Abstract
Background
Research on quality of life (QoL) among women with breast cancer has often examined the impact of coping strategies on QoL. However, the transactional model of stress and coping would argue that QoL can impact coping. This reciprocal relationship between QoL and coping has been inadequately studied.
Purpose
This study examined reciprocal relationships over 18 months between QoL and coping (positive and negative coping) among women with breast cancer.
Methods
Three-wave cross-lagged structural equation modelling (SEM) analysis was used over 3 timepoints post-diagnosis (T1–T3; N=637, 577, 553, respectively).
Results
SEM results revealed a significant reciprocal relationship between negative coping and QoL, indicating that negative coping predicted subsequent QoL, which in turn predicted later negative coping. Although QoL at cancer diagnosis predicted subsequent positive coping, we did not find a reciprocal relation between QoL and positive coping.
Conclusion
Findings expand our knowledge of the relation between QoL and coping by suggesting the reciprocal relationship between negative coping and QoL among women with breast cancer.
Keywords: Breast cancer survivors, Quality of life, Coping, Reciprocal relationship
INTRODUCTION
Breast cancer is the most commonly diagnosed non-skin cancer among women in the U.S., with 231,840 estimated new cases expected in 2015 (1). Substantial improvements in early detection and breast cancer treatment have resulted in improved 5-year relative survival rates leading to over 3.1 million breast cancer survivors currently in the U.S (2). With improved survival, quality of life (QoL) issues during and after treatment have become increasingly important to women (3, 4). A substantial body of research focuses on understanding factors related to QoL, with coping strategies being one factor receiving considerable attention (5–13).
Previous research has generally shown that positive or adaptive types of coping strategies are linked with better QoL, whereas negative or less adaptive coping strategies are associated with poorer outcomes (5–8, 14, 15). Specifically, cross-sectional studies have shown that positive-adaptive types of coping strategies such as planning-problem solving (8, 15), positive reframing (5, 8, 15), and acceptance (16) are associated with better psychological and physical well-being. In contrast, negative or passive types of coping, including self-blame (17–19), denial (15, 17), behavioral disengagement (15), cognitive escape-avoidance (20), and emotional suppression and keeping to self (5, 17) are related to poorer psychosocial adjustment and greater depressive symptoms. Relatively few longitudinal studies have investigated the effects of coping on subsequent QoL, but findings are similar to those of cross-sectional studies (6, 11, 21–23). For example, one longitudinal study of 70 women with stage I and II breast cancer found that acceptance coping at cancer diagnosis was associated with better psychological well-being (e.g., decreased depression and increased positive mood) one year later, whereas avoidance coping was linked to greater fear of recurrence (21). Similarly, a study of 55 women with early stage breast cancer found that use of avoidance-based coping (e.g., resigned acceptance and cognitive avoidance) at cancer diagnosis and treatment was associated with worse psychological outcomes (e.g., depression and anger) at 3-year follow-up (23).
Although research generally focuses on the effects of coping on QoL, the Lazarus and Folkman (24) transactional theory of stress and coping would suggest that a reverse causal association may also exist. The transactional theory is a framework for assessing ways people cope with stress (e.g., cancer diagnosis and treatment) and hypothesizing how these processes influence a person’s emotional and adaptational outcomes (e.g., QoL) (24). According to the transactional theory, coping is not static, but changes over time (24). The theory also states that the overall process is recursive and posits a mutually reciprocal and dynamic interplay of factors in the process. The transactional theory would suggest that outcomes such as QoL are influenced by an individual’s coping, and that in turn, QoL may also influence subsequent coping.
Three longitudinal studies have provided empirical evidence supporting the reciprocal process between QoL and coping strategies (6, 12, 25). A study of 267 younger breast cancer survivors showed that coping strategies predicted subsequent QoL, which then predicted coping 6 months later (12). Two prospective longitudinal studies of 59 (6) and 131 (25) women with early stage breast cancer found bidirectional causality between coping and psychological distress over a 12-month period (6, 25). These previous studies, however, had several limitations, including small sample sizes (6, 25) and/or restriction to younger women (12). These studies also focused only on the early months post-treatment and thus did not provide data on longer-term relationships. Further, methodological limitations of these studies include the use of separate regression models for QoL and for coping, which do not permit simultaneous analysis of bidirectional causality (6, 25), and separate observations for each coping subscale (6, 12, 25).
To further clarify the dynamic interplay between QoL and coping, the present research addresses these limitations by including a wide age range of women, a larger sample size of 637 women, a longer period of time post-treatment, and by using a more comprehensive reciprocal modeling analysis (cross-lagged structural equation model) in which bidirectional causality can be simultaneously tested within a model. Since a breast cancer survivor’s QoL changes over time (26, 27) and coping processes may be amenable to change (28, 29), it is important to understand the dynamic process between coping and QoL. Understanding the potential reciprocity in this relationship could contribute to building a more coherent body of knowledge in QoL and coping research.
Study Objectives
Our first objective of the current study is to examine the time course of QOL and coping at three time points over an 18 month period following breast cancer diagnosis. The second objective is to examine the longitudinal reciprocal relationships between coping strategies and QoL over this time period. Specifically, we use a cross-lagged modeling approach to test the hypothesis that prior positive/negative coping will influence later QoL and prior QoL will influence later positive/negative coping.
METHODS
Study Sample
This is a secondary analysis of a longitudinal study that examined age-related differences in adjustment to breast cancer. Participants were recruited from two research sites, Memorial Sloan Kettering Cancer Center and the University of Texas-Southwestern Center for Breast Care, from 2002 to 2006. The data were collected in four waves: the first survey (within 8 months of breast cancer diagnosis), and at 6, 12, and 18 months following completion of the first survey. Participants completed a self-administered questionnaire that included questions on quality of life and coping at each assessment point. A medical chart review was performed 1 year after the first survey to obtain treatment-related data. Study eligibility criteria included age 18 or over (though no one was under age 25), first time diagnosis of breast cancer, and ability to speak and read English. Study design and sample criteria have been previously detailed (30).
The current analyses used data from the first survey (T1; 0–8 months post diagnosis, N=637), the 12-month later survey (T2; 12–20 months post diagnosis, N=577), and the 18-month later survey (T3; 18–26 months post diagnosis, N=553). Importantly, our analyses include the time period of 18–26 months post diagnosis as the transition following active treatment has been recognized as an important phase in the trajectory of cancer survivorship (31). We excluded the 6-month survey data since many women were still in active treatment at this time and we wanted to avoid overlap with active treatment at T1. Our analyses excluded women who were in active treatment at T2 or T3 (n=13) and women who did not receive breast surgery (e.g., mastectomy or lumpectomy) (n=1) and those who received reconstruction after lumpectomy without mastectomy (n=2).
Measures
Quality of life was measured by the Functional Assessment of Cancer Therapy-Breast (FACT-B) (32) which is comprised of 35 items that assess five QoL domains: physical well-being, social/family well-being, emotional well-being, functional well-being and an —additional concerns domain specific to breast cancer. Each item is rated on a 5-point Likert scale ranging from 0 (not at all) to 4 (very much) and the total FACT-B score ranges from 0 to 140. The domain scores are obtained by summing the responses, with higher scores representing better QOL. Cronbach’s alpha for the domain scores ranged from .69 to .90.
Coping was measured using the Brief COPE (33) that assesses 14 different coping strategies: self-distraction, active coping, denial, substance use, use of emotional support, use of instrumental support, behavioral disengagement, venting, positive reframing, planning, humor, acceptance, religion, and self-blame. Each coping strategy is measured by two items, each scored 1–4 (from not doing at all to doing a lot). Scores on the two items are summed and divided by 2 to yield a mean subscale score. Cronbach’s alpha for the subscales ranged from .46 (behavioral disengagement) to .82 (use of emotional support).
Higher-order exploratory factor analyses (EFA) on our data were conducted to determine the underlying factor structure. Consistent with the two-factor model proposed by Carver (33), the EFA results suggested that a two-factor solution provided the most interpretable and parsimonious description across all surveys. The first latent factor was defined by active coping, use of emotional support, use of instrumental support, and positive reframing and is called —positive coping. The second latent factor, defined by denial, behavioral disengagement, and self-blame, is called —negative coping. Other coping strategies did not consistently load on either factor across assessments and were therefore excluded.
Demographic and cancer related characteristics were included as covariates: age at diagnosis, cancer stage, time since breast cancer diagnosis, mastectomy (yes/no), and chemotherapy (yes/no).
Analyses
Descriptive Analyses
Descriptive statistics were used to summarize the demographic and medical characteristics of the sample. The linear mixed model analysis was used to assess the overall QoL, mean QoL domain, and coping subscale scores over the three time points: T1, T2, and T3.
Structural Equation Modeling (SEM) Analyses
Study main variables, QoL and coping strategies, were treated as latent variables estimated by multiple observed indicators. The benefits of using a latent variable structural equation modeling (SEM) approach include simultaneously testing the relationships among multiple observed indicators and latent variables, and accounting for measurement errors in both latent and observed variables (34–36).
Measurement model
Prior to testing the cross-lagged model, a confirmatory factor analysis (CFA) was conducted to test the measurement model that defines the relations between observed indicators and latent constructs (35). Three latent constructs (QoL, positive coping, and negative coping) at each time point, 5 indicators for QoL, 4 indicators for positive coping, and 3 indicators of negative coping were included in the measurement model. The indicators of positive and negative coping were derived from the earlier higher-order EFA. The measurement model allowed the latent constructs to be correlated. The measurement errors between same observed indicators over time were also allowed to be correlated. In longitudinal analysis, establishing measurement invariance is necessary to ensure the instrument measures the same construct across assessment points (37). In order to test for measurement invariance, all parameters of the same indicator were constrained to be equal across measurement points (37).
Cross-lagged model
To examine the reciprocal relationships between QoL and positive coping and between QoL and negative coping strategies over time, a three-wave cross-lagged SEM model was then tested. Since the time interval varies between the two sets of waves (12 months vs. 6 months), the paths in the cross-lagged model were not constrained to be equal across measurement points. Age, cancer stage, time since diagnosis, adjuvant therapy (chemotherapy and radiation), and surgery (lumpectomy only, mastectomy without reconstruction, and mastectomy with breast reconstruction) were included as covariates in the cross-lagged model.
For descriptive statistics, SPSS 22.0 was used. The SEM analyses were conducted using Mplus 7.11 (38), with full information maximum likelihood (FIML) as the method of handling missing data. A non-significant χ2 value (p > .05) is an indicator of good fit with the data, however, it is highly sensitive with a large sample and rejects the model inadequately when large samples are used (39). In this study, the model-data fit was evaluated using multiple fit indices: χ2 goodness of fit statistic, the comparative fit index (CFI), the Tucker-Lewis Index (TLI), and the root mean square error of approximation (RMSEA) (36, 40). CFI and TLI values above .90 and RMSEA values below .08 (36) were used as indicators of acceptable model fit.
RESULTS
Sample Characteristics
A total of 637 eligible breast cancer survivors were identified at T1. The sample was predominantly white (89.6%) and well educated (62.3% completed college), with higher household income (42.7% had household income >$100,000) (Table 1). The mean age at T1 was 55.5 years (range 26–97 years) and the majority of respondents (71.9%) were married or partnered. More than half of the respondents (52.4%) were diagnosed with stage I breast cancer, 72.2% received adjuvant radiation therapy and 66.4% received chemotherapy. The majority (64.2%) received lumpectomy only and 35.8% received mastectomy. The mean time since breast cancer diagnosis at T1 was 4.5 months.
Table 1.
Characteristics of Study Participants at T1 (N = 637)
| Characteristics | N (%) | Mean (SD) | Range |
|---|---|---|---|
| Age (years) | 55.5 (12.6) | 25.6 – 97.1 | |
| Ethnicity | |||
| White | 571 (89.6) | ||
| Black | 35 (5.5) | ||
| Other | 31 (4.9) | ||
| Education | |||
| High school graduate or less | 80 (12.6) | ||
| Some college/vocational | 160 (25.1) | ||
| College/post-college graduate | 397 (62.3) | ||
| Household Income | |||
| < $20,000 | 44 (6.9) | ||
| $20,000–$49,999 | 109 (17.1) | ||
| $50,000–$100,000 | 192 (30.1) | ||
| > $100,000 | 272 (42.7) | ||
| Employment status | |||
| Full-time employed | 181 (28.4) | ||
| Part-time employed | 83 (13.0) | ||
| Unemployed/retired | 373 (58.6) | ||
| Marital status | |||
| Married/partner | 458 (71.9) | ||
| Non-married | 179 (28.1) | ||
| Time since diagnosis (months) | 4.5 (1.3) | .10 – 7.3 | |
| Cancer stage | |||
| 1 | 334 (52.4) | ||
| 2 | 253 (39.7) | ||
| 3 | 50 (7.8) | ||
| Adjuvant therapy | |||
| Radiation (yes) | 460 (72.2) | ||
| Chemotherapy (yes) | 423 (66.4) | ||
| Surgery | |||
| Lumpectomy only (yes) | 409 (64.2) | ||
| Mastectomy without reconstruction (yes) | 133 (20.9) | ||
| Mastectomy with reconstruction (yes) | 95 (14.9) | ||
QoL and coping strategies over time
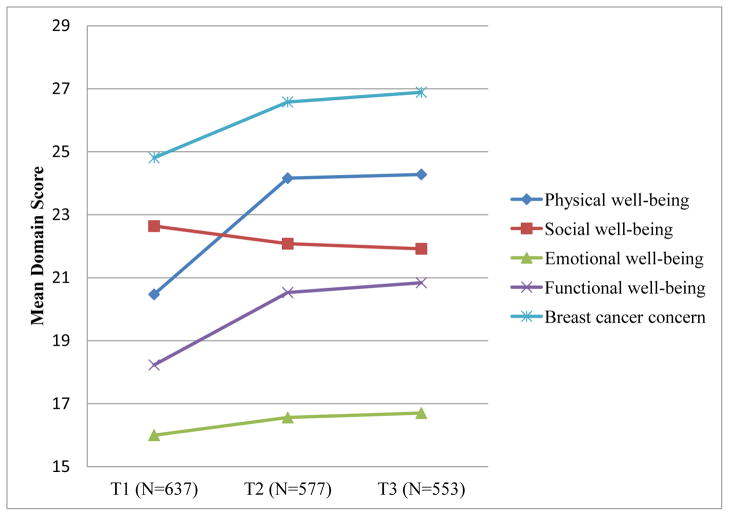
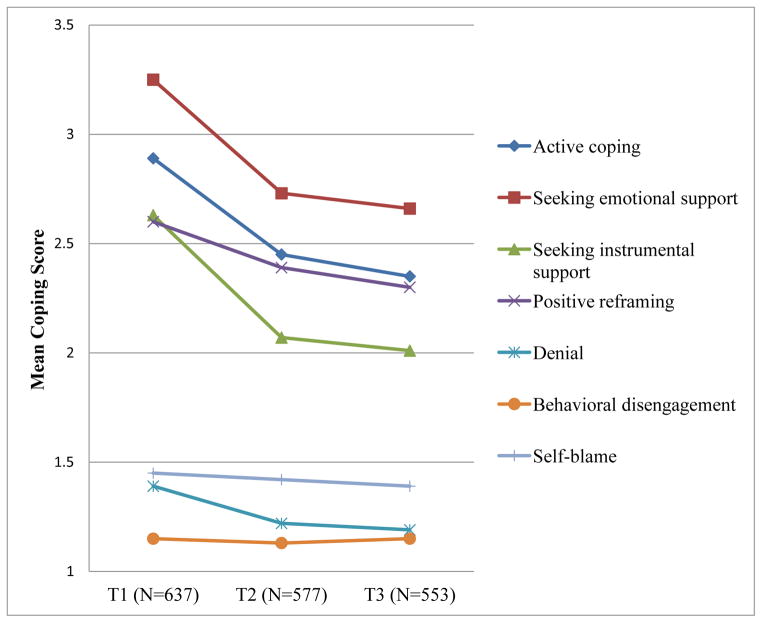
Before proceeding with SEM results, we first examined FACT-B domain and total scores, and coping subscale scores over the three points of assessment. Total FACT-B scores improved over time (T1 = 102.0, T2 = 109.9, and T3 = 110.6, respectively) with significant increases from T1 to T2. Mean QoL scores for all domains, except social well-being, significantly improved from T1 to T2 (p <.01 or below), but showed no additional improvement from T2 to T3 (all ps > .05) (Figure 1). Although social well-being scores decreased over time, this change was not statistically significant. Seeking emotional support was the most frequently used coping strategy. Our study sample tended to utilize positive coping strategies (e.g., active coping, seeking emotional and instrumental support, and positive reframing) more often than negative coping strategies (denial, behavioral disengagement, and self-blame). Use of positive coping strategies, as well as use of denial, significantly decreased from T1 to T2 (all ps < .05), but did not decline significantly between T2 and T3 (all ps > .05) (Figure 2). Behavioral disengagement and self-blame were used less frequently and did not change significantly over time (all ps > .05).
Figure 1.
Mean FACT-B domain scores by survey time points
Figure 2.
Mean coping subscale scores by survey time points. Positive coping includes active coping, seeking emotional support, seeking instrumental support, and positive reframing. Negative coping includes denial, behavioral disengagement, and self-blame.
Measurement model
The measurement model over 3 waves of longitudinal data on QoL, positive, and negative coping strategies was tested. The measurement model had a good fit to the data (χ2 (522) = 1278.03, p = .000, CFI = .93, TLI = .91, and RMSEA = .047). The parameter-constrained model which assesses measurement invariance also yielded a good fit to the data, (χ2 (540) = 1360.43, p = .000, CFI = .92, TLI = .91, and RMSEA = .048). Although the change in χ2 between the measurement and the parameter-constrained model was statistically significant (Δχ2 = 82.4, Δdf = 18, p = .00), the changes in other fit indices were negligible (ΔCFI < .01, ΔTLI < .01, and ΔRMSEA < .001), supporting invariance of measurement over time. All factor loadings from observed indicators to each latent construct were significant, with standardized path coefficients ranging from .52 to .84 for QoL, .50 to .78 for positive coping, and .37 to .67 for negative coping.
Cross-lagged model
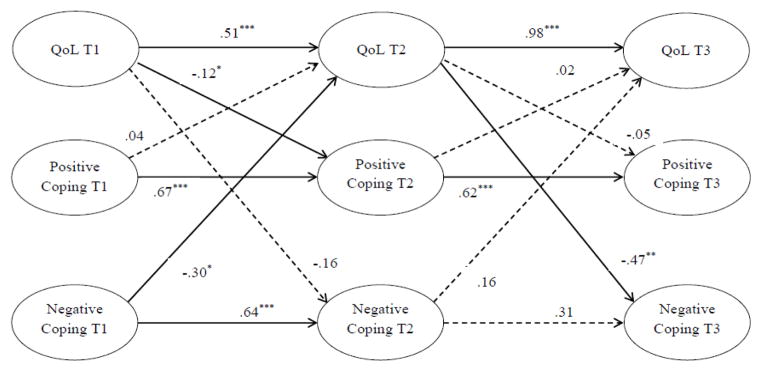
The cross-lagged model examined the reciprocal relationship between QoL and positive coping and between QoL and negative coping across three waves. The stability effects which refer to the autoregressive effects of each latent construct over time (i.e., QoL, positive, and negative coping), were also examined. Covariates included age, cancer stage, time since diagnosis, adjuvant therapy (chemotherapy, radiation), and surgery (lumpectomy only, mastectomy without reconstruction, and mastectomy with reconstruction). Figure 3 depicts the results of the structural model testing. The model yielded an adequate fit to the data: χ2 (733) = 1677.73, p = .000, CFI = .91, TLI = .90, and RMSEA = .045.
Figure 3.
Structural model of QoL, positive, and negative coping strategies. Solid lines represent statistically significant paths (p < .05). The dashed lines indicate statistically not significant paths (p > .05). Factor loadings of the latent constructs and measurement errors are not presented. Age, cancer stage, time since diagnosis, adjuvant therapy (chemotherapy, radiation), and surgery (lumpectomy only, mastectomy without reconstruction, and mastectomy with breast reconstruction) were included as covariates in the analysis.
*p <.05. **p <.01. ***p <.001.
The paths from prior QoL to subsequent QoL were highly significant, with .51 for the path from T1 to T2 and .98 for the path from T2 to T3, indicating that the stability effect of QoL was stronger between T2 and T3 than between T1 and T2. A high stability effect for positive coping was observed over both timepoints (.67 and .62, respectively). The path from T1 negative coping to T2 negative coping was statistically significant (.64), whereas the path from T2 to T3 (.31) was not.
Three statistically significant cross-lagged effects were identified. First, negative coping at T1 predicted worse QoL at T2, β = −.30, p < .05. However, this cross-lagged path was not found from T2 to T3. Second, another significant cross-lagged relationship was found from T2 QoL to T3 negative coping, β = −.47, p < .01. That is, women who had better QoL at T2 reported less frequent use of negative coping at T3. The cross-path from T1 QoL to T2 negative coping was not significant, suggesting women’s T1 QoL was not associated with subsequent use of negative coping earlier on. Lastly, T1 QoL had a small but statistically significant causal influence on subsequent use of positive coping, β = −.12, p < .05, suggesting that better QoL at T1 predicted less subsequent use of positive coping. However, the cross path was not replicated from T2 QoL to T3 positive coping. All other cross-paths did not reach significance.
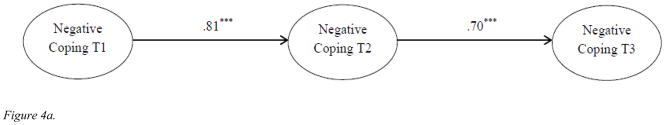
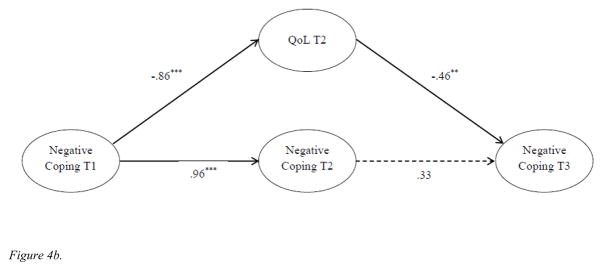
The insignificant stability of negative coping between T2 and T3 was unexpected given the stability of coping from T2 to T3 in Figure 2. To further investigate what might account for this finding, two separate structural models were tested. We first tested the autoregressive model for negative coping only (Figure 4a) and found significant stability coefficients for the two time intervals (β = .81, p < .001; β = .70, p < .001, respectively). However, when T2 QoL was included as a mediator of the relationship between T1 and T3 negative coping (Figure 4b), the stability coefficient between T2 and T3 was non-significant (β = .33, p > .05). The direct paths from T1 negative coping to T2 QoL and T2 QoL to T3 negative coping were both statistically significant (β = −.86, p < .001 and β = −.46, p < .01, respectively). These findings indicated that stability effect of negative coping in our full model was at least partially mediated by T2 QoL. The results of these additional analyses confirmed the significant effect of T2 QoL on T3 negative coping and the insignificant path of T2 and T3 negative coping.
Figure 4.
Figure 4a. Autoregressive models of negative coping over time. Age, cancer stage, time since diagnosis, adjuvant therapy (chemotherapy, radiation), and surgery (lumpectomy only, mastectomy without reconstruction, and mastectomy with breast reconstruction) were included as covariates.
***p <.001.
Figure 4b. Autoregressive models of negative coping over time and the effect of 12-month QoL. Age, cancer stage, time since diagnosis, adjuvant therapy (chemotherapy, radiation), and surgery (lumpectomy only, mastectomy without reconstruction, and mastectomy with breast reconstruction) were included as covariates. The dashed lines indicate statistically not significant paths (p > .05).
**p < .01. ***p< .001.
DISCUSSION
Study results showed that physical, emotional, and functional well-being and breast cancer concerns all improved from T1 to T2 (corresponding to a time period of 1–8 months post diagnosis to 12–20 months post diagnosis), but then stabilized or improved only slightly afterwards. However social well-being showed a different pattern with a decrease from T1 to T2. Our findings are similar to earlier studies that found improvement in overall QoL over one year (41–43), but no significant changes in most QoL domains between 1- and 2-years (44) or beyond (43). The decline in social/family well-being from end of treatment to the 12 or 24-month follow-up period has also been found by others (42, 45) and reinforces the feeling expressed by survivors that they experience a decline in support from family and friends when they are further from diagnosis (46). The overall QoL and change in QoL scores over time for this study population has been found to be comparable to other studies (12, 47, 48). Consistent with previous studies (6, 25, 49), the use of coping strategies decreased from cancer diagnosis to one year later or remained stable over time; we also found that women with breast cancer used positive coping strategies more frequently, whereas use of negative coping strategies were relatively infrequent (6, 25, 50, 51).
We also found that greater use of negative coping strategies at cancer diagnosis was associated with subsequently poorer QoL soon after diagnosis, as found in previous longitudinal investigations (6, 12, 25). Adding to this research, we also found that lower QoL was associated with greater negative coping even further from diagnosis. These consistent findings suggested that the longitudinal reciprocal relationship exists between negative coping and QoL in women with breast cancer.
In this study, we observed that better QoL at T1 predicted less use of positive coping at T2, suggesting women who had better QoL during diagnosis and treatment of breast cancer made less use of positive coping strategies (e.g., active coping, positive reframing, and seeking emotional and instrumental support). This finding replicated and extended previous research indicating that better post-surgery QoL predicts less subsequent use of positive type of coping (e.g., seeking social support) (12). It is likely that survivors who have better QoL may need to use fewer coping strategies. However, contrary to our hypothesis, positive coping was not a significant predictor of later QoL at any time point. Although a number of cross-sectional and longitudinal studies have shown that greater use of positive/adaptive types of coping is related to better QoL (5, 7, 8, 11), other studies have not found a relationship (49, 50). Possible explanations for these inconsistent findings may be the variation across studies in the constructs used to measure positive types of coping strategies, variation in the higher order coping subscales of the Brief COPE, our latent variable approach, and/or sample characteristics. In sum, although we hypothesized a reciprocal relationship between positive coping and QoL, positive coping yielded no significant effect on the subsequent QoL and no evidence was found to support the reciprocal relationship. Our finding is similar to previous studies that have shown no significant reciprocal or bidirectional relationship between QoL and positive types of coping during slightly shorter time period (6, 12, 25).
To our knowledge, the current study is the first to investigate the dynamic relationship between QoL and coping among women with breast cancer, using a three-wave latent factor SEM approach. Study findings add to existing research by simultaneously examining the longitudinal reciprocal relationships between QoL and positive/negative coping. Our findings suggest that in addition to the usual view that coping strategies impact QoL, and QoL also influences coping strategies. The findings provide further support for the Lazarus and Folkman transactional theory of stress and coping and extend the application of this theoretical approach to breast cancer population. This alternative point of view would be useful for researchers trying to better understand the relationship between coping and QoL. It is recommended that future research examine the dynamic nature of coping and QoL in cancer survivors.
The significant reciprocal process between negative coping and QoL has several implications for clinical practice. Although previous studies have suggested that interventions focused on positive coping might improve QoL (52, 53), our findings suggest that interventions targeted at reducing negative coping strategies may be particularly beneficial to improve QoL in cancer survivors. Findings also suggest that interventions should target survivors who have poor QoL. Additionally, psycho-educational interventions are needed to break the cycle of negative coping and poor QoL throughout the survival period.
The main strengths of our study include its longitudinal design and use of a comprehensive analytical model allowing for simultaneous examination of the causality between positive/negative coping and QoL. In addition, the study observed coping and QoL substantially over a longer time period than in previous research. A key limitation is that the study sample was predominantly White and well-educated. The underrepresentation of ethnic minority women and low socioeconomic women might limit the generalizability of our results. Another limitation is the different time length between measurement points. Twelve months elapsed between T1 and T2 and there were only 6 months between T2 and T3. This may explain why there was greater change in both QoL and coping from T1 to T2 than from T2 to T3. The two-item scales for each coping measure and low to moderate alpha coefficients for some of the subscales are also the limitations of this study. The use of these very short scales may not be sensitive enough to detect subtle changes in coping responses over time.
In conclusion, our findings provide converging evidence for the longitudinal reciprocal relationship between QoL and negative coping, but not between QoL and positive coping. Taken together, these findings contribute to our knowledge about the reciprocal pathways and highlight an additional avenue for future research.
Acknowledgments
Research reported in this study was supported by the Department of Defense, Grant #DAMD 17-01-0447 and the National Institute of Health/National Cancer Institute, Grant #R25CA122061 (PI: Nancy Avis, PhD).
Footnotes
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards
Authors Paek, Ip, Levine and Avis declare that they have no conflicts of interest to disclose. All study procedures were conducted in accordance with the ethical standards of the Institutional Review Boards of all participating institutions.
References
- 1.American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015. [Google Scholar]
- 2.American Cancer Society. Cancer Treatment and Survivorship Fact and Figures 2014–2015. Atlanta, GA: American Cancer Society; 2014. [Google Scholar]
- 3.Montazeri A. Health-related quality of life in breast cancer patients: A bibliographic review of the literature from 1974 to 2007. J Exp & Clin Canc Res. 2008;27(1):32–63. doi: 10.1186/1756-9966-27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mols F, Vingerhoets AJJM, Coebergh JW, van de Poll-Franse LV. Quality of life among long-term breast cancer survivors: A systematic review. Eur J Cancer. 2005;41(17):2613–2619. doi: 10.1016/j.ejca.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 5.Avis NE, Crawford S, Manuel J. Quality of life among younger women with breast cancer. J Clin Oncol. 2005;23:3322–3330. doi: 10.1200/JCO.2005.05.130. [DOI] [PubMed] [Google Scholar]
- 6.Carver CS, Pozo C, Harris SD, et al. How coping mediates the effect of optimism on distress: A study of women with early stage breast cancer. J Pers Soc Psychol. 1993;65(2):375–390. doi: 10.1037//0022-3514.65.2.375. [DOI] [PubMed] [Google Scholar]
- 7.Kershaw T, Northouse L, Kritpracha C, Schafenacker A, Mood D. Coping strategies and quality of life in women with advanced breast cancer and their family caregivers. Psychol Health. 2004;19(2):139–155. [Google Scholar]
- 8.Holland KD, Holahan CK. The Relation of Social Support and Coping to Positive Adaptation to Breast Cancer. Psychol Health. 2003;18(1):15–29. [Google Scholar]
- 9.Ransom S, Jacobsen PB, Schmidt JE, Andrykowski MA. Relationship of Problem-Focused Coping Strategies to Changes in Quality of Life Following Treatment for Early Stage Breast Cancer. J Pain Symptom Manag. 2005;30(3):243–253. doi: 10.1016/j.jpainsymman.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Kenne Sarenmalm E, Öhlén J, Jonsson T, Gaston-Johansson F. Coping with Recurrent Breast Cancer: Predictors of Distressing Symptoms and Health-Related Quality of Life. J Pain Symptom Manag. 2007;34(1):24–39. doi: 10.1016/j.jpainsymman.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Stanton AL, Danoff-Burg S, Cameron CL, et al. Emotionally expressive coping predicts psychological and physical adjustment to breast cancer. J Consult Clin Psych. 2000;68(5):875–882. [PubMed] [Google Scholar]
- 12.Danhauer S, Crawford S, Farmer D, Avis N. A longitudinal investigation of coping strategies and quality of life among younger women with breast cancer. J Behav Med. 2009;32(4):371–379. doi: 10.1007/s10865-009-9211-x. [DOI] [PubMed] [Google Scholar]
- 13.Gall TL, Guirguis-Younger M, Charbonneau C, Florack P. The trajectory of religious coping across time in response to the diagnosis of breast cancer. Psycho-Oncology. 2009;18(11):1165–1178. doi: 10.1002/pon.1495. [DOI] [PubMed] [Google Scholar]
- 14.Bishop SR, Warr D. Coping, Catastrophizing and Chronic Pain in Breast Cancer. J Behav Med. 2003;26(3):265–281. doi: 10.1023/a:1023464621554. [DOI] [PubMed] [Google Scholar]
- 15.Ben-Zur H, Gilbar O, Lev S. Coping with breast cancer: patient, spouse, and dyad models. Psychosom Med. 2001;63(1):32–39. doi: 10.1097/00006842-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro SL, Lopez AM, Schwartz GE, et al. Quality of life and breast cancer: Relationship to psychosocial variables. J Clin Psychol. 2001;57(4):501–519. doi: 10.1002/jclp.1026. [DOI] [PubMed] [Google Scholar]
- 17.David D, Montgomery GH, Bovbjerg DH. Relations between coping responses and optimism–pessimism in predicting anticipatory psychological distress in surgical breast cancer patients. Pers Indiv Differ. 2006;40(2):203–213. doi: 10.1016/j.paid.2005.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman L, Romero C, Elledge R, et al. Attribution of Blame, Self-forgiving Attitude and Psychological Adjustment in Women with Breast Cancer. J Behav Med. 2007;30(4):351–357. doi: 10.1007/s10865-007-9108-5. [DOI] [PubMed] [Google Scholar]
- 19.Kim J, Han JY, Shaw B, McTavish F, Gustafson D. The Roles of Social Support and Coping Strategies in Predicting Breast Cancer Patients’ Emotional Well-being: Testing Mediation and Moderation Models. J Health Psychol. 2010;15(4):543–552. doi: 10.1177/1359105309355338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lehto U-S, Ojanen M, Kellokumpu-Lehtinen P. Predictors of quality of life in newly diagnosed melanoma and breast cancer patients. Ann Oncol. 2005;16(5):805–816. doi: 10.1093/annonc/mdi146. [DOI] [PubMed] [Google Scholar]
- 21.Stanton AL, Danoff-burg S, Huggins ME. The first year after breast cancer diagnosis: hope and coping strategies as predictors of adjustment. Psycho-Oncology. 2002;11(2):93–102. doi: 10.1002/pon.574. [DOI] [PubMed] [Google Scholar]
- 22.Sears SR, Stanton AL, Danoff-Burg S. The yellow brick road and the emerald city: Benefit finding, positive reappraisal coping and posttraumatic growth in women with early-stage breast cancer. Health Psychol. 2003;22(5):487–497. doi: 10.1037/0278-6133.22.5.487. [DOI] [PubMed] [Google Scholar]
- 23.Hack TF, Degner LF. Coping responses following breast cancer diagnosis predict psychological adjustment three years later. Psycho-Oncology. 2004;13(4):235–247. doi: 10.1002/pon.739. [DOI] [PubMed] [Google Scholar]
- 24.Lazarus RS, Folkman S. Stress, appraisal, and coping. New York: Springer; 1984. [Google Scholar]
- 25.Culver JL, Arena PL, Antoni MH, Carver CS. Coping and distress among women under treatment for early stage breast cancer: comparing african americans, hispanics and non-hispanic whites. Psycho-Oncology. 2002;11(6):495–504. doi: 10.1002/pon.615. [DOI] [PubMed] [Google Scholar]
- 26.Holzner B, Kemmler G, Kopp M, et al. Quality of Life in Breast Cancer Patients—Not Enough Attention for Long-Term Survivors? Psychosomatics. 2001;42(2):117–123. doi: 10.1176/appi.psy.42.2.117. [DOI] [PubMed] [Google Scholar]
- 27.Ganz PA, Guadagnoli E, Landrum MB, Lash TL, Rakowski W, Silliman RA. Breast cancer in older women: quality of life and psychosocial adjustment in the 15 months after diagnosis. J Clin Oncol. 2003;21(21):4027–4033. doi: 10.1200/JCO.2003.08.097. [DOI] [PubMed] [Google Scholar]
- 28.Antoni MH, Lehman JM, Kilbourn KM, et al. Cognitive-behavioral stress management intervention decreases the prevalence of depression and enhances benefit finding among women under treatment for early-stage breast cancer. Health Psychol. 2001;20(1):20–32. doi: 10.1037//0278-6133.20.1.20. [DOI] [PubMed] [Google Scholar]
- 29.David WK, Sidney B, Graeme CS, et al. Cognitive-existential group psychotherapy for women with primary breast cancer: A randomised controlled trial. Psycho-Oncology. 2003;12(6):532–546. doi: 10.1002/pon.683. [DOI] [PubMed] [Google Scholar]
- 30.Avis NE, Levine B, Naughton MJ, Case LD, Naftalis E, Van Zee KJ. Age-related longitudinal changes in depressive symptoms following breast cancer diagnosis and treatment. Breast Cancer Res Tr. 2013;139(1):199–206. doi: 10.1007/s10549-013-2513-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hewitt MGS, Stovall E. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, D.C: The National Academics Press; 2006. [Google Scholar]
- 32.Brady MJ, Cella DF, Mo F, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J Clin Oncol. 1997;15(3):974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 33.Carver C. You want to measure coping but your protocol’ too long: Consider the brief cope. Int J Behav Med. 1997;4(1):92–100. doi: 10.1207/s15327558ijbm0401_6. [DOI] [PubMed] [Google Scholar]
- 34.Muthen BO. Beyond SEM: General latent variable modeling. Behaviormetrika. 2002;29(1):81–117. [Google Scholar]
- 35.Byrne BM. Structural equation modeling with Mplus: Basic concepts, applications, and programming. Routledge: 2011. [Google Scholar]
- 36.Kline RB. Principles and practice of structural equation modeling. 3. New York, NY: The Guilford Press; 2010. [Google Scholar]
- 37.Pitts SC, West SG, Tein J-Y. Longitudinal measurement models in evaluation research: Examining stability and change. Eval Program Plann. 1996;19(4):333–350. [Google Scholar]
- 38.Muthén LK, Muthén BO. Mplus user’s guide. Los Angeles, CA: Muthén & Muthén; 2012. [Google Scholar]
- 39.Bentler PM, Bonett DG. Significance tests and goodness of fit in the analysis of covariance structures. Psychol Bull. 1980;88(3):588–606. [Google Scholar]
- 40.Hu L-t, Bentler PM. Fit indices in covariance structure modeling: Sensitivity to underparameterized model misspecification. Psychol Methods. 1998;3(4):424. [Google Scholar]
- 41.Härtl KPHD, Schennach RMD, Müller MPHD, et al. Quality of Life, Anxiety, and Oncological Factors: A Follow-Up Study of Breast Cancer Patients. Psychosomatics. 2010;51(2):112–123. doi: 10.1176/appi.psy.51.2.112. [DOI] [PubMed] [Google Scholar]
- 42.Schreier A, Williams S. Anxiety and quality of life of women receive radiation or chemotherapy for breast cancer. Oncol Nurs Forum. 2004;31:127– 130. doi: 10.1188/04.ONF.127-130. [DOI] [PubMed] [Google Scholar]
- 43.Ritz L, Nissen M, Swenson K, et al. Effects of advanced nursing care on quality of life and cost outcomes in women diagnosed with breast cancer. Oncol Nurs Forum. 2000;27:923– 932. [PubMed] [Google Scholar]
- 44.Ganz P, Coscarelli A, Fred C, Kahn B, Polinsky M, Petersen L. Breast cancer survivors: Psychosocial concerns and quality of life. Breast Cancer Res Tr. 1996;38(2):183–199. doi: 10.1007/BF01806673. [DOI] [PubMed] [Google Scholar]
- 45.Taira N, Shimozuma K, Shiroiwa T, et al. Associations among baseline variables, treatment-related factors and health-related quality of life 2 years after breast cancer surgery. Breast Cancer Res Tr. 2011;128(3):735–747. doi: 10.1007/s10549-011-1631-y. [DOI] [PubMed] [Google Scholar]
- 46.Arora NK, Finney Rutten LJ, Gustafson DH, Moser R, Hawkins RP. Perceived helpfulness and impact of social support provided by family, friends, and health care providers to women newly diagnosed with breast cancer. Psycho-Oncology. 2007;16(5):474–486. doi: 10.1002/pon.1084. [DOI] [PubMed] [Google Scholar]
- 47.Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized Controlled Trial of Exercise Training in Postmenopausal Breast Cancer Survivors: Cardiopulmonary and Quality of Life Outcomes. J Clin Oncol. 2003;21(9):1660–1668. doi: 10.1200/JCO.2003.04.093. [DOI] [PubMed] [Google Scholar]
- 48.Eton DT, Cella D, Yost KJ, et al. A combination of distribution- and anchor-based approaches determined minimally important differences (MIDs) for four endpoints in a breast cancer scale. J Clin Epidemiol. 2004;57(9):898–910. doi: 10.1016/j.jclinepi.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 49.McCaul KD, Sandgren AK, King B, O’Donnell S, Branstetter A, Foreman G. Coping and adjustment to breast cancer. Psycho-Oncology. 1999;8(3):230–236. doi: 10.1002/(SICI)1099-1611(199905/06)8:3<230::AID-PON374>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 50.Yang H-C, Brothers BM, Andersen BL. Stress and Quality of Life in Breast Cancer Recurrence: Moderation or Mediation of Coping? Ann Behav Med. 2008;35(2):188–197. doi: 10.1007/s12160-008-9016-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heim E, Valach L, Schaffner L. Coping and Psychosocial Adaptation: Longitudinal Effects Over Time and Stages in Breast Cancer. Psychosom Med. 1997;59(4):408–418. doi: 10.1097/00006842-199707000-00011. [DOI] [PubMed] [Google Scholar]
- 52.Stanton AL. Psychosocial Concerns and Interventions for Cancer Survivors. J Clin Oncol. 2006;24(32):5132–5137. doi: 10.1200/JCO.2006.06.8775. [DOI] [PubMed] [Google Scholar]
- 53.Al-Azri M, Al-Awisi H, Al-Moundhri M. Coping With a Diagnosis of Breast Cancer-Literature Review and Implications for Developing Countries. Breast J. 2009;15(6):615–622. doi: 10.1111/j.1524-4741.2009.00812.x. [DOI] [PubMed] [Google Scholar]