Abstract
Objectives
To develop a fully-automated algorithm to process axial magnetic resonance imaging (MRI) slices for quantifying abdominal visceral, subcutaneous and total adipose tissues, i.e. VAT, SAT and TAT, without human intervention or prior knowledge.
Materials and Methods
Fat regions in single MRI slice or sequence (20 slices) were identified with image processing techniques including region-growing, inhomogeneity correction, fuzzy c-means clustering, and active contours segmentation. The MR images of 85 subjects (60 males and 25 females), whose body mass index (BMI) values ranged from 19.96 to 40.35 kg/m2, were analyzed using the fully-automated algorithm—the automatic method developed in the research and the widely used semi-automated software (sliceOmatic® Tomovision, Inc.)—the reference method.
Results
The proposed automated method showed good performance against the reference method to quantify adipose tissues in both single umbilical slice and MRI sequence. The square of the Pearson correlation coefficients (R2) based on the results generated from the two methods for VAT/SAT/TAT were 0.977/0.998/0.997 for single slice data and 0.995/0.999/0.999 for volumetric data. The intra-class correlation of VAT between the three operators was 0.939 in the reference method, which was improved to 0.999 in the automatic method. The adipose tissue measurements in the slice at Lumbar 3 vertebra have the highest correlation with the total fat volumes across the entire abdomen.
Conclusion
The fully-automated algorithm presented in the paper provides an accurate and reliable assessment of abdominal fat without human intervention.
Keywords: T1-weighted MRI, abdominal adipose tissue, image processing
Introduction
Currently, 33.6% of adults are overweight and 34.9% are obese in the United States (Ogden CL et al., 2014). Excessive abdominal adiposity, including both visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT), has adverse effects on health as it increases risks of diseases. Specifically, VAT is a major predictor for metabolic syndrome, diabetes mellitus type 2, cardiovascular disease and certain types of cancers (Klein, 2004; Ibrahim, 2010; Baglioni et al., 2012). SAT is a better indicator of insulin resistance (Preis et al., 2010; Frederiksen et al., 2009). Since SAT and VAT have different health implications, assessing VAT and SAT separately is more important than just measuring total adipose tissue (TAT). Total body fat can be assessed by indirect methods including air displacement plethysmography (ADP) (Fields et al., 2002), underwater weighing (UWW) (Wang et al., 1989), and bioelectrical impedance analysis (BIA) (Kuczmarski, 1996). Although these methods are relatively accessible, they are unable to discriminate visceral and subcutaneous fat depots.
Magnetic resonance imaging (MRI) (Thomas et al., 1998) and computed tomography (CT) (Tokunaga et al., 1983) are the more advanced methods that can be used to accurately measure VAT and SAT. A newly developed dual-energy X-ray absorptiometry (DXA) (Haarbo et al., 1991) has been used to estimate VAT based on some abdominal geometric assumptions (Kaul et al., 2012). MRI and CT allow a direct differentiation of VAT from SAT, and are often considered as gold standards in adiposity quantification (Klein, 2004; Ibrahim, 2010). T1-weighted MRI is a commonly used tool to measure adiposity, as fat tissues show higher signal intensity and appear brighter in T1-weighted images. The slow molecular motion of a fat nucleus causes a quick regaining of longitudinal magnetization, resulting in short longitudinal relaxation time and higher image intensity (Lancaster et al., 1991; Abate et al., 1994). Although CT can detect internal fat, the subject has to be exposed to ionizing radiation (Tokunaga et al., 1983). Thus, MRI is a safer and more favorable method for adiposity measurement (Martin and Semelka, 2006).
MRI slices have been analyzed with manual, semi- and fully- automated methods to extract SAT and VAT regions. A typical two-step manual assessment requires trained experts to draw boundaries of the whole abdominal area and delineate areas of VAT and SAT separately (Jin et al., 2003). This manual process is very time-consuming and subjective. Semi-automated methods employ adjustable thresholds, boundary enhancement and other image processing tools to help observers identify adipose tissues (Gronemeyer et al., 2000; Machann et al., 2005). These software packages, such as sliceOmatic, Analyze and ImageJ, are less laborious and more objective than the manual segmentation method, but still require more than 10 minutes on average for a trained expert to process one single slice (Bonekamp et al., 2008). A whole abdominal scan typically consists of 15 to 50 slices, imposing a substantial image-processing task when processed by a semi-automated segmentation method. Both manual and semiautomated methods require users’ interventions, which may cause inter- and intra-observer variations that undermine the data reliability (Thörmer et al., 2013). In order to circumvent the above disadvantages, user-independent and fully-automated MR image processing methods have been investigated (Thörmer et al., 2013; Kullberg et al., 2007; Zhou et al., 2011; Positano et al., 2004; Demerath et al., 2007). However, three major challenges still exist. Inhomogeneity of image intensity in T1-weighted MRI slices has not been thoroughly studied (Thörmer et al., 2013). In addition, prior knowledge is required to train the automated algorithm, limiting usage to a small range of body types and fat amount (Kullberg et al., 2007). Another challenge is the presence of validation cohorts with a wide range of BMIs, various body sizes and fat depots to confirm the robustness of the algorithms.
In this study, we aim to develop a novel MR image processing algorithm in order to provide a solution to abdominal adiposity assessment without human intervention or prior knowledge. The new algorithm integrates intensity correction, image clustering and image segmentation methods. This fully-automated algorithm eliminates observer’s bias and increases reliability by avoiding human supervision. The algorithm performance was evaluated on 85 subjects having a wide range of BMIs, and compared to a semi-automated method–sliceOmatic (Version 4.3).
Methods
Magnetic resonance imaging
A total of 85 subjects (60 male and 25 female) underwent abdominal MRI test via a 3.0T General Electric scanner (GE Health Care, Milwaukee, WI). Subject characteristics are shown in Table 1. Those with any serious illness, metallic fragments or implants were eliminated due to the risks involved with MR imaging. Female subjects who were pregnant, or were lactating were excluded due to University regulations. Demographic and health history questionnaires were completed and anthropometric measurements, including weight, height, and body tape circumferences were also taken from all the subjects. This study was approved by the Institutional Review Board of the University of Texas at Austin and the informed consent for every subject was obtained.
Table 1.
Characteristics of subjects
| Sex | Subjects (n) | BMI (kg/m2) Mean (range) |
BMI Classification | Age (years) Mean (range) |
|---|---|---|---|---|
| Male | 60 | 28.07 (19.96 – 40.35) | Normal to Obese Class III | 35 (19–60) |
| Female | 25 | 29.30 (20.92–40.1) | Normal to Obese Class III | 36 (19–61) |
BMI: body mass index.
Participants were positioned on the center of the MRI magnet and a 4-second 3-plane localizer scan was conducted for visualization of anatomical landmarks. Participants were asked to place their arms on the sides of the trunk without hard pressing against the abdominal area. For each subject, a total of 20 axial slices covering the abdominal area were obtained with 140 ms repetition time, 2.1 ms echo time, 80° flip angle, 8 mm slice thickness, and 2 mm gap between slices. An acquisition matrix of 512×192, a reconstruction matrix of 512× 512 and a field of view, varying from 400 mm×400 mm to 480 mm×480 mm, depending on body size, were utilized. The voxel volume can be obtained by dividing the field of view with the size of the reconstruction matrix, then multiplying the slice thickness. The voxel volume on a single slice in this study varied from 4.88 mm3 to 7.03 mm3.
Fully-automated algorithm
Step 1: Abdominal tissue mask computation
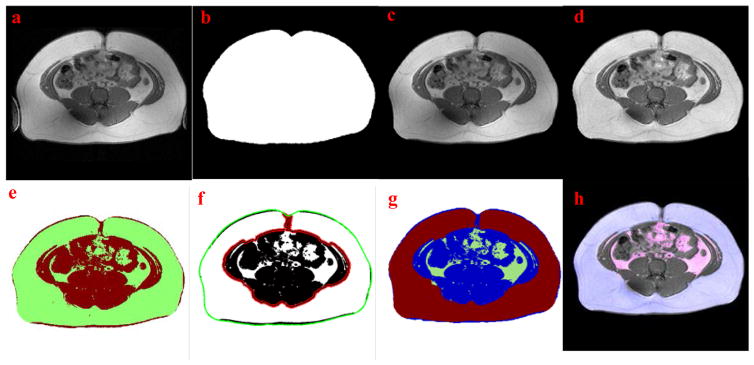
In order to separate abdominal tissue voxels from air background and unimportant limbs, a region growing method was employed. This process started from four corners of the original MRI slice where air background is always present. For each growing region iteration, mean value (μb) and standard deviation (σb) of the air background voxels were calculated and used as a standard to determine the category of each voxel in the new growing region. If the new voxel value was less than μb + 3σb, the voxel was classified as background; otherwise, the voxel was determined as tissue. Image morphological algorithms were employed to close the image and fill the holes. Then, the size of each tissue areas in the image was measured. Only the largest tissue area representing the abdomen was retained. Smaller areas containing the limbs were removed. The original T1-weighed abdominal MRI slice with unwanted limbs on two sides from a male subject is shown in Fig. 1a. Fig. 1b shows a tissue mask and Fig. 1c shows a processed image with the retained abdominal tissue after limbs were removed. This mask constrains the calculation area for the following image processing steps, accelerating computation and reducing MRI artifacts/noise outside the abdomen
Figure 1.
Examples of the fully-automated fat assessment processing. (a) Original MRI slice with limbs on two sides. (b) Abdominal tissue mask after region growing. (c) Clean image with limbs removed by (b). (d) Intensity corrected image. (e) Discrimination of fat and non-fat tissue using FCM clustering. (f) Segmentation of VAT and SAT using active contour, the initial and final contour are marked with green and red lines, respectively. (g) Segmentation result: green - visceral fat, red - subcutaneous fat and blue - non-fat tissue. (h) Final assessment result (Overlaying segmentation result on original MRI slice): pink -VAT and blue- SAT.
Step 2: Intensity inhomogeneity correction
Spatial inhomogeneity of the MRI coil sensitivity and main magnetic field gives rise to the artifact of inhomogeneous signal intensities across MRI slices (Vovk et al., 2007). Therefore, intensity correction step is critical for MRI voxel intensity-based computation, especially for T1-weighted images. The model to describe the bias field causing such inhomogeneity can be summarized as: Ib=IcB+n, where Ib is the observed inhomogeneous MR image, B is the bias field with intensity variation, Ic is the corrected or true image of interest, and n is the additive noise.
The original T1-weighed MR image (Fig. 1a) has noticeable inhomogeneous intensity, especially within the SAT. Without correction, such variations within the same tissue compartment (i.e., fat or muscle) often mislead conventional segmentation process. In this algorithm, the correction of the bias field was implemented using a modified version of “ local entropy minimization with a bicubic spline model ” (LEMS) proposed by Salvado (Salvado et al., 2006). First, the initial bias field B0 was predicted by fitting a 2D polynomial function to Ib using voxels above the average intensity of Ib. The corresponding corrected image was then calculated by using Ic0= Ib/B0. Ic0 and B0 were separated into small knots which were ordered by the B0 values of each knot center. Entropy optimization started at the knot with the highest B0 value with its eight neighboring knots denoted as Region1 (R1) because it may contain adipose tissue, which is of our interest. The center B0 value was updated to ensure the lowest local entropy in Ic0 within R1. The second optimization step within Region2 (R2) started at the knot with the second highest B0 value. If the average intensity value of the corrected Ic0 in R2 was similar to that in R1, then two regions (R1∪R2) were merged before the optimization. Otherwise, R2 was optimized independently. This adaptive piecewise optimizing process continued until all knots were processed, and the final image was the corrected image Ic. The modified LEMS model proposed in this study improves the homogeneity of the MR image in the same tissue compartments while preventing the over-correction in non-fat area (Fig. 1d). Details about knot’s definition and its size choosing criteria can be found in Ref. (Salvado et al., 2006).
Step 3: Image clustering
Image clustering algorithm needs to be employed to automatically classify MR image into fat and non-fat tissues after the intensity correction. The fuzzy c-means (FCM) algorithm with Euclidian norm was used in this study since it does not require prior information and is computationally efficient. The energy function to be minimized during the FCM iterations is defined as (Yang et al., 2009): , where N is the total number of voxels of the MR image, K is the number of clusters (K=3 in this study—fat tissue, non-fat tissue and background), yi is the voxel intensity and ck is the center intensity of the kth cluster. Iterative optimization of the above objective function was carried out with the update of fuzzy cluster membership of each voxel and the center of each cluster. Iteration stopped when the centers and the membership were stabilized, or the allowed iteration was reached. Fig. 1e exemplifies the clustering result of one MR image after inhomogeneity correction. The green color indicates fat tissues while the red color shows muscle and other non-fat tissues.
Step 4: Image segmentation
Image segmentation is a procedure to further distinguish VAT from SAT by locating the abdominal muscle wall (i.e. target contour). “Chan-Vese” active contour algorithm is a well-known image segmentation method which defines the contour by balancing a shrinking force and an expanding force of the contour defined by regional average intensities (Chan and Vese, 2001). Because the algorithm is not based on image gradient like other traditional segmentation methods, it is insensitive to noisy voxels in an MRI slice. In order to prevent human interference, the “Chan-Vese” algorithm was modified in this study. The abdominal contour obtained in the previous region-growing step was utilized as the initial contour (green line in Fig. 1f). Iteration stopped automatically when the contour was stabilized or the maximum iteration number was achieved. As a result, the optimized contour encompasses the non-fat region (red line in Fig. 1f). As shown in Fig. 1g, fat voxels inside the final contour are classified as VAT (green) while other fat voxels are counted as SAT (red). Final computation result is shown in Fig. 1h where the segmentation result is overlaid on the original corrected MR image. Pink denotes VAT and blue indicates SAT.
Step 5: Volumetric analysis
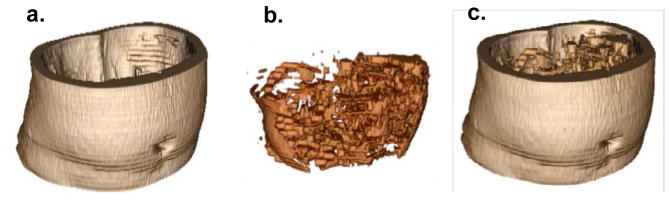
The MRI sequence (20 slices per subject) was analyzed slice by slice with the algorithm to quantify the abdominal adiposity based on volumetric data. Among the MRI sequence of a subject, the middle slice was chosen as a starting slice and its calculated bias field provided an initial value for the rest of the slices for the subject. Then, the active contour calculated in each slice was used as the initial contour in the adjacent slices. From one slice, VAT (cm3) and SAT (cm3) were calculated by multiplying the number of voxels by the voxel volume. TAT (cm3) was the sum of the VAT and SAT volumes. After the twenty slices were processed, the 3D images indicating the total abdominal VAT and SAT volumes were constructed by stacking up the VAT and SAT from each slice according to the slice’s position and thickness (see Fig. 2), and the total volumetric VAT, SAT and TAT measurements were obtained by summing the fat volumes of each slice in the sequence. Fig. 2a, Fig. 2b and Fig. 2c are the SAT, VAT and TAT volumes reconstructed from multiple slices in one MRI sequence.
Figure 2.
Visualization of volumetric abdominal adiposity reconstructed from all twenty MRI slices. (a) SAT, (b) VAT and (c) Combined SAT and VAT.
Semi-automated segmentation
In order to obtain the reference adiposity data to validate the proposed automatic method, a semi-automated MRI processing method, sliceOmatic, was utilized, as it is recognized as an easy-to-use method for fat assessment (Bonekamp et al., 2008). This method was referred to as the reference method in the study. Mathematical morphology functions in sliceOmatic were used to segment an MRI slice into a number of sub-regions by computing the watershed line of the image gradient (Fig. 3a). Then the operators manually filled those separated regions with appropriate tags for visceral fat (green) and subcutaneous fat (red). A semi-automatically segmented result using sliceOmatic is shown in Fig. 3b and compared to its corresponding result using the fully-automated algorithm in Fig. 3c.
Figure 3.
(a) Manual tagging processing in sliceOmatic, (b) Segmentation result after semi-automated processing and (c) Segmentation results after fully-automated processing.
In the first stage of the study, only the single umbilical slices of the 85 subjects were analyzed with sliceOmatic because of its subjective and time-consuming nature. Three trained operators analyzed the 60 male subjects and two trained operators did the 25 female subjects. The SAT, VAT and TAT in this single slice evaluation from all the operators were averaged to reduce the subjectivity. In addition, the umbilical slice was used for the single slice evaluation because it is easy to be located and compared among the subjects with different body shapes and heights. In the second stage, 20 subjects (10 male and 10 female) were randomly selected for their MRI sequence analysis with the reference method. For each sequence, the twenty slices across a subject’s abdomen area were processed by one trained operator. The volumetric adiposity data were obtained by summing the VAT and SAT measured from each slice. This volumetric evaluation was only performed on the 20 subjects due to the heavy workload for an operator to segment the slices when using sliceOmatic.
Statistical analysis
The SAT, VAT and TAT volumes measured by the automatic and the reference methods were statistically analyzed by using Pearson correlations and Bland-Altman plots to evaluate the accuracy of the automated algorithm. The umbilical slices from the 60 male and 25 female subjects were utilized for the single slice evaluation, while 20 randomly selected subjects were used for the abdominal MRI sequence evaluation. The inter-observer reliability of the semiautomated (reference) method was assessed using the intra-class correlation (ICC, single score, two-way random) of the three independent operators’ segmented adiposity volumes from umbilical slices of male subjects (McGraw and Wong, 1996). For comparison, the reliability of the automated algorithm also was tested by processing the same slice twice.
The VAT and SAT volumes assessed from one of multiple slices at different anatomical locations of a subject, i.e. umbilicus, lumbar 2 (L2) vertebra, L3, L4 and L5, were compared to the total abdominal adiposity of the slices covered from L2 to L5 to explore the relevancy of each slice. The fat distribution across the abdomen was also analyzed by examining the fat depots assessed at different anatomical locations. All these analyses were performed using Matlab (MathWorks Inc, Natic, MA) and SPSS Statistics (IBM SPSS, Chicago, IL). A P-value less than 0.05 was adopted for the significance level.
Results
For the single slice (umbilical) evaluation, an operator needed approximately 5 minutes to analyze one umbilical MRI slice when using sliceOmatic (reference), and 20 to 103 seconds when using the automated algorithm (automatic) depending on the trans-axial size of a subject. For the MRI sequence evaluation in the second stage, the operator needed about 1.5 hour to analyze the 20 MRI slices in one sequence with the reference method, but only 10 to 20 minutes to process a sequence with the automatic method. Thus, the fully-automated algorithm has tremendously increased the efficiency of extracting adiposity data from an MRI slice, which makes the MRI sequence evaluation of a large subject set more attainable.
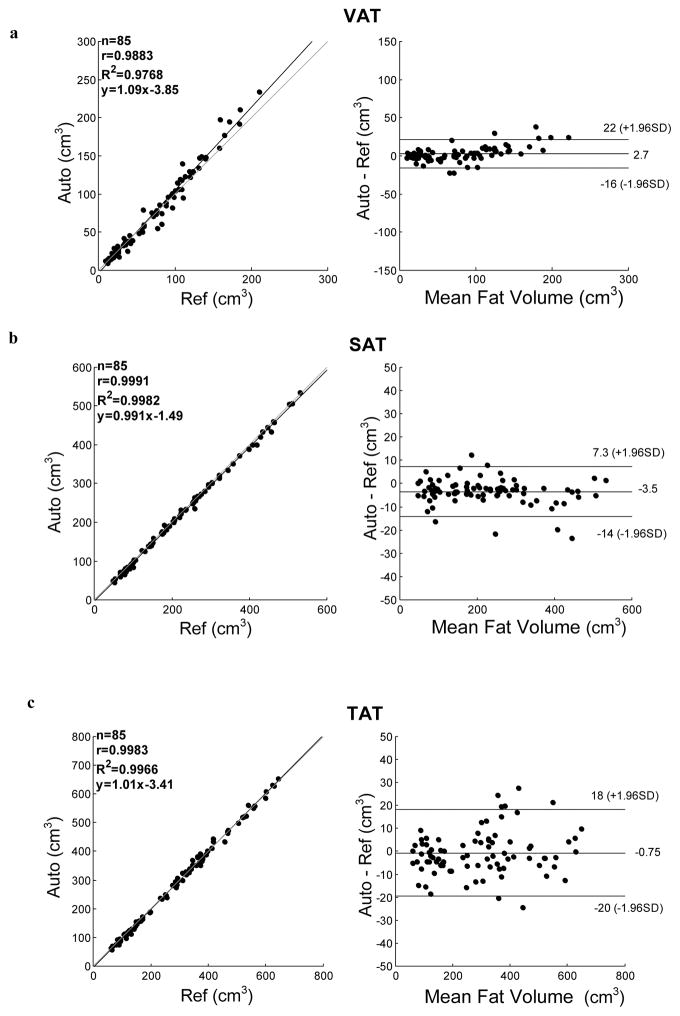
Fig. 4 plots the VAT, SAT and TAT measurements from the umbilical slices made by the reference method (the x-axis) and the automatic method (the y-axis). The square of the Pearson correlation coefficient (R2) between the two sets of measurements is displayed also on the left side of each plot in the figure. The strongest correlations occurred in SAT (R2=0.998, slope=1.01) and TAT (R2=0.997, slope=0.988) measurements between the two methods (Fig. 4b and Fig. 4c). A strong correlation (R2=0.977, slope=1.09) also existed in VAT measurements (Fig. 4a). The Bland-Altman plots (Bland and Altman, 1986) of the three volume measurements from the automatic method in comparison with the reference method also shown on the right side of the Fig. 4, in which the x-axis is the average of the fat volumes assessed from the reference method and the automatic method. The y-axis is the measurement difference between the two methods. Measurement differences between the two methods primarily lied within the 95% limits and no substantial difference was found between the two methods.
Figure 4.
Linear correlation (left) and Bland-Altman (right) plots for the slice-wise evaluation based on 85 umbilical slices from 85 subjects. Differences between automated (Auto) and semiautomated (Ref) assessment of (a) VAT (b) SAT and (c) TAT are shown. Upper- and lower- limits represent 95% of differences. Mean difference is indicated as the solid bar in the middle of Bland-Altman plots.
In order to assess the accuracy of the single slice evaluation made by the automatic method, mean difference between the two methods, Auto-Ref, and their relative difference, (Auto-Ref)/Ref, were calculated and shown in Table 2. The results show that the VAT measured by the automatic method is slightly higher than those measured by the reference method, while the SAT exhibited an opposite trend.
Table 2.
Accuracy and inter-observer reliability
| Single-slicea | Sequenceb | ICCa | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| (Auto-Ref)c (cm3) | (Auto-Ref)/Ref (%) | Auto-Ref (cm3) | (Auto-Ref)/Ref (%) | Ref | Auto | |
| VAT | 2.72±9.63 | 2.57±14.20 | −38.10±127.04 | −1.35±9.14 | 0.9393* | 0.9999* |
| SAT | −3.47±5.38 | −1.94±3.53 | −130.50±50.42 | −4.47±3.50 | 0.9989* | 0.9999* |
| TAT | −0.75±9.64 | −0.75±4.3 | −92.20±135.74 | −3.46±4.49 | 0.9900* | 0.9999* |
Measurements based on the single umbilical slices from subjects (n=85).
Measurements based on the MRI sequences (20 slices/sequence) from randomly selected subjects (n=20).
Accuracy data are given as mean ± standard deviation in cm3 and percentage (%).
VAT: visceral adipose tissue; SAT: subcutaneous adipose tissue; TAT: total abdominal adipose tissue; Auto: the automatic method; Ref: the reference method; ICC: intra-class correlation;
P<0.001.
Reliability for the semi-automated segmentation and the fully-automated algorithm were analyzed on the dataset of male subjects with an umbilical slice (Table 2). The ICC for the manual segmentation from the three independent observers using sliceOmatic were 0.9989, 0.9393 and 0.9900 for SAT, VAT and TAT, respectively. Since no user intervention is needed for automated processing, there will be no difference in results if more than one user were involved. In this study, one operator used the fully-automated algorithm to process the same slices twice. The ICC for SAT, VAT and TAT was all above 0.999. Thus, these results indicate that the reliability of the fully-automated algorithm for SAT, VAT and TAT assessment is higher than that of the semi-automated segmentation.
For the MRI sequence evaluation based on 20 subjects (20 slices/subject), accuracy also was analyzed by the mean difference and the relative difference (Table 2). For volumetric VAT, the mean difference is 38.1±127.04 (cm3) and the relative difference is 1.35±9.14 (%). For volumetric SAT, the mean difference is −130.5±50.42 (cm3), and the relative difference is −4.47±3.50 (%) which are higher than that of VAT, for volumetric TAT, the mean difference is −92.20±135.74 (cm3) and the relative difference is −3.46±4.49 (%). The R2 between the two sets of measurements of VAT, SAT, and TAT are 0.995, 0.999 and 0.999 respectively.
Table 3 presents the fat volumes determined by the two methods for both genders. As previously indicated (Katsiki et al., 2011), this study also revealed noticeable differences in fat distributions between men and women. Based on the measurements from umbilical slices, female subjects tended to accumulate more subcutaneous fat, but less visceral fat, than male subjects.
Table 3.
Adipose tissue volumes from umbilical slices of male (n=60) and female (n=25) subjects
| Sex | VAT | SAT | TAT | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Ref | Auto | Ref | Auto | Ref | Auto | |
| Male | 74.77±53.46 | 77.0±58.89 | 196.71±122.33 | 192.64±122.29 | 271.48±158.71 | 272.52±161.62 |
| Female | 68.05±43.02 | 69.36±47.90 | 280.03±118.53 | 273.86±116.07 | 348.08±156.26 | 343.23±157.01 |
VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue; TAT, total abdominal adipose tissue; Auto: volumes measured by the automatic method; Ref: volumes measured by the reference method; Data are given as mean ± standard deviation in cm3.
We also explored the associations between the VAT, SAT and TAT measured from the single umbilical slice by the automatic method and the traditional anthropometric measurements, such as BMI, waist circumference (WC) and waist-hip-ratio (WHR) (see Table 4). Among the three anthropometric parameters, WHR and BMI possessed, respectively, the highest and the lowest correlations with VAT (r, Pearson correlation coefficient). WC provided the highest correlation with SAT and TAT. The WHR was the only measurement that was significantly correlated with the ratio of VAT/SAT, which represents the relative amount of the two fat types.
Table 4.
Relationship between traditional anthropometric measurements and fat volumes measured from umbilical slices, using the automatic method (n=60)
| Fat Volumes
|
||||
|---|---|---|---|---|
| VAT | SAT | TAT | VAT/SAT | |
| BMI | r=0.59(P<0.001) | r=0.79(P<0.001) | r=0.81(P<0.001) | r=0.16(P>0.05) |
| WC | r=0.72(P<0.001) | r=0.94(P<0.001) | r=0.97(P<0.001) | r=0.25(P>0.05) |
| WHR | r=0.77(P<0.001) | r=0.77(P<0.001) | r=0.86(P<0.001) | r=0.38(P<0.005) |
r: Pearson correlation coefficient; VAT: visceral adipose tissue; SAT: subcutaneous adipose tissue; TAT: total abdominal adipose tissue; BMI: body mass index; WC: waist circumference; WHR: waist hip ratio.
In order to determine which slice carried the highest correlation with the total abdominal adiposity, the VAT, SAT and TAT measured from one of the MRI slices at different anatomical locations, i.e., umbilicus, L2 to L5 vertebra, were compared with the total VAT, SAT and TAT measured from all slices between L2 and L5 for the 60 male subjects (Table 5). The data showed that the VAT was more likely to be concentrated between L3 and L4 slices, while SAT existed primarily at the lower position of the abdomen, with the L5 slice containing the most SAT. The VAT and SAT measured from the L3 slice had the highest correlations with the total abdominal VAT (r=0.99) and SAT (r=0.99). Among all locations, the umbilical slice showed the lowest correlation (r=0.89) of the total abdominal VAT.
Table 5.
Mean fat volumes and associations between fat on single slice and total abdominal adiposity (n=60)
| Fat depots | Total | Umbilicus | L2 | L3 | L4 | L5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Mean volume (cm3) | Mean volume (cm3) | r | Mean volume (cm3) | r | Mean volume (cm3) | r | Mean volume (cm3) | r | Mean volume (cm3) | r | |
| VAT | 1.29×103 | 77.95 | 0.89 | 88.13 | 0.97 | 99.31 | 0.99 | 91.36 | 0.98 | 65.42 | 0.92 |
| SAT | 2.49×103 | 191.74 | 0.99 | 113.74 | 0.98 | 149.40 | 0.99 | 190.06 | 0.99 | 198.02 | 0.99 |
| TAT | 3.78×103 | 269.69 | 0.97 | 201.87 | 0.97 | 248.71 | 0.99 | 281.42 | 0.99 | 263.44 | 0.97 |
L: lumbar vertebra; Total: fat depots measured on slices from L2– L5;
r: Pearson correlation coefficients measured between fat volume assessed from a single slice and total fat volume from L2– L5.
All measurements of adipose tissues were assessed by the automatic method.
Discussion
A reliable method for fat quantification is important due to the high prevalence of obesity. We have demonstrated a fully-automated algorithm which is able to assess the abdominal adipose tissue on T1-weighted MRI slices, with a high degree of accuracy and reliability. The proposed algorithm was validated in a dataset of both male and female subjects, with a wide range of BMIs and fat depots.
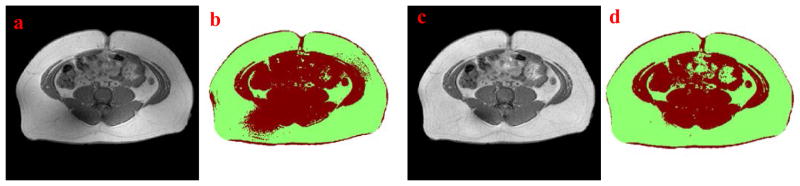
Major challenges that exist in the automation of T1-weighted MRI segmentation of the abdominal area include inhomogeneity correction in slices, fat/non-fat classifications, delineation of abdominal wall, and varying body size, shape and internal fat distribution. In our dataset, the inhomogeneity was more common in obese subjects and may have caused errors in the clustering and classification procedures. In the presented algorithm, a modified LEMS model was utilized to correct the shading effect to ensure the homogeneity in the same tissue type. Fig. 5a is an original MRI slice after the region growing procedure with the voxels outside of the abdominal area removed. Variations in signal intensity of the subcutaneous fat resulted in an inaccurate clustering computation in Fig. 5b. The LEMS model proposed by Salvado was used originally to correct spinal MRI (Salvado et al., 2006). When the LEMS approach was applied to the abdominal MRI with a large amount of fat issue, the dark areas containing muscle and bones were frequently over-corrected. This may impact the following clustering procedure. The modified LEMS model proposed in this study is able to prevent the over-correction while keeping homogeneity within the same type of tissue (Fig. 5c). This ensures accurate segmentation of fat from non-fat tissue (Fig. 5d).
Figure 5.
(a) Original MRI slice after region growing, (b) Clustered result based on (a), (c) Intensity corrected image, and (d) Clustered result based on (c)
Intermuscular fat and vertebral bone marrow fat were not separated from abdominal fat in this study since such fat have been proved to be either positively correlated with visceral fat volume or associated with increased risk of developing obesity-related health issues (Addison et al., 2014; Bredella et al., 2011).
Three trained operators in this study enhanced the accuracy of the semi-automated measurements. The lower operators agreement was similar to what has been reported elsewhere (Thörmer et al., 2013). In contrast, the reliability of the fully-automated algorithm was excellent because human interventions in MRI processing were eliminated.
The accuracy of the fully-automated algorithm was evaluated with both single-slice and MRI sequence data. First, the single umbilical slice was analyzed to assess the accuracy of the algorithm for the 85 subjects that had a wide range of BMI and body shapes. Then, the MRI sequences were analyzed to validate the algorithm with multiple MRI slices that covered the entire abdominal area with diverse organ and fat contents.
Overall, the fat amount assessed using the fully-automated algorithm is in a high agreement with that obtained from sliceOmatic. The VAT volumes measured automatically were slightly higher than the reference values, possibly because it is hard to identify tiny and spread adipose tissues, such as intramuscular fat and bone marrow, in the sliceOmatic. On the other hand, it is easier to select bulky SAT area as a whole, even if there are tiny non-fat tissues present in the sliceOmatic. This is attributed to the higher SAT measurements of the sliceOmatic. A systematic trend was observed nevertheless in the Bland-Altman plot of the VAT measurement. Data points were tightly clustered on the left side of the plot and spread out on the right side. This trend indicates that the deviation between the two methods might be increased in subjects with larger VAT volumes. A similar but less obvious trend was observed in the Bland-Altman plot of TAT measurement. The difference between the two TAT measurements was elevated in the middle of the plot. In the future, more subjects should be tested in order to reduce the sampling bias that might have caused these systematic trends. It is noted also that the equality lines in the three Bland-Altman plots were all within the 95% confidence intervals. This implies that the systematic differences between the automatic method and the reference method were not significant (Giavarina, 2015).
Compared to the results in the previous studies, our fat assessment results showed either equal or higher accuracy. For instance, Thörmer et al (2013) evaluated a fully-automated method in 20 morbidly obese subjects, and reported the Pearson correlation coefficients were 0.966, 0.960 and 0.978 for SAT, VAT and TAT, respectively. Kullberg et al (2009) presented an automated approach which underestimated the VAT by 4.7%. Bryan et al reported a slice-wise analysis similar to our automated algorithm, with R2 being 0.997 for VAT and 0.987 for SAT (Addeman et al., 2015). Finally, Positano et al (2004) explored an automated approach in which the Pearson correlation coefficients were 0.992 and 0.960 for SAT and VAT, respectively. Yet, we could not make direct comparisons with the above studies due to differences in imaging protocols, segmentation approaches and numbers of slices.
The present research study also found that the VAT and SAT depots were not equally associated with the traditional adiposity predictors such as BMI, WC and WHR. Table 6 exemplifies this finding with two subjects who had similar WCs. The left image is from an individual who had higher BMI and was classified as overweight (BMI>25). The image on the right is from a subject with a lower and normal BMI, but a higher WHR. The right subject’s VAT is twice that of the left subject, while the SAT is slightly smaller. Thus, BMI and WC do not indicate the true abdominal VAT volume as compared to WHR, and are less relevant to internal fat conditions.
Table 6.
Anthropometric and adipose measurements comparison between two male subjects
| Subject 1 | Subject 2 | |
|---|---|---|
| BMI (kg/m2) | 28.67 | 23.49 |
| WC (cm) | 99 | 100 |
| WHR | 0.93 | 1.00 |
| VAT (cm3)* | 32.90 | 128.44 |
| SAT (cm3)* | 201.59 | 171.20 |
| Umbilical slice |
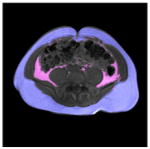
|
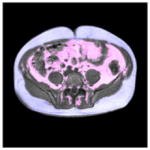
|
Measurements based on single umbilical slice by using the automatic method; BMI: body mass index; WC: waist circumference; WHR: waist hip ratio; VAT: visceral adipose tissue; SAT: subcutaneous adipose tissue; TAT: total abdominal adipose tissue.
In the single slice evaluation on slices at different anatomical locations, we found the umbilical slice is not the most representative of the total abdominal adiposity, which is consistent with the previous studies presented by Shen et al (2004, 2012). As opposed to a single slice, the MRI sequence provides a more accurate assessment of the abdominal adiposity because it contains variations in fat distribution across slices (Lee et al., 2004; Demerath et al., 2007).
Due to the limitation of the T1-weighted MRI, the partial volume effect was not considered and this may have caused an inaccurate estimation of fat tissue. To deal with this partial volume effect, advanced imaging modalities such as water-saturated images (Zhou et al., 2011), Dixon MRI sequences (Ma, 2008) and IDEAL (Iterative Decomposition with Echo Asymmetry and Least squares estimation) technique (Reeder et al., 2007) could be useful. Image processing algorithms based on these advanced MRI techniques have been also proposed for abdominal adiposity assessment. For example, Borga et al (2015) reported an automated muscle-fat quantification software with the utilization of the IDEAL technique. Schaudinn et al (2015) explored the use of a semi-automated program for VAT segmentation on Dixon MRI sequences. Despite the advantages of the Dixon MRI, T1-weighted MRI still remains popular because of its simpler operations and less scanning time, which is critical for certain applications (Hu et al., 2015). The proposed algorithm has been validated only on the T1-weighted MRI slices acquired from one institution. An area for future investigation is to adapt the algorithm for different imaging protocols, such as T2-weighted images, chemical (frequency) selective images, Dixon sequences and even CT scans, as the condition allows.
In conclusion, this paper presented a fully-automated MR image processing algorithm that can be used to identify and measure VAT, SAT and TAT volumes from a single slice or a multiple-slice sequence. The new algorithm was validated with the MRI slices of 85 subjects who had a wide range of BMIs, body shapes, sizes and ages. The improved accuracy and reliability of the VAT, SAT and TAT data indicated that the fully-automated algorithm is more robust and effective than a manual or semi-automated method.
Acknowledgments
Grant Support: This research was supported by grant R21DK081206 from the National Institutes of Health.
The authors acknowledge Drs. Reese Pepper and Jane Lee for their contributions in the MRI data collection and Ms. Bingfei Gu for her assistance in manual image analysis in this study.
Footnotes
Author contributions:
JS developed and tested the algorithm, analyzed the data and drafted the manuscript. BX supervised the project, finalized the manuscript and served as a corresponding author. JF supervised the collection of MRI data, edited the manuscript and provided critical comments.
Conflict of interest statement:
The authors declare no conflict of interest.
Literature Cited
- Abate N, Burns D, Peshock RM, Garg A, Grundy SM. Estimation of adipose tissue mass by magnetic resonance imaging: validation against dissection in human cadavers. J Lipid Res. 1994;35:1490–1496. [PubMed] [Google Scholar]
- Addeman BT, Kutty S, Perkins TG, Soliman AS, Wiens CN, McCurdy CM, Beaton MD, Hegele RA, McKenzie CA. Validation of volumetric and single-slice MRI adipose analysis using a novel fully automated segmentation method. J Magn Reson Imaging. 2015;41:233–241. doi: 10.1002/jmri.24526. [DOI] [PubMed] [Google Scholar]
- Addison O, Marcus RL, LaStayo PC, Ryan AS. Intermuscular fat: a review of the consequences and causes. Int J Endocrinol. 2014;2014:e309570. doi: 10.1155/2014/309570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglioni S, Cantini G, Poli G, Francalanci M, Squecco R, Di Franco A, Borgogni E, Frontera S, Nesi G, Liotta F, Lucchese M, Perigli G, Francini F, Forti G, Serio M, Luconi M. Functional differences in visceral and subcutaneous fat pads originate from differences in the adipose stem cell. PLoS ONE. 2012;7:e36569. doi: 10.1371/journal.pone.0036569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- Bonekamp S, Ghosh P, Crawford S, Solga S, Horska A, Brancati F, Diehl A, Smith S, Clark J. Quantitative comparison and evaluation of software packages for assessment of abdominal adipose tissue distribution by magnetic resonance imaging. Int J Obes 2005. 2008;32:100–111. doi: 10.1038/sj.ijo.0803696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borga M, Thomas EL, Romu T, Rosander J, Fitzpatrick J, Dahlqvist Leinhard O, Bell JD. Validation of a fast method for quantification of intra-abdominal and subcutaneous adipose tissue for large-scale human studies. NMR Biomed. 2015;28:1747–1753. doi: 10.1002/nbm.3432. [DOI] [PubMed] [Google Scholar]
- Bredella MA, Torriani M, Ghomi RH, Thomas BJ, Brick DJ, Gerweck AV, Rosen CJ, Klibanski A, Miller KK. Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity. 2011;19:49–53. doi: 10.1038/oby.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan TF, Vese LA. Active contours without edges. IEEE Trans Image Process. 2001;10:266–277. doi: 10.1109/83.902291. [DOI] [PubMed] [Google Scholar]
- Demerath EW, Shen W, Lee M, Choh AC, Czerwinski SA, Siervogel RM, Towne B. Approximation of total visceral adipose tissue with a single magnetic resonance image. Am J Clin Nutr. 2007;85:362–368. doi: 10.1093/ajcn/85.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields DA, Goran MI, McCrory MA. Body-composition assessment via air-displacement plethysmography in adults and children: a review. Am J Clin Nutr. 2002;75:453–467. doi: 10.1093/ajcn/75.3.453. [DOI] [PubMed] [Google Scholar]
- Frederiksen L, Nielsen TL, Wraae K, Hagen C, Frystyk J, Flyvbjerg A, Brixen K, Andersen M. Subcutaneous Rather than Visceral Adipose Tissue Is Associated with Adiponectin Levels and Insulin Resistance in Young Men. J Clin Endocrinol Metab. 2009;94:4010–4015. doi: 10.1210/jc.2009-0980. [DOI] [PubMed] [Google Scholar]
- Giavarina D. Understanding Bland Altman analysis. Biochem Medica. 2015;25:141–151. doi: 10.11613/BM.2015.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronemeyer SA, Steen RG, Kauffman WM, Reddick WE, Glass JO. Fast adipose tissue (FAT) assessment by MRI. Magn Reson Imaging. 2000;18:815–818. doi: 10.1016/s0730-725x(00)00168-5. [DOI] [PubMed] [Google Scholar]
- Haarbo J, Gotfredsen A, Hassager C, Christiansen C. Validation of body composition by dual energy X-ray absorptiometry (DEXA) Clin Physiol. 1991;11:331–341. doi: 10.1111/j.1475-097x.1991.tb00662.x. [DOI] [PubMed] [Google Scholar]
- Hu HH, Chen J, Shen W. Segmentation and quantification of adipose tissue by magnetic resonance imaging. Magn Reson Mater Phys Biol Med. 2015:1–18. doi: 10.1007/s10334-015-0498-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- Jin Y, Imielinska CZ, Laine AF, Udupa J, Shen W, Heymsfield SB. Segmentation and evaluation of adipose tissue from whole body MRI scans. In: Ellis RE, Peters TM, editors. Medical Image Computing and Computer-Assisted Intervention - MICCAI 2003 Lecture Notes in Computer Science. Springer; Berlin Heidelberg: 2003. pp. 635–642. [Google Scholar]
- Katsiki N, Ntaios G, Vemmos K. Stroke, obesity and gender: A review of the literature. Maturitas. 2011;69:239–243. doi: 10.1016/j.maturitas.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Kaul S, Rothney MP, Peters DM, Wacker WK, Davis CE, Shapiro MD, Ergun DL. Dual-Energy X-Ray Absorptiometry for Quantification of Visceral Fat. Obes Silver Spring Md. 2012;20:1313–1318. doi: 10.1038/oby.2011.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S. The case of visceral fat: argument for the defense. J Clin Invest. 2004;113:1530–1532. doi: 10.1172/JCI22028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczmarski RJ. Bioelectrical impedance analysis measurements as part of a national nutrition survey. Am J Clin Nutr. 1996;64:453S–458S. doi: 10.1093/ajcn/64.3.453S. [DOI] [PubMed] [Google Scholar]
- Kullberg J, Ahlström H, Johansson L, Frimmel H. Automated and reproducible segmentation of visceral and subcutaneous adipose tissue from abdominal MRI. Int J Obes 2005. 2007;31:1806–1817. doi: 10.1038/sj.ijo.0803671. [DOI] [PubMed] [Google Scholar]
- Kullberg J, Johansson L, Ahlström H, Courivaud F, Koken P, Eggers H, Börnert P. Automated assessment of whole-body adipose tissue depots from continuously moving bed MRI: a feasibility study. J Magn Reson Imaging JMRI. 2009;30:185–193. doi: 10.1002/jmri.21820. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Ghiatas AA, Alyassin A, Kilcoyne RF, Bonora E, Defronzo RA. Measurement of abdominal fat with T1-weighted MR images. J Magn Reson Imaging. 1991;1:363–369. doi: 10.1002/jmri.1880010315. [DOI] [PubMed] [Google Scholar]
- Lee S, Janssen I, Ross R. Interindividual variation in abdominal subcutaneous and visceral adipose tissue: influence of measurement site. J Appl Physiol. 2004;97:948–954. doi: 10.1152/japplphysiol.01200.2003. [DOI] [PubMed] [Google Scholar]
- Machann J, Thamer C, Schnoedt B, Haap M, Haring H-U, Claussen CD, Stumvoll M, Fritsche A, Schick F. Standardized assessment of whole body adipose tissue topography by MRI. J Magn Reson Imaging. 2005;21:455–462. doi: 10.1002/jmri.20292. [DOI] [PubMed] [Google Scholar]
- Ma J. Dixon techniques for water and fat imaging. J Magn Reson Imaging JMRI. 2008;28:543–558. doi: 10.1002/jmri.21492. [DOI] [PubMed] [Google Scholar]
- Martin DR, Semelka RC. Health effects of ionising radiation from diagnostic CT. The Lancet. 2006;367:1712–1714. doi: 10.1016/S0140-6736(06)68748-5. [DOI] [PubMed] [Google Scholar]
- McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychol Methods. 1996;1:30–46. [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. PRevalence of childhood and adult obesity in the united states, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Positano V, Gastaldelli A, Sironi A maria, Santarelli MF, Lombardi M, Landini L. An accurate and robust method for unsupervised assessment of abdominal fat by MRI. J Magn Reson Imaging. 2004;20:684–689. doi: 10.1002/jmri.20167. [DOI] [PubMed] [Google Scholar]
- Preis SR, Massaro JM, Robins SJ, Hoffmann U, Vasan RS, Irlbeck T, Meigs JB, Sutherland P, D’Agostino RB, Sr, O’Donnell CJ, Fox CS. Abdominal subcutaneous and visceral adipose tissue and insulin resistance in the Framingham heart study. Obes Silver Spring Md. 2010;18:2191–2198. doi: 10.1038/oby.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeder SB, McKenzie CA, Pineda AR, Yu H, Shimakawa A, Brau AC, Hargreaves BA, Gold GE, Brittain JH. Water-fat separation with IDEAL gradient-echo imaging. J Magn Reson Imaging JMRI. 2007;25:644–652. doi: 10.1002/jmri.20831. [DOI] [PubMed] [Google Scholar]
- Sadananthan SA, Prakash B, Leow MK-S, Khoo CM, Chou H, Venkataraman K, Khoo EYH, Lee YS, Gluckman PD, Tai ES, Velan SS. Automated segmentation of visceral and subcutaneous (deep and superficial) adipose tissues in normal and overweight men. J Magn Reson Imaging. 2015;41:924–934. doi: 10.1002/jmri.24655. [DOI] [PubMed] [Google Scholar]
- Salvado O, Hillenbrand C, Zhang Shaoxiang, Wilson DL. Method to correct intensity inhomogeneity in MR images for atherosclerosis characterization. IEEE Trans Med Imaging. 2006;25:539–552. doi: 10.1109/TMI.2006.871418. [DOI] [PubMed] [Google Scholar]
- Schaudinn A, Linder N, Garnov N, Kerlikowsky F, Blüher M, Dietrich A, Schütz T, Karlas T, Kahn T, Busse H. Predictive accuracy of single- and multi-slice MRI for the estimation of total visceral adipose tissue in overweight to severely obese patients. NMR Biomed. 2015;28:583–590. doi: 10.1002/nbm.3286. [DOI] [PubMed] [Google Scholar]
- Shen W, Chen J, Gantz M, Velasquez G, Punyanitya M, Heymsfield SB. A Single MRI Slice Does Not Accurately Predict Visceral and Subcutaneous Adipose Tissue Changes During Weight Loss. Obes Silver Spring Md. 2012;20:2458–2463. doi: 10.1038/oby.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge M-P, Albu J, Heymsfield SB, Heshka S. Visceral adipose tissue: relations between single-slice areas and total volume. Am J Clin Nutr. 2004;80:271–278. doi: 10.1093/ajcn/80.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas EL, Saeed N, Hajnal JV, Brynes A, Goldstone AP, Frost G, Bell JD. Magnetic resonance imaging of total body fat. J Appl Physiol Bethesda Md 1985. 1998;85:1778–1785. doi: 10.1152/jappl.1998.85.5.1778. [DOI] [PubMed] [Google Scholar]
- Thörmer G, Bertram HH, Garnov N, Peter V, Schütz T, Shang E, Blüher M, Kahn T, Busse H. Software for automated MRI-based quantification of abdominal fat and preliminary evaluation in morbidly obese patients. J Magn Reson Imaging. 2013;37:1144–1150. doi: 10.1002/jmri.23890. [DOI] [PubMed] [Google Scholar]
- Tokunaga K, Matsuzawa Y, Ishikawa K, Tarui S. A novel technique for the determination of body fat by computed tomography. Int J Obes. 1983;7:437–445. [PubMed] [Google Scholar]
- Vovk U, Pernus F, Likar B. A Review of methods for correction of intensity inhomogeneity in MRI. IEEE Trans Med Imaging. 2007;26:405–421. doi: 10.1109/TMI.2006.891486. [DOI] [PubMed] [Google Scholar]
- Wang J, Heymsfield SB, Aulet M, Thornton JC, Pierson RN. Body fat from body density: underwater weighing vs. dual-photon absorptiometry. Am J Physiol - Endocrinol Metab. 1989;256:E829–E834. doi: 10.1152/ajpendo.1989.256.6.E829. [DOI] [PubMed] [Google Scholar]
- Yang D, Zheng J, Nofal A, Deasy J, El Naqa IM. Techniques and software tool for 3D multimodality medical image segmentation. J Radiat Oncol Inform. 2009;1:1–22. [Google Scholar]
- Zhou A, Murillo H, Peng Q. Novel segmentation method for abdominal fat quantification by MRI. J Magn Reson Imaging JMRI. 2011;34:852–860. doi: 10.1002/jmri.22673. [DOI] [PubMed] [Google Scholar]