Abstract
Deficits in prefrontal cortical (PFC) function have been consistently reported in individuals with cocaine use disorders (iCUD), and have separately been shown to improve with longer-term abstinence. However, it is less clear whether the PFC structural integrity possibly underlying these deficits is also modulated by sustained reduction in drug use in iCUD. Here, T1-weighted magnetic resonance imaging scans were acquired, and performance on a neuropsychological test battery was assessed, in 19 initially abstinent treatment-seeking iCUD, first at baseline and then after six months of significantly reduced or no drug use (follow-up). A comparison cohort of 12 healthy controls was also scanned twice with a similar inter-scan interval. The iCUD showed increased gray matter volume in the left inferior frontal gyrus and bilaterally in the ventromedial prefrontal cortex at follow-up compared to baseline; healthy controls, as expected, showed no changes over this same time period. The iCUD also showed improved decision making and cognitive flexibility, with the latter correlated significantly with the gray matter volume increases in the inferior frontal gyrus. Given its association with improved cognitive function, the longitudinal recovery in cortical gray matter volume, particularly in regions where structure and function are adversely affected by chronic drug use, reflects a quantifiable positive impact of significantly reduced drug use on cortical structural integrity.
Introduction
Drug addiction is a neuropsychiatric disorder characterized by a recurring desire to continue taking the drug despite harmful consequences, driven at least in part by disrupted function of the prefrontal cortex (PFC) (Goldstein and Volkow, 2002, 2011). These functional deficits are associated with structural abnormalities such as reductions in PFC gray matter volume (GMV), with effects shown across most substance use disorders in cross-sectional studies (e.g., Alia-Klein et al., 2011; Franklin et al., 2002; Tanabe et al., 2009). In these studies, regions within the PFC that have consistently been implicated include the inferior frontal gyrus (IFG), anterior cingulate cortex (ACC), the ventromedial PFC (vmPFC), the dorsolateral PFC (dlPFC), and the orbitofrontal cortex (OFC), which subserve different dimensions of executive functions, such as inhibitory control (Brass and Haggard, 2007; Kuhn et al., 2009), emotion-influenced decision-making (Hansel and von Kanel, 2008), and adaptive learning (Kringelbach, 2005). The extent of these decreases in PFC GMV has been shown to covary with lifetime drug use (Alia-Klein et al., 2011) and its severity (Ersche et al., 2011). These findings possibly reflect the deleterious effects of chronic drug use on neuronal structure (Boess et al., 2000), which could compromise relevant cognitive functions (e.g., decision-making, regulatory top-down control and cognitive flexibility) culminating in the maintenance or exacerbation of drug-seeking and drug-taking behaviors. Alternatively, these findings could also point to predisposing factors that contribute to both reductions in PFC GMV and the propensity for drug use and addiction. The documentation of longitudinal changes in these measures, as a function of treatment or reduced drug use, could further be helpful in determining the direction of effect.
Longitudinal test-retest functional magnetic resonance imaging (fMRI) studies show normalization of functional response to inhibitory control tasks in the PFC [encompassing the IFG, ACC, and medial and lateral PFC regions] and in midbrain at follow-up compared to baseline in individuals with substance use disorder (e.g., Moeller et al., 2012). Similarly, longitudinal structural MRI studies suggest abstinence-mediated recovery of GMV in the ACC and insula in 3-month abstinent alcoholics (Demirakca et al., 2011), anterior PFC with even longer abstinence (91-336 days of sobriety) from alcohol use (Pfefferbaum et al., 1995), and of GMV in the IFG, insula, precuneus, angular and superior temporal gyri and occipital lobe after a month of methamphetamine abstinence (Morales et al., 2012). Similar abstinence-mediated PFC GMV increases have been reported in cross-sectional studies, showing that in cocaine users, longer abstinence from drugs have shown greater GMV in PFC and temporal cortical regions (Connolly et al., 2013; Hanlon et al., 2011). Thus, results from these studies suggest that both structural and functional recoveries may be possible with reduced drug use, although important gaps in the literature remain.
In particular, no longitudinal research to date has been undertaken to study the impact of significantly reduced drug use on GMV in iCUD. Moreover, prior studies have not directly addressed the question of whether the PFC structural recovery that accompanied abstinence was also associated with improved PFC-mediated executive function. Finally, prior studies have not typically recruited community samples engaged in treatment-as-usual, which represents the preponderance of abstinent addicted individuals at treatment-initiation, a considerable proportion of whom relapse within the first 12 months of treatment (Dennis et al., 2007). To fill these gaps, the current longitudinal within-subjects MRI study investigated GMV in initially abstinent treatment-seeking iCUD at baseline (after detoxification; ≥3 weeks after last drug use) and at 6-month follow-up. We hypothesized that treatment-seeking iCUD would show increased PFC GMV from baseline to 6-month follow-up in parallel with reduced or eliminated drug use over this same time period. We further hypothesized that longitudinal changes in PFC GMV in the iCUD would be associated with improved executive functioning on a neuropsychological test battery that specifically targets PFC functions, thereby providing a behavioral functional correlate to the GMV effects.
Materials and Methods
Participants
Participants were 19 right-handed treatment-seeking iCUD (13 males; 42.58 ± 7.63 years old) (Table 1). All iCUD were referred from three addiction treatment facilities located in the New York Tri-State area: Phoenix House (N=8), Samaritan Village (N=6), and the Yale Cocaine Research Clinic (N=5), and were free of sustained/maintenance medication for >30 days prior to and throughout the study. Further exclusionary criteria were history of head trauma and/or loss of consciousness (>30 min) or other neurological diseases of central origin (including seizures), current medical diseases that require hospitalization or regular monitoring, scores of two standard deviations below the norm on a verbal intelligence measure [measured via the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999)], and contraindications to MRI. All participants provided written consent to participate in the study in accordance with the Stony Brook University Institutional Review Board and associated treatment facility’s Institutional Review Board.
Table 1.
Demographic and clinical characteristics of the study sample
| iCUD (N = 19) Baseline |
iCUD (N = 19) 6-Month Follow-Up |
Controls (N = 12) Baseline |
|
|---|---|---|---|
| Demographics | |||
| Age (years) | 42.58 ± 7.63 | – | 39.33 ± 8.66 |
| Gender (male/female) | 13/6 | – | 11/1 |
| Race (African-American/Hispanic/Other) | 12/3/4 | – | 6/1/5 |
| Education (years) | 12.68 ± 2.75 | – | 12.71 ± 1.60 |
| Non-Verbal IQ (WASI-II MRS) | 9 ± 3.65 | – | 10.92 ± 3.34 |
| State Depression (BDI-II)a | 6.84 ± 7.69 | 5.68 ± 8.21 | 1.50 ± 2.47 |
| Drug Use | |||
| Cigarette Use (current/past/none)a | 13/3/3 | 3/1/8 | |
| Current Cigarette Use (number of cigarettes/day) | 3.58 ± 5.18 | 4.89 ± 6.01 | 2 ± 5.78 |
| Quantity of Alcohol Use in Last Year (oz./week) | 57.58 ± 77.45 | 47.63 ± 55.37 | 32.44 ± 26.11 |
| Lifetime Duration of Cocaine Use (years) | 12.74 ± 7.42 | – | – |
| Abstinence from Cocaine (days)b | 149.32 ± 189.46 | 271.95 ± 202.56 | – |
| Self-reported Craving (CCQ) | 8.95 ± 8.55 | 5.42 ± 6.15 | – |
iCUD vs. Controls (Baseline), p < 0.05.
iCUD baseline vs. iCUD follow-up, p < 0.05.
WASI-II MRS, Wechsler Abbreviated Scale of Intelligence-II Matrix Reasoning Scale; BDI-II, Beck Depression Inventory-II; CCQ, Cocaine Craving Questionnaire.
Values are frequencies or means ± SD.
Toxicology screens were performed on both study days, and all were negative for psychoactive drugs and their metabolites (cocaine, methamphetamine/amphetamines, phencyclidine, benzodiazepines, cannabis, opiates, and barbiturates), except for one participant (N=1) whose urine was positive for cocaine and who reported using cocaine three days prior to the follow-up scan day. We tracked abstinence between scanning sessions using a three-tiered tracking system, which we also used in our prior longitudinal research to track 6-month clinical outcomes in iCUD (Moeller et al., 2012). This tracking system consisted of: (i) Monthly phone calls to all participants and their designated collaterals (e.g., family member, counselor) – for those participants who remained in residential care for the duration of the 6-month study (N=6), the treatment center (Samaritan Village) was consulted directly; (ii) The Timeline Follow-back Calendar (CL-90) (Miller and Del Boca, 1994), a retrospective calendar that assesses drug use (including number of days and amount used) in the past 90 days; (iii) A retrospective questionnaire regarding the last date of cocaine use. Participants were informed about the importance of reliable self-reporting, and had no incentive for denial or biased reporting since neither inclusion into the study nor compensation were contingent on abstinence [nevertheless, there was one participant with a discrepancy between self-report (reporting abstinence) and a collateral report (reporting probable cocaine use); as a conservative measure, this participant was coded as having used cocaine]. This approach revealed that 11 iCUD remained abstinent (352.73 ± 189.73 days of abstinence at 6-month follow-up study session) while eight iCUD had between one and five lapses where cocaine was used between the two sessions (160.88 ± 172.50 days of abstinence at follow-up session). Nevertheless, combined, both groups (abstinent and lapsed iCUD) had longer duration of abstinence at the 6-month follow-up compared to baseline [t(18)=3.67, p=0.002], reflecting that iCUD were abstinent for a significantly longer duration at follow-up than they were at baseline and suggesting that all iCUD were successful at reducing, if not eliminating, their drug use.
To attribute any observed changes in GMV in the iCUD specifically to the significantly reduced or no drug-use and not to the elapsed time or other incidental factors between the baseline and follow-up scans, 12 healthy controls (HC; 11 male; 39.33 ± 8.66 years old) were also scanned twice at comparable inter-scan intervals (p>0.4) (Table 1). These HC participants did not differ from the iCUD on any demographic variables listed in Table 1 (ps>0.2), with the exception of history of cigarette smoking (p=0.03; only three HC were current smokers) and state depression (p=0.02). All HC underwent the same screening procedures as iCUD (e.g., interviews, toxicology screens, and questionnaires) on each scan day.
Psychiatric Interview Measures
The clinical diagnostic interview, conducted in all participants, consisted of: (i) Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (First et al., 1996); (ii) Addiction Severity Index (McLellan et al., 1992), a semi-structured interview instrument that assesses the severity as well as recent and lifetime history of alcohol and drug-related problems as they relate to seven problem areas (medical, employment, legal, alcohol, other drug use, family-social functioning, and psychological status); (iii) 18-item Cocaine Selective Severity Assessment Scale (Kampman et al., 1998), designed to evaluate cocaine abstinence/withdrawal signs and symptoms (i.e., sleep impairment, anxiety, energy levels, craving, and depressive symptoms) 24 hours prior to the interview; (iv) 3-item Severity of Dependence Scale (Gossop et al., 1992); and (v) 5-item Cocaine Craving Questionnaire (Tiffany et al., 1993). The Beck Depression Inventory (BDI-II) was used to measure severity of depressive symptoms (Beck et al., 1996).
This interview revealed that, at baseline, the iCUD had current cocaine dependence (N=14), or cocaine dependence in partial (N=4) or sustained (N=1) remission (mostly related to the length of time they had been abstinent prior to beginning the study). Current comorbidities in this sample (again related to the length of abstinence prior to beginning the study) included mild marijuana and ecstasy abuse (N=1), alcohol use disorder (N=3), opiate use disorder (N=1), unipolar depression (N=1), anti-social personality disorder (N=4), and post-traumatic stress disorder (N=1); all other comorbidities were in partial or sustained remission. At follow-up, all iCUD were in partial (N=14) or sustained (N=5) remission for cocaine use disorder, which further highlights the reduction in symptom severity associated with reduced or no drug use from baseline to follow-up.
Neuropsychological Test Battery
During each study session, 17 of 19 iCUD also completed a neuropsychological test battery designed to assess executive functions that are primarily mediated by distinct PFC subregions. The battery included the (i) Wisconsin Card Sorting Test (WCST) (Heaton, 1999), which tracks cognitive flexibility and has been previously linked with lateral (both dorsal and ventral) PFC function (see Nyhus and Barcelo, 2009, for review); (ii) modified color-word Stroop task (Golden, 1978), which has classically been used to behaviorally assess cognitive control in correlation with activity in the IFG, ACC, insula, and premotor cortex (MacDonald et al., 2000); (iii) Attention Network Test (Fan et al., 2002), which can be used to assess conflict monitoring mediated by the rostral ACC and the lateral PFC (Fan et al., 2005); and the (iv) Iowa Gambling Task (IGT) (Bechara et al., 1994), which is used to assess decision-making and is associated with activity in the medial OFC, vmPFC and ACC (Bechara et al., 1994). Together, these tests were used in the current study to profile the behavioral representation of possible changes in PFC function.
Dependent variables included, respectively: (i) WCST percent correct responses (representing the percentage of correct cards over the number of cards played) and percent perseverative errors (the concentration of perseverative errors in relation to overall test performance); (ii) the age-corrected Stroop interference score, calculated by subtracting a predicted color (C) word (W) score (predicted CW=C×W/C+W) from the raw CW score (interference = raw CW - predicted CW); (iii) reaction time from the ANT Conflict subscale, computed by subtracting the mean reaction time of all congruent conditions from the mean reaction time of all incongruent conditions; and (iv) IGT net score, which is computed by subtracting disadvantageous card deck choices from the total advantageous choices, as done previously in our group (Woicik et al., 2009).
MRI Image Acquisition
During each study day, all participants (CUD and HC) completed a T1-weighted MRI scan. MRI scanning was performed on a 4T whole-body Varian/Siemens MRI scanner, equipped with self-shielded whole-body Siemens Sonata EPI hardware (maximum 44mT/m gradient strength per channel, 176 mT/m/ms slew rate) and a standard quadrature head resonator. Earplugs and headphones minimized the interference effect of scanner noise. Anatomical MRI started with the acquisition of a sagittal T1-weighted gradient-echo localizer (TE/TR 10/100 ms, 5 mm slice thickness, 20 cm FOV, matrix size = 256 × 192, 128 phase encoding steps, 13 sec scan time). This was followed by an axial T1-weighted 3D-MDEFT (three-dimensional-modified driven equilibrium Fourier transform) sequence (TE/TR 7/15 ms, 0.94 × 0.94 × 1 mm3 spatial resolution, 256 readout and 192 × 96 phase-encoding steps, partial k-space acquisition, 16 minute scan time) (Deichmann et al., 2004), and a modified T2-weighted hyper-echo sequence (Hennig and Scheffler, 2001). Images were reconstructed in IDL (Interactive Data Language, Research Systems, Boulder, CO) using a Hamming filter, a phase correction method that produces minimal ghost artifacts, as well as an iterative phase correction to partially recover signal loss due to susceptibility effects. Anatomical images (both T1- and T2-weighted) were reviewed by a neurologist to rule out gross morphological abnormalities.
MRI Data Processing
Data from the T1-weighted images were preprocessed and analyzed using statistical parametric mapping (SPM8, Wellcome Department of Cognitive Neurology, London UK; www.fil.ion.ucl.ac.uk/spm) (Friston et al., 1995) and the longitudinal processing pipeline as offered in the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm/) running on MATLAB v7.13 (Mathworks Inc., Natick, MA).
Specifically, as part of the VBM8 toolbox longitudinal pipeline, all images were realigned to anterior-posterior commissure line. Next, follow-up scan was registered to the baseline scan separately for each participant. After correcting intrasubject biases, scans were segmented for gray matter, white matter and cerebrospinal fluid tissue types followed by linear (i.e., affine) and nonlinear (i.e., DARTEL) registration. Next, the tissue segments were modulated by nonlinear normalization parameters to account for individual brain size differences. The segmentation procedure was further refined by accounting for partial volume effects (Tohka et al., 2004), by applying adaptive maximum a posteriori estimations (Rajapakse et al., 1997), and by applying a hidden Markov random-field model (Cuadra et al., 2005). Finally, the realigned and normalized gray matter segments were smoothed with a 10 mm3 full-width-half-maximum (FWHM) Gaussian kernel. These smoothed GMV maps (at baseline and follow-up) for each participant were then fed into a ?exible factorial design with three factors (subjects, group and time) to assess Group and Time main effects and their interaction.
Statistical Analysis
The whole-brain statistical analysis on GMV maps was conducted with a 2 (Group: iCUD versus HC) × 2 (Time: baseline versus follow-up) flexible factorial design, which yielded Group and Time main effects and a Group-by-Time interaction. Significant interaction effects were investigated further with whole-brain t-tests with age and gender entered as nuisance regressors. Since the gray matter maps were modulated during MRI data processing, the statistical correction for total intercranial volume became redundant and therefore, was not used as a covariate. The statistical significance threshold was set at p<.05 [corrected for multiple comparisons using the False Discovery Rate (FDR)] with a minimum cluster size of 50 contiguous voxels for all analyses. Labels for the resulting coordinates were inspected with the Anatomy toolbox for SPM (Anatomy toolbox; Institute of Neuroscience and Medicine, Jülich, Germany). The GMV of the entire significant cluster around the peak voxel was extracted using EasyROI (http://www.sbirc.ed.ac.uk/cyril/cp_download.html). This approach resulted in GMV values for each participant in each of these regions, allowing the measures to be used for subsequent correlation analyses in SPSS (IBM SPSS, Version 21; IBM Corp., Armonk, NY, USA). Since eight iCUD lapsed during the study (reflecting the norm in a community sample of treatment-seekers), we also performed a secondary 2 (Scan: baseline and follow-up) × 2 (Subgroup: lapsed and non-lapsed) mixed ANOVA in SPSS to test whether abstinence modulated the extent of these GMV changes over time. Although alcohol use did not differ between baseline and follow-up (p=0.46, Table 1) in the iCUD, we examined the Spearman rank correlations to investigate whether alcohol use influenced longitudinal changes in GMV.
Performance scores of the neuropsychological tests were compared between baseline and follow-up only in iCUD by either using a paired t-test if the variables were normally distributed or a Wilcoxon Rank Sum Test if the variables were non-normally distributed. Of greater interest for our purposes, correlational analyses were used to investigate the association between changes in GMV and changes in neuropsychological performance over the course of the significantly reduced drug use period. To protect against Type I errors, Bonferroni-corrected significance level was required for all correlations between GMV and task behaviors, while p<0.05 is reported as a trend. In all correlation analyses, lapse status (yes/no) was controlled using partial correlations.
Results
MRI Results
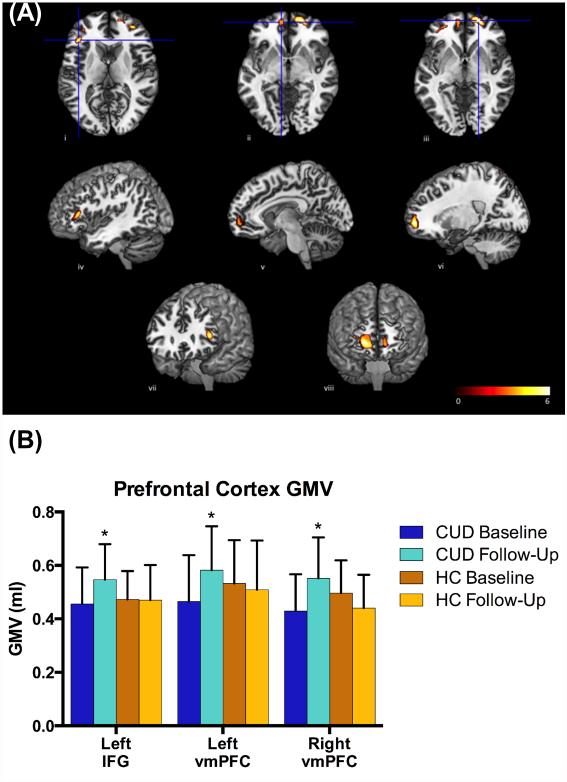
The whole-brain 2 × 2 flexible factorial design yielded a significant Group main effect, such that HC showed greater GMV in the left cuneus (pFDR<0.001) and the right precuneus (pFDR<0.001) compared to iCUD, and iCUD showed greater GMV in the right supplementary motor (pFDR<0.001), the left medial superior frontal (pFDR<0.001), and the right parietal/occipital cortices (pFDR=0.036). The Time main effect and the Group-by-Time interaction did not yield signficant clusters at the preset threshold (pFDR<0.05). However, at a lower threshold (puncorrected<0.005), the right middle occipital cortex, the right IFG, and the right dorsal ACC as well as bilateral vmPFC showed a trend for Group-by-Time interaction. Indeed, planned comparisons revealed that the longitudinal comparison in the iCUD (6-Month Follow-Up > Baseline) yielded increased GMV in the left IFG (pFDR=0.032) and in the bilateral vmPFC (pFDR=0.032; extending to the lateral OFC; Table 2; Figure 1). The parallel comparison (6-Month Follow-Up > Baseline) in the HC did not yield any region with significant changes in GMV (pFDR>0.1). There were no regions showing significant decreases in GMV at the 6-month follow-up compared to baseline.
Table 2.
Brain regions with statistically significant changes in regional gray matter volumes.
| Anatomical Label | Hemisphere | BA | X | Y | Z | voxels | Z-Score |
|---|---|---|---|---|---|---|---|
| Group Main Effect: HC > iCUD (pFDR < 0.05) | |||||||
| Cuneus | Left | 19 | −17 | −77 | 20 | 2672 | 5.24 |
| Precuneus | Right | 40 | 23 | −54 | 33 | 3902 | 4.82 |
| Group Main Effect: iCUD > HC (pFDR < 0.05) | |||||||
| Supplementary motor | Right | 6 | 36 | −11 | 68 | 771 | 5.66 |
| Medial Superior Frontal | Left | 8 | 2 | 36 | 45 | 908 | 5.46 |
| Parietal/Occipital | Right | 7 | 30 | −57 | 54 | 207 | 4.59 |
| Group-by-Time Interaction (puncorrected < 0.005) | |||||||
| Middle Occipital cortex | Right | 30 | −70 | 39 | 969 | 3.73 | |
| Inferior Frontal Gyrus | Right | 48 | 17 | 3 | 482 | 3.69 | |
| Dorsal ACC | Right | 3 | 30 | 43 | 387 | 3.44 | |
| Ventromedial PFC | Left | −35 | 54 | 4 | 51 | 2.92 | |
| Ventromedial PFC | Right | 23 | 60 | 4 | 313 | 2.90 | |
| Planned Comparisons | |||||||
| iCUD: Follow-Up > Baseline (pFDR < 0.05) | |||||||
| Inferior Frontal Gyrus | Left | 45 | −44 | 30 | 9 | 425 | 4.02 |
| Ventromedial PFC | Right | 11 | 20 | 59 | −3 | 693 | 4.26 |
| Ventromedial PFC | Left | 11 | −9 | 56 | −5 | 127 | 3.56 |
| HC: Follow-Up > Baseline (pFDR < 0.05) | |||||||
| None | |||||||
Note: x, y, and z coordinates given in MNI space (Montreal Neurological Institute)
Figure 1.
(A) Clusters in the left IFG (i, iv, and vii), left vmPFC (ii, v, and viii), and right vmPFC (iii, vi, viii) that showed significant increase from baseline to follow-up in iCUD. For display purposes, clusters are shown using a threshold of p<0.001 (uncorrected) and k=20, though all shown clusters are above the specified statistical threshold (pFDR<0.05). (B) The average gray matter volume (GMV) at baseline and follow-up in iCUD and HC, showing longitudinal increase only in iCUD and not in HC. Note that there were no significant between-group GMV differences at baseline or at follow-up. * p<0.05
Exploratory 2×2-mixed ANOVAs (separately for the left IFG and bilateral vmPFC) were carried out to study the effect of lapse in abstinence between baseline and follow-up scans. These analyses did not show significant Scan nor Subgroup main effects, nor a significant Scan-by-Subgroup interaction (p>0.05), suggesting that several lapses do not necessarily derail GMV recovery in iCUD.
Neuropsychological Test Battery Results
The iCUD showed increased task accuracy on the WCST from baseline to follow-up [t16=2.31, p=0.0343]. Additionally, the iCUD showed a significant increase in net IGT score from baseline to follow-up [t16=2.709, p=0.0152]. No other performance scores on any other task changed significantly (p>0.1) (Table 3; Figure 2).
Table 3.
Neuropsychological performance of iCUD at baseline and follow-up
| iCUD (N = 17) Baseline |
iCUD (N = 17) 6-Month Follow-Up |
|
|---|---|---|
| Wisconsin Card Sorting Test | ||
| Percentage of correct responsesa | 69.65 ± 15.31 | 77.47 ± 11.01 |
| Percentage of perseverative errors | 15.29 ± 8.83 | 10.71 ± 5.98 |
| Color-Word Stroop Task | ||
| Interference score | 2.39 ± 13.325 | 1.06 ± 7.57 |
| Attention Network Test | ||
| Conflict monitoring score | 140.57 ± 78.41 | 132.79 ± 48.43 |
| Iowa Gambling Task | ||
| Net score (Advantageous – Disadvantageous)a | −9.88 ± 10.59 | 18.35 ± 8.07 |
iCUD (Baseline) vs. iCUD (6-Month Follow-Up), p ± 0.05; Values are means ± SD.
Figure 2.
Performance on Iowa Gambling Task (IGT), and Wisconsin Card Sorting Task (WCST) showing longitudinal increase from baseline to follow-up in iCUD (also see Table 3). * p<0.05
Correlations
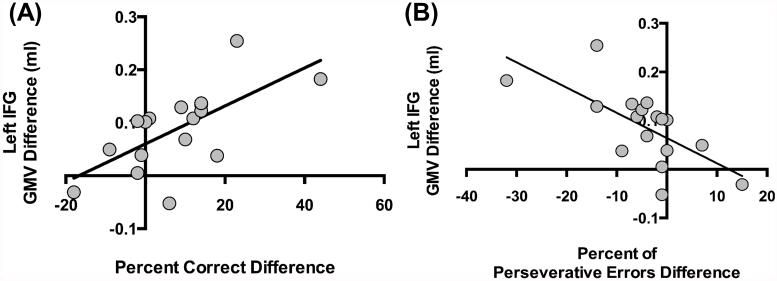
For the correlational analyses between the change in GMV (in clusters that reached significance in the planned baseline to follow-up analysis) and the respective change in neuropsychological task performance, the Bonferroni-corrected significance threshold of p<0.003 (3 GMV ROIs × 5 dependent variables from the neuropsychological test battery) was used. The results showed that the increase in IFG GMV was significantly correlated with the increase in WCST accuracy (rs=0.68, p=0.0029) and the decrease in WCST percent perseverative errors (rs=−0.71, p=0.0014) (Figure 3).
Figure 3.
In iCUD, increase in gray matter volume (GMV) of the left inferior frontal gyrus (IFG) from baseline to follow-up is correlated with (A) increased task accuracy (rs=0.66, p=0.004), and with (B) decreased percent of preservative errors (rs=−0.75, p=0.001) on the Wisconsin Card Sorting Test (WCST), such that the greater the increase in IFG GMV at follow-up, the greater the overall WCST accuracy and lower commission of perseverative error, respectively, in initially-abstinent iCUD who decreased their drug use over a 6-month follow-up.
Additionally, for the correlation between GMV and neuropsychological performance variables with drug use variables, the Bonferroni-corrected significance threshold of p<0.001 [(3 GMV ROIs + 5 dependent variables from the neuropsychological test battery) × 5 continuous drug use variables (Table 1)] was used. The results showed that at baseline only, GMV in the left IFG in iCUD was marginally correlated with lifetime duration of cocaine use (rs=−0.64, p=0.0289).
Lastly, Spearman rank correlations between the difference in alcohol use from baseline to follow-up and the respective change in GMV in clusters that reached significance and longitudinal changes in neuropsychological performance did not reveal significant correlations (p>0.122). Thus, the longitudinal GMV increases in the left IFG and bilateral vmPFC as well as the improvement in WCST and IGT performance were not associated with change in alcohol use in iCUD.
Discussion
The main purpose of this study was to evaluate longitudinal GMV changes in a community sample of treatment-seeking iCUD over a period of reduced or no drug use, and to ascertain if these changes were associated with changes in neuropsychological function, especially those that are dependent on the PFC. The current results show GMV increases as possibly indicating recovery in the left IFG and bilateral vmPFC from baseline to 6-month follow-up only in the iCUD, and not in the HC who were scanned during similar time intervals for control purposes. These longitudinal findings corroborate results from previous studies showing PFC GMV recovery after at least one month of abstinence in methamphetamine-dependent smokers (Morales et al., 2012), in cocaine users (Hanlon et al., 2011), and in treatment-seeking alcoholics using region-of-interest analyses (Demirakca et al., 2011). Our results advance research in this field by showing these longitudinal effects over a longer-term (~6 months) reduced or eliminated cocaine use in treatment-seeking iCUD using statistically robust whole-brain analyses. Furthermore, the GMV recovery found in the iCUD at 6-month follow-up in our study was observed irrespective of lapse status, providing hope for a favorable rehabilitation prognosis even for the iCUD who do not maintain complete abstinence.
The current results further advance the field by showing concomitant improvements in cognitive flexibility (i.e., increased accuracy on the WCST) and in advantageous decision making (i.e., increased net IGT score), which are neuropsychological functions mediated by lateral and medial PFC subregions, respectively. Executive dysfunction (assessed via the Frontal Systems Behavior Scale) (Gjini et al., 2014) and reduced performance on the IGT (Balconi et al., 2014) have been previously reported in iCUD (as compared to HC). Some of these cognitive impairments are ameliorated by significant decreases (>70%) in cocaine use or even recover fully after complete cessation of cocaine use (Vonmoos et al., 2014). Our study shows that such longitudinal improvement in cognitive flexibility (as measured by accuracy and percent perseverative errors on the WCST) was significantly associated with the respective increase in IFG GMV in the iCUD. This association corroborates a previous cross-sectional study in methamphetamine abuse, where short-term (<6 months) compared to long-term (≥6 months) abstinent methamphetamine abusers showed greater WCST performance impairment (as measured by total errors) as well as significantly lower right middle frontal cortical GMV (Kim et al., 2006). Thus, the current results of recovery in the PFC structural integrity and improved performance on PFC-mediated neurocognitive functions are consequential as they suggest that both structural and functional recovery of the PFC are putatively mediated by a significant reduction in drug use in initially abstinent treatment-seeking iCUD. Indeed the marginal correlation between the left IFG GMV with lifetime duration of cocaine use supports this conclusion.
The neurobiological underpinnings of the current PFC structural recovery remain to be studied further. One possibility, however, is that such recovery could be attributed to cellular changes in glutamate, γ-Aminobutyric acid (GABA), and peptide transmission in the PFC (Kalivas, 2007), which have been deemed essential for the process of recovery and prolonged abstinence in drug addiction (Garavan and Weierstall, 2012). Specifically, preclinical studies show neurochemical changes in the PFC and hippocampus with drug-administration and its recovery with abstinence and posit that these changes result from a shift in the steady-state equilibrium of the glutamate, glutamine and GABA metabolic cycle in this region (Deschaux et al., 2014; Gao et al., 2007). Similarly, a recent study using MR spectroscopy of the ACC has shown that while glutamate, N-acetylaspartate (a measure of neuronal viability), and choline-containing metabolites (a measure of glial or cell synthesis/turnover) are reduced in alcohol-dependent individuals compared to light/non-drinking controls at baseline, the concentration of these neurochemicals increases from one to five weeks of abstinence (Mon et al., 2012). While intriguing, the exact mechanisms underlying PFC GMV recovery with abstinence remain to be determined in human cocaine addiction.
Limitations of our study include a small sample size, especially of the HC, which may explain the absence of group differences, or group by time interaction, in the left IFG and bilateral vmPFC. Although our primary effects indicating GMV improvement in iCUD were detected with a within-subjects longitudinal design, which is more robust against the effects of limited sample sizes than a cross-sectional between-subjects design, future studies with larger sample sizes are warranted to replicate these findings. Future studies should also further investigate the effect of relapse status at multiple time-points in the longitudinal time-frame, such that non-linear trajectories of GMV recovery could also be studied. Repeated neuropsychological testing conducted with the same battery which may enhance practice effects also presents a limitation. Future studies could employ alternative versions of these tasks at baseline and follow-up. However, of note is that practice effects on these tests are not expected to last for six months and that improved performance was observed only in two of the four selected tests, suggesting that practice effects may not have unduly influenced these results. Lastly, some iCUD (N=6, combined) had comorbid psychopathologies other than substance use disorders at baseline, which might have influenced the current results, and is needed to be fully characterized in future studies. However, the incidence of these comorbidities and depressive symptoms (assessed via BDI) did not change from baseline to follow-up.
Taken together, the results of the current study showed recovery of GMV in the left IFG and associated cognitive flexibility, as well as recovery of GMV in the bilateral vmPFC, and associated decision-making, after six months of significant reduction in drug use in treatment-seeking iCUD. The IFG and vmPFC play a key role in executive functions that have been consistently shown to be impaired in iCUD, and in turn may contribute to maintenance or even exacerbation of drug use. This study therefore provides a basis for investigating long-term treatment effects on GMV in iCUD, and their relationship with clinical symptoms, which may inform substance abuse prognosis and future treatment efforts. Overall, the current results suggest that some of the negative morphological (and potentially associated functional) PFC effects often observed with chronic drug use can be rescued with significantly reducing or quitting drug use.
Acknowledgments
This study was supported by grants from the National Institute on Drug Abuse (to MAP: F32DA033088; to SJM: 1K01DA037452; to RZG: R01DA023579) and from the National Institute on Mental Health (to NAK: R01MH090134). We gratefully acknowledge the contributions from Patricia Woicik, Dardo Tomasi, Ruiliang Wang and Pias Malaker for their help with data acquisition and analysis. We also acknowledge the support from Rajita Sinha (Yale School of Medicine), Deni Carise (Phoenix House Foundation, Inc.) and Janetta Astone-Twerell (Samaritan Village, Inc.) for their support in participant referral and recruitment.
Footnotes
Authors Contribution
MAP, SJM, NAK, and RZG were responsible for study concept and design. MAP, FDU, AP, and SJM performed data analysis and interpretations of findings. MAP and TM contributed to the acquisition of imaging data. MAP, FDU, SJM, and RZG drafted the manuscript, and all authors contributed to the final version.
References
- Alia-Klein N, Parvaz MA, Woicik PA, Konova AB, Maloney T, Shumay E, Wang R, Telang F, Biegon A, Wang GJ, Fowler JS, Tomasi D, Volkow ND, Goldstein RZ. Gene x disease interaction on orbitofrontal gray matter in cocaine addiction. Archives of general psychiatry. 2011;68:283–294. doi: 10.1001/archgenpsychiatry.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balconi M, Finocchiaro R, Campanella S. Reward sensitivity, decisional bias, and metacognitive deficits in cocaine drug addiction. Journal of addiction medicine. 2014;8:399–406. doi: 10.1097/ADM.0000000000000065. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory Manual. 2nd San Antonio; The Psychological Corporation: 1996. [Google Scholar]
- Boess F, Ndikum-Moffor FM, Boelsterli UA, Roberts SM. Effects of cocaine and its oxidative metabolites on mitochondrial respiration and generation of reactive oxygen species. Biochemical pharmacology. 2000;60:615–623. doi: 10.1016/s0006-2952(00)00355-5. [DOI] [PubMed] [Google Scholar]
- Brass M, Haggard P. To do or not to do: the neural signature of self-control. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:9141–9145. doi: 10.1523/JNEUROSCI.0924-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly CG, Bell RP, Foxe JJ, Garavan H. Dissociated grey matter changes with prolonged addiction and extended abstinence in cocaine users. PloS one. 2013;8:e59645. doi: 10.1371/journal.pone.0059645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadra MB, Cammoun L, Butz T, Cuisenaire O, Thiran JP. Comparison and validation of tissue modelization and statistical classification methods in T1-weighted MR brain images. IEEE transactions on medical imaging. 2005;24:1548–1565. doi: 10.1109/TMI.2005.857652. [DOI] [PubMed] [Google Scholar]
- Deichmann R, Schwarzbauer C, Turner R. Optimisation of the 3D MDEFT sequence for anatomical brain imaging: technical implications at 1.5 and 3 T. NeuroImage. 2004;21:757–767. doi: 10.1016/j.neuroimage.2003.09.062. [DOI] [PubMed] [Google Scholar]
- Demirakca T, Ende G, Kammerer N, Welzel-Marquez H, Hermann D, Heinz A, Mann K. Effects of alcoholism and continued abstinence on brain volumes in both genders. Alcoholism, clinical and experimental research. 2011;35:1678–1685. doi: 10.1111/j.1530-0277.2011.01514.x. [DOI] [PubMed] [Google Scholar]
- Dennis ML, Foss MA, Scott CK. An eight-year perspective on the relationship between the duration of abstinence and other aspects of recovery. Evaluation review. 2007;31:585–612. doi: 10.1177/0193841X07307771. [DOI] [PubMed] [Google Scholar]
- Deschaux O, Vendruscolo LF, Schlosburg JE, Diaz-Aguilar L, Yuan CJ, Sobieraj JC, George O, Koob GF, Mandyam CD. Hippocampal neurogenesis protects against cocaine-primed relapse. Addiction biology. 2014;19:562–574. doi: 10.1111/adb.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Barnes A, Jones PS, Morein-Zamir S, Robbins TW, Bullmore ET. Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain : a journal of neurology. 2011;134:2013–2024. doi: 10.1093/brain/awr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. NeuroImage. 2005;26:471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. J Cogn Neurosci. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL. User's Guide for the Structured Clinical Interview for Axis I DSM-IV Disorders - Research Version. Biometrics Research; New York: 1996. al. e. [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O'Brien CP, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R. Analysis of fMRI time-series revisited. Neuroimage. 1995;2:45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- Gao HC, Xiang Y, Sun NL, Zhu H, Wang YQ, Liu ML, Ma YY, Lei H. Metabolic changes in rat prefrontal cortex and hippocampus induced by chronic morphine treatment studied ex vivo by high resolution H-1 NMR spectroscopy. Neurochem Int. 2007;50:386–394. doi: 10.1016/j.neuint.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Garavan H, Weierstall K. The neurobiology of reward and cognitive control systems and their role in incentivizing health behavior. Preventive medicine. 2012;55(Suppl):S17–23. doi: 10.1016/j.ypmed.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Gjini K, Qazi A, Greenwald MK, Sandhu R, Gooding DC, Boutros NN. Relationships of behavioral measures of frontal lobe dysfunction with underlying electrophysiology in cocaine-dependent patients. The American journal on addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 2014;23:265–271. doi: 10.1111/j.1521-0391.2014.12095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden C. Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Stoelting Company; Wood Dale, IL: 1978. [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossop M, Griffiths P, Powis B, Strang J. Severity of dependence and route of administration of heroin, cocaine and amphetamines. Br J Addict. 1992;87:1527–1536. doi: 10.1111/j.1360-0443.1992.tb02660.x. [DOI] [PubMed] [Google Scholar]
- Hanlon CA, Dufault DL, Wesley MJ, Porrino LJ. Elevated gray and white matter densities in cocaine abstainers compared to current users. Psychopharmacology (Berl) 2011;218:681–692. doi: 10.1007/s00213-011-2360-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel A, von Kanel R. The ventro-medial prefrontal cortex: a major link between the autonomic nervous system, regulation of emotion, and stress reactivity? Biopsychosoc Med. 2008;2:21. doi: 10.1186/1751-0759-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK. Wisconsin Card Sorting Test: Computer Version 3 for Windows. Psychological Assessment Resource; Odessa, FL: 1999. [Google Scholar]
- Hennig J, Scheffler K. Hyperechoes. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2001;46:6–12. doi: 10.1002/mrm.1153. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Neurobiology of cocaine addiction: implications for new pharmacotherapy. The American journal on addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 2007;16:71–78. doi: 10.1080/10550490601184142. [DOI] [PubMed] [Google Scholar]
- Kampman KM, Volpicelli JR, McGinnis DE, Alterman AI, Weinrieb RM, D'Angelo L, Epperson LE. Reliability and validity of the Cocaine Selective Severity Assessment. Addict Behav. 1998;23:449–461. doi: 10.1016/s0306-4603(98)00011-2. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Lyoo IK, Hwang J, Chung A, Hoon Sung Y, Kim J, Kwon DH, Chang KH, Renshaw PF. Prefrontal grey-matter changes in short-term and long-term abstinent methamphetamine abusers. Int J Neuropsychopharmacol. 2006;9:221–228. doi: 10.1017/S1461145705005699. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kuhn S, Haggard P, Brass M. Intentional inhibition: how the "veto-area" exerts control. Human brain mapping. 2009;30:2834–2843. doi: 10.1002/hbm.20711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Miller WR, Del Boca FK. Measurement of drinking behavior using the Form 90 family of instruments. Journal of studies on alcohol Supplement. 1994;12:112–118. doi: 10.15288/jsas.1994.s12.112. [DOI] [PubMed] [Google Scholar]
- Moeller SJ, Tomasi D, Woicik PA, Maloney T, Alia-Klein N, Honorio J, Telang F, Wang GJ, Wang R, Sinha R, Carise D, Astone-Twerell J, Bolger J, Volkow ND, Goldstein RZ. Enhanced midbrain response at 6-month follow-up in cocaine addiction, association with reduced drug-related choice. Addict Biol. 2012;17:1013–1025. doi: 10.1111/j.1369-1600.2012.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mon A, Durazzo TC, Meyerhoff DJ. Glutamate, GABA, and other cortical metabolite concentrations during early abstinence from alcohol and their associations with neurocognitive changes. Drug Alcohol Depend. 2012;125:27–36. doi: 10.1016/j.drugalcdep.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales AM, Lee B, Hellemann G, O'Neill J, London ED. Gray-matter volume in methamphetamine dependence: cigarette smoking and changes with abstinence from methamphetamine. Drug and alcohol dependence. 2012;125:230–238. doi: 10.1016/j.drugalcdep.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyhus E, Barcelo F. The Wisconsin Card Sorting Test and the cognitive assessment of prefrontal executive functions: a critical update. Brain and cognition. 2009;71:437–451. doi: 10.1016/j.bandc.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcoholism, clinical and experimental research. 1995;19:1177–1191. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- Rajapakse JC, Giedd JN, Rapoport JL. Statistical approach to segmentation of single-channel cerebral MR images. IEEE transactions on medical imaging. 1997;16:176–186. doi: 10.1109/42.563663. [DOI] [PubMed] [Google Scholar]
- Tanabe J, Tregellas JR, Dalwani M, Thompson L, Owens E, Crowley T, Banich M. Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biological psychiatry. 2009;65:160–164. doi: 10.1016/j.biopsych.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST, Singleton E, Haertzen CA, Henningfield JE. The development of a cocaine craving questionnaire. Drug Alcohol Depend. 1993;34:19–28. doi: 10.1016/0376-8716(93)90042-o. [DOI] [PubMed] [Google Scholar]
- Tohka J, Zijdenbos A, Evans A. Fast and robust parameter estimation for statistical partial volume models in brain MRI. Neuroimage. 2004;23:84–97. doi: 10.1016/j.neuroimage.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Vonmoos M, Hulka LM, Preller KH, Minder F, Baumgartner MR, Quednow BB. Cognitive impairment in cocaine users is drug-induced but partially reversible: evidence from a longitudinal study. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:2200–2210. doi: 10.1038/npp.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- Woicik PA, Moeller SJ, Alia-Klein N, Maloney T, Lukasik TM, Yeliosof O, Wang GJ, Volkow ND, Goldstein RZ. The neuropsychology of cocaine addiction: recent cocaine use masks impairment. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34:1112–1122. doi: 10.1038/npp.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]