Abstract
Objectives
We sought to describe blood pressure changes after antiretroviral therapy (ART) initiation and evaluate the association of markers of inflammation with incident hypertension in a cohort of HIV-individuals in Uganda.
Methods
We used mixed effects linear regression to model changes in systolic blood pressure (BP) over time among a cohort of HIV-infected individuals initiating ART in Uganda. After exclusion of participants with pre-existing hypertension, we identified participants with normal BP throughout follow-up (controls) and those with elevated BP on ≥3 consecutive visits (cases). Prior to ART initiation, participants had testing for lnterleukin-6, kynurenine/tryptophan ratio, lipopolysaccharide, soluble CD14, soluble CD163, and D-dimer and those with viral suppression at six months during ART had repeat tests. We fit logistic regression models to estimate associations between biomarkers and risk of incident hypertension.
Results
In the entire cohort, systolic BP increased by 9.6 mmHg/year (95% CI 7.3 - 11.8) in the first six months of ART, then plateaued. Traditional factors: male gender (AOR 2.76, 95% CI 1.34-5.68), age (AOR 1.09, 95% CI 1.04-1.13), overweight (AOR 4.48, 95%CI 1.83-10.97), and a CD4 count < 100 cells (AOR 3.08, 95% CI 1.07-8.89) were associated with incident hypertension. After adjusting for these, D-dimer levels at month 6 were inversely associated with incident hypertension (AOR 0.61, 95% CI 0.37-0.99). Although not significant, similar associations were seen with sCD14 and Kynurenine/Tryptophan (K/T) ratio.
Conclusion
Blood pressure increases early after ART initiation in Ugandans. Traditional risk factors, rather than immune activation were associated with incident hypertension in this population.
Keywords: D-dimer, Immune activation, Hypertension, Antiretroviral therapy, Africa
Background
Early HIV infection is characterized by a dramatic depletion of CD4+ T cells in the gut, leading to depletion of the immunity at the gastrointestinal mucosal surface1. This heightens translocation of microbial products, particularly lipopolysaccharide (LPS), into the systemic circulation2-4. The resultant immune activation escalates monocyte activation 2,5, often measured by the level of circulating soluble CD14 (sCD14) and soluble CD163 (sCD163). Although these inflammatory markers have been associated with an increased risk of hypertension in the general population 6,7, few data are available on associations between ART, inflammation, and hypertension in HIV-infected individuals.
Data on the epidemiology of hypertension among HIV-infected persons in sub-Saharan Africa have demonstrated conflicting findings. While a number of studies have reported high incidence rates 8-12, others suggest that people with HIV are at lower risk of hypertension than matched HIV uninfected counterparts 13-16. However, these studies are confounded by the inconsistent inclusion of patients with and without AIDS as well as the variable use and duration of ART in the cohorts17.
It remains unclear whether HIV-mediated systemic inflammation and immune activation increases hypertension risk. If they did, then inflammatory biomarkers may be good candidates for identifying HIV-infected patients at risk of hypertension. Furthermore, a better understanding of these relationships could help identify pathophysiologic mechanisms of cardiovascular disease in this population. We therefore sought to describe blood pressure changes after ART initiation in a cohort of HIV-individuals initiating ART in Uganda, and hypothesized that biomarkers of inflammation (lnterleukin-6 [IL6]), indoleamine 2,3-dioxygenase-1 (IDO) activity as measured by kynurenine/tryptophan [K/T] ratio), microbial translocation (plasma lipopolysaccharide [LPS]), monocyte activation (soluble CD14, soluble CD163), and altered coagulation (D-dimer), would be associated with incident hypertension.
Methods
Study population
We conducted a closed cohort study nested within the Uganda AIDS Rural Treatment Outcomes (UARTO) cohort, which has been described in detail previously 18. The UARTO cohort is a population-based prospective cohort study of HIV-infected adults, aged at least 18 years, living within a 60-kilometer radius around the Mbarara Regional Referral Hospital Immune Suppression Syndrome (ISS) clinic. Participants were enrolled just prior to initiation of ART and were observed every 3-4 months to collect biologic and socio-behavioral data. By November 2014, 771 individuals were enrolled in UARTO and 94% initiated ART within four days of enrolment. Enrollment started in 2005, and follow-up for health events was completed in September 2015. For this analysis, study participants were observed from enrollment into the UARTO cohort until the earliest of the date of hypertension diagnosis or their last recorded clinic visit with a blood pressure measurement. Of note, all UARTO participants received biomarker testing prior to ART initiation but only those with HIV viral load suppression (< 400 viral copies/mL) at 6 months of ART had repeat biomarker tests done. Therefore, to describe the mean change in blood pressure, all UARTO cohort data was used. However, only the data of those with pre-ART and on-ART biomarkers data were used to determine the association of biomarker levels with incident hypertension in an unmatched case-control design.
The ethical review boards at Mbarara University of Science & Technology, University of California San Francisco, Partners Healthcare, and Uganda National Council of Science and Technology, approved the conduct of this study. All participants signed written informed consent.
Data collection
Biomarker measurements
At enrollment into UARTO (just prior to ART initiation) and six months later, participants underwent blood draw for CD4+ T cell count and inflammatory biomarker testing. A trained laboratory technician collected about 40-mL of venous blood into sodium heparin-lined vacutainers. All plasma samples were drawn in the morning before 9:30am. Blood samples were centrifuged immediately after blood draw, and derivative plasma aliquots were then preserved at -80°C until shipment for laboratory testing at the University of California San Francisco, California, USA.
Thawed cryopreserved plasma from the pre-ART and 6 months time points were assessed for several biomarkers. Plasma lipopolysaccharide (LPS) levels were measured from Anticoagulant Citrate Dextrose (ACD) - anticoagulated plasma with the Limulus amebocyte assay as described previously19. We used liquid chromatography–tandem mass spectrometry to assess kynurenine and tryptophan levels20. Cryopreserved plasma was also assessed for soluble CD14 (sCD14, R&D Systems), soluble CD163 (sCD163, Trillium Diagnostics), interleukin-6 (IL-6, Human IL-6 Ultra-Sensitive Kit, Meso Scale Diagnostics), and D-dimer (Diagnostico Stago). We chose biomarkers based on prior data which observed important relationships between each of these markers and cardiovascular disease outcomes, mortality, or both 2,21-24.
Blood pressure measurements
Separate from the UARTO study visits, participants were seen at the Immune Suppression Syndrome (ISS) Clinic at Mbarara Regional Referral Hospital for routine clinical care every 2 weeks to 3 months, as determined by clinical staff. At the ISS clinic, trained nurses measured blood pressure at each visit using an aneroid sphygmomanometer (Welch Allyn® Tycos 767 Series; Skaneateles Falls, New York, USA), with normal (22–32 cm) cuff sizes. We defined hypertension as occurrence of a systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg on at least three consecutive clinic visits or prescription of antihypertensive medications after ART initiation.
Weight and height were measured using standardized scales (seca 762, Hanover, USA) and roll-up measuring stadiometers (seca 206, Hanover, USA), respectively. Height was measured to the nearest 0.1 cm after removal of shoes. Weight was measured to the nearest kilogram after the participants had removed their shoes, as well as any heavy clothing. We used height and weights to calculate body mass indexes (BMI) as weight (in kilograms) divided by the square of height (in metres squared) for all participants, and categorized BMI as less than 25, 25 to 29.9, or 30 or greater.
We set implausible values as missing, including systolic blood pressure recorded as >300 or <50, diastolic blood pressure recorded as >200 or <30mmHg and weight <25kg or >200kg. In the ISS clinic, clinical data were collected on paper forms and subsequently entered into an Open Medical Records System electronic database as part of the International Epidemiologic Databases to Evaluate AIDS Project25.
Statistical analysis
We conducted two principal analyses. In the first, we included all participants in the cohort with biomarker data and at least one blood pressure measurement after ART initiation. We fit mixed effects linear regression models with systolic blood pressure as the dependent variable, duration of ART as a fixed effect variable with spline breakpoints at 6 months, 1 year, and 5 years, and a random effect by participant to estimate changes in mean systolic blood pressure over time from after ART initiation.
We then conducted a nested case-control analysis to estimate associations between inflammatory markers at pre-ART and 6-month on ART time points with incident hypertension. We restricted this analysis to study participants who had three or more consecutive visits with high blood pressure during follow-up (cases) or those who had no visits with high blood pressure during follow-up (controls). We plotted mean levels of biomarkers at pre-ART and months 6 to show change in biomarkers over 6 months. We fit logistic regression models to calculate odds ratios for incident hypertension, including traditional risk factors (age, gender, BMI, smoking history, and pre-treatment CD4 count); and then added each biomarker at the pre-ART and 6 month visit individually into the model. Models at the pre-ART visit were adjusted for viral load, whereas those at 6 months were not because biomarker testing at 6 months of ART was restricted to participants with a suppressed HIV RNA viral load (< 400 copies/mL). Biomarkers were modeled as changes in each interquartile range (IQR) of the log-transformed value.
Sensitivity analysis
We performed sensitivity analyses to assess the robustness of our findings. First, we altered our definition of the outcome to include either prevalent or incident hypertension (as opposed to incident hypertension alone). Then, we altered our definition of a case to include any participant who was prescribed any anti-hypertensive medication at least once during follow-up. All analyses were performed with STATA® version 13 (College Station, Texas, USA).
Results
As of November 2014, 771 participants had been enrolled into the UARTO cohort study. We excluded 225 who had no biomarker testing available and 10 participants with no blood pressure measurements (Figure 1). Among the remaining 536 participants, females constituted the majority (70%) and the median age at ART initiation was 34 years IQR (29 - 39) (Table 1).
Figures 1. Study profile showing selection of subjects.
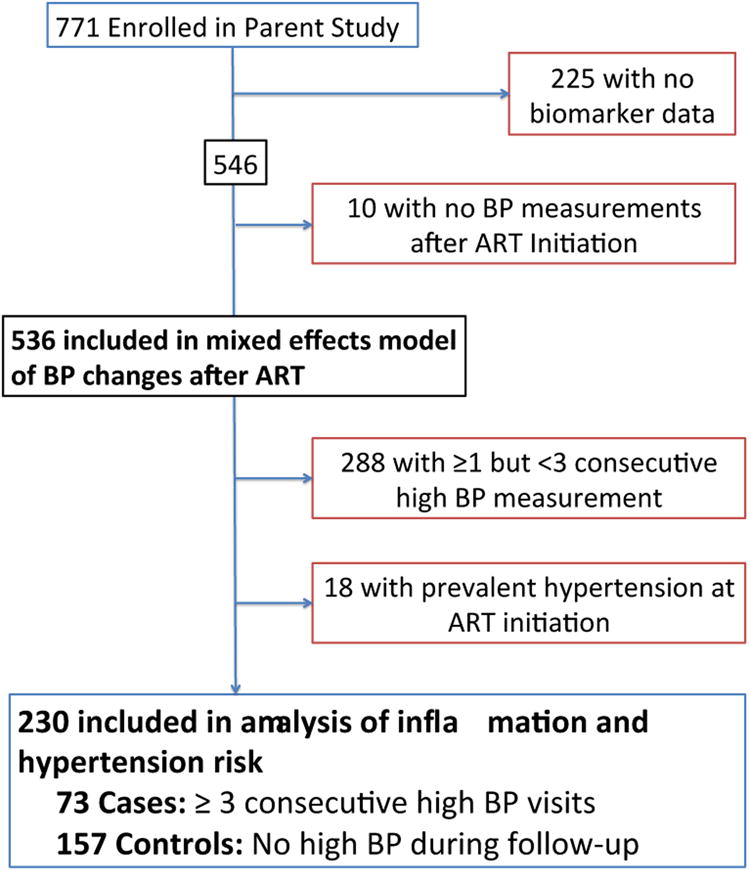
Table 1. Baseline characteristics.
| Characteristic | Cases n=73 (31%) |
Controls, n=157 (69%) |
|---|---|---|
| Male gender, n (%) | 30 (41.10) | 40 (25.48) |
|
| ||
| Age at ART initiation, median (IQR) | 36 (32 - 43) | 33 (28 - 38) |
|
| ||
| Smoking Status | ||
|
| ||
| Never | 53 (72.60) | 119 (75.80) |
| Past or current | 18 (26.14) | 36 (22.93) |
|
| ||
| BMI (Kg/m2) categories, n (%) | ||
|
| ||
| < 25 | 50 (68.49) | 128 (81.53) |
| 25 - 29.9 | 15 (20.55) | 17 (10.83) |
| ≥ 30 | 4 (5.48) | 2 (1.27) |
| Missing | 4 (5.48) | 10 (6.37) |
|
| ||
| CD4+ T cell counts (cells/mm3) at ART initiation, n (%) | ||
|
| ||
| < 100 | 29 (39.73) | 48 (30.57) |
| 100 – 249 | 35 (47.95) | 82 (52.23) |
| ≥ 250 | 9 (12.33) | 27 (17.20) |
| Missing | 1 (1.37) | 4 (2.55) |
|
| ||
| HIV viral load (Log10/mm3), median (IQR) | 5.09 (4.72 - 5.63) | 5.07 (4.60 - 5.50) |
|
| ||
| Duration of observation until censor (years), median (IQR) | 7.29 (6.37 - 7.87) | 5.76 (1.59 - 7.14) |
|
| ||
| Clinic visits with blood pressure measurement during observation, median (IQR) | 35 (29 - 38) | 27 (13 - 36) |
BMI: Body mass index; ART: Antiretroviral therapy; CD4: cluster differentiation cells; IQR: interquartile range
In the total cohort of 536 participants, systolic blood pressure increased significantly at 9.6 mmHg/year (95%CI 7.3 - 11.8) in the first six months after ART initiation (Figure 2). However, blood pressure plateaued thereafter, with total average increase in systolic blood pressure of 2.3 mmHg/year (95%CI 2.1 - 2.5) from years 1 through 5 of observation, and only 0.7 mmHg/year thereafter (95%CI 0.3 - 1.0). We found no difference in mean systolic blood pressure between those with undetectable HIV viremia versus detectable HIV viremia at 6 months (p=0.18). In contrast, increasing weight from ART initiation to 6 months time point was significantly correlated with increasing systolic blood pressure over first 6 months (R2 = 0.14, p <0.0001).
Figure 2. Change in mean systolic blood pressure by ART duration for patients who developed hypertension and those who remained normotensive during follow-up.
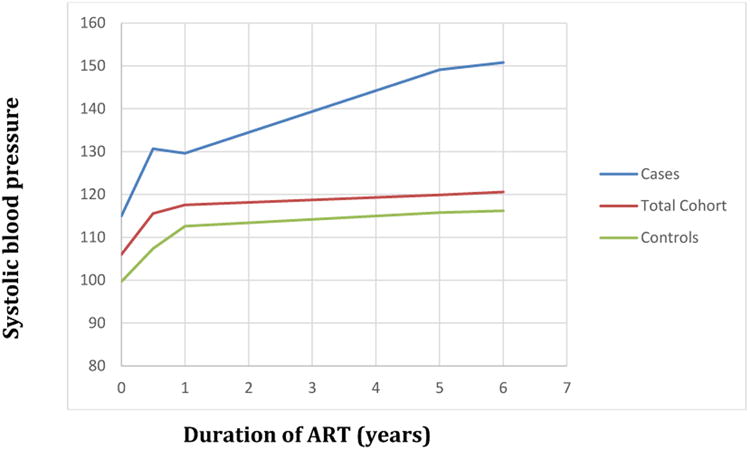
Mixed effects linear regression model with random effect by participant and fixed effect by hypertension status and ART duration with spline terms at 6months, 1, and 5 years. Total cohort consisted of 536, cases were 73, and controls were 157.
In the restricted case-control analysis, we excluded 18 participants with prevalent hypertension before ART initiation and 288 participants who had at least one but never 3 consecutive visits with hypertension during follow-up. We identified 73 participants who had ≥3 consecutive high blood pressure visits after ART initiation (cases), and 157 participants who never had elevated blood pressure readings on a minimum 3 consecutive visits after ART initiation (controls) (Table 1).
We found significant associations between traditional risk factors and incident hypertension in multivariable logistic regression models for each year increase in age (AOR 1.09, 95% CI 1.04 - 1.13, p<0.001), male gender (AOR 2.76, 95% CI 1.34 - 5.68, p=0.006), overweight (AOR 4.48, 95%CI 1.83 - 10.97, p=0.001), obesity (AOR 8.36, 95% CI 1.27 - 54.79, p=0.027), and a low pre-ART CD4+ T cell count (< 100 cells) at ART initiation (AOR 3.08, 95% CI 1.07 - 8.89, p=0.037) with incident hypertension (Table 2).
Table 2. Association between traditional risk factors, biomarkers and incident hypertension.
| Characteristic | Univariable models | Multivariable models | ||
|---|---|---|---|---|
|
| ||||
| *OR (95%CI) | p-Value | *AOR (95%CI) | p-Value | |
| Age (each year) | 1.07 (1.04 - 1.11) | <0.001 | 1.09 (1.04 - 1.13) | <0.001 |
|
| ||||
| Male gender | 2.04 (1.13 - 3.67) | 0.018 | 2.85 (1.37 - 5.94) | 0.005 |
|
| ||||
| Ever smoked | 1.12 (0.58 - 2.15) | 0.728 | -- | -- |
|
| ||||
| Body mass index (kg/m2) at ART initiation | ||||
|
| ||||
| <25 | REF | -- | REF | -- |
| 25 - 29.9 | 2.26 (1.05 - 4.87) | 0.037 | 4.47 (1.82 - 10.96) | 0.001 |
| ≥ 30 | 5.12 (0.91 - 28.84) | 0.064 | 8.30 (1.26 - 54.79) | 0.028 |
|
| ||||
| CD4+ T cell counts (cells/mm3) at ART Initiation | ||||
|
| ||||
| ≥ 250 | REF | -- | REF | -- |
| 100 - 249 | 1.23 (0.50 - 3.01) | 0.655 | 1.43 (0.51 - 4.01) | 0.500 |
| <100 | 1.74 (0.69 - 4.39) | 0.243 | 3.42 (1.09 - 10.74) | 0.035 |
|
| ||||
| Log10 pre-ART HIV viralload (each viral copy/mL) | 1.12 (0.75 - 1.66) | 0.592 | 0.88 (0.53 - 1.43) | 0.624 |
| Log10 sCD163 | ||||
|
| ||||
| ART initiation | 1.11 (0.78 - 1.59) | 0.590 | 1.01 (0.65 - 1.59) | 0.947 |
| 6 months post-ART initiation | 1.14 (0.73 - 1.79) | 0.600 | 1.07 (0.64 - 1.78) | 0.801 |
|
| ||||
| Log10 sCD14 | ||||
|
| ||||
| ART initiation | 1.24 (0.81 - 1.89) | 0.312 | 1.26 (0.72 - 2.20) | 0.420 |
| 6 months post-ART initiation | 0.73 (0.49 - 1.07) | 0.105 | 0.79 (0.50 - 1.27) | 0.340 |
|
| ||||
| Log10 lipopolysaccharide | ||||
|
| ||||
| ART initiation | 0.94 (0.64 - 1.38) | 0.767 | 1.07 (0.69 - 1.66) | 0.760 |
| 6 months post-ART initiation | 1.06 (0.69 - 1.63) | 0.707 | 1.13 (0.69 - 1.86) | 0.624 |
|
| ||||
| Log10 K/T ratio | ||||
|
| ||||
| ART initiation | 0.87 (0.59 - 1.30) | 0.509 | 0.89 (0.54 - 1.46) | 0.638 |
| 6 months post-ART initiation | 0.74 (0.49 - 1.09) | 0.129 | 0.91 (0.58 - 1.42) | 0.683 |
|
| ||||
| Log10 IL6 | ||||
|
| ||||
| ART initiation | 1.12 (0.81 - 1.56) | 0.483 | 1.12 (0.73 - 1.74) | 0.600 |
| 6 months post-ART initiation | 1.02 (0.73 - 1.43) | 0.916 | 0.98 (0.66 - 1.47) | 0.934 |
|
| ||||
| Log10 D-dimer | ||||
|
| ||||
| ART initiation | 0.87 (0.59 - 1.28) | 0.491 | 0.85 (0.54 - 1.32) | 0.460 |
| 6 months post-ART initiation | 0.62 (0.39 - 0.97) | 0.038 | 0.61 (0.37 - 0.99) | 0.049 |
Odds ratios (OR) or Adjusted Odds ratios (OR) of having the given predictor value compared to the reference value by hypertension status.
Adjusted Odds ratios (AOR) of having the given predictor value compared to the reference value by hypertension status. For biomarkers at ART initiation, models are adjusted for age, gender, BMI, pre-ART CD4+ T cell count, and Log10 pre-ART HIV RNA viral load. For biomarkers at 6 months post-ART initiation, models are adjusted for age, gender, BMI, and pre-ART CD4+ T cell count since all participants had viral suppression.
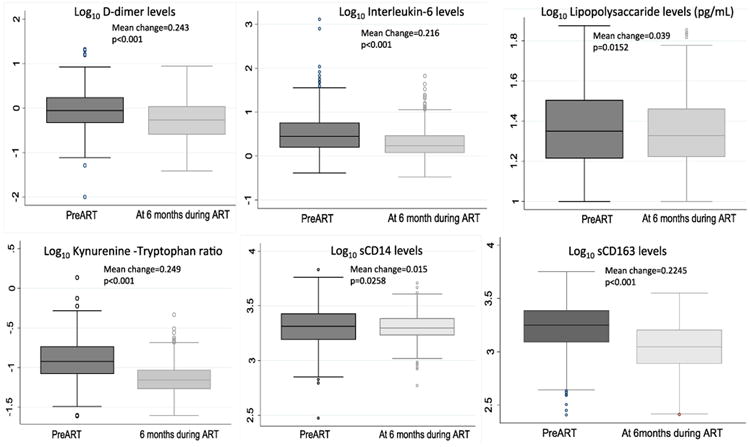
At month 6 of ART-mediated viral suppression, all biomarkers had declined from pre-ART levels. The greatest mean change from pre-ART to 6 months levels were observed in D-dimer, K/T ratio, interleukin-6, and sCD163 (Figure 3). In multivariable logistic regression models adjusted for known cardiovascular risk factors, each unit interquartile range decrease in D-dimer levels at 6 months (while taking ART) was associated with an increased risk of incident hypertension (AOR 0.61, 95% CI 0.37 - 0.99, p=0.049). Although we found no other significant associations between other biomarkers and incident hypertension (Table 2), the estimates indicated that lower levels of IL-6 and sCD14 at 6 months were associated with an increased risk of incident hypertension.
Figure 3. Change in biomarker levels from preART to 6months time point for all participants.
Sensitivity analyses
Anti-hypertensive medications were prescribed at approximately 1% of all observed visits (220/18,698) and were ever prescribed in only 30% (22/74) of patients with three or more consecutive visits with high blood pressure; 21 of these were prescribed antihypertensive medication after ART initiation and were already considered cases. In comparison, 7 participants among controls were ever prescribed antihypertensive medication (3 before starting ART and 4 after starting ART) and an additional 18 participants who had been neither classified as cases nor controls in the main models were prescribed antihypertensive medication at least once after starting ART. After reclassifying these 22 participants as cases, we found no significant differences in estimates between models with or without antihypertensive medication use as part of the definition of incident hypertension.
Discussion
In this study of HIV-infected individuals taking ART in southwestern Uganda, we found that blood pressure increases rapidly in the months immediately following initiation of antiretroviral therapy. We hypothesize that this represents an ART-mediated “return to health” 26, which may in turn unmask other mechanisms of hypertension risk such as increased body mass index and lipid abnormalities. Although our study did not have an HIV-uninfected group for comparison, the elevations we observed in blood pressure are comparable to those described in HIV cohorts 27-29 though greater than what would be expected over the same period in the general population 30. In support of this ART-mediated “return to health” hypothesis, we observed a positive correlation between weight gain and systolic blood pressure shortly after ART initiation. Alternatively, ART might directly mediate vascular remodeling which has also been demonstrated in other metabolic disorders and is associated with risk of hypertension 31,32.
Although we document increasing blood pressure after ART initiation, our finding should be taken in light of population-based surveys from sub-Saharan Africa, which have suggested that people with HIV appear to be at lower risk of hypertension than matched HIV uninfected counterparts 13-16. Given that we did not have an HIV-uninfected comparison group in our study, we cannot rule out the possibility that blood pressure remains lower in the HIV-infected population despite the increases we documented. Alternatively, because prior studies tended to assess HIV-infected patients irrespective of ART status, they may not have noted the “return to health” phenomenon we report here.
To the best of our knowledge, ours is the first report from a large cohort demonstrating no statistical association between biomarkers of immune activation and risk of incident hypertension after adjusting for traditional risk factors. Indeed, we found that D-dimers levels at month 6 of ART (IL-6 and sCD14 to a lesser extent) were inversely associated with risk of incident hypertension. These findings contrast those from a Norwegian HIV cohort that showed increased inflammation (LPS and sCD14) predicted hypertension 33. However, participants in that study had less advanced HIV disease at ART initiation compared to those in our study. Our findings therefore, suggest that – if anything – lower inflammation is associated with higher blood pressure, implying distinct pathophysiologic mechanisms of hypertension in this population. Whereas HIV associated inflammation and immune activation are known to contribute to arterial stiffness 34 and atheroma formation22,35,36, similar pathways are not known to be primarily responsible for hypertension. In fact, certain immune activation pathways that are increased by microbial products in HIV infection like the kynurenine pathway of tryptophan catabolism may be causally associated with vasodilation 37.
In contrast, our findings of increasing age, male gender, overweight and obesity, lower pre-ART CD4+ T cell counts, are consistent with prior studies 13,38-40. These data reaffirm that, unlike atherosclerosis, risk factors for hypertension among those with HIV are largely similar to the known risk factors in those without HIV 8,13,41. We did find a paradoxical association between low pre-ART CD4+ T cell count and hypertension. This suggests that the mechanism by which low pre-ART CD4 is associated with hypertension may not be mediated by chronic immune activation42,43. Whereas further data will be required to help elucidate these mechanisms, it could be explained by the possibility that immunodeficiency and immune activation affect hypertension risk through fundamentally distinct pathways 14,44,45.
Our data should be interpreted in the context of the study design. Our results could be biased by both exposure and outcome misclassification. We attempted to minimize this through rigorous laboratory quality assurance protocols and by using strict criteria for our definitions of both cases and controls, respectively. Differential misclassification of inflammatory markers is also unlikely because laboratory testing of biomarkers was performed without knowledge of participant blood pressure results, which further mitigates risk of systematic bias in our results.
Our study was observational without a randomized intervention, and thus our results could be susceptible to both measured and unmeasured confounders. Confounding by other conditions such as diabetes mellitus 39, dyslipidemia, and chronic kidney disease, which were not captured in the database, offers a particularly important challenge in the context of hypertension. These conditions are known to directly or indirectly cause hypertension and may also be associated with immune activation. We attempted to mitigate such confounding by adjusting for surrogates common to the above potentially confounding conditions (e.g. age, sex, and BMI). However, we lacked data on renal function or glucose intolerance and so were unable to assess other putative pathways between inflammation and hypertension risk. The findings described are largely generalizable to those HIV-infected individuals achieving viral suppression within 6 months of ART.
Conclusion
We found significant elevations in blood pressure among HIV-infected persons in the first six months after initiation of ART. This effect might be mediated by an ART-induced “return to health”, as evidenced by our observation that the risk of incident hypertension was positively associated with traditional risk factors (age, male gender, and BMI), but not elevated levels of biomarkers of immune activation. In fact, we found evidence that HIV-associated immune activation, inflammation and altered coagulation may be inversely related to risk of incident hypertension, such that lower levels of D-dimer at 6 months after ART initiation were associated with an increased risk of incident hypertension. Taken together, the lack of an association between incident hypertension and inflammatory markers may suggest that traditional cardiovascular risk factors are the main predictors of hypertension in this population. If corroborated, our results support the integration of strategies for hypertension prevention and management into routine HIV care. Focusing attention on traditional cardiovascular risk factors might be the most appropriate method to affect hypertension related morbidity and mortality in HIV-infected individuals in this setting.
Acknowledgments
The authors would like to thank the ISS clinic patients at Mbarara Regional Referral Hospital and Bernard Lown Scholars in Cardiovascular Health Program at Harvard T. H Chan School of Public Health.
sources of funding: This study was supported by the National Institute of Health (R01 MH054907, K23 MH099916, R56AI100765, R21AI078774, U01 AI069919, UM1 CA181255), Doris Duke Charitable Foundation (Clinical Scientist Development Award 2008047), the Sullivan Family Foundation, and the Canada-Africa Prevention Trials (CAPT) network. The funders had no role in study design, conduct, data analysis, or manuscript production.
Footnotes
Disclosure of Previous Presentations: Part of this work was presented as an oral abstract at IDWeek 2015, San Diego, October 2015
Conflicts of Interest: All authors report no conflicts of interest.
Financial Disclosure: The authors prepared this report in their roles as employees of their respective institutions. They have no financial or other potential conflict of interest with regard to this manuscript.
Author Contributions: S.O., S.B.A., J.N.M., P.W.H., and M.J.S., conceptualized and designed this study; M.K., B.B.M., W.R.M., Y.H., K.W., T.P.B., T.P.R., D.R.B., A.M.R., and J.W.H., collected data; S.O., S.B.A., M.K., W.R.M., Y.B., B.B.M., J.E.H., Y.H., K.W., T.P.B., T.P.R., D.R.B., A.M.R., J.W.H., and M.J.S analyzed, interpreted data, wrote and approved manuscript
References
- 1.Klatt NR, Funderburg NT, Brenchley JM. Microbial translocation, immune activation, and HIV disease. Trends Microbiol. 2013;21(1):6–13. doi: 10.1016/j.tim.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12(12):1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 3.Marchetti G, Cozzi-Lepri A, Merlini E, et al. Microbial translocation predicts disease progression of HIV-infected antiretroviral-naive patients with high CD4+ cell count. AIDS. 2011;25(11):1385–1394. doi: 10.1097/QAD.0b013e3283471d10. [DOI] [PubMed] [Google Scholar]
- 4.Ancuta P, Kamat A, Kunstman KJ, et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One. 2008;3(6):e2516. doi: 10.1371/journal.pone.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blodget E, Shen C, Aldrovandi G, et al. Relationship between microbial translocation and endothelial function in HIV infected patients. PLoS One. 2012;7(8):e42624. doi: 10.1371/journal.pone.0042624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niskanen L, Laaksonen DE, Nyyssönen K, et al. Inflammation, abdominal obesity, and smoking as predictors of hypertension. Hypertension. 2004;44(6):859–865. doi: 10.1161/01.HYP.0000146691.51307.84. [DOI] [PubMed] [Google Scholar]
- 7.Engström G, Lind P, Hedblad B, Stavenow L, Janzon L, Lindgärde F. Long-term effects of inflammation-sensitive plasma proteins and systolic blood pressure on incidence of stroke. Stroke. 2002;33(12):2744–2749. doi: 10.1161/01.str.0000034787.02925.1f. [DOI] [PubMed] [Google Scholar]
- 8.Okello S, Kanyesigye M, Muyindike WR, et al. Incidence and predictors of hypertension in adults with HIV-initiating antiretroviral therapy in south-western Uganda. J Hypertens. 2015;33(10):2039–2045. doi: 10.1097/HJH.0000000000000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malaza A, Mossong J, Bärnighausen T, Newell ML. Hypertension and obesity in adults living in a high HIV prevalence rural area in South Africa. PloS one. 2012;7(10):e47761. doi: 10.1371/journal.pone.0047761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotwani P, Kwarisiima D, Clark TD, et al. Epidemiology and awareness of hypertension in a rural Ugandan community: a cross-sectional study. BMC public health. 2013;13(1):1151. doi: 10.1186/1471-2458-13-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mateen FJ, Kanters S, Kalyesubula R, et al. Hypertension prevalence and Framingham risk score stratification in a large HIV-positive cohort in Uganda. Journal of hypertension. 2013;31(7):1372–1378. doi: 10.1097/HJH.0b013e328360de1c. [DOI] [PubMed] [Google Scholar]
- 12.Bloomfield GS, Hogan JW, Keter A, et al. Hypertension and obesity as cardiovascular risk factors among HIV seropositive patients in Western Kenya. PloS one. 2011;6(7):e22288. doi: 10.1371/journal.pone.0022288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krauskopf K, Van Natta ML, Danis RP, et al. Correlates of hypertension in patients with AIDS in the era of highly active antiretroviral therapy. Journal of the International Association of Providers of AIDS Care. 2013;12(5):325–333. doi: 10.1177/2325957413491432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fourie CMT, Schutte AE, Smith W, Kruger A, van Rooyen JM. Endothelial activation and cardiometabolic profiles of treated and never-treated HIV infected Africans. Atherosclerosis. 2015;240 doi: 10.1016/j.atherosclerosis.2015.03.015. 154e160. [DOI] [PubMed] [Google Scholar]
- 15.Peck RN, Shedafa R, Kalluvya S, et al. Hypertension, kidney disease, HIV and antiretroviral therapy among Tanzanian adults: a cross-sectional study. BMC Med. 2014;12(1):125. doi: 10.1186/s12916-014-0125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwarisiima Dalsone, Kotwani Prashant, Sang Norton, et al. Population-based Assessment of Hypertension among HIV Patients in Rural Uganda. CROI; Seattle, Washington, USA: 2015. [Google Scholar]
- 17.Dillon DG, Gurdasani D, Riha J, et al. Association of HIV and ART with cardiometabolic traits in sub-Saharan Africa: a systematic review and meta-analysis. Int J Epidemiol. 2013;42(6):1754–1771. doi: 10.1093/ije/dyt198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunt PW, Cao HL, Muzoora C, et al. Impact of CD8+ T cell activation on CD4+ T cell recovery and mortality in HIV-infected Ugandans initiating antiretroviral therapy. AIDS. 2011;25(17):2123. doi: 10.1097/QAD.0b013e32834c4ac1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byakwaga H, Boum Y, Huang Y, et al. The kynurenine pathway of tryptophan catabolism, CD4+ T-cell recovery, and mortality among HIV-infected Ugandans initiating antiretroviral therapy. The Journal of infectious diseases. 2014;210(3):383–391. doi: 10.1093/infdis/jiu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez P, Tsai AC, Muzoora C, et al. Reversal of the Kynurenine pathway of tryptophan catabolism may improve depression in ART-treated HIV-infected Ugandans. J Acquir Immune Defic Syndr. 2014;65(4):456–462. doi: 10.1097/QAI.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang W, Lederman MM, Hunt P, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis. 2009;199(8):1177–1185. doi: 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ford ES, Greenwald JH, Richterman AG, et al. Traditional risk factors and D-dimer predict incident cardiovascular disease events in chronic HIV infection. AIDS. 2010;24(10):1509. doi: 10.1097/QAD.0b013e32833ad914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duprez DA, Neuhaus J, Kuller LH, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One. 2012;7(9):e44454. doi: 10.1371/journal.pone.0044454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunt PW, Sinclair E, Rodriguez B, et al. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. The Journal of infectious diseases. 2014;210(8):1228–1238. doi: 10.1093/infdis/jiu238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Were MC, Shen C, Bwana M, et al. Creation and evaluation of EMR-based paper clinical summaries to support HIV-care in Uganda, Africa. Int J Med Inform. 2010;79(2):90–96. doi: 10.1016/j.ijmedinf.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palacios R, Santos J, García A, et al. Impact of highly active antiretroviral therapy on blood pressure in HIV-infected patients. A prospective study in a cohort of naive patients. HIV Med. 2006;7:10–15. doi: 10.1111/j.1468-1293.2005.00333.x. [DOI] [PubMed] [Google Scholar]
- 27.Palacios R, Santos J, Garcia A, et al. Impact of highly active antiretroviral therapy on blood pressure in HIV- infected patients. A prospective study in a cohort of naive patients. HIV medicine. 2006;7(1):10–15. doi: 10.1111/j.1468-1293.2005.00333.x. [DOI] [PubMed] [Google Scholar]
- 28.Botha S, Fourie CM, van Rooyen JM, Kruger A, Schutte AE. Cardiometabolic Changes in Treated Versus Never Treated HIV-Infected Black South Africans: The PURE Study. Heart, Lung and Circulation. 2014;23(2):119–126. doi: 10.1016/j.hlc.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 29.Crane HM, Van Rompaey SE, Kitahata MM. Antiretroviral medications associated with elevated blood pressure among patients receiving highly active antiretroviral therapy. Aids. 2006;20(7):1019–1026. doi: 10.1097/01.aids.0000222074.45372.00. [DOI] [PubMed] [Google Scholar]
- 30.Franklin SS, Gustin W, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure The Framingham Heart Study. Circulation. 1997;96(1):308–315. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- 31.Dillon DG, Gurdasani D, Riha J, et al. Association of HIV and ART with cardiometabolic traits in sub-Saharan Africa: a systematic review and meta-analysis. International journal of epidemiology. 2013;42(6):1754–1771. doi: 10.1093/ije/dyt198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mutimura E, Stewart A, Rheeder P, Crowther NJ. Metabolic function and the prevalence of lipodystrophy in a population of HIV-infected African subjects receiving highly active antiretroviral therapy. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2007;46(4):451–455. doi: 10.1097/qai.0b013e318158c0a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manner I, Baekken M, Kvale D, et al. Markers of microbial translocation predict hypertension in HIV- infected individuals. HIV medicine. 2013;14(6):354–361. doi: 10.1111/hiv.12015. [DOI] [PubMed] [Google Scholar]
- 34.Schillaci G, Maggi P, Madeddu G, et al. Symmetric ambulatory arterial stiffness index and 24-h pulse pressure in HIV infection: results of a nationwide cross-sectional study. Journal of hypertension. 2013;31(3):560–567. doi: 10.1097/HJH.0b013e32835ca949. [DOI] [PubMed] [Google Scholar]
- 35.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473(7347):317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 36.Croce K, Libby P. Intertwining of thrombosis and inflammation in atherosclerosis. Curr Opin Hematol. 2007;14(1):55–61. doi: 10.1097/00062752-200701000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Liu H, McKenzie G, et al. Kynurenine is an endothelium-derived relaxing factor produced during inflammation. Nat Med. 2010;16(3):279–285. doi: 10.1038/nm.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manner Ingjerd W, Trøseid Marius, Oektedalen Olav, Baekken Morten, Os Ingrid. Low Nadir CD4 Cell Count Predicts Sustained Hypertension in HIV-Infected Individuals. J Clin Hypertens. 2013;15(2):101–106. doi: 10.1111/jch.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gazzaruso C, Bruno R, Garzaniti A, et al. Hypertension among HIV patients: prevalence and relationships to insulin resistance and metabolic syndrome. Journal of hypertension. 2003;21(7):1377–1382. doi: 10.1097/01.hjh.0000059071.43904.dc. [DOI] [PubMed] [Google Scholar]
- 40.Bergersen B, Sandvik L, Dunlop O, Birkeland K, Bruun J. Prevalence of hypertension in HIV-positive patients on highly active retroviral therapy (HAART) compared with HAART-naive and HIV-negative controls: results from a Norwegian study of 721 patients. Eur J Clin Microbiol Infect Dis. 2003;22(12):731–736. doi: 10.1007/s10096-003-1034-z. [DOI] [PubMed] [Google Scholar]
- 41.Thiebaut R, El-Sadr WM, Friis-Moller N, et al. Predictors of hypertension and changes of blood pressure in HIV-infected patients. Antiviral therapy. 2005;10(7):811–823. doi: 10.1177/135965350501000706. [DOI] [PubMed] [Google Scholar]
- 42.Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. Journal of Infectious Diseases. 2003;187(10):1534–1543. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 43.Fernandez S, Price P, McKinnon EJ, Nolan RC, French MA. Low CD4+ T-cell counts in HIV patients receiving effective antiretroviral therapy are associated with CD4+ T-cell activation and senescence but not with lower effector memory T-cell function. Clinical Immunology. 2006;120(2):163–170. doi: 10.1016/j.clim.2006.04.570. [DOI] [PubMed] [Google Scholar]
- 44.Gibellini D, Borderi M, Clo A, et al. HIV-related mechanisms in atherosclerosis and cardiovascular diseases. J Cardiovasc Med. 2013;14(11):780–790. doi: 10.2459/JCM.0b013e3283619331. [DOI] [PubMed] [Google Scholar]
- 45.Hsue PY, Deeks SG, Hunt PW. Immunologic basis of cardiovascular disease in HIV-infected adults. The Journal of infectious diseases. 2012;205(3):S375–382. doi: 10.1093/infdis/jis200. [DOI] [PMC free article] [PubMed] [Google Scholar]


