Abstract
Purpose
A giant cell tumor (GCT) of bone presenting in the distal radius is rare, however, when they occur, Campanacci Grade III tumors can present formidable reconstructive challenges. They are associated with a high local recurrence rate with intralesional treatment, therefore approaches to reconstruct the wrist after en bloc resection warrant study.
Questions
We asked: (1) What are the functional outcomes after en bloc resection and reconstruction of the wrist with a unipolar prosthesis in patients with Grade III GCT of the distal radius? (2) What complications occur with use of a unipolar prosthesis in these patients? (3) What are the oncologic outcomes with using en bloc resection and reconstruction with a custom unipolar wrist hemiarthroplasty for Grade III GCTs of the distal radius?
Methods
We retrospectively analyzed 10 patients with Campanacci Grade III GCTs of the distal radius treated by a unipolar prosthesis after wide resection of the tumor between January 2008 and October 2013. During that period, all patients at our medical group who presented with a Grade III GCT of the distal radius were treated with wide resection and reconstruction using a custom unipolar implant. Pre- and postoperative pain at rest were assessed according to a 10-cm VAS score. The functional outcomes of the wrist were assessed using the modified Mayo wrist score, and the degenerative changes were evaluated radiographically by a new rating system based on the Knirk and Jupiter scale. We also analyzed tumor recurrence, metastases, and complications associated with the reconstruction procedure. All patients were available for followup at a mean of 52 months (range, 24–90 months).
Results
Although the complication rate associated with prosthetic arthroplasty was relatively high (six of 10), none of our patients experienced severe complications. Two patients reported having occasional pain of the involved wrist at the time of final followup (VAS, preoperative versus postoperative: 0 versus 3; 5 versus 2, respectively). The mean modified Mayo wrist score was 68 (range, 45–90). Degenerative changes were found in three wrists (Grade 1, two patients; Grade 2, one patient). Aseptic loosening occurred in one patient and wrist subluxation occurred in two patients. Lung metastases or local tumor recurrence were not observed.
Conclusions
Because of the proportion of patients who had complications and progressive degeneration with this approach, we recommend first exploring alternatives to reconstruction with custom unipolar wrist hemiarthroplasty after resection of Grade III GCTs of the distal radius, such as fibular autografting. However, this technique provides an alternative for patients with concerns regarding possible morbidity associated with autografting, and for situations when allograft is not available.
Level of Evidence
Level IV, therapeutic study.
Introduction
A giant cell tumor (GCT) of bone is a benign lesion, usually epiphyseal in location, in patients in the 20- to 40-year age group [2, 24]. It is locally aggressive with a tendency for local recurrence (20%–50%) and a low incidence of lung metastases (2%) [5, 10, 13]. After the distal femur and proximal tibia, the distal radius is the third most commonly involved site (10% of cases) [13]. For patients with Campanacci Grades I and II lesions, intralesional curettage and cementation is the most common treatment. However, there is a high risk of local recurrence after this treatment method for patients with more-aggressive (Grade III) GCTs [1, 33]. En bloc resection of Campanacci Grade III tumors with reconstruction is associated with a lower risk of local recurrence than curettage for these aggressive lesions [6, 9, 32, 34].
However, reconstruction of defects that remain after en bloc resection of GCTs of the distal radius is challenging because of high functional demands of the wrist. In the past, numerous procedures including arthrodesis, osteoarticular allografts, fibular autograft, and prosthetic replacement have been used for reconstructing these bone defects [6, 9, 15, 19, 29, 32]. Although there are a few reports of prosthetic arthroplasty after resection of the distal radius, most have been case reports [8, 11, 14, 15, 25]. One other group [35] specifically evaluated this treatment approach. However, they looked at a mixed group of Grade II and Grade III lesions; we believe that Grade II lesions may be better treated with curettage, and that using this approach in the less-aggressive tumors may have resulted in overestimation of the treatment’s efficacy. We therefore wished to evaluate custom unipolar arthroplasty in a group of patients with Grade III GCTs of the distal radius.
Specifically, we asked: What are the functional outcomes after en bloc resection and reconstruction of the wrist with a unipolar prosthesis in patients with Grade III GCTs of the distal radius? (2) What complications occur with use of a unipolar prosthesis in these patients? (3) What are the oncologic outcomes with using en bloc resection and reconstruction with a custom unipolar wrist hemiarthroplasty for Grade III GCTs of the distal radius?
Patients and Methods
Clinical Data
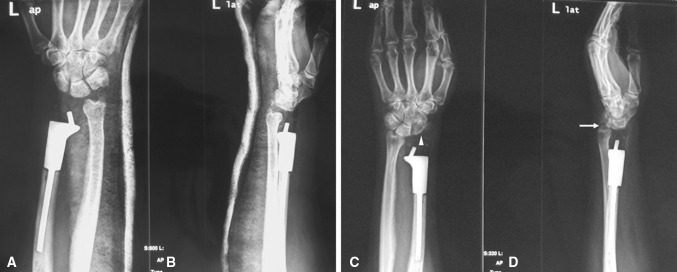
Ten patients (seven men, three women; mean age, 39 years; range, 20–59 years) who underwent prosthetic replacement after wide resection of GCTs of the distal radius between January 2008 and October 2013 were enrolled in this study. During that period, all patients at our medical group who presented with a Campanacci Grade III GCT of the distal radius were treated with en bloc resection and reconstruction using a custom unipolar implant. The pathology specimens for all patients were obtained by preoperative needle biopsy, and evaluated by an experienced bone pathologist (TG). Of the 10 patients, the distal radius prosthetic reconstruction was used as the initial treatment in seven and as a revision procedure in three with recurrent disease after curettage and bone grafting (Fig. 1). All patients underwent preoperative plain radiographs of the wrist and chest, CT and MRI of the wrist, and bone scans when appropriate. We included only patients with Campanacci Grade III tumors in this study. Patients either had a primary GCT of the distal radius or recurrent GCTs with cortex or soft tissue invasion (Table 1). We selected an orthopaedic surgeon (FP) who was not on our research team to perform the clinical and radiologic assessments. All patients were assessed for pain, ROM, and grip strength of the involved wrist. Pre- and postoperative pain at rest was assessed according to a 10-cm VAS score, and the functional outcomes were assessed by the wrist-specific modified Mayo wrist score at the time of final followup [7]. The degenerative changes of the wrist were evaluated radiographically according to a new rating system based on Knirk and Jupiter scale [18] (Table 2). All patients were evaluated every 3 months during the first year of followup and every 6 months thereafter with a physical examination, VAS, functional evaluation of the wrist, and plain radiographs of the wrist and chest.
Fig. 1A–B.
Preoperative (A) AP and (B) lateral view radiographs are shown of a recurrent giant cell tumor of the distal radius.
Table 1.
Summary data for all patients
| Patient | Age (years) | Sex | Side | Occupation | Campanacci grade | Initial treatment |
|---|---|---|---|---|---|---|
| 1 | 32 | Male | Left | Architect | III | Prosthetic arthroplasty |
| 2 | 37 | Male | Left | Bus driver | III (recurrence) | Curettage and bone grafting |
| 3 | 59 | Female | Right | Retired | III | Prosthetic arthroplasty |
| 4 | 37 | Male | Right | Building worker* | III | Prosthetic arthroplasty |
| 5 | 22 | Female | Left | Restaurant waiter | III | Prosthetic arthroplasty |
| 6 | 20 | Male | Left | Undergraduate | III | Prosthetic arthroplasty |
| 7 | 35 | Male | Left | Farmer | III | Prosthetic arthroplasty |
| 8 | 41 | Male | Right | Teacher | III | Prosthetic arthroplasty |
| 9 | 56 | Female | Left | Hotel manager | III (recurrence) | Curettage and bone grafting |
| 10 | 48 | Male | Right | Vehicle mechanic* | III (recurrence) | Curettage and bone grafting |
* Two patients did not return to their prior occupations.
Table 2.
Degree of degeneration
| Grade | Radius translocation distance* (mm) | Radiologic findings (AP and lateral radiographs) |
|---|---|---|
| 0 | 0 | None |
| 1 | 0–1 | Slight joint space narrowing |
| 2 | 1–2 | Marked joint space narrowing, osteoporosis of carpal bones |
| 3 | > 2 | Bone on bone, osteoporosis of carpal bones, osteophyte formation |
* Difference between distance from osteotomy plane of the radius to the lunate bone, of two times.
No patient was lost to followup, and all were available for followup at a mean of 52 months (range, 24–90 months).
Materials
The prosthesis was customized for each patient and produced (LDK Co, Ltd, Haidian, Beijing, China) according to a preoperative detailed design. It took approximately 2 weeks to manufacture the prosthesis, during which time the patients were treated with NSAIDs if they had pain. Measurements for exact manufacture of the prosthesis were taken from radiographs of the contralateral and/or ipsilateral forearm. The prosthesis is composed of a macromolecular polyethylene epiphysis and cobalt-chromium-molybdenum (Co-Cr-Mo) metal stem (Fig. 2). Nonabsorbable polyester sutures (EthibondTM size 2; Johnson & Johnson, Ltd, New Brunswick, NJ, USA) were used for reconstructing ligaments.
Fig. 2.
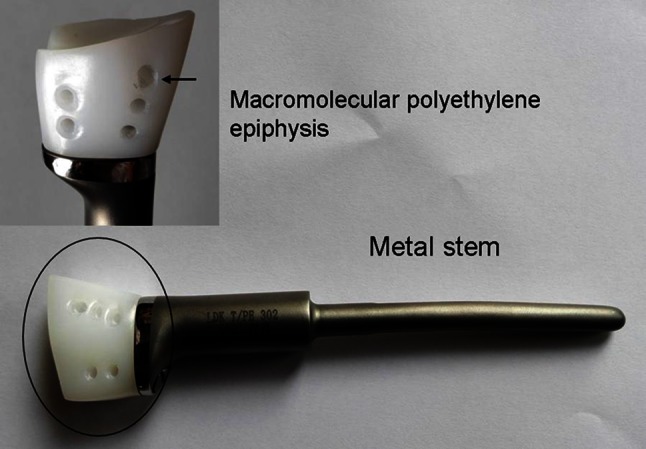
The custom unipolar distal radius prosthesis composed of a macromolecular polyethylene epiphysis (inset in upper left corner) and cobalt-chromium-molybdenum metal stem are shown. Five pores (arrow) were reserved in the macromolecular polyethylene epiphysis.
Surgical Technique
This study was approved and monitored by the institutional review board of our hospital. All patients were allowed to weigh the risks and benefits of prosthetic arthroplasty before signing informed consent.
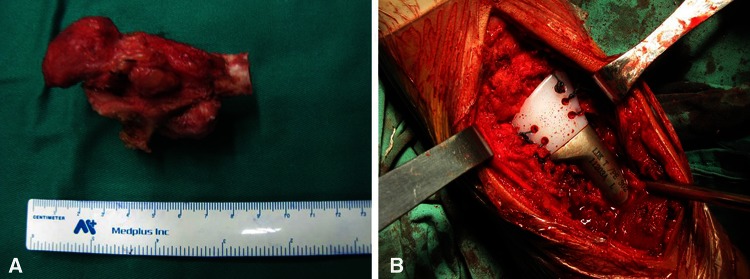
The surgical procedure was performed through a dorsal approach with the patient under general anesthesia. Soft tissue dissection was dependent on the presence or absence of tumor compromise, and the previous biopsy track and hematoma were excised in continuity with removed soft tissues. A jigsaw was used to make an accurate osteotomy when the proposed level of radius resection was identified (Fig. 3A). After proper location of the prosthesis, the prosthesis with appropriate dimensions obtained from preoperative radiographs, was implanted in the radius using polymethylmethacrylate cement. The remaining dorsal radiocarpal ligaments and triangular fibrocartilage complex were sutured to the reserved pores in the macromolecular polyethylene epiphysis by a nonabsorbable suture to enhance stability of the radioulnar and radiocarpal joints (Fig. 3B).
Fig. 3A–B.
A custom unipolar hemiarthroplasty after en bloc resection of a GCT of the distal radius was performed. (A) An en bloc resection specimen, including 8 cm of the distal radius, is shown (B) To enhance stability of radioulnar and radiocarpal joints, the remaining dorsal radiocarpal ligaments and triangular fibrocartilage complex were sutured to the five reserved pores in the macromolecular polyethylene epiphysis.
Results
The mean modified Mayo wrist score used to determine the functional outcome was 68 (range, 45–90). All patients could perform routine daily activities (such as cooking, grooming, and dressing) without difficulty. Although the complication rate associated with prosthetic arthroplasty was relatively high, none of the patients in this study experienced severe complications. Two patients reported mild occasional pain of the involved joint at the latest followup (VAS, preoperative versus 36 months postoperative: 0 versus 3; preoperative versus 60 months postoperative: 5 versus 2, respectively); they reported that the pain was relieved by NSAIDs and it had no major effect on their quality of the life. At the time of final followup, all patients showed some limitation in ROM of the involved wrist compared with the contralateral wrist. The mean ROM of the wrist after distal radius reconstruction was 22° active extension (range, 15°–39°), 20° flexion (range, 10°–38°), 36° pronation (range, 22°–60°), and 38° supination (range, 15°–65°). Grip strength was 75%–100% of the contralateral normal hand in five patients, 50%–75% in three, and 25%–50% in two. The mean grip strength of the surgically treated limb was 68% of the normal hand. Although there are some movement limitations in the involved wrists, eight of the 10 patients returned to their prior occupations (Table 1).
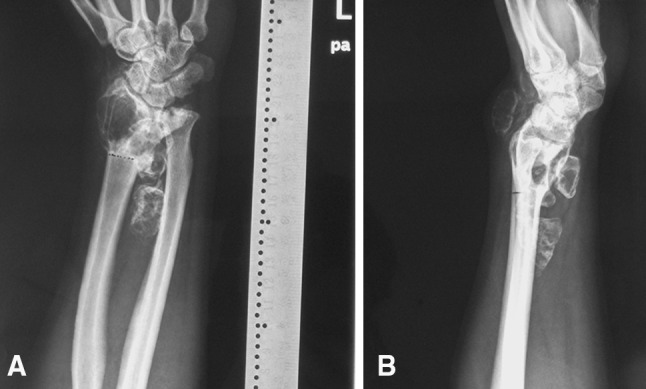
Aseptic loosening occurred 3 years after surgery in one patient, and although radiologic evidence of loosening was observed, the patient was asymptomatic. Wrist subluxation occurred 3 months after surgery in two patients. Both patients were able to perform most of their daily activities without pain and did not have any additional surgery. At the latest followup, neither patient had pain. Different degrees of degenerative changes (Grade 0, seven patients; Grade 1, two patients; Grade 2, one patient) were seen in the involved wrists at the time of final followup (Fig. 4). We observed no infections or periprosthetic fractures in this small series.
Fig. 4A–D.
Seven-day postsurgical (A) AP and (B) lateral radiographs are shown. (C) AP and (D) lateral radiographs also were obtained 56 months after surgery. Ulnar translocation (arrow) and osteoporosis of the scaphoid (arrowhead) were seen at the time of final followup.
No local tumor recurrence or lung metastases were observed in this series.
Discussion
A GCT of the long bones is a locally aggressive lesion with a tendency for local recurrence, especially when located at the distal end of the radius [2, 26]. The primary aim of treatment of GCTs of the distal radius is to completely remove the tumor, reduce the chances of recurrence, and retain maximum possible function of the involved wrist. Primary en bloc tumor resection, especially for Campanacci Grade III GCTs, has been advocated to minimize the risk of recurrence [1, 34]. The use of prosthetic arthroplasty for reconstruction of the resulting distal radial defects has been reported with varying success [8, 11, 14, 15, 25, 35]. However, the information provided by these reports is limited because of the small number of patients. Ten patients with Campanacci Grade III GCTs of the distal radius underwent custom unipolar wrist hemiarthroplasty to determine whether this approach offers an effective treatment. We found that although function generally was regained using this approach, a high proportion of patients had complications. For this reason, we do not consider it as a first-line treatment, and have focused our efforts on assessing fibular autograft, which seems to be a promising alternative.
This study has some limitations. First, the patients have relatively short followup, therefore it is possible that more complications or problems might arise as we follow these patients for a longer time. Second, we had no control group; however, the proportion of patients in this series who had complications was high enough that we believe alternative approaches—perhaps including fibular autografting—need to be explored to reconstruct the wrist after en bloc resection for GCT. The small number of patients is another limitation, and more experience with the use of an implant such as this will be necessary before it can be widely used. However, Campanacci Grade III GCTs are rare, so a multiinstitutional study would be needed to do this, and, again, because of the complications we observed, we do not believe that this approach should be the first-line treatment. We had few patients in this study, so it is likely that a study with more patients might show some local recurrence or metastatic disease.
We observed that the functional results with the en bloc approach and custom unipolar prosthesis allowed patients to return to reasonable function and good pain control. The prosthesis can replace large bone defects, restore radiocarpal joint anatomy, and preserve wrist function while avoiding complications seen with bulk allografts or autografts [8, 11, 14, 15, 25, 35]. Since Gold’s report in 1957 [12], many types of prostheses have been used for reconstructing defects after en bloc resection of the distal radius, which obtained reasonable functional outcome (Table 3). Although our results were encouraging, reconstruction with the custom unipolar prosthesis did not produce functional outcomes similar to those reported for fibular autograft or osteoarticular allograft [3, 9, 16, 19, 29]. Numerous reconstructive methods for large bone defects after en bloc resection of the distal radius have been described, including arthrodesis of the wrist; osteoarticular allograft; nonarthrodesed, nonvascularized or vascularized fibula graft; and prosthetic arthroplasty [6, 8, 9, 11, 14, 19, 25, 29, 32]. Although radiocarpal arthrodesis using various bone grafts can produce good stability, movement of the wrist is sacrificed [6, 20]. Arthrodesis is still an option if the prosthetic arthroplasty fails. Reconstruction using osteoarticular allograft seems promising; however, limited availability of allograft and lack of specialized bone-bank facilities in many countries may greatly limit this technique [4, 9]. Because of the anatomic similarities between the distal radius and the proximal fibula, the fibular autograft has been the preferred technique [21, 29, 30]. In children, the fibular autograft reconstruction can have an excellent outcome because of joint-surface remodeling of the proximal fibular epiphysis [17]. In contrast, in adults, the absence of joint-surface remodeling results in radiocarpal joint incongruity and consequently osteoarthritis and movement limitation [22, 29]. The average ROM of the wrist after reconstruction using the fibular autograft has been reported as 20°–38° flexion, 20°–50° extension, 30°–80° pronation, and 15°–52° supination [21, 28, 30]. Despite osteoarthritic changes and decreased ROM, most patients in those studies [21, 28, 30] had little limitation in daily activities. However, the technique has been associated with potential complications such as nonunion, delayed union of the graft, collapse of the grafted fibular head and donor-site morbidity [28, 31].
Table 3.
Prosthetic arthroplasty for GCTs of the distal radius
| Study | Patients | Prosthesis | Followup (months) | Recurrence rate | Flexion/extension | Supination/pronation | Complications | Functional scores |
|---|---|---|---|---|---|---|---|---|
| Gold [12] 1957 | 1 | Distal radius prosthesis | 59 | 0 | NA | NA | The fracture of the metallic stem | NA |
| Hariri et al. [14] 2013 | 1 | Mega wrist prosthesis | 33 | 0 | 20°/70 | 60°/70° | Dorsal subluxation of the ulnar head | QuickDASH score, 52.27/100 Enneking score, 83% |
| Hatano et al. [15] 2006 | 2 | Distal radius ceramic prosthesis | 168, 120 | 0 | 15°/30° 15°/35° |
45°/30° 50°/60° |
DJD (2), radiolucent line of bone-cement interface (2) | Enneking score, 83% Enneking score, 83% |
| Natarajan et al. [25] 2009 | 24* | Custom-made bipolar hinge prosthesis | 78 (range, 24–156) | 0 | 25° (range, 5°–40°)/20° (range 10°–50°) | 40° (range, 5°–80°)/60° (range, 25°–85°) | Skin flap necrosis (2), infection (2), aseptic loosening (2) | Averaged MSTS score, 75% |
| Gokaraju et al. [11] 2009 | 1 | Predominantly titanium prosthesis | 56 | 0 | 20°/40° | Pronation full while supination 45° | Mild degenerative changes of the carpal bones | Full DASH score, 10.3/100 |
| Zhang et al. [35] 2015 | 11 | Custom-made distal radius prosthesis | 55.5 (range, 24–83) | 9.09 | 30° (range, 15°–45°)/40.9° (range, 20°–60°) | 46.4° (range, 20°–65°)/38.2° (range, 10°–60°) | Superficial infection (1), pain (1) | MSTS score, 80 (range, 63.3–93.3) |
| Current study | 10 | Custom-made distal radius prosthesis | 52 (range, 24–90) | 0 | 20°(range, 10°–38°)/22°(range, 15°–39°) | 38° (range, 15°–65°)/36° (range, 22°–60°) | Pain (2), DJD (3), aseptic loosening (1), wrist subluxation (2) | Modified Mayo wrist score, 68 (range, 45–90) |
GCT = giant cell tumor; * of 24 patients, 16 had giant cell tumors; DJD = degenerative joint disease; NA = not applicable; MSTS = Musculoskeletal Tumor Society.
Although the new prosthesis is also a unipolar design, the rate of complications is substantially lower than that of a unipolar ceramic prosthesis [15]. Furthermore, the macromolecular polyethylene carpus reconstruction provided acceptable functional results at the latest followup. Compared with other hinged prostheses, the nonhinged wrist prostheses may have a lower risk of loosening because of the complex motion mode of the wrist axis [14]. Zhang et al. [35] treated 11 patients with GCTs of the distal radius using custom unipolar prostheses with the articular surface of the macromolecular polyethylene liner, and obtained reasonable functional outcomes at an average followup of 55.5 months. These results indicate that macromolecular polyethylene may be more suitable for articular surface reconstruction than ceramic material. Inconsistent with the findings of Zhang et al. [35], different degrees of degenerative changes were observed in the reconstructed wrist at the medium-term followup (52 months) in our study, which might be related to progressive radius and ulnar translocation resulting from a relatively short prosthesis and reduced wrist motion. A previous study showed that wrist subluxation often occurred after prosthetic arthroplasty, which might limit wrist function [15]. To decrease the ratio of wrist subluxation, five pores were reserved in the macromolecular polyethylene epiphysis to reattach the dorsal radiocarpal and distal radioulnar joint ligamentous structures. Our findings showed that wrist subluxation, which might be related to relatively extensive soft tissue involvement, was seen in only two patients during the early stages of this study.
We observed no local recurrences or metastases in our patients. This is in keeping with the findings of others who observed that resection was associated with a lower local recurrence rate than curettage [10, 13, 19, 23, 35]. Since the incidence of metastatic disease is very low (close to 2%), we would not expect to find pulmonary metastases in such a small group of patients and we did not do routine bone scans to look for multicentric disease or bone metastases [5, 27, 35].
We present some preliminary results of use of a unipolar distal radius prosthesis reconstruction for Campanacci Grade III GCTs of the distal radius. Our patients achieved reasonable functional outcomes. Because of the proportion of patients who had complications and progressive degeneration with this approach, we recommend first exploring alternatives to reconstruction with custom unipolar wrist hemiarthroplasty after resection of Grade III GCTs of the distal radius, such as fibular autografting. A larger study is needed to confirm our observations, ideally comparing this approach with other types of reconstruction. This prosthesis has the disadvantage of being a custom-made prosthesis, which might not be available in all countries, but our preliminary results with its use make it an option for surgeons to consider. Until larger studies are done, we do not consider custom unipolar implants to be a first-line treatment, but they are an option for patients concerned with possible morbidity associated with autografting, and for situations when allograft is not available.
Acknowledgments
We thank Tao Guo MD (Department of Pathology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology) for performing pathologic examination of all specimens, and Feifei Pu MD, PhD (Department of Orthopedics, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology) for performing the clinical and radiologic assessments of all patients.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article. This study was approved and monitored by the institutional review board of our hospital.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
A comment to this article is available at http://dx.doi.org/10.1007/s11999-016-5052-4.
A comment to this article is available at http://dx.doi.org/10.1007/s11999-016-5076-9.
References
- 1.Abat F, Almenara M, Peiro A, Trullols L, Bague S, Gracia I. Giant cell tumour of bone: a series of 97 cases with a mean follow-up of 12 years. Rev Esp Cir Ortop Traumatol. 2015;59:59–65. doi: 10.1016/j.recot.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Amanatullah DF, Clark TR, Lopez MJ, Borys D, Tamurian RM. Giant cell tumor of bone. Orthopedics. 2014;37:112–120. doi: 10.3928/01477447-20140124-08. [DOI] [PubMed] [Google Scholar]
- 3.Bassiony AA. Giant cell tumour of the distal radius: wide resection and reconstruction by non-vascularised proximal fibular autograft. Ann Acad Med Singapore. 2009;38:900–904. [PubMed] [Google Scholar]
- 4.Bianchi G, Donati D, Staals EL, Mercuri M. Osteoarticular allograft reconstruction of the distal radius after bone tumour resection. J Hand Surg Br. 2005;30:369–373. doi: 10.1016/j.jhsb.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am. 1987;69:106–114. [PubMed] [Google Scholar]
- 6.Chalidis BE, Dimitriou CG. Modified ulnar translocation technique for the reconstruction of giant cell tumor of the distal radius. Orthopedics. 2008;31:608. doi: 10.3928/01477447-20080601-05. [DOI] [PubMed] [Google Scholar]
- 7.Cooney WP, Linscheid RL, Dobyns JH. Triangular fibrocartilage tears. J Hand Surg Am. 1994;19:143–154. doi: 10.1016/0363-5023(94)90238-0. [DOI] [PubMed] [Google Scholar]
- 8.Damert HG, Altmann S, Kraus A. Custom-made wrist prosthesis in a patient with giant cell tumor of the distal radius. Arch Orthop Trauma Surg. 2013;133:713–719. doi: 10.1007/s00402-013-1692-y. [DOI] [PubMed] [Google Scholar]
- 9.Duan H, Zhang B, Yang HS, Liu YH, Zhang WL, Min L, Tu CQ, Pei FX. Functional outcome of en bloc resection and osteoarticular allograft reconstruction with locking compression plate for giant cell tumor of the distal radius. J Orthop Sci. 2013;18:599–604. doi: 10.1007/s00776-013-0394-1. [DOI] [PubMed] [Google Scholar]
- 10.Eckardt JJ, Grogan TJ. Giant cell tumor of bone. Clin Orthop Relat Res. 1986;204:45–58. [PubMed] [Google Scholar]
- 11.Gokaraju K, Sri-Ram K, Donaldson J, Parratt MT, Blunn GW, Cannon SR, Briggs TW. Use of a distal radius endoprosthesis following resection of a bone tumour: a case report. Sarcoma. 2009;2009:938295. doi: 10.1155/2009/938295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gold AM. Use of a prosthesis for the distal portion of the radius following resection of a recurrent giant-cell tumor. J Bone Joint Surg Am. 1957;39:1374–1380. [PubMed] [Google Scholar]
- 13.Goldenberg RR, Campbell CJ, Bonfiglio M. Giant-cell tumor of bone: an analysis of two hundred and eighteen cases. J Bone Joint Surg Am. 1970;52:619–664. [PubMed] [Google Scholar]
- 14.Hariri A, Facca S, Di Marco A, Liverneaux P. Massive wrist prosthesis for giant cell tumour of the distal radius: a case report with a 3-year follow-up. Orthop Traumatol Surg Res. 2013;99:635–638. doi: 10.1016/j.otsr.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Hatano H, Morita T, Kobayashi H, Otsuka H. A ceramic prosthesis for the treatment of tumours of the distal radius. J Bone Joint Surg Br. 2006;88:1656–1658. doi: 10.1302/0301-620X.88B12.17989. [DOI] [PubMed] [Google Scholar]
- 16.Humail SM, Ghulam MK, Zaidi IH. Reconstruction of the distal radius with non-vascularised fibular graft after resection of giant cell tumour of bone. J Orthop Surg (Hong Kong). 2014;22:356–359. doi: 10.1177/230949901402200318. [DOI] [PubMed] [Google Scholar]
- 17.Innocenti M, Delcroix L, Manfrini M, Ceruso M, Capanna R. Vascularized proximal fibular epiphyseal transfer for distal radial reconstruction. J Bone Joint Surg Am. 2004;86:1504–1511. doi: 10.2106/00004623-200407000-00021. [DOI] [PubMed] [Google Scholar]
- 18.Knirk JL, Jupiter JB. Intra-articular fractures of the distal end of the radius in young adults. J Bone Joint Surg Am. 1986;68:647–659. [PubMed] [Google Scholar]
- 19.Kocher MS, Gebhardt MC, Mankin HJ. Reconstruction of the distal aspect of the radius with use of an osteoarticular allograft after excision of a skeletal tumor. J Bone Joint Surg Am. 1998;80:407–419. doi: 10.2106/00004623-199803000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Kumar N. Limb preservation in recurrent giant cell tumour of distal end of radius with fibular graft fracture: role of ulnocarpal arthrodesis. Hand Surg. 2015;20:307–309. doi: 10.1142/S0218810415720107. [DOI] [PubMed] [Google Scholar]
- 21.Legname M, Barbary S, Dautel G. Distal radius reconstruction using a split vascularized fibula: two cases following giant cell tumor resection. Orthop Traumatol Surg Res. 2011;97:762–765. doi: 10.1016/j.otsr.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Maruthainar N, Zambakidis C, Harper G, Calder D, Cannon SR, Briggs TW. Functional outcome following excision of tumours of the distal radius and reconstruction by autologous non-vascularized osteoarticular fibula grafting. J Hand Surg Br. 2002;27:171–174. doi: 10.1054/jhsb.2001.0707. [DOI] [PubMed] [Google Scholar]
- 23.McLean JM, Clayer M, Stevenson AW, Samson AJ. A modified ulnar translocation reconstruction technique for Campanacci grade 3 giant cell tumors of the distal radius using a clover leaf plate. Tech Hand Up Extrem Surg. 2014;18:135–142. doi: 10.1097/BTH.0000000000000053. [DOI] [PubMed] [Google Scholar]
- 24.Mendenhall WM, Zlotecki RA, Scarborough MT, Gibbs CP, Mendenhall NP. Giant cell tumor of bone. Am J Clin Oncol. 2006;29:96–99. doi: 10.1097/01.coc.0000195089.11620.b7. [DOI] [PubMed] [Google Scholar]
- 25.Natarajan MV, Chandra BJ, Viswanath J, Balasubramanian N, Sameer M. Custom prosthetic replacement for distal radial tumours. Int Orthop. 2009;33:1081–1084. doi: 10.1007/s00264-009-0732-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Donnell RJ, Springfield DS, Motwani HK, Ready JE, Gebhardt MC, Mankin HJ. Recurrence of giant-cell tumors of the long bones after curettage and packing with cement. J Bone Joint Surg Am. 1994;76:1827–1833. doi: 10.2106/00004623-199412000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Purohit S, Pardiwala DN. Imaging of giant cell tumor of bone. Indian J Orthop. 2007;41:91–96. doi: 10.4103/0019-5413.32037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saikia KC, Borgohain M, Bhuyan SK, Goswami S, Bora A, Ahmed F. Resection-reconstruction arthroplasty for giant cell tumor of distal radius. Indian J Orthop. 2010;44:327–332. doi: 10.4103/0019-5413.65134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saini R, Bali K, Bachhal V, Mootha AK, Dhillon MS, Gill SS. En bloc excision and autogenous fibular reconstruction for aggressive giant cell tumor of distal radius: a report of 12 cases and review of literature. J Orthop Surg Res. 2011;6:14. doi: 10.1186/1749-799X-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taraz-Jamshidi MH, Gharadaghi M, Mazloumi SM, Hallaj-Moghaddam M, Ebrahimzadeh MH. Clinical outcome of en-block resection and reconstruction with nonvascularized fibular autograft for the treatment of giant cell tumor of distal radius. J Res Med Sci. 2014;19:117–121. [PMC free article] [PubMed] [Google Scholar]
- 31.Usui M, Murakami T, Naito T, Wada T, Takahashi T, Ishii S. Some problems in wrist reconstruction after tumor resection with vascularized fibular-head graft. J Reconstr Microsurg. 1996;12:81–88. doi: 10.1055/s-2007-1006458. [DOI] [PubMed] [Google Scholar]
- 32.Wang T, Chan CM, Yu F, Li Y, Niu X. Does wrist arthrodesis with structural iliac crest bone graft after wide resection of distal radius giant cell tumor result in satisfactory function and local control? Clin Orthop Relat Res. 2016 Jan 4. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 33.Wysocki RW, Soni E, Virkus WW, Scarborough MT, Leurgans SE, Gitelis S. Is intralesional treatment of giant cell tumor of the distal radius comparable to resection with respect to local control and functional outcome? Clin Orthop Relat Res. 2015;473:706–715. doi: 10.1007/s11999-014-4054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu S, Yu X, Xu M, Fu Z. Inactivated autograft-prosthesis composite has a role for grade III giant cell tumor of bone around the knee. BMC Musculoskelet Disord. 2013;14:319. doi: 10.1186/1471-2474-14-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang S, Xu MT, Wang XQ, Wang JJ. Functional outcome of en bloc excision and custom prosthetic replacement for giant cell tumor of the distal radius. J Orthop Sci. 2015;20:1090–1097. doi: 10.1007/s00776-015-0763-z. [DOI] [PubMed] [Google Scholar]