Abstract
The precise wiring of the nervous system is a combined outcome of progressive and regressive events during development. Axon guidance and synapse formation intertwined with cell death and neurite pruning sculpt the mature circuitry. It is now well recognized that pruning of dendrites and axons as means to refine neuronal networks, is a wide spread phenomena required for the normal development of vertebrate and invertebrate nervous systems. Here we will review the arising principles of cellular and molecular mechanisms of neurite pruning. We will discuss these principles in light of studies in multiple neuronal systems, and speculate on potential explanations for the emergence of neurite pruning as a mechanism to sculpt the nervous system.
Keywords: Neuronal remodeling, Axon pruning, Axon degeneration, Neurodevelopment
Introduction
Since it was proposed in the middle of the 20th century, Roger Sperry’s chemoaffinity hypothesis [1] has been validated in many neuronal systems across various model organisms [2–4]. In its core, Sperry’s hypothesis postulated that each growing axon has a unique molecular identity that determines its attraction or repulsion from distinct local cues. Thus, according to Sperry, each axon “knows” where to go during the initial wiring of the nervous system or even when the circuit has to reassemble itself after injury. The discovery of the key families of cues that govern axon guidance, such as netrins, slits, semaphorins, and ephrins has provided the kind of molecular proof that the chemoaffinity hypothesis lacked for many years [5, 6]. Despite its beauty and simplicity, it has become evident in recent years that the chemoaffinity theory provides only a partial answer to how the wiring of the nervous system is established in vivo during development. Mounting evidence from multiple systems suggest that an exuberant network of neurons and connections is generated during the early stages of development and later remodeled by a wide variety of cellular strategies, as part of the normal course of network establishment and refinement and that this is not an anecdotal phenomenon [7–9]. These regressive events eliminate cells, synapses, and long stretches of axons in a precise and timely fashion during development and are essential to sculpt the mature nervous system of both vertebrates and invertebrates. The elimination of these exuberant connections is largely timed to postnatal development in vertebrates but the precise timing varies among organisms [7]. In humans, more than half of the neural connections formed during embryonic development are eliminated within the first 2 years of life and then further remodeled during puberty [10]. In insects, however, pruning takes place during metamorphosis when large-scale rearrangements occur within the entire nervous system [11].
The cellular and molecular mechanisms of neuronal remodeling are only starting to be elucidated. As with many phenomena in neurobiology, in retrospect we now realize that Ramon y Cajal made the initial observations recognizing these regressive events more than 100 years ago when he followed the development of avian Purkinje and mammalian granule cells. He noticed that these cells form an exuberant number of dendrites during development, many of which are later eliminated, in what he called “process resorption” (Fig. 1; [12]). In this review, we provide an overview of some classical examples in developmental neuronal remodeling and use these examples to discuss various cellular and molecular pathways that have emerged from classical and more recent studies, which have been performed mostly in the mouse and the fly. We do not aim to be comprehensive but rather provide our own view of the current principles arising from many studies in a wide variety of neuronal systems. As a consequence, we will neither discuss all of the neuronal systems that undergo remodeling nor all of the important work done in the field.
Fig. 1.
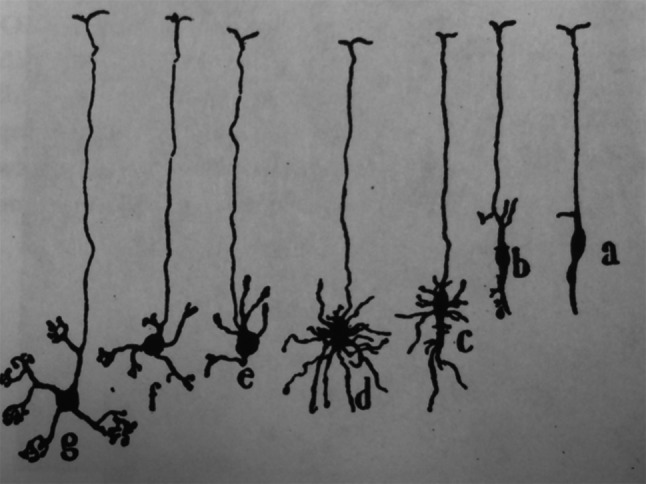
Early observations of neuronal remodeling. A drawing by Santiago Ramon y Cajal highlighting process elimination during granule cell development. As development proceeds (a–g), granule cells initially form exuberant dendrites (d) that are later eliminated (resorbed, in Ramon y Cajal’s own words; e) and undergo regrowth and maturation (f, g). Figure taken from [12], with permission from Oxford University Press (UK)
The term “developmental neuronal remodeling” is often used to describe a relatively wide range of biological processes including synapse elimination or strengthening, stereotypic and non-stereotypic axon elimination, and programmed cell death of specific neuronal populations. Several terms have been used extensively to describe these processes such as axon pruning, elimination, and degeneration. In this review, we will focus on processes that occur on the scale of axons and dendrites but not on the scale of individual synapses. Additionally, we will focus on remodeling of connections that do not involve neuronal cell death. (Other reviews focus on aspects of synapse elimination at the neuromuscular junction, climbing fibers or in the visual cortex [13–15]). For the sake of this review, we would like to begin by defining a few terms (shown in italics) to clarify their use in the context of this review. We define pruning of axons and dendrites as the process of neurite elimination that occurs during normal development and adult nervous system reorganization. Stereotyped pruning is a process in which one can predict the identity of the axonal or dendritic branches destined for elimination as well as the developmental stage in which this elimination will occur. In other words, pruning occurs with a temporal, cell specific, and spatial stereotypy in each and every individual. Examples for this type of pruning are the remodeling of layer 5 corticospinal (CST) connections in mammals and mushroom body (MB) neurons in Drosophila, both discussed in depth later in the review. In contrast, non-stereotyped pruning usually entails adaptation of the circuit to limiting factors or neuronal activity. A classic example is competition for neurotrophic factors in which sensory axons that were not able to make a functional connection and thus failed to internalize those factors, are eliminated [16]. We will be using neurite elimination as a broad term that includes pruning but also describes the elimination of axons in artificial conditions in vivo or in vitro. A classical example for this would be the axon elimination of explanted neurons (rat, mouse and chick) following the deprivation of nerve growth factor (NGF). The full extent by which axon elimination of artificial connections in vitro resembles axon pruning during development is not clear yet. Although axon degeneration is a term that can sometimes be used to describe pruning and subsequent elimination, in this review, we will use degeneration to describe pathological conditions during disease and following injury (for example, in Wallerian degeneration—see more below) while we will use fragmentation followed by clearance to describe developmental neurite pruning.
To many readers, generating an excess of neuronal connections only to get rid of them later might seem like a wasteful and inefficient strategy. Given our brain’s complexity, however, one explanation is that exuberant connections are needed to provide robustness in light of initial errors in connectivity. This might be true for non-stereotypic pruning where many axons might be “competing” for trophic signals and only one or few “win” while the “losing” axons are eliminated. However, many of the examples that we will review here focus on stereotyped pruning where one can predict which branch or axon will be eliminated even before the process has begun. The answer to “why” stereotyped pruning occurs as an evolutionary solution remains a mystery. In our concluding remarks, we will provide some speculations.
Cellular mechanisms of neurite pruning
Pruning by local fragmentation
For many years, it was known that nervous system development includes late regressive events. Indeed, about 50 % of murine neurons die during development and in early post-natal life [8]. One of the first studies to show that neuronal connections are eliminated during the normal course of development without the death of the soma was the discovery that layer 5 (L5) cortical neurons undergo selective and stereotypic axon elimination during early postnatal life (Fig. 2). Classic experiments by O’Leary, Stanfield and colleagues [17] using anterograde and retrograde labeling, first in rats and then in mice and other rodents [18, 19], have beautifully demonstrated that L5 cortical neurons from the motor and visual regions initially send identical projections to various targets including the spinal cord and superior colliculus [7, 20]. This “improper” connectivity is later resolved by selective axon elimination that takes place at the second to third week in rats [20]. Anterograde labeling followed by careful microscopic analysis has revealed that the exuberant connections undergo fragmentation [18] and are later removed by an unknown mechanism. The normal course of development of the layer 5 neuronal connectivity highlights the stereotypy of the process, the long stretches of axons that are eliminated in a timely fashion and the topographic restriction of the process implying that the borders of the axon fragmentation need to be extremely sharp.
Fig. 2.
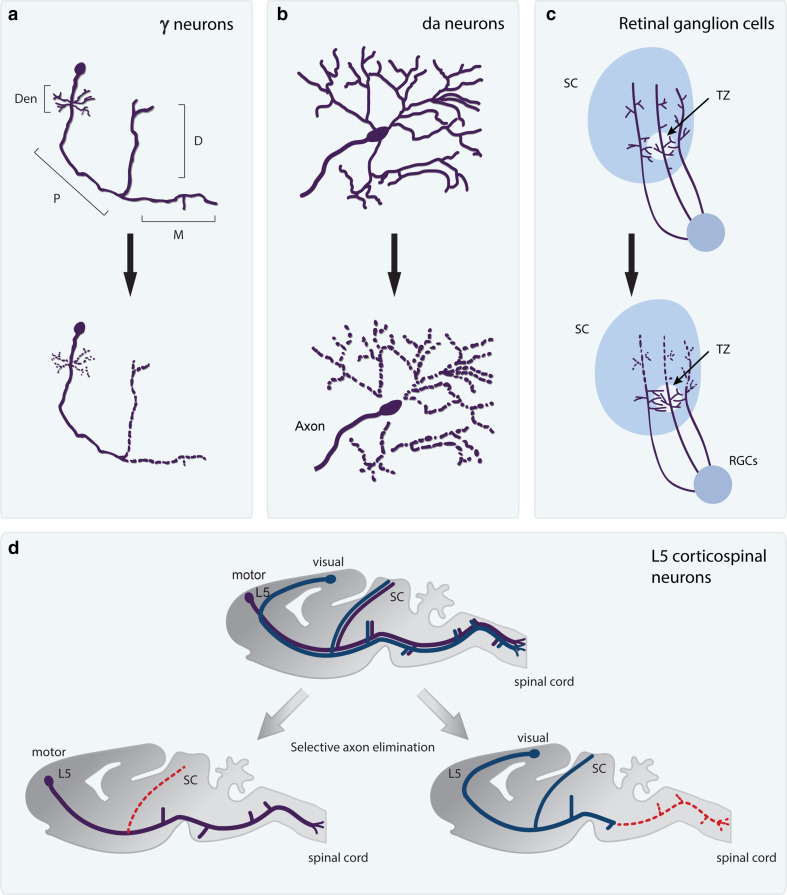
Pruning by local fragmentation. Localized neurite fragmentation is a widespread mechanism to eliminate unwanted connections. a Drosophila MB γ neurons eliminate dendrites and some parts of their axons in a stereotypic manner via localized fragmentation. While the dorsal (D) and medial (M) axonal branches as well as the dendrite (Den) undergo pruning, the peduncular axon (P) remains intact. b Dendrites of larval fly sensory da neurons are eliminated via severing following by localized fragmentation. The axons of these da neurons remain intact. c Murine retinal ganglion cell (RGCs) axons projecting to the superior colliculus (SC) overshoot their target zone (TZ) and then eliminate unnecessary connections via fragmentation. d Murine Layer 5 (L5) cortical neurons from the motor and visual regions initially send out identical projections to the spinal cord and visual areas including the superior colliculus (SC). Later, they undergo selective axon elimination via localized fragmentation (dashed red line) such that L5 cortical neurons from the visual area retain only their visual connections and vice versa. The schematics depicting RGCs and L5 cortical neurons were modified with permission from Luo and O’Leary [7]
Another example for temporally and spatially regulated developmental axon pruning that takes place by local fragmentation is the formation of the retinotopic map of retinal ganglion cells (RGC) at the superior colliculus (SC). Work pioneered by O’Leary and colleagues has shown that RGCs initially send long axonal processes that extend throughout the length of the SC [21]. In a process that is reviewed elsewhere [22], and is mediated by Ephrin-ephReceptor (ephR) counter gradients, each RGC sends an axon that extends almost through the entire SC, and later initiates sprouting in an area that will become the ‘target zone’. Soon thereafter, the overshot axon undergoes localized fragmentation that is instrumental in the formation of a stereotypical retinotopic mapping in the SC (Fig. 2). Interestingly, RGCs also remodel their connections with thalamic neurons in the dorsal lateral geniculate nucleus (dLGN) [23, 24] although the cellular mechanisms, fragmentation or retraction, remains unclear.
This type of timely and restricted axon fragmentation is observed in invertebrates as well. The massive stereotypic changes that occur in all tissues during metamorphosis in insects serve as an excellent model to study neuronal remodeling [11, 25, 26]. Neuronal remodeling of Drosophila mushroom body (MB) γ neurons is an attractive system as it involves temporal, spatial and cell-type stereotypy. The original observations that implicated that MB development includes axonal loss were elucidated from counting axon profiles in EM micrographs at various points during development [27]. Since then, techniques such as mosaic analysis with a repressible cell marker (MARCM) were developed and have revolutionized our ability to visualize and manipulate neurons in vivo in up to a single cell resolution [28]. Using MARCM, Luo and colleagues [29–31] have described the cellular sequence of events in great details: During the larval stages, MB γ neurons extend axons that bifurcate to both medial and dorsal lobes. At the onset of metamorphosis and in a timed and coordinated fashion, γ neurons prune their axons up to a specific branch point. By looking at single cell clones at different times during development, Watts and colleagues [30] have shown that MB γ neurons undergo localized fragmentation during development but the mechanism that limits this to only specific parts of the axons and dendrites is still not known. Later during development, γ axons regrow to an adult-specific, medial lobe. Thus, this is another example that demonstrates the need to eliminate long (‘long’ being relative to the organism size) stretches of axons in a tightly regulated temporal and spatial manner.
Both layer five cortical neuron and MB γ neuron remodeling involve axon pruning by localized axon fragmentation. However, in both systems it is not clear how this is achieved—does the fragmentation begin from the proximal or distal part of the axon or does it occur in a coordinated fashion all along the axonal branch? What determines the limit of fragmentation? Is there active protection of the axon that needs to remain intact, a specific tag on the border or an initial cut to define the fragmentation limit and if so by which mechanism? Genetic as well as live imaging experiments in both systems should help shed light on these open questions.
The mechanisms of neurite fragmentation are better understood, however, in another model of neuronal remodeling, that of the dendritic arborization (da) neurons in Drosophila [31–33]. The dendrites of these sensory neurons cover the larval body wall in a nicely tiled manner [34]. During metamorphosis, the dendrites, but not the axon of these neurons undergo stereotyped pruning. A combination of descriptive as well as molecular studies have suggested that the dendritic tree is first severed from its cell body and subsequently undergoes fragmentation in a process that, at least in part, depends on local caspase activity [32, 35, 36]. Imaging of glial cells has shown that they are localized to the sites of severing, although the precise role that they play in this process is unclear [37]. These findings propose that dendrite pruning in this system involves a ‘Wallerian degeneration’ like process (see more below). However, the extent of the molecular similarity between dendrite remodeling of da neurons and Wallerian degeneration is not yet clear. Whether other examples of developmental pruning involve neurite severing and whether axonal or dendritic severing is a common mechanism to define the limit of fragmentations remains to be further explored.
A major limitation to delineating the precise mechanism in which axon fragmentation occurs in vivo is the lack of good imaging systems that allow time-lapse imaging. Indeed, one of the reasons that da dendritic remodeling is better understood is the ability to perform live imaging due to the superficial location of the dendrites on the larval body wall. While transparent model organisms such as zebrafish have been used to study axon degeneration following injury [38, 39] and non-stereotyped remodeling during tiling of sensory axons [40] we are not aware of a defined system to study stereotypic developmental axon remodeling in this transparent animals. This, and other systems of live imaging, may help to discover the temporal and spatial aspects of axons fragmentation during development.
Axon elimination by fragmentation
Axon fragmentation as a mechanism to eliminate neurites is also common in cell-culture systems. When cultured dorsal root ganglion (DRG) or sympathetic neurons are deprived of trophic factors they undergo axonal fragmentation that eventually leads to cell death [41]. If grown in complex chambers, such as Campenot or microfluidics chambers, that allow the specific neurotrophic deprivation of axons but not of cell bodies, only axons undergo fragmentation while the cell bodies are spared [42]. Although these compartmentalized culture systems are widely used to study axon elimination in vitro, the exact developmental process that they mimic is less clear. One developmental process that resembles axon elimination following trophic deprivation is that of the elimination of sympathetic axons that innervate the eye [43]. These neurons initially extend axon branches to both the anterior and the posterior eye compartments. Later on, axon branches are eliminated without cell death through local fragmentation, such that each neuron projects to only one eye compartment [43]. More recently it was demonstrated that sensory axons that innervate the male mammary gland are pruned due to local deprivation of the trophic factor BDNF [44]. The cellular mechanism by which these axons are eliminated is not known. Finally, in dying-back neurodegenerative diseases such as amyotrophic lateral sclerosis (ALS; Lou Gehrig’s disease), spinal muscular atrophy, spinocerebellar disorders, and peripheral neuropathies, axon degeneration precedes cell death, thus resembling DRG axon elimination following trophic deprivation [7]. However, the dynamics of axon elimination and whether it occurs via fragmentation have not been well characterized in these pathological conditions.
Pruning by retraction
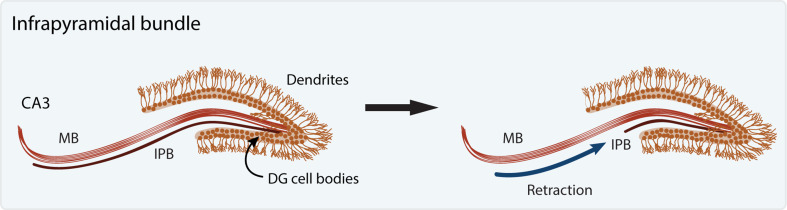
Axon retraction involves the reabsorption of axons and processes into the proximal part of the axon without the generation of fragments. While axon retraction has seemingly been documented, many times it is assumed as the most likely mechanism when no fragments are observed. For example, axon pruning of the hippocampal infrapyramidal bundle (IPB) in mammals is currently assumed to occur via axon retraction although this is mostly based on lack of fragmentation and direct evidence for retraction is lacking (Fig. 3; [45]). In contrast, direct evidence for axon retraction was obtained by long-term 2-photon in vivo imaging by Svoboda and colleagues [46]. Focusing on mouse thalamocortical and Cajal-Retzius neurons, the study revealed that while Cajal-Retzius neurons underwent small-scale axon elimination via retraction, thalamocortical neurons employed axon fragmentation to get rid of large stretches of axons and retraction to eliminate short pieces of axons. Thus, the current dogma is that long-range axon elimination occurs mainly via axon fragmentation and small-scale axon elimination occurs mainly via retraction [7].
Fig. 3.
Pruning by local retraction. Hippocampal granule cells in the dentate gyrus (DG) initially extend two axonal bundles, the main and infrapyramidal bundles (MB and IPB, respectively), that innervate the CA3 pyramidal cells. Subsequently the IPB is eliminated via apparent retraction while the MB remains intact
Pruning by axosome shedding
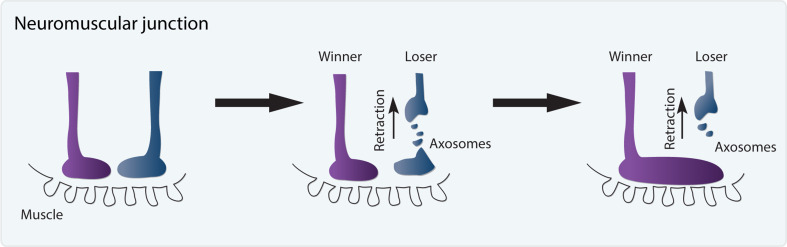
During development of the mammalian neuromuscular junction (NMJ) pervasive synapse elimination occurs after birth amounting to the elimination of ~90 % of existing synapses [47, 48]. This non-stereotypic remodeling is crucial for the maturation of the NMJ in which immature junctions that are poly-innervated by about ten axons mature into mono-innervated junctions [48]. Initially thought to be the hallmark of synapse retraction, new imaging techniques allowed Bishop, Misgeld and colleagues to visualize that small pieces of pruned axons were left behind in a process that they called axosome shedding [49] (Fig. 4). Axosomes are then engulfed by neighboring glia [49] and sent to degradation via the lysosomal pathway [50]. The fact that a process that was initially thought to involve axon retraction but with better imaging techniques was found to involve a strategy which is essentially a hybrid of retraction and fragmentation, raises the possibility that other events currently recognized as axon retraction might actually encompass axosome shedding. Interestingly, remodeling of the fly NMJ during metamorphosis, which involves the initial dismantling of postsynpase followed by dismantling of the presynapse, likely also involves axon retraction coupled with some form of axosome shedding [51, 52].
Fig. 4.
Pruning by axosome shedding. Motor axons compete for target innervation (muscle) during the formation of the neuromuscular junction (NMJ). The losing synapse is pruned by axosome shedding while the winning synapse expands to occupy the full junction
Wallerian degeneration and pruning
When Augustus Waller made his discovery in 1850, that axons severed from their cell body undergo fragmentation, the notion was that this process was passive wasting away due to lack of nutrients and energy. The serendipitous finding of the Wallerian degeneration slow (Wlds) mutant mouse [53] changed this dogma and highlighted Wallerian degeneration (WD) as an active process in which the axon is actively dismantled. While we will touch briefly on WD, good reviews focusing on the axonal [54] and glial [55] aspects of WD have been published elsewhere. Interestingly, axon fragmentation of WD is a biphasic process, beginning with a latent phase whose length varies between organisms, followed by a rapid catastrophic axonal fragmentation phase [38, 56–58]. It is not yet known whether developmental axon pruning can also be divided into such distinct phases.
WD is a highly conserved process and is studied in multiple organisms ranging from mice to zebrafish and Drosophila. In the fly, models to study WD include the olfactory receptor neuron axons, which can be separated from their cell body by tearing the antenna or maxillary pulps [18, 58, 59], larval motoneurons [58] and axons innervating the wing, which can be cut and directly visualized in live animals [60, 61]. Being able to compare WD and developmental axon pruning within the same organism is advantageous since using the same alleles and transgenes ensures consistency. While some molecular aspects are shared between these two systems, such as the involvement of the ubiquitin proteasome system and cell engulfment [18, 59], Wlds does not confer axon protection during MB pruning [18]. In contrast, Wlds does provide robust protection to the axons of trophic deprived DRG neurons [62] and a modest protection to dendrite pruning of da neurons, functioning in parallel to the apoptotic machinery [63, 64]. Several published and unpublished data suggest the existence of various molecules that are important for WD but not MB pruning and vice versa. For example, Sarm and Highwire that are both required for WD [65, 66] are not required during pruning of MB neurons [30, 65]. Similarly, Plum, a TGF-β accessory receptor (see more below) or the ecdysone receptor (EcR-B1) that are both required for MB pruning [28, 67] are not required for WD (Schuldiner, unpublished observation; [18]). Thus, the molecular similarities and differences between WD and various types of developmental neuronal remodeling remain to be further studied.
Fragment clearance of pruned connections
Axon pruning via axon fragmentation or by axosome shedding results in axonal fragments left behind. These, in turn, need to be cleared away in an efficient manner. The emerging theme from many studies in mammals and flies suggests that glia and other neighboring cells, such as epidermal cells, function as phagocytes to engulf the degenerated material.
As detailed above, axon pruning of fly MB neurons occurs via localized axon fragmentation. Two studies from the Ito and Luo labs have shown that glial membranes infiltrate the degenerating lobes [68, 69]. Subsequently, axonal fragments are engulfed by glia and undergo lysosomal degradation [68, 69]. Inhibiting endocytosis in glia by over expression of the temperature sensitive dominant negative allele of shibire (the Drosophila ortholog of dynamin) resulted in clearance defects [68]. In a follow-up study, both groups showed that the Drosophila homolog of the Ced-1 engulfment receptor, Draper, is required for glia-neuron recognition and clearance [18, 70].
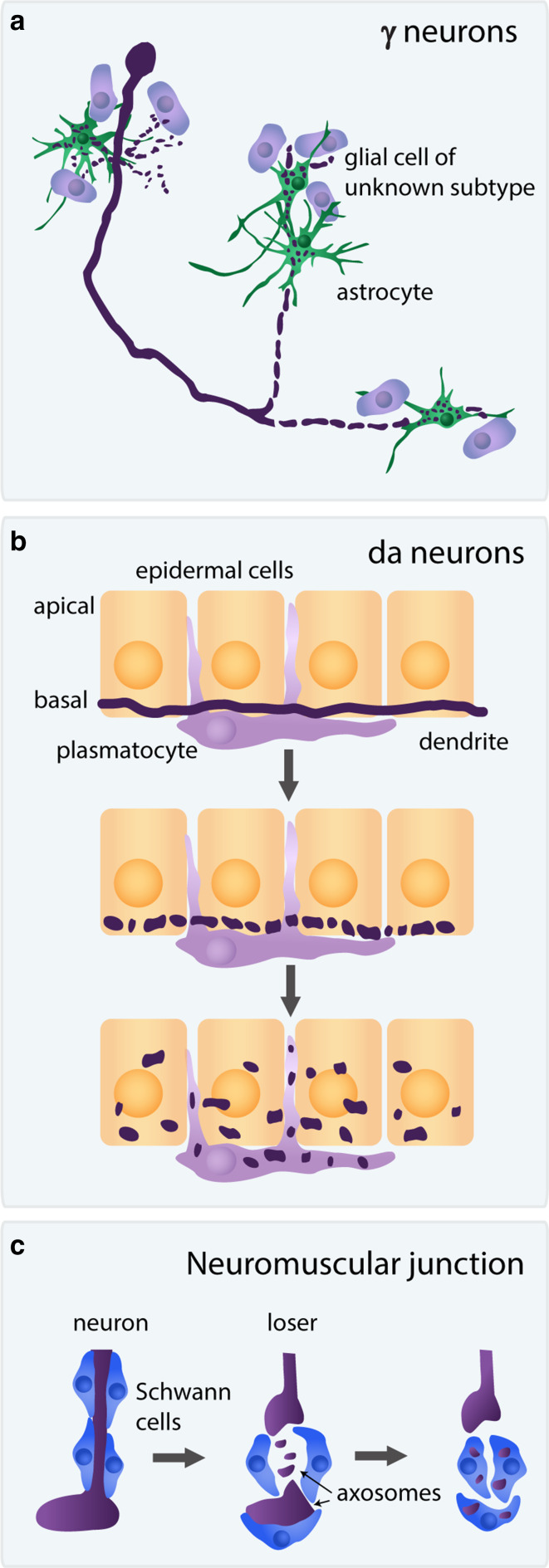
Glial sub-classification in Drosophila is primitive relative to their mammalian counterparts and is mostly based on morphology. Drosophila glia belong to three main groups: Surface-associated glia, which form the blood brain barrier (BBB), cortex associated glia, and neuropil associated glia which can be further divided into wrapping, ensheathing, and astrocyte-like glia [71, 72]. Previously, Freeman and colleagues have found that ensheathing glia function as phagocytes of degenerating olfactory axons undergoing WD [73]. In contrast, two recent studies, have found that astrocytes are the major glial cell type that engulfs debris of MB axons undergoing pruning, despite the fact that there are few astrocytes in close proximity to the degenerating lobes during remodeling [74, 75] (Fig. 5a).
Fig. 5.
Pruned neurites are cleared via engulfment by neighboring cells. a Fragmented Drosophila MB γ axons are engulfed and cleared by astrocytes (green). The role or subtype of other glial cells in the vicinity of the MB (purple) is currently not known. b Fragmented Drosophila dendrites of da neurons are engulfed predominantly by epidermal cells (yellow) and to a lesser extent also by plasmatocytes (purple). c Remnants of mouse motoneuron axosomes are engulfed by neighboring Schwann cells
Following dendrite severing and local degeneration, the dendritic debris of Drosophila da neurons is also removed in a timely fashion. Early studies have reported that plasmatocytes, phagocytic blood cells, are responsible for engulfing dendritic debris and in a few cases were seen to “attack” intact branches [32]. A recent study, however, suggests that the main cell type that is responsible for engulfing and clearing the degenerated dendrites are actually epidermal cells [76]. Whether the epidermal cells play a passive role by clearing the debris or might also be important for the induction of dendrite pruning is not yet clear. Additionally, the potential phagocytic role of glia around the da neurons has not been studied, to the best of our knowledge (Fig. 5b).
Compared to our rather detailed understanding of the roles of glia and epidermal cells in fragment removal in Drosophila and the roles of glia in synapse elimination in mammals, we know much less about how fragments of axons and dendrites are eliminated in mammals. Classical descriptive work regarding developmental axon elimination in the corpus callosum in cats suggest that similar cellular mechanisms are at play [77]. During postnatal development, the number of axons crossing between brain hemispheres via the corpus callosum decreases [78]. EM studies show that astrocytes and microglia intermingle with what seems to be degenerating axons during development [77]. Apart from this descriptive study, however, we know virtually nothing about the cellular and molecular mechanism of neurite fragment clearance in mammalian systems.
In contrast, we know much more about the role of microglia, astrocytes, and Schwann cells in the local pruning of synapses in the mammalian visual system, NMJ, and hippocampus [79]. At the NMJ, Schwann cells enwrap the axonal retraction bulb as well as engulf the axosomes left behind [49] which are then degraded by the lysosomal pathway within the Schwann cells [50] (Fig. 5c). Microglia were shown to play a key role in synapse remodeling in the hippocampus [80] and in the mouse visual system [81] in a process that depends on neuronal activity as well as glial expression of proteins that belong to the complement system. A recent study, however, has reported that in addition to microglia, astrocytes also play instructive roles in the elimination of synapses of retinoganglion cells (RGC) in the dorsal lateral geniculate nucleus (dLGN) of the thalamus [82]. The precise contribution of microglia and astrocytes to the elimination of synapses at the dLGN and their potential interaction remains unknown but one can speculate that both cell types participate in somewhat redundant engulfment processes, explaining the partial phenotypes when one cell type is eliminated [81, 82].
On the molecular level one of the key missing parts of the puzzle is the “eat me” signal on the axonal fragments that signals to the engulfing cell. One attractive candidate is phosphatidylserine (PS), which is the prime “eat me” signal on apoptotic cells [83]. Indeed, Draper, which is known to play a key role in the engulfment of apoptotic cells, was recently shown to bind directly to PS [84]. More recently, the mammalian engulfment receptors MERTK, which recognizes PS through the adaptor proteins GAS6 and PROTS1 [85], and MEGF10, the ced-1/Draper mammalian ortholog, were shown to mediate synapse elimination during RGC refinement at the dLGN [82]. However, whether pruned axonal fragments are indeed marked for clearance by PS remains to be determined.
Molecular control of pruning
Initiation of the pruning process—signaling by pruning receptors
How does a neuron “know” that the time has come to prune its neurite/s and how is the specific neurite branch selected? Although one can imagine a cell-autonomous developmental program that can govern these decisions in neurons, it seems that involvement of cell–cell signaling can facilitate these temporal and locally defined decisions. Indeed, several receptors have been identified in various systems that regulate the initiation of the pruning process. In general, the pruning receptors identified so far can be divided into three main categories: axon guidance receptors, receptors of the TGF-β family, and death receptors (Fig. 6).
Fig. 6.
Pruning receptors and signaling pathways. A schematic representation of molecules that are important for neuronal remodeling. Axon guidance receptors (Plexin-A3, Neuropillin-2, and eprin-B3), receptors of the TGF-β family, and death receptors (p75, DR6) induce pruning through diverse signaling pathways. These include the apoptotic machinery, actin regulators and transcription factors
Pruning by axon guidance receptors
Molecules originally identified as regulators of axonal guidance are now well recognized as multifactorial proteins, controlling different aspects of the wiring process as well as development of other tissues. Members of two major guidance molecule families, the semaphorins and ephrins, have been implicated in axon pruning. Studies in the mouse by Tessier-Lavigne and colleagues and Cheng and colleagues have found that stereotyped pruning of the InfraPyramidal Bundle (IPB) in the hippocampus and the corticospinal tract (CST) from layer 5 of the visual cortex are regulated by signaling complexes that are composed of Neuropilin-2 (Nrp2) and Plexin-A (PlxA) [45, 86, 87]. The ligand of the Nrp2/PlxA3 complex in the hippocampus was identified as Semaphorin3F (Sema3F), which is expressed in sporadic interneurons adjacent to the IPB and in its absence the IPB does not prune normally [45]. The expression of Sema3F by these interneurons is correlated with the onset of the pruning process, raising the hypothesis that Sema3F expression and binding by its receptor complex regulates pruning initiation. Whether the responding axons have to be primed in parallel to the expression of Sema3F and whether forced expression of Sema3F at an earlier developmental stage will induce pruning of these axons is currently unknown. Based on its expression pattern, Sema3F is also likely to trigger pruning of layer 5 visual neurons projecting to the CST, although genetic proof is still lacking [88]. Interestingly, it seems that semaphorin signaling does not function alone in promoting IPB pruning as other studies have also implicated reverse ephrin signaling in this process [89]. Although ephrins were initially described as ligands for the Eph reporters, it is now well recognized that reverse signaling by Eph receptors into ephrin-expressing cells play crucial roles during neurodevelopment [90]. By analyzing ephrin-B3 (EB3) null animals or animals that express a truncated form of EB3, lacking its cytoplasmic domain, Henkemeyer and colleagues have determined that EB3 reverse signaling is required for efficient pruning of the IPB [89]. Unlike the case of Sema3F, the expression of EB3 or its ligands do not seem to be developmentally regulated in a tight manner. Therefore, how EB3 signaling is restricted to a specific developmental stage is not clear. Additionally, the relationship between semaphorin and ephrin signaling and whether they play instructive versus permissive roles is not yet clear. One possibility is that signaling from the Sema3F receptor complex indirectly controls the reverse signaling by EB3. Another speculative and non-mutually exclusive possibility is that while Sema3F plays an instructive role, EB3 signaling is permissive in nature. More experiments are necessary to address these issues in detail.
Pruning by TGF-β receptors
In Drosophila, signaling by the TGF-β receptor complex controls developmental pruning of both MB axons [91] and da dendrites [31] (Fengwei Yu, personal communication). In MB neurons, the TGF-β receptor complex includes the type I receptor Baboon (Babo) and either one of the type II receptors Punt (Put) or Wishful thinking (Wit) [91]. In an elegant study, Lee and colleagues have found that the TGF-β ligand Myoglianin (Myo) is secreted by neighboring glia, mostly cortex glia and to a lesser extent also astrocytes [92]. More recently, we found that a novel immunoglobulin superfamily protein that we named Plum facilitates the signaling of the TGF-β receptor complex [67]. Although the biochemical basis for this facilitation is not completely understood, it appears that Plum might be involved in regulating the availability of the TGF-β ligand, Myo, to the canonical receptor complex.
The canonical TGF-β signaling pathway requires dSmad2 (SmoX; [91] ) and dSmad4 (Medea; Schuldiner, unpublished observations) to initiate the pruning program, largely through transcriptional induction of the steroid hormone Ecdysone Receptor-B1 (EcR-B1). However, there is no clear evidence that EcR-B1 is a direct target of the Smads and other direct Smad responsive genes that are required for pruning have not been identified. Additionally, it is not clear whether EcR-B1 activation is the only important outcome of TGF- β signaling in the context of pruning. While TGF-β signaling does regulate the expression of EcR-B1 within MB γ neurons, the precise temporal regulation is achieved by the systemic release of the steroid hormone ecdysone, which in turn modulates the activity of EcR-B1. Interestingly, activation of the Babo and Plum containing TGF-β receptor complex by Myo also regulates the elimination of ectopic larval connection at the fly NMJ via transcriptional control of EcR-B1, drawing a mechanistic connection between seemingly different developmental remodeling processes, as NMJ refinement is not stereotypic [67].
Pruning by death receptors
Axon elimination might be seen as the “death” of an axon. Indeed, this notion is supported by the finding that the apoptotic machinery is required for pruning in some, but not all, neuronal systems (see more below and reviewed in [93]) and is further strengthened by the discovery that two receptors belonging to the TNF superfamily, DR6/TNFSF21 and p75/TNFRSF16, are required for efficient pruning in the peripheral nervous system [43, 94].
The p75 neurotrophin receptor (P75NTR) has been implicated in multiple aspects of vertebrate neurobiology including axonal growth, neuronal cell death, and survival [95, 96]. The clearest example for the role of p75 in developmental axon pruning comes from the sympathetic system. Mouse sympathetic neurons initially project axons to both the anterior and posterior sides of the eye. During postnatal stages, they undergo refinement to establish a network in which each neuron innervates either the anterior or the posterior side of the eyes, but not both. By retrograde labeling of sympathetic neurons of the superior cervical ganglion (SCG) before and after refinement, Miller and colleagues found that this refinement involves axonal pruning that depends on p75 signaling which is triggered by activity dependent expression and axonal secretion of its ligand, brain derived neurotrophic factor (BDNF) [43]. Moreover, using a sophisticated culture system, the authors found that p75 is upregulated in unstimulated axons, which might provide a mechanism by which BDNF, secreted from active axons, acts specifically on inactive, ‘losing’ axons. Interestingly, a similar mechanism was proposed to control the outcome of induced axon competition in the mature mouse olfactory bulb [97]. To induce competition, the authors silenced synaptic activity by inducing the expression of the tetanus toxin light chain, which inhibits neurosecretory activity, in a small random subset of olfactory neurons. This block of activity induces the pruning of these axons by local degeneration. Importantly, when most of the olfactory neurons were silenced, no pruning was detected suggesting that competition, rather then lack of activity by itself induces the pruning process. Although both BDNF and p75 were suggested to regulate the pruning of these olfactory neurons following competition, the mechanistic details and the relevance to the sympathetic neuronal pruning remain to be further studied.
In the mouse sympathetic system, Miller and colleagues found that the p75 regulated pruning activity is mediated, at least in part, by suppressing survival signaling by the tropomyosin receptor kinase-A (TrkA), the receptor of NGF. Therefore, pruning might be an outcome of ‘reading’ NGF vs BDNF signals that is concomitantly translated into levels of TrkA signaling. This, however, seems unlikely to be the only mechanism, as p75 is known to activate the apoptotic system by itself and recent experiments in adult septal cholinergic axons revealed that p75 induces axon degeneration via direct interaction with RhoGDI and activation of Rho [98].
The second TNF receptor that was implicated in pruning is the death receptor 6 (DR6), which was initially identified in an expressed sequence tag (EST) search for TNF receptor-related molecules, and was shown to regulate lymphocyte differentiation in the adult animal [99]. Interestingly, expression of DR6 is developmentally regulated in various murine neuronal types as they differentiate both in the CNS and the PNS [94]. In vitro experiments demonstrated that ablation of DR6 delays axonal elimination in response to trophic withdrawal of multiple neuronal types. Moreover, the stereotyped pruning of RGC axons in the superior colliculus (SC) is attenuated in DR6 knockout mice, suggesting that DR6 might function as a global facilitator of axonal pruning [94]. How DR6 and p75 signaling relate to each other in vivo is not known and whether they induce pruning by similar intracellular mechanisms also remains to be determined.
Downstream elements: proteolytic systems
The destructive nature of neurite pruning ignited the idea that proteolytic systems might play an active role in the dismantling of axons and dendrites. Studies on the stereotyped axon pruning of MB neurons and dendrite pruning of sensory da neurons in Drosophila provided the first evidence for this hypothesis. Mutations in the single fly E1 enzyme of the ubiquitin system (Uba1) or in subunits of the proteasome blocked pruning of MB axons and da dendrites [30, 100]. Importantly, the expression of EcR-B1 in these mutants was normal, suggesting that the ubiquitin–proteasome system (UPS) operates either in parallel to or downstream of the EcR-B1 pathway [30]. Additional studies in da neurons demonstrated that the ubiquitin E2 protein UbcD1 is required for pruning. UbcD1 regulates the self-ubiquitination and proteasomal degeneration of the caspase inhibitor DIAP1 [36]. More recently, the SCF ubiquitin ligase complex Cullin1/Roc1a/SkpA/Slimb has also been shown to control da dendrite pruning, presumably through the degradation of Akt and attenuation of the insulin receptor signaling pathway to TOR, suggesting that at least in these neurons the insulin pathway antagonizes pruning [101]. The relative contribution of the DIAP1 vs Cullin1 dependent proteasome degradation remains to be determined.
In contrast to our knowledge about the roles of the UPS in the pruning of fly da neuron dendrites, the exact function of the UPS in the pruning of fly MB axons is less clear. Although the Cullin1/Roc1a/SkpA/Slimb complex has been implicated in the pruning of MB axons [101], the insulin pathway does not seem to antagonize pruning of MB γ neurons nor does the TOR pathway ([102]; Schuldiner, unpublished observations) Likewise, DIAP1 is not required for MB axon, or dendrite, pruning ([30]; Schuldiner, unpublished observations]). Whether the UPS functions at the initiation or execution of pruning or at both steps still needs to be further delineated. The studies on the role of UPS in pruning of mammalian axons have been limited so far to pharmacological inhibition of axonal elimination upon trophic-factor withdrawal [103]. There is no genetic support yet for these experiments and the ubiquitin system components involved remain unidentified.
Another set of proteases that have a major role in developmental pruning and axonal elimination are the caspases. Although initially discovered as executers of apoptotic cell death, it is now well established that these family of proteases are regulators of cell morphogenesis [104, 105]. Early studies on fly da neurons provided clear evidence that the initiator caspase, Dronc (the Casapses-9 homolog), and the executer caspases, Drice and DCP-1 (homologs of Casapse-3), are essential for developmental da dendrite pruning [35, 36, 63]. In contrast, these caspases are not required for axon or dendrite pruning of fly MB neurons (Schuldiner, unpublished observation) and activated caspases were not observed during MB remodeling [70]. Therefore, caspases do not seem to play a role in all of the developmental pruning processes, even within the same organism.
Studies in vertebrates have lagged behind but recently a well-defined role for caspases has been established both in axonal elimination upon trophic deprivation and developmental pruning. Caspase-9 and Caspase-3 were both found to be required for axonal elimination of sensory and sympathetic neurons upon trophic withdrawal in vitro [106, 107]. This requirement was shown in both global deprivations, in which cell death accompanies axonal elimination, as well as in axonal restricted deprivation using compartmentalized chambers. In contrast, Caspase-6 mediates axonal elimination only in the axon-only deprivation paradigm [107]. In addition to these studies it was demonstrated that Caspase-3 and -6 are needed for efficient developmental pruning of RGCs axons in the SC [106]. The mechanisms that allow the neuron to spatially control these death proteases are largely unknown.
Recent work has suggested upstream divergence between death and pruning, as death induction requires the apoptosis protease activating factor 1 (Apaf-1) while axon elimination does not, although both require the pro-apoptotic protein BAX [63, 94, 107]. Additional mechanisms involve the axonal local translation of the anti-apoptotic protein Bclw and axonal restriction of caspase activity by X-linked inhibitor of apoptosis protein (XIAP), the homolog of the fly DIAP1 [107–109].
Lastly, recent studies have provided strong evidence for the role of the calcium activated proteases calpains in neurite pruning [110, 111]. In the mouse, calapin activity is regulated through their intrinsic inhibitor calpastatin. Down regulation of calpastatin accelerated while overexpression of calpastatin inhibited axonal punning, both in vitro and in vivo [110]. In Drosophila, calcium transients through a voltage-gated calcium channel in da dendrites were postulated to activate calpains, which facilitate efficient pruning [111]. Interestingly while in the mouse the activation of calpains is downstream to caspase-3, in the fly caspases and calpains seem to be operating in parallel. Although the substrates of calpains in neurite pruning are not known, strong candidates are the axonal cytoskeletal elements and specifically neurofilaments in the mouse [112].
Overall, the emerging picture is that the neuron employs multiple proteolytic systems to initiate and execute the efficient breakdown of axons and dendrites. Importantly, as inhibition of each system interferes with pruning, it seems that each has its unique role and probably specific substrates, which remain to be discovered.
Downstream elements: kinases (IKK, GSK3, DLK/JNK)
Several protein kinases have also been implicated in axon pruning. The most compelling evidence exists for GSK3α and GSK3β, both required for axon degeneration in response to trophic deprivation in vitro, but only GSK3β plays a role during mouse RGCs axonal pruning in vivo [113]. GSK3s are negative regulators of many microtubule (MT) polymerization factors, controlling both their MT binding ability as well as their stability, which may be a key step in MT breakdown during pruning (see below). The importance of the DLK-JNK pathway for axonal breakdown has been demonstrated in vitro in response to trophic deprivation [114]. Interestingly, this pathway also controls neuronal cell death in response to trophic withdrawal, however, while JNK mediated cell death depends on the transcription factor c-jun, axon pruning seems to be c-jun independent [114]. Lastly, the fly IκB kinase (IKK) related kinase, Ik2, is required for dendrite pruning of da neurons [115]. This family of kinases may have a conserved function in pruning as pharmacological and in vitro knockdown experiments provided evidence that the mammalian IKK is required for axon degeneration upon trophic withdrawal [116].
Downstream elements: breakdown of the axonal cytoskeleton
The neuronal cytoskeleton consists of three major cytoskeletal elements: neurofilaments (NFs), actin, and microtubules. NFs have classically been considered to consist of three subunits, termed NF-H, NF-M, and NF-L, corresponding to heavy, medium, and light in reference to their molecular mass. NFs provide the structural support necessary to sustain axons whose volume is often thousands of times larger than the volume of the soma [117]. In addition, they increase the axonal caliber of myelinated axons and consequently their conduction velocity [118]. Actin filaments are thin polymers of the 42 kD protein actin. In mature axons, the actin filaments provide membrane stability and a transport substrate. In developing neurons, dynamic regulation of actin filaments is responsible for initiating, stimulating, and guiding axons as neural circuits are formed [119]. Microtubule (MT) is a tube constructed from parallel linear polymers—protofilaments. Each protofilament consists of heterodimers, α and β—tubulin, that are assembled head to tail in a polar fashion [120]. In neurons, MTs are essential for structural support, localization of organelles and intracellular trafficking. MTs are highly dynamic polymers whose assembly and disassembly is controlled by β tubulin guanosine triphosphate (GTP) hydrolyzation [121]. However, a multitude of associated proteins are able to fine-tune these dynamics so that MTs are assembled and disassembled at the appropriate time and in a specific place and compartment within the neuron.
Based on these functions it comes as no surprise that it is well believed that the breakdown of the axon cytoskeleton is a key step in the pruning process. Indeed, the disruption of the MTs was found to be one of the first steps during axonal pruning that occur by fragmentation in flies and in cultured mammalian neurons [30, 32, 103]. Moreover, MT destabilization is sufficient to induce axon fragmentation of cultured neurons [122]. Furthermore, the MT stabilizing agent paclitaxel is sufficient to protect MT depolymerization as well as axon fragmentation following trophic deprivation in vitro [123]. Interestingly, disruption of actin or NFs does not affect the distribution of MTs and other axon cytoskeleton structures [124]. Overall, these experiments suggested that breakdown of MT is a key step in axonal pruning. The molecular machinery that executes this breakdown is largely unknown. The Drosophila Katanin-like molecule, Kat60L, was recently found to be essential for MT disruption during dendrite severing of the da neurons but not for MB axon pruning [115]. In mouse sensory axons, the MT depolymerization kinesin Kif2a was found to regulate MTs breakdown in response to trophic deprivation in vitro. Remarkably, Kif2a KO mice manifest skin hyperinnervation suggesting that Kif2a is required for axon elimination in vivo [123]. Importantly, MT depolymerization is also promoted by degradation of MT polymerization factors such as Tau and Collapsin response mediator proteins CRMPs [123, 125, 126].
While MTs breakdown appears as a key step in pruning by fragmentation, it appears that actin dynamics drive retraction. By assessing the levels of the active (GTP bound) vs. inactive (GDP bound) forms of the actin regulator Rac1, Riccomagno et al. [127] have shown that Rac1 activity is attenuated during the pruning of the mouse IPB. Moreover, the authors suggest that this attenuation of Rac1 activity, which is essential for pruning of the IPB in vivo, is driven by the binding of the Sema3F receptor Neuropilin-2 to RacGAP β2-chimaerin [127]. Interestingly, β2-chimaerin is not required for axonal repulsion by Sema3F, suggesting that it is a specific pruning mediator [127].
Interestingly, ephrin reverse signaling was also shown to be required for IPB pruning [89]. In this context, ephrin reverse signaling activates Rac1 by binding to and recruiting the adaptor protein Grb4 and subsequently the RacGEF DOCK180 [89]. Why opposing modulations of Rac1 by Sema3F and ephrin reverse signaling are both required for pruning of the IPB is confusing and remains to be addressed. One possibility is that high and low Rac1 activities are required in distinct axonal domains. Alternatively, Rac1 function might be differentially required on a temporal axis; For example, the active form of Rac1, which was shown to control trafficking of Plexin [128], the semaphorin receptor, might be required at an early step to setup Plexin localization. Subsequently, and upon Sema3F stimulation, an attenuation of Rac1 activity might be required for modulating actin dynamics and axonal retraction. The importance of actin dynamics for pruning is also supported by the fact the MICAL, which depolymerizes actin through direct oxidation, is required for the pruning of fly da dendrites [129], although changes in actin dynamics in this pruning process have not been demonstrated.
Overall, it is clear that disassembly of the neuronal cytoskeleton is a key step in pruning. However, our current view is still fragmented, as the mediators of the cytoskeleton components breakdown have been discovered in only a handful of axonal elimination paradigms. Whether the role of these regulators is conserved across paradigms and what is their relative contribution to the overall pruning process remains to be discovered.
Transcriptional control of neurite pruning
Although axonal pruning is often spatially tightly regulated such that only specific axons or dendrites are eliminated, there is strong evidence that it is also controlled by the cell body, which activates a transcriptional pruning program. In flies, mounting evidence highlights the steroid hormone Ecdysone Receptor-B1 (EcR-B1) as the master regulator of axon and dendrite pruning. One of the key EcR-B1 targets, implicated in both da dendrite pruning and MB axon pruning is the Sox14 transcription factor [129]. Sox14, in turn, binds to the MICAL promoter and activates its transcription. In contrast to Sox14, MICAL is important for da dendrite but not MB axon pruning [129], suggesting that SOX14 may activate a different pruning program in different neurons. Another EcR-B1 target is headcase (hdc), a protein that is required for da dendrite severing, likely functioning in parallel to Sox14 [130]. Recent data has uncovered Cullin1 as a potential EcR-B1 target required for pruning, thereby linking between EcR-B1 and the UPS (see also the section above on the role of the UPS). While EcR-B1 seems to be required for remodeling of all neurons tested so far in Drosophila [31, 131], its precise downstream target spectrum and their specific functions in the different systems remains to be further investigated. Because the activity of EcR-B1 is so important, several layers of regulation control its own expression. The cohesin complex seems to function in postmitotic neurons to regulate the levels of EcR-B1 expression, likely by controlling chromatin structure [132]. A nuclear receptor network comprised by Ftz-F1 and Hr39 are also required for controlling EcR-B1 expression providing the means for feedback regulation [133]. Finally, spatial and perhaps also temporal regulation is provided by the TGF-β signaling cascade (as discussed above). Despite all these tight regulations, the ectopic expression of EcR-B1 in α’/β’ neurons, that do not normally undergo pruning, is not sufficient to induce their fragmentation (Schuldiner, unpublished observations) suggesting that additional safeguard mechanisms exist, in addition to those controlling EcR-B1 expression.
In contrast to flies, not much is known in mammals about the transcriptional control of remodeling. Moreover, remodeling in mammals occurs on a wider time frame, thus a hormonal regulation of remodeling, as a general phenomenon is less likely. Interestingly, however, recent work has demonstrated that sex hormone regulation of BDNF signaling directs the pruning of sensory axons in the male mammary gland [44]. Moreover, remodeling in humans occurs in two main phases, during the first 2 years of life as well as during puberty [10] therefore raising the exciting speculation that hormones might contribute to different aspects of remodeling.
Whether transcription factors control pruning in an instructive or permissive manner remains to be further studied. In the fly, it seems that EcR-B1 plays a permissive role although this has not been fully tested as a constantly active version of EcR is currently lacking. In contrast, it seems that the inhibition of BDNF signaling and pruning of sensory axons by the androgen receptor (AR) is instructive in mammals. An additional enigma is how a transcription factor would control a process that is tightly spatially regulated. More studies in the future should shed light on the involvement of processes such as localized translation, cargo trafficking, and localized signaling.
Concluding remarks
Studies in different model organisms combining both in vitro and in vivo models of neurite pruning have provided great knowledge on the commonality of this developmental process as well as the mechanisms that control it. Yet we are still at the beginning of the journey to reach a comprehensive mechanistic understanding of developmental pruning and its role in neuro-developmental disorders. So far only a handful of intracellular regulators and executers have been discovered, and their mechanism of action is poorly understood. For example, although the UPS has been implicated in several pruning models, studies have so far failed to generate an integrated picture of the target proteins that are degraded as well as their functional role. Thus, it is still unclear how the UPS integrates information and sends the execution instructions in the various model systems and whether any of these will be conserved. Moreover, the current data are still consistent with two main models—in one, the UPS is required to target a key molecule whose degradation initiates pruning; in the second, the UPS targets many proteins whose sum degradation results in pruning. More work in many systems is required to decipher which model is better supported.
Likewise, caspase activation seems to play a key role in multiple models of pruning but not in all. While the evidence of the role of caspases is direct and strong in developmental dendrite pruning of da neurons in Drosophila and axon fragmentation following trophic deprivation in vitro, the extent of their role in other remodeling events remains to be determined. For example, there is strong evidence that pruning of MB neurons is caspase-independent ([30, 70], Schuldiner unpublished observations). The substrates for these caspases during neurite pruning are not known—cytoskeletal components being one attractive but yet unsupported candidate. Moreover, if indeed the degradation of these basic axonal components by caspases is essential for pruning, it will be interesting to uncover the equivalent function in caspase-independent remodeling events.
One additional interesting question is whether and how the caspases regulate pruning vs apoptosis. Recent studies suggest that the threshold of caspase activation determines whether it affects morphogenesis or activates cell death [134]. An alternative method to direct the neuron to pruning and not to apoptosis would be to physically restrict caspase activation, which is consistent with da dendrite pruning in which the process seemingly begins with severing. What is the mechanism to spare the cell body from caspase activation in the various models of pruning needs further exploration. Interestingly, a recent study that focused on optical induction of local caspase activation resulting in synapse elimination has highlighted caspase inhibitors and the proteasome as potential candidates to restrict caspase activity and prevent cell death [135].
One area, which still lacks convergence, is the signaling pathways of the pruning receptors. While in some systems receptors of the Semaphorins or ephrin receptors are involved, in others it is receptors from the TNF family such as P75 and DR6 while in others it is receptors of the TGF-β family. Is each case of pruning initiated by a different signal or are we yet to discover some common signal? Because these events occur in different developmental contexts, we predict that different extracellular signals might be involved in initiating pruning in different cases. That said, the TGF-β signaling pathway driving the expression of the steroid hormone receptor EcR-B1 is required for pruning of da dendrites, MB axons, projection neuron axons and ectopic NMJ connections, spanning different developmental time points and different pruning ‘types’. Interestingly, the TGF-β pathway was recently found to regulate neuronal C1q expression, which in turn is important for synaptic elimination by microglia at the developing mouse dLGN [136]. Whether this is the key ‘pruning initiator’ in Drosophila and whether the TGF-β pathway is important in other mammalian systems remains to be further explored.
We expect convergence to be more apparent in the downstream events, such as caspase activation or other proteolytic systems that will be identified. One interesting new direction that might provide a common mechanism is the role of calcium [110–112], but the mechanism of action needs to be further delineated in the various systems.
Another aspect of pruning that remains unexplored, mostly in mammals, is the nature of the transcriptional pruning programs. Recent technological breakthroughs in genomic research allow, in principle, a single cell analysis of these programs. A detailed description of the transcriptional profiles of identifiable neurons in vivo during development will be a huge step towards identifying specific pathways convergence points with different systems.
Speculative thoughts on the logic of neurite pruning as a wiring strategy
A question that is often raised when people are introduced to the idea that development of the nervous system involves regressive events in large scale such as pruning is—why? Why waste organismal resources to generate too many neurons? Why waste cellular resources to generate too many axons and dendrites? Obviously, we don’t have the answers to these questions and the “why” question cannot really be adequately addressed in experimental biology. Nonetheless, we would like to raise the hypothesis that the reasons ‘why’ neuronal networks develop like this are different for each neuronal system. We want to provide a few speculative reasons that might explain why evolution sculpted neurodevelopment in this way by addressing specific examples.
Pruned neurites are important for pre-remodeled network: in some cases the neurons before and after remodeling might be integrated into different neuronal networks and might thus be functionally important for the behavior of the animal. One clear example is the remodeling of da neurons in Drosophila—while the soma and axon remain intact, dendrites remodel to reflect the metamorphing animal and thus new connection. MB neurons might be another case—larval MB γ neurons do form synapses as demonstrated by EM [69] and thus might form functional networks. Interestingly, embryonic projection neurons (PNs) are likely the pre-synaptic partners of these larval MB neurons suggesting that γ neurons are used twice—once to mediate information transfer from the larval antennal lobe (AL—the fly equivalent of the olfactory bulb) and later to mediate information transfer from the adult AL.
Pruned neurites play anatomical roles: In some cases, the pre-remodeled neurons might function as pioneering neurons that subsequently guide other neurons. MB γ neurons are the first MB neuronal type to be born. Later, α′/β′ neurons grow along the existing axonal bundle. Although this was not directly tested, the γ neurons might thus serve as growing templates for the α′/β′ neurons.
Exuberant connections are made to ensure competition and correct functional connectivity: There are several examples now that axon–axon competition might play a role in non-stereotypic axon pruning such as the development of the NMJ, the desegregation of eye specific inputs in the dLGN, and the formation of ocular dominance columns (not discussed here). In all of these cases, the formation of exuberant connections forms a platform in which experience dependent remodeling can sculpt the mature neuronal network. A variation of this idea would be the trophic withdrawal dependent pruning. In these systems, trophic signals are available in limiting amounts that allow the formation of specific number of connections. The initial formation of more axons merely ensures correct connectivity even if some mistakes are made in earlier steps of the wiring process. The in vitro trophic deprivation systems mimic this physiological setting and have contributed significantly to our ability to study mechanisms of axon fragmentation. However, while in the in vitro system axon fragmentation and cell death can be easily categorized as two distinct events, the situation in vivo is less clear.
Over-branching as a mechanisms to sense gradients: When RGC extend their axons to their targets in the superior colliculus (SC) they form excessive branches that most likely assist them in measuring local gradients of Ephrins subsequently allowing them to match their connection with the appropriate target in what would form the target zone.
Simplified developmental programs: The creation of branches that are later eliminated might be a result of evolutionary constraints on development. Layer 5 cortical neurons from the visual and motor cortex initially form identical connections that span into the spinal cord. Perhaps, one developmental program for all L5 cortical neurons followed by remodeling is a simpler, or more evolutionarily stable, option than defining a distinct guidance process for these two neuronal populations.
The diversity in our speculative ‘why’ may be in the basis for the diversity in the ‘how’ neurites are pruned, as discussed throughout this review. Perhaps, because punning has evolved in different systems to account for different needs, not one single pruning program has evolved but rather multiple ways of achieving the same outcome.
Although it is likely that convergence in some of the downstream mechanisms will be discovered, it is clear that there are different roads leading to Rome. Traveling along these roads in the coming years and exploring new ones as we incorporate and study new model systems of neurite pruning will be an exciting journey.
Acknowledgments
We thank M. Maor-Nof and S. Yaniv for critical reading of the manuscript and Z. Schoenmann for assistance with the graphics. Research in our laboratories is currently supported by the Israeli Science Foundaton (ISF) and Minerva foundation (A.Y.) and the European Research Council (erc), Israeli Science Foundation (ISF) and Minerva foundation (O.S). O.S. is an incumbent of the Aser Rothstein Career Development Chair.
Abbreviations
- ALS
Amyotrophic lateral sclerosis
- BDNF
Brain derived neurotrophic factor
- CRMPs
Collapsin response mediator proteins
- CST
Corticospinal tract
- da
Dendritic arborization
- dLGN
Dorsal lateral geniculate nucleus
- DRG
Dorsal root ganglia
- EcR
Ecdysone receptor
- GAP
GTPase activating protein
- GEF
GTP exchange factor
- IPB
Infrapyramidal bundle
- MARCM
Mosaic analysis with a repressible cell marker
- MB
Mushroom body
- MTs
Microtubules
- NFs
Neurofilaments
- NGF
Nerve growth factor
- NMJ
Neuromuscular junction
- PS
Phosphatidylserine
- RGCs
Retinal ganglion cells
- SC
Superior colliculus
- SCG
Superior cervical ganglion
- TGF-β
Transforming growth factor-β
- UPS
Ubituitin proteasome System
- WD
Wallerian degeneration
Contributor Information
Oren Schuldiner, Email: oren.schuldiner@weizmann.ac.il.
Avraham Yaron, Email: avraham.yaron@weizmann.ac.il.
References
- 1.Sperry RW. Chemoaffinity in the orderly growth of nerve fiber patterns and connections. Proc Natl Acad Sci USA. 1963;50:703–710. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raper JA, Bastiani M, Goodman CS. Pathfinding by neuronal growth cones in grasshopper embryos. II. Selective fasciculation onto specific axonal pathways. J Neurosci Off J Soc Neurosci. 1983;3(1):31–41. doi: 10.1523/JNEUROSCI.03-01-00031.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas JB, Bastiani MJ, Bate M, Goodman CS. From grasshopper to Drosophila: a common plan for neuronal development. Nature. 1984;310(5974):203–207. doi: 10.1038/310203a0. [DOI] [PubMed] [Google Scholar]
- 4.Tessier-Lavigne M, Placzek M, Lumsden AG, Dodd J, Jessell TM. Chemotropic guidance of developing axons in the mammalian central nervous system. Nature. 1988;336(6201):775–778. doi: 10.1038/336775a0. [DOI] [PubMed] [Google Scholar]
- 5.Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298(5600):1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- 6.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274(5290):1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 7.Luo L, O’Leary DD. Axon retraction and degeneration in development and disease. Annu Rev Neurosci. 2005;28:127–156. doi: 10.1146/annurev.neuro.28.061604.135632. [DOI] [PubMed] [Google Scholar]
- 8.Cowan WM, Fawcett JW, O’Leary DD, Stanfield BB. Regressive events in neurogenesis. Science. 1984;225(4668):1258–1265. doi: 10.1126/science.6474175. [DOI] [PubMed] [Google Scholar]
- 9.Innocenti GM, Price DJ. Exuberance in the development of cortical networks. Nat Rev Neurosci. 2005;6(12):955–965. doi: 10.1038/nrn1790. [DOI] [PubMed] [Google Scholar]
- 10.Thompson RA, Nelson CA. Developmental science and the media. Early brain development. Am Psychol. 2001;56(1):5–15. doi: 10.1037/0003-066x.56.1.5. [DOI] [PubMed] [Google Scholar]
- 11.Truman JW. Metamorphosis of the central nervous system of Drosophila . J Neurobiol. 1990;21(7):1072–1084. doi: 10.1002/neu.480210711. [DOI] [PubMed] [Google Scholar]
- 12.Ramon Y, Cajal S. Histology of the nervous system. New-York: Oxford University Press; 1995. [Google Scholar]
- 13.Chung WS, Barres BA. Selective remodeling: refining neural connectivity at the neuromuscular junction. PLoS Biol. 2009;7(8):e1000185. doi: 10.1371/journal.pbio.1000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugihara I. Organization and remodeling of the olivocerebellar climbing fiber projection. Cerebellum. 2006;5(1):15–22. doi: 10.1080/14734220500527385. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto K, Kano M. Synapse elimination in the developing cerebellum. Cell Mol Life Sci. 2013;70(24):4667–4680. doi: 10.1007/s00018-013-1405-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies AM. The neurotrophic hypothesis: where does it stand? Philos Trans R Soc Lond B Biol Sci. 1996;351(1338):389–394. doi: 10.1098/rstb.1996.0033. [DOI] [PubMed] [Google Scholar]
- 17.Stanfield BB, O’Leary DD, Fricks C. Selective collateral elimination in early postnatal development restricts cortical distribution of rat pyramidal tract neurones. Nature. 1982;298(5872):371–373. doi: 10.1038/298371a0. [DOI] [PubMed] [Google Scholar]
- 18.Hoopfer ED, McLaughlin T, Watts RJ, Schuldiner O, O’Leary DD, Luo L. Wlds protection distinguishes axon degeneration following injury from naturally occurring developmental pruning. Neuron. 2006;50(6):883–895. doi: 10.1016/j.neuron.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 19.O’Leary DD, Stanfield BB. A transient pyramidal tract projection from the visual cortex in the hamster and its removal by selective collateral elimination. Brain Res. 1986;392(1–2):87–99. doi: 10.1016/0165-3806(86)90235-x. [DOI] [PubMed] [Google Scholar]
- 20.O’Leary DD, Koester SE. Development of projection neuron types, axon pathways, and patterned connections of the mammalian cortex. Neuron. 1993;10(6):991–1006. doi: 10.1016/0896-6273(93)90049-w. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura H, O’Leary DD. Inaccuracies in initial growth and arborization of chick retinotectal axons followed by course corrections and axon remodeling to develop topographic order. J Neurosci Off J Soc Neurosci. 1989;9(11):3776–3795. doi: 10.1523/JNEUROSCI.09-11-03776.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feldheim DA, O’Leary DD. Visual map development: bidirectional signaling, bifunctional guidance molecules, and competition. Cold Spring Harb Perspect Biol. 2010;2(11):a001768. doi: 10.1101/cshperspect.a001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shatz CJ, Sretavan DW. Interactions between retinal ganglion cells during the development of the mammalian visual system. Annu Rev Neurosci. 1986;9:171–207. doi: 10.1146/annurev.ne.09.030186.001131. [DOI] [PubMed] [Google Scholar]
- 24.Josten NJ, Huberman AD. Milestones and mechanisms for generating specific synaptic connections between the eyes and the brain. Curr Top Dev Biol. 2010;93:229–259. doi: 10.1016/B978-0-12-385044-7.00008-4. [DOI] [PubMed] [Google Scholar]
- 25.Thummel CS. Flies on steroids—Drosophila metamorphosis and the mechanisms of steroid hormone action. Trends Genet. 1996;12(8):306–310. doi: 10.1016/0168-9525(96)10032-9. [DOI] [PubMed] [Google Scholar]
- 26.Schubiger M, Wade AA, Carney GE, Truman JW, Bender M. Drosophila EcR-B ecdysone receptor isoforms are required for larval molting and for neuron remodeling during metamorphosis. Development. 1998;125(11):2053–2062. doi: 10.1242/dev.125.11.2053. [DOI] [PubMed] [Google Scholar]
- 27.Technau G, Heisenberg M. Neural reorganization during metamorphosis of the corpora pedunculata in Drosophila melanogaster . Nature. 1982;295(5848):405–407. doi: 10.1038/295405a0. [DOI] [PubMed] [Google Scholar]
- 28.Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22(3):451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 29.Lee T, Lee A, Luo L. Development of the Drosophila mushroom bodies: sequential generation of three distinct types of neurons from a neuroblast. Development. 1999;126(18):4065–4076. doi: 10.1242/dev.126.18.4065. [DOI] [PubMed] [Google Scholar]
- 30.Watts RJ, Hoopfer ED, Luo L. Axon pruning during Drosophila metamorphosis: evidence for local degeneration and requirement of the ubiquitin-proteasome system. Neuron. 2003;38(6):871–885. doi: 10.1016/s0896-6273(03)00295-2. [DOI] [PubMed] [Google Scholar]
- 31.Yu F, Schuldiner O. Axon and dendrite pruning in Drosophila . Curr Opin Neurobiol. 2014;27C:192–198. doi: 10.1016/j.conb.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams DW, Truman JW. Cellular mechanisms of dendrite pruning in Drosophila: insights from in vivo time-lapse of remodeling dendritic arborizing sensory neurons. Development. 2005;132(16):3631–3642. doi: 10.1242/dev.01928. [DOI] [PubMed] [Google Scholar]
- 33.Parrish JZ, Emoto K, Kim MD, Jan YN. Mechanisms that regulate establishment, maintenance, and remodeling of dendritic fields. Annu Rev Neurosci. 2007;30:399–423. doi: 10.1146/annurev.neuro.29.051605.112907. [DOI] [PubMed] [Google Scholar]
- 34.Jan YN, Jan LY. Branching out: mechanisms of dendritic arborization. Nat Rev Neurosci. 2010;11(5):316–328. doi: 10.1038/nrn2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams DW, Kondo S, Krzyzanowska A, Hiromi Y, Truman JW. Local caspase activity directs engulfment of dendrites during pruning. Nat Neurosci. 2006;9(10):1234–1236. doi: 10.1038/nn1774. [DOI] [PubMed] [Google Scholar]
- 36.Kuo CT, Zhu S, Younger S, Jan LY, Jan YN. Identification of E2/E3 ubiquitinating enzymes and caspase activity regulating Drosophila sensory neuron dendrite pruning. Neuron. 2006;51(3):283–290. doi: 10.1016/j.neuron.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 37.Han C, Jan LY, Jan YN. Enhancer-driven membrane markers for analysis of nonautonomous mechanisms reveal neuron-glia interactions in Drosophila . Proc Natl Acad Sci USA. 2011;108(23):9673–9678. doi: 10.1073/pnas.1106386108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin SM, O’Brien GS, Portera-Cailliau C, Sagasti A. Wallerian degeneration of zebrafish trigeminal axons in the skin is required for regeneration and developmental pruning. Development. 2010;137(23):3985–3994. doi: 10.1242/dev.053611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenberg AF, Wolman MA, Franzini-Armstrong C, Granato M. In vivo nerve-macrophage interactions following peripheral nerve injury. J Neurosci Off J Soc Neurosci. 2012;32(11):3898–3909. doi: 10.1523/JNEUROSCI.5225-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sagasti A, Guido MR, Raible DW, Schier AF. Repulsive interactions shape the morphologies and functional arrangement of zebrafish peripheral sensory arbors. Curr Biol CB. 2005;15(9):804–814. doi: 10.1016/j.cub.2005.03.048. [DOI] [PubMed] [Google Scholar]
- 41.Deckwerth TL, Johnson EM., Jr Temporal analysis of events associated with programmed cell death (apoptosis) of sympathetic neurons deprived of nerve growth factor. J Cell Biol. 1993;123(5):1207–1222. doi: 10.1083/jcb.123.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campenot RB. Development of sympathetic neurons in compartmentalized cultures. II. Local control of neurite survival by nerve growth factor. Development Biol. 1982;93(1):13–21. doi: 10.1016/0012-1606(82)90233-0. [DOI] [PubMed] [Google Scholar]
- 43.Singh KK, Park KJ, Hong EJ, Kramer BM, Greenberg ME, Kaplan DR, Miller FD. Developmental axon pruning mediated by BDNF-p75NTR-dependent axon degeneration. Nat Neurosci. 2008;11(6):649–658. doi: 10.1038/nn.2114. [DOI] [PubMed] [Google Scholar]
- 44.Liu Y, Rutlin M, Huang S, Barrick CA, Wang F, Jones KR, Tessarollo L, Ginty DD. Sexually dimorphic BDNF signaling directs sensory innervation of the mammary gland. Science. 2012;338(6112):1357–1360. doi: 10.1126/science.1228258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bagri A, Cheng HJ, Yaron A, Pleasure SJ, Tessier-Lavigne M. Stereotyped pruning of long hippocampal axon branches triggered by retraction inducers of the semaphorin family. Cell. 2003;113(3):285–299. doi: 10.1016/s0092-8674(03)00267-8. [DOI] [PubMed] [Google Scholar]
- 46.Portera-Cailliau C, Weimer RM, De Paola V, Caroni P, Svoboda K. Diverse modes of axon elaboration in the developing neocortex. PLoS Biol. 2005;3(8):e272. doi: 10.1371/journal.pbio.0030272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tapia JC, Wylie JD, Kasthuri N, Hayworth KJ, Schalek R, Berger DR, Guatimosim C, Seung HS, Lichtman JW. Pervasive synaptic branch removal in the mammalian neuromuscular system at birth. Neuron. 2012;74(5):816–829. doi: 10.1016/j.neuron.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 48.Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annu Rev Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- 49.Bishop DL, Misgeld T, Walsh MK, Gan WB, Lichtman JW. Axon branch removal at developing synapses by axosome shedding. Neuron. 2004;44(4):651–661. doi: 10.1016/j.neuron.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 50.Song JW, Misgeld T, Kang H, Knecht S, Lu J, Cao Y, Cotman SL, Bishop DL, Lichtman JW. Lysosomal activity associated with developmental axon pruning. J Neurosci Off J Soc Neurosci. 2008;28(36):8993–9001. doi: 10.1523/JNEUROSCI.0720-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boulanger A, Farge M, Ramanoudjame C, Wharton K, Dura JM. Drosophila motor neuron retraction during metamorphosis is mediated by inputs from TGF-beta/BMP signaling and orphan nuclear receptors. PLoS One. 2012;7(7):e40255. doi: 10.1371/journal.pone.0040255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Z, Chen Y, Wang D, Wang S, Zhang YQ. Distinct presynaptic and postsynaptic dismantling processes of Drosophila neuromuscular junctions during metamorphosis. J Neurosci Off J Soc Neurosci. 2010;30(35):11624–11634. doi: 10.1523/JNEUROSCI.0410-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mack TG, Reiner M, Beirowski B, Mi W, Emanuelli M, Wagner D, Thomson D, Gillingwater T, Court F, Conforti L, Fernando FS, Tarlton A, Andressen C, Addicks K, Magni G, Ribchester RR, Perry VH, Coleman MP. Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nat Neurosci. 2001;4(12):1199–1206. doi: 10.1038/nn770. [DOI] [PubMed] [Google Scholar]
- 54.Coleman MP, Freeman MR. Wallerian degeneration, wld(s), and nmnat. Annu Rev Neurosci. 2010;33:245–267. doi: 10.1146/annurev-neuro-060909-153248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vargas ME, Barres BA. Why is Wallerian degeneration in the CNS so slow? Annu Rev Neurosci. 2007;30:153–179. doi: 10.1146/annurev.neuro.30.051606.094354. [DOI] [PubMed] [Google Scholar]
- 56.Bareyre FM, Kerschensteiner M, Misgeld T, Sanes JR. Transgenic labeling of the corticospinal tract for monitoring axonal responses to spinal cord injury. Nat Med. 2005;11(12):1355–1360. doi: 10.1038/nm1331. [DOI] [PubMed] [Google Scholar]
- 57.Beirowski B, Adalbert R, Wagner D, Grumme DS, Addicks K, Ribchester RR, Coleman MP. The progressive nature of Wallerian degeneration in wild-type and slow Wallerian degeneration (WldS) nerves. BMC Neurosci. 2005;6:6. doi: 10.1186/1471-2202-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiong X, Collins CA. A conditioning lesion protects axons from degeneration via the Wallenda/DLK MAP kinase signaling cascade. J Neurosci Off J Soc Neurosci. 2012;32(2):610–615. doi: 10.1523/JNEUROSCI.3586-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.MacDonald JM, Beach MG, Porpiglia E, Sheehan AE, Watts RJ, Freeman MR. The Drosophila cell corpse engulfment receptor Draper mediates glial clearance of severed axons. Neuron. 2006;50(6):869–881. doi: 10.1016/j.neuron.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 60.Neukomm LJ, Burdett TC, Gonzalez MA, Zuchner S, Freeman MR. Rapid in vivo forward genetic approach for identifying axon death genes in Drosophila . Proc Natl Acad Sci USA. 2014;111(27):9965–9970. doi: 10.1073/pnas.1406230111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fang Y, Soares L, Teng X, Geary M, Bonini NM. A novel Drosophila model of nerve injury reveals an essential role of Nmnat in maintaining axonal integrity. Curr Biol CB. 2012;22(7):590–595. doi: 10.1016/j.cub.2012.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deckwerth TL, Johnson EM., Jr Neurites can remain viable after destruction of the neuronal soma by programmed cell death (apoptosis) Dev Biol. 1994;165(1):63–72. doi: 10.1006/dbio.1994.1234. [DOI] [PubMed] [Google Scholar]
- 63.Schoenmann Z, Assa-Kunik E, Tiomny S, Minis A, Haklai-Topper L, Arama E, Yaron A. Axonal degeneration is regulated by the apoptotic machinery or a NAD+-sensitive pathway in insects and mammals. J Neurosci. 2010;30(18):6375–6386. doi: 10.1523/JNEUROSCI.0922-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tao J, Rolls MM. Dendrites have a rapid program of injury-induced degeneration that is molecularly distinct from developmental pruning. J Neurosci Off J Soc Neurosci. 2011;31(14):5398–5405. doi: 10.1523/JNEUROSCI.3826-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Osterloh JM, Yang J, Rooney TM, Fox AN, Adalbert R, Powell EH, Sheehan AE, Avery MA, Hackett R, Logan MA, MacDonald JM, Ziegenfuss JS, Milde S, Hou YJ, Nathan C, Ding A, Brown RH, Jr, Conforti L, Coleman M, Tessier-Lavigne M, Zuchner S, Freeman MR. dSarm/Sarm1 is required for activation of an injury-induced axon death pathway. Science. 2012;337(6093):481–484. doi: 10.1126/science.1223899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xiong X, Hao Y, Sun K, Li J, Li X, Mishra B, Soppina P, Wu C, Hume RI, Collins CA. The Highwire ubiquitin ligase promotes axonal degeneration by tuning levels of Nmnat protein. PLoS Biol. 2012;10(12):e1001440. doi: 10.1371/journal.pbio.1001440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu XM, Gutman I, Mosca TJ, Iram T, Ozkan E, Garcia KC, Luo L, Schuldiner O. Plum, an immunoglobulin superfamily protein, regulates axon pruning by facilitating TGF-beta signaling. Neuron. 2013;78(3):456–468. doi: 10.1016/j.neuron.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Awasaki T, Ito K. Engulfing action of glial cells is required for programmed axon pruning during Drosophila metamorphosis. Curr Biol CB. 2004;14(8):668–677. doi: 10.1016/j.cub.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 69.Watts RJ, Schuldiner O, Perrino J, Larsen C, Luo L. Glia engulf degenerating axons during developmental axon pruning. Curr Biol CB. 2004;14(8):678–684. doi: 10.1016/j.cub.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 70.Awasaki T, Tatsumi R, Takahashi K, Arai K, Nakanishi Y, Ueda R, Ito K. Essential role of the apoptotic cell engulfment genes draper and ced-6 in programmed axon pruning during Drosophila metamorphosis. Neuron. 2006;50(6):855–867. doi: 10.1016/j.neuron.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 71.Awasaki T, Lai SL, Ito K, Lee T. Organization and postembryonic development of glial cells in the adult central brain of Drosophila . J Neurosci Off J Soc Neurosci. 2008;28(51):13742–13753. doi: 10.1523/JNEUROSCI.4844-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stork T, Bernardos R. Freeman MR (2012) Analysis of glial cell development and function in Drosophila . Cold Spring Harb Protoc. 2012;1:1–17. doi: 10.1101/pdb.top067587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Doherty J, Logan MA, Tasdemir OE, Freeman MR. Ensheathing glia function as phagocytes in the adult Drosophila brain. J Neurosci Off J Soc Neurosci. 2009;29(15):4768–4781. doi: 10.1523/JNEUROSCI.5951-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hakim Y, Yaniv SP, Schuldiner O. Astrocytes play a key role in Drosophila mushroom body axon pruning. PLoS One. 2014;9(1):e86178. doi: 10.1371/journal.pone.0086178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tasdemir-Yilmaz OE, Freeman MR. Astrocytes engage unique molecular programs to engulf pruned neuronal debris from distinct subsets of neurons. Genes Dev. 2014;28(1):20–33. doi: 10.1101/gad.229518.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Han C, Song Y, Xiao H, Wang D, Franc NC, Jan LY, Jan YN. Epidermal cells are the primary phagocytes in the fragmentation and clearance of degenerating dendrites in Drosophila . Neuron. 2014;81(3):544–560. doi: 10.1016/j.neuron.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Berbel P, Innocenti GM. The development of the corpus callosum in cats: a light- and electron-microscopic study. J Comp Neurol. 1988;276(1):132–156. doi: 10.1002/cne.902760109. [DOI] [PubMed] [Google Scholar]
- 78.Innocenti GM. Growth and reshaping of axons in the establishment of visual callosal connections. Science. 1981;212(4496):824–827. doi: 10.1126/science.7221566. [DOI] [PubMed] [Google Scholar]
- 79.Schafer DP, Stevens B. Phagocytic glial cells: sculpting synaptic circuits in the developing nervous system. Curr Opin Neurobiol. 2013;23(6):1034–1040. doi: 10.1016/j.conb.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333(6048):1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 81.Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74(4):691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, Joung J, Foo LC, Thompson A, Chen C, Smith SJ, Barres BA. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013;504(7480):394–400. doi: 10.1038/nature12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nat Rev Immunol. 2007;7(12):964–974. doi: 10.1038/nri2214. [DOI] [PubMed] [Google Scholar]
- 84.Tung TT, Nagaosa K, Fujita Y, Kita A, Mori H, Okada R, Nonaka S, Nakanishi Y. Phosphatidylserine recognition and induction of apoptotic cell clearance by Drosophila engulfment receptor Draper. J Biochem. 2013;153(5):483–491. doi: 10.1093/jb/mvt014. [DOI] [PubMed] [Google Scholar]
- 85.Burstyn-Cohen T, Lew ED, Traves PG, Burrola PG, Hash JC, Lemke G. Genetic dissection of TAM receptor-ligand interaction in retinal pigment epithelial cell phagocytosis. Neuron. 2012;76(6):1123–1132. doi: 10.1016/j.neuron.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Low LK, Liu XB, Faulkner RL, Coble J, Cheng HJ. Plexin signaling selectively regulates the stereotyped pruning of corticospinal axons from visual cortex. Proc Natl Acad Sci US A. 2008;105(23):8136–8141. doi: 10.1073/pnas.0803849105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu XB, Low LK, Jones EG, Cheng HJ. Stereotyped axon pruning via plexin signaling is associated with synaptic complex elimination in the hippocampus. J Neurosci Off J Soc Neurosci. 2005;25(40):9124–9134. doi: 10.1523/JNEUROSCI.2648-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Low LK, Liu XB, Faulkner RL, Coble J, Cheng HJ. Plexin signaling selectively regulates the stereotyped pruning of corticospinal axons from visual cortex. Proc Natl Acad Sci USA. 2008;105(23):8136–8141. doi: 10.1073/pnas.0803849105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu NJ, Henkemeyer M. Ephrin-B3 reverse signaling through Grb4 and cytoskeletal regulators mediates axon pruning. Nat Neurosci. 2009;12(3):268–276. doi: 10.1038/nn.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Klein R. Eph/ephrin signaling in morphogenesis, neural development and plasticity. Curr Opin Cell Biol. 2004;16(5):580–589. doi: 10.1016/j.ceb.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 91.Zheng X, Wang J, Haerry TE, Wu AY, Martin J, O’Connor MB, Lee CH, Lee T. TGF-beta signaling activates steroid hormone receptor expression during neuronal remodeling in the Drosophila brain. Cell. 2003;112(3):303–315. doi: 10.1016/s0092-8674(03)00072-2. [DOI] [PubMed] [Google Scholar]
- 92.Awasaki T, Huang Y, O’Connor MB, Lee T. Glia instruct developmental neuronal remodeling through TGF-beta signaling. Nat Neurosci. 2011;14(7):821–823. doi: 10.1038/nn.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maor-Nof M, Yaron A. Neurite pruning and neuronal cell death: spatial regulation of shared destruction programs. Curr Opin Neurobiol. 2013;23(6):990–996. doi: 10.1016/j.conb.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 94.Nikolaev A, McLaughlin T, O’Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457(7232):981–989. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 95.Schecterson LC, Bothwell M. Neurotrophin receptors: old friends with new partners. Dev Neurobiol. 2010;70(5):332–338. doi: 10.1002/dneu.20767. [DOI] [PubMed] [Google Scholar]
- 96.Ibanez CF, Simi A. p75 neurotrophin receptor signaling in nervous system injury and degeneration: paradox and opportunity. Trends Neurosci. 2012;35(7):431–440. doi: 10.1016/j.tins.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 97.Cao L, Dhilla A, Mukai J, Blazeski R, Lodovichi C, Mason CA, Gogos JA. Genetic modulation of BDNF signaling affects the outcome of axonal competition in vivo. Curr Biol CB. 2007;17(11):911–921. doi: 10.1016/j.cub.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Park KJ, Grosso CA, Aubert I, Kaplan DR, Miller FD. p75NTR-dependent, myelin-mediated axonal degeneration regulates neural connectivity in the adult brain. Nat Neurosci. 2010;13(5):559–566. doi: 10.1038/nn.2513. [DOI] [PubMed] [Google Scholar]
- 99.Pan G, Bauer JH, Haridas V, Wang S, Liu D, Yu G, Vincenz C, Aggarwal BB, Ni J, Dixit VM. Identification and functional characterization of DR6, a novel death domain-containing TNF receptor. FEBS Lett. 1998;431(3):351–356. doi: 10.1016/s0014-5793(98)00791-1. [DOI] [PubMed] [Google Scholar]
- 100.Kuo CT, Jan LY, Jan YN. Dendrite-specific remodeling of Drosophila sensory neurons requires matrix metalloproteases, ubiquitin-proteasome, and ecdysone signaling. Proc Natl Acad Sci USA. 2005;102(42):15230–15235. doi: 10.1073/pnas.0507393102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wong JJ, Li S, Lim EK, Wang Y, Wang C, Zhang H, Kirilly D, Wu C, Liou YC, Wang H, Yu F. A Cullin1-based SCF E3 ubiquitin ligase targets the InR/PI3K/TOR pathway to regulate neuronal pruning. PLoS Biol. 2013;11(9):e1001657. doi: 10.1371/journal.pbio.1001657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yaniv SP, Issman-Zecharya N, Oren-Suissa M, Podbilewicz B, Schuldiner O. Axon regrowth during development and regeneration following injury share molecular mechanisms. Curr Biol CB. 2012;22(19):1774–1782. doi: 10.1016/j.cub.2012.07.044. [DOI] [PubMed] [Google Scholar]
- 103.Zhai Q, Wang J, Kim A, Liu Q, Watts R, Hoopfer E, Mitchison T, Luo L, He Z. Involvement of the ubiquitin-proteasome system in the early stages of wallerian degeneration. Neuron. 2003;39(2):217–225. doi: 10.1016/s0896-6273(03)00429-x. [DOI] [PubMed] [Google Scholar]
- 104.Feinstein-Rotkopf Y, Arama E (2009) Can’t live without them, can live with them: roles of caspases during vital cellular processes. Apoptosis [DOI] [PubMed]
- 105.Kuranaga E, Miura M. Nonapoptotic functions of caspases: caspases as regulatory molecules for immunity and cell-fate determination. Trends Cell Biol. 2007;17(3):135–144. doi: 10.1016/j.tcb.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 106.Simon DJ, Weimer RM, McLaughlin T, Kallop D, Stanger K, Yang J, O’Leary DD, Hannoush RN, Tessier-Lavigne M. A caspase cascade regulating developmental axon degeneration. J Neurosci Off J Soc Neurosci. 2012;32(49):17540–17553. doi: 10.1523/JNEUROSCI.3012-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cusack CL, Swahari V, Hampton Henley W, Michael Ramsey J, Deshmukh M. Distinct pathways mediate axon degeneration during apoptosis and axon-specific pruning. Nat Commun. 2013;4:1876. doi: 10.1038/ncomms2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Unsain N, Higgins JM, Parker KN, Johnstone AD, Barker PA. XIAP Regulates Caspase Activity in Degenerating Axons. Cell Rep. 2013;4(4):751–763. doi: 10.1016/j.celrep.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 109.Cosker KE, Pazyra-Murphy MF, Fenstermacher SJ, Segal RA. Target-derived neurotrophins coordinate transcription and transport of bclw to prevent axonal degeneration. J Neurosci Off J Soc Neurosci. 2013;33(12):5195–5207. doi: 10.1523/JNEUROSCI.3862-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang J, Weimer RM, Kallop D, Olsen O, Wu Z, Renier N, Uryu K, Tessier-Lavigne M. Regulation of axon degeneration after injury and in development by the endogenous calpain inhibitor calpastatin. Neuron. 2013;80(5):1175–1189. doi: 10.1016/j.neuron.2013.08.034. [DOI] [PubMed] [Google Scholar]
- 111.Kanamori T, Kanai MI, Dairyo Y, Yasunaga K, Morikawa RK, Emoto K. Compartmentalized calcium transients trigger dendrite pruning in Drosophila sensory neurons. Science. 2013;340(6139):1475–1478. doi: 10.1126/science.1234879. [DOI] [PubMed] [Google Scholar]
- 112.George EB, Glass JD, Griffin JW. Axotomy-induced axonal degeneration is mediated by calcium influx through ion-specific channels. J Neurosci Off J Soc Neurosci. 1995;15(10):6445–6452. doi: 10.1523/JNEUROSCI.15-10-06445.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen M, Maloney JA, Kallop DY, Atwal JK, Tam SJ, Baer K, Kissel H, Kaminker JS, Lewcock JW, Weimer RM, Watts RJ. Spatially coordinated kinase signaling regulates local axon degeneration. J Neurosci Off J Soc Neurosci. 2012;32(39):13439–13453. doi: 10.1523/JNEUROSCI.2039-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ghosh AS, Wang B, Pozniak CD, Chen M, Watts RJ, Lewcock JW. DLK induces developmental neuronal degeneration via selective regulation of proapoptotic JNK activity. J Cell Biol. 2011;194(5):751–764. doi: 10.1083/jcb.201103153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee HH, Jan LY, Jan YN. Drosophila IKK-related kinase Ik2 and Katanin p60-like 1 regulate dendrite pruning of sensory neuron during metamorphosis. Proc Natl Acad Sci U S A. 2009;106(15):6363–6368. doi: 10.1073/pnas.0902051106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gerdts J, Sasaki Y, Vohra B, Marasa J, Milbrandt J. Image-based screening identifies novel roles for IkappaB kinase and glycogen synthase kinase 3 in axonal degeneration. J Biol Chem. 2011;286(32):28011–28018. doi: 10.1074/jbc.M111.250472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shea TB, Lee S. The discontinuous nature of neurofilament transport accommodates both establishment and repair of the axonal neurofilament array. Cytoskeleton (Hoboken) 2013;70(2):67–73. doi: 10.1002/cm.21087. [DOI] [PubMed] [Google Scholar]
- 118.Perrot R, Berges R, Bocquet A, Eyer J. Review of the multiple aspects of neurofilament functions, and their possible contribution to neurodegeneration. Mol Neurobiol. 2008;38(1):27–65. doi: 10.1007/s12035-008-8033-0. [DOI] [PubMed] [Google Scholar]
- 119.Letourneau PC. Actin in axons: stable scaffolds and dynamic filaments. Results Probl Cell Differ. 2009;48:65–90. doi: 10.1007/400_2009_15. [DOI] [PubMed] [Google Scholar]
- 120.Amos LA, Schlieper D. Microtubules and maps. Adv Protein Chem. 2005;71:257–298. doi: 10.1016/S0065-3233(04)71007-4. [DOI] [PubMed] [Google Scholar]
- 121.Weisenberg RC, Deery WJ. Role of nucleotide hydrolysis in microtubule assembly. Nature. 1976;263(5580):792–793. doi: 10.1038/263792a0. [DOI] [PubMed] [Google Scholar]
- 122.Luduena RF, Anderson WH, Prasad V, Jordan MA, Ferrigni KC, Roach MC, Horowitz PM, Murphy DB, Fellous A. Interactions of vinblastine and maytansine with tubulin. Ann N Y Acad Sci. 1986;466:718–732. doi: 10.1111/j.1749-6632.1986.tb38454.x. [DOI] [PubMed] [Google Scholar]
- 123.Maor-Nof M, Homma N, Raanan C, Nof A, Hirokawa N, Yaron A. Axonal pruning is actively regulated by the microtubule-destabilizing protein kinesin superfamily protein 2A. Cell Rep. 2013;3(4):971–977. doi: 10.1016/j.celrep.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 124.Walker KL, Yoo HK, Undamatla J, Szaro BG. Loss of neurofilaments alters axonal growth dynamics. J Neurosci Off J Soc Neurosci. 2001;21(24):9655–9666. doi: 10.1523/JNEUROSCI.21-24-09655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wakatsuki S, Saitoh F, Araki T. ZNRF1 promotes Wallerian degeneration by degrading AKT to induce GSK3B-dependent CRMP2 phosphorylation. Nat Cell Biol. 2011;13(12):1415–1423. doi: 10.1038/ncb2373. [DOI] [PubMed] [Google Scholar]
- 126.Touma E, Kato S, Fukui K, Koike T. Calpain-mediated cleavage of collapsin response mediator protein(CRMP)-2 during neurite degeneration in mice. Eur J Neurosci. 2007;26(12):3368–3381. doi: 10.1111/j.1460-9568.2007.05943.x. [DOI] [PubMed] [Google Scholar]
- 127.Riccomagno MM, Hurtado A, Wang H, Macopson JG, Griner EM, Betz A, Brose N, Kazanietz MG, Kolodkin AL. The RacGAP beta2-Chimaerin selectively mediates axonal pruning in the hippocampus. Cell. 2012;149(7):1594–1606. doi: 10.1016/j.cell.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Turner LJ, Nicholls S, Hall A. The activity of the plexin-A1 receptor is regulated by Rac. J Biol Chem. 2004;279(32):33199–33205. doi: 10.1074/jbc.M402943200. [DOI] [PubMed] [Google Scholar]
- 129.Kirilly D, Gu Y, Huang Y, Wu Z, Bashirullah A, Low BC, Kolodkin AL, Wang H, Yu F. A genetic pathway composed of Sox14 and Mical governs severing of dendrites during pruning. Nat Neurosci. 2009;12(12):1497–1505. doi: 10.1038/nn.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Loncle N, Williams DW. An interaction screen identifies headcase as a regulator of large-scale pruning. J Neurosci Off J Soc Neurosci. 2012;32(48):17086–17096. doi: 10.1523/JNEUROSCI.1391-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Marin EC, Watts RJ, Tanaka NK, Ito K, Luo L. Developmentally programmed remodeling of the Drosophila olfactory circuit. Development. 2005;132(4):725–737. doi: 10.1242/dev.01614. [DOI] [PubMed] [Google Scholar]
- 132.Schuldiner O, Berdnik D, Levy JM, Wu JS, Luginbuhl D, Gontang AC, Luo L. piggyBac-based mosaic screen identifies a postmitotic function for cohesin in regulating developmental axon pruning. Dev Cell. 2008;14(2):227–238. doi: 10.1016/j.devcel.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Boulanger A, Clouet-Redt C, Farge M, Flandre A, Guignard T, Fernando C, Juge F, Dura JM. ftz-f1 and Hr39 opposing roles on EcR expression during Drosophila mushroom body neuron remodeling. Nat Neurosci. 2011;14(1):37–44. doi: 10.1038/nn.2700. [DOI] [PubMed] [Google Scholar]
- 134.Florentin A, Arama E. Caspase levels and execution efficiencies determine the apoptotic potential of the cell. J Cell Biol. 2012;196(4):513–527. doi: 10.1083/jcb.201107133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Erturk A, Wang Y, Sheng M. Local pruning of dendrites and spines by caspase-3-dependent and proteasome-limited mechanisms. J Neurosci Off J Soc Neurosci. 2014;34(5):1672–1688. doi: 10.1523/JNEUROSCI.3121-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bialas AR, Stevens B. TGF-beta signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat Neurosci. 2013;16(12):1773–1782. doi: 10.1038/nn.3560. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]