Abstract
Introduction:
Active surveillance (AS) is a strategy for the management of low-risk prostate cancer (PCa). However, few studies have assessed the uptake of AS at a population level and none of these were based on a Canadian population. Therefore, our objectives were to estimate the proportion of men being managed by AS in Ontario and to assess the factors associated with its uptake.
Methods:
This was a retrospective, population-based study using administrative databases from the province of Ontario to identify men ≤75 years diagnosed with localized PCa between 2002 and 2010. Descriptive statistics were used to estimate the proportion of men managed by AS, whereas mixed models were used to assess the factors associated with the uptake of AS.
Results:
45 691 men met our inclusion criteria. Of these, 18% were managed by AS. Over time, the rates of AS increased significantly from 11% to 21% (p<0.001). Older age, residing in an urban centre, being diagnosed in the later years of the study period, having a neighborhood income in the highest quintile, and being managed by urologists were all associated with greater odds of receiving AS.
Conclusions:
There has been a steady increase in the uptake of AS between 2002 and 2010. However, only 18% of men diagnosed with localized PCa were managed by AS during the study period. The decisions to adopt AS were influenced by several individual and physician characteristics. The data suggest that there is significant opportunity for more widespread adoption of AS.
Introduction
Since the introduction of prostate-specific antigen (PSA)-based screening, there has been an increase in the incidence of prostate cancer (PCa).1,2 However, this increase is mostly driven by an increase in the diagnosis of clinically insignificant cancers.3 Thus, the management of PCa has been associated with considerable overtreatment. Active surveillance (AS) has been proposed as a strategy to decrease overtreatment4–10 and is now recognized as a management option by a number of evidence-based guidelines.11–13
Although several prospective series have reported on its safety,4–10 few studies have reported on the uptake of AS at a population level.14–23 No previous population-based study has evaluated the proportion of men being managed by AS in Canada. In other areas of PCa management, there are significant differences between Canada and other countries. Although a recent single-institution series from the University of Ottawa has examined the treatment patterns of men diagnosed with low-risk PCa,24 there remains a need to better understand the rates of AS use and the factors related to its adoption, outside of single-institution series. We hypothesized that the rates of AS increased throughout the study period.
Methods
Participants
This was an institutional review board-approved, population-based, retrospective study that identified, using administrative databases, men aged 18–75 years who were diagnosed with adenocarcinoma of the prostate between January 1, 2002 and December 31, 2010 in Ontario. We excluded men whose diagnostic procedure was not a transrectal ultra-sound-guided biopsy (TRUSB) or a transurethral resection of the prostate (TURP). Men who died or who received primary medical or surgical castration and/or palliative radiotherapy within the first year after diagnosis were also excluded.
All medical procedures in Ontario are reimbursed by a single payer system (Ontario Health Insurance Plan [OHIP]). All OHIP fee codes used are listed in Appendix 1 (available at www.cuaj.ca). We linked these OHIP codes to the Ontario Cancer Registry, the Registered Persons Database, and the Ontario Drug Database to identify the management of subjects diagnosed with PCa (data cutoff December 31, 2013). As there are no codes to differentiate between radiotherapy given with curative or palliative intent, we defined the latter as therapy given within one month or ≥6 months after castration. All localized PCa were included in this study, regardless of the risk-group stratification.25
Treatment groups
Subjects were allocated to one of four groups. The ones who received definitive therapies (i.e., surgery, external beam radiotherapy, or brachytherapy) within the first year following diagnosis without a second TRUSB beforehand were allocated to the definitive treatment group. The remaining men were considered to be in the expectant/observation group that was then subdivided into AS, watchful waiting (WW), and delayed treatments.
The AS group was composed of individuals who had undergone a second TRUSB (confirmatory biopsy) following diagnosis, before any definitive treatments were instituted or before castration. The remaining patients were allocated to the WW/delayed treatment group, which consisted of men who had no subsequent repeat TRUSB or treatments other than castration or palliative radiotherapy (WW) and of men who received definitive therapies >12 months after diagnosis (delayed treatment).
Variables
Using the databases, we determined individual-, physician-and institution-level characteristics. The individual-level characteristics included age at diagnosis, year of diagnosis, neighbourhood income quintile (hereinafter referred to as simply income quintile), and the area of residency. The Aggregated Diagnosis Groups (ADG) score, derived from the Johns Hopkins University Adjusted Clinical Groups Case-Mix system, was used to measure comorbidity.26
Physician- and institution-level characteristics included the treating physician’ speciality and his/her annual new PCa-related case volume, as well as the type of treating centre and its annual new PCa-related case volume. The treating physician was defined as the physician who claimed the most PCa-related visits for each individual during the first year after diagnosis, while the treating institution was defined as the institution where the patient received the majority of his PCa care during the same timeframe.
Outcomes
The primary outcome was to determine the proportion of men with localized PCa managed by AS during the study period. Secondary outcomes were to estimate the uptake of AS over time and to estimate the characteristics associated with the uptake of AS.
Statistical analysis
Descriptive statistics were used to describe the cohort. Medians and interquartile range (IQR) were reported for continuous variables, while proportions were used to report categorical variables. Medians were compared using Wilcoxon or the Kruskal-Wallis sum of rank tests, where appropriate. Chi-squared analyses were used to compare categorical variables, while the Chi-square test for trend was used to estimate whether there was a significant increase in the adoption of AS over time.
Baseline characteristics associated with the adoption of AS were evaluated using a non-linear mixed model adjusted for a priori defined covariates based on previous studies (Appendix 2 at www.cuaj.ca) and adjusted for physician-and institution-level clusters assuming cross-classified data (i.e., physicians could work in more than one institution).27 Estimates in the multivariable models are reported as odds ratios (ORs) with corresponding 95% confidence intervals (CIs). Physician- and institution-attributable intra-class correlations were obtained by calculating the ratio of the between-cluster variance to the total variance.28 Five models specified for each of the outcomes were constructed to account for explained and unexplained variances.
Sensitivity analyses were also performed using three different definitions to identify men managed by AS (Appendix 3 at www.cuaj.ca). All statistical analyses were performed using SAS 9.4 and R version 3.1.3 statistical software. All analyses were two-sided, and p values less than 0.05 were considered statistically significant, with the exception of when multiple comparisons were required, at which time a Bonferroni correction was used.29
Results
A total of 79 498 men diagnosed with PCa between 2002 and 2010 were identified, of which 33 807 were excluded for various reasons (Appendix 4 at www.cuaj.ca). The final cohort was composed of 45 691 men. The characteristics of these men and their treating physicians (n=424) and institutions (n=215) are listed in Table 1.
Table 1.
Individual-, physician- and institution-level characteristics according to treatment groupsa
| Expectant therapy (n=13 872) | |||||
|---|---|---|---|---|---|
|
| |||||
| Variables |
Total (n=45 691) n (%) |
Active surveillance (n=8079) n (%) |
Watchful waiting (n=4570) n (%) |
Delayed treatment (n=1223) n(%) |
Definitive treatment (n=31 819) n (%) |
| Individual-level characteristics | |||||
|
| |||||
| Year of diagnosis | |||||
| 2002–2004 | 12 554 (28) | 1637 (28) | 1204 (26) | 396 (32) | 9713 (29.3) |
| 2005–2007 | 15 937 (25) | 2917 (33) | 1497 (33) | 430 (35) | 11 093 (34.9) |
| 2008–2010 | 17200 (38) | 3525 (39) | 1869 (41) | 397 (33) | 11 409 (35.9) |
| Age group (years old) | |||||
| Less or equal to 55 | 6148 (14) | 948 (12) | 343 (8) | 120 (10) | 4737 (14.9) |
| 56–65 | 19 430 (43) | 3369 (42) | 1337 (29) | 472 (39) | 14 252 (44.8) |
| 66–75 | 20 113 (44) | 3762 (47) | 2890 (63) | 631 (52) | 12 830 (40.3) |
| Diagnostic procedure | |||||
| Biopsy | 43 670 (96) | 7975 (99) | 3232 (71) | 1084 (89) | 31 379 (71.9) |
| TURP | 2021 (4) | 104 (1) | 1338 (29) | 139 (11) | 440 (21.8) |
| ADG score, median (IQR) | 16 (7–22) | 16 (7–23) | 17 (9–26) | 17 (8–23) | 15 (7–22) |
| Survival status | |||||
| Alive | 42 592 (93) | 7746 (96) | 3982 (87) | 1127 (92) | 29 737 (94) |
| Died | 3099 (7) | 333 (4) | 588 (13) | 96 (8) | 2082 (7) |
| Prostate cancer death | 396 (0.9) | 13 (0.2) | 45 (1) | 15 (1) | 323 (1) |
| Income quintile | |||||
| First (lowest) | 6428 (14) | 1061 (13) | 778 (17) | 189 (16) | 4400 (14) |
| Second | 8408 (18) | 1442 (18) | 923 (20) | 238 (20) | 5805 (18) |
| Third | 8974 (20) | 1485 (18) | 893 (20) | 244 (20) | 6352 (20) |
| Fourth | 9937 (22) | 1734 (22) | 928 (20) | 247 (20) | 7028 (22) |
| Fifth (highest) | 11 791 (26) | 2327 (29) | 1025 (22) | 302 (25) | 8137 (26) |
| Rural | |||||
| Yes | 6653 (15) | 817 (10) | 709 (16) | 211 (17) | 4916 (16) |
| No | 39 003 (85) | 7257 (90) | 3857 (84) | 1012 (83) | 26 877 (85) |
|
| |||||
| Physician-level characteristics | |||||
|
| |||||
| Type of primary physician | |||||
| Urologist | 30 552 (67) | 5323 (66) | 3866 (85) | 1030 (84) | 20 333 (64) |
| Radiation oncologist | 14 986 (33) | 2705 (34) | 627 (14) | 190 (16) | 11 464 (36) |
| Physician volume per year | |||||
| 1st quartile (lowest) | 11 278 (25) | 1799 (22) | 1724 (38) | 450 (37) | 7305 (23) |
| 2nd quartile | 11 327 (25) | 1794 (22) | 1323 (29) | 358 (30) | 7852 (25) |
| 3rd quartile | 11 152 (24) | 1807 (22) | 813 (18) | 215 (18) | 8317 (26) |
| 4th quartile (highest) | 11 781 (26) | 2628 (33) | 633 (14) | 197 (16) | 8323 (26) |
|
| |||||
| Institution-level characteristics | |||||
|
| |||||
| Institution volume per year | |||||
| 1st quartile (lowest) | 10 954 (24) | 1522 (19) | 1389 (30) | 380 (31) | 7663 (24) |
| 2nd quartile | 10 824 (24) | 1676 (21) | 1390 (30) | 297 (24) | 7561 (24) |
| 3rd quartile | 11 315 (25) | 1490 (18) | 892 (20) | 285 (23) | 8648 (27) |
| 4th quartile (highest) | 11 497 (25) | 2952 (37) | 503 (11) | 192 (16) | 7850 (25) |
| Type of centre | |||||
| Non-cancer centre | 19 444 (43) | 3147 (39) | 2340 (51) | 636 (52) | 13 321 (42) |
| Cancer centre | 25 151 (55) | 4493 (56) | 1735 (38) | 518 (42) | 18 405 (58) |
All adjusted p values were significant (p<0.001) when the active surveillance group was compared to the watchful waiting group, to the delayed treatment group, and to the definitive treatment group, with the exception of the type of centre variable comparison between the AS group and the definitive treatment group (p=0.2). ADG: Aggregated Diagnosis Groups; IQR: interquartile range; TURP: transurethral resection of the prostate.
Of the men included in this study, 70% (n=31 819) opted for upfront definitive therapies, whereas the remaining patients (n=13 872) were managed, at least initially, expectantly. Of these, 58% (n=8079), 33% (n=4570), and 9% (n=1223) were managed by AS, WW, and delayed definitive treatment, respectively. The proportion of men managed by AS represented 18% of the total cohort (Table 2). Over time, the proportion of men managed expectantly increased significantly (p<0.001; Appendix 5 at www.cuaj.ca). This increase was mainly driven by an increase in the number of men managed by AS, which increased from 11% in 2002 to 21% in 2010 (p<0.001).
Table 2.
Type of management according to year of diagnosis (n=45 691)
| Year of diagnosis | Active surveillance n (%) | Watchful waiting n (%) | Delayed treatment n (%) | Definitive treatment n (%) | Total per year n (%) |
|---|---|---|---|---|---|
| 2002 | 436 (11) | 397 (10) | 135 (3) | 3044 (76) | 4012 (9) |
| 2003 | 545 (14) | 388 (10) | 124 (3) | 2952 (74) | 4009 (9) |
| 2004 | 656 (15) | 419 (9) | 137 (3) | 3321 (73) | 4533 (10) |
| 2005 | 801 (17) | 433 (9) | 148 (3) | 3403 (71) | 4785 (11) |
| 2006 | 998 (19) | 519 (10) | 133 (3) | 3716 (69) | 5366 (12) |
| 2007 | 1118 (19) | 545 (9) | 149 (3) | 3974 (69) | 5786 (13) |
| 2008 | 1104 (20) | 567 (10) | 145 (3) | 3773 (68) | 5589 (12) |
| 2009 | 1215 (21) | 640 (11) | 156 (3) | 3835 (66) | 5846 (13) |
| 2010 | 1206 (21) | 662 (12) | 96 (2) | 3701 (66) | 5765 (13) |
| Total | 8079 (18) | 4570 (10) | 1223 (3) | 31 819 (70) | 45 691 (100) |
Cochrane-Armitage test for trend p value <0.001.
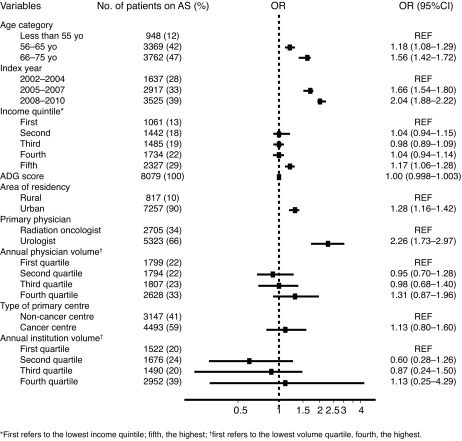
In multivariable analysis, older age, residing in an urban centre, being diagnosed in the later years of the study period, having an average neighbourhood income in the highest quintile, and being primarily managed by an urologist were all associated with greater odds of receiving AS. A forest plot summary of the effects of each covariate included in the full model (Model 5) is presented in Fig. 1.
Fig. 1.
Forest plot of the odds ratio (OD) for each covariate included in the multivariable analysis – uptake of active surveillance. ADG: Aggregated Diagnosis Groups; AS: active surveillance.
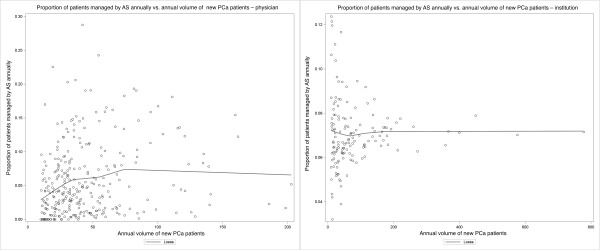
Despite adding all individual-, physician-, and institution-level characteristics (Model 5; Appendix 6 at www.cuaj.ca), there remained significant variance between physicians (14%) and institutions (36%). All three sensitivity analyses yielded similar results, with the exception of higher comorbidity, which was associated with lower odds of adopting AS using the most liberal definition of AS (Appendix 7 at www.cuaj.ca). There was marked heterogeneity between physicians with regard to the annual proportion of new patients managed by AS. Such heterogeneity was also observed, but to a lesser degree, among the treating institutions (Fig. 2).
Fig. 2.
Scatter plot of the proportion of patients managed by active surveillance annually vs. the annual volume of new prostate cancer patients (A) for each physician (minimum of 10 new case/year); (B) for each institution (minimum of 10 new case/year). AS: active surveillance; PCa: prostate cancer.
Discussion
In this first Canadian population-based study on AS, 18% of men diagnosed with localized PCa between 2002 and 2010 were managed by this approach. Since 2002, the use of AS has increased by approximately 1% per year to reach a rate of 21% in 2010. This supports the fact that there is a growing acceptance of AS and likely represents an underestimation of the true proportion of men managed by AS, as the study was not restricted to low-risk PCa.18,20,23 Assuming that 50% of subject had low-risk disease15 and that the majority of patients included in our AS group were indeed low-risk, one could postulate that approximately 36% of patients with low-risk disease were treated by this approach during the study period. These rates were similar to those in other population-based studies, which varied from 10–38%11,16–18,20–22 and in line with the recent single-institution series by Cristea et al.24 Differences in study methodology (any-risk cohort vs. low-risk cohort; pooling AS and WW together vs. presenting them separately) and the countries’ healthcare systems could explain the divergent rates. Given the similarities of our single-payer healthcare system with that of Sweden, we expected our rates to more closely resemble theirs.19,20 In the Swedish study, which excluded men with high-risk diseases, 38% of Swedish men were managed expectantly between 1998 and 2011.19 Rates of AS for the period covering 2007 and 2011 were 59% and 41% for the very-low and low- and intermediate-risk groups, respectively. Although a direct comparison with our study is difficult because our cohort included men with high-risk PCa and restricted the age to ≤75 years (the Swedish trial included 10% of men >75 years of age), our rates were comparable.
The factors associated with the uptake of AS in this study were similar to those previously identified.14,18,19 Increasing age was strongly associated with a greater likelihood of being managed by AS. This may reflect a degree of discomfort either from physicians, patients, or both, with AS as a safe option for younger and healthier men. Contrary to previous findings, we identified that men living in an urban area and men with the highest income quintile were more likely to receive AS.14,30 This may be explained by the universal access to healthcare as opposed to a system in which care is more accessible to higher socio-economic groups. The lack of financial incentive to treat a patient with radical therapies in Canada could also be a plausible explanation as to why men treated in urban centres were more likely to undergo AS. Physicians working in designated cancer centres, which are usually located in urban centres, may have also adopted AS earlier than their other colleagues. Although plausible, this factor was not found to be significantly associated with the uptake of AS in our study.
A major strength of our study is that we used administrative data that encompasses the care of the entire Ontario population. Thus, whereas a study based on Surveillance, Epidemiology, and End Results (SEER) only included Medicare patients ≥65 years of age, our study included all men ≤75 years of age. This study also has several limitations. First, a repeat biopsy was used as a surrogate to identify patients who were managed by AS. Although patients should undergo a confirmatory biopsy (generally within the first year), some refuse.25 To partially account for this, we used a minimum look-forward window of three years to identify a repeat biopsy. In addition, we also used several sensitivity analyses to validate our findings. Furthermore, the fact that we could not adjust for risk-group classification represents a significant limitation, as high-risk PCa and, for the most part, intermediate-risk PCa men are not generally considered candidates for AS. Thus, our estimate of the rate of AS is likely conservative and our interpretation of the identified factors associated with the uptake is limited by this confounder.
In spite of these limitations, the study is the first one that attempts to estimate the proportion of men managed with AS in Canada. It supports a greater acceptance of AS as a management option during the study period, but highlights the need for more widespread adoption. In this era of personalized medicine and concerns regarding overtreatment, this study provides a starting point for further studies that should aim toward estimating the ideal proportion of patients (benchmark) with low-risk PCa that should be managed by AS.
Conclusion
Between 2002 and 2010, 18% of men diagnosed with localized PCa in Ontario were managed by AS. Over the years, there has been a steady increase in the uptake of AS, which attests to the growing acceptance of this management option. The decision to adopt AS was influenced by several individual and physician characteristics. Further research is underway to better understand the forces influencing care and the rigour with which AS is being provided. The data suggest that there is significant opportunity for more widespread adoption of AS in Ontario.
Footnotes
Competing interests: Dr. Alibhai has received grants from Sanofi. The remaining authors report no competing personal or financial interests.
This paper has been peer-reviewed.
References
- 1.American Cancer Society Cancer Facts and Statistics. 2015. Available at http://www.cancer.org/research/cancerfactsstatistics/index. Accessed August 29, 2016.
- 2.Public Health Agency of Canada Public Health Portal. Available at http://www.cancer.ca/en/cancer-information/cancer-101/canadian-cancer-statistics-publication. Accessed August 29, 2016.
- 3.Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: Importance of methods and context. J Natl Cancer Inst. 2009;101:374–83. doi: 10.1093/jnci/djp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dall’Era M, Konety B, Cowan J, et al. Active surveillance for the management of prostate cancer in a contemporary cohort. Cancer. 2008;112:2664–70. doi: 10.1002/cncr.23502. [DOI] [PubMed] [Google Scholar]
- 5.Eggener S, Mueller A, Berglund R, et al. A multi-institutional evaluation of active surveillance for low-risk prostate cancer. J Urol. 2013;189:S19–26. doi: 10.1016/j.juro.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 6.Klotz L, Vesprini D, Sethukavalan P, et al. Long-term followup of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015;33:272–7. doi: 10.1200/JCO.2014.55.1192. [DOI] [PubMed] [Google Scholar]
- 7.Selvadurai E, Singhera M, Thomas K, et al. Medium-term outcomes of active surveillance for localized prostate cancer. Eur Urol. 2013;64:981–7. doi: 10.1016/j.eururo.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 8.Soloway M, Soloway C, Eldefrawy A, et al. Careful selection and close monitoring of low-risk prostate cancer patients on active surveillance minimizes the need for treatment. Eur Urol. 2010;58:831–5. doi: 10.1016/j.eururo.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 9.Bul M, van den Bergh R, Zhu X, et al. Outcomes of initially expectantly managed patients with low- or intermediate-risk screen-detected localized prostate cancer. BJU Int. 2012;110:1672–7. doi: 10.1111/j.1464-410X.2012.11434.x. [DOI] [PubMed] [Google Scholar]
- 10.van den Bergh R, Roemeling S, Roobol M, et al. Outcomes of men with screen-detected prostate cancer eligible for active surveillance who were managed expectantly. Eur Urol. 2009;55:1–8. doi: 10.1016/j.eururo.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Carter H, Albertsen P, Barry M, et al. Early detection of prostate cancer: AUA guideline. J Urol. 2013;190:419–26. doi: 10.1016/j.juro.2013.04.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heidenreich A, Abrahamsson P, Artibani W, et al. Early detection of prostate cancer: European Association of Urology recommendation. Eur Urol. 2013;64:347–54. doi: 10.1016/j.eururo.2013.06.051. [DOI] [PubMed] [Google Scholar]
- 13.Izawa J, Klotz L, Siemens D, et al. Prostate cancer screening Canadian guidelines. Can Urol Assoc J. 2011;5:235–40. doi: 10.5489/cuaj.11134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chamie KWS, Hu J. Population-based assessment of determining treatments for prostate cancer. JAMA Oncol. 2015;1:60–7. doi: 10.1001/jamaoncol.2014.192. [DOI] [PubMed] [Google Scholar]
- 15.Cooperberg M, Broeing J, Carroll P. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28:1117–23. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooperberg MR, Carroll PR. Trends in management for patients with localized prostate cancer, 1990–2013. JAMA. 2015;314:80–2. doi: 10.1001/jama.2015.6036. [DOI] [PubMed] [Google Scholar]
- 17.Filson C, Schroeck F, Ye Z, et al. Variation in use of active surveillance among men undergoing expectant treatment for early stage prostate cancer. J Urol. 2014;192:75–81. doi: 10.1016/j.juro.2014.01.105. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman K, Niu J, Shen Y, et al. Physician variation in management of low-risk prostate cancer: A population-based cohort study. JAMA Intern Med. 2014;174:1450–9. doi: 10.1001/jamainternmed.2014.3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loeb S, Berglund A, Stattin P. Population-based study of use and determinants of active surveillance and watchful waiting for low and intermediate risk prostate cancer. J Urol. 2013;190:1742–9. doi: 10.1016/j.juro.2013.05.054. [DOI] [PubMed] [Google Scholar]
- 20.Loeb S, Folkvaljon Y, Makarov D, et al. Five-year nationwide followup study of active surveillance for prostate cancer. Eur Urol. 2015;67:233–8. doi: 10.1016/j.eururo.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahmood U, Levy L, Nguyen P, et al. Current clinical presentation and treatment of localized prostate cancer in the U.S. J Urol. 2014;182:1650–6. doi: 10.1016/j.juro.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weerakoon M, Papa N, Lawrentschuk N, et al. The current use of active surveillance in an Australian cohort of men: A pattern of care analysis from the Victorian Prostate Cancer Registry. BJU Int. 2015;115:50–6. doi: 10.1111/bju.13049. [DOI] [PubMed] [Google Scholar]
- 23.Womble P, Montie J, Ye Z, et al. Contemporary use of initial active surveillance among men in Michigan with low-risk prostate cancer. Eur Urol. 2015;67:44–50. doi: 10.1016/j.eururo.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 24.Cristea O, Lavallée LT, Montroy J, et al. Active surveillance in Canadian men with low-grade prostate cancer. CMAJ. 2016. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 25.Mohler J, Armstrong A, Bahnson R, et al. Prostate cancer, version 3.2012: Featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2012;10:1081–7. doi: 10.6004/jnccn.2012.0114. [DOI] [PubMed] [Google Scholar]
- 26.Austin P, van Walraven C. The mortality risk score and the ADG score. Two points-based scoring systems for the Johns Hopkins aggregated diagnosis groups to predict mortality in a general adult population cohort in Ontario, Canada. Med Care. 2011;49:940–7. doi: 10.1097/MLR.0b013e318229360e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leckie G. Cross-classified multilevel models - Concepts. LEMMA VLE. 2013;12:1–60. [Google Scholar]
- 28.Browne W, Subramanian S, Jones K, et al. Variance partitioning in multilevel logistic models that exhibit overdispersion. J R Stat Soc A. 2005;168:599–613. doi: 10.1111/j.1467-985X.2004.00365.x. [DOI] [Google Scholar]
- 29.Cabin RJ, Mitchell RJ. To Bonferroni or not to Bonferroni: When and how are the questions. Bulletin of the Ecological Society of America. 2000;81:246–8. [Google Scholar]
- 30.Mishra M, Shen X, Den R, et al. Patterns of care for elderly men diagnosed with favourable-risk prostate cancer from 2004–2008: A population-based analysis. Am J Clin Oncol. 2013;36:606–11. doi: 10.1097/COC.0b013e318261056c. [DOI] [PubMed] [Google Scholar]