Abstract
Cold acclimation (CA) results in alteration of the plasma membrane (PM) lipid composition in plants, which plays a crucial role in the acquisition of freezing tolerance via membrane stabilization. Recent studies have indicated that PM structure is consistent with the fluid mosaic model but is laterally non-homogenous and contains microdomains enriched in sterols, sphingolipids and specific proteins. In plant cells, the function of these microdomains in relation to CA and freezing tolerance is not yet fully understood. The present study aimed to investigate the lipid compositions of detergent resistant fractions of the PM (DRM) which are considered to represent microdomains. They were prepared from leaves of low-freezing tolerant oat and high-freezing tolerant rye. The DRMs contained higher proportions of sterols, sphingolipids and saturated phospholipids than the PM. In particular, one of the sterol lipid classes, acylated sterylglycoside, was the predominant sterol in oat DRM while rye DRM contained free sterol as the major sterol. Oat and rye showed different patterns (or changes) of sterols and 2-hydroxy fatty acids of sphingolipids of DRM lipids during CA. Taken together, these results suggest that CA-induced changes of lipid classes and molecular species in DRMs are associated with changes in the thermodynamic properties and physiological functions of microdomains during CA and hence, influence plant freezing tolerance.
Keywords: Cold acclimation; Oat (Avena sativa); Rye (Secale cereale); Freezing tolerance; Plasma membrane; Detergent resistant membrane (Microdomain, Lipid raft); Sterol; Sphingolipid; Phospholipid
Introduction
Cold acclimation (CA) is one of the most important processes for adaptation to freezing temperatures in plants. During this process, the plasma membrane (PM) composition changes dynamically, which is crucial for acquisition of freezing tolerance. The PM is composed of a variety of proteins and lipids which are considered to be distributed homogeneously and to move relatively freely [40]. However, recent studies have raised the possibility that PM contains microdomains in which components are restricted in their movement. Subsequent biochemical studies proposed that microdomains can be extracted as detergent resistant membrane fractions (DRM) and suggested that DRM-associated proteins and lipids are important for determining the structure and function of microdomains [38,15,54,8,26]. In fact, previous studies, using proteomics techniques, identified DRM-enriched proteins in oat and rye PM and revealed their dynamic responses to CA [42,43]. The results suggested that some microdomain proteins associated with CA in the two plant species contribute to their vastly different freezing tolerance.
In addition to proteins, many studies have suggested that lipids are necessary for microdomain functions. Simons and Ikonen considered the lateral organization of raft-like lipid microdomains to be due to preferential packing of sphingolipids and cholesterol in specific small regions [38,40]. Sphingolipids, in fact, form nanodomains in phosphatidylcholine (PC)-based lipid bilayers and cholesterol fills the intermolecular spaces of sphingolipids [35,37]. Thus, microdomain formation and stabilization may be driven by lipid–lipid and lipid–protein interactions in addition to protein–protein interactions [11,27,38,39]. In plants, some studies have examined the significance of lipids in microdomains. Borner et al. [4] and Mongrand et al. [31] first quantified lipid class and fatty acid composition using DRM as microdomain-enriched fractions. In the same way, the DRM lipid compositions of maize embryos and bean leaves have been characterized [6,7]. Furt et al. [12] reported that phosphatidylinositol-4,5-bisphosphate forms a cluster-like structure that is not influenced by sterol depletion, and that phosphoinositide metabolism-related enzyme activities in DRMs are higher than in the PM.
Lipid changes in DRMs were reported in Arabidopsis leaves during CA [30]. Although free sterol proportions changed in DRMs of the Arabidopsis PM during CA [30], the authors performed only lipid composition measurements at the lipid class level, and did not examine the molecular species in each lipid class. In the present study, we took biochemical and lipidomic approaches to determine the lipid compositions in oat and rye DRMs during CA. Considering a previous study that dealt with PM lipid compositions in oat and rye during CA [44], we aimed to (a) compare lipid compositions between the PM and DRMs, (b) investigate compositional changes of PM and DRM lipids during CA and (c) compare lipid composition and CA-induced changes between low freezing-tolerant oat and high freezing-tolerant rye. The physical properties of membrane lipids influence their stability and are important for the acquisition of plant freezing tolerance during CA [13,41,46]. Therefore, both the physical properties of the PM and microdomains and the biochemical functions of microdomains during CA will be discussed by comparing oat and rye.
Materials and methods
Plant Materials and Isolation of DRM Fractions
Non-acclimated and cold-acclimated oat (Avena sativa L. cv. New almighty) and rye (Secale cereale cv. Maskateer) were grown under conditions reported by Takahashi et al. [42]. PM and DRM isolation were performed in accordance with Takahashi et al. [42].
Total Lipid Extraction and Thin Layer Chromatography
Extraction of total lipids from PM and DRM fractions was carried out according to the method of Bligh and Dyer [3]. Isopropanol (2.5 mL) was added to 1 mL of PM or DRM suspensions. Subsequently, 1.25 mL of chloroform was added twice and samples in test tubes were mixed well. After adding 1.25 mL of 0.9% (w/v) NaCl, samples were incubated for 15 min at room temperature. Test tubes were then centrifuged at 196 × g for 5 min to induce phase separation and the lower phase was collected in a new test tube. To re-extract lipids, chloroform (1.5 mL) was added to the remaining upper phase, test tubes were then centrifuged (784 × g for 10 min) and the lower phase was combined with the previously collected lower phase. After adding 2 mL of chloroform/isopropanol/0.9% (w/v) NaCl (3:47:48, v/v/v) to the combined lower phase, samples were centrifuged at 196 × g for 5 min and the lower phase was collected in a new test tube. The lower phase was then dried at 40°C under N2 gas flow and after adding an aliquot of chloroform, it was stored at −20°C under N2 gas until use.
Thin layer chromatography (TLC) analysis of total lipids was conducted using silica gel plates (Silica gel 60, 0.25-mm thickness, Merck, Darmstadt, Germany) with chloroform/methanol/water (65:25:4, v/v/v) as the developing solvent. Subsequently, solvent on thin layer plates was removed completely and lipids were visualized by spraying with 0.1% (w/v) primuline in acetone (Sigma-Aldrich, St Louis, MO, USA).
Quantification of Sterols, Glucocerebrosides and Phospholipids
Silica gel of the spot corresponding to free sterol (FS), sterylglycoside (SG), acylated sterylglycoside (ASG), glucocerebrosides (GlcCer) and phospholipids (PLs) were collected and subjected to quantification of each lipid class.
Sterol quantification was carried out according to the method of Zlatkis and Zak (1969) with a slight modification [55]. Briefly, 1.1 mL of acetic acid was added to the silica powder in sample tubes and sonicated. An aliquot (1 mL) of o-phthalaldehyde in acetic acid (0.1%, w/v) and 1 mL of concentrated sulfuric acid were added successively and mixed well. These additions generated heat. After cooling the mixture at room temperature for 15 min, sample tubes were centrifuged at 196 × g for 10 min to precipitate silica powder. Absorbance of supernatants at 550 nm was measured. Standard curves where generated from absorbances of cholesterol standards in acetic acid in the range of 0 to 0.15 µM.
GlcCer quantification was performed as sugar determination according to the method of DuBois et al. (1956) [9]. Briefly, 1 mL of water was added to silica powder in sample tubes and then sonicated. Subsequently, 0.5 mL of 5% (w/v) phenol and 2.5 mL of concentrated sulfuric acid were successively added and mixed well. These additions generated heat. After cooling the mixture at room temperature for 15 min, sample tubes were centrifuged at 196 × g for 10 min. Supernatants were collected and absorbance at 485 nm was measured. Standard curves where generated from absorbances of glucose standards in the range of 0 to 0.15 µM.
PL was determined as phosphate according to the method of Marinetti (1962) [29]. Briefly, 50 µL of water and 0.5 mL of 70% perchloric acid were added to sample tubes containing silica gel and mixed well. Subsequently, tubes were incubated at 200°C for 30 min. After cooling to room temperature, 3 mL of water and 0.5 mL of 2.5% (w/v) ammonium molybdate, 0.2 mL of Fiske-SubbaRow reagent (30 g of sodium hydrogen sulfite, 1 g of sodium sulfite and 1 g of 1-amino-2-naphthol-4-sulfonic acid in 200 mL of water) were added in sequence and mixed well. Tubes were boiled for 7 min, cooled to room temperature and centrifuged at 196 × g for 10 min. Supernatants were collected and absorbance at 700 nm was measured. Standard curves where generated from absorbances of KH2PO4 standards in the range of 0 to 0.25 µM.
Determination of Molecular Species of Sterols, Glucocerebrosides and Phospholipids
Total lipid fractions obtained from 50 µg of PM protein were separated into neutral lipid (e.g. FS), glycolipid (SG, ASG and GlcCer), and PLs using silica gel column chromatography according to the method of Lynch and Steponkus [28]. Each separated fraction was dried by blowing N2 gas, dissolved in 1 mL chloroform/acetic acid (100:1, v/v) and transferred to a Sep-Pak Silica Classic Cartridge (Waters, Milford, MA, USA) coupled to a grass syringe barrel. Neutral lipids were eluted with 10 mL chloroform/acetic acid (100:1, v/v). Glycolipids were then eluted by sequential addition of 5 mL acetone and 5 mL acetone/acetic acid (100:1, v/v). Subsequently, phospholipids were eluted by addition of 7.5 mL methanol/chloroform/water (100:50:40, v/v/v). Water/chloroform (12:9, v/v) was added to phospholipid fractions and the fractions were centrifuged at 1000 × g for 5 min. The resultant lower phase was collected and dried under N2 gas. The three lipid fractions were dissolved in chloroform/acetic acid (100:1, v/v) and stored at −20°C under N2 gas until use.
For determination of molecular species of FS, neutral lipid fractions were dried again and dissolved in 100 µL chloroform and transferred into a vial insert (Shimadzu Scientific, Kyoto, Japan). After evaporating chloroform from the vial insert, lipids were dissolved in 5 µL chloroform. Subsequently, the sample (0.5 µL) was injected into a GC-18A gas chromatograph (Shimadzu Scientific) equipped with a dimethyl polysiloxane coated TC-1 capillary column (0.25 mm × 15 m; GL Science, Tokyo, Japan). Lipids were detected using a hydrogen flame ionization detector (FID). Temperature of the column was increased at 3°C/min from 180°C to 240°C. Both injector and detector temperatures were set at 250°C. Each sterol species was identified by the retention time of peaks corresponding to the following standards: cholesterol, campesterol, stigmasterol and sitosterol (Wako Chemicals, Tokyo, Japan).
GlcCer molecular species analysis was carried out according to the method of Imai et al [19]. First, total lipid extracts were subjected to mild alkaline hydrolysis to remove glycerolipids. The completely dried total lipid extracts were dissolved in 133 µL of 0.4 M KOH in methanol at 37°C for 1 h and neutralized with 5 µL of HCl. Subsequently, 2666 µL of chloroform, 2533 µL of methanol and 1333 µL of water were sequentially added to samples and resultant lower phases were collected. Sample fractions containing GlcCer was transferred to a Sep-Pak Plus Silica Cartridge (Waters, Milford, MA, USA) coupled to a glass syringe barrel and chloroform/acetic acid (100:1, v/v) was passed through the column. The fractions containing GlcCer were then eluted with chloroform/methanol (2:1, v/v) and injected into a LC-10AT pump (Shimadzu Scientific) coupled with ACQUITY TQD tandem quadrupole mass spectrometer (Waters) as described in Watanabe et al. [45]. Samples were separated on a 3 µm TSKgel ODS-100Z column (Tosoh, Tokyo, Japan) eluted with a gradient from 80% solvent A (methanol/formic acid, 1000/1, v/v)/20% solvent B (water/formic acid, 1000/1, v/v) to 100% solvent A for 30 min and then solvent A for 70 min at a flow rate of 200 µL/min. The following conditions were used for detection: capillary voltage, 3 kV; desolvation gas flow, 600 L/h; nebulizer gas flow, 50 L/h; source temp., 120°C; and collision gas flow, 0.3 mL/min (4–5 mbar). Identification of various GlcCer molecular species was carried out based on 30 pairs of precursor ions [M + H]+ and product ions of sphingoid base moieties as listed in Watanabe et al. [45] in the positive ionization MRM mode.
Determination of molecular species of SG and ASG was carried out by direct-infusion electrospray ionization triple quadrupole mass spectrometry according to Schrick et al. [36] with minor modifications. An aliquot of each sample (0.2 mL) was combined with 0.15 nmol PG (di20:0 [phytanoyl]) as an internal standard in a total sample volume of 1 mL of chloroform/methanol/300 mM ammonium acetate in water (300/665/35, v/v/v). The sample was directly infused at 30 µL per min. The analytical parameters and data processing were as described previously [36].
Determination of phospholipid species was performed using direct-infusion electrospray ionization triple quadrupole mass spectrometry according to the method described in the supplemental data of Xiao et al. [52] with minor modifications. The samples were dissolved in 1 mL chloroform. An aliquot of extract (200 µL) in chloroform was used. Precise amounts of internal standards, obtained and quantified as previously described [49], were added in the following quantities (with some small variation in amounts in different batches of internal standards): 0.27 nmol PC (di12:0), 0.27 nmol PC (di24:1), 0.27 nmol Lyso PC (13:0), 0.27 nmol Lyso PC (19:0), 0.14 nmol PE (di12:0), 0.14 nmol PE (di23:0), 0.14 nmol Lyso PE (14:0), 0.14 nmol Lyso PE (18:0), 0.14 nmol PG (di14:0), 0.14 nmol PG (di20:0 [phytanoyl]), 0.14 nmol Lyso PG (14:0), 0.14 nmol Lyso PG (18:0), 0.10 nmol PI (16:0–18:0), 0.07 nmol PI (di18:0), 0.09 nmol PS (di14:0), 0.09 nmol PS (di20:0 [phytanoyl]), 0.14 nmol PA (di14:0), 0.14 nmol PA (di20:0 [phytanoyl]), 0.22 nmol DGDG (16:0–18:0), 0.32 nmol DGDG (di18:0), 0.90 nmol MGDG (16:0–18:0), and 0.18 nmol MGDG (di18:0). The samples and internal standard mixture were combined with solvents composed of chloroform/methanol/300 mM ammonium acetate in water (300/665/35, v/v/v) at a final volume of 0.8 mL. Samples were then directly infused at 30 µL per min. The analytical parameters and data processing protocol were the same as described previously [52]. The mean unsaturation index was calculated as the average number of double bonds per fatty acid present ([the percentage of each lipid] × [number of double bonds / number of fatty acid species per lipid]) as described by Lee et al [24].
Results
Determination of Lipid Profiles in Oat and Rye DRM during CA
DRM fractions were extracted from purified PM fractions by adding Triton X-100 as described in Peskan et al. [34]. A white layer was observed at the interface of the 30% and 35% (w/w) sucrose layers. As previously described [42], the recovery rates of DRMs from PM fractions (based on protein amount) significantly decreased in both oat and rye DRM during CA (from 13.86% to 8.16% in oat and 11.73% to 7.17% in rye).
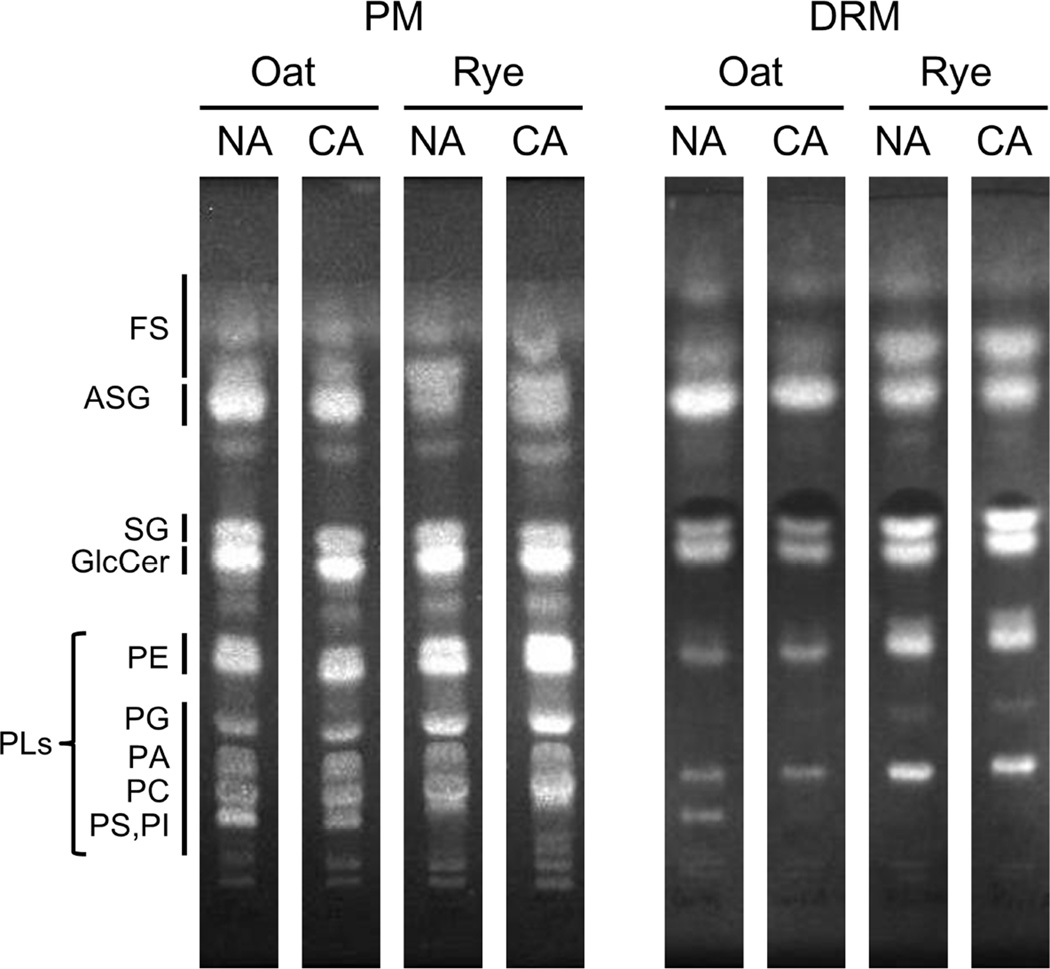
In the present study, we extracted lipids from PM and DRM fractions corresponding to 100 µg protein each, and subsequently separated the extracted lipids with TLC. Comparing the patterns of PM and DRM lipids with those of commercial marker lipids showed that the PM and DRM fractions contained FS, ASG, SG and PLs (PE, phosphatidylethanolamine; PG, phosphatidyl glycerol; PA, phosphatidic acid; PC, phosphatidylcholine; PS, phosphatidylserine; PI, phosphatidylinositol) as major lipid components (Fig. 1). DRM fractions contained smaller amounts of PLs than PM fractions. Three sterol classes (FS, ASG and SG) were more brightly stained in DRMs than the PM. As shown in Fig. 1, ASG and FS seemed to be the major components in oat and rye DRMs, respectively. Sterols, glycolipids and PLs in each spot from the TLC plates were quantified by colorimetric assays for sterol, sugar and phosphate moieties, respectively. PE, PG, PA, PC, PS and PI were combined for quantification because they were difficult to separate into distinct spots.
Fig. 1. TLC patterns of PM and DRM fractions in oat and rye.
PM and DRM fractions isolated from oat and rye leaves were examined by TLC with developing solvent composed by chloroform/methanol/water (65:25:4, v/v/v). FS, free sterol; ASG, acylated sterylglycoside; SG, sterylglycoside; GlcCer, glucocerebroside; PE, phosphatidylethanolamine; PG, phosphatidylglycerol; PA, phosphatidic acid; PC, phosphatidylcholine; PS, phosphatidylserine; PI, phosphatidylinositol.
The quantification of lipids (mol% of total lipids [nmol/100 µg protein]) in NA and CA oat is described in Table 1. The proportions of sterols and PLs were quite different between the PM and DRMs in oat. For sterols, the mol% of sterols in the total lipids accounted for 42.6% and 73.6% in the PM and DRMs, respectively. The major sterol component was ASG (28.5% in PM and 50.3% in DRMs). In contrast to sterols, the proportion of PLs in the PM was greater than in DRMs (41.9% in PM and 11.4% in DRMs). The GlcCer proportion in the PM was similar to that in DRMs (15.5% in PM vs 15.0% DRMs). These results indicate that, in oat DRMs, sterols are enriched and PLs are depleted.
Table 1. Overall compositions of lipid classes in oat PM and DRM.
The results are mol% calculated as (mole of each lipid classes)/(mole of total lipids). The value of nmol lipid/100 µg protein is indicated in parentheses. Each value is the mean ± SD (n=3–5).
| PM | DRM | |||
|---|---|---|---|---|
| Lipid | NA (n=3) | CA (n=4) | NA (n=3) | CA (n=5) |
| Sterols | 42.6±3.1 (96±23) |
37.7±2.5 (70±10) |
73.6±3.8 (175±37) |
66.0±3.7* (188±28) |
| FS | 10.2±2.5 (22±4) |
7.4±0.7* (14±1*) |
21.1±2.5 (50±6) |
14.4±2.8* (40±6) |
| ASG | 28.5±2.2 (65±18) |
24.8±2.4 (46±8) |
50.3±4.2 (121±32) |
48.7±2.6 (139±25) |
| SG | 3.9±1.2 (9±4) |
5.6±1.1 (10±2) |
2.2±0.8 (5±1) |
2.9±0.7 (8±3) |
| GlcCer | 15.5±0.7 (35±8) |
12.6±0.9* (24±6) |
15.0±4.2 (35±10) |
18.3±4.7 (53±22) |
| PLs | 41.9±1.2 (94±22) |
49.7±2.2* (94±19) |
11.4±1.0 (28±11) |
15.7±0.8* (46±12) |
| Total | 100 (225±41) |
100 (188±29) |
100 (238±42) |
100 (287±48) |
p<0.05 by student’s t-test comparing NA and CA.
FS, free sterol; ASG, acylated sterylglycoside; SG, sterylglycoside; GlcCer, glucocerebroside; PLs, phospholipids.
In rye, similar to oat, DRMs contained much higher amounts of sterols and lower amounts of PLs than the PM (Table 2). In rye, however, FS was the major form of sterol in both the PM and DRMs, accounting for 50.5% of the total lipids in DRMs. The proportion of PLs was lower in DRMs than in the PM (48.0% in PM vs 20.6% in DRMs). Although DRMs tended to contain a slightly higher proportion of GlcCer than the PM (15.6% in DRMs vs 13.4% in PM), the difference was not significant. Overall, rye DRMs had higher sterols and lower PLs than the PM, and had a different sterol composition from oat DRMs.
Table 2. Overall compositions of lipid classes in rye PM and DRM.
The results are mol% calculated as (mole of each lipid classes)/(mole of total lipids). The value of nmol lipid/100 µg protein is indicated in parentheses. Each value is the mean ± SD (n=3–5).
| PM | DRM | |||
|---|---|---|---|---|
| Lipid | NA (n=4) | CA (n=3) | NA (n=4) | CA (n=5) |
| Sterols |
39.2±2.1 (105±16) |
40.1±1.8 (122±42) |
63.8±4.6 (156±64) |
64.5±3.3 (190±37) |
| FS | 32.9±1.9 (87±13) |
33.8±5.0 (100±22) |
50.5±2.4 (123±48) |
51.7±4.1 (153±36) |
| ASG | 2.9±1.5 (8±4) |
2.9±2.3 (10±11) |
9.6±1.1 (24±10) |
5.8±2.3* (16±4) |
| SG | 3.2±1.1 (10 4) |
3.4±2.5 (12±12) |
3.7±1.5 (9±6) |
6.9±1.2* (20±6*) |
| GlcCer |
13.4±1.5 (34±2) |
7.8±1.8* (25±14) |
15.6±3.7 (37±17) |
14.4±3.1 (42±11) |
| PLs |
48.0±0.9 (128±17) |
52.1±0.7* (158±52) |
20.6±1.0 (48 15) |
21.2±3.0 (64±29) |
| Total |
100 (267±28) |
100 (305±87) |
100 (241±79) |
100 (296±61) |
p<0.05 by student’s t-test comparing NA and CA.
FS, free sterol; ASG, acylated sterylglycoside; SG, sterylglycoside; GlcCer, glucocerebroside; PLs, phospholipids.
After CA treatment, the total amount of sterols in the oat PM decreased from 42.6% to 37.7% (Table 1). Sterols in oat DRMs also decreased from 73.6% to 66.0% (Table 1). During CA, the proportions of FS in the oat PM significantly decreased from 10.2% to 7.4%, respectively, while no changes were observed in the rye PM (Tables 1 and 2). In oat DRMs, FS significantly decreased from 21.1% to 14.4% during CA. Similarly, ASG in rye DRMs significantly decreased from 9.6% to 5.8% during CA (Tables 1 and 2). GlcCer decreased from 15.5% to 12.6% and 13.8% to 7.8% in the oat and rye PMs, respectively, during CA (Tables 1 and 2). However, there were no statistically significant changes of GlcCer in either oat or rye DRMs during CA (Tables 1 and 2). Slight increases in PL were observed in PM and DRM in both species. (Tables 1 and 2).
Sterol Compositions of the PM and DRM in Oat and Rye during CA
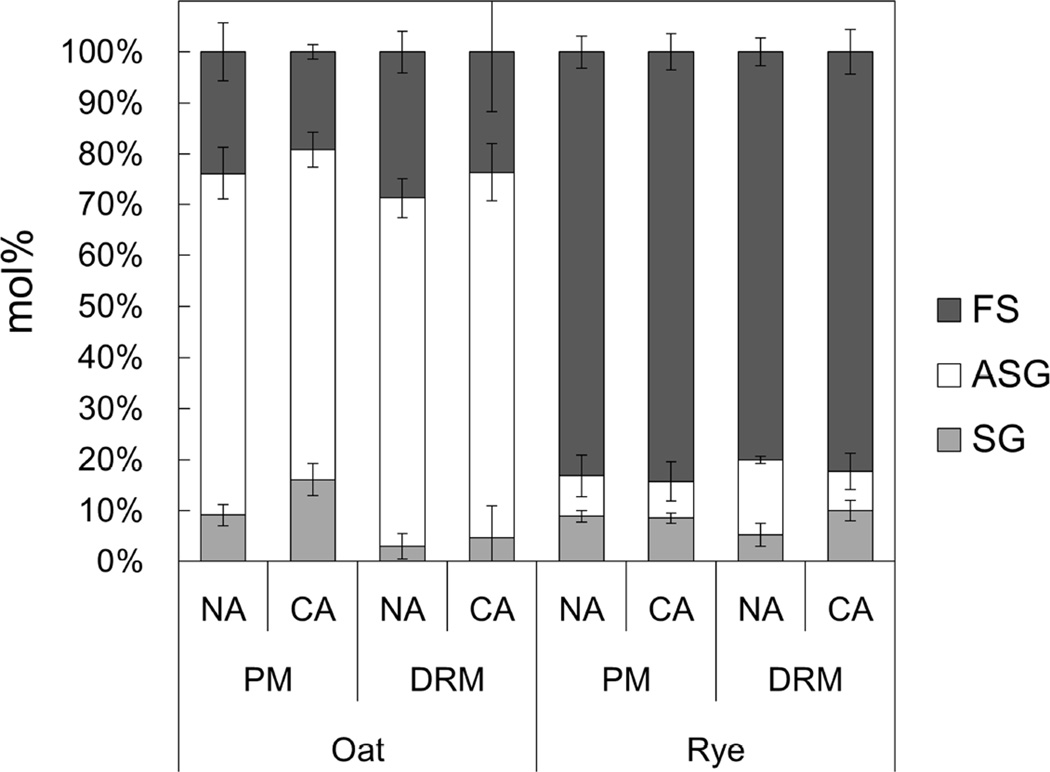
Fig. 2 shows the sterol compositions in the oat and rye PM and DRMs during CA. In both the PM and DRMs, the sterol composition in oat was quite different from that in rye. In oat, ASG was the major sterol in both the PM and DRMs while FS was abundant in the rye PM and DRMs. In the oat PM and DRMs, the proportion of FS decreased but that of SG increased during CA. In contrast, the proportion of ASG in rye DRMs decreased from 14.7% to 7.7% but the proportion of SG increased from 5.3% to 10.0%.
Fig. 2. Sterol compositions of PM and DRM fractions in oat and rye.
Sterol compositions of PM and DRM were quantified and Y-axis of the graph is mol% of each sterol in the total sterol content. The content of each group was determined colorimetrically after TLC, as shown in Fig. 1. Error bars indicate standard deviations (n=3–5). FS, free sterol; ASG, acylated sterylglycoside; SG, sterylglycoside.
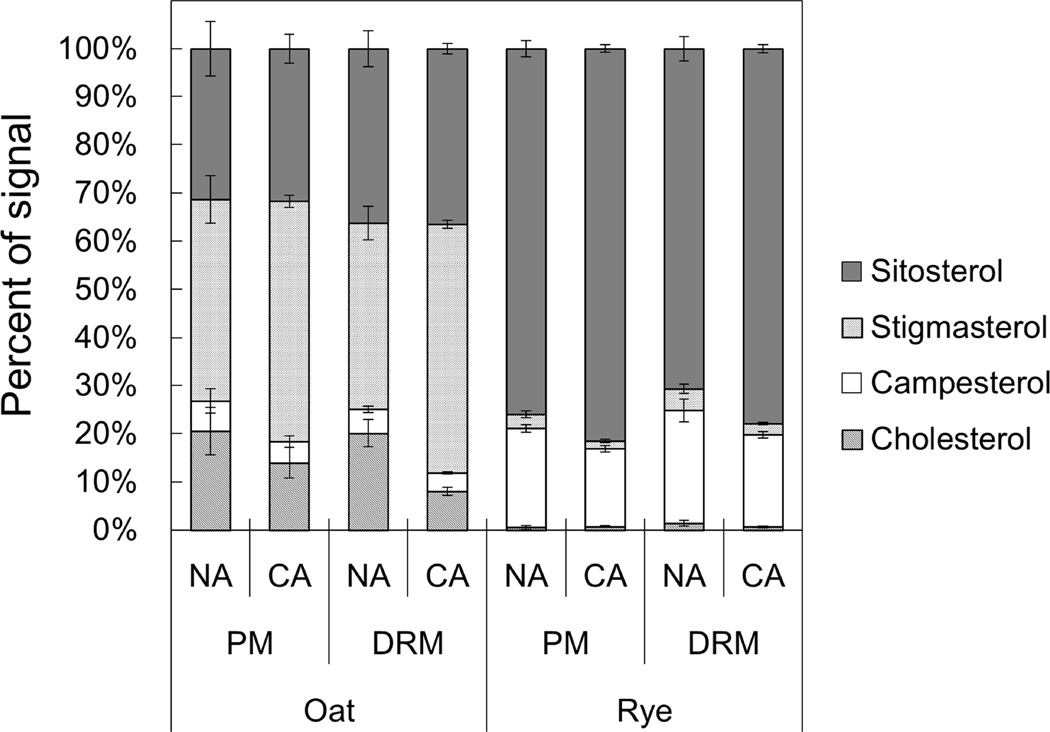
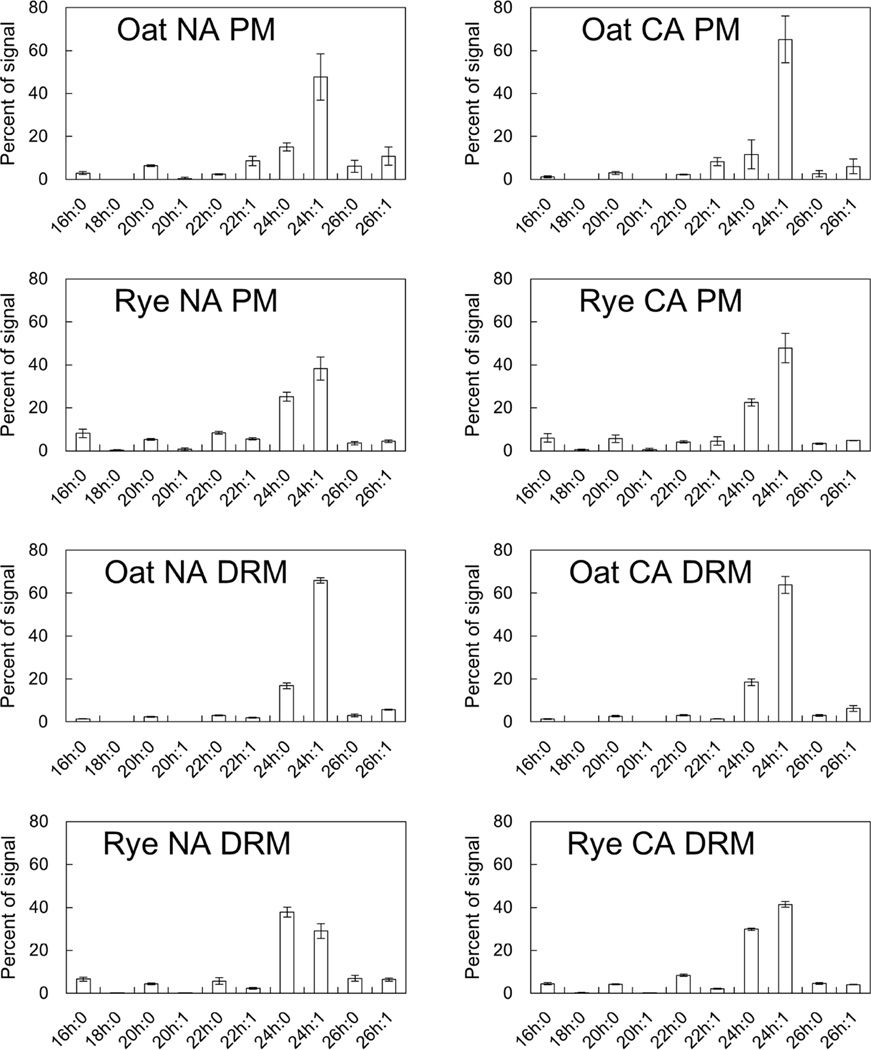
To obtain detailed FS compositional data, we performed gas chromatography analysis. We examined four major sterol species, sitosterol, stigmasterol, campesterol and cholesterol, and calculated the proportion of each sterol in the total FS (Fig. 3, Supplemental Table 1). Oat FS were mainly composed of sitosterol, stigmasterol and cholesterol. On the other hand, in the rye PM and DRMs, sitosterol and campesterol were the predominant sterol species. Although campesterol, stigmasterol and cholesterol were significantly enriched in rye NA DRMs compared with the rye NA PM (1.14-, 1.53- and 2.54-fold, respectively), the general FS composition was similar between the rye PM and DRMs.
Fig. 3. Molecular species compositions of FS in PM and DRM fractions.
Molecular species compositions of FS in PM and DRM were quantified by GC analysis. Y-axis of the graph is proportions of signals derived from each sterol species in total FS signals. Error bars indicate standard deviations (n=4–8).
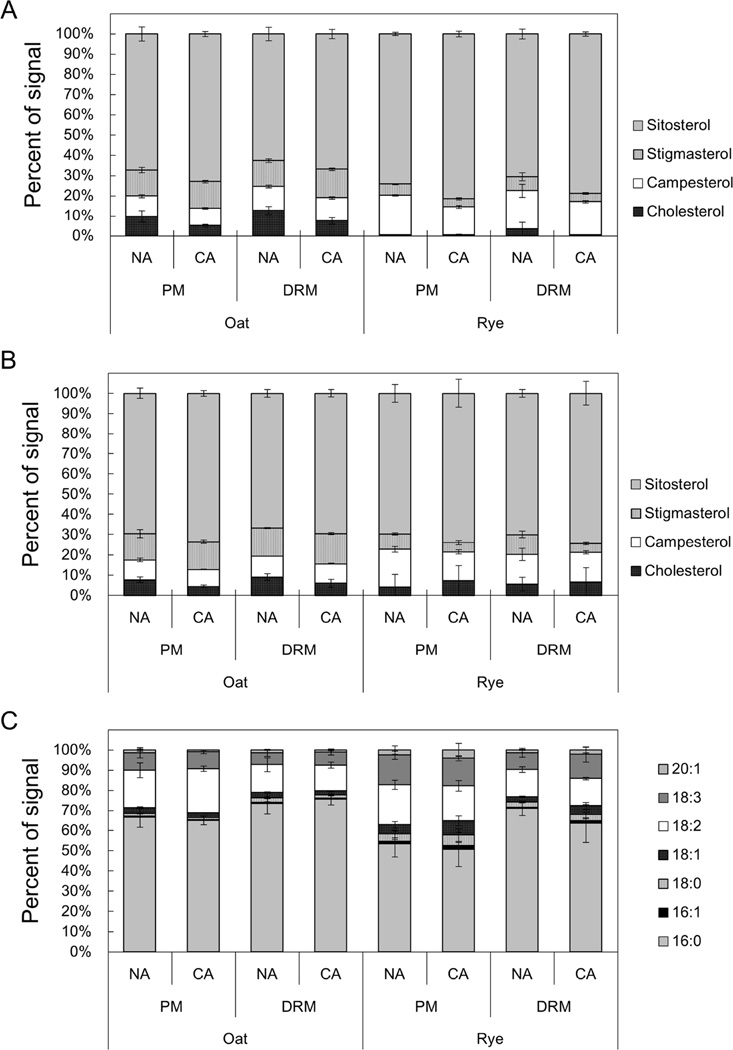
For SG and ASG, we used a newly developed quantification method with electrospray ionization tandem (triple quadrupole) mass spectrometry (ESI-MS/MS) as reported by Schrick et al. [36] and calculated the proportions of four major sterol species in the total SG and ASG fractions (Fig. 4, Supplemental Table 1). Although, we could not make a simple comparison between FS and SG/ASG compositions because of the different analysis methods, the proportions of stigmasterol in oat SG and ASG were 24.9 to 37.3% lower than those in FS. In both oat and rye, the predominant sterol species in SG and ASG was sitosterol and most of the CA-induced changes were common among FS, SG and ASG (Figs. 4A and 4B).
Fig. 4. Sterol and fatty acyl compositions of SG and ASG in PM and DRM fractions.
SG and ASG in PM and DRM were quantified by direct infusion MS/MS analysis. The Y-axis of the graph represents the proportion of signals derived from each sterol or acyl species in the total SG or ASG signals. Error bars indicate standard deviations (n=4). Molecular species compositions of the sterol part of SG and ASG are shown in A and B, respectively. C shows molecular species compositions of acyl chains of ASG.
In addition to sterol composition, we determined the compositions of the acyl chains bound to ASGs (Fig. 4C). The predominant acyl species in ASGs was 16:0 in both oat and rye. In both species, DRMs contained significantly higher proportions of 16:0 than the PM. Specifically, rye DRMs had 1.33 and 1.26 times higher proportions of 16:0 than the PM at NA or CA conditions. Although the proportions of unsaturated acyl chains such as 16:1, 18:1, 18:2, 18:3 and 20.1 were higher in the rye PM than the oat PM (32.0% in oat PM vs 43.1% in rye PM), no remarkable changes were observed during CA in either the oat or rye PM. During CA, unsaturated lipid species such as 18:3 and 18:1 slightly increased (1.27-fold) and the proportion of 16:0 decreased (0.89-fold) in rye DRMs.
GlcCer Compositions of the PM and DRMs in Oat and Rye during CA
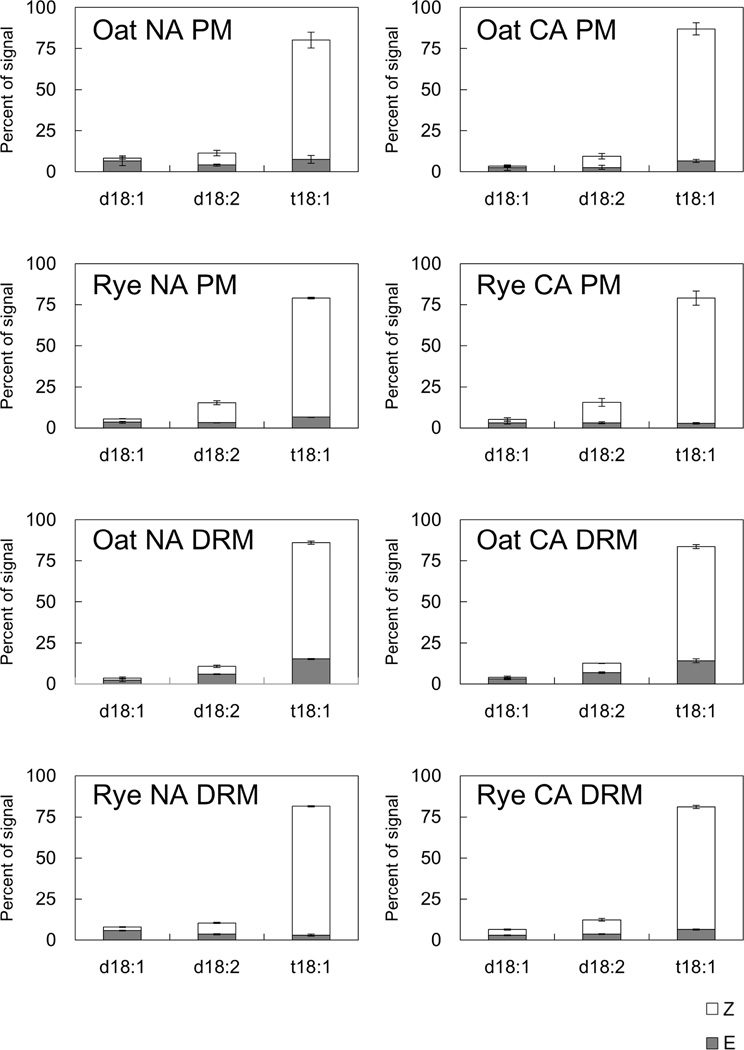
GlcCer is composed of a C18 sphingoid base and a 2-hydroxy fatty acid. First, we measured the sphingoid base, which contains three major species (d18:1, 8-sphingadienine; d18:2, 4,8-sphingadienine; t18:1, 4-hydroxy-8-sphingenine) with cis-8 (Z) and trans-8 (E) isomers [17], and calculated the sphingoid base compositions against the total sphingoid base content in oat and rye (Fig. 5, Supplemental Table 2). t18:1 (Z) was the predominant sphingoid base in the PM and DRMs isolated from both oat and rye. During CA, in both oat and rye DRMs, there were no remarkable changes in sphingoid base composition during CA.
Fig. 5. Compositions of C18 sphingoid bases of GlcCer in PM and DRM fractions.
Compositions of C18 sphingoid bases of GlcCer in PM and DRM were quantified by LC-MS/MS analysis. Y-axis of the graph indicates proportions of signals derived from each sphingoid base species in total GlcCer signals. Error bars indicate standard deviations (n=3). Z and E mean cis-8 and trans-8 isomers, respectively. d18:1, 8-sphingadienine; d18:2, 4,8-sphingadienine; t18:1, 4-hydroxy-8-sphingenine.
The 2-hydroxy fatty acid analysis revealed that the proportions of two major components, 24h:0 and 24h:1, were different between oat and rye (Fig. 6, Supplemental Table 2). In the NA PM, 24h:0 accounted for a higher proportion in rye than in oat (25.1% and 15.0%, respectively). When compared in the PM and DRMs, oat had a higher proportion of 24h:1 in DRMs than in the PM (65.8% and 47.6%, respectively). On the other hand, rye DRMs contained a higher proportion of 24h:0 than the PM (37.8% and 25.1%, respectively) and the proportion of 24h:1 was lower in DRMs than in the PM (29.0% and 38.3%, respectively). No obvious shifts of 2-hydroxy fatty acid composition were observed in the oat or rye PM during CA. Although the 2-hydroxy fatty acid composition in oat DRMs was unchanged during CA, rye DRMs showed a decrease in 24h:0 (37.8% to 29.9%) and an increase in 24h:1 (29.0% to 41.5%) during CA.
Fig. 6. Compositions of 2-hydroxy fatty acids of GlcCer in PM and DRM fractions.
Compositions of 2-hydroxy fatty acids of GlcCer in PM and DRM were quantified by LC-MS/MS analysis. Y-axis of the graph indicates proportions of signals derived from each 2-hydroxy fatty acid in total GlcCer signals. Error bars indicate standard deviations (n=3).
PL Compositions of the PM and DRMs in Oat and Rye during CA
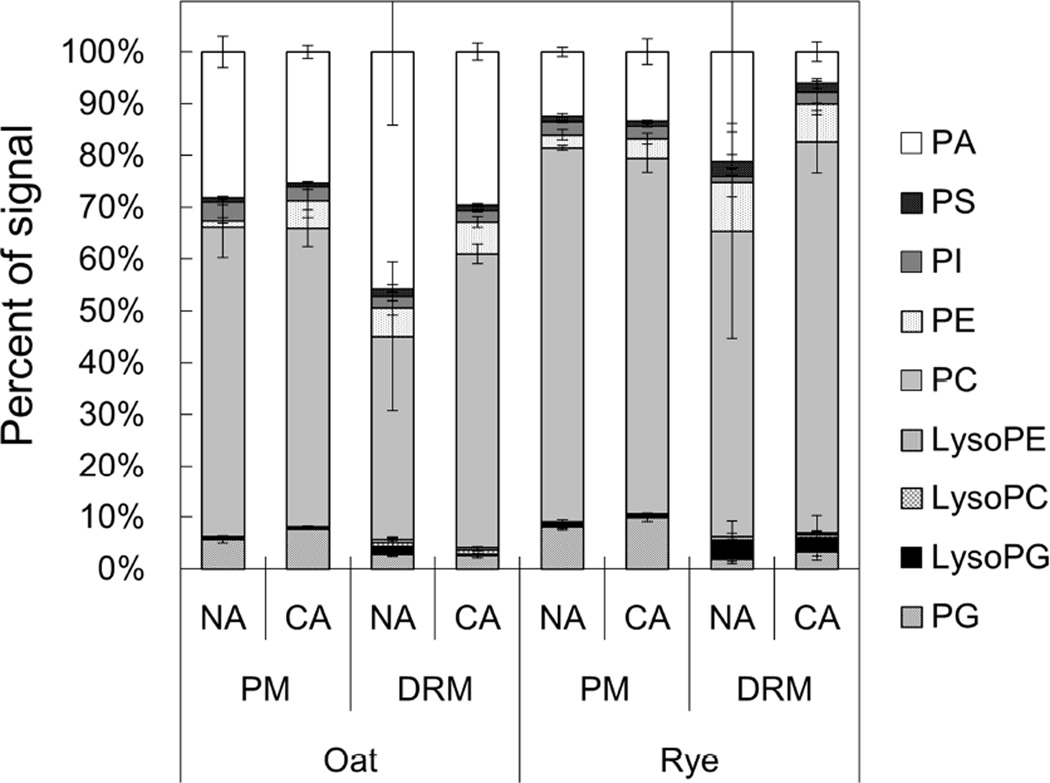
We next determined the molecular species composition of PA, PS, PI, PE, PC, PG, LysoPE, LysoPC and LysoPG, and then calculated the proportion of each of these PL classes (Fig. 7, Supplemental Table 3). In all samples, PA and PC were the predominant PLs and accounted for more than 80.3%. The proportion of PC in NA DRMs was lower than that in the PM in both oat and rye (39.4% in oat DRMs and 59.1% in rye DRMs). Conversely, PE was enriched in DRMs and the DRM/PM ratios for PE were 4.6 and 3.8 in oat and rye, respectively. After CA, the PC proportion increased by 1.44-fold and the PA proportion decreased by 0.65-fold in oat DRMs. Similarly to oat, the PA and PC proportions changed by 1.28- and 0.29-fold, respectively, in rye DRMs.
Fig. 7. PL classes of PM and DRM fractions in oat and rye.
PL compositions of PM and DRM were quantified by direct infusion MS/MS analysis and the Y-axis of the graph represents proportions of signals derived from each PL class in total PL signals. Error bars indicate standard deviations (n=4). PA, phosphatidic acid; PS, phosphatidylserine; PI, phosphatidylinositol; PE, phosphatidylethanolamine; PC, phosphatidylcholine; LysoPE, lysophosphatidylethanolamine; LysoPC, lysophosphatidylcholine; LysoPG, lysophosphatidylglycerol; PG, phosphatidylglycerol.
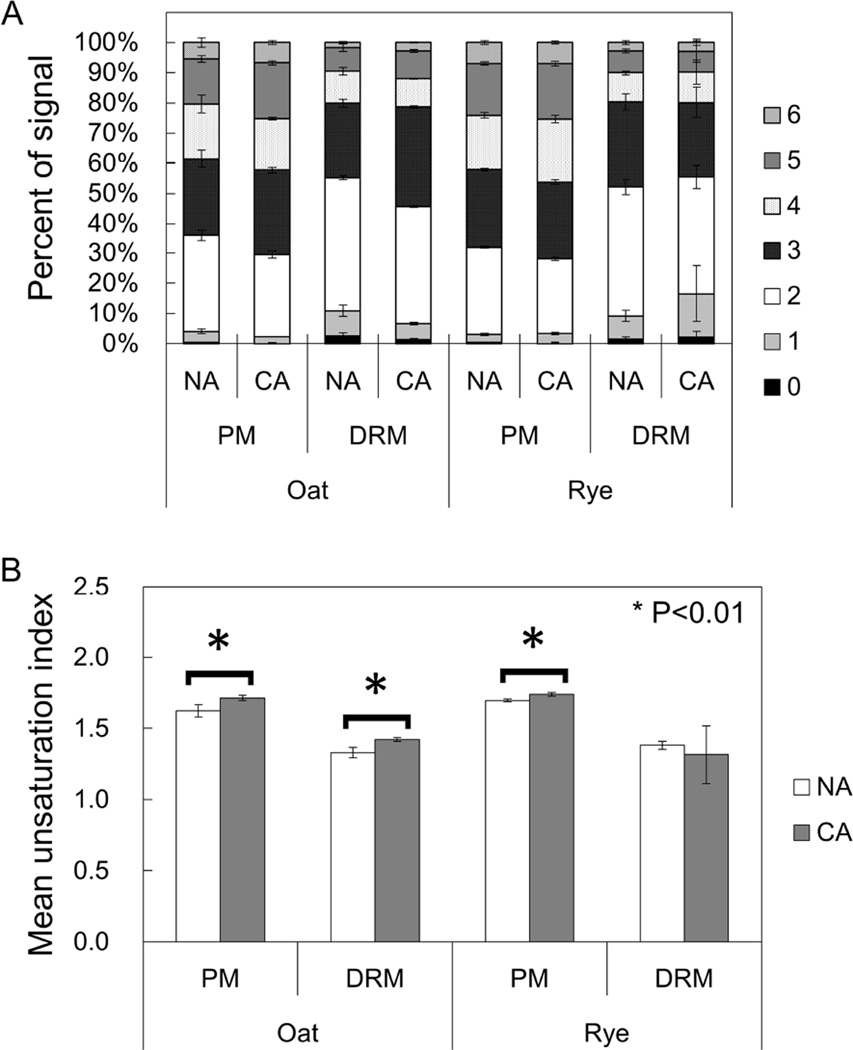
To examine the unsaturation levels of acyl chain in PLs in oat and rye, we computed the proportions of each PL molecular species with 0–6 double bonds (Fig. 8A). In NA oat and rye, the pair of fatty acids in a single PL molecule mainly contained 2, 3, 4, or 5 double bonds with smaller amounts of 0, 1, and 6 double bonds. For example, PC (34:2), PC (34:3) and PC (36:4) in NA oat and PC (34:2), PC (36:4) and PC (36:5) in NA rye were the three most abundant PL species. NA DRMs contained more low-unsaturated PL species than PM such as PLs containing two double bonds in both oat and rye (1.39- and 1.49-fold greater in NA DRMs than in NA PM in oat and rye, respectively). In oat PM and DRM, less unsaturated PLs decreased and higher unsaturated ones increased during CA. For example, the proportion of PLs containing three double bonds in oat was 1.33-fold greater in CA DRM than in NA DRM. However, rye DRM did not show any statistically significant changes in the proportions of each PL during CA.
Fig. 8. Unsaturation levels of fatty acid in PLs in PM and DRM fractions.
(A) PL molecules were categorized based on the sum of unsaturated bonds in two acyl chains. (B) The Mean unsaturation index was calculated for PM and DRM fractions in oat and rye. Error bars indicate standard deviations (n=4). *p<0.01 by student’s t-test.
Based on the results in Fig. 8A, we calculated the mean unsaturation index of PLs in each fraction and plant species during CA (Fig. 8B). When comparing the PM and DRMs under NA, mean unsaturation index in the PM were greater than those in DRMs in both oat and rye (1.22- and 1.23-fold in oat and rye, respectively) which means DRMs contain much more saturated PLs than PM. In the PM, the unsaturation index significantly increased in both oat and rye from 1.63 to 1.71 and from 1.70 to 1.74 during CA. In DRMs, however, there were no statistical changes in rye during CA even though oat DRMs showed an increased unsaturation level from 1.33 to 1.42.
Discussion
Previously, PM lipid compositions in oat (cv. Ogle) and rye (cv. Puma) was determined and discussed in relation to differences in the freezing tolerance of the two species after CA [44]. Webb et al. [48] reported that freeze-induced lesions of the PM occurred at different temperatures in oat and rye and structural changes of the PM such as lamellar to HII phase transitions were observed under freezing temperature in oat protoplast. In the present study, the lipid composition of microdomain-enriched DRM fractions and the PM was determined in oat and rye both before and after CA and are discussed from the viewpoint of the properties of each lipid species and the influence of these lipids on freezing tolerance with reference to previous studies.
Microdomain Compositions in Oat and Rye before CA
In the present study, DRM fractions were isolated as microdomain-enriched fractions by detergent treatment of the PM and subsequent sucrose-density gradient centrifugation. PM and DRM lipid separation and subsequent quantification revealed that sterols were highly enriched but PLs were depleted in the DRM fractions in both oat and rye before CA treatment (Fig. 1, Tables 1 and 2). Previous studies with Arabidopsis, tobacco and leek also showed lower proportions of PLs and higher proportions of sphingolipids and sterols in DRM fractions than PM fractions [4,23,31], which is generally consistent with the present study. In the present study, however, a sphingolipid, GlcCer, did not show statistically significant differences between the PM and DRMs in either oat or rye (Tables 1 and 2). Because sphingolipids can be relatively tightly associated with each other and have a high melting point due to the long chain fatty acid in their molecular structure and high hydrophobicity, they form a lipid nanodomain with low fluidity that differs from the fluid phase mainly composed of PLs and probably contribute to microdomain formation [38]. On the other hand, sterols are also considered to be a major component of microdomains because they have an affinity to GlcCer and fill the intermolecular spaces of GlcCer [38]. Thus, these results indicate that sterols may be a particularly important constituent of microdomains to determine their functions and structure in oat and rye.
Alterations of PM and Microdomain Compositions in Oat and Rye during CA
The relationship between PM lipid composition and freezing tolerance in oat (cv. Ogle) and rye (cv. Puma) has been investigated and discussed previously [44,48]. These studies demonstrated that freeze-induced dehydration results in ultrastructural changes associated with the PM, fracture-jump lesions and lamellar-to-hexagonal II (HII) phase transitions, and that changes of PM lipid compositions decrease the occurrence of these membrane-associated lesions. Although CA treatment resulted in many compositional changes in the PM, the patterns of PM lipid changes (e.g. an increase of PLs and a decrease of GlcCer in oat) were common in the previous and present studies [44]. In oat and rye, the proportions of PLs and GlcCer in the PM changed, which may influence lipid bilayer hydration because PLs and GlcCer are highly and poorly hydrated lipid classes, respectively [47]. Hydration of the PM surface is important for preventing HII phase formation, which is an inter-bilayer event that occurs when interaction between the PM and various closely-positioned endomembranes triggered by freeze-induced dehydration becomes stronger [13,48]. Therefore, greater hydration of the PM surface in both oat and rye achieved by increasing PLs and decreasing GlcCer during CA may contribute to increasing PM stability under freezing conditions. Additionally, because the PL/GlcCer ratio was higher in rye (6.68) than in oat (3.94), the rye PM may have a lower incidence of HII phase formation and more stable membrane structure than the oat PM under freezing temperatures.
In oat DRMs, the proportion of sterols significantly decreased (by 7.6%) and the proportion of PLs increased (by 4.3%) during CA, while the GlcCer proportion did not change (Table 1). Conversely, rye DRMs did not show any significant changes in the proportions of sterols, GlcCer or PLs. The proportion of GlcCer in both oat and rye did not change in DRMs during CA, but was decreased in the PM, which may be related to the fact that GlcCer is the main component of microdomains [38]. The same tendency was also observed in Arabidopsis DRMs [30]. The proportion of sterols decreased in oat DRMs (73.6% to 66.0%) during CA but did not change in rye DRMs (63.8% to 64.5%). Plant sterols are considered to promote lipid domain formation [53] and also to be important for regulating the temperature sensitivity of raft-like membrane structures [2]. Although the proportions of PLs increased by 4.3% during CA in oat DRMs (11.4% to 15.7%), rye DRMs did not show a change in PL proportion and maintained a higher proportion of PLs than oat DRMs after CA (20.6% in oat and 21.2% in rye after CA). As described above, PLs are important for maintaining PM stability under freezing conditions [13,48]. Thus, the high proportion of PLs in rye DRMs may contribute to maintain and/or change microdomain properties during freezing.
Alterations of Sterol Compositions in Oat and Rye during CA
ASG and FS were the predominant sterols in the oat and rye PM, respectively, which was also observed in DRM samples (Fig. 2). In DRMs, the proportion of ASG increased in oat (68.2% to 71.7%) but decreased in rye (14.7% to 7.7%) during CA. Webb et al. [46] revealed that ASG is more effective than FS for HII phase formation in lipid mixtures of dioleoyl-PC (DOPC) and dioleoyl-PE (DOPE) under dehydration conditions. ASGs contain a sterol ring, sugar and acyl group, and are more hydrophobic than other sterols [46]. Therefore, the different proportions of ASGs between oat and rye DRM may result in different membrane behavior under freeze-induced dehydration and ultimately influence plant freezing tolerance. The biosynthesis of SG and ASG is catalyzed by UDP-glucose:sterol glucosyltransferases (UGTs, EC 2.4.1.173, 22, 23). Thus, the activity of UGTs possibly contributes to the different ASG proportions in oat and rye.
In addition to sterol lipid classes, the molecular species of sterols were determined before and after CA (Figs. 3 and 4). In oat and rye, sitosterol was commonly the predominant phytosterol among FSs, SGs and ASGs (Figs. 3, 4A and 4B). Just for a reference, the proportion of each sterol species in total sterol content (FS, SG and ASG in combination) was calculated although it is not scientifically sound to combine the data obtained by different quantification methods for each sterol class (data not shown). Results showed that sitosterol accounted for 58.0% to 81.0% of total sterol content in samples obtained from both oat and rye, which means that biosynthesis of each sterol species is relatively similar in oat and rye while sterol modification processes such as glycosylation and acylation for SG and ASG biosynthesis might be an important difference between the two plant species as we mentioned above. However, Oat FS contained more stigmasterol than rye FS in NA DRMs (38.7% in oat and 4.5% in rye) and DRMs prepared from NA rye had more sitosterol than NA oat DRMs (36.3% in oat and 70.7% in rye) although the SG and ASG sterol compositions were only slightly different between oat and rye. Although no studies have examined the effects of sitosterol on lipid mixtures under freeze-induced dehydration conditions in comparison with stigmasterol, the vast differences in sterol species may contribute to the difference in freezing tolerance between oat and rye.
The acyl chain lipid species of ASGs were also determined (Fig. 4C). The acyl chain compositions in ASGs did not change considerably during CA in oat and rye. DRM ASGs contained more saturated fatty acids (16:0 and 18:0) than PM ASGs in NA oat (68.0% in PM and 75.8% in DRMs) and the same was true in NA rye (56.9% in PM and 73.6% in DRMs). Given that microdomains are considered to be enriched in hydrophobic and high-melting point lipids such as sphingolipids and sterols [38], saturated acyl chain ASGs may also tend to gather in microdomain areas in the PM.
Sterols are also important for the modulation of H+-ATPase activity [14]. H+-ATPase is known as a DRM-enriched protein [30,42,43] and is enriched in microdomains of the yeast Saccharomyces cerevisiae [1]. H+-ATPase activity is up-regulated by cold and may be involved in the regulation of intracellular pH and membrane potential [20]. Thus, it is possible that differences in sterol composition in microdomains may affect the activity of microdomain-associated proteins, and sterol-enriched microdomains may have an important role for the modulation of protein structure and function as a functional scaffold during CA.
Alterations of GlcCer Compositions in Oat and Rye during CA
Among the two major parts of GlcCer, the C18 sphingoid base showed no significant changes during CA in both oat and rye (Fig. 5). t18:1 was the predominant C18 sphingoid base in both species as reported in Brassicaceae [18]. The overall profiles of sphingoid base compositions were similar between oat and rye and the maximum amount of CA-induced changes was less than 4.2% (d18:1[E] in the oat PM; Fig. 5) even though data from Kawaguchi et al. [21] indicated that higher proportions of t18:1 (Z) were positively correlated with freezing tolerance in grapevine leaves. On the other hand, the 2-hydroxy fatty acid composition was different in oat and rye during CA (Fig. 6). In both oat and rye, 24h:0 and 24h:1 were the major 2-hydroxy fatty acid species. When comparing the PM and DRMs under NA conditions, the 2-hydroxy fatty acid composition in oat DRMs was similar to that in the oat PM. In rye, DRMs contained more 24h:0 and less 24h:1 than the PM. Although the total GlcCer proportions in rye did not differ between the PM and DRMs (Table 2), rye DRMs were enriched in 24h:0-containing GlcCer, which has a relatively higher melting point than 24h:1. During CA, there were no notable changes of 2-hydroxy fatty acid composition in the oat and rye PMs or oat DRMs. Only rye DRMs showed CA-induced changes in 2-hydroxy fatty acid composition (37.8% to 29.9% for 24h:0 and 29.0% to 41.5% for 24h:1). Similar to the acyl chain changes in ASGs, these changes may increase the unsaturation index of fatty acids and affect the physical properties of microdomains in the PM under freezing conditions. Therefore, the 2-hydroxy fatty acid composition of microdomains and its changes during CA may be involved in the higher freezing tolerance in rye.
In addition, sphingolipids have numerous other functions in plant cells. A sphingolipid metabolite, sphingosine-1-phosphate, is responsive to drought-induced signal transduction in guard cells [33,50] and sphingolipid fatty acid 2-hydroxylase (FAH) in Arabidopsis is required for oxidative stress responses [32]. The relationship between the 2-hydroxy fatty acid composition in microdomains and many signaling pathways derived from sphingolipids may be involved in the CA mechanism and the acquisition of freezing tolerance. In fact, transient formation of phytosphingosine phosphate is involved in cold response via nitric oxide and AtMPK6 in Arabidopsis [5,10].
Alterations of PL Compositions in Oat and Rye during CA
When looking at the composition of PL species in Fig. 7, PC and PA were the two most abundant PL species. However, Uemura and Steponkus reported that PC and PE were the predominant PL species in oat and rye PMs [44]. This difference may have been caused by endogenous phospholipase D (PLD) activity during the PM preparation process. PLD has the ability to produce PA mainly from PC and PE and some PLDs such as PLDγ1 and PLDδ prefer PE rather than PC [25]. These PLDs require micromolar levels of Ca2+ for PA-producing activity and are located in intracellular membranes and the PM [25]. Although we added EGTA to chelate Ca2+ and performed PM preparation on ice to repress PLD activity, PL analysis is a long process from PM preparation to PL extraction/quantification. During this process, PE may have been degraded and changed to PA by PLD activity. Nevertheless, PC is still the predominant PL species in both the PM and DRMs (Fig. 7). A previous study of tobacco leaves and maize BY-2 cells revealed that PC is one of the major PL species in both the PM and DRMs [12]. Therefore, PC is universal in monocotyledonous and dicotyledonous plants as the predominant PL in the PM and microdomains. In human cells, extracellular leaflets of the PM are considered to be enriched in sphingomyelin and cytoplasmic leaflets are composed of PC, PE and PS as the major components of lipid rafts [22].
PLs containing double or triple unsaturated fatty acids were abundant in both the PM and DRMs (Fig. 8A). A previous study [44] found that 16:0/18:2 and 16:0/18:3 fatty acid pairs were the major combinations in PC and PE. The present study revealed that PC (34:2) and PC (34:3) were abundant PLs in both oat and rye, accounting for 16.8% and 11.1% of the total PLs, respectively, in the PM fraction prepared from NA oat (Supplemental Table 3). Therefore, the fatty acid composition profile in our study is in agreement with the previous study.
In the PM, PC (36:4) and PC (36:5) accounted for large proportions as major PL species in both NA oat and rye (e.g. 10.9% for PC [36:4] and 8.8% for PC [36:5] in the oat NA PM; Supplemental Table 3). In DRM factions, however, these two PLs were much less abundant (less than 5%) and CA treatment increased their proportions (e.g. 1.63-fold for PC [36:4] and 2.47-fold for PC [36:5] in oat; Supplemental Table 3). Phase transitions of membranes can be promoted by saturated fatty acids at low temperature [16,51] and microdomain-enriched DRMs contained relatively larger proportions of saturated fatty acids than the PM (Fig. 8B). Although saturated fatty acids tend to be associated with sterols and other microdomain components [39,53], plants may be able to decrease saturated fatty acids in microdomain by increase the availability of unsaturated PLs such as PC (36:4) and PC (36:5) in PM during CA in order to avoid phase transitions in microdomains under low temperature. The overall unsaturation index of PLs, in fact, increased in the oat and rye PM and oat DRMs during CA in agreement with the relationship between the physicochemical properties of unsaturated PLs and membrane stability at low temperature (Fig. 8B). In rye DRMs, however, the mean unsaturation index of PLs did not change during CA. Rye DRMs were rich in FS, which is less effective for freeze-induced phase transitions in membranes than ASG [46], and even the acyl chain of ASGs in rye was relatively enriched in unsaturated species in comparison with oat (Figs. 2 and 4C). Considering these factors in oat and rye, sterol compositions favorable for PM stability at freezing temperatures may be necessary to maintain and/or change physical property and function of microdomain toward acquisition of freezing tolerance during CA.
Supplementary Material
Acknowledgments
We are grateful to Dr. Andre Laroche (Agriculture and Agri-Food Canada) for providing rye seeds. Determinations of sterylglycoside, acylated sterylglycoside and phospholipid lipid species described in this work were performed at the Kansas Lipidomics Research Center (KLRC) Analytical Laboratory, where instrument acquisition and lipidomics method development were supported by National Science Foundation (DBI 0521587, DBI1228622), Kansas Technology Enterprise Corporation, K-IDeA Networks of Biomedical Research Excellence (INBRE) of National Institute of Health (P20GM103418), and Kansas State University. We thank Mary Roth of KLRC for technical assistance and Ruth Welti for reading the manuscript. We also thank Dirk Hincha and Ellen Zuther (Max-Planck Institute of Molecular Plant Physiology) for English proof-reading of the manuscript and helpful advice. This study was in part supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (#247373 to DT, #25292205 to YK and #22120003 and #24370018 to MU).
Abbreviations
- CA
cold acclimation
- NA
non-acclimation
- PM
plasma membrane
- DRM
detergent-resistant membrane fraction of the plasma membrane
- PC
phosphatidylcholine
- TLC
thin layer chromatography
- FS
free sterol
- SG
sterylglycoside
- ASG
acylated sterylglycoside
- GlcCer
glucocerebroside
- PL
phosholipid
- PE
phosphatidylethanolamine
- PC
phosphatidylcholine
- PG
phosphatidylglycerol
- PA
phosphatidic acid
- PS
phosphatidylserine
- PI
phosphatidylinositol
- DGDG
digalactosyldiacylglycerol
- MGDG
monogalactosyldiacylglycerol
- LysoPE
lyso-phosphatidylethanolamine
- LysoPC
lyso-phosphatidylcholine
- LysoPG
lyso- phosphatidylglycerol
- HII
hexagonal II
- DOPC
dioleoyl-PC
- DOPE
dioleoyl-PE
- UGT
UDP-glucose: sterol glucosyltransferases
- FAH
fatty acid 2-hydroxylase
- PLD
phospholipase D
- d18:1
8-sphingadienine
- d18:2
4, 8-sphingadienine
- t18:1
4-hydroxy-8-sphingenine
- ANOVA
analysis of variance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supporting information
Supplemental Table 1. Sterol compositions in oat and rye during CA.
Supplemental Table 2. GlcCer compositions in oat and rye during CA.
Supplemental Table 3. PLs compositions in oat and rye during CA.
References
- 1.Bagnat M, Chang A, Simons K. Plasma membrane proton ATPase Pma1p requires raft association for surface delivery in yeast. Mol. Biol. Cell. 2001;12:4129–4138. doi: 10.1091/mbc.12.12.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck JG, Mathieu D, Loudet C, Buchoux S, Dufourc EJ. Plant sterols in “rafts”: a better way to regulate membrane thermal shocks. FASEB J. 2007;21:1714–1723. doi: 10.1096/fj.06-7809com. [DOI] [PubMed] [Google Scholar]
- 3.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 4.Borner GHH, Sherrier DJ, Weimar T, Mchaelson LV, Hawkins ND, MacAskill A, Napier JA, Beale MH, Lilley KS, Dupree P. Analysis of detergent-resistant membranes in Arabidopsis: evidence for plasma membrane lipid rafts. Plant Physiol. 2005;137:104–116. doi: 10.1104/pp.104.053041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cantrel C, Vazquez T, Puyaubert J, Rezé N, Lesch M, Kaiser WM, Dutilleul C, Guillas I, Zachowski A, Baudouin E. Nitric oxide participates in cold-responsive phosphosphingolipid formation and gene expression in Arabidopsis thaliana. New Phytol. 2011;189:415–427. doi: 10.1111/j.1469-8137.2010.03500.x. [DOI] [PubMed] [Google Scholar]
- 6.Carmona-Salazar L, El Hafidi M, Enríquez-Arredondo C, Vázquez-Vázquez C, González de la Vara LE, Gavilanes-Ruíz M. Isolation of detergent-resistant membranes from plant photosynthetic and non-photosynthetic tissues. Anal. Biochem. 2011;417:220–227. doi: 10.1016/j.ab.2011.05.044. [DOI] [PubMed] [Google Scholar]
- 7.Carmona-Salazar L, El Hafidi M, Gutiérrez-Nájera N, Noyola-Martínez L, González-Solís A, Gavilanes-Ruíz M. Fatty acid profiles from the plasma membrane and detergent resistant membranes of two plant species. Phytochemistry. 2015;109:25–35. doi: 10.1016/j.phytochem.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 8.Douglass AD, Vale RD. Single-molecule microscopy reveals plasma membrane microdomains created by protein-protein networks that exclude or trap signaling molecules in T cells. Cell. 2005;121:937–950. doi: 10.1016/j.cell.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956;28:350–356. [Google Scholar]
- 10.Dutilleul C, Benhassaine-Kesri G, Demandre C, Rézé N, Launay A, Pelletier S, Renou J-P, Zachowski A, Baudouin E, Guillas I. Phytosphingosine-phosphate is a signal for AtMPK6 activation and Arabidopsis response to chilling. New Phytol. 2012;194:181–191. doi: 10.1111/j.1469-8137.2011.04017.x. [DOI] [PubMed] [Google Scholar]
- 11.Friedrichson T, Kurzchalia TV. Microdomains of GPI-anchored proteins in living cells revealed by crosslinking. Nature. 1998;394:802–805. doi: 10.1038/29570. [DOI] [PubMed] [Google Scholar]
- 12.Furt F, Konig S, Bessoule JJ, Sargueil F, Zallot R, Stanislas T, Noirot E, Lherminier J, Simon-Plas F, Heilmann I, Mongrand S. Polyphosphoinositides are enriched in plant membrane rafts and form microdomains in the plasma membrane. Plant Physiol. 2010;152:2173–2187. doi: 10.1104/pp.109.149823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordon-Kamm WJ, Steponkus PL. Lamellar-to-hexagonalII phase transitions in the plasma membrane of isolated protoplasts after freeze-induced dehydration. Proc. Natl. Acad. Sci. U. S. A. 1984;81:6373–6377. doi: 10.1073/pnas.81.20.6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grandmougin-Ferjani A, Schuler-Muller I, Hartmann M-A. Sterol modulation of the plasma membrane H+-ATPase activity from corn roots reconstituted into soybean lipids. Plant Physiol. 1997;113:163–174. doi: 10.1104/pp.113.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holopainen JM, Subramanian M, Kinnunen PKJ. Sphingomyelinase induces lipid microdomain formation in a fluid phosphatidylcholine/sphingomyelin membrane. Biochemistry. 1998;37:17562–17570. doi: 10.1021/bi980915e. [DOI] [PubMed] [Google Scholar]
- 16.Iba K. Acclimative response to temperature stress in higher plants: approaches of gene engineering for temperature tolerance. Annu. Rev. Plant Biol. 2002;53:225–245. doi: 10.1146/annurev.arplant.53.100201.160729. [DOI] [PubMed] [Google Scholar]
- 17.Imai H, Hattori H, Watanabe M. An improved method for analysis of glucosylceramide species having cis-8 and trans-8 isomers of sphingoid bases by LC–MS/MS. Lipids. 2012;47:1221–1229. doi: 10.1007/s11745-012-3725-7. [DOI] [PubMed] [Google Scholar]
- 18.Imai H, Morimoto Y, Tamura K. Sphingoid base composition of monoglucosylceramide in Brassicaceae. J. Plant Physiol. 2000;157:453–456. [Google Scholar]
- 19.Imai H, Yamamoto K, Shibahara A, Miyatani S, Nakayama T. Determining double-bond positions in monoenoic 2-hydroxy fatty acids of glucosylceramides by gas chromatography-mass spectrometry. Lipids. 2000;35:233–236. doi: 10.1007/BF02664774. [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa M, Yoshida S. Seasonal changes in plasma membranes and mitochondria isolated from Jerusalem artichoke tubers: possible relationship to cold hardiness. Plant Cell Physiol. 1985;26:1331–1344. [Google Scholar]
- 21.Kawaguchi M, Imai H, Naoe M, Yasui Y, Ohnishi M. Cerebrosides in grapevine leaves: distinct composition of sphingoid bases among the grapevine species having different tolerances to freezing temperature. Biosci. Biotechnol. Biochem. 2000;64:1271–1273. doi: 10.1271/bbb.64.1271. [DOI] [PubMed] [Google Scholar]
- 22.Kiessling V, Wan C, Tamm LK. Domain coupling in asymmetric lipid bilayers. Biochim. Biophys. Acta. 2009;1788:64–71. doi: 10.1016/j.bbamem.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laloi M, Perret A-M, Chatre L, Melser S, Cantrel C, Vaultier M-N, Zachowski A, Bathany K, Schmitter J-M, Vallet M, Lessire R, Hartmann M-A, Moreau P. Insights into the role of specific lipids in the formation and delivery of lipid microdomains to the plasma membrane of plant cells. Plant Physiol. 2006;143:461–472. doi: 10.1104/pp.106.091496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee J, Welti R, Roth M, Schapaugh WT, Li J, Trick HN. Enhanced seed viability and lipid compositional changes during natural ageing by suppressing phospholipase Dα in soybean seed. Plant Biotechnol. J. 2012;10:164–173. doi: 10.1111/j.1467-7652.2011.00650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li M, Hong Y, Wang X. Phospholipase D- and phosphatidic acid-mediated signaling in plants. Biochim. Biophys. Acta. 2009;1791:927–935. doi: 10.1016/j.bbalip.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 26.Lingwood D, Simons K. Detergent resistance as a tool in membrane research. Nat. Protoc. 2007;2:2159–2165. doi: 10.1038/nprot.2007.294. [DOI] [PubMed] [Google Scholar]
- 27.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 28.Lynch DV, Steponkus PL. Plasma membrane lipid alterations associated with cold acclimation of winter rye seedlings (Secale cereale L. cv Puma) Plant Physiol. 1987;83:761–767. doi: 10.1104/pp.83.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marinetti GV. Chromatographic separation, identification and analysis of phosphatides. J. Lipid Res. 1962;3:1–12. [Google Scholar]
- 30.Minami A, Fujiwara M, Furuto A, Fukao Y, Yamashita T, Kamo M, Kawamura Y, Uemura M. Alterations in detergent-resistant plasma membrane microdomains in Arabidopsis thaliana during cold acclimation. Plant Cell Physiol. 2009;50:341–359. doi: 10.1093/pcp/pcn202. [DOI] [PubMed] [Google Scholar]
- 31.Mongrand S, Morel J, Laroche J, Claverol S, Carde J-P, Hartmann M-A, Moreau P. Lipid rafts in higher plant cells: purification and characterization of Triton X-100-insoluble microdomains from tobacco plasma membrane. J. Biol. Chem. 2004;279:36277–36286. doi: 10.1074/jbc.M403440200. [DOI] [PubMed] [Google Scholar]
- 32.Nagano M, Takahara K, Fujimoto M, Tsutsumi N, Uchimiya H, Kawai-Yamada M. Arabidopsis sphingolipid fatty acid 2-hydroxylases (AtFAH1 and AtFAH2) are functionally differentiated in fatty acid 2-hydroxylation and stress responses. Plant Physiol. 2012;159:1138–1148. doi: 10.1104/pp.112.199547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ng CK, Carr K, McAinsh MR, Powell B, Hetherington AM. Drought-induced guard cell signal transduction involves sphingosine-1-phosphate. Nature. 2001;410:596–599. doi: 10.1038/35069092. [DOI] [PubMed] [Google Scholar]
- 34.Peskan T, Westermann M, Oelmüller R. Identification of low-density Triton X-100-insoluble plasma membrane microdomains in higher plants. Eur. J. Biochem. 2000;267:6989–6995. doi: 10.1046/j.1432-1327.2000.01776.x. [DOI] [PubMed] [Google Scholar]
- 35.Sankaram MB, Thompson TE. Interaction of cholesterol with various glycerophospholipids and sphingomyelin. Biochemistry. 1990;29:10670–10675. doi: 10.1021/bi00499a014. [DOI] [PubMed] [Google Scholar]
- 36.Schrick K, Shiva S, Arpin JC, Delimont N, Isaac G, Tamura P, Welti R. Steryl glucoside and acyl steryl glucoside analysis of Arabidopsis seeds by electrospray ionization tandem mass spectrometry. Lipids. 2012;47:185–193. doi: 10.1007/s11745-011-3602-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silvius JR. Cholesterol modulation of lipid intermixing in phospholipid and glycosphingolipid mixtures. Evaluation using fluorescent lipid probes and brominated lipid quenchers. Biochemistry. 1992;31:3398–3408. doi: 10.1021/bi00128a014. [DOI] [PubMed] [Google Scholar]
- 38.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 39.Simons K, Sampaio JL. Membrane organization and lipid rafts. Cold Spring Harb. Perspect. Biol. 2011;3:a004697–a004697. doi: 10.1101/cshperspect.a004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972;175:720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- 41.Steponkus PL. Role of the plasma membrane in freezing injury and cold acclimation. Annu. Rev. Plant Physiol. 1984;35:543–584. [Google Scholar]
- 42.Takahashi D, Kawamura Y, Uemura M. Changes of detergent-resistant plasma membrane proteins in oat and rye during cold acclimation: association with differential freezing tolerance. J. Proteome Res. 2013;12:4998–5011. doi: 10.1021/pr400750g. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi D, Kawamura Y, Yamashita T, Uemura M. Detergent-resistant plasma membrane proteome in oat and rye: similarities and dissimilarities between two monocotyledonous plants. J. Proteome Res. 2012;11:1654–1665. doi: 10.1021/pr200849v. [DOI] [PubMed] [Google Scholar]
- 44.Uemura M, Steponkus PL. A contrast of the plasma membrane lipid composition of oat and rye leaves in relation to freezing tolerance. Plant Physiol. 1994;104:479–496. doi: 10.1104/pp.104.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watanabe M, Miyagi A, Nagano M, Kawai-Yamada M, Imai H. Characterization of glucosylceramides in the Polygonaceae, Rumex obtusifolius L. injurious weed. Biosci. Biotechnol. Biochem. 2011;75:877–881. doi: 10.1271/bbb.100802. [DOI] [PubMed] [Google Scholar]
- 46.Webb MS, Irving TC, Steponkus PL. Effects of plant sterols on the hydration and phase behavior of DOPE/DOPC mixtures. Biochim. Biophys. Acta. 1995;1239:226–238. doi: 10.1016/0005-2736(95)00147-u. [DOI] [PubMed] [Google Scholar]
- 47.Webb MS, Irving TC, Steponkus PL. Cerebrosides alter the lyotropic and thermotropic phase transitions of DOPE:DOPC and DOPE:DOPC:sterol mixtures. Biochim. Biophys. Acta. 1997;1326:225–235. doi: 10.1016/s0005-2736(97)00026-6. [DOI] [PubMed] [Google Scholar]
- 48.Webb MS, Uemura M, Steponkus PL. A comparison of freezing injury in oat and rye: two cereals at the extremes of freezing tolerance. Plant Physiol. 1994;104:467–478. doi: 10.1104/pp.104.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Welti R, Li W, Li M, Sang Y, Biesiada H, Zhou H-E, Rajashekar CB, Williams TD, Wang X. Profiling membrane lipids in plant stress responses: role of phospholipase Dα in freezing-induced lipid changes in Arabidopsis. J. Biol. Chem. 2002;277:31994–32002. doi: 10.1074/jbc.M205375200. [DOI] [PubMed] [Google Scholar]
- 50.Worrall D, Ng CK-Y, Hetherington AM. Sphingolipidsm, new players in plant signaling. Trends Plant Sci. 2003;8:317–320. doi: 10.1016/S1360-1385(03)00128-6. [DOI] [PubMed] [Google Scholar]
- 51.Wu J, Browse J. Elevated levels of high-melting-point phosphatidylglycerols do not induce chilling sensitivity in an Arabidopsis mutant. Plant Cell. 1995;7:17–27. doi: 10.1105/tpc.7.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiao S, Gao W, Chen Q-F, Chan S-W, Zheng S-X, Ma J, Wang M, Welti R, Chye M-L. Overexpression of Arabidopsis acyl-CoA binding protein ACBP3 promotes starvation-induced and age-dependent leaf senescence. Plant Cell. 2010;22:1463–1482. doi: 10.1105/tpc.110.075333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu X, Bittman R, Duportail G, Heissler D, Vilcheze C, London E. Effect of the structure of natural sterols and sphingolipids on the formation of ordered sphingolipid/sterol domains (rafts): comparison of cholesterol to plant, fungal, and disease-associated sterols and comparison of sphingomyelin, cerebrosides, and ceramide. J. Biol. Chem. 2001;276:33540–33546. doi: 10.1074/jbc.M104776200. [DOI] [PubMed] [Google Scholar]
- 54.Zacharias DA, Violin JD, Newton AC, Tsien RY. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science. 2002;296:913–916. doi: 10.1126/science.1068539. [DOI] [PubMed] [Google Scholar]
- 55.Zlatkis A, Zak B. Study of a new cholesterol reagent. Anal. Biochem. 1969;29:143–148. doi: 10.1016/0003-2697(69)90017-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.