Abstract
Unified theories of addiction are challenged by differing drug-seeking behaviors and neurobiological adaptations across drug classes, particularly for narcotics and psychostimulants. We previously showed that protracted abstinence to opiates leads to despair behavior and social withdrawal in mice, and we identified a transcriptional signature in the extended amygdala that was also present in animals abstinent from nicotine, i9-tetrahydrocannabinol (THC) and alcohol. Here we examined whether protracted abstinence to these 4 drugs would also share common behavioral features, and eventually differ from abstinence to the prototypic psychostimulant cocaine. We found similar reduced social recognition, increased motor stereotypies, and increased anxiety with relevant c-fos response alterations in morphine, nicotine, THC and alcohol abstinent mice. Protracted abstinence to cocaine, however, led to strikingly distinct, mostly opposing adaptations at all levels, including behavioral responses, neuronal activation and gene expression. Together, these data further document the existence of common hallmarks for protracted abstinence to opiates, nicotine, THC and alcohol that develop within motivation/emotion brain circuits. In our model however, these do not apply to cocaine, supporting the notion of unique mechanisms in psychostimulant abuse.
Keywords: Social interaction, gene expression, extended amygdala
INTRODUCTION
Drug abuse is a chronic neuropsychiatric disorder characterized by loss of control over consumption despite negative consequences (APA, 2013). Quitting drug use represents a true challenge for addicted individuals, as remaining drug-free for an extended period of time leads to a negative emotional state, anxiety disorders and social withdrawal (Goodwin et al., 2002; McGregor et al., 2008; Wise and Koob, 2014), making protracted abstinence difficult to sustain and relapse more likely to occur (O'Brien, 2005). The neurobiological mechanisms involved in long-term drug abstinence, however, remain poorly understood. Further, unified theories of addiction are challenged by differing drug-seeking behaviors and brain adaptations across drug classes, particularly for narcotics and psychostimulants (Badiani et al., 2011; Pickens et al., 2011). As a consequence, behavioral deficits and molecular mechanisms underlying protracted abstinence may differ also, but this has not been tested in animal models.
In previous reports, we developed a mouse model of protracted abstinence to morphine (Goeldner et al., 2011) and heroin (Lutz et al., 2014), and showed the development of depressive-like symptoms and social withdrawal. These behavioral modifications differed across mouse strains (Ayranci et al., 2015) and were enhanced or reduced by genetic inactivation of delta and kappa opioid receptors, respectively (Lutz et al., 2014). We also tested gene expression for a large set of genes in the extended amygdala (EA), a key site contributing to hedonic and stress-related dysfunction in drug abuse. We found that specific transcriptional modifications develop after prolonged withdrawal from chronic morphine exposure, and intriguingly, further identified a transcriptional signature that develop upon protracted abstinence morphine, as well as nicotine, n9-tetrahydrocannabinol (THC) and alcohol (Le Merrer et al., 2012). Abstinence from these 4 drugs of abuse, therefore, leads to common molecular neuroadaptations, which may underlie a unitary mechanism contributing to the negative affect in protracted abstinence. Whether behavioral modifications also generalize across the four drugs and whether both behavioral and neurobiological hallmarks of protracted abstinence apply to psychostimulants, however, remained to be investigated.
In the present study, we evaluated whether mice abstinent for four weeks from chronic morphine, nicotine, THC, alcohol, and also cocaine, display modified behavioral responses in multiple tests extending our previous work. Behavioral assays were chosen as to specifically challenge functions of striatum and EA, where gene expression was modified in our previous study (Le Merrer et al., 2012): social interaction, nest building, motor stereotypies, and anxiety-like behavior in marble burying and novelty-suppressed feeding tests. We also evaluated neuronal reactivity following the latter approach/avoidance conflict task using Fos immunolabelling across multiple brain regions. We finally directly compared morphine and cocaine abstinence in further behavioral testing and gene expression analysis. Together, the data show a sharp contrast between morphine, nicotine, THC and alcohol abstinent mice on one-hand and cocaine abstinent animals on the other hand.
MATERIALS AND METHODS
Subjects
For all experiments, we used C57BL/6J male mice aged 8-10 weeks (Charles River, Lyon, France). Except otherwise stated, animals were group housed and maintained on a 12hr light/dark cycle (lights on at 7:00 AM) at controlled temperature (21±1°C); food and water were available ad libitum. Animals housed in the same cage received the same treatment. Experimental procedures were reviewed and approved by the Comité Régional d’Ethique en Matière d’Expérimentation Animale de Strasbourg (CREMEAS, 2003-10-08-[1]-58).
Drug treatments
Morphine, nicotine, THC and alcohol treatments were performed as described previously (Le Merrer et al., 2012). Doses and paradigm for cocaine administration (25 mg/kg, twice a day) were chosen to ensure significant locomotor sensitization (Figure S1). Details about drugs and treatment paradigms are available in Supplement 1.
Behavioral experiments
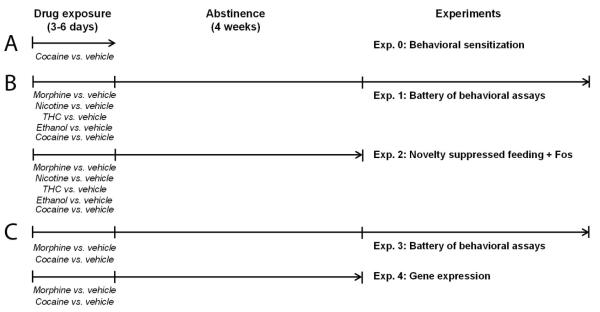
Detailed protocols for behavioral tests are described in Supplement 1. Figure 1 recapitulates experiments (Exp.) performed in our study and their time line. Testing order for batteries of behavioral assays is depicted in Figures S3A and S4A. Social behavior was explored using the direct social interaction, nest building and three-chamber tests, stereotyped behavior was assessed by scoring motor stereotypies and analyzing alternation patterns in the Y-maze, anxiety-like behavior was evaluated in the marble burying, novelty-suppressed feeding and elevated plus-maze tests. Locomotor activity was recorded using video-tracking to assess sensitization. Behavioral scoring was performed blind to the experimental groups.
Figure 1. Summary and time line of the experiments.
We performed three series of experiments in the present study. (A) We verified the development of behavioral sensitization after cocaine treatment in a dedicated cohort of naïve animals (Exp. 0). (B) We compared the effects of protracted abstinence (4 weeks) from morphine, THC, nicotine, alcohol and cocaine versus corresponding vehicle groups in a battery of behavioral tests (Exp. 1) and in the novelty suppressed feeding (NFS) test followed by immunohistochemical assessment of brain Fos expression (Exp. 2). (C) Finally we further compared long-term consequences of morphine versus cocaine abstinence or vehicle treatment on behavior (Exp. 3) and gene expression in the extended amygdala (Exp. 4). The order of behavioral assays in Exp. 1 and 3 was identical for all tested cohorts (see Figures S3A and S4A). Exp.: experiment.
Fos immunohistochemistry
Brains were removed from anesthetized and perfusion-fixed mice (0.9% NaCl followed by 4% paraformaldehyde in PB 0.1 M, pH 7.4), post-fixed for 48 hours, cryoprotected in 30% sucrose / PB overnight at 4°C and stored at -80°C until 50 μm frontal sections were cut on a cryostat. Immunohistochemistry was performed on free-floating sections using a standard avidin-biotin peroxidase method (Becker et al., 2014). Slides were digitized using a Hamamatsu Nanozoomer 2-HT whole slide scanner (Hamamatsu Photonics, Hamamatsu, Japan) at 20x magnification. Frames were acquired using NDP View software, and Fos-positive nuclei were counted using ImageJ software (NIH). Fos-immunoreactivity was assessed bilaterally in 26 cerebral regions (Paxinos and Franklin, 2001) on two consecutive sections per animal (see Figure S2). To express the obtained data (Fos-positive nuclei/mm2) as a fold-change, the number of Fos positive nuclei for each brain region of each abstinent animal was divided by the mean number of positive nuclei measured in the same brain area of the corresponding control (vehicle) group. The result was transformed using the following formula: if x<1, y=1-1/x; if x>1, y=x-1 (x: Fos count; y: transformed data).
Real-time quantitative PCR analysis
Separate cohorts of morphine, cocaine and vehicle abstinent animals were prepared for qRT-PCR experiments. Brains were removed and placed into a brain matrix (ASI Instruments, Warren, MI, USA). Bed Nucleus of the Stria Terminalis (BNST) and Central Nucleus of the Amygdala (CeA) were punched bilaterally out from 1mm-thick slices. Tissues were immediately frozen on dry ice and kept at -80°C until use. BNST and CeA punches from two mice were pooled in EA samples (n=5 samples/treatment). RNA was extracted and purified using the MIRNeasy mini-kit (Qiagen, Courtaboeuf, France). cDNA was synthetized using the first-strand Superscript II kit (Invitrogen®, Life Technologies, Saint Thomas, France) (Le Merrer et al., 2012). qRT-PCR was performed in quadruplets on a LightCycler 480 Real-Time PCR (Roche, Manheim, Germany) using iQ-SYBR Green supermix (Bio-Rad, Marnes-la-Coquette, France) kit with 0.25μl cDNA in a 12.5μl final volume. Gene-specific primers were designed using Primer3 software to obtain a 100- to 150-bp product (Table S1). Relative expression ratios were normalized to the level of actin and the 2–ΔΔCt method was applied to evaluate differential expression level. Primer sequences are displayed in Table S1.
Statistics
Statistical analyses were performed using Statistica 9.0 software (StatSoft, Maisons-Alfort, France). For all comparisons, values of p<0.05 were considered as significant. Statistical significance in behavioral and immunohistochemical experiments was assessed using one or two-way analysis of variance (drug, stimulus and treatment effects) followed by Newman-Keules post-hoc test, except for nesting for which significance was tested using the non-parametric Kruskal-Wallis’ analysis of variance. A standard principal component analysis (PCA) was performed on behavioral and Fos count data (Becker et al., 2014). Loadings for each extracted principal component (PC) are quoted in Table S2. We considered the two first extracted PCs (PC1 and PC2) for schematic representation. Significance of Fos and qRT-PCR results was assessed after transformation using a one-sample t-test, as previously described (Becker et al., 2014; Le Merrer et al., 2013). Unsupervised clustering analysis was performed on transformed immunohistochemical and qRT-PCR data using complete linkage with correlation distance (Pearson correlation) for drug and brain region (Cluster 3.0 and Treeview software).
RESULTS
Mice abstinent from morphine, nicotine, alcohol or THC, but not cocaine, show deficient social interaction, motor stereotypies and exacerbated anxiety
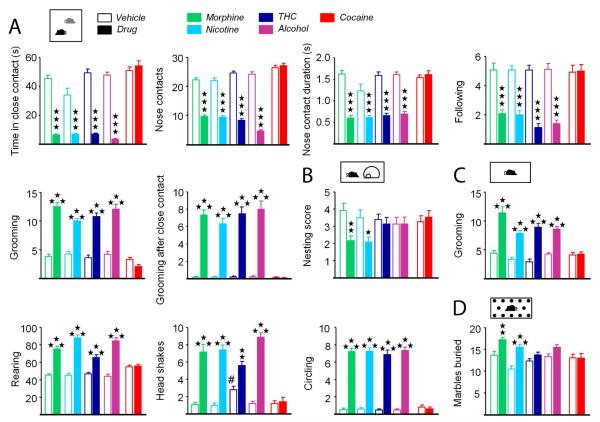
We assessed social behavior in abstinent mice using two tests (Exp. 1). During dyadic interaction, mice abstinent from chronic morphine, nicotine, THC and alcohol, but not cocaine, spent less time in close contact with a matched conspecific (statistics in Table S3), due to reduced number and duration of nose and paw contacts, initiated less following sequences and groomed more often, especially after a close contact, than vehicle-treated animals (Figures 2A and S3B). Consistent with deficient social behavior, morphine and nicotine abstinent mice displayed impaired ability to build a nest (morphine: H1,24=9.7, p=0.001; nicotine: H1,24= 5.9, p<0.05)(Figure 2B).
Figure 2. Mice made abstinent from morphine, nicotine, THC, alcohol but not cocaine show social interaction deficit, motor stereotypies and increased marble burying.
(A) Social interaction test performed after a 4-week abstinence period from chronic, morphine, nicotine, THC, alcohol and cocaine. Six parameters are shown. (B) Nest building behavior. (C) Motor stereotypies assessed by grooming, rearing episodes, head shakes and circling behavior. (D) Marble burying test. Data are presented as mean ± SEM. Stars: abstinence effect, compared to vehicle group. Hash symbol: vehicle effect, compared to morphine vehicle. One symbol p<0.05, two symbols p<0.01, three symbols p<0.001. See more parameters in Figure S3.
We measured the occurrence of spontaneous motor stereotypies in abstinent animals (Exp. 1). When isolated in a standard cage, mice abstinent from chronic morphine, nicotine, THC and alcohol, but not cocaine, displayed more frequent rearing, grooming, headshakes and circling than vehicle controls. Frequency of spontaneous burying was increased in nicotine and alcohol abstinent animals, time spent burying was decreased in morphine and THC abstinent mice, but duration of individual burying episodes was reduced in all abstinent animals except cocaine-treated mice (see Table S3, Figures 2C and S3C). Of note, vehicle-treated animals in the THC group displayed stereotyped head-shaking, as a likely result of the presence of 5% ethanol in the vehicle solution.
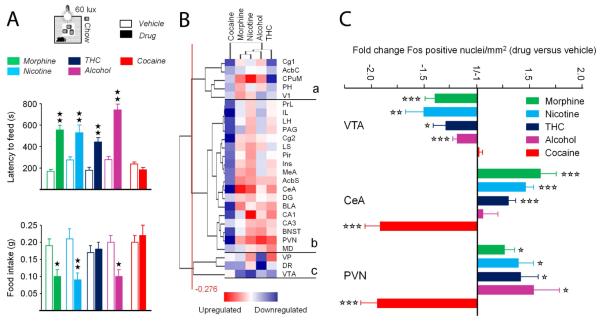
We evaluated anxiety levels in abstinent animals using two tests. In the marble burying test (Exp.1), mice previously exposed to morphine or nicotine buried more marbles than their respective vehicle controls (Figure 2D). In the novelty-suppressed feeding (NSF) test (Exp. 2), a conflict test challenging approach/avoidance behavior (Aupperle and Paulus, 2010), mice abstinent from chronic morphine, nicotine, THC and alcohol, but not cocaine, showed higher latencies to reach and eat food pellets in the center of the arena. Mice previously exposed to morphine, nicotine and alcohol, but not THC and cocaine, ate less chow when returned to their home cage (Figure 3A).
Figure 3. Morphine, nicotine, THC and alcohol, but not cocaine, abstinent mice display exacerbated conflict anxiety associated with modified neuronal reactivity in brain regions controlling anxiety and motivation.
(A) Novelty-suppressed feeding test (60 lux) performed in abstinent mice at (B) Cluster analysis of Fos expression data. Cluster (c) gathers key brain regions involved in the control of anxiety, where Fos levels where mostly increased in morphine, nicotine, THC and alcohol abstinent animals. (C) Fold change number of Fos positive nuclei in the VTA, CeA and PVN in abstinent animals. Data in (A) are presented as mean± SEM. Solid stars: abstinence effect, compared to vehicle group. Open stars: abstinence effect, as compared to no regulation. One symbol p<0.05, two symbols p<0.01, three symbols p<0.001. CeA: central amygdala; PVN: paraventricular hypothalamus; VTA: ventral tegmental area. See Table S4 for complete list of abbreviations.
In these animals, we used Fos immunostaining to map neuronal activation induced by NSF. To correlate behavioral and Fos responses, we performed a Principal Component Analysis on NSF data. Consistent with previous results (Becker et al., 2014), we observed that Fos labeling in the VTA correlated with food intake, while staining in the CeA clustered with latency to eat, except for cocaine abstinent animals (Figure S3). Projection in the subjects’ space (Figure S4) clearly dissociated drug abstinent from control mice, with a shift towards high Fos expression in anxiety-related structures for morphine, nicotine, THC and alcohol abstinent mice, and a shift towards increased Fos levels in the VTA for cocaine abstinent animals. We then expressed immunohistochemical data as fold change versus respective vehicle control groups (Table S4), and identified brain regions with similar Fos expression pattern using clustering analysis. Hierarchical clustering (Figure 3B) organized Fos levels across brain regions in three main clusters. In cluster (a), Fos expression was decreased for cocaine and THC-treated animals while mostly increased in morphine, nicotine and alcohol abstinent mice. Cluster (b) gathered brain regions where Fos expression was increased for animals previously exposed to alcohol, morphine, nicotine and THC, and down-regulated in cocaine abstinent mice. Among these regions, the amygdala nuclei (CeA, Medial nucleus of the Amygdala -MeA, Basolateral nucleus of the Amygdala -BLA), BNST, shell of the Nucleus Accumbens (AcbS), Paraventricular Nucleus (PVN) of the hypothalamus and dorsal hippocampus (CA1, CA3 and Dentate Gyrus-DG) play a critical role in modulating anxiety levels. Lastly, Fos expression was decreased under most conditions in brain regions clustering in (c), including the Ventral Tegmental Area (VTA) and Dorsal Raphe (DR). Notably, in this last region, Fos expression was down-regulated for animals previously exposed to alcohol, morphine, nicotine and THC, and not cocaine. Thus morphine, nicotine, THC and alcohol abstinent mice show increased Fos levels in anxiety-related brain structures and decreased Fos expression in the reward/approach-related VTA and DR, whereas cocaine animals mostly display reduced Fos staining following the NSF test (Figure 3C).
Further comparison of morphine and cocaine abstinence at behavioral level reveals both differences and similarities
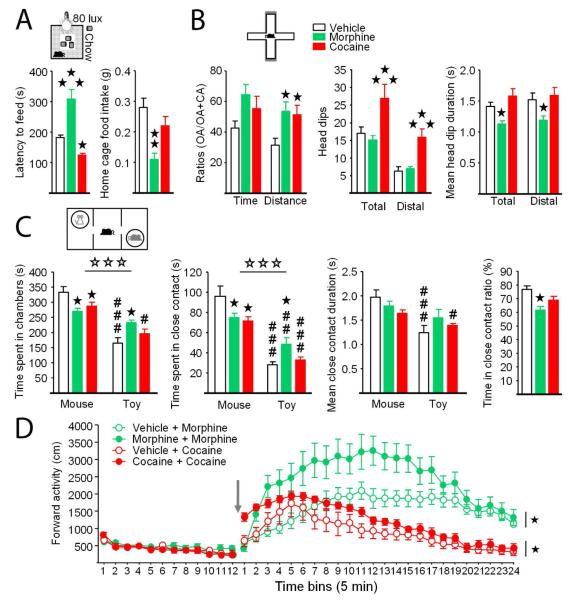
We designed a novel series of experiments to further explore differences between morphine and cocaine abstinent mice (Exp. 3). We first used a more challenging version of the NSF test (80 lux instead of 60 lux) to better dissociate anxiety levels in morphine and cocaine abstinent mice, in accordance with Fos immunochemistry results. Under these conditions, we confirmed increased feeding latencies in mice previously exposed to morphine, and detected lowered latencies in cocaine-treated mice. Evocative of elevated anxiety levels, food intake was reduced in morphine abstinent animals while no modification was detected in cocaine abstinent mice (Figure 4A, Table S5). We then tested morphine and cocaine abstinent mice in the EPM, to assess exploration-dependent anxiety in these animals. In this test, morphine- and cocaine-exposed groups both showed increased distance ratios and a tendency for increased time ratios, suggestive of lowered anxiety levels as compared to vehicle abstinent controls. Ethological parameters (Cole and Rodgers, 1993) were consistently increased in cocaine abstinent mice. However, such an increase was not observed in morphine abstinent animals, and duration of head dips was even reduced, evocative of higher anxiety levels in these mice (Figures 3B and S3B, Table S4).
Figure 4. Comparing morphine and cocaine abstinent mice reveals convergences and differences at behavioral level.
(A) Novelty-suppressed feeding test performed under more challenging conditions (80 lux) reveals decreased anxiety in cocaine abstinent mice. (B) In the elevated plus-maze test, morphine and cocaine abstinent mice travelled more in open arms, but only the later showed increased number of head-dips whereas these were shorter in morphine abstinent mice. (C) Three-chamber test shows decreased social preference in morphine and cocaine-treated animals, with only morphine abstinent mice spending more time exploring the toy as compared to vehicle-treated animals. (D) Locomotor sensitization was assessed by injecting morphine to morphine and vehicle abstinent mice, and cocaine to cocaine and vehicle abstinent mice (see time line in Figure S4). Arrow: drug injection. Data are presented as mean ± SEM. Stars: abstinence effect, compared to vehicle group. Hash symbol: stimulus effect, toy vs. mouse. One symbol p<0.05, two symbols p<0.01, three symbols p<0.001. See also Figure S4.
Because we found impaired direct social interactions in morphine, but not cocaine, abstinent mice (Fig 1), we assessed social preference in these animals using the three-chamber test. Remarkably, morphine- and cocaine-exposed mice similarly spent less time in the chamber where the stimulus mouse was confined and closely exploring this mouse than vehicle-treated mice. However, only morphine abstinent mice concomitantly spent more time in the chamber of the toy mouse and exploring this toy, with equivalent duration of close contacts with the stimulus mouse or toy. This produced a significant decrease of the ratio of time spent in close contact in these animals, while this parameter was not significantly modified in cocaine abstinent mice (Figures 4C and S4C, Table S5).
Finally, we tested whether after a four-week period of abstinence mice previously exposed to morphine or cocaine would still display locomotor sensitization to the effects of this drug. After a 60-min habituation, acute administration of morphine (10 mg/kg) in morphine abstinent mice resulted in greater locomotor activity than in vehicle abstinent animals. Similarly, cocaine injection (25 mg/kg) produced a greater stimulant effect on locomotor activity in cocaine abstinent animals than in vehicle abstinent controls (Figure 4D, Table S5).
Comparison of morphine and cocaine abstinence at transcriptional level reveals opposing adaptations in the EA
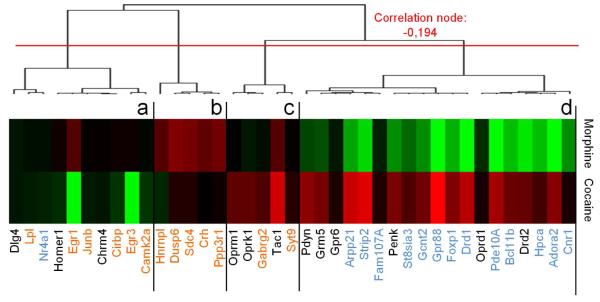
We compared the effects of a history of morphine or cocaine exposure at transcriptional level in the EA (Exp. 4). We focused on two of the gene networks that we had previously identified as contributing to most of the variance of gene expression under abstinent conditions (Le Merrer et al., 2012): a huntingtin (HTT)-centered network (Adora2a, Arpp21, Bcl11b, Cnr1, Drd1, Fam107a, Strip2, Foxp1, GPR88, Gcnt2, Nr4a1, Pde10a, Hpca, St8sia3), found commonly down-regulated in abstinent animals, and a CREB/ERK-centered network (Camk2a, Crh, Gabrg2, Sdc4, Cirbp, Dusp6, Egr1, Egr3, Hnrnpl, Junb, Lpl, Ppp3r1, Syt9). In addition, we assessed transcription levels of several striatal/EA neurotransmission gene markers (Chrm4, Dlg4, Drd2, Gpr6, Grm5, Oprk1, Oprm1, Oprd1, Pdyn, Penk, Tac1). Hierarchical clustering including all qRT-PCR data organized gene expression in 4 main clusters (Figure 5, Table S6). Cluster (a) grouped genes with down-regulated expression in cocaine abstinent animals. Gene expression was upregulated under morphine conditions in cluster (b) whereas levels of expression were higher under cocaine conditions in cluster (c). Finally, cluster (d) gathered genes with down-regulated expression under morphine abstinence and up-regulated expression in cocaine abstinence. Most strikingly, all genes from HTT-centered network but one (Nr4a1) were found in this cluster, together with several genes coding for key actors of striatal/EA neurotransmission (Drd2, Oprd1, Penk, Gpr6, Grm5, Pdyn). Genes belonging to the CREB/ERK-centered network were found across clusters (a)-(c). Together, our transcriptional data show that morphine and cocaine abstinence result in highly contrasted transcriptional regulations for a collection of HTT-related genes expressed in the MSNs of the EA.
Figure 5. Morphine and cocaine abstinence produced opposite transcriptional regulations of a set of HTT-related genes in the EA.
Hierarchical clustering of gene expression. Cluster (d) gathers genes with down-regulated expression after morphine abstinence and up-regulated expression after cocaine abstinence. All genes of the HTT-centered network (highlighted in blue) but one (Nr4a1) are found in this cluster, together with several genes coding for key actors of striatal/EA neurotransmission (Drd2, Oprd1, Penk, Gpr6, Grm5, Pdyn). Genes belonging to the CREB/ERK-centered network (highlighted in orange) are spread across the first three clusters. Full gene names are displayed in Table S1.
DISCUSSION
One original aspect of our study lies in the comparison of 5 different drugs of abuse for their long-lasting behavioral effects upon cessation of drug exposure, revealing clearly distinct profiles. Indeed, we show for the first time that mice kept abstinent during four weeks, after chronic morphine, nicotine, THC and alcohol, share similar long-term deficient direct social interaction, increased motor stereotypies and exacerbated anxiety, with consistent Fos expression patterns. In contrast, we show that cocaine abstinence produces only a moderate decrease in social interest, reduces anxiety levels, supported by a relevant Fos expression pattern, and results in transcriptional regulations opposing those induced by morphine abstinence.
We first demonstrate that abstinence from morphine, nicotine, THC, alcohol and, to a lesser extent, cocaine, leads to enduring impairments of social abilities. Morphine abstinent mice displayed impaired direct social interaction, deficient nest building and compromised social preference, indicative of altered social behavior. These results match previous reports that protracted abstinence from chronic escalating opiate treatment produces social behavior deficits in rodents (Ayranci et al., 2014; Goeldner et al., 2011; Lutz et al., 2014; Zanos et al., 2014), as well as clinical reports describing social cognition/judgment deficits in patients under opioid maintenance (Johnson et al., 2014; McDonald et al., 2013). Alcohol, nicotine and THC abstinent mice also exhibited reduced social interaction. Previous studies revealed social behavior deficits in early alcohol withdrawn rats (Broadwater et al., 2011; Overstreet et al., 2002), in primates under chronic alcohol (Shively et al., 2002) as well as in nicotine abstinent rats (Aydin et al., 2012). In the clinics, impaired social cognition was reported in patients with a drinking history (Valmas et al., 2014). Tobacco smokers and abstinent marijuana smokers appear more prone to display aggressive behavior (Bernstein et al., 2014; Budney et al., 2001), which may compromise social relationships. In cocaine abstinent animals, we found no modification of direct social interaction, but a moderately decreased interest for the social stimulus in the three-chamber test. This alteration fits clinical findings that social abilities are blunted in cocaine users, although one cannot exclude that multiple drug use, a major concern when studying addiction, have contributed to such behavioral deficit (Preller et al., 2014). To pinpoint neurobiological substrates differentially recruited after long-term abstinence, we mapped neuronal activation induced by an approach/avoidance conflict task, the NSF. As regards approach, morphine, nicotine, THC and alcohol, but not cocaine, abstinent mice displayed lower Fos stimulation in the VTA following NSF. Diminished neuronal activation in this region, a likely result of sustained stimulation during drug exposure and withdrawal (Luscher and Malenka, 2011), reduces striatal dopamine (DA) release and may contribute to lower reward sensitivity in addiction (Volkow et al., 2010). Remarkably, decreased DA release in the nucleus accumbens (NAc) was evidenced as a long-term consequence of adolescent binge alcohol exposure (Zandy et al., 2015). Moreover, social interactions recruit brain reward circuitry (Becker et al., 2014; Gunaydin et al., 2014; Trezza et al., 2010). Therefore, in our study, blunted VTA activation in abstinent mice, with the exception of cocaine treated animals, may account for their social interaction deficits, together with decreased reactivity in the DR, as social reward involves serotonin release in the NAc (Dolen et al., 2013).
Next, we evidence for the first time that mice made abstinent from morphine, nicotine, THC and alcohol develop spontaneous motor stereotypies. Repeated morphine administration was shown to induce motor stereotypies (Pollock and Kornetsky, 1989; Walter and Kuschinsky, 1989) and opiate abstinence to produce excessive grooming and deficient spatial alternation, suggestive of stereotypes/perseverative behavior (Goeldner et al., 2011; Lutz et al., 2014). Concerning alcohol, early withdrawal causes stereotypic behavior in mice (Becker et al., 1987). In the clinics, substance abuse induce movement disorders, including stereotypies (Brust, 2010), and stereotyped behavior may contribute to poor cognitive flexibility (Baldacchino et al., 2012; Lundqvist, 2005). Finally, we failed to detect cocaine abstinence-induced motor stereotypies despite clinical reports of such behaviors in cocaine users (Fasano et al., 2008), although here again multiple drug abuse should be considered.
Third, we show increased anxiety levels for morphine, nicotine, THC and alcohol, but not cocaine, abstinent animals. For morphine, we detected increased marble burying and augmented latencies to feed in the NFS test in morphine abstinent mice, suggestive of elevated anxiety. Morphine abstinent animals explored the open arms more than saline counterparts in the EPM test, pointing conversely to reduced anxiety. Head-dipping frequency and duration, however, were not concomitantly increased, as expected in mice with low levels of anxiety (Sorregotti et al., 2013). These results evoke a mu opioid receptor (MOR)-mediated mechanism, as similar discrepancies are observed in mice lacking MORs (Becker et al., 2014; Filliol et al., 2000; Ide et al., 2010), and rats injected with a MOR antagonist in the CeA (Wilson and Junor, 2008). Paradoxical or limited effects in anxiety tests based on exploration may account for previous failure to detect modified anxiety levels in morphine abstinent animals (Goeldner et al., 2011; Lutz et al., 2013) despite high frequency of comorbid anxiety in patients with a history of opiate abuse (Fatseas et al., 2010). Regarding nicotine, THC and alcohol, abstinent animals buried more marbles (for the former) and showed longer latency to eat food pellets in the NFS test (all three drugs), pointing to increased anxiety levels. These results are consistent with previous reports of increased anxiety under early nicotine withdrawal (Cippitelli et al., 2011; Cohen et al., 2015) and in alcohol abstinent rats (Economidou et al., 2011; Gillett et al., 2013; Zhao et al., 2007). In the clinics, patients with a history of alcohol drinking display anxiety disorders and biased perception of emotions towards negative feeling (Townshend and Duka, 2003; Trick et al., 2014). Consistent with behavioral data, Fos immunochemistry performed after NFS revealed increased neuronal reactivity in anxiety-related brain regions for morphine, alcohol, THC and nicotine abstinent mice, most strikingly in the CeA and PVN. Interestingly, elevated cortisol levels, as a possible result of excessive PVN reactivity, were detected after 30 days of abstinence in heroin addicts (Shi et al., 2009). In contrast, cocaine abstinence resulted in decreased anxiety levels in the NSF test under challenging conditions (80 lux) and in the EPM. In the literature, anxiety was reported as increased after acute cocaine withdrawal (Perrine et al., 2008) and either unchanged (Craige et al., 2015) or increased (El Hage et al., 2012) following a period of abstinence. Discrepancies may result from the use of different cocaine exposure paradigms, anxiety tests and species. In agreement with decreased anxiety levels in cocaine abstinent mice, though, we evidenced decreased Fos immunoreactivity throughout anxiety-related brain circuitry following NSF, consistent with our qRT-PCR data showing decreased early gene expression (Egr1 and Egr3) in EA and previous report in early cocaine withdrawal (El Hage et al., 2012). In the clinics, blunted anxiety could contribute to exacerbated risk-taking behavior in psychostimulant users (Gorini et al., 2014; Morley et al., 2015). Our data thus suggest that chronic exposure to morphine, nicotine, THC or alcohol long-lastingly compromises social behavior, motor patterns and emotional responses, together with neuronal reactivity in circuits underlying approach and anxiety, while such alterations are not observed, or milder, after cocaine abstinence.
Over the last decades, a unitary view of addiction has emerged, nurtured by influential theories highlighting shared psychological processes and neurobiological substrates across addictions to different drugs of abuse. Although these theories have allowed significant advances in the field, they ignore noticeable differences, especially in opiate versus psychostimulant (cocaine) addiction, or alcohol addiction versus addiction to other drugs (Badiani et al., 2011; Ozburn et al., 2015). Our data identify further differences between cocaine addiction and addiction to other drugs, while they substantiate commonalities between opiate, alcohol, THC and nicotine abuse. Limitations of our study, however, lie in the use of a single paradigm of administration for each drug, selected for its ability to induce a withdrawal syndrome of comparable intensity in morphine, nicotine, THC and alcohol-treated mice (Le Merrer et al., 2012). Furthermore, paradigms differed across drugs, due to differences in solubility, kinetics and reinforcing properties. Convergence in the long-term behavioral (this study) and transcriptional (Le Merrer et al., 2012) consequences of drug exposure under such conditions is thus remarkable, but will require further investigation by varying administration protocols (doses, routes of administration, sequences of injection/exposure, active versus passive exposure). As regards cocaine, the administration paradigm was chosen based on its ability to induce robust behavioral sensitization; selected doses were higher than these required to produce withdrawal-induced anxiety (El Hage et al., 2012), making unlikely that differences in the long-term behavioral effects between cocaine and other drugs were due to insufficient exposure. Further work will nevertheless be necessary to explore the effects of cocaine following various administration protocols. Finally, abstinence to all these drugs will need to be assessed at additional time points. In the case of opiates, social withdrawal, not detected after 1 week of morphine or heroine abstinence, was evidenced after 4 weeks, and maintained up to 7 weeks for heroin (Goeldner et al., 2011; Lutz et al., 2014). Comparison with alcohol, THC, nicotine and cocaine abstinence could reveal differences in time course, with possible later onset for cocaine. Keeping in mind such limits, though, our results point substantial differences in the long-term consequences of psychostimulant versus opiate addiction.
Crucial differences between addiction to psychostimulants versus other drugs reside notably in their neurobiological substrates (Badiani et al., 2011). Of interest in the context of abstinence, morphine and cocaine differentially alter MSN morphology in the NAc when drug exposure ceases, with morphine abstinence reducing whereas cocaine abstinence increases spine density (Diana et al., 2006; Dobi et al., 2011; Lee et al., 2006; Spiga et al., 2014). Accordingly, our qRT-PCR data in the EA show opposite transcriptional regulation after morphine versus cocaine abstinence for a huntingtin (HTT)-related network of genes (Le Merrer et al., 2012) and several marker genes, all sharing common enriched expression in MSNs (Doyle et al., 2008). MSNs, abundant in striatal and striatal-like regions (Kawaguchi, 1997; Sun and Cassell, 1993), play a crucial role in every stage of the addiction process (Koob et al., 2014; McNally, 2014; Nieh et al., 2013; Shalev et al., 2002; Volkow and Baler, 2014). Thus differential enduring effects of psychostimulants versus opiates on these neurons and, more specifically, on the two main MSNs populations, D1 and D2 dopamine receptor bearing neurons (Dobi et al., 2011; Enoksson et al., 2012; Lee et al., 2006; Lobo et al., 2013), may have crucial implications for long-term vulnerability to these drugs. Most interestingly, such contrasting effects could account for setting-driven differential reinstatement of heroin versus cocaine-seeking (Montanari et al., 2015). Conversely, shared long-lasting effects on MSNs (Ehlinger et al., 2014; Le Merrer et al., 2012; Peterson et al., 2015; Smith et al., 2015) represent a plausible substrate for commonalities between alcohol, THC, nicotine and morphine addiction. Involvement of MORs represents another major neurobiological difference between opiate and psychostimulant addiction, as required for the former (as well as alcohol, THC and nicotine abuse) and not for the later (Badiani et al., 2011; Le Merrer et al., 2009; Ozburn et al., 2015). Interestingly, our gene expression analysis also reveals opposite effects of morphine versus cocaine abstinence on the expression of opioid genes. Transcription of Penk (coding Proenkephalin) and Pdyn (Prodynorphin) was reduced or tended to be in morphine abstinent mice, suggestive of low opioid peptide release, as recently evidenced in the striatum of alcoholic patients (Sarkisyan et al., 2015). Such a decrease may lead to diminished MOR activity and therefore account for common behavioral features between morphine abstinent and MOR knockout mice (Becker et al., 2014). Conversely, MOR transcription was increased in cocaine abstinent mice, consistent with previous report (Unterwald et al., 1992), as well as Penk and Pdyn transcription, suggesting that opioid tone is high in these animals, as recently demonstrated in the ventral pallidum (Kupchik et al., 2014). Elevated opioid tone might have protected cocaine abstinent animals from social behavior deficits.
In conclusion, our study not only highlights commonalities in the behavioral and neurobiological consequences of long-term abstinence from morphine, nicotine, THC and alcohol but also evidences clear differences with cocaine abstinence, thus challenging unitary theories of addiction. These differences may have crucial clinical and therapeutic implications. They could account for differential influence of environment (home versus outside) on drug taking and relapse (Caprioli et al., 2009; Montanari et al., 2015), maybe in relation with distinct long-term effects of social abilities. They could also explain why efficient pharmacotherapies for addiction to opiates or other drugs of abuse have limited effects on cocaine addiction (Badiani et al., 2011; Somaini et al., 2011; Soyka and Mutschler, 2015). Importantly, they might provide useful cues for the development of novel pharmacological and/or cognitive behavioral therapeutic strategies for addiction, targeting better the specific needs of each patient depending on the abused drug.
Supplementary Material
ACKNOWLEDGEMENTS
We thank P. Chu Sin Chung, A.C. Meirsman, A. Parlog and A. Stephan for their assistance, and G. Duval and D. Memedov for animal care. This work was supported by the Centre National de la Recherche Scientifique (CNRS), Institut National de la Santé et de la Recherche Médicale (INSERM), Université de Strasbourg and Institut de Recherches Scientifiques sur les Boissons (IREB). JLM acknowledges postdoctoral fellowship from the Fondation Université de Strasbourg, generously granted by Pierre Fabre Laboratories. We thank the National Institutes of Health (NIAAA #16658 and NIDA # 005010) for financial support.
Footnotes
AUTHOR CONTRIBUTIONS
J.A.J.B., J.L.M. and B.L.K. designed the experiments. J.A.J.B. and J.L.M. performed and analyzed behavioral, immunohistochemical and pharmacological experiments. J.A.J.B. and J.L.M. performed and analyzed qRT-PCR experiments. J.A.J.B., J.L.M. and B.L.K. interpreted the results. J.A.J.B., B.L.K. and J.L.M. wrote the article. All three authors contributed equally to this work, critically reviewed content and approved final version for publication.
References
- APA . Diagnostic and statistical manual of mental disorders: DSM-5. American Psychiatric Association; 2013. [Google Scholar]
- Aupperle RL, Paulus MP. Neural systems underlying approach and avoidance in anxiety disorders. Dialogues Clin Neurosci. 2010;12:517–531. doi: 10.31887/DCNS.2010.12.4/raupperle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydin C, Oztan O, Isgor C. Nicotine-induced anxiety-like behavior in a rat model of the novelty-seeking phenotype is associated with long-lasting neuropeptidergic and neuroplastic adaptations in the amygdala: effects of the cannabinoid receptor 1 antagonist AM251. Neuropharmacology. 2012;63:1335–1345. doi: 10.1016/j.neuropharm.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayranci G, Befort K, Lalanne L, Kieffer BL, Lutz PE. Dissociation of heroin-induced emotional dysfunction from psychomotor activation and physical dependence among inbred mouse strains. Psychopharmacology (Berl) 2014 doi: 10.1007/s00213-014-3826-5. [DOI] [PubMed] [Google Scholar]
- Ayranci G, Befort K, Lalanne L, Kieffer BL, Lutz PE. Dissociation of heroin-induced emotional dysfunction from psychomotor activation and physical dependence among inbred mouse strains. Psychopharmacology (Berl) 2015;232:1957–1971. doi: 10.1007/s00213-014-3826-5. [DOI] [PubMed] [Google Scholar]
- Badiani A, Belin D, Epstein D, Calu D, Shaham Y. Opiate versus psychostimulant addiction: the differences do matter. Nature reviews Neuroscience. 2011;12:685–700. doi: 10.1038/nrn3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldacchino A, Balfour DJ, Passetti F, Humphris G, Matthews K. Neuropsychological consequences of chronic opioid use: a quantitative review and meta-analysis. Neurosci Biobehav Rev. 2012;36:2056–2068. doi: 10.1016/j.neubiorev.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Becker HC, Anton RF, Randall CL. Stereotypic wall climbing in mice during ethanol withdrawal: a new measure of physical dependence. Alcohol. 1987;4:443–447. doi: 10.1016/0741-8329(87)90083-8. [DOI] [PubMed] [Google Scholar]
- Becker JA, Clesse D, Spiegelhalter C, Schwab Y, Le Merrer J, Kieffer BL. Autistic-like syndrome in mu opioid receptor null mice is relieved by facilitated mGluR4 activity. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:2049–2060. doi: 10.1038/npp.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein MH, Colby SM, Bidwell LC, Kahler CW, Leventhal AM. Hostility and cigarette use: a comparison between smokers and nonsmokers in a matched sample of adolescents. Nicotine Tob Res. 2014;16:1085–1093. doi: 10.1093/ntr/ntu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwater M, Varlinskaya EI, Spear LP. Chronic intermittent ethanol exposure in early adolescent and adult male rats: effects on tolerance, social behavior, and ethanol intake. Alcoholism, clinical and experimental research. 2011;35:1392–1403. doi: 10.1111/j.1530-0277.2011.01474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brust JC. Substance abuse and movement disorders. Mov Disord. 2010;25:2010–2020. doi: 10.1002/mds.22599. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, Novy PL. Marijuana abstinence effects in marijuana smokers maintained in their home environment. Arch Gen Psychiatry. 2001;58:917–924. doi: 10.1001/archpsyc.58.10.917. [DOI] [PubMed] [Google Scholar]
- Caprioli D, Celentano M, Dubla A, Lucantonio F, Nencini P, Badiani A. Ambience and drug choice: cocaine- and heroin-taking as a function of environmental context in humans and rats. Biological psychiatry. 2009;65:893–899. doi: 10.1016/j.biopsych.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Astarita G, Duranti A, Caprioli G, Ubaldi M, Stopponi S, Kallupi M, Sagratini G, Rodriguez de Fonseca F, Piomelli D, Ciccocioppo R. Endocannabinoid regulation of acute and protracted nicotine withdrawal: effect of FAAH inhibition. PLoS One. 2011;6:e28142. doi: 10.1371/journal.pone.0028142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A, Treweek J, Edwards S, Leao RM, Schulteis G, Koob GF, George O. Extended access to nicotine leads to a CRF1 receptor dependent increase in anxiety-like behavior and hyperalgesia in rats. Addict Biol. 2015;20:56–68. doi: 10.1111/adb.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JC, Rodgers RJ. An ethological analysis of the effects of chlordiazepoxide and bretazenil (Ro 16-6028) in the murine elevated plus-maze. Behavioural pharmacology. 1993;4:573–580. [PubMed] [Google Scholar]
- Craige CP, Lewandowski S, Kirby LG, Unterwald EM. Dorsal raphe 5-HT(2C) receptor and GABA networks regulate anxiety produced by cocaine withdrawal. Neuropharmacology. 2015;93:41–51. doi: 10.1016/j.neuropharm.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana M, Spiga S, Acquas E. Persistent and reversible morphine withdrawal-induced morphological changes in the nucleus accumbens. Annals of the New York Academy of Sciences. 2006;1074:446–457. doi: 10.1196/annals.1369.045. [DOI] [PubMed] [Google Scholar]
- Dobi A, Seabold GK, Christensen CH, Bock R, Alvarez VA. Cocaine-induced plasticity in the nucleus accumbens is cell specific and develops without prolonged withdrawal. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:1895–1904. doi: 10.1523/JNEUROSCI.5375-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501:179–184. doi: 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JP, Dougherty JD, Heiman M, Schmidt EF, Stevens TR, Ma G, Bupp S, Shrestha P, Shah RD, Doughty ML, Gong S, Greengard P, Heintz N. Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell. 2008;135:749–762. doi: 10.1016/j.cell.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D, Cippitelli A, Stopponi S, Braconi S, Clementi S, Ubaldi M, Martin-Fardon R, Weiss F, Massi M, Ciccocioppo R. Activation of brain NOP receptors attenuates acute and protracted alcohol withdrawal symptoms in the rat. Alcoholism, clinical and experimental research. 2011;35:747–755. doi: 10.1111/j.1530-0277.2010.01392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlinger DG, Bergstrom HC, Burke JC, Fernandez GM, McDonald CG, Smith RF. Adolescent nicotine-induced dendrite remodeling in the nucleus accumbens is rapid, persistent, and D1-dopamine receptor dependent. Brain structure & function. 2014 doi: 10.1007/s00429-014-0897-3. [DOI] [PubMed] [Google Scholar]
- El Hage C, Rappeneau V, Etievant A, Morel AL, Scarna H, Zimmer L, Berod A. Enhanced anxiety observed in cocaine withdrawn rats is associated with altered reactivity of the dorsomedial prefrontal cortex. PLoS One. 2012;7:e43535. doi: 10.1371/journal.pone.0043535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoksson T, Bertran-Gonzalez J, Christie MJ. Nucleus accumbens D2- and D1-receptor expressing medium spiny neurons are selectively activated by morphine withdrawal and acute morphine, respectively. Neuropharmacology. 2012;62:2463–2471. doi: 10.1016/j.neuropharm.2012.02.020. [DOI] [PubMed] [Google Scholar]
- Fasano A, Barra A, Nicosia P, Rinaldi F, Bria P, Bentivoglio AR, Tonioni F. Cocaine addiction: from habits to stereotypical-repetitive behaviors and punding. Drug Alcohol Depend. 2008;96:178–182. doi: 10.1016/j.drugalcdep.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Fatseas M, Denis C, Lavie E, Auriacombe M. Relationship between anxiety disorders and opiate dependence--a systematic review of the literature: implications for diagnosis and treatment. J Subst Abuse Treat. 2010;38:220–230. doi: 10.1016/j.jsat.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gaveriaux-Ruff C, Dierich A, LeMeur M, Valverde O, Maldonado R, Kieffer BL. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- Gillett K, Harshberger E, Valdez GR. Protracted withdrawal from ethanol and enhanced responsiveness stress: regulation via the dynorphin/kappa opioid receptor system. Alcohol. 2013;47:359–365. doi: 10.1016/j.alcohol.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeldner C, Lutz PE, Darcq E, Halter T, Clesse D, Ouagazzal AM, Kieffer BL. Impaired emotional-like behavior and serotonergic function during protracted abstinence from chronic morphine. Biological psychiatry. 2011;69:236–244. doi: 10.1016/j.biopsych.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin RD, Stayner DA, Chinman MJ, Wu P, Tebes JK, Davidson L. The relationship between anxiety and substance use disorders among individuals with severe affective disorders. Compr Psychiatry. 2002;43:245–252. doi: 10.1053/comp.2002.33500. [DOI] [PubMed] [Google Scholar]
- Gorini A, Lucchiari C, Russell-Edu W, Pravettoni G. Modulation of risky choices in recently abstinent dependent cocaine users: a transcranial direct-current stimulation study. Front Hum Neurosci. 2014;8:661. doi: 10.3389/fnhum.2014.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, Lammel S, Mirzabekov JJ, Airan RD, Zalocusky KA, Tye KM, Anikeeva P, Malenka RC, Deisseroth K. Natural neural projection dynamics underlying social behavior. Cell. 2014;157:1535–1551. doi: 10.1016/j.cell.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide S, Sora I, Ikeda K, Minami M, Uhl GR, Ishihara K. Reduced emotional and corticosterone responses to stress in mu-opioid receptor knockout mice. Neuropharmacology. 2010;58:241–247. doi: 10.1016/j.neuropharm.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B, Ulberg S, Shivale S, Donaldson J, Milczarski B, Faraone SV. Fibromyalgia, autism, and opioid addiction as natural and induced disorders of the endogenous opioid hormonal system. Discov Med. 2014;18:209–220. [PubMed] [Google Scholar]
- Kawaguchi Y. Neostriatal cell subtypes and their functional roles. Neurosci Res. 1997;27:1–8. doi: 10.1016/s0168-0102(96)01134-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, Schmeichel B, Vendruscolo LF, Wade CL, Whitfield TW, Jr., George O. Addiction as a stress surfeit disorder. Neuropharmacology. 2014;76(Pt B):370–382. doi: 10.1016/j.neuropharm.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupchik YM, Scofield MD, Rice KC, Cheng K, Roques BP, Kalivas PW. Cocaine dysregulates opioid gating of GABA neurotransmission in the ventral pallidum. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:1057–1066. doi: 10.1523/JNEUROSCI.4336-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Merrer J, Becker JA, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89:1379–1412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Merrer J, Befort K, Gardon O, Filliol D, Darcq E, Dembele D, Becker JA, Kieffer BL. Protracted abstinence from distinct drugs of abuse shows regulation of a common gene network. Addict Biol. 2012;17:1–12. doi: 10.1111/j.1369-1600.2011.00365.x. [DOI] [PubMed] [Google Scholar]
- Le Merrer J, Rezai X, Scherrer G, Becker JA, Kieffer BL. Impaired Hippocampus-Dependent and Facilitated Striatum-Dependent Behaviors in Mice Lacking the Delta Opioid Receptor. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KW, Kim Y, Kim AM, Helmin K, Nairn AC, Greengard P. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:3399–3404. doi: 10.1073/pnas.0511244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Zaman S, Damez-Werno DM, Koo JW, Bagot RC, DiNieri JA, Nugent A, Finkel E, Chaudhury D, Chandra R, Riberio E, Rabkin J, Mouzon E, Cachope R, Cheer JF, Han MH, Dietz DM, Self DW, Hurd YL, Vialou V, Nestler EJ. DeltaFosB induction in striatal medium spiny neuron subtypes in response to chronic pharmacological, emotional, and optogenetic stimuli. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:18381–18395. doi: 10.1523/JNEUROSCI.1875-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist T. Cognitive consequences of cannabis use: comparison with abuse of stimulants and heroin with regard to attention, memory and executive functions. Pharmacol Biochem Behav. 2005;81:319–330. doi: 10.1016/j.pbb.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz PE, Ayranci G, Chu-Sin-Chung P, Matifas A, Koebel P, Filliol D, Befort K, Ouagazzal AM, Kieffer BL. Distinct mu, delta, and kappa opioid receptor mechanisms underlie low sociability and depressive-like behaviors during heroin abstinence. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:2694–2705. doi: 10.1038/npp.2014.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz PE, Reiss D, Ouagazzal AM, Kieffer BL. A history of chronic morphine exposure during adolescence increases despair-like behaviour and strain-dependently promotes sociability in abstinent adult mice. Behav Brain Res. 2013;243:44–52. doi: 10.1016/j.bbr.2012.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald S, Darke S, Kaye S, Torok M. Deficits in social perception in opioid maintenance patients, abstinent opioid users and non-opioid users. Addiction. 2013;108:566–574. doi: 10.1111/add.12040. [DOI] [PubMed] [Google Scholar]
- McGregor IS, Callaghan PD, Hunt GE. From ultrasocial to antisocial: a role for oxytocin in the acute reinforcing effects and long-term adverse consequences of drug use? British journal of pharmacology. 2008;154:358–368. doi: 10.1038/bjp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally GP. Extinction of drug seeking: Neural circuits and approaches to augmentation. Neuropharmacology. 2014;76(Pt B):528–532. doi: 10.1016/j.neuropharm.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Montanari C, Stendardo E, De Luca MT, Meringolo M, Contu L, Badiani A. Differential vulnerability to relapse into heroin versus cocaine-seeking as a function of setting. Psychopharmacology (Berl) 2015;232:2415–2424. doi: 10.1007/s00213-015-3877-2. [DOI] [PubMed] [Google Scholar]
- Morley KI, Lynskey MT, Moran P, Borschmann R, Winstock AR. Polysubstance use, mental health and high-risk behaviours: Results from the 2012 Global Drug Survey. Drug Alcohol Rev. 2015;34:427–437. doi: 10.1111/dar.12263. [DOI] [PubMed] [Google Scholar]
- Nieh EH, Kim SY, Namburi P, Tye KM. Optogenetic dissection of neural circuits underlying emotional valence and motivated behaviors. Brain research. 2013;1511:73–92. doi: 10.1016/j.brainres.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien CP. Anticraving medications for relapse prevention: a possible new class of psychoactive medications. The American journal of psychiatry. 2005;162:1423–1431. doi: 10.1176/appi.ajp.162.8.1423. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Accentuated decrease in social interaction in rats subjected to repeated ethanol withdrawals. Alcoholism, clinical and experimental research. 2002;26:1259–1268. doi: 10.1097/01.ALC.0000023983.10615.D7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozburn AR, Janowsky AJ, Crabbe JC. Commonalities and Distinctions Among Mechanisms of Addiction to Alcohol and Other Drugs. Alcoholism, clinical and experimental research. 2015;39:1863–1877. doi: 10.1111/acer.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The Mouse Brain in Stereotaxic coordinates. second edition ed Academic Press; 2001. [Google Scholar]
- Perrine SA, Sheikh IS, Nwaneshiudu CA, Schroeder JA, Unterwald EM. Withdrawal from chronic administration of cocaine decreases delta opioid receptor signaling and increases anxiety- and depression-like behaviors in the rat. Neuropharmacology. 2008;54:355–364. doi: 10.1016/j.neuropharm.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson VL, McCool BA, Hamilton DA. Effects of ethanol exposure and withdrawal on dendritic morphology and spine density in the nucleus accumbens core and shell. Brain research. 2015;1594:125–135. doi: 10.1016/j.brainres.2014.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends in neurosciences. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock J, Kornetsky C. Evidence for the role of dopamine D1 receptors in morphine induced stereotypic behavior. Neuroscience letters. 1989;102:291–296. doi: 10.1016/0304-3940(89)90094-3. [DOI] [PubMed] [Google Scholar]
- Preller KH, Herdener M, Schilbach L, Stampfli P, Hulka LM, Vonmoos M, Ingold N, Vogeley K, Tobler PN, Seifritz E, Quednow BB. Functional changes of the reward system underlie blunted response to social gaze in cocaine users. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:2842–2847. doi: 10.1073/pnas.1317090111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkisyan D, Hussain MZ, Watanabe H, Kononenko O, Bazov I, Zhou X, Yamskova O, Krishtal O, Karpyak VM, Yakovleva T, Bakalkin G. Downregulation of the endogenous opioid peptides in the dorsal striatum of human alcoholics. Front Cell Neurosci. 2015;9:187. doi: 10.3389/fncel.2015.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Shi J, Li SX, Zhang XL, Wang X, Le Foll B, Zhang XY, Kosten TR, Lu L. Time-dependent neuroendocrine alterations and drug craving during the first month of abstinence in heroin addicts. Am J Drug Alcohol Abuse. 2009;35:267–272. doi: 10.1080/00952990902933878. [DOI] [PubMed] [Google Scholar]
- Shively CA, Grant KA, Register TC. Effects of long-term moderate alcohol consumption on agonistic and affiliative behavior of socially housed female cynomolgus monkeys (Macaca fascicularis) Psychopharmacology (Berl) 2002;165:1–8. doi: 10.1007/s00213-002-1223-y. [DOI] [PubMed] [Google Scholar]
- Smith KC, Ehlinger DG, Smith RF. Adolescent nicotine alters dendritic morphology in the bed nucleus of the stria terminalis. Neuroscience letters. 2015;590:111–115. doi: 10.1016/j.neulet.2015.01.056. [DOI] [PubMed] [Google Scholar]
- Somaini L, Donnini C, Raggi MA, Amore M, Ciccocioppo R, Saracino MA, Kalluppi M, Malagoli M, Gerra ML, Gerra G. Promising medications for cocaine dependence treatment. Recent Pat CNS Drug Discov. 2011;6:146–160. doi: 10.2174/157488911795933893. [DOI] [PubMed] [Google Scholar]
- Sorregotti T, Mendes-Gomes J, Rico JL, Rodgers RJ, Nunes-de-Souza RL. Ethopharmacological analysis of the open elevated plus-maze in mice. Behav Brain Res. 2013;246:76–85. doi: 10.1016/j.bbr.2013.02.035. [DOI] [PubMed] [Google Scholar]
- Soyka M, Mutschler J. Treatment-refractory substance use disorder: Focus on alcohol, opioids, and cocaine. Prog Neuropsychopharmacol Biol Psychiatry. 2015 doi: 10.1016/j.pnpbp.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Spiga S, Mulas G, Piras F, Diana M. The “addicted” spine. Front Neuroanat. 2014;8:110. doi: 10.3389/fnana.2014.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N, Cassell MD. Intrinsic GABAergic neurons in the rat central extended amygdala. J Comp Neurol. 1993;330:381–404. doi: 10.1002/cne.903300308. [DOI] [PubMed] [Google Scholar]
- Townshend JM, Duka T. Mixed emotions: alcoholics' impairments in the recognition of specific emotional facial expressions. Neuropsychologia. 2003;41:773–782. doi: 10.1016/s0028-3932(02)00284-1. [DOI] [PubMed] [Google Scholar]
- Trezza V, Baarendse PJ, Vanderschuren LJ. The pleasures of play: pharmacological insights into social reward mechanisms. Trends Pharmacol Sci. 2010;31:463–469. doi: 10.1016/j.tips.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trick L, Kempton MJ, Williams SC, Duka T. Impaired fear recognition and attentional set-shifting is associated with brain structural changes in alcoholic patients. Addict Biol. 2014;19:1041–1054. doi: 10.1111/adb.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unterwald EM, Horne-King J, Kreek MJ. Chronic cocaine alters brain mu opioid receptors. Brain research. 1992;584:314–318. doi: 10.1016/0006-8993(92)90912-s. [DOI] [PubMed] [Google Scholar]
- Valmas MM, Mosher Ruiz S, Gansler DA, Sawyer KS, Oscar-Berman M. Social cognition deficits and associations with drinking history in alcoholic men and women. Alcoholism, clinical and experimental research. 2014;38:2998–3007. doi: 10.1111/acer.12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Baler RD. Addiction science: Uncovering neurobiological complexity. Neuropharmacology. 2014;76(Pt B):235–249. doi: 10.1016/j.neuropharm.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F, Baler R. Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain's control circuit. Bioessays. 2010;32:748–755. doi: 10.1002/bies.201000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter S, Kuschinsky K. Conditioning of morphine-induced locomotor activity and stereotyped behaviour in rats. J Neural Transm Gen Sect. 1989;78:231–247. doi: 10.1007/BF01249232. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Junor L. The role of amygdalar mu-opioid receptors in anxiety-related responses in two rat models. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:2957–2968. doi: 10.1038/sj.npp.1301675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Koob GF. The development and maintenance of drug addiction. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:254–262. doi: 10.1038/npp.2013.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandy SL, Matthews DB, Tokunaga S, Miller AD, Blaha CD, Mittleman G. Reduced dopamine release in the nucleus accumbens core of adult rats following adolescent binge alcohol exposure: age and dose-dependent analysis. Psychopharmacology (Berl) 2015;232:777–784. doi: 10.1007/s00213-014-3712-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Georgiou P, Wright SR, Hourani SM, Kitchen I, Winsky-Sommerer R, Bailey A. The oxytocin analogue carbetocin prevents emotional impairment and stress-induced reinstatement of opioid-seeking in morphine-abstinent mice. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:855–865. doi: 10.1038/npp.2013.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Weiss F, Zorrilla EP. Remission and resurgence of anxiety-like behavior across protracted withdrawal stages in ethanol-dependent rats. Alcoholism, clinical and experimental research. 2007;31:1505–1515. doi: 10.1111/j.1530-0277.2007.00456.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.