson RA. Cinnamon extract and polyphenols affect the expression of tristetraprolin, insulin receptor, and glucose transporter 4 in mouse 3T3-L1 adipocytes. Arch Biochem Biophys. 2007;459(2):214–222. doi: 10.1016/j.abb.2006.12.034. [DOI] [PubMed] [Google Scholar]
- 19.Ranasinghe P, Jayawardana R, Galappaththy P, Constantine GR, de Vas Gunawardana N, Katulanda P. Efficacy and safety of 'true' cinnamon (Cinnamomum zeylanicum) as a pharmaceutical agent in diabetes: a systematic review and meta-analysis. Diabet Med. 2012;29(12):1480–1492. doi: 10.1111/j.1464-5491.2012.03718.x. [DOI] [PubMed] [Google Scholar]
- 20.Jarvill-Taylor KJ, Anderson RA, Graves DJ. A hydroxychalcone derived from cinnamon functions as a mimetic for insulin in 3T3-L1 adipocytes. J Am Coll Nutr. 2001;20(4):327–336. doi: 10.1080/07315724.2001.10719053. [DOI] [PubMed] [Google Scholar]
- 21.Khan A, Safdar M, Ali Khan MM, Khattak KN, Anderson RA. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care. 2003;26(12):3215–3218. doi: 10.2337/diacare.26.12.3215. [DOI] [PubMed] [Google Scholar]
- 22.Crawford P. Effectiveness of cinnamon for lowering hemoglobin A1C in patients with type 2 diabetes: a randomized, controlled trial. J Am Board Fam Med. 2009;22(5):507–512. doi: 10.3122/jabfm.2009.05.080093. [DOI] [PubMed] [Google Scholar]
- 23.Mang B, Wolters M, Schmitt B, et al. Effects of a cinnamon extract on plasma glucose, HbA, and serum lipids in diabetes mellitus type 2. Eur J Clin Invest. 2006;36(5):340–344. doi: 10.1111/j.1365-2362.2006.01629.x. [DOI] [PubMed] [Google Scholar]
- 24.Stoecker BJ, Zhan Z, Luo R, et al. innamon extract lowers blood glucose in hyperglycemic subjects. (abstract). FASEB J. 2010;24:722.721. [Google Scholar]
- 25.Blevins SM, Leyva MJ, Brown J, Wright J, Scofield RH, Aston CE. Effect of cinnamon on glucose and lipid levels in non insulin-dependent type 2 diabetes. Diabetes Care. 2007;30(9):2236–2237. doi: 10.2337/dc07-0098. [DOI] [PubMed] [Google Scholar]
- 26.Suppapitiporn S, Kanpaksi N, Suppapitiporn S. The effect of cinnamon cassia powder in type 2 diabetes mellitus. J Med Assoc Thai. 2006;89(Suppl 3):S200–205. [PubMed] [Google Scholar]
- 27.Vanschoonbeek K, Thomassen BJ, Senden JM, Wodzig WK, van Loon LJ. Cinnamon supplementation does not improve glycemic control in postmenopausal type 2 diabetes patients. J Nutr. 2006;136(4):977–980. doi: 10.1093/jn/136.4.977. [DOI] [PubMed] [Google Scholar]
- 28.Akilen R, Tsiami A, Devendra D, Robinson N. Cinnamon in glycaemic control: Systematic review and meta analysis. Clin Nutr. 2012;31(5):609–615. doi: 10.1016/j.clnu.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Allen RW, Schwartzman E, Baker WL, Coleman CI, Phung OJ. Cinnamon use in type 2 diabetes: an updated systematic review and meta-analysis. Ann Fam Med. 2013;11(5):452–459. doi: 10.1370/afm.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baker WL, Gutierrez-Williams G, White CM, Kluger J, Coleman CI. Effect of cinnamon on glucose control and lipid parameters. Diabetes Care. 2008;31(1):41–43. doi: 10.2337/dc07-1711. [DOI] [PubMed] [Google Scholar]
- 31.Davis PA, Yokoyama W. Cinnamon intake lowers fasting blood glucose: meta-analysis. J Med Food. 2011;14(9):884–889. doi: 10.1089/jmf.2010.0180. [DOI] [PubMed] [Google Scholar]
- 32.Leach MJ, Kumar S. Cinnamon for diabetes mellitus. Cochrane Database Syst Rev. 2012;9:CD007170. doi: 10.1002/14651858.CD007170.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akilen R, Tsiami A, Devendra D, Robinson N. Glycated haemoglobin and blood pressure-lowering effect of cinnamon in multi-ethnic Type 2 diabetic patients in the UK: a randomized, placebo-controlled, double-blind clinical trial. Diabet Med. 2010;27(10):1159–1167. doi: 10.1111/j.1464-5491.2010.03079.x. [DOI] [PubMed] [Google Scholar]
- 34.Khan R, Khan Z, Shah S. Cinnamon may reduce glucose, lipid and cholesterol level in type 2 diabetic individuals. Pakistan J Nutr. 2010;9(5):430–433. [Google Scholar]
- 35.Lu T, Sheng H, Wu J, Cheng Y, Zhu J, Chen Y. Cinnamon extract improves fasting blood glucose and glycosylated hemoglobin level in Chinese patients with type 2 diabetes. Nutr Res. 2012;32(6):408–412. doi: 10.1016/j.nutres.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Mirfeizi M, Mehdizadeh Tourzani Z, Mirfeizi SZ, et al. Controlling diabetes mellitus type 2 with herbal medicines: A triple blind, randomized clinical trial of efficacy and safety. J Diabetes. 2015 Sep 11; doi: 10.1111/1753-0407.12342. doi: 10.1111/1753-0407.12342. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 37.Vafa M, Mohammadi F, Shidfar F, et al. Effects of cinnamon consumption on glycemic status, lipid profile and body composition in type 2 diabetic patients. Int J Prev Med. 2012;3(8):531–536. [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma P, Sharma S, Agrawal RP, Agrawal V, Singhal S. A randomised double blind placebo control trial of cinnamon supplementation on glycemic control and lipid profile in type 2 diabetes mellitus. Aust J Herbal Med. 2012;24(1):4–9. [Google Scholar]
- 39.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 40.Nathan DM, Bayless M, Cleary P, et al. Diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: advances and contributions. Diabetes. 2013;62(12):3976–3986. doi: 10.2337/db13-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kirkham S, Akilen R, Sharma S, Tsiami A. The potential of cinnamon to reduce blood glucose levels in patients with type 2 diabetes and insulin resistance. Diabetes Obes Metab. 2009;11(12):1100–1113. doi: 10.1111/j.1463-1326.2009.01094.x. [DOI] [PubMed] [Google Scholar]
- 42.National Center for Complementary and Integrative Health (NCCIH) [December, 2015];Cinnamon. https://nccih.nih.gov/health/cinnamon. Published 2011.
- 43.Nahas R, Goguen J. Natural health products. Can J Diabetes. 2013;37(Suppl 1):S97–99. doi: 10.1016/j.jcjd.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 44.Bailey RL, Gahche JJ, Miller PE, Thomas PR, Dwyer JT. Why US adults use dietary supplements. JAMA Intern Med. 2013;173(5):355–361. doi: 10.1001/jamainternmed.2013.2299. [DOI] [PubMed] [Google Scholar]
- 45.Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 46.Natural Medicines Comprehensive Database . Cassia Cinnamon. Therapeutic Research Faculty; Stockton, CA: 1995-2015. [December 12, 2015]. http://naturaldatabase.therapeuticresearch.com/nd/Search.aspx?cs=CEPDA&s=ND&pt=100&sh=15&id=1002. [Google Scholar]
- 47.Food and Drug Administration . Spices and other natural seasonings and flavorings. US Department of Health and Human Services.; 2015. Substances Generally Regarded As Safe. 21CFR182.10. [Google Scholar]
- 48.Blumenthal M, Busse WR, Goldberg A, Gruenwald J, Hall T, Riggins CW. The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines. American Botanical Council; Austin, TX: 1998. [Google Scholar]
- 49.Dugoua JJ, Seely D, Perri D, et al. From type 2 diabetes to antioxidant activity: a systematic review of the safety and efficacy of common and cassia cinnamon bark. Can J Physiol Pharmacol. 2007;85(9):837–847. doi: 10.1139/Y07-080. [DOI] [PubMed] [Google Scholar]
- 50.Born SL, Api AM, Ford RA, Lefever FR, Hawkins DR. Comparative metabolism and kinetics of coumarin in mice and rats. Food Chem Toxicol. 2003;41(2):247–258. doi: 10.1016/s0278-6915(02)00227-2. [DOI] [PubMed] [Google Scholar]
- 51.Felter SP, Vassallo JD, Carlton BD, Daston GP. A safety assessment of coumarin taking into account species-specificity of toxicokinetics. Food Chem Toxicol. 2006;44(4):462–475. doi: 10.1016/j.fct.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 52.American Herbal Products Association . American Herbal Products Association's Botanical Safety Handbook. Second Edition CRC Press; Boca Raton, FL: 2013. [Google Scholar]