Abstract
Objective
An unsettling aspect of the US opioid epidemic is the high rate of in utero exposure, especially since most of these pregnancies are unintended, due in part to low rates of effective contraceptive use among opioid-using women. This study tested an intervention informed by behavioral economic theory and aimed at promoting effective contraceptive use among opioid-maintained women at risk of unintended pregnancy in the Burlington, VT area between 2011–2013.
Methods
Thirty-one women were assigned (initial 5 consecutively, subsequent 26 randomly) to either usual care or an experimental intervention. Participants in usual care received condoms, a dose of emergency contraception, and referral to local providers. Participants in the experimental condition received usual care plus the World Health Organization’s contraception initiation protocol, including free prescription contraceptives, and financial incentives for attending 13 follow-up visits over 6 months to help manage side effects and other issues.
Results
Significantly more women in the experimental vs. usual care control conditions initiated prescription contraceptive use (100% vs. 29%) and reported prescription contraceptive use at 1-month (63% vs. 13%), 3-month (88% vs. 20%), and 6-month (94% vs. 13%) assessments. None of the experimental condition participants became pregnant during the 6-month protocol vs. three women (20%) in the control condition.
Conclusions
These results provide the first experimental evidence supporting the efficacy of an intervention for increasing prescription contraceptive use among opioid-maintained women at risk of unintended pregnancy.
Keywords: Opioid substitution therapy, unintended pregnancy, contraceptives, birth control, long-acting reversible contraceptives (LARCs), behavioral economics, incentives
A particularly unsettling aspect of the current US opioid epidemic is the high rate of in utero exposure and associated adverse health and economic outcomes, with the cost of acute medical care for these opioid-exposed neonates estimated at nearly $67,000 per infant (Patrick et al., 2015). Further, nearly 80% of these pregnancies are unintended (Black et al., 2012; Burns et al., 2011; Heil et al., 2011; Jones et al., 2011), due in part to alarmingly low rates of effective contraceptive use among opioid-using women (<10%; see review by Terplan et al., 2015).
One important but under-researched approach to reducing this problem is increasing the use of more effective contraceptives among opioid-dependent women at risk of unintended pregnancy. We know of no controlled studies on promoting effective contraceptive use in this vulnerable population. There have been many interventions aimed at increasing condom use to reduce HIV and other sexually transmitted infections (STIs), but results have been modest (see meta analyses by Semaan et al., 2002 and Copenhaver et al., 2006) and coital methods like condoms are only moderately effective contraceptives (15% pregnancy rate with typical use in the first year; World Health Organization (WHO), 2007). Behavioral economic theory suggests at least one important contributor to their lesser efficacy: condoms are used at the time of intercourse, when sexual arousal increases impulsive choice, undermining men’s intentions to use condoms (Ariely & Lowenstein, 2006). In contrast, choices regarding use of the more effective prescription contraceptives (birth control pills, patch, ring, injection, intrauterine devices (IUDs), and implants, with an average 4% pregnancy rate; WHO, 2007) are made temporally distant from intercourse and are thus more likely to be made with a rational eye towards longer-term outcomes. However, it requires substantially more effort to initiate prescription contraceptives. Women need to find a provider they are comfortable with and schedule and attend an appointment with that provider. At the appointment, women are often required to undergo examinations or tests that are not necessary to safely prescribe contraceptives (e.g., pelvic exam, Pap smear; Henderson et al., 2010). In addition, out-of-pocket expenses for such appointments average $40 (Davis & Carper, 2012). Women who choose an IUD or implant are frequently required to schedule an additional visit to have the method inserted for administrative reasons (e.g., insurance policies, outdated STI testing protocols; Bergin et al., 2012). Those who choose pills, patch, or ring are often instructed to wait for menses to start the method despite the demonstrated safety and efficacy of “quick start” protocols that allow most women to start immediately (see review by Brahmi & Curtis, 2013). Once a prescription contraceptive method is initiated, women may also face difficulties in continuing it. Side effects such as bleeding irregularities are the most common reason women discontinue prescription contraceptives (Daniels et al., 2013; Grunloh et al., 2013). Women who choose pills, patch, or ring are also often required to refill prescriptions at frequent intervals and these methods plus injections require women to manage use (e.g., taking a pill, changing a patch or ring, returning to the provider for the next injection). In addition, out-of-pocket costs across such methods average $9/month (Becker & Polsky, 2015).
The present study tested an intervention informed by behavioral economic theory and aimed at promoting more effective contraceptive use among opioid-maintained women at risk of unintended pregnancy. Even among maintained women, unintended pregnancy rates average 77% (Black et al., 2012; Welle-Strand et al., 2013) and use of effective contraceptives is low (36%; Black et al., 2012; Harding & Ritchie, 2003; Morrison et al., 1995; Ralph & Spigner, 1986). The intervention had two components. The first component, the WHO’s Decision-Making Tool for Family Planning Clients and Providers (WHO and Johns Hopkins Bloomberg School of Public Health, 2005), aimed to promote contraceptive initiation. Many of the elements incorporated into the WHO’s Decision-Making Tool, such as providing contraception without requiring a physical exam and dispensing/inserting the chosen method at the same visit, are consistent with the behavioral economic strategy of reducing barriers to initiating healthier behaviors. The second component of the intervention, financial incentives provided contingent on attendance at follow-up visits, aimed to promote prescription contraceptive continuation.
Financial incentives are a widely used behavioral economic strategy that has been reliably shown to increase attendance at counseling sessions, medical appointments, and other health-related appointments among substance abusers (see meta-analysis by Lussier et al., 2006). In the present study, increasing attendance at follow-up visits gave staff an opportunity to help participants manage contraceptive side effects and problem-solve adherence problems, provide free refills of the chosen contraceptive method, and assist with switching methods when indicated. In addition, the intervention was delivered in a clinic that was co-located with an agonist maintenance clinic in an effort to further reduce barriers to healthy choices. This report describes results obtained in a preliminary controlled trial examining the efficacy of this treatment.
Method
Participants
Potential participants were recruited in and around opioid maintenance treatment programs in the greater Burlington, VT area between October 2011 and September 2013. Participants were screened for initial eligibility via a study prescreen completed by phone or in person, followed by an in-person screen. This screen included demographic, general medical history, and reproductive history questionnaires developed in our clinic, and a urine pregnancy test. A modified version of the Time-Line Followback (TLFB) interview adapted to assess sexual activity and contraceptive use was used to collect this information for the 90 days prior to screening (Weinhardt, 2002; Weinhardt et al., 1998). The screen also included a 32-item survey designed to assess knowledge, attitudes and beliefs regarding pregnancy risk and contraceptive methods (National Campaign to Prevent Teen and Unplanned Pregnancy, 2009). Other instruments included in the screen were the Addiction Severity Index – Fifth Edition (McLellan et al., 1992; Makela, 2004), Risk Assessment Battery – sexual practices section only (University of Pennsylvania HIV/AIDS Prevention Research Division, 1995), Beck Depression Inventory (Beck et al., 1961, 1988), Barrett Impulsivity Scale -11 (Patton et al., 1995; Stanford et al. 2009), and computerized measures of delay discounting (Johnson & Bickel, 2002; Johnson & Bruner, 2012, 2013; Kowal et al., 2007; Richards et al., 1999). Potential participants were compensated $35 for completing the screen.
Women who were eligible for the study (1) were between 18–44 years of age, (2) were pre-menopausal and had no history of a tubal ligation or hysterectomy, (3) had had heterosexual vaginal intercourse in the past 3 months, (4) were at least 6 months postpartum since their last pregnancy, (5) were not planning to become pregnant in the next 6 months, (6) were medically eligible to use prescription contraceptives, (7) reported no use of birth control pills, patch, ring, implants, or IUDs in the last 7 days or no depot injections in the last 3 months, (8) were in opioid maintenance treatment for at least the past 30 days, (9) were not facing imminent incarceration, and (10) were English-speaking. All participants provided written informed consent and the study was approved by the University of Vermont Institutional Review Board.
Procedures
Conditions
Usual care control
Participants assigned to the usual care control condition received an informational booklet about birth control methods (Planned Parenthood’s “Facts About Birth Control”) and a list of nearby providers of contraceptive services. Participants in this condition were also offered condoms and a dose of emergency contraception (i.e., Plan B or ella).
Experimental
Participants in the experimental condition received the same treatment as the usual care control condition, but with two additional components. Regarding the first component, participants in the experimental condition met with an advanced practice registered nurse after their screening assessment. Using the World Health Organization’s (WHO) Contraceptive Decision-Making Tool for Family Planning Clients and Providers as a guide, the participant and nurse practitioner worked to come to a mutual decision regarding the best contraceptive method for the participant’s current circumstances. Participants could choose oral contraceptive pills, Ortho Evra transdermal patches, NuvaRing vaginal ring, depot medroxyprogesterone acetate (DMPA) intramuscular injection, intrauterine device (IUD; i.e., Mirena or ParaGard), or subcutaneous implant (i.e., Implanon or Nexplanon). While diaphragms are prescription contraceptives, they were not offered to participants due to their relatively high pregnancy rate (16%; WHO, 2007). Participants who decided to initiate a prescription contraceptive were provided with educational counseling about their chosen method and were given the method at no cost. Pills, patches, and rings were dispensed immediately and participants were encouraged to initiate these methods in the clinic. Injections were administered immediately by study medical personnel. For IUDs or implants, arrangements were made for the insertion to be performed by study medical personnel at a nearby university-affiliated clinic as soon as possible (median delay of 17 days). Regarding the second component, participants in the experimental condition were scheduled to attend 13 follow-up visits over the next 6 months. At follow-up visits, a urine pregnancy test was performed and participants updated their TLFB of sexual behavior and contraceptive use. Participants who had initiated a prescription contraceptive method were asked about any side effects and assistance managing side effects was provided. Participants who had not initiated a prescription contraceptive method were offered additional assistance in selecting a method and could start a method at any of these follow-up visits. Participants received vouchers for attending follow-up visits starting at $15 for the first visit and increasing by $2.50 for each consecutive visit attended. If a participant was more than 2 days late for a follow-up visit, their next voucher value was reset to $15, but two consecutive on-time visits returned the voucher value to the level previously attained. Incentive amount was based on prior treatment development work with similarly disadvantaged populations by our group (e.g., Higgins et al., 1991, 2004; Dunn et al., 2008). Earnings could not be lost once earned and money was never provided directly to participants. Instead, voucher earnings could be used to purchase retail items in the community. A staff member made all purchases. Follow-up visits were scheduled weekly for the first 2 months, every other week for the next 2 months, and monthly for the last 2 months. The frequency and duration of follow-up visits was based on prescription contraceptive discontinuation rates in the general population, which are steepest in the first few weeks after method initiation, then decline progressively in subsequent months before leveling off around six months after method initiation (Nelson et al., 2008).
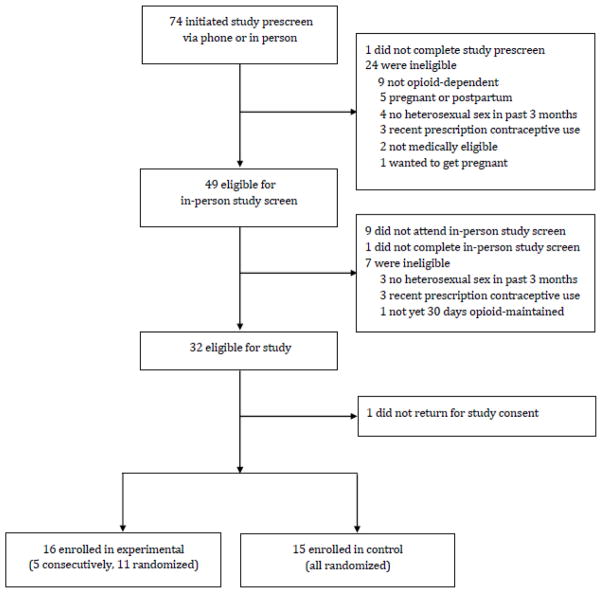
The first five participants were assigned to the experimental condition consecutively to allow study staff to become accustomed to the intervention. The subsequent 26 participants were randomly assigned by research staff using a minimization allocation procedure to either experimental or usual care control conditions, balancing on the following characteristics known to affect prescription contraceptive use: (1) age (< 35 vs. ≥35 years); (2) smoking status; (3) opioid maintenance medication (methadone vs. buprenorphine); (4) intent to start a prescription contraceptive; and (5) whether or not they had one steady heterosexual partner. The flow of participants through screening and randomization is shown in Figure 1.
Figure 1.
The flow of participants through the study. Participants were opioid-maintained women at risk of unintended pregnancy in the greater Burlington, VT area between 2011–2013.
All participants completed a modified version of the screen at 1-, 3-, and 6-months after trial entry and were compensated $35. Research staff conducting these assessments were not blind to treatment condition.
Data Analysis
Demographic characteristics were compared between conditions using t-tests for continuous variables and chi-square tests for discrete variables. The percentage of participants who ever initiated a prescription contraceptive method was compared between conditions using Fisher’s Exact test. Prescription contraceptive continuation was measured at the 1-, 3-, and 6-month assessments and was defined as reporting adherence to the directions of their prescription contraceptive for the seven days prior to the assessment on the TLFB interview. In cases when the seven days prior to the assessment overlapped with a placebo phase for pill users, adherence was considered reaching the placebo phase on time based on the date the method was initiated and remaining adherent with placebo phase directions. Point-prevalence data were compared between conditions using Fisher’s Exact tests; the primary outcome for the trial was point-prevalence prescription contraceptive adherence at the 6-month assessment. Fisher’s Exact tests were also used to compare conditions at each assessment on the collective percentage of participants who used injections, IUDs, and implants, as administration/insertion of these methods was performed and therefore verified by study medical personnel for experimental condition participants. Fisher’s Exact tests comparing conditions at each assessment were also conducted excluding the initial five subjects assigned to the experimental condition as consecutive admissions. While the study was not powered to detect differences between conditions on pregnancy rates, experimental and control conditions were compared using a Fisher’s Exact test. TLFB data were also used to characterize condom use prior to trial entry and during the intervention period within each condition.
Results
Demographic and Other Characteristics
Demographic and other characteristics of enrolled participants are summarized in Table 1. On average, participants were 30 years old and had a high school education and most (89%) were Caucasian. While the majority of participants (68%) had never been married, most (84%) reported having one steady, male sexual partner. Ninety percent of participants were maintained on methadone and 10%, buprenorphine. Most participants (84%) were current smokers. Regarding reproductive history, 90% of participants had been pregnant before. Participants averaged 3.3 pregnancies to date and 87% reported having at least one unintended pregnancy. Fifty-five percent of all of these pregnancies ended in a live birth, 25% in miscarriage/stillbirth, and 20% in abortion. All participants had used a prescription contraceptive at some time in their life. There were no significant differences between conditions for any of the demographic or other characteristics assessed in the trial screen.
Table 1.
Demographic and Other Participant Characteristics
| Characteristic | Overall (N = 31) | Experimental (n = 16) | Usual Care (n = 15) | p value |
|---|---|---|---|---|
| Demographic and other characteristics | ||||
| Age (years) | 29.6 ± 4.8 | 29.5 ± 5.1 | 29.7 ± 4.5 | .96 |
| Race (% Caucasian) | 89 | 87 | 93 | .58 |
| Education (years) | 12.2 ± 1.2 | 12.4 ± 1.0 | 11.9 ± 1.4 | .21 |
| Unemployed (%) | 87 | 81 | 93 | .32 |
| Never married (%) | 68 | 69 | 67 | .90 |
| Has steady male sexual partner (%) | 84 | 81 | 87 | .68 |
| Agonist medication (% methadone) | 90 | 88 | 93 | .58 |
| Current smoker (%) | 84 | 81 | 87 | .68 |
| Reproductive and contraceptive history | ||||
| Age first had intercourse | 14.6 ± 2.3 | 14.2 ± 2.7 | 14.9 ± 1.9 | .38 |
| Pregnancies in lifetime | 3.3 ± 2.2 | 3.6 ± 2.8 | 2.9 ± 1.5 | .40 |
| Live deliveries | 1.8 ± 1.1 ±1.2 | 1.7 ± 1.4 | 1.8 ± 1.1 | .80 |
| Miscarriages/stillbirths | 0.8 ± 1.1 | 1.0 ± 0.7 | 0.7 ± 0.9 | .43 |
| Abortions | 0.7 ± 0.7 | 0.9 ± 0.8 | 0.4 ± 0.6 | .08 |
| Unintended pregnancy in lifetime (%) | 87 | 88 | 87 | .94 |
| Ever used prescription contraceptives | 100 | 100 | 100 | .99 |
Note: Values represent mean ± SD unless otherwise specified. Significance levels based on two-sample t-tests for continuous variables and chi square tests for categorical variables. Participants(%) (%) were 31 opioid-maintained women at risk of unintended pregnancy in the greater Burlington, VT area between 2011–2013.
As part of the survey that assessed knowledge, attitudes and beliefs regarding pregnancy risk and contraceptive methods, all participants were asked how much they knew about 11 different contraceptive methods. Only 3% of participants answered that they knew “a lot” or “everything” about implants and 29%, “a lot” or “everything” about IUDs, the two most effective prescription methods. Despite this, one of the most frequently endorsed barriers to seeking further information about contraception, STIs, and pregnancy was “I think I know enough; I don’t need more information” (39%). The other two most commonly cited barriers of nine listed were “It’s too time-consuming” (39%), and “It’s too difficult to find the right resource” (35%). Regarding sources of information about the prevention of pregnancy or STIs, only 48% said they trusted health care professionals the most and even fewer (19%) agreed with the statement “My doctor has helped me select the best birth control method for me.” More generally, the majority of participants (65%) agreed with the statement “I am a planner in most aspects of my life,” but most (71%) also endorsed the statement “In life, things just seem to happen to me.”
Study Visit Attendance (Experimental Condition Only)
Participants assigned to the experimental condition attended 197/208 (95%) of scheduled study visits within ≤ 1 day of the scheduled time.
Assessment Compliance
High levels of compliance were obtained with assessments, with 100% of participants in both conditions interviewed at each of the three assessment points.
Prescription Contraceptive Initiation and Continuation
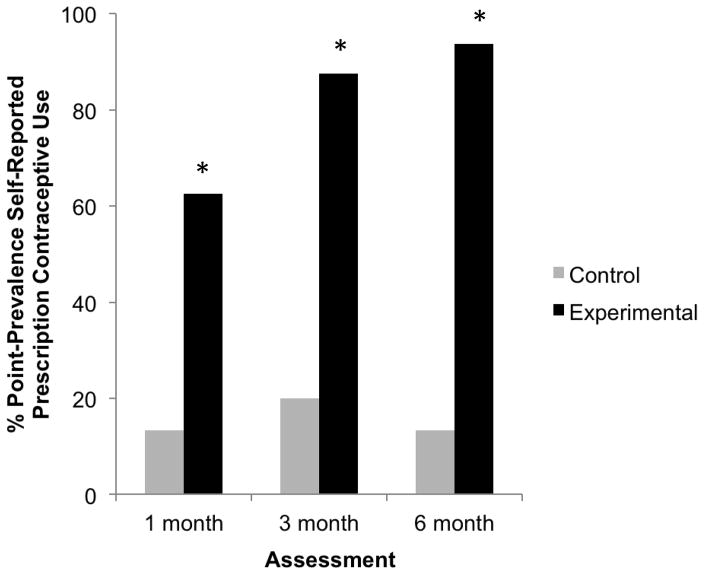
Prescription contraceptive initiation was significantly higher in the experimental vs. usual care conditions (100% vs. 29%, p < .01). Regarding prescription contraceptive continuation, seven-day point prevalence prescription contraceptive adherence was significantly higher in the experimental vs. usual care conditions at the 1- (63% vs. 13%; p < .01), 3- (88% vs. 20%; p < .001), and 6-month (94% vs. 13%; p < .001) assessments (Figure 2). Treatment effects changed little when analyses were restricted to the collective use of injections, IUDs, and implants, with higher rates in the experimental vs. usual care conditions at the 1- (19% vs. 0%; p = .26), 3- (44% vs. 7%; p = .04), and 6-month (56% vs. 7%; p < .01) assessments. Similarly, when the initial five subjects assigned to the experimental condition as consecutive admissions were excluded, adherence remained higher in the experimental vs. usual care conditions at the 1- (45% vs. 13%; p = .09), 3- (82% vs. 20%; p < .01), and 6-month (91% vs. 13%; p < .001) assessments.
Figure 2.
Seven-day point-prevalence self-reported prescription contraceptive use at the 1-, 3-, and 6-month assessments. Grey and black bars represent data from the usual care control and experimental conditions, respectively. Asterisks indicate significant differences between conditions (p < .05). Participants were 31 opioid-maintained women at risk of unintended pregnancy in the greater Burlington, VT area between 2011–2013.
Pregnancy
There was a trend towards statistical significance in terms of pregnancy rates, with 0% vs. 20% of women in the experimental vs. usual care conditions, respectively, becoming pregnant during the 6-month study period (p = .10). Of the three who became pregnant, two did not report any prescription contraceptive use during the study period and one reported that she had been using pills, but still became pregnant.
Condom Use
Condom use did not change from the period prior to trial entry to the intervention period for most participants in the two study conditions (12/16 (75%) experimental and 13/15 (86%) usual care control condition participants) because most (12 experimental and 10 usual care control) did not report any condom use at all during either period despite being at risk for an unintended pregnancy. Among the experimental condition participants, 3/16 (19%) decreased their condom use from one period to the other, and 1/16 (6%) increased her use. Further examination of TLFB data of the three participants who decreased their use indicated that all three adopted a prescription contraceptive method and that two of the three each had sex with the same partner prior to and throughout the study period, suggesting they had been protected from pregnancy and were at relatively low risk for STIs. Among usual care control condition participants, 1/15 (7%) decreased her use, and 1/15 (7%) increased her use. The participant who decreased her use also had sex with the same partner prior to and throughout the study period but did not report any other contraceptive use, suggesting she remained at high risk of unintended pregnancy.
Discussion
To our knowledge, the present study is the first controlled trial of an intervention aimed at promoting effective contraceptive use among opioid-maintained women. Regarding prescription contraceptive initiation, three times more women in the experimental condition initiated a prescription contraceptive as compared to women in the control condition. Regarding continuation, 5- to 7-times more women in the experimental condition were using prescription contraceptives at each assessment as compared to women in the control condition. While we were not powered to detect differences in pregnancy rates, there was a non-significant trend toward fewer pregnancies in the experimental compared to the control condition.
The intervention largely functioned as planned. Many participants reported in the screen that it was too time consuming to seek additional information about contraception and other reproductive health issues. Responses to other survey items also suggest that opioid-maintained women don’t see traditional health care professionals as the best resource for information about contraception and other reproductive health issues. In the present study, likely because of reduction of these and other barriers afforded by the WHO protocol, all women in the experimental condition initiated a prescription contraceptive method. Women in the experimental intervention also attended nearly all of the scheduled study visits in a timely manner, likely due to the incentives offered. This gave study staff the opportunity to check in with participants about method use, help manage side effects, and assess adherence. Where either side effects or adherence were a problem, participants were offered the opportunity to change methods. Indeed, 4/16 (25%) of participants in the experimental condition changed methods during the study and three of the four changed from “effective” prescription method (pills, ring; 8% pregnancy rate) to “very effective” prescription methods (IUDs, implants; <1% pregnancy rate).
Across conditions, 71% of participants did not report any condom use prior to or during study participation despite being at risk of unintended pregnancy, underscoring the urgent need for other approaches to reduce the high rate of unplanned pregnancy among opioid-dependent women. In the few cases where experimental condition participants reported that their condom use decreased, it was typically by a woman in a monogamous relationship who had recently adopted a prescription contraceptive method, suggesting that the intervention did not inadvertently increase risk of STIs among participants assigned to this condition.
Cost is an obvious practical issue with regard to the intervention. We have not yet quantified the total cost per patient of the present intervention beyond the financial incentive costs, nor have we dissociated clinical costs from those associated with researching the intervention. However, considering the substantial excess health care costs associated with opioid-exposed pregnancies, such interventions are likely to be cost-effective. For example, Patrick and colleagues (2015) recently estimated that the cost of acute medical care for these neonates is nearly $67,000 per infant.
Although the results obtained in the experimental condition in the present study are encouraging, the small sample size could adversely affect the precision of the estimate of the magnitude of the treatment effects. The absence of significant differences in subject characteristics between the treatment conditions argues against this, but only a replication using a fully randomized design can adequately address that possibility. A larger, randomized trial comparing these same treatments is ongoing in our clinic. This ongoing trial will also isolate the effects of the two intervention components. We also relied on self-reported contraceptive use for our primary analysis and assigned the initial five participants to the experimental condition as consecutive admissions rather than by random assignment, which could introduce bias; however, the significant treatment effects observed even when analyses were restricted to use of more verifiable methods or with those five women excluded suggests that any such influence was minimal.
These limitations notwithstanding, we consider these initial experimental results encouraging regarding the efficacy of this intervention for promoting effective contraceptive use among opioid-maintained women at risk for unintended pregnancy.
Highlights.
Around 80% of pregnancies among opioid-maintained women are unintended.
We know of no controlled studies to promote effective contraceptives in this group.
An intervention informed by behavioral economics significantly improved outcomes.
Effective contraceptive initiation and continuation were significantly increased.
The results provide experimental evidence of the efficacy of this intervention.
Acknowledgments
We thank Betsy Bahrenburg, R.N., Ira Bernstein, M.D., Lori Daily, A.P.R.N., Robert Hayward, M.D., Hendree Jones, Ph.D., Patricia Livingston, A.P.R.N., Laura Solomon, Ph.D., Lydia Southworth, B.A., and Elizabeth Wegner, M.D. for their assistance in completing the study.
Funding
This work was supported by grants from the National Institute on Drug Abuse (R34DA030534 and T32DA07242).
Footnotes
Conflict Of Interest
The authors declare there are no conflicts of interest.
ClinicalTrials.gov Identifier: NCT0142506
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ariely D, Lowenstein G. The heat of the moment: The effect of sexual arousal on sexual decision making. J Behav Dec Making. 2006;19:87–98. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961 Jun;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988;8(1):77–100. [Google Scholar]
- Becker NV, Polsky D. Women saw large decrease in out-of-pocket spending for contraceptives after ACA mandate removed cost sharing. Health Aff (Millwood) 2015 Jul;34(7):1204–11. doi: 10.1377/hlthaff.2015.0127. [DOI] [PubMed] [Google Scholar]
- Bergin A, Tristan S, Terplan M, Gilliam ML, Whitaker AK. A missed opportunity for care: two-visit IUD insertion protocols inhibit placement. Contraception. 2012 Dec;86(6):694–7. doi: 10.1016/j.contraception.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Black KI, Stephens C, Haber PS, Lintzeris N. Unplanned pregnancy and contraceptive use in women attending drug treatment services. Aust N Z J Obstet Gynaecol. 2012 Apr;52(2):146–50. doi: 10.1111/j.1479-828X.2012.01413.x. [DOI] [PubMed] [Google Scholar]
- Brahmi D, Curtis KM. When can a woman start combined hormonal contraceptives (CHCs)? A systematic review. Contraception. 2013 May;87(5):524–38. doi: 10.1016/j.contraception.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Burns L, Conroy E, Moore EA, Hutchinson D, Haber PS. Psychosocial characteristics and obstetric health of women attending a specialist substance use antenatal clinic in a large metropolitan hospital. Int J Pediatr. 2011;2011:729237. doi: 10.1155/2011/729237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copenhaver MM, Johnson BT, Lee IC, Harman JJ, Carey MP SHARP Research Team. Behavioral HIV risk reduction among people who inject drugs: meta-analytic evidence of efficacy. J Subst Abuse Treat. 2006 Sep;31(2):163–71. doi: 10.1016/j.jsat.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels K, Mosher WD, Jones J. Contraceptive methods women have ever used: United States, 1982–2010. Natl Health Stat Report. 2013 Feb 14;(62):1–15. [PubMed] [Google Scholar]
- Davis K, Carper K. Statistical brief #381. Agency for Healthcare Research and Quality; Rockville, MD: Aug, 2012. Use and expenses for office-based physician visits by specialty, 2009: Estimates for the U.S. civilian noninstitutionalized population. [Google Scholar]
- Dunn KE, Sigmon SC, Thomas CS, Heil SH, Higgins ST. Voucher-based contingent reinforcement of smoking abstinence among methadone-maintained patients: a pilot study. J Appl Behav Anal. 2008 Winter;41(4):527–38. doi: 10.1901/jaba.2008.41-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunloh DS, Casner T, Secura GM, Peipert JF, Madden T. Characteristics associated with discontinuation of long-acting reversible contraception within the first 6 months of use. Obstet Gynecol. 2013 Dec;122(6):1214–21. doi: 10.1097/01.AOG.0000435452.86108.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C, Ritchie J. Contraceptive practice of women with opiate addiction in a rural centre. Aust J Rural Health. 2003 Jan;11(1):2–6. doi: 10.1046/j.1440-1584.2003.00477.x. [DOI] [PubMed] [Google Scholar]
- Heil SH, Jones HE, Arria A, Kaltenbach K, Coyle M, Fischer G, Stine S, Selby P, Martin PR. Unintended pregnancy in opioid-abusing women. J Subst Abuse Treat. 2011 Mar;40(2):199–202. doi: 10.1016/j.jsat.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson JT, Sawaya GF, Blum M, Stratton L, Harper CC. Pelvic examinations and access to oral hormonal contraception. Obstet Gynecol. 2010 Dec;116(6):1257–64. doi: 10.1097/AOG.0b013e3181fb540f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Delaney DD, Budney AJ, Bickel WK, Hughes JR, Foerg F, Fenwick JW. A behavioral approach to achieving initial cocaine abstinence. Am J Psychiatry. 1991 Sep;148(9):1218–24. doi: 10.1176/ajp.148.9.1218. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Solomon LJ, Bernstein IM, Lussier JP, Abel RL, Lynch ME, Badger GJ. A pilot study on voucher-based incentives to promote abstinence from cigarette smoking during pregnancy and postpartum. Nicotine Tob Res. 2004 Dec;6(6):1015–20. doi: 10.1080/14622200412331324910. [DOI] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK. Within-subject comparison of real and hypothetical money rewards in delay discounting. J Exp Anal Behav. 2002 Mar;77(2):129–46. doi: 10.1901/jeab.2002.77-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Bruner NR. The Sexual Discounting Task: HIV risk behavior and the discounting of delayed sexual rewards in cocaine dependence. Drug Alcohol Depend. 2012 Jun 1;123(1–3):15–21. doi: 10.1016/j.drugalcdep.2011.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Bruner NR. Test-retest reliability and gender differences in the sexual discounting task among cocaine-dependent individuals. Exp Clin Psychopharmacol. 2013 Aug;21(4):277–86. doi: 10.1037/a0033071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HE, O’Grady KE, Tuten M. Reinforcement-based treatment improves the maternal treatment and neonatal outcomes of pregnant patients enrolled in comprehensive care treatment. Am J Addict. 2011 May-Jun;20(3):196–204. doi: 10.1111/j.1521-0391.2011.00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal BP, Yi R, Erisman AC, Bickel WK. A comparison of two algorithms in computerized temporal discounting procedures. Behav Processes. 2007 Jun;75(2):231–6. doi: 10.1016/j.beproc.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006 Feb;101(2):192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- Makela K. Studies of the reliability and validity of the Addiction Severity Index. Addiction. 2004 Apr;99(4):398–418. doi: 10.1111/j.1360-0443.2003.00665.x. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9(3):199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Morrison CL, Ruben SM, Beeching NJ. Female sexual health problems in a drug dependency unit. Int J STD AIDS. 1995 May-Jun;6(3):201–3. doi: 10.1177/095646249500600311. [DOI] [PubMed] [Google Scholar]
- The National Campaign to Prevent Teen and Unplanned Pregnancy. Magical Thinking: Young Adults’ Attitudes and Beliefs about Sex, Contraception, and Unplanned Pregnancy, Results from a Public Opinion Survey. Washington, DC: Author; 2008. Retrieved December 30, 2015 from https://thenationalcampaign.org/resource/magical-thinking. [Google Scholar]
- Nelson AL, Westhoff C, Schnare SM. Real-world patterns of prescription refills for branded hormonal contraceptives: a reflection of contraceptive discontinuation. Obstet Gynecol. 2008 Oct;112(4):782–7. doi: 10.1097/AOG.0b013e3181875ec5. [DOI] [PubMed] [Google Scholar]
- Patrick SW, Davis MM, Lehman CU, Cooper WO. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012. J Perinatol. 2015 Aug;35(8):667. doi: 10.1038/jp.2015.63. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995 Nov;51(6):768–74. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Ralph N, Spigner C. Contraceptive practices among female heroin addicts. Am J Public Health. 1986 Aug;76(8):1016–7. doi: 10.2105/ajph.76.8.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JB, Zhang L, Mitchell SH, de Wit H. Delay or probability discounting in a model of impulsive behavior: effect of alcohol. J Exp Anal Behav. 1999 Mar;71(2):121–43. doi: 10.1901/jeab.1999.71-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semaan S, Des Jarlais DC, Sogolow E, Johnson WD, Hedges LV, Ramirez G, Flores SA, Norman L, Sweat MD, Needle R. A meta-analysis of the effect of HIV prevention interventions on the sex behaviors of drug users in the United States. J Acquir Immune Defic Syndr. 2002 Jul 1;30( Suppl 1):S73–93. [PubMed] [Google Scholar]
- Stanford MS, Mathias CW, Dougherty DM, Lake SL, Anderson NE, Patton JH. Fifty years of the Barratt Impulsiveness Scale: An update and review. Pers Individ Dif. 2009;47:385–395. [Google Scholar]
- Terplan M, Hand DJ, Hutchinson M, Salisbury-Afshar E, Heil SH. Contraceptive use and method choice among women with opioid and other substance use disorders: A systematic review. Prev Med. 2015 Nov;80:23–31. doi: 10.1016/j.ypmed.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- University of Pennsylvania HIV/AIDS Prevention Research Division. Risk Assessment Battery. 1995 Instrument retrieved from: http://www.med.upenn.edu/hiv/rab_download.html Psychometrics retrieved from: http://www.med.upenn.edu/hiv/rab_psychometrics.html.
- Weinhardt LS. Effects of a detailed sexual behavior interview on perceived risk of HIV infection: preliminary experimental analysis in a high risk sample. J Behav Med. 2002 Apr;25(2):195–203. doi: 10.1023/a:1014888905882. [DOI] [PubMed] [Google Scholar]
- Weinhardt LS, Carey MP, Maisto SA, Carey KB, Cohen MM, Wickramasinghe SM. Reliability of the timeline follow-back sexual behavior interview. Ann Behav Med. 1998 Winter;20(1):25–30. doi: 10.1007/BF02893805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welle-Strand GK, Skurtveit S, Jones HE, Waal H, Bakstad B, Bjarkø L, Ravndal E. Neonatal outcomes following in utero exposure to methadone or buprenorphine: a National Cohort Study of opioid-agonist treatment of Pregnant Women in Norway from 1996 to 2009. Drug Alcohol Depend. 2013 Jan 1;127(1–3):200–6. doi: 10.1016/j.drugalcdep.2012.07.001. [DOI] [PubMed] [Google Scholar]
- World Health Organization Department of Reproductive Health and Research (WHO/RHR) and Johns Hopkins Bloomberg School of Public Health/ Center for Communication Programs (CCP), INFO Project. Family Planning: A Global Handbook for Providers. Baltimore and Geneva: CCP and WHO; 2007. [Google Scholar]
- World Health Organization and Johns Hopkins Bloomberg School of Public Health, Center for Communication Programs. Information and Knowledge for Optimal Health (INFO) Decision-making tool for family planning clients and providers. Baltimore, Maryland: INFO; Geneva: WHO; 2005. [Google Scholar]