Abstract
Background
Obesity, physical inactivity and poor diet quality have been associated with increased risk of breast cancer-specific and all-cause mortality as well as treatment-related side-effects in breast cancer survivors. Weight loss intervention trials in breast cancer survivors have shown that weight loss is safe and achievable; however, few studies have examined the benefits of such interventions on a broad range of outcomes and few have examined factors important to translation (e.g. feasible delivery method for scaling up, assessment of sustained changes, cost-effectiveness). The Living Well after Breast Cancer randomized controlled trial aims to evaluate a 12-month telephone-delivered weight loss intervention (versus usual care) on weight change and a range of secondary outcomes including cost-effectiveness.
Methods/design
Women (18–75 years; body mass index 25–45 kg/m2) diagnosed with stage I-III breast cancer in the previous 2 years are recruited from public and private hospitals and through the state-based cancer registry (target n = 156). Following baseline assessment, participants are randomized 1:1 to either a 12-month telephone-delivered weight loss intervention (targeting diet and physical activity) or usual care. Data are collected at baseline, 6-months (mid-intervention), 12-months (end-of-intervention) and 18-months (maintenance). The primary outcome is change in weight at 12-months. Secondary outcomes are changes in body composition, bone mineral density, cardio-metabolic and cancer-related biomarkers, metabolic health and chronic disease risk, physical function, patient-reported outcomes (quality of life, fatigue, menopausal symptoms, body image, fear of cancer recurrence) and behaviors (dietary intake, physical activity, sitting time). Data collected at 18-months will be used to assess whether outcomes achieved at end-of-intervention are sustained six months after intervention completion. Cost-effectiveness will be assessed, as will mediators and moderators of intervention effects.
Discussion
This trial will provide evidence needed to inform the wide-scale provision of weight loss, physical activity and dietary interventions as part of routine survivorship care for breast cancer survivors.
Trial registration
Australian and New Zealand Clinical Trial Registry (ANZCTR) - ACTRN12612000997853 (Registered 18 September 2012).
Electronic supplementary material
The online version of this article (doi:10.1186/s12885-016-2858-0) contains supplementary material, which is available to authorized users.
Keywords: Breast cancer survivors, Physical activity, Diet, Nutrition, Lifestyle intervention
Background
Breast cancer is the most common invasive cancer diagnosed among women in developed countries and the second most common cause of cancer death [1]. High incidence and high overall survival (between 80 and 90 % relative 5-year survival in most developed countries [1]) have resulted in a growing number of breast cancer survivors worldwide. Addressing survivorship issues for these women is important for improving quality of life and health outcomes, and for reducing burden on the health care system.
Excess body weight, physical inactivity, and poor diet quality are prevalent among breast cancer survivors, both prior to and following diagnosis and treatment, with over 60 % of survivors overweight or obese; over 60 % insufficiently active; and, over 80 % consuming inadequate amounts of fruit and vegetables [2–5]. These factors have been associated with poor outcomes (breast cancer-specific and all-cause mortality) [6–9] and an increased risk of treatment-related side-effects [10–15]. Obesity management has been identified as a priority area for cancer survivors, with breast cancer survivors being a sub-group where an obesity link with cancer progression appears particularly important [16].
A small but growing number of weight loss intervention trials in breast cancer survivors [17–20] have shown that modest weight loss is safe and achievable, and can improve some treatment-related side-effects as well as women’s quality of life in the short-term. However, a number of gaps in this evidence base remain, including: i) understanding the benefits of weight loss across a broader range of outcomes, i.e., assessment of hard endpoints (such as survival), intermediate biomarkers, co-morbidities, and patient-reported outcomes; ii) evaluating interventions that are: feasible to deliver and implement in routine practice, convenient and flexible to the patient, and that result in sustained behavior and weight change; iii) assessing economic outcomes; and, iv) identifying sub-groups of the population who benefit the most from particular interventions to inform a personalised approach to weight management [16, 21]. Comparisons of interventions against usual care are still warranted, particularly when examining patient-reported outcomes and treatment-related side-effects, as these may naturally improve over time following treatment completion. Comparison of cost-effectiveness against current practice (i.e., usual care) also is needed to inform translation into practice and allocation of scarce health care resources.
The Living Well after Breast Cancer trial aims to address a number of these gaps. This randomized controlled trial is evaluating a telephone-delivered weight loss intervention versus usual care in women following treatment for breast cancer. Specifically, the trial aims to:
evaluate the effect of the intervention compared with usual care on percent change in weight at end-of-intervention (primary outcome); and changes in body composition, bone mineral density, cardio-metabolic and cancer-related biomarkers, metabolic health and chronic disease risk, physical function, patient-reported outcomes (quality of life, fatigue, menopausal symptoms, body image, fear of cancer recurrence) and behaviors (dietary intake, physical activity, sitting time) (secondary outcomes);
assess whether changes in the primary and secondary outcomes are sustained six months after the end of the intervention;
evaluate the cost-effectiveness of the weight loss intervention compared to usual care;
identify subgroups who achieve the greatest benefit from the intervention (based on demographic, social and clinical characteristics, cancer-related characteristics and genomic profiles); and
explore mediators and moderators of the intervention on primary and secondary outcomes to understand how the intervention worked.
Methods
Study design
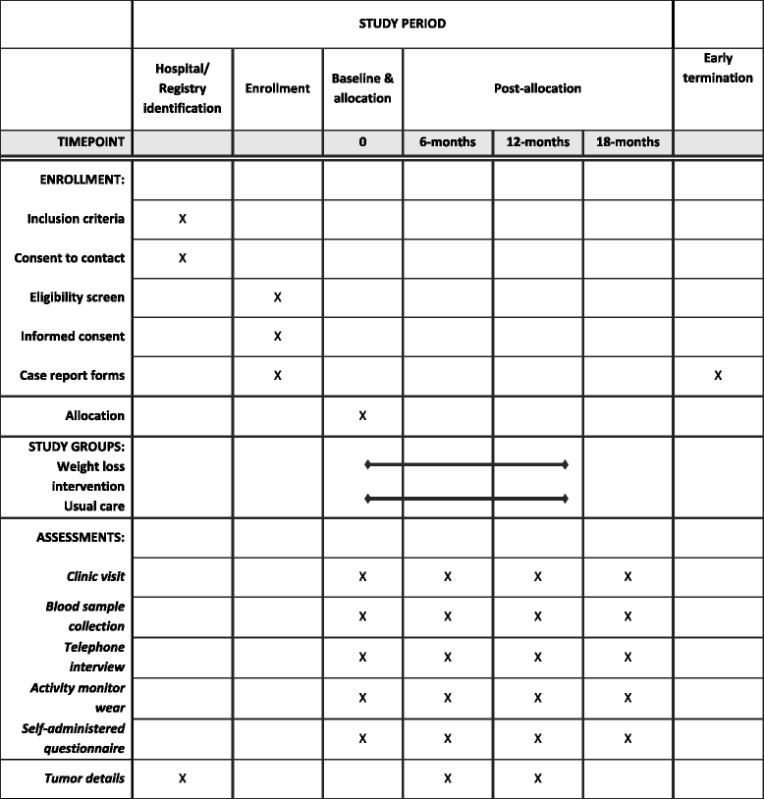
Living Well after Breast Cancer is a two-arm parallel group randomized controlled trial evaluating a 12-month telephone-delivered weight loss intervention versus usual care in women diagnosed with breast cancer. An overview of the study design and the schedule for enrollment and study assessments is shown in Table 1.
Table 1.
Schedule of enrollment, intervention, and assessment
Ethical approval was granted from the human research ethics committees of Greenslopes Private Hospital (12/26), Royal Brisbane and Women’s Hospital (HREC/12/QRBW/149), St. Vincent’s Health & Aged Care (13/02); and The University of Queensland Medical Research Ethics Committee (2012000944). A copy of the latest version of the study protocol approved by the ethics committees is included as Additional File 1. Approval was also granted from the Queensland Health Director General for accessing confidential information through the state-based cancer registry (RD004777). The trial was prospectively registered with the Australian New Zealand Clinical Trials Registry (www.anzctr.org.au; ACTRN12612000997853).
Eligibility criteria
Inclusion criteria are: female, stage I-III breast cancer diagnosed within the previous 2 years (based on cancer registry pathology data), aged 18–75 years, body mass index (BMI) 25–45 kg/m2, and completed primary treatment (i.e., surgery, chemotherapy or radiotherapy). Continued hormonal treatment is permitted. Exclusion criteria are: pregnant; contraindications to participating in an unsupervised program (e.g., unstable heart disease, breathing problems requiring hospitalization in the last 6 months, undergoing dialysis, planning a knee or hip replacement in the next 6 months, regular use of a mobility aid); taking pharmacological doses of warfarin; greater than 5 % weight loss in previous 6 months; insufficient English to complete assessments and participate in the intervention; unable to travel to Brisbane to complete study assessments; or self-reporting depression, anxiety or other mental health condition as a current significant problem that would interfere with study participation. Women who develop a recurrence during the study period are withdrawn from the study.
Participant recruitment
Participants were recruited through seven Brisbane hospital sites (Royal Brisbane and Women’s Hospital, Redcliffe Hospital, Mater Public Hospital, Mater Private Hospital, Greenslopes Private Hospital, North West Private Hospital, and Holy Spirit Northside Private Hospital; between October 2012 and June 2013), and through the state-based cancer registry (between July 2013 and December 2014). At hospital sites, nursing staff (e.g., breast care nurses, cancer care coordinators) provided potential participants with a study information packet during a routine consultation and briefly informed them of the study. The information packet contained a patient information brochure and consent to contact form with a reply-paid envelope. Interested women returned the form to their nurse or clinician or posted it directly to the research team. At one hospital site, nursing staff identified potentially eligible women from hospital records and posted the study information packet following confirmation of vital status against the state-based death register. Potentially eligible women diagnosed with breast cancer (based on age, stage of disease and residing within a 100 km radius of Brisbane) between 1 July 2013 and 30 June 2014, were identified through the Queensland Cancer Registry. Consistent with protocols for recruitment through the cancer registry, oncology care physicians identified from registry notifications, were first sent a letter to gain consent for patient contact. Physicians providing approval signed a letter informing their patient of the study, which was posted along with the patient information brochure, the consent to contact form and a reply-paid envelope. Additional recruitment methods included: posters placed in participating hospitals, word-of-mouth, and institution newsletters.
Screening and consent
Women who are interested in hearing further about the study (i.e., return the consent to contact form) are posted an information sheet and consent form and are telephoned by study staff to explain the study, answer any questions, and screen for eligibility. Screening for BMI is based on self-reported height and weight. Women still undergoing primary treatment, but who are interested in the study, are contacted after treatment completion. Those who are eligible and interested provide signed informed consent.
Randomization, allocation and blinding
The randomization sequence was generated using a computer-generated randomization program, with uneven block sizes ranging from four to 10 (www.randomization.com), by a staff member not otherwise involved with the study, and remained concealed from the study team. Following completion of baseline data collection, the project manager is notified of individuals’ allocation to study groups (randomized 1:1 into intervention or usual care) by the staff member responsible for the allocation sequence. In instances where participants have family or close friends already participating in the trial, participants are manually allocated (yoked) to the same study group as their family member or friend in order to prevent potential contamination. All assessors are blinded to participants’ study group allocation.
Weight loss intervention
The weight loss intervention is based on clinical practice guidelines for overweight and obesity [22, 23] and recommendations for cancer survivors [24, 25], and has been previously pilot tested [26, 27]. The intervention uses a combined approach of increasing physical activity, reducing energy intake and behavior therapy (i.e., use of behavior change strategies), delivered by lifestyle coaches (accredited practising dietitians with additional study-specific training in exercise promotion), and aims for modest weight loss of between 5 and 10 %.
Intervention targets
Physical activity
Participants are encouraged to gradually increase activity, aiming for at least 210 min per week of planned (aerobic) activity at a moderate-to-vigorous intensity (30 min each day; if possible, increasing to 45–60 min/day), and 2–3 sessions of resistance exercise per week. These recommendations are consistent with physical activity guidelines for weight loss and weight loss maintenance, both generally [28] and specifically among cancer survivors [24, 25, 29]. Rather than being provided with a structured exercise program, participants identify planned activities that they enjoy and that can easily be incorporated into their lifestyle (e.g., walking, swimming, exercise classes), to meet the aerobic activity target. Participants who choose to do their resistance exercises at home are provided with detailed instructions and diagrams on home-based resistance exercises, including exercises using dumbbells and some without. In addition, participants are encouraged to increase their incidental/everyday activity (e.g., gardening, taking the stairs, housework), and reduce their sitting time (i.e., to get up and move at least every 30 min and to aim for no more than 2 h per day of screen time, outside of work hours). Participants are provided with a pedometer and encouraged to achieve 10,000 steps each day. This is consistent with evidence regarding the health consequences, notably cardio-metabolic, regarding high levels of sedentary time [30, 31], and the health benefits of increasing time spent in physical activities of any intensity [32, 33].
Dietary intake
Participants are encouraged to reduce energy intake by approximately 2000 kJ per day through a prescribed recommended kilojoule intake (between 5,000 and 7,500 kJ/day) based on age and baseline weight [34]. They are also encouraged to improve diet quality. Intervention strategies focus on portion control (by reducing portion size or number of serves) and reducing energy density, along with self-monitoring of food (and energy) intake. In addition, participants are encouraged to aim for: five serves per day of vegetables and two serves per day of fruit; total fat intake ≤30 % of energy; saturated fat intake <7 % of energy; and, limit alcohol intake to one standard drink per day (with at least two alcohol-free days per week), consistent with general dietary recommendations for weight management [22, 23] as well as specific recommendations for cancer survivors [24, 25].
Behavior therapy
Behavior change strategies and principles used to guide the intervention are evidence-based and derived from Social Cognitive Theory [35], which emphasizes self-monitoring, goal setting, problem solving, social support, stimulus control, positive self-talk and self-reward.
Intervention Protocol
The intervention is delivered entirely remotely, with no face-to-face contact, and involves: telephone coaching calls, a posted workbook and materials, and optional supportive text-messages. Intervention participants also receive a copy of a newsletter from the national breast cancer consumer organisation and a study newsletter after each assessment. In addition, participants are provided with written feedback following each assessment comparing their assessment results (body composition, dietary intake, physical activity, blood test results) to recommendations. Telephone coaches use baseline feedback to assess participants’ status at the commencement of the intervention and feedback from follow-up assessments to monitor their progress. The 12-month intervention includes: an initial intensive 6-month phase, followed by a 6-month extended care phase (see Table 2).
Table 2.
Overview of intervention content
| Intervention phase & call frequency | Purpose | Objectives |
|---|---|---|
| Initial phase | ||
| 6 weekly calls Optional text (SMS) messages |
Building rapport Education Engagement Skill-building |
• Program overview • Feedback on baseline assessment to build motivation • Understand importance of physical activity and diet in weight loss management • Build patient engagement through homework and self-monitoring • Use of behavior change skills and action plans: goal setting, self-monitoring, problem solving, rewarding success • Review progress, reinforce success & problem-solve barriers • Ongoing education |
| 10 fortnightly calls Optional text (SMS) messages |
Establish behavior change & achieve weight loss Skill-building |
• Reflect on progress (changes made) and outcomes/benefits experienced • Progress goals • Continued use of behavior change skills: goal setting, self-monitoring, problem solving, rewarding success, managing slips • Ongoing education |
| Extended care phase | ||
| 6 monthly calls Optional structured text (SMS) messages |
Consolidation Maintaining changes |
• Encourage participants to direct sessions • Reinforce successes and review benefits • Behavior change skills: relapse prevention and maintaining motivation • Plans and strategies for maintaining weight loss and behavior changes |
SMS short-messaging service
Initial intensive phase of intervention (months 1–6)
The focus of the initial intensive phase (weekly and fortnightly coaching calls over 6-months) is to build rapport, provide education about the importance of physical activity, healthy eating and weight management (by working through workbook content both during and in-between calls), encourage skill building through self-monitoring and goal setting, and work towards behavior change and weight loss (see Table 2). During this phase, participants receive up to 16 calls (6 × weekly calls, 10 × fortnightly calls) as well as optional text messages. Telephone call timing is scheduled based on participant preference (day vs. night). Participants also receive a detailed workbook, set of digital scales, measuring tape, pedometer, calorie counter book and self-monitoring diary, which is referred to throughout the intervention.
Lifestyle coaches use a motivational interviewing counseling style [36], with a semi-structured approach in relation to both call contact and content (e.g., the order in which intervention targets are addressed) based on participant preference. The protocol for each call includes: assessment of progress; problem solving; advice/education; collaborative goal setting; and development of a behaviorally-specific action plan. Call outlines and checklists are used by coaches to facilitate intervention fidelity. Fortnightly supervision meetings and audio-recording of randomly selected calls are used to monitor and maintain intervention fidelity, provide coaches with feedback throughout the intervention delivery period, and to discuss participants with particular challenges.
Participants also have the option to receive mobile phone text messages during this initial phase, starting from the second phone call. This aspect of the intervention is based on prior research on the use of mobile phone text messages for supporting and maintaining behavior changes and weight loss [27, 37]. In the initial phase, the participant, in consultation with the coach, determines the content and timing of the text messages. The texts are created (maximum of 160 characters) and sent using a web-based platform that enables the coaches to pre-schedule texts to send at specific times and days. Coaches received training on how to introduce the text messages and were provided with examples on the types of content they could include. All messages are personalized with the participant’s first name and signed off by the coach.
Extended care phase of intervention (months 7–12)
The focus of the 6-month extended care phase (6 × monthly calls) is to review progress, problem solve, and identify barriers and solutions to maintaining weight loss, physical activity and dietary changes. During this phase participants are encouraged to receive text messages between calls (regardless of whether they received texts during the initial phase). During the extended care phase, a more structured approach for text content and timing is used, with the texts designed to target specific behavioral skills: 1) prompting self-monitoring of weight; 2) behavioral goal setting; and, 3) prompting real-time behaviors (see Table 3 for examples). Each text type has a suggested frequency (Table 3), but is tailored to the participant’s preferences. The total number of text messages that can be received is in the range of two to 16 per fortnight (i.e. every two weeks). Participants who opt to receive the text messages during this phase first complete a scripted interview to collect information for tailoring the text messages. Text message content is tailored to: the names of the participant and coach, behavioral goals, rewards for reaching goals, identified barriers (and solutions) for reaching goals, preparatory behaviors to reach their goals, and outcome expectancies. Throughout this phase, tailoring information for the text messages is updated as requested by the participants or suggested by the coach.
Table 3.
Examples of types of structured text messages that could be received during the extended care phase of the intervention (months 7 – 12)
| Text message type | Behavior change strategies targeted | Example text messages | Frequency |
|---|---|---|---|
| Self-monitoring weight | Self-regulation; Satisfaction with perceived outcomes | Keeping track of ur weight is important 2 maintain progress & catch ‘slips’ Jane. Weigh yourself today & write it down in ur Living Well Diary. Jenny | 1 per week or fortnight |
| Goal check | Self-regulation; Satisfaction with perceived outcomes | Hi Jane. Did u achieve ur goal 2 have 3 alcohol free days this week? Text me back yes or no so I know how u r going. Jenny | 1 per week or fortnight per goal |
| Goal check reply | Self-regulation; Outcome expectancy; Satisfaction with perceived outcomes; Self efficacy; Social support | Wonderful news Jane! Remember how good u feel achieving ur goal & use this as motivation on ur ‘off’ days. Keep it up! Jenny | Only sent if participant responds to goal check |
| Behavior prompt | Think ahead Jane. U want 2 do 30 min on the treadmill 6× this week so make sure u set the alarm for the morning. Jenny | Up to 2 messages per week per goal |
Usual care
Participants in the usual care group continue to receive their standard medical care. In addition, these participants are posted materials after each of their study assessments (baseline, 6-months, 12-months, 18-months), which includes brief written feedback from their study assessment, a copy of a newsletter from the national breast cancer consumer organisation and a study newsletter. The feedback following study assessments is similar to that provided to intervention participants with the exception that participants’ results are not compared to national and study recommendations.
Data collection
Data are collected from all participants at baseline, 6-months (mid-intervention), 12-months (end-of-intervention) and 18-months (follow-up after 6-months of no contact) by research staff blinded to participants’ study group (see Table 1). Each assessment involves: a clinic visit, blood sample collection, two telephone interviews, a self-administered questionnaire, and objective monitoring of physical activity by accelerometry for 7 days. The clinic visit includes objective measurements of height, weight, waist and hip circumferences, body composition, bone mineral density, blood pressure, and performance-based measures of physical function. Participants attend their local pathology collection centre after at least a 10-h fast to have blood samples (18 mL) taken by trained phlebotomists. Assays are conducted on fresh samples with serum samples also frozen at −80 °C for batch assays. Telephone interviews collect data on: physical activity levels, dietary intake, breast cancer-related information, and demographic and health characteristics. The self-administered questionnaire collects information on patient-reported outcomes and a range of constructs related to social-cognitive theory. Physical activity is also objectively measured via two activity monitors (one worn on the hip and one on the thigh), fitted at the clinic visit. Tumor characteristic data are obtained from pathology notifications within the cancer registry. Data related to intervention delivery are tracked in the study database. This includes data on call outcomes (call completion versus missed calls), call duration and call content (via a checklist of topics). A summary of study outcomes and data collection methods and tools is shown in Table 4. All staff received detailed training in data collection protocols and are blinded to participants’ study arm. Where appropriate, measurements are taken at least in duplicate.
Table 4.
Primary and secondary outcomes and assessment methods
| Outcome | Collection method | Assessment tool |
|---|---|---|
| Primary outcome | ||
| Weight | Clinic visit | Tanita BWB-600 Wedderburn Scales |
| Secondary outcomes | ||
| Anthropometry | ||
| Waist circumference | Clinic visit | Non-expandable tape measure |
| Hip circumference | Clinic visit | Non-expandable tape measure |
| Body composition & densitometry | ||
| Body composition (FM, FFM, LBM) | Clinic visit | Lunar Prodigy DXA - Total body and regional |
| Bone mineral density | Clinic visit | Lunar Prodigy DXA – Anterior-posterior lumbar spine (L1-L4); Bilateral proximal femur. |
| Cardio-metabolic & cancer-related biomarkers | ||
| Glucose, lipids, HbA1c | Fasting blood test | Standard assays on fresh blood |
| Other cardio-metabolic and cancer-related blood markers | Fasting blood test | Stored serum, plasma, buffy coat |
| Metabolic health & chronic disease risk | ||
| Blood pressure | Clinic visit | Welch Allyn 300 Series Vital Signs Monitor |
| Physical functioning | ||
| Hand grip strength | Clinic visit | Smedley dynamometer |
| Timed chair stands | Clinic visit | 5 stopwatch timed sit-to-stand transitions |
| Patient-reported outcomes | ||
| Quality of life | SAQ | PROMIS Global Health Scale [50] |
| Fatigue | SAQ | Functional Assessment of Chronic Illness Therapy – Fatigue Scale (FACTIT-Fatigue) [51] |
| Menopausal symptoms | SAQ | Greene Climacteric Scale [53] |
| Body image | SAQ | Body Image and Relationships Scale (BIRS) [56] |
| Fear of cancer recurrence | SAQ | Concerns about Recurrence Questionnaire – 4-items (CARQ-4) [58] |
| Arthralgia | SAQ | Breast Cancer Prevention Trial Symptom Scale – Musculoskeletal Pain subscale [59] |
| Peripheral neuropathy | SAQ | Patient Neurotoxicity Questionnaire (PNQ) [61, 62] |
| Behavioral outcomes | ||
| Dietary intake | Telephone interview | 2 × 24-h dietary recalls |
| Physical activity | Objectively collected, Telephone interview | Actigraph GT3X+ tri-axial accelerometer Active Australia Survey [69] |
| Sitting time | Objectively collected | activPAL3TM monitor |
DXA Dual-energy X-ray Absorptiometry, FFM fat-free mass, FM fat mass, HbA1c, glycated hemoglobin, LBM, lean body mass, SAQ, self-administered questionnaire
Primary outcome
The primary outcome is change in weight (% initial body weight). At each visit, weight is measured in duplicate to the nearest 0.1 kg, without shoes or heavy clothing, using calibrated scales (Tanita BWB-600 Wedderburn Scales, Australia), with the mean of the two values recorded.
Secondary outcomes
Secondary outcomes include: anthropometry (waist and hip circumference), body composition, bone mineral density, cardio-metabolic and cancer-related blood markers, metabolic health (blood pressure and metabolic syndrome), performance-based measures of physical function, patient-reported outcomes (quality of life, fatigue, menopausal symptoms, body image, fear of cancer recurrence, arthralgia, chemotherapy-induced peripheral neuropathy), behavioral changes (dietary intake, physical activity, sitting time), and cost-effectiveness.
Anthropometry
Height is measured in duplicate to the nearest 0.1 cm using a stadiometer (Magnimeter, Raven Equipment, UK) at baseline. Waist and hip circumference are measured in duplicate to the nearest 0.5 cm using a non-expandable tape measure at the superior border of the iliac crest [38] and the greatest gluteal protuberance, respectively, following a normal expiration. A third measurement is taken if measures differ by more than 1.0 cm. The mean of all measurements taken is used.
Body composition & densitometry
Body composition and bone mineral density are acquired by Lunar Prodigy Dual-energy X-ray Absorptiometry (DXA; GE Medical Systems, LUNAR, Madison, WI, USA), using the manufacturer’s standard procedures. Daily calibration of the DXA is performed on the morning prior to each measurement using an aluminium spine phantom. All images are acquired and analysed by a trained technician (DSKB) using the manufacturer’s proprietary software (enCORE, version 14.1). Measures of body composition (fat mass (FM, g), percent fat (% region), fat free mass (FFM, g), and lean body mass (LBM, g; proxy for muscle mass)) are acquired from the total body scans. Total body scans provide measures of whole and regional body composition. Appendicular lean mass (LBM in arms and legs separated from trunk LBM) is calculated and used in the assessment of sarcopenia [39, 40].
Bone mineral density (BMD, g/cm2), bone mineral content (g) and bone area (cm2) for total body, anterior-posterior lumbar spine (LS 1–4) and bilateral proximal femur sites are measured. All BMD values are calculated as T- and Z-scores using the Geelong Osteoporosis Study reference database [41], for all sites.
Cardio-metabolic and cancer-related biomarkers
Glucose, glycated haemoglobin (HbA1c), total cholesterol, high density lipoprotein (HDL) cholesterol, low density lipoprotein (LDL) cholesterol and triglycerides are measured in fresh blood. HbA1c is measured from whole blood samples by the high performance liquid chromatography method (ion-exchange with ultraviolet detection; D-100 analyser, Biorad Laboratories, Hercules, CA, USA). Glucose, triglycerides, total cholesterol and HDL cholesterol are measured by an enzymatic colorimetric assay with Abbott c16000 Clinical Chemistry Analyzer (Abbott Diagnostics, Abbott Park, IL, USA). LDL cholesterol is calculated using the Friedewald equation [42].
Aliquots of serum, plasma and buffy coat are stored frozen at −80 °C to allow analysis of other relevant blood markers – for example insulin, adipokines (total and high molecular weight adiponectin, leptin), inflammatory markers (e.g., high-sensitivity C-reactive Protein), and genomic markers (e.g., microRNAs). All samples will be measured in duplicate with repeated samples from individuals assayed together to avoid batch variation.
Metabolic health & chronic disease risk
Blood pressure is measured seated using an automated blood pressure monitor (300 Series Vital Signs Monitor, Welch Allyn, Beaverton, OR, USA) with appropriately sized cuff. Measurements are taken in duplicate, with a third taken if the first two differ by ≥10 mmHg systolic or ≥6 mmHg diastolic, or if the first two readings are more than 140 mmHg/90 mmHg. The mean of the readings is recorded.
Comorbidities are assessed using the Charlson Comorbidity Index based on self-reported diagnosis of 13 conditions during the telephone interview [43, 44]. Participants also self-report if they have ever been diagnosed with hypertension [45]. Metabolic syndrome is classified according to the International Diabetes Federation worldwide consensus definition [46] and examined continuously [47, 48]. Current use of blood pressure, lipid lowering and/or diabetes medications are self-reported during the telephone interview. Comorbidities and medication use are assessed at baseline and each follow-up visit.
Performance-based measures of physical function
Hand grip strength and timed chair stands are used to assess upper and lower body function, respectively, as they have shown good to excellent reliability and high discrimination across different functional levels [49]. Bi-lateral hand grip strength (kg) is measured using a handheld dynamometer (Smedley, Scandidact, Denmark), with participants standing in neutral position with elbow flexed at 90°, the forearm in neutral and wrist held between 0 and 30° dorsiflexion and 0–15° ulnar deviation. Three measurements are conducted on each hand, alternating hands with a rest period in between measurements to prevent fatigue. Five chair stands are timed using a stopwatch. Participants are asked to perform, as quickly and ably as possible, five repetitions from a seated position to a fully standing position with arms crossed over their chest.
Patient-reported outcomes
Quality of life
is measured using the 10-item Patient Reported Outcome Measurement Information System (PROMIS) Global Health Scale which asks participants to evaluate their general health across five domains (physical function, fatigue, pain, emotional distress and social health) as well as general health perceptions. Items are scored into a Global Physical Health component and Global Mental Health component with higher scores indicating better functioning. The component scores have shown good internal consistency and good relative validity compared to EQ-5D [50].
Fatigue
is measured using the 13-item Functional Assessment of Chronic Illness Therapy – Fatigue Scale (FACIT-Fatigue) which assesses fatigue over the last seven days on a 5-point scale (0 = not at all to 4 = very much) [51]. Items are summed, giving a score of 0–52, with higher scores indicating lower fatigue. Fatigue is classed as present if the FACIT-Fatigue score is <34, corresponding to ICD-10 criteria for fatigue [52].
Menopausal symptoms
are measured using the 21-item Greene Climacteric Scale [53], which assesses the extent to which participants are affected by specified menopausal symptoms at present. Items are answered on a modified 5-point response scale from ‘none’ to ‘very severe.’ Responses of ‘severe’ and ‘very severe’ are collapsed to correspond to ‘extremely’ on the original 4-point response scale. Items are summed to create a total score as well as three subscale scores – psychological symptoms, somatic symptoms, vasomotor symptoms – with higher scores indicating more severe symptoms [53]. The total scale has shown excellent internal consistency, subscale scores have shown good two week test-retest reliability and are sensitive to change [54, 55].
Body image
is measured using the 32-item Body Image and Relationships Scale (BIRS), a scale developed specifically for women who have been diagnosed and treated for breast cancer [56]. The BIRS has shown good reliability, convergent and divergent validity, and sensitivity to change [56, 57]. Items are scored on a 5-point scale (1 = strongly disagree to 5 = strongly agree), with items summed to yield a total score and three subscale scores – strength and health; social barriers; sexuality and appearance – with higher scores indicating greater impairment.
Fear of cancer recurrence
is measured using the 11-point 4-item Concerns about Recurrence Questionnaire (CARQ-4) [58]. The four items are summed, giving a score of 0–40, with higher scores indicating greater fear. The CARQ-4 has shown good two week, test-retest reliability, and demonstrated concurrent and convergent validity with good correlations against the Fear of Cancer Recurrence Inventory and moderate correlations with measures of depression and anxiety [58].
Arthralgia
is measured using the Breast Cancer Prevention Trial Symptom Scale - Musculoskeletal Pain subscale, which includes three items assessing general aches and pains, joint pains and muscle stiffness over the past 4 weeks [59]. Items are scored on a 5-point severity scale from 0 ‘not at all’ to 4 ‘extremely,’ with scores from the three items averaged such that higher scores indicate a greater degree of being bothered by pain. The subscale has shown good internal consistency and is sensitive to change [59, 60].
Chemotherapy-induced peripheral neuropathy (CIPN)
is measured using the Patient Neurotoxicity Questionnaire (PNQ) [61, 62] in the subset of participants who were treated with chemotherapy. The PNQ includes two items assessing the presence and severity of sensory and motor disturbances over the past 7 days. Each item is rated on a 5-point scale from 0 ‘no neuropathy’ to 4 ‘severe neuropathy,’ with each item considered separately [63]. The PNQ scores show good concurrent validity against more detailed scales of CIPN [62] and are sensitive to changes over time [62, 63].
Behavioral outcomes
Physical activity and sedentary time are measured objectively at each assessment with the tri-axial Actigraph GT3X+ accelerometer (ActiGraph, Pensacola, Florida) and the activPAL3TM monitor (PAL Technologies Limited, Glasgow, UK), each worn for seven consecutive days. Participants record sleep time and monitor removal times in a logbook. The Actigraph GT3X+ is worn positioned over the right hip via an adjustable elastic belt. Participants are asked to wear this monitor during all waking hours and to remove it only for sleep and during times the monitor could be damaged (e.g., during water-based activities). The activPAL is waterproofed, attached to the anterior mid-line of the right thigh using a hypoallergenic adhesive patch and worn continuously across the 7-day wear period, for 24 h per day (during waking and sleeping hours). Additional patches are provided to replace as necessary. The activPAL monitor records triaxial acceleration at 10Hz, from which thigh position, and the start and end of each period of time spent sitting/reclining, standing and stepping, are determined, along with transitions from sitting to standing and stepping speed. The activPAL has been shown to be a valid monitor for measuring sedentary behavior and is sensitive to detecting change [64]. The Actigraph GT3X+ monitor has shown acceptable relative validity when compared to oxygen consumption [65].
Raw GT3X+ data are collected at 30Hz and downloaded in Actilife (v 6.6.3). Both 10-s and 60-s epoch files are processed in SAS version 9.4. Non-wear time (estimated as blocks of ≥60 min of 0 counts per minute (cpm) with up to 2 min with counts 1–49 cpm) is removed [66]. Non-wear (invalid) days are also removed (<10 h wear or before/after the monitoring period based on the logbook). When quality controls (data visualisation and the logbook data) indicate participants wore the monitor to bed, self-reported sleep and naps are also removed. All minutes with ≥1952 cpm [67] are classed as moderate- to vigorous-intensity physical activity (MVPA), then summed for each day and averaged across valid days (i.e., ≥10 h of wear).
The activPAL data are downloaded using activPAL Professional 7.3.32 software (PAL Technologies Limited, Glasgow, UK). Recorded bouts of activity (sitting/reclining, standing and stepping) are processed in SAS version 9.4, using the monitor and logbook data with quality controls (data visualisation and cross-checking against the logbook). Unreported wake/sleep times are estimated by staff from times when movement first began/last ceased. All time during bouts that are ≥50 % during a self-reported sleep, removal or nap period are initially classed as sleep, removal, or a nap. Sleep periods are then adjusted to begin/end with the first/last sitting/reclining bout of ≥20 min duration during each period initially identified as sleep. Total sitting time (i.e., sitting/reclining during waking wear time) is summed for each day then averaged on valid days. Days are defined from wake on one day until wake the next day. Days are classed as valid if removals constituted <20 % of waking hours, and, when sleep/wake times are not reported, if waking wear time was ≥10 h. These methods are consistent with previous reports [68].
Physical activity is also self-reported using the Active Australia Survey, an 8-item questionnaire which assesses times spent walking, in moderate and in vigorous activities, and doing household and gardening activities, over the past week [69]. As per standard scoring protocols, self-reported MVPA is calculated as the sum of time spent walking, in moderate activities, and in vigorous activities (weighted by two), with truncation at 1680 min per week to reduce over-reporting. The Active Australia Survey has been shown to be valid, reliable, and responsive to intervention change [70–73]. The number of days and amount of time in the past week spent specifically walking for exercise [74], as well as undertaking strength or resistance based exercises, are also collected during the telephone interview.
Dietary intake is assessed using two unprompted 24-h dietary recall interviews (recalling one weekday and one weekend day) conducted using FoodWorks® Interview (version 1, 2009, Xyris Software, Brisbane, Australia), based on a 5-stage multi-pass method [75]. Participants are provided with a food model booklet to assist in estimating portion sizes. Energy and nutrient intakes are derived from dietary intake data using FoodWorks® Professional Edition (version 6, 2009, Xyris, Brisbane, Australia) nutritional analysis software, using the average of intakes from recalled days. Daily fruit and vegetable intake are also assessed using two items, which have been shown to be reliable and valid when compared to blood biomarkers [76, 77].
Potential mediating and moderating variables
Demographics
Demographic and social characteristics collected during the baseline telephone interview include: highest educational attainment, employment status, household income, ethnicity, country of birth, marital status, children living at home and smoking status. Employment and smoking status are both re-assessed at each follow-up assessment. Age at baseline is determined from date of birth on pathology records. Participants’ residential postcode was used to assess area-level socio-economic position and geographical location/remoteness [78, 79].
At each assessment all participants self-report whether they have used any particular tools to assist with weight loss over the previous 6 months. This includes meal replacements, food delivery (pre-prepared meals) programs, other commercial weight loss programs (e.g. Weight Watchers), bariatric surgery or weight loss medications.
Cancer and treatment-related information
Cancer-related details are obtained from pathology records in the cancer registry and include: date of diagnosis; tumor size, type and histological grade; surgery details; receptor status (estrogen, progesterone and HER2/neu); and, lymph node involvement. Participants report menopausal status at diagnosis; treatments received (surgery, chemotherapy, radiation therapy, endocrine therapy, reconstructive surgery); treatment completion dates; and, presence (either current or past) of lymphedema.
Depression and anxiety
Participants self-report whether they have ‘ever been diagnosed with depression or anxiety.’ Depressive symptoms are also assessed using the 8-item PROMIS Short Form v1.0 – Depression 8b tool, which asks about negative mood, views of self and social cognition, decreased positive affect, and engagement over the last seven days on a 5-point scale (1 = never to 5 = always). Scores are summed (giving a score of 8–40) and converted to a T-score (M = 50, SD = 10) based on the U.S. general population [80]. This depression scale has demonstrated good reliability and validity [81].
Theory-based constructs
Data on constructs related to Social Cognitive Theory, and targeted as part of the intervention are collected using the self-administered questionnaire. Each of these constructs are assessed separately for physical activity and dietary intake, using items adapted from previous tools which have been described in detail elsewhere [37]. The constructs measured include outcome expectancies [82, 83], satisfaction with outcomes [84], self-regulation [85], self-efficacy [86, 87], social support [88], and perceived environmental opportunity [89–91].
Adverse events
Data on adverse outcomes are collected at each follow-up assessment for all participants. Adverse outcomes are defined to participants as any new health problem such as: a breast cancer recurrence; other cancer diagnosis; diagnosis of other medical condition; period of hospitalisation; muscle injury or bone/joint problems; new symptoms; or, worsening of pre-existing conditions. Adverse events spontaneously reported either during intervention contacts or in between assessments are also recorded. The severity (5-categories ‘mild’ through to 'fatal') and relatedness to the intervention (5-categories ‘clearly not related’ through to ‘clearly related’) are recorded for all adverse events reported. The relatedness to the intervention is reported by the participant in discussion with the interviewer, as well as with consultation with the treating physician and principal investigator if needed. Unintentional weight loss is also assessed for all participants at each follow-up assessment.
Retention strategies
A number of strategies are used to maximise retention over the 18-month study period. Of key importance is the development of good rapport with study staff (project manager, lifestyle coaches and assessors). Intervention calls and assessments are scheduled at times convenient to the participant. Participants travelling 50 km or further (round trip) to attend the clinic visit are provided with a gift card as reimbursement for their travel costs. Car parking costs are covered for all participants. Participants are contacted 2–4 weeks prior to their due date for the assessment to schedule their clinic visit and other aspects of their assessment. A 45-day window is allowed for the completion of the study assessment. Where necessary, participants who plan to be travelling, or are otherwise not available to complete all of the assessment during the scheduled window, coordinate with the project manager to complete their assessment earlier. All participants receive a study newsletter following each assessment and all participants are posted birthday cards. Feedback from the study assessments (e.g., bone mineral density DXA scan results) is provided to treating doctors as requested. Participants provide multiple contact details (e.g., mobile, home and/or work phone and email address) along with details of an emergency contact (family member or friend) to minimize loss to follow-up.
Data management
Data sheets are stored in locked filing cabinets and electronic data are stored in password-protected files on a secure network system. Access to the study database is restricted. All data are double-key entered and checked for inconsistencies. Where appropriate, the database includes automatic range checks. In addition, all data are checked and cleaned prior to analysis.
Statistical analyses
Sample size
The sample size calculation is based on the between-group difference in weight change (the primary outcome) 12 months after study entry, with a minimum clinically important difference (MCID) in weight change of 5 % of initial body weight [24]. Conservatively, assuming a standard deviation (SD) of change of 8.5 % [92], then with alpha = 0.05 and 90 % power we will need to collect 12-month data on 62 participants in each group (124 total). Allowing for 20 % attrition at 12 months, 78 per group (156 participants in total) will need to be recruited. With a sample size of 62 per group we will have 80 % power to detect effect sizes of 0.5 (i.e., minimum between group difference of half a SD) for the secondary outcomes. Of the secondary outcomes, only fatigue (FACIT-Fatigue) and hand grip strength (hand held dynamometer) have established MCIDs; 3 units and 3 kg, respectively [93, 94]. For these secondary outcomes, with our sample size of 62 per group we will have approximately 35 % power for detecting the MCID for fatigue and 96 % power for detecting the MCID for hand grip strength, assuming SDs of these outcomes in breast cancer survivors of 10.5 units [26, 95] and 4.5 kg [96], respectively.
Primary and secondary outcomes
Summary descriptive statistics for demographic, social and clinical data at baseline will be reported by allocated study treatment. Continuous data will be summarized descriptively using either mean and SD, or median and inter-quartile range, depending on the distribution of the variable of interest. Categorical data will be presented as frequencies and percentages. Comparisons between the treatment groups will be conducted to assess the degree to which comparability of randomization was achieved. The baseline between-group difference in the potentially influential confounding variables (stage of disease, menopausal status, treatment, pre-diagnosis weight, employment status and educational level) will be examined. If any of these variables are statistically significant at p < 0.001 they will be included as co-variables in all outcome analyses.
The primary study outcome is the change in weight (expressed as % of initial body weight) at 12 months. The mean difference between treatment groups will be calculated using linear mixed models with treatment group (intervention/usual care) and time (0/12/18 months) entered as fixed effects and patient entered as a random effect. We will include an interaction effect between treatment group and time in order to investigate change in weight. The corresponding 95 % Wald confidence interval and p-value will be reported. For secondary outcomes, the effect estimates will be presented as a mean difference, which will be calculated using a mixed effects linear regression model. For all models the corresponding 95 % Wald confidence interval and p-value will be reported. Statistical significance will be set at p <0.05 (two-tailed) and there will be no adjustment for multiple comparisons. All analyses will be intention-to-treat, with all evaluable data analysed according to the treatment group allocated.
To examine the sensitivity of results to attrition, analyses will be re-run after imputing missing data. The type of imputation will be decided after the characteristics of participants with missing data are compared against the characteristics of those with complete data. Either a single imputation method (using last observation carried forward) or a multiple imputation method (using chained equations) will be selected. Further, per-protocol analyses will be performed to compare those completing at least 75 % of intervention calls with those completing less intervention calls. Stratified analyses exploring effects on secondary outcomes based on amount of weight loss achieved (≥5 % weight loss vs. < 5 % weight loss) will be conducted.
Moderators and mediators of outcome
Exploratory analyses will be conducted to determine whether there is moderation or mediation of intervention effects. Moderator analysis will determine whether intervention effects differ across demographic (e.g., age, menopausal status) and breast cancer (e.g., receptor status/subtype, chemotherapy treatment, fear of cancer recurrence) characteristics and will be performed by considering the statistical significance of an interaction between a potential moderator and the intervention using a Wald test. Mediator analysis will determine whether theoretically-driven constructs and mechanisms for behavior change do in fact mediate the intervention effects. Potential mediators will be assessed using path analysis. Point estimates and bootstrap confidence intervals of path coefficients and the product of the mediated path coefficients will be used to determine the potency, certainty, and direction of any mediation effect.
Cost-effectiveness
Intervention costs
Costs to deliver the intervention, not including the research/assessment components, are used in the cost analysis. They are tracked during trial implementation and include the cost of coach time, intervention materials (workbook, self-monitoring diary, pedometer, digital scale, measuring tape, kilojoule-calorie counter book) and related infrastructure (i.e., office space, telephones, computers and call costs).
Incremental cost-effectiveness/analysis
The cost-effectiveness analysis will present results as cost per health-adjusted life year (HALY) taking usual care as comparison. An existing micro-simulation model with a lifetime horizon (developed for the ACE Prevention project [97, 98]) will be adapted for this purpose. Epidemiological data will be updated to 2013 using estimates for Australia from the Global Burden of Disease study [99]. Demographic and health-related data collected at baseline and changes in weight and clinical biomarkers will be used to estimate lifetime risks of cardiovascular disease (based on sex, age, clinical biomarkers and body composition) using calibrated Framingham equations [100]. Other non-breast cancer health risks (diabetes mellitus, osteoarthritis, colon cancer, endometrial cancer and kidney cancer) will be modeled assuming that age-specific Australian average rates apply. Breast cancer recurrence risks and mortality will be modeled based on best available evidence at the time of conducting the modeling. We will take into account that after the 18-month assessment, weight is regained at a rate of 0.02–0.03 kg/m2 per month [101] and assume commensurate waning of the effects on clinical biomarkers. Years spent with disease will be valued less than healthy years by applying disability weights [102]. All of this will be used to calculate lifetime HALYs for all participants in the intervention and usual care groups. In both approaches, one-way sensitivity analysis and a combination of parametric and non-parametric bootstrapping will be applied. Net costs will be estimated from intervention costs and the modeled difference in health care costs in the remaining lifetime between the intervention and control groups. In both analyses, costing will take a health sector perspective that includes costs to patients and government. Costs associated with delivering the intervention (e.g. coach time, intervention materials) and costs incurred by participants (e.g., time) will be based on trial information. Costs associated with the development of the intervention will not be included. Costs of disease treatment will be taken from the Australian Institute of Health and Welfare’s Disease Costs and Impacts Studies. As per common convention, participants’ time will be valued at 25 % of the wage rate [103, 104].
Discussion
The Living Well after Breast Cancer trial is the first large-scale randomized controlled trial of a weight loss intervention versus usual care in Australian breast cancer survivors. This trial adds to the international evidence in breast cancer survivors on the effectiveness of telephone-delivered weight loss interventions [17, 18, 105–107], which have greater potential for scaling-up into routine practice [21, 108]. Further, this trial will provide important evidence on the effect of weight loss on a broad range of secondary outcomes, some of which have not been examined in this population to date. Pertinent to the uptake of such interventions as part of routine follow-up care for breast cancer survivors, this trial will include an analysis on the cost-effectiveness of the intervention.
An additional strength of this study is the recruitment of a broad sample of breast cancer survivors through hospital clinics and the population-based cancer registry, including both pre- and post-menopausal breast cancer survivors and women with pre-existing comorbidities. Participants were recruited as close to the end of treatment as possible, which differs from many other weight loss intervention trials in breast cancer survivors, where participants were on average 3–5 years post-diagnosis at study baseline [18, 19].
The trial is powered based on the primary outcome of weight change. With the sample size it is powered to detect medium or larger effect sizes in secondary outcomes. For some secondary outcomes (e.g. fatigue), smaller effect sizes may be clinically meaningful but the trial will be underpowered to detect these. As a number of the secondary outcomes have not been previously examined in the context of a weight loss intervention in breast cancer survivors, results from this trial will provide preliminary evidence on the effect of weight loss on these outcomes. Due to the nature of the data collection procedures, recruitment was limited to women living within traveling distance of the capital city, therefore, results may not generalize to women living in regional and rural areas.
This trial will provide novel evidence on effectiveness and cost-effectiveness of a telephone-delivered weight loss intervention for breast cancer survivors. Findings from this trial will help address a number of gaps identified by the American Society of Clinical Oncology [16] and add to the evidence needed to inform the provision of weight loss interventions to breast cancer survivors as part of routine survivorship care.
Acknowledgements
We wish to thank the study participants, nursing staff from recruiting hospitals and surgeons and oncologists who gave permission for us to contact their patients. We would also like to thank Breast Cancer Network Australia for endorsing this study. We are extremely grateful to our consumer advocates, Lorraine Woods and Fiona Evans, for their invaluable input to this study. We would also like to thank staff from the Children’s Nutrition Research Centre and Queensland MetroSouth Pathology for assisting with data collection. Finally, we would like to thank the project staff for their integrity and commitment: Fiona Heys, Jodie Jetann, Zoe Thomson, Charlotte Brakenridge, Erin Robson, Megan Whelan, Kym Spathonis, Genevieve Maher, Cathy Swart and Charani Kiriwandeniya.
Funding
This study is funded by an Australian National Health and Medical Research Council (NHMRC) Project Grant (GNT1024739). Reeves is funded by a National Breast Cancer Foundation research fellowship (ECF-13-09). Terranova is funded by a postgraduate scholarship from The University of Queensland. Healy and Janda are funded by NHMRC Career Development Fellowships (GNT108029 and GNT1045247). Eakin is funded by an NHMRC Senior Research Fellowship in Population Health (GNT1041789). Winkler is supported by a NHMRC Centre for Research Excellence Grant (GNT1057608). The funders had no role in the design of the study, collection, analysis or interpretation of the data, or preparation of the manuscript.
Availability of data and materials
Not applicable.
Authors’ contributions
MMR, NM, IJH, TV and EGE are chief investigators and SPL, BSF, GNH, EAHW, MJ, JLV, JP and WD-W are associate investigators on the funded study. They were extensively involved in the study concept and design. RSW contributed to statistical aspects of the study design. COT, JME, JRJ, and DSKB assisted with supervision of the project, recruitment, intervention delivery and data collection. All authors were involved with drafting and critically revising the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The trial was approved by the following ethics committess: Greenslopes Private Hospital (12/26); Royal Brisbane and Women’s Hospital (HREC/12/QRBW/149); St. Vincent’s Health & Aged Care (13/02); and The University of Queensland Medical Research Ethics Committee (2012000944). All participants provided written, signed informed consent.
Abbreviations
- ACTRN
Australian clinical trials registration number
- BIRS
Body Image and Relationships Scale
- BMD
Bone mineral density
- BMI
Body mass index
- CARQ-4
Concerns about Recurrence Questionnaire – 4 items
- CIPN
Chemotherapy-induced peripheral neuropathy
- Cpm
Counts per minute
- DXA
Dual-energy X-ray Absorptiometry
- FACIT
Functional Assessment of Chronic Illness Therapy
- FFM
Fat free mass
- FM
Fat mass
- HALY
Health-adjusted life year
- HbA1c
Glycated haemoglobin
- HDL
High density lipoprotein
- LBM
Lean body mass
- LDL
Low density lipoprotein
- LS
Lumbar spine
- MCID
Minimum clinically important difference
- MVPAm
Moderate-vigorous intensity physical activity
- PNQ
Patient Neurotoxicity Questionnaire
- PROMIS
Patient reported outcome measurement information system
- SD
Standard deviation
Additional file
Study Protocol. (DOCX 159 kb)
Contributor Information
Marina M. Reeves, Email: m.reeves@sph.uq.edu.au
Caroline O. Terranova, Email: c.terranova@uq.edu.au
Jane M. Erickson, Email: j.erickson@sph.uq.edu.au
Jennifer R. Job, Email: j.job@uq.edu.au
Denise S. K. Brookes, Email: d.brookes@uq.edu.au
Nicole McCarthy, Email: NMcCarthy@iconcancercare.com.au.
Ingrid J. Hickman, Email: i.hickman@uq.edu.au
Sheleigh P. Lawler, Email: s.lawler@sph.uq.edu.au
Brianna S. Fjeldsoe, Email: b.fjeldsoe@sph.uq.edu.au
Genevieve N. Healy, Email: g.healy@uq.edu.au
Elisabeth A. H. Winkler, Email: e.winkler@sph.uq.edu.au
Monika Janda, Email: m.janda@qut.edu.au.
J. Lennert Veerman, Email: l.veerman@sph.uq.edu.au.
Robert S. Ware, Email: r.ware@sph.uq.edu.au
Johannes B. Prins, Email: john.prins@mater.uq.edu.au
Theo Vos, Email: tvos@uw.edu.
Wendy Demark-Wahnefried, Email: demark@uab.edu.
Elizabeth G. Eakin, Email: e.eakin@sph.uq.edu.au
References
- 1.Youlden DR, Cramb SM, Dunn NA, Muller JM, Pyke CM, Baade PD. The descriptive epidemiology of female breast cancer: an international comparison of screening, incidence, survival and mortality. Cancer Epidemiol. 2012;36(3):237–48. doi: 10.1016/j.canep.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Blanchard CM, Courneya KS, Stein K. Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society’s SCS-II. J Clin Oncol. 2008;26(13):2198–204. doi: 10.1200/JCO.2007.14.6217. [DOI] [PubMed] [Google Scholar]
- 3.Hair BY, Hayes S, Tse CK, Bell MB, Olshan AF. Racial differences in physical activity among breast cancer survivors: implications for breast cancer care. Cancer. 2014;120(14):2174–82. doi: 10.1002/cncr.28630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiralerspong S, Kim ES, Dong W, Feng L, Hortobagyi GN, Giordano SH. Obesity, diabetes, and survival outcomes in a large cohort of early-stage breast cancer patients. Ann Oncol. 2013;24(10):2506–14. doi: 10.1093/annonc/mdt224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mason C, Alfano CM, Smith AW, Wang CY, Neuhouser ML, Duggan C, et al. Long-term physical activity trends in breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2013;22(6):1153–61. doi: 10.1158/1055-9965.EPI-13-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan DS, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25(10):1901–14. doi: 10.1093/annonc/mdu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.George SM, Ballard-Barbash R, Shikany JM, Caan BJ, Freudenheim JL, Kroenke CH, et al. Better postdiagnosis diet quality is associated with reduced risk of death among postmenopausal women with invasive breast cancer in the women’s health initiative. Cancer Epidemiol Biomarkers Prev. 2014;23(4):575–83. doi: 10.1158/1055-9965.EPI-13-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhong S, Jiang T, Ma T, Zhang X, Tang J, Chen W, et al. Association between physical activity and mortality in breast cancer: a meta-analysis of cohort studies. Eur J Epidemiol. 2014;29(6):391–404. doi: 10.1007/s10654-014-9916-1. [DOI] [PubMed] [Google Scholar]
- 9.Lahart IM, Metsios GS, Nevill AM, Carmichael AR. Physical activity, risk of death and recurrence in breast cancer survivors: a systematic review and meta-analysis of epidemiological studies. Acta Oncol. 2015;54(5):635–54. doi: 10.3109/0284186X.2014.998275. [DOI] [PubMed] [Google Scholar]
- 10.Demark-Wahnefried W, Campbell KL, Hayes SC. Weight management and its role in breast cancer rehabilitation. Cancer. 2012;118(Suppl 8):S2277–S87. doi: 10.1002/cncr.27466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.George SM, Alfano CM, Neuhouser ML, Smith AW, Baumgartner RN, Baumgartner KB, et al. Better postdiagnosis diet quality is associated with less cancer-related fatigue in breast cancer survivors. J Cancer Surviv. 2014;8(4):680–7. doi: 10.1007/s11764-014-0381-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imayama I, Alfano CM, Neuhouser ML, George SM, Wilder Smith A, Baumgartner RN, et al. Weight, inflammation, cancer-related symptoms and health related quality of life among breast cancer survivors. Breast Cancer Res Treat. 2013;140(1):159–76. doi: 10.1007/s10549-013-2594-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidt ME, Chang-Claude J, Seibold P, Vrieling A, Heinz J, Flesch-Janys D, et al. Determinants of long-term fatigue in breast cancer survivors: results of a prospective patient cohort study. Psychooncology. 2015;24(1):40–6. doi: 10.1002/pon.3581. [DOI] [PubMed] [Google Scholar]
- 14.Gho SA, Steele JR, Jones SC, Munro BJ. Self-reported side effects of breast cancer treatment: a cross-sectional study of incidence, associations, and the influence of exercise. Cancer Causes Control. 2013;24(3):517–28. doi: 10.1007/s10552-012-0142-4. [DOI] [PubMed] [Google Scholar]
- 15.Wayne SJ, Baumgartner K, Baumgartner RN, Bernstein L, Bowen DJ, Ballard-Barbash R. Diet quality is directly associated with quality of life in breast cancer survivors. Breast Cancer Res Treat. 2006;96(3):227–32. doi: 10.1007/s10549-005-9018-6. [DOI] [PubMed] [Google Scholar]
- 16.Ligibel JA, Alfano CM, Courneya KS, Demark-Wahnefried W, Burger RA, Chlebowski RT, et al. American society of clinical oncology position statement on obesity and cancer. J Clin Oncol. 2014;32(31):3568–74. doi: 10.1200/JCO.2014.58.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodwin PJ, Segal RJ, Vallis M, Ligibel JA, Pond GR, Robidoux A, et al. Randomized trial of a telephone-based weight loss intervention in postmenopausal women with breast cancer receiving letrozole: the LISA trial. J Clin Oncol. 2014;32(21):2231–9. doi: 10.1200/JCO.2013.53.1517. [DOI] [PubMed] [Google Scholar]
- 18.Harrigan M, Cartmel B, Loftfield E, Sanft T, Chagpar AB, Zhou Y, et al. Randomized trial comparing telephone versus in-person weight loss counseling on body composition and circulating biomarkers in women treated for breast cancer: The Lifestyle, Exercise, and Nutrition (LEAN) study. J Clin Oncol. 2016;34(7):669–76. doi: 10.1200/JCO.2015.61.6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reeves MM, Terranova CO, Eakin EG, Demark-Wahnefried W. Weight loss intervention trials in women with breast cancer: a systematic review. Obes Rev. 2014;15(9):749–68. doi: 10.1111/obr.12190. [DOI] [PubMed] [Google Scholar]
- 20.Rock CL, Flatt SW, Byers TE, Colditz GA, Demark-Wahnefried W, Ganz PA, et al. Results of the Exercise and Nutrition to Enhance Recovery and Good Health for You (ENERGY) Trial: a behavioral weight loss intervention in overweight or obese breast cancer survivors. J Clin Oncol. 2015;33(28):3169–76. doi: 10.1200/JCO.2015.61.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ligibel JA, Alfano CM, Hershman D, Ballard RM, Bruinooge SS, Courneya KS, et al. Recommendations for obesity clinical trials in cancer survivors: American Society of Clinical Oncology statement. J Clin Oncol. 2015;33(33):3961–7. doi: 10.1200/JCO.2015.63.1440. [DOI] [PubMed] [Google Scholar]
- 22.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. Circulation. 2014;129(25 Suppl 2):S102–38. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Health and Medical Research Council. Clinical practice guidelines for the management of overweight and obesity in adults, adolescents and children in Australia. Melbourne: National Health and Medical Research Council; 2013.
- 24.Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243–74. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 25.World Cancer Research Fund, American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington DC: 2007.
- 26.Reeves MM, Winkler EAH, McCarthy N, Lawler SP, Terranova CO, Hayes SC, Janda M, Demark-Wahnefried W, Eakin EG. The Living Well after Breast Cancer™ Pilot Trial: A weight loss intervention for women following treatment for breast cancer. Asia Pac J Clin Oncol. 2016; (in press; accepted 11/09/2016). [DOI] [PubMed]
- 27.Spark LC, Fjeldsoe BS, Eakin EG, Reeves MM. Efficacy of a text message-delivered extended contact intervention on maintenance of weight loss, physical activity, and dietary behavior change. JMIR Mhealth Uhealth. 2015;3(3):e88. doi: 10.2196/mhealth.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. American College of Sports Medicine position stand - appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41(2):459–71. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 29.Hayes SC, Spence RR, Galvao DA, Newton RU. Australian Association for Exercise and Sport Science position stand: optimising cancer outcomes through exercise. J Sci Med Sport. 2009;12(4):428–34. doi: 10.1016/j.jsams.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Biswas A, Oh PI, Faulkner GE, Bajaj RR, Silver MA, Mitchell MS, et al. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta-analysis. Ann Intern Med. 2015;162(2):123–32. doi: 10.7326/M14-1651. [DOI] [PubMed] [Google Scholar]
- 31.Wilmot EG, Edwardson CL, Achana FA, Davies MJ, Gorely T, Gray LJ, et al. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis. Diabetologia. 2012;55(11):2895–905. doi: 10.1007/s00125-012-2677-z. [DOI] [PubMed] [Google Scholar]
- 32.Aune D, Norat T, Leitzmann M, Tonstad S, Vatten LJ. Physical activity and the risk of type 2 diabetes: a systematic review and dose–response meta-analysis. Eur J Epidemiol. 2015;30(7):529–42. doi: 10.1007/s10654-015-0056-z. [DOI] [PubMed] [Google Scholar]
- 33.Woodcock J, Franco OH, Orsini N, Roberts I. Non-vigorous physical activity and all-cause mortality: systematic review and meta-analysis of cohort studies. Int J Epidemiol. 2011;40(1):121–38. doi: 10.1093/ije/dyq104. [DOI] [PubMed] [Google Scholar]
- 34.Institute of Medicine of the National Academies. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients). 2005. Available from URL: http://www.nap.edu/openbook.php?record_id=10490&page=R1. Accessed 02 Nov 2015. [DOI] [PubMed]
- 35.Bandura A. Social foundations of thought and action: a social cognitive theory. Englewood Cliffs: Prentice Hall; 1986. [Google Scholar]
- 36.Emmons KM, Rollnick S. Motivational interviewing in health care settings. Opportunities and limitations. Am J Prev Med. 2001;20(1):68–74. doi: 10.1016/S0749-3797(00)00254-3. [DOI] [PubMed] [Google Scholar]
- 37.Fjeldsoe B, Phongsavan P, Bauman A, Goode A, Maher G, Eakin E. ‘Get Healthy, Stay Healthy’: protocol for evaluation of a lifestyle intervention delivered by text-message following the Get Healthy Information and Coaching Service(R) BMC Public Health. 2014;14:112. doi: 10.1186/1471-2458-14-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ross R, Berentzen T, Bradshaw A, Janssen I, Kahn H, Katzmarzyk P, et al. Does the relationship between waist circumference, morbidity and mortality depend on measurement protocol for waist circumference? Obes Rev. 2008;9(4):312–25. doi: 10.1111/j.1467-789X.2007.00411.x. [DOI] [PubMed] [Google Scholar]
- 39.Villasenor A, Ballard-Barbash R, Baumgartner K, Baumgartner R, Bernstein L, McTiernan A, et al. Prevalence and prognostic effect of sarcopenia in breast cancer survivors: the HEAL Study. J Cancer Surviv. 2012;6(4):398–406. doi: 10.1007/s11764-012-0234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. 2010;39(4):412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henry MJ, Pasco JA, Pocock NA, Nicholson GC, Kotowicz MA. Reference ranges for bone densitometers adopted Australia-wide: Geelong osteoporosis study. Australas Radiol. 2004;48(4):473–5. doi: 10.1111/j.1440-1673.2004.01351.x. [DOI] [PubMed] [Google Scholar]
- 42.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 43.Braithwaite D, Moore DH, Satariano WA, Kwan ML, Hiatt RA, Kroenke C, et al. Prognostic impact of comorbidity among long-term breast cancer survivors: results from the LACE study. Cancer Epidemiol Biomarkers Prev. 2012;21(7):1115–25. doi: 10.1158/1055-9965.EPI-11-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patnaik JL, Byers T, Diguiseppi C, Denberg TD, Dabelea D. The influence of comorbidities on overall survival among older women diagnosed with breast cancer. J Natl Cancer Inst. 2011;103(14):1101–11. doi: 10.1093/jnci/djr188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCaskill-Stevens W, Abrams JS. Comorbidities in the aging breast cancer population: are current assessments leading to improved outcomes? J Natl Cancer Inst. 2011;103(14):1072–3. doi: 10.1093/jnci/djr239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 47.Johnson JL, Slentz CA, Houmard JA, Samsa GP, Duscha BD, Aiken LB, et al. Exercise training amount and intensity effects on metabolic syndrome (from Studies of a Targeted Risk Reduction Intervention through Defined Exercise) Am J Cardiol. 2007;100(12):1759–66. doi: 10.1016/j.amjcard.2007.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas GA, Alvarez-Reeves M, Lu L, Yu H, Irwin ML. Effect of exercise on metabolic syndrome variables in breast cancer survivors. Int J Endocrinol. 2013;2013:168797. doi: 10.1155/2013/168797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Curb JD, Ceria-Ulep CD, Rodriguez BL, Grove J, Guralnik J, Willcox BJ, et al. Performance-based measures of physical function for high-function populations. J Am Geriatr Soc. 2006;54(5):737–42. doi: 10.1111/j.1532-5415.2006.00700.x. [DOI] [PubMed] [Google Scholar]
- 50.Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res. 2009;18(7):873–80. doi: 10.1007/s11136-009-9496-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13(2):63–74. doi: 10.1016/S0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 52.Van Belle S, Paridaens R, Evers G, Kerger J, Bron D, Foubert J, et al. Comparison of proposed diagnostic criteria with FACT-F and VAS for cancer-related fatigue: proposal for use as a screening tool. Support Care Cancer. 2005;13(4):246–54. doi: 10.1007/s00520-004-0734-y. [DOI] [PubMed] [Google Scholar]
- 53.Greene JG. Constructing a standard climacteric scale. Maturitas. 1998;29(1):25–31. doi: 10.1016/S0378-5122(98)00025-5. [DOI] [PubMed] [Google Scholar]
- 54.Somunkiran A, Erel CT, Demirci F, Senturk ML. The effect of tibolone versus 17beta-estradiol on climacteric symptoms in women with surgical menopause: a randomized, cross-over study. Maturitas. 2007;56(1):61–8. doi: 10.1016/j.maturitas.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 55.Greene JG. Menopause Symptoms: Climacteric Scale. 2015. Available from URL: http://www.menopausematters.co.uk/greenescale.php. Accessed 15 Mar 2016.
- 56.Hormes JM, Lytle LA, Gross CR, Ahmed RL, Troxel AB, Schmitz KH. The body image and relationships scale: development and validation of a measure of body image in female breast cancer survivors. J Clin Oncol. 2008;26(8):1269–74. doi: 10.1200/JCO.2007.14.2661. [DOI] [PubMed] [Google Scholar]
- 57.Speck RM, Gross CR, Hormes JM, Ahmed RL, Lytle LA, Hwang WT, et al. Changes in the Body Image and Relationship Scale following a one-year strength training trial for breast cancer survivors with or at risk for lymphedema. Breast Cancer Res Treat. 2010;121(2):421–30. doi: 10.1007/s10549-009-0550-7. [DOI] [PubMed] [Google Scholar]
- 58.Thewes B, Zachariae R, Christensen S, Nielsen T, Butow P. The Concerns About Recurrence Questionnaire: validation of a brief measure of fear of cancer recurrence amongst Danish and Australian breast cancer survivors. J Cancer Surviv. 2015;9(1):68–79. doi: 10.1007/s11764-014-0383-1. [DOI] [PubMed] [Google Scholar]
- 59.Stanton AL, Bernaards CA, Ganz PA. The BCPT symptom scales: a measure of physical symptoms for women diagnosed with or at risk for breast cancer. J Natl Cancer Inst. 2005;97(6):448–56. doi: 10.1093/jnci/dji069. [DOI] [PubMed] [Google Scholar]
- 60.Swenson KK, Nissen MJ, Henly SJ, Maybon L, Pupkes J, Zwicky K, et al. Identification of tools to measure changes in musculoskeletal symptoms and physical functioning in women with breast cancer receiving aromatase inhibitors. Oncol Nurs Forum. 2013;40(6):549–57. doi: 10.1188/13.ONF.549-557. [DOI] [PubMed] [Google Scholar]
- 61.Hausheer FH, Schilsky RL, Bain S, Berghorn EJ, Lieberman F. Diagnosis, management, and evaluation of chemotherapy-induced peripheral neuropathy. Semin Oncol. 2006;33(1):15–49. doi: 10.1053/j.seminoncol.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 62.Shimozuma K, Ohashi Y, Takeuchi A, Aranishi T, Morita S, Kuroi K, et al. Feasibility and validity of the Patient Neurotoxicity Questionnaire during taxane chemotherapy in a phase III randomized trial in patients with breast cancer: N-SAS BC 02. Support Care Cancer. 2009;17(12):1483–91. doi: 10.1007/s00520-009-0613-7. [DOI] [PubMed] [Google Scholar]
- 63.Shimozuma K, Ohashi Y, Takeuchi A, Aranishi T, Morita S, Kuroi K, et al. Taxane-induced peripheral neuropathy and health-related quality of life in postoperative breast cancer patients undergoing adjuvant chemotherapy: N-SAS BC 02, a randomized clinical trial. Support Care Cancer. 2012;20(12):3355–64. doi: 10.1007/s00520-012-1492-x. [DOI] [PubMed] [Google Scholar]
- 64.Kozey-Keadle S, Libertine A, Lyden K, Staudenmayer J, Freedson PS. Validation of wearable monitors for assessing sedentary behavior. Med Sci Sports Exerc. 2011;43(8):1561–7. doi: 10.1249/MSS.0b013e31820ce174. [DOI] [PubMed] [Google Scholar]
- 65.Kelly LA, McMillan DG, Anderson A, Fippinger M, Fillerup G, Rider J. Validity of actigraphs uniaxial and triaxial accelerometers for assessment of physical activity in adults in laboratory conditions. BMC Med Phys. 2013;13(1):5. doi: 10.1186/1756-6649-13-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Winkler EA, Gardiner PA, Clark BK, Matthews CE, Owen N, Healy GN. Identifying sedentary time using automated estimates of accelerometer wear time. Br J Sports Med. 2012;46(6):436–42. doi: 10.1136/bjsm.2010.079699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30(5):777–81. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 68.Healy GN, Winkler EA, Owen N, Anuradha S, Dunstan DW. Replacing sitting time with standing or stepping: associations with cardio-metabolic risk biomarkers. Eur Heart J. 2015;36(39):2643–9. doi: 10.1093/eurheartj/ehv308. [DOI] [PubMed] [Google Scholar]
- 69.Australian Institute of Health and Welfare. The Active Australia Survey: A Guide and Manual for Implementation, Analysis and Reporting. Canberra: 2003.
- 70.Brown WJ, Burton NW, Marshall AL, Miller YD. Reliability and validity of a modified self-administered version of the Active Australia physical activity survey in a sample of mid-age women. Aust N Z J Public Health. 2008;32(6):535–41. doi: 10.1111/j.1753-6405.2008.00305.x. [DOI] [PubMed] [Google Scholar]
- 71.Brown WJ, Trost SG, Bauman A, Mummery K, Owen N. Test-retest reliability of four physical activity measures used in population surveys. J Sci Med Sport. 2004;7(2):205–15. doi: 10.1016/S1440-2440(04)80010-0. [DOI] [PubMed] [Google Scholar]
- 72.Lee WY, Clark BK, Winkler E, Eakin EG, Reeves MM. Responsiveness to Change of Self-Report and Device-Based Physical Activity Measures in the Living Well With Diabetes Trial. J Phys Act Health. 2015;12(8):1082–7. doi: 10.1123/jpah.2013-0265. [DOI] [PubMed] [Google Scholar]
- 73.Reeves MM, Marshall AL, Owen N, Winkler EA, Eakin EG. Measuring physical activity change in broad-reach intervention trials. J Phys Act Health. 2010;7(2):194–202. doi: 10.1123/jpah.7.2.194. [DOI] [PubMed] [Google Scholar]
- 74.Johnson MF, Sallis JF, Hovell MF. Self-report assessment of walking: effects of aided recall instructions and item order. Meas Phys Educ Exerc Sci. 2000;4(3):141–55. doi: 10.1207/S15327841Mpee0403_1. [DOI] [Google Scholar]
- 75.Conway JM, Ingwersen LA, Vinyard BT, Moshfegh AJ. Effectiveness of the US Department of Agriculture 5-step multiple-pass method in assessing food intake in obese and nonobese women. Am J Clin Nutr. 2003;77(5):1171–8. doi: 10.1093/ajcn/77.5.1171. [DOI] [PubMed] [Google Scholar]
- 76.Coyne T, Ibiebele TI, McNaughton S, Rutishauser IHE, O’Dea K, Hodge AM, et al. Evaluation of brief dietary questions to estimate vegetable and fruit consumption using serum carotenoids and red-cell folate. Public Health Nutr. 2005;8(3):298–308. doi: 10.1079/PHN2004688. [DOI] [PubMed] [Google Scholar]
- 77.Rutishauser IHE, Webb K, Abraham B, Allsopp R. Evaluation of short dietary questions from the 1995 National Nutrition Survey. Canberra: Australian Government Department of Health and Ageing; 2001. [Google Scholar]
- 78.Australian Bureau of Statistics . Australian Standard Geographical Classification (ASGC) Remoteness Area Correspondences, 2006. Canberra: ABS; 2011. [Google Scholar]
- 79.Australian Bureau of Statistics . Census of Population and Housing: Socio-Economic Indexes for Areas (SEIFA), Australia, 2011. Canberra: ABS; 2013. [Google Scholar]
- 80.PROMIS. Depression: A brief guide to the PROMIS depression instruments. Available from URL: http://www.assessmentcenter.net/documents/PROMIS%20Depression%20Scoring%20Manual.pdf. Accessed 30 Aug 2015.
- 81.Amtmann D, Kim J, Chung H, Bamer AM, Askew RL, Wu S, et al. Comparing CESD-10, PHQ-9, and PROMIS depression instruments in individuals with multiple sclerosis. Rehabil Psychol. 2014;59(2):220–9. doi: 10.1037/a0035919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rodgers WM, Brawley LR. The role of outcome expectancies in participation motivation. J Sport Exerc Psychol. 1991;13:411–27. doi: 10.1123/jsep.13.4.411. [DOI] [Google Scholar]
- 83.Zunft HJ, Friebe D, Seppelt B, de Graaf C, Margetts B, Schmitt A, et al. Perceived benefits of healthy eating among a nationally-representative sample of adults in the European Union. Eur J Clin Nutr. 1997;51(Suppl 2):S41–6. [PubMed] [Google Scholar]
- 84.Courneya KS, Jones LW, Mackey JR, Fairey AS. Exercise beliefs of breast cancer survivors before and after participation in a randomized controlled trial. Int J Behav Med. 2006;13(3):259–64. doi: 10.1207/s15327558ijbm1303_10. [DOI] [PubMed] [Google Scholar]
- 85.Petosa SP. Use of social cognitive theory to explain exercise behavior among adults. Ohio: The Ohio State University; 1993. [Google Scholar]
- 86.Linde JA, Rothman AJ, Baldwin AS, Jeffery RW. The impact of self-efficacy on behavior change and weight change among overweight participants in a weight loss trial. Health Psychol. 2006;25(3):282–91. doi: 10.1037/0278-6133.25.3.282. [DOI] [PubMed] [Google Scholar]
- 87.Marcus BH, Selby VC, Niaura RS, Rossi JS. Self-efficacy and the stages of exercise behavior change. Res Q Exerc Sport. 1992;63(1):60–6. doi: 10.1080/02701367.1992.10607557. [DOI] [PubMed] [Google Scholar]
- 88.Sallis JF, Grossman RM, Pinski RB, Patterson TL, Nader PR. The development of scales to measure social support for diet and exercise behaviors. Prev Med. 1987;16(6):825–36. doi: 10.1016/0091-7435(87)90022-3. [DOI] [PubMed] [Google Scholar]
- 89.Alexander A, Bergman P, Hagströmer M, Sjöström M. IPAQ environmental module; reliability testing. J Public Health. 2006;14(2):76–80. doi: 10.1007/s10389-005-0016-2. [DOI] [Google Scholar]
- 90.Dibsdall LA, Lambert N, Bobbin RF, Frewer LJ. Low-income consumers’ attitudes and behaviour towards access, availability and motivation to eat fruit and vegetables. Public Health Nutr. 2003;6(2):159–68. doi: 10.1079/PHN2002412. [DOI] [PubMed] [Google Scholar]
- 91.Moore LV, Diez Roux AV, Nettleton JA, Jacobs DR., Jr Associations of the local food environment with diet quality--a comparison of assessments based on surveys and geographic information systems: the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2008;167(8):917–24. doi: 10.1093/aje/kwm394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jen KL, Djuric Z, DiLaura NM, Buison A, Redd JN, Maranci V, et al. Improvement of metabolism among obese breast cancer survivors in differing weight loss regimens. Obes Res. 2004;12(2):306–12. doi: 10.1038/oby.2004.38. [DOI] [PubMed] [Google Scholar]
- 93.Cella D, Yount S, Sorensen M, Chartash E, Sengupta N, Grober J. Validation of the Functional Assessment of Chronic Illness Therapy Fatigue Scale relative to other instrumentation in patients with rheumatoid arthritis. J Rheumatol. 2005;32(5):811–9. [PubMed] [Google Scholar]
- 94.Fisher MI, Davies C, Beuthin C, Colon G, Zoll B, Pfalzer L. Breast Cancer EDGE Task Force outcomes - clinical measures of strength and muscular endurance: a systematic review. Rehabil Oncol. 2014;32(4):6–15. [Google Scholar]
- 95.Hayes SC, Rye S, Disipio T, Yates P, Bashford J, Pyke C, et al. Exercise for health: a randomized, controlled trial evaluating the impact of a pragmatic, translational exercise intervention on the quality of life, function and treatment-related side effects following breast cancer. Breast Cancer Res Treat. 2013;137(1):175–86. doi: 10.1007/s10549-012-2331-y. [DOI] [PubMed] [Google Scholar]
- 96.Vardar Yagli N, Sener G, Arikan H, Saglam M, Inal Ince D, Savci S, et al. Do yoga and aerobic exercise training have impact on functional capacity, fatigue, peripheral muscle strength, and quality of life in breast cancer survivors? Integr Cancer Ther. 2015;14(2):125–32. doi: 10.1177/1534735414565699. [DOI] [PubMed] [Google Scholar]
- 97.Forster M, Veerman JL, Barendregt JJ, Vos T. Cost-effectiveness of diet and exercise interventions to reduce overweight and obesity. Int J Obes. 2011;35(8):1071–8. doi: 10.1038/ijo.2010.246. [DOI] [PubMed] [Google Scholar]
- 98.Vos T, Carter R, Barendregt J, Mihalopoulos C, Veerman JL, Magnus A, et al. Assessing Cost-Effectiveness in Prevention (ACE-Prevention): final report. Melbourne: The University of Queensland, Brisbane and Deakin University; 2010. [Google Scholar]
- 99.Murray CJ, Barber RM, Foreman KJ, Abbasoglu Ozgoren A, Abd-Allah F, Abera SF, et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet. 2015;386(10009):2145–91. doi: 10.1016/S0140-6736(15)61340-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.D’Agostino RB, Russell MW, Huse DM, Ellison RC, Silbershatz H, Wilson PW, et al. Primary and subsequent coronary risk appraisal: new results from the Framingham study. Am Heart J. 2000;139(2 Pt 1):272–81. doi: 10.1016/S0002-8703(00)90236-9. [DOI] [PubMed] [Google Scholar]
- 101.Dansinger ML, Tatsioni A, Wong JB, Chung M, Balk EM. Meta-analysis: the effect of dietary counseling for weight loss. Ann Intern Med. 2007;147(1):41–50. doi: 10.7326/0003-4819-147-1-200707030-00007. [DOI] [PubMed] [Google Scholar]
- 102.Salomon JA, Haagsma JA, Davis A, de Noordhout CM, Polinder S, Havelaar AH, et al. Disability weights for the Global Burden of Disease 2013 study. Lancet Glob Health. 2015;3(11):e712–23. doi: 10.1016/S2214-109X(15)00069-8. [DOI] [PubMed] [Google Scholar]
- 103.Jacobs P, Fassbender K. The measurement of indirect costs in the health economics evaluation literature. A review. Int J Technol Assess Health Care. 1998;14(4):799–808. doi: 10.1017/S0266462300012095. [DOI] [PubMed] [Google Scholar]
- 104.Vos T, Carter R, Doran C, Anderson I, Lopez AD, Wilson A. Assessing Cost-Effectiveness in the Prevention of Non-Communicable Disease (ACE–Prevention) Economic Evaluation Protocol. Brisbane: The University of Queensland; 2007. [Google Scholar]
- 105.Befort CA, Klemp JR, Austin HL, Perri MG, Schmitz KH, Sullivan DK, et al. Outcomes of a weight loss intervention among rural breast cancer survivors. Breast Cancer Res Treat. 2012;132(2):631–9. doi: 10.1007/s10549-011-1922-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Djuric Z, DiLaura NM, Jenkins I, Darga L, Jen CK, Mood D, et al. Combining weight-loss counseling with the weight watchers plan for obese breast cancer survivors. Obes Res. 2002;10(7):657–65. doi: 10.1038/oby.2002.89. [DOI] [PubMed] [Google Scholar]
- 107.Harris MN, Swift DL, Myers VH, Earnest CP, Johannsen NM, Champagne CM, et al. Cancer survival through lifestyle change (CASTLE): a pilot study of weight loss. Int J Behav Med. 2013;20(3):403–12. doi: 10.1007/s12529-012-9234-5. [DOI] [PubMed] [Google Scholar]
- 108.Eakin EG, Hayes SC, Haas MR, Reeves MM, Vardy JL, Boyle F, et al. Healthy Living after Cancer: a dissemination and implementation study evaluating a telephone-delivered healthy lifestyle program for cancer survivors. BMC Cancer. 2015;15(1):992. doi: 10.1186/s12885-015-2003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


