Abstract
Purpose of Review
Hematopoietic stem cells (HSCs) are a population of cells in the bone marrow (BM) which can self-renew, differentiate into late lineage progenitors, or remain quiescent. HSCs exist alongside several cell types in the BM microenvironment which comprise the stem cell niche. These cells regulate HSC function and can contribute to leukemogenesis. In this review we will discuss recent advances in this field.
Recent Findings
In the vascular niche, arteriolar and sinusoidal zones appear to play distinct roles in HSC function. Endothelial cells modulate HSC function via Notch and other signaling pathways. In the endosteal niche multiple cell types regulate HSCs. Osteoblasts promote HSC quiescence via secreted factors and possibly physical interactions, while adipocytes may oppose HSC quiescence. The balance of these opposing factors depends on metabolic cues. Feedback from HSC-derived cells including macrophages and megakaryocytes also appear to regulate HSC quiescence. Dysfunction of the BM microenvironment including MSC-derived stromal cells and the sympathetic nervous system can induce or alter the progression of hematologic malignancies.
Summary
Many cell types in the BM microenvironment affect HSC function and contribute to malignancy. Further understanding how HSCs are regulated by the microenvironment has clinical implications for stem cell transplantation and other therapies for hematologic malignancies.
Keywords: Hematopoietic stem cell, Niche, Microenvironment, Leukemogenesis
Introduction
Renewal of blood cells depends on hematopoietic stem cells (HSCs), a self-renewing population of cells in the bone marrow (BM) which give rise to late lineage progenitors and ultimately terminally differentiated mature cells. Differentiation of HSCs must be balanced against preservation of HSC capacity for self-renewal and quiescence to maintain adequate supplies of blood cells throughout the life of the organism. Failure to achieve this balance can give rise to stem cell exhaustion and BM failure or hematological disorders.
HSCs exist in the BM along with several other cell types which collectively comprise the BM microenvironment. Regulation of HSC function by microenvironmental factors including vascular anatomy and signaling from other cell types is a growing area of research. In this review we discuss recent advancements in our understanding of microenvironmental effects on normal HSC function and leukemogenesis.
The Vascular Niche
The BM microenvironment is highly vascularized with arterioles and capillaries which drain into densely organized sinusoids [1]. Arterioles are associated with more sympathetic nerve fibers and smooth muscle cells than are sinusoids [1], and arteriolar and venous endothelial cells have differing gene expression profiles [2]. In addition to vascular endothelial cells [3], blood vessels in the BM are closely associated with perivascular cells which regulate HSC function including nonmyelinating Schwann cells [4], Nestin+ mesenchymal stem cells (MSCs) [5], Leptin receptor-expressing (LepR+) cells [6], and cells expressing stem cell factor (Scf) [7].
How the unique properties of arterioles and sinusoids relate to HSC distribution and quiescence is controversial. Although HSCs are seen in close proximity to sinusoids more often than to arterioles, this may be due to the dense, regular distribution of sinusoids rather than a true association. A 2013 study showed that HSC proximity to sinusoids was statistically indistinguishable from random distribution, while HSCs were significantly more proximal to arterioles than predicted by random distribution [1]. In the same study, the authors showed that mesenchymal stem cells (MSCs) expressing Nestin-GFP at high levels and the pericyte marker NG2 were located exclusively along arterioles, and depletion of these cells induced HSC mobilization. Nestin+ MSCs have previously been shown to be an important component of the HSC niche [5, 8]. These results suggest that HSCs are spatially associated with arterioles and that the arteriolar niche promotes HSC quiescence.
Another study examining HSC proximity to blood vessels showed different results. The authors again found that absolute distances of HSCs to sinusoids did not differ from random distribution, but HSCs being closer to sinusoids than to other vessel types was seen more frequently than predicted by random spots [9**]. This was the case for both dividing and non-dividing HSCs. In the same study, the authors found that perisinusoidal LepR+ cells, rather than NG2+Nestinhigh cells, were spatially associated with HSCs and secreted Scf and CXCL12, both of which promote HSC quiescence [7, 10]. This is consistent with a previous study which showed that Scf is expressed by perivascular stromal and vascular endothelial cells, particularly those surrounding sinusoids, and that deletion of Scf from endothelial cells or LepR+ cells significantly decreased BM HSC populations and repopulating ability in transplantation studies but had no effect when deleted from osteoblasts and Nestin+ stromal cells [11]. Together, these results suggest that the peri-sinusoidal zone creates a niche that promotes HSC quiescence.
Further characterization of factors surrounding the arteriolar and sinusoidal niches may help clarify these disparate findings. Hypoxia is thought to be an important contributor to stem cell quiescence [12]. There is evidence that the peri-sinusoidal zone is more hypoxic than other areas of the BM, with a 2014 study showing greater local oxygen concentrations and Nestin expression near arterioles [13]. It is possible that using stromal Nestin expression as a marker for the HSC niche may be problematic, however, as Nestin transgene expression may vary regionally across different populations of perivascular stromal cells in the BM [11].
Endothelial cells also regulate HSCs in the vascular niche via multiple mechanisms in addition to Scf secretion. E-selectin expressed by BM endothelial cells promotes HSC cycling and proliferation at the expense of population maintenance, and its knockout or pharmacologic blockade promotes HSC retention in the vascular niche [14]. Endothelial Jagged-1-Notch signaling regulates HSC cycling as well. Previous studies indicate that Notch activation in HSCs promotes HSC population maintenance [15, 16] and expansion after pharmacologic insult [17]. Endothelial cell-specific knockout of Jagged-1, a ligand for Notch pathway receptors, decreased populations of long-term HSCs (LT-HSCs) in the BM, increased HSC cell cycling, and led to premature exhaustion of HSCs after sublethal irradiation [18]. While endothelial E-selectin and Jagged-1 expression are important regulators of HSC function, their relative expression levels by arteriolar and sinusoidal endothelial cells is not well established. Recent evidence and the association of hypoxia and Scf expression in the sinusoidal zone seem to suggest that it promotes HSC quiescence, however, in light of contradictory evidence further studies are needed to more accurately define the HSC vascular niche.
The Endosteal Niche
The endosteal niche is also thought to be an important site of HSC quiescence. Several mesenchymal stem cell (MSC)-derived cell types in this niche regulate HSC function including CXCL12-abundant reticular (CAR) cells [19], osteoblasts [15], and spindle-shaped N-cadherin+CD45− osteoblastic (SNO) cells [20].
CXCL12 is expressed in multiple stromal cell types and is an important regulator of HSC retention through its receptor CXCR4 [21]. Selective deletion of CXCL12 from CAR cells, osteoblasts, endothelial cells, and Nestin+ mesenchymal progenitors resulted in loss of HSC quiescence or repopulating ability [10], and its expression in Nestin+ MSCs is important in establishing the HSC niche during development [8]. CAR cells, a population of osterix-expressing adipo-osteogenic progenitor cells found in the endosteal and perivascular niches, are a major source of Scf and CXCL12 in the BM and are important in maintaining HSC populations in an undifferentiated state [22]. Recent studies have shown that the transcription factor Foxc1 is important in promoting CAR expression of Scf and CXCL12 and inhibiting adipogenic processes in CAR progenitors; deletion of Foxc1 in murine mesenchymal cells resulted in increased BM adipocyte populations, depletion of CAR cells, decreased Scf and CXCL12, and decreased HSC populations in vivo [23].
Other studies have further demonstrated the importance of relative populations of osteoblasts and adipocytes in HSC function. A 2015 study showed that high fat diet alters gut microbiota, which increased the population of adipocytes and decreased the population of osteoblasts in the BM [24**]. This change in populations of MSC-derived cells, mediated by PPARγ, was associated with increased LT-HSC cell cycling, increase in multipotent progenitors, and diminished hematologic recovery following pharmacologic myelosuppression. Increased levels of leptin in the BM were seen in high fat diet-fed mice with decreased levels of Jagged-1, Notch2, Tie2, and CXCL12. These results are consistent with previous observations that leptin promotes lymphopoiesis and myelopoiesis [25].
Osteoblasts regulate HSC function directly or indirectly through several mechanisms including promotion of HSC quiescence via Jagged-1/Notch [15], angiopoietin-1/Tie2 [26], thrombopoietin [27], and osteopontin [28]. Consistent with the above studies, a recent study showed that ablation of osteoblasts in adult mice resulted in increased cell cycling of HSCs and decreased repopulating capacity in transplantation studies [29]. Together, these studies suggest that MSC-derived CAR cells and osteoblasts promote HSC maintenance while adipocytes and leptin promote HSC cycling and differentiation. The finding that MSC fate can be influenced by the metabolic environment of the organism, and that this fate can have significant effects on HSC quiescence, may be clinically relevant to many disease processes. Underscoring this idea is the observation that obesity may be a risk factor for hematologic malignancies [30].
In addition to secreted factors, osteoblasts may regulate HSC quiescence and retention in the BM via direct interactions with cadherins [31]. Previous reports suggest that LT-HSCs localize to SNO cells via N-cadherin [20]. The importance of these interactions in maintaining HSC quiescence is unclear, however, as studies have yielded conflicting results. Some studies suggest that N-cadherin expression in HSCs promotes quiescence via decreased β-catenin signaling [32], that knockdown of N-cadherin in HSCs is associated with increased cycling [32], and that HSCs home to N-cadherin+ osteoblasts on transplantation into irradiated hosts [33]. Other studies have shown that Cdh2 ablation in hematopoietic or osteolineage cells has no discernable effects on hematopoiesis [34, 35]. However, these studies do not exclude the possibility of compensatory functions of other cell adhesion molecules (CAMs) in the setting of N-cadherin deficiency.
Studies of other adhesion proteins have supported the role of direct cell-cell interactions in mediating HSC quiescence in the endosteal niche. Wnt signaling has been shown to be important in regulating HSC function, with a shift from canonical to non-canonical Wnt signaling being associated with increased HSC self-renewal and decreased repopulating ability [36]. Wnt regulation of HSCs appears to be mediated in part through its effects on HSC adhesion in the niche. Two downstream effectors of Wnt, β-catenin and the phosphatase PTPN13, are important in this process [37, 38*]. Knockdown of these effectors in murine Lin− cells increased the fraction of LT-HSCs, increased HSC and progenitor adhesion in the BM, decreased cell cycling and proliferation, and was associated with upregulation of several CAM genes (ITGA4, CDH1, CDH12, NCAM2, and RELN) [38*]. Notably, this effect was seen in vivo in transplantations studies and in co-culture with MSCs, but not in isolated Lin− cultures or co-cultures with MSCs in transwell devices, demonstrating the importance of physical interactions of HSCs with niche stromal cells. In the same study the authors showed that PTPN13 and β-catenin levels as well as CAM expression in HEL cells were modulated by TPO, CXCL12, and Scf, suggesting that these factors may act at least in part through cell adhesion.
While the importance of MSC-derived cells in the endosteal niche is well established, there is growing evidence that feedback from HSC-derived cells themselves is important in regulating HSC function. Endosteal macrophages play a role in supporting the HSC niche and maintaining local osteoblast populations [39] and can promote erythropoiesis in the setting of hemolytic anemia by interactions with erythroid precursors via the adhesion molecule VCAM-1 [40]. More recent studies have also suggested that megakaryocytes in the endosteal niche have dynamic effects on HSCs. In one study, megakaryocytes promoted HSC quiescence by secreting CXCL4 [41]. A separate study showed that megakaryocytes can both promote quiescence via secretion of transforming growth factor β1 (TGF-β1) under homeostatic conditions and promote HSC expansion and cycling after pharmacologic myelosuppression via increased expression of fibroblast growth factor-1 (FGF-1) [42].
The Nervous System
There is mounting evidence for the important role the sympathetic nervous system plays in regulation of HSCs in the BM microenvironment. Studies have shown that HSCs express catecholamine receptors [43] and that β2-adrenergic activity promotes HSC motility and proliferation [44]. Circadian variations in norepinephrine secretion and β3-adrenergic activity in BM stromal cells also appear to mediate HSC mobilization via downregulation of CXCL12 [45]. In addition to catecholaminergic signaling by neurons, glial fibrillary acidic protein (GFAP)-expressing nonmyelinating Schwann cells induce HSC quiescence by activating latent TGF-β in the niche, which promotes intracellular Smad activity in HSCs [4]. Neuropeptide Y also appears to be important in maintaining sympathetic nerve fibers and HSC populations in the BM, likely through modulation of BM macrophage activity [46]. The importance of the nervous system in regulating HSC function has become increasingly clear in recent years as studies have shown associations between neuropathy and progression of hematologic malignancies.
Microenvironmental factors contributing to malignancy
Hematologic malignancies arise from a leukemogenic mutation of a stem cell or progenitor creating a subset of self-renewing leukemic stem cells (LSCs) in the BM which are responsible for the generation of bulk leukemic blasts [47]. There is growing evidence that the BM microenvironment is important in leukemogenesis and in regulating LSC function. In 2007 two studies showed that microenvironmental dysfunction is capable of inducing myeloproliferation or neoplastic transformation by extrinsic influences on HSCs through retinoblastoma inactivation [48] and retinoic acid receptor gamma deletion [49] in the microenvironment. Recent studies have elaborated on this idea and revealed several mechanisms by which the microenvironment can induce or alter the natural history of hematologic malignancies.
Just as obstetrics-expressing osteolineage cells play critical roles in regulating normal HSC quiescence and proliferation, they are also important in malignancy. In one study, deletion of the microRNA endonuclease Dicer1 in osteolineage cells with Osx-GFP-Cre disrupted progenitor differentiation into osteoblasts and was associated with myelodysplasia and neoplastic disease [50]. Another study showed that constitutive β-catenin activation in osteoblasts led to increased Jagged-1 expression in osteoblasts and Notch activation in HSCs, which induced leukemia [51]. The transcription factor FoxO1 was important in downstream signaling of the constitutively active β-catenin [52]. While these studies illustrate the role of Notch activity in HSCs in leukemogenesis, other studies have shown that decreased Notch activity in BM stromal cells similarly promote malignancy. In one such study deletion of the Notch downstream effector RBPJ in BM stromal cells with Mx1-Cre or endothelial cells with Tie2-Cre induced myeloid cell mobilization and myeloproliferative disease via upregulation of microRNA-155 and NF-κB activation, which were associated with increased granulocyte colony stimulating factor (G-CSF) and tumor necrosis factor α (TNFα) expression in stromal cells [53].
Osteoblasts can modulate disease progression in the setting of existing malignancy as well. In one study, myeloproliferative neoplasm (MPN) development in a mouse model of CML induced expansion of abnormal osteoblasts which overexpressed TGFβ and inflammatory cytokines and showed attenuated Notch expression, which was associated with impaired functioning of normal HSCs but not of LSCs [54]. Osteoblast ablation in a mouse model of chronic myelogenous leukemia (CML) led to accelerated disease progression [29], suggesting that complete loss of osteoblast activity, including Jagged-1 signaling, in the BM contributes to leukemic cell expansion. Interestingly, a recent study also showed that osteoblasts promote dormancy in myeloma cells while osteoclasts reverse this effect [55*]. This is clinically relevant as it may partly explain the therapeutic effect of bisphosphonates in multiple myeloma [55*].
Together these findings indicate that balanced osteoblastic function is essential in maintaining normal HSC function, and that aberrant overactivity or absence of osteoblastic input promotes hematologic disease. The multiple roles of osteoblasts in hematologic diseases are illustrated by the findings that increased TGF-β production and activation of osteoblasts by constitutively active osteoblast parathyroid hormone receptors attenuates BCR-ABL1-induced MPN but enhances MLL-AF9-induced AML in mouse transplantation models using human cell lines [56]. In addition to evidence supporting the importance of endothelial cells and other cells in the BM in disease progression, these results suggest that microenvironmental stromal cells could be an important target for therapy.
Several recent studies have also illustrated the impact of BM nervous system dysfunction in hematologic malignancies. One study showed that MLL-AF9-induced AML induced sympathetic neuropathy in the BM, which was associated with increased leukemic infiltration into the BM and proliferation and differentiation of Nestin-GFP+ cells toward the osteoblast lineage. Blockade of β2-adrenergic tone in these mice was associated with greater leukemic cell proliferation and reduced host survival [57]. Another study examining JAK2(V617F)-induced MPN showed that mutant HSCs damaged the BM sympathetic nervous system including Schwann cells by overproduction of interleukin-1β, which was associated with depletion of Nestin-GFP+ cells and MPN progression [58]. Importantly, in this study β3-adrenergic agonists restored Nestin-GFP+ cell populations and prevented MPN progression, illustrating the importance of BM sympathetic tone in maintaining a healthy HSC microenvironment. These studies show that sympathetic tone is an important regulator of BM stromal cells as well as HSCs directly, and that neuropathy can contribute to leukemia progression by multiple mechanisms.
Conclusion
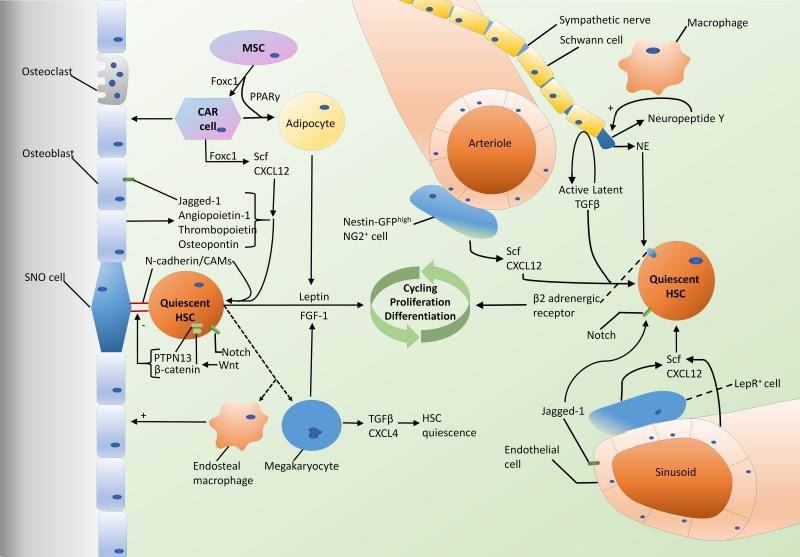
Microenvironmental regulation of HSC quiescence, self-renewal, and mobilization is highly complex (see the graphic abstract in Figure 1). There are in fact multiple microenvironments within the BM with distinct mechanistic and anatomic relationships to HSCs, and several of these relationships remain controversial. The HSC microenvironment is a growing area of research and in recent years there have been great advancements in our understanding of HSC regulation in healthy as well as disease states. Better understanding of microenvironmental contributions to malignancy would have important clinical implications, as microenvironmental dysfunction can affect engraftment of healthy donor stem cells or give rise to donor-derived hematologic malignancies following stem cell transplantation. The vascular and endosteal niches, nervous system, cell signaling pathways, and metabolic influences may provide many opportunities for intervention in hematologic malignancies.
Figure 1. The BM HSC microenvironment.
In the osteoblastic niche near the bone surface several stromal cells as well as HSC-derived cells regulate HSC function. Scf, CXCL12, and several other secreted factors from stromal cells, in addition to direct interactions via CAMs promote HSC quiescence. Wnt effectors PTPN13 and β-catenin and signals from adipocytes and megakaryotes including leptin and FGF-1, respectively, may promote HSC differentiation and expansion. In the vascular niche there is controversy over the roles of arteriolar and sinusoidal zones, with evidence supporting the association of both with the quiescent HSC niche. Endothelial cells, sympathetic nerves, Schwann cells, macrophages, LepR+ cells, and Nestinhigh NG2+ stromal cells all play a role in regulating HSC function via multiple mechanisms including Scf and CXCL12 secretion, catecholaminergic signaling, TGFβ, and the Notch pathway.
Key Points.
The BM microenvironment plays an essential role in the regulation of HSC self-renewal, differentiation, and maintenance.
Different niches regulate different sub-populations of HSCs.
Aberrant niches cause various blood disorders, including hematological malignancies.
Acknowledgements
The authors wish to extend their apologies to the many colleagues whose work could not be cited due to space limitations. This work was supported by The National Institutes of Health grant HL068212 (to C.K.Q.).
Footnotes
Conflicts of Interest
There are no conflicts of interest.
References and Recommended Reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, Mizoguchi T, Wei Q, Lucas D, Ito K, Mar JC, Bergman A, Frenette PS. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502(7473):637–643. doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chi JT, et al. Endothelial cell diversity revealed by global expression profiling. Proc Natl Acad Sci U S A. 2003;100:10623–10628. doi: 10.1073/pnas.1434429100. 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiel MJ, Yilmaz O H, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 4.Yamazaki S, Ema H, Karlsson G, Yamaguchi T, Miyoshi H, Shioda S, Taketo MM, Karlsson S, Iwama A, Nakauchi H. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell. 2011;147(5):1146–1158. doi: 10.1016/j.cell.2011.09.053. [DOI] [PubMed] [Google Scholar]
- 5.Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma'ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou BO, Yue R, Murphy MM, Peyer JG, Morrison SJ. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 2014;15:154–168. doi: 10.1016/j.stem.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oguro H, Ding L, Morrison SJ. SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors. Cell Stem Cell. 2013;13:102–116. doi: 10.1016/j.stem.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isern J, García-García A, Martín AM, Arranz L, Martín-Pérez D, Torroja C, Sánchez-Cabo F, Méndez-Ferrer S. The Neural Crest Is a Source of Mesenchymal Stem Cells with Specialized Hematopoietic Stem Cell Niche Function. ELife. 2014;3(2014):E03696. doi: 10.7554/eLife.03696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9**.Acar M, Kocherlakota KS, Murphy MM, Peyer JG, Oguro H, Inra CN, Jaiyeola C, Zhao Z, Luby-Phelps K, Morrison SJ. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature. 2015;526(7571):126–130. doi: 10.1038/nature15250. [This study suggests that quiescent HSCs are more closely associated with BM sinusoids rather than with arterioles and that LepR+ cells play an important role in maintaining HSC quiescence, in contrast to previous studies.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenbaum A, Hsu YM, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN, Nagasawa T, Link DC. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495(7440):227–230. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding L, Saunders T, Enikolopov G, Morrison S. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481(7382):457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suda T, Takubo K, Semenza GL. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell. 2011 doi: 10.1016/j.stem.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Spencer JA, Ferraro F, Roussakis E, Klein A, Wu J, Runnels JM, Zaher W, Mortensen LJ, Alt C, Turcotte R, Yusuf R, Côté D, Vinogradov SA, Scadden DT, Lin CP. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014;508(7495):269–273. doi: 10.1038/nature13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winkler IG, Barbier V, Nowlan B, Jacobsen RN, Forristal CE, Patton JT, Magnani JL, Lévesque JP. Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nature medicine. 2012;11:1651–1657. doi: 10.1038/nm.2969. [DOI] [PubMed] [Google Scholar]
- 15.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;6960:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 16.Duncan AW, Rattis FM, DiMascio LN, Congdon KL, Pazianos G, Zhao C, Yoon K, Cook JM, Willert K, Gaiano N, Reya T. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nature Immunology. 2005;6(3):314–322. doi: 10.1038/ni1164. [DOI] [PubMed] [Google Scholar]
- 17.Butler JM, Nolan DJ, Vertes EL, Varnum-Finney B, Kobayashi H, Hooper AT, Seandel M, Shido K, White IA, Kobayashi M, Witte L, May C, Shawber C, Kimura Y, Kitajewski J, Rosenwaks Z, Bernstein ID, Rafii S. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6(3):251–264. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poulos MG, Guo P, Kofler NM, Pinho S, Gutkin MC, Tikhonova A, Aifantis I, Frenette PS, Kitajewski J, Rafii S, Butler JM. Endothelial Jagged-1 is necessary for homeostatic and regenerative hematopoiesis. Cell Reports. 2013;4(5):1022–1034. doi: 10.1016/j.celrep.2013.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25(6):977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425(6960):836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 21.Ara T, Tokoyoda K, Sugiyama T, Egawa T, Kawabata K, Nagasawa T. Long-term hematopoietic stem cells require stromal cell-derived factor-1 for colonizing bone marrow during ontogeny. Immunity. 2003;19(2):257–267. doi: 10.1016/s1074-7613(03)00201-2. [DOI] [PubMed] [Google Scholar]
- 22.Omatsu Y, Sugiyama T, Kohara H, Kondoh G, Fujii N, Kohno K, Nagasawa T. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010;33(3):387–399. doi: 10.1016/j.immuni.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 23.Omatsu Y, Seike M, Sugiyama T, Kume T, Nagasawa T. Foxc1 is a critical regulator of haematopoietic stem/progenitor cell niche formation. Nature. 2013;508(7497):536–540. doi: 10.1038/nature13071. [DOI] [PubMed] [Google Scholar]
- 24**.Luo Y, Chen GL, Hannemann N, Ipseiz N, Krönke G, Bäuerle T, Munos L, Wirtz S, Schett G, Bozec A. Microbiota from Obese Mice Regulate Hematopoietic Stem Cell Differentiation by Altering the Bone Niche. Cell Metabolism. 2015;22(5):886–894. doi: 10.1016/j.cmet.2015.08.020. [This study shows that metabolic cues influence BM microenvironmental stromal cell populations and that increased MSC differentiation toward adipocytes rather than osteoblasts induced by high fat diet can disrupt HSC quiescence.] [DOI] [PubMed] [Google Scholar]
- 25.Claycombe K, King L, Fraker P. A role for leptin in sustaining lymphopoiesis and myelopoiesis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(6):2017–2021. doi: 10.1073/pnas.0712053105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118(2):149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Yoshihara H, Arai F, Hosokawa K, Hagiwara T, Takubo K, Nakamura Y, Gomei Y, Iwasaki H, Matsuoka S, Miyamoto K, Miyazaki H, Takahashi T, Suda T. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell. 2007;1(6):685–697. doi: 10.1016/j.stem.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 28.Nilsson SK, Johnston HM, Whitty GA, Williams B, Webb RJ, Denhardt DT, Bertoncello I, Bendall LJ, Simmons PJ, Haylock DN. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood. 2005;4:1232–1239. doi: 10.1182/blood-2004-11-4422. [DOI] [PubMed] [Google Scholar]
- 29.Bowers M, Zhang B, Ho Y, Agarwal P, Chen C, Bhatia R. Osteoblast ablation reduces normal long-term hematopoietic stem cell self-renewal but accelerates leukemia development. Blood. 2015;125(17):2678–2688. doi: 10.1182/blood-2014-06-582924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poynter JN, Richardson M, Blair CK, Roesler MA, Hirsch BA, Nguyen P, Cioc A, Warlick E, Cerhan JR, Ross JA. Obesity over the life course and risk of acute myeloid leukemia and myelodysplastic syndromes. Cancer epidemiology. 2015:134–140. doi: 10.1016/j.canep.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wein F, Pietsch L, Saffrich R, Wuchter P, Walenda T, Bork S, Horn P, Diehlmann A, Eckstein V, Ho AD, Wagner W. N-cadherin is expressed on human hematopoietic progenitor cells and mediates interaction with human mesenchymal stromal cells. Stem Cell Res. 2010;4(2):129–139. doi: 10.1016/j.scr.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Hosokawa K, Arai F, Yoshihara H, Iwasaki H, Nakamura Y, Gomei Y, Suda T. Knockdown of N-cadherin suppresses the long-term engraftment of hematopoietic stem cells. Blood Journal of the American Society of Hematology. 2010;116(4):554–563. doi: 10.1182/blood-2009-05-224857. [DOI] [PubMed] [Google Scholar]
- 33.Xie Y, Yin T, Wiegraebe W, He XC, Miller D, Stark D, Perko K, Alexander R, Schwartz J, Grindley JC, Park J, Haug JS, Wunderlich JP, Li H, Zhang S, Johnson T, Feldman RA, Li L. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature. 2009;457(7225):97–101. doi: 10.1038/nature07639. [DOI] [PubMed] [Google Scholar]
- 34.Kiel M, Radice G, Morrison S. Lack of evidence that hematopoietic stem cells depend on N-cadherin-mediated adhesion to osteoblasts for their maintenance. Cell Stem Cell. 2007;1(2):204–217. doi: 10.1016/j.stem.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Greenbaum A, Revollo L, Woloszynek J, Civitelli R, Link D. N-cadherin in osteolineage cells is not required for maintenance of hematopoietic stem cells. Blood Journal of the American Society of Hematology. 2012;120(2):295–302. doi: 10.1182/blood-2011-09-377457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Florian MC, Nattamai KJ, Dörr K, Marka G, Uberle B, Vas V, Eckl C, Andrä I, Schiemann M, Oostendorp RA, Scharffetter-Kochanek K, Kestler HA, Zheng Y, Geiger H. A canonical to non-canonical Wnt signalling switch in haematopoietic stem-cell ageing. Nature. 2013;7476:392–396. doi: 10.1038/nature12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sardina JL, López-Ruano G, Prieto-Bermejo R, Sánchez-Sánchez B, Pérez-Fernández A, Sánchez-Abarca LI, Pérez-Simón JA, Quintales L, Sánchez-Yagüe J, Llanillo M, Antequera F, Hernández-Hernández A. PTPN13 regulates cellular signalling and β-catenin function during megakaryocytic differentiation. Biochimica Et Biophysica Acta. : International Journal of Biochemistry and Biophysics. 2014;1843(12):2886–2899. doi: 10.1016/j.bbamcr.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 38*.López-Ruano G, Prieto-Bermejo R, Ramos TL, San-Segundo L, Sánchez-Abarca LI, Sánchez-Guijo F, Pérez-Simón JA, Sánchez-Yagüe J, Llanillo M, Hernández-Hernández Á . PTPN13 and β-Catenin Regulate the Quiescence of Hematopoietic Stem Cells and Their Interaction with the Bone Marrow Niche. Stem Cell Reports. 2015;5(4):516–531. doi: 10.1016/j.stemcr.2015.08.003. [This study demonstrates the importance of downstream effectors of the Wnt signaling pathway and of physical interactions between HSCs and neighboring stromal cells in leukemogenesis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winkler IG, Sims NA, Pettit AR, Barbier V, Nowlan B, Helwani F, Poulton IJ, van Rooijen N, Alexander KA, Raggatt LJ, Lévesque JP. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;23:4815–4828. doi: 10.1182/blood-2009-11-253534. [DOI] [PubMed] [Google Scholar]
- 40.Chow A, Huggins M, Ahmed J, Hashimoto D, Lucas D, Kunisaki Y, Pinho S, Leboeuf M, Noizat C, van Rooijen N, Tanaka M, Zhao ZJ, Bergman A, Merad M, Frenette PS. CD169â+ macrophages provide a niche promoting erythropoiesis under homeostasis and stress. Nature medicine. 2013;4:429–436. doi: 10.1038/nm.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bruns I, Lucas D, Pinho S, Ahmed J, Lambert MP, Kunisaki Y, Scheiermann C, Schiff L, Poncz M, Bergman A, Frenette PS. Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nature medicine. 2014;11:1315–1320. doi: 10.1038/nm.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao M, Perry JM, Marshall H, Venkatraman A, Qian P, He XC, Ahamed J, Li L. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nature medicine. 2014;11:1321–1326. doi: 10.1038/nm.3706. [DOI] [PubMed] [Google Scholar]
- 43.Spiegel A, Shivtiel S, Kalinkovich A, Ludin A, Netzer N, Goichberg P, Azaria Y, Resnick I, Hardan I, Ben-Hur H, Nagler A, Rubinstein M, Lapidot T. Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling. Nature immunology. 2007;10:1123–1131. doi: 10.1038/ni1509. [DOI] [PubMed] [Google Scholar]
- 44.Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;2:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 45.Méndez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;7186:442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 46.Park MH, Jin HK, Min WK, Lee WW, Lee JE, Akiyama H, Herzog H, Enikolopov GN, Schuchman EH, Bae JS. Neuropeptide Y regulates the hematopoietic stem cell microenvironment and prevents nerve injury in the bone marrow. The EMBO journal. 2015;12:1648–1660. doi: 10.15252/embj.201490174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature medicine. 1997;3(7):730–7. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 48.Walkley CR, Shea JM, Sims NA, Purton LE, Orkin SH. Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell. 2007;6:1081–1095. doi: 10.1016/j.cell.2007.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walkley CR, Olsen GH, Dworkin S, Fabb SA, Swann J, McArthur GA, Westmoreland SV, Chambon P, Scadden DT, Purton LE. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency. Cell. 2007;6:1097–1110. doi: 10.1016/j.cell.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raaijmakers MH, Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA, Ebert BL, Al-Shahrour F, Hasserjian RP, Scadden EO, Aung Z, Matza M, Merkenschlager M, Lin C, Rommens JM, Scadden DT. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010;7290:852–857. doi: 10.1038/nature08851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kode A, Manavalan JS, Mosialou I, Bhagat G, Rathinam CV, Luo N, Khiabanian H, Lee A, Murty VV, Friedman R, Brum A, Park D, Galili N, Mukherjee S, Teruya-Feldstein J, Raza A, Rabadan R, Berman E, Kousteni S. Leukaemogenesis induced by an activating β-catenin mutation in osteoblasts. Nature. 2014;7487:240–244. doi: 10.1038/nature12883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kode A, Mosialou I, Manavalan SJ, Rathinam CV, Friedman RA, Teruya-Feldstein J, Bhagat G, Berman E, Kousteni S. FoxO1-dependent induction of acute myeloid leukemia by osteoblasts in mice. Leukemia. 2015;1:1–13. doi: 10.1038/leu.2015.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang L, Zhang H, Rodriguez S, Cao L, Parish J, Mumaw C, Zollman A, Kamoka MM, Mu J, Chen DZ, Srour EF, Chitteti BR, HogenEsch H, Tu X, Bellido TM, Boswell HS, Manshouri T, Verstovsek S, Yoder MC, Kapur R, Cardoso AA, Carlesso N. Notch-dependent repression of miR-155 in the bone marrow niche regulates hematopoiesis in an NF-κB-dependent manner. Cell stem cell. 2014;1:51–65. doi: 10.1016/j.stem.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schepers K, Pietras EM, Reynaud D, Flach J, Binnewies M, Garg T, Wagers AJ, Hsiao EC, Passegué E. Myeloproliferative neoplasia remodels the endosteal bone marrow niche into a self-reinforcing leukemic niche. Cell stem cell. 2013;3:285–299. doi: 10.1016/j.stem.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55*.Lawson MA, McDonald MM, Kovacic N, Hua Khoo W, Terry RL, Down J, Kaplan W, Paton-Hough J, Fellows C, Pettitt JA, Neil Dear T, Van Valckenborgh E, Baldock PA, Rogers MJ, Eaton CL, Vanderkerken K, Pettit AR, Quinn JM, Zannettino AC, Phan TG, Croucher PI. Osteoclasts control reactivation of dormant myeloma cells by remodeling the endosteal niche. Nature communications. 2015;8983 doi: 10.1038/ncomms9983. [This study suggests that osteoblasts can promote dormancy in myeloma cells and that this state can be reversed by osteoclasts, demonstrating a clinically important role of microenvironmental stromal cells in modulating disease course.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krause DS, Fulzele K, Catic A, Sun CC, Dombkowski D, Hurley MP, Lezeau S, Attar E, Wu JY, Lin HY, Divieti-Pajevic P, Hasserjian RP, Schipani E, Van Etten RA, Scadden DT. Differential regulation of myeloid leukemias by the bone marrow microenvironment. Nature medicine. 2013;11:1513–1517. doi: 10.1038/nm.3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hanoun M, Zhang D, Mizoguchi T, Pinho S, Pierce H, Kunisaki Y, Lacombe J, Armstrong SA, Dührsen U, Frenette PS. Acute myelogenous leukemia-induced sympathetic neuropathy promotes malignancy in an altered hematopoietic stem cell niche. Cell stem cell. 2014;3:365–375. doi: 10.1016/j.stem.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arranz L, Sánchez-Aguilera A, Martín-Pérez D, Isern J, Langa X, Tzankov A, Lundberg P, Muntión S, Tzeng YS, Lai DM, Schwaller J, Skoda RC, Méndez-Ferrer S. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature. 2014;7512:78–81. doi: 10.1038/nature13383. [DOI] [PubMed] [Google Scholar]