Abstract
Prenatal alcohol exposure can interfere with endocrine function and have sex-specific effects on behavior. Disrupted development of the pituitary gland, which has been observed in rodent studies, may account for some of these effects. To determine if gestational exposure to alcohol produces measureable changes in the pituitary in human adolescents, we manually traced the pituitary in T1-weighted structural magnetic resonance images (MRI) from adolescents with (15 males, 11 females) and without (16 males, 11 females) heavy prenatal alcohol exposure. Pituitary gland volume and maximum signal intensity were examined for group differences. Control female adolescents presented with significantly greater pituitary volume compared to males, as has been previously reported. However, this sexual dimorphism was absent in adolescents with histories of prenatal alcohol exposure. Alcohol-exposed adolescents, regardless of sex, demonstrated reduced pituitary maximum signal intensity compared to controls. The lack of a sex difference in pituitary volumes within the alcohol-exposed group suggests such exposure may interfere with adolescent typical sexual dimorphism of the pituitary. Signal intensity in the posterior pituitary may reflect vasopressin storage. Our findings suggest vasopressin activity should be evaluated in alcohol-exposed adolescents.
Keywords: fetal, prenatal, alcohol, pituitary, sex
Introduction
There are well-documented detrimental effects of prenatal alcohol exposure on embryonic and fetal development. Fetal alcohol spectrum disorders (FASD) encompass the range of outcomes that may occur as a result of alcohol teratogenesis, including growth retardation, birth defects, facial dysmorphia, and central nervous system dysfunction. Exposure to alcohol during prenatal development disrupts midline expansion, sonic hedgehog signaling, and neural crest development, which makes the midline craniofacial and brain structures especially vulnerable (Sulik et al. 1984, 1986; Johnson et al., 1996; Smith et al., 2014).
The pituitary gland is a midline endocrine structure located along the base of the brain. Pituitary abnormalities have been noted in autopsy examinations of individuals with prenatal alcohol exposure (Coulter et al., 1993). Animal models support this effect and are dependent on the timing (gestational day: GD) of the prenatal alcohol exposure. Following exposure, the pituitary of fetal mice was absent (GD7: Godin et al., 2010), disproportionately enlarged (GD8: Parnell et al., 2009; GD10: O'Leary-Moore et al., 2010), or dysplastic (GD9: Kotch and Sulik, 1992). Alcohol’s interference with the development of the pituitary gland could have lasting effects on endocrine function. An alteration of the hormonal milieu has been noted in infants and children with prenatal alcohol exposure. Higher basal cortisol levels were observed in 2-month old infants exposed to alcohol or cigarettes during gestation (Ramsay et al., 1996) and the amount of alcohol consumed during pregnancy was positively related to both the basal cortisol level and the post-stress cortisol response in 13-month old infants (Jacobson et al., 1999). School age children (6–14 years) with FASD displayed elevated salivary cortisol levels in the afternoon and bedtime as compared to controls (Keiver et al., 2015).
The effects of prenatal alcohol exposure on pituitary gland can be probed using magnetic resonance imaging (MRI). The volume of the pituitary gland, which fluctuates along with hormonal changes, has been postulated as an index of hormonal status in humans (MacMaster et al., 2006). During adolescence, coinciding with pubertal development, the structure of the pituitary gland changes in shape and increases in volume (Elster et al., 1990; Kato et al., 2002; MacMaster et al., 2007) and there is a positive linear relationship between pituitary volume and levels of the gonadal hormones and sex steroids (Peper et al., 2010). Girls have a larger adolescent increase in pituitary volume than do boys (Elster et al., 1990; Kato et al., 2002; MacMaster et al., 2007) and pituitary volume also surges during pregnancy (Dinç et al., 1998). Additionally, MRI measurements of the pituitary can provide insight into vasopressin levels stored in its posterior lobe. On T1-weighted MRI the neurohypophysis (i.e., posterior lobe of the pituitary) is hyperintense (i.e., a bright spot), but the signal intensity diminishes after water deprivation (Lee et al., 2001) or infusion of hypertonic saline into the blood stream (Teshima et al., 2008). The neurohypophyseal signal intensity change corresponds to blood plasma levels of vasopressin (Lee et al., 2001; Teshima et al., 2008). This suggests that T1-weighted MRI signal intensity of the neurohypophysis can be used to estimate vasopressin storage.
T1-weighted MRI was used to examine pituitary volume and signal intensity in male and female adolescents with and without histories of heavy prenatal alcohol exposure. We predicted that prenatal alcohol exposure would result in reduced pituitary volume and signal intensity.
Materials and Methods
Subjects
Twenty-six adolescents with histories of heavy prenatal alcohol exposure (AE; 15 males, 11 females) and 27 controls (CON; 16 males, 11 females) completed magnetic resonance imaging as part of ongoing studies at the Center for Behavioral Teratology at San Diego State University. As part of these ongoing studies, Full Scale IQ scores (FSIQ, from the Wechsler Intelligence Scale for Children; Wechsler, 1991; Wechsler, 2004), socioeconomic status index scores (SES, measured by the Hollingshead Four Factor Index of Social Status; Hollingshead, 1975), and presence of psychopathology (defined as meeting criteria for any Axis I disorder based on caregiver report with the clinician assisted Computerized Diagnostic Interview Schedule for Children Version IV [DISC]; Shaffer et al., 2000) were available for all participants.
Study inclusion criteria required subjects to be age 13–18y and fluent English speakers. A confirmed history of heavy prenatal alcohol exposure was required for inclusion in the AE group. Exposure was established via the review of medical, adoption, or social service records or maternal self-report. If review of records indicated that the mother was alcohol abusing or dependent during pregnancy, this was considered evidence of heavy prenatal alcohol exposure. Direct maternal report was not available for 24 out of 26 of our subjects, as these children no longer resided with their biological mothers. However, maternal report was available for two children and for these cases heavy alcohol exposure (defined as average maternal consumption of 4 or more drinks per occasion at least once a week or an average of 14 drinks per week on several occasions during pregnancy) was confirmed. In addition, children were examined by a pediatric dysmorphologist (KL Jones). A fetal alcohol syndrome (FAS) diagnosis was not required for inclusion in the AE group, but was indicated by 2 or more facial features and either growth restriction or microcephaly (Mattson et al., 2010). Nine adolescents in the AE group (35%) received an FAS diagnosis using these research criteria. For inclusion in the CON group, subjects must have minimal exposure to alcohol, defined as maternal consumption of no more than 1 alcoholic drink per week on average and never more than 2 drinks per occasion during pregnancy. Screening for prenatal alcohol exposure in the CON group was often determined through direct maternal report, as 25 out of 27 control subjects resided with their biological mothers.
Exclusion criteria for both groups included head trauma with loss of consciousness for more than 30 minutes, contraindication for MRI scanning (e.g., irremovable metal in the body or claustrophobia), or serious medical or psychiatric conditions that would prevent participation. The Institutional Review Boards of San Diego State University and the University of California, San Diego approved all study procedures. Parents or guardians signed a written informed consent/parental permission form and adolescent subjects signed an age-appropriate assent document. Subjects received a financial incentive for participation.
MRI data acquisition and processing
High-resolution T1-weighted sagittal volumes were acquired for 53 subjects on a 3T GE Signa Excite scanner (General Electric, Milwaukee, WI) using an 8-channel head coil. To maximize our sample size, images from two separate MRI projects were combined. Images were acquired for 40 subjects with the following scan parameters: TR, 8 ms; TE, 3.1 ms; flip angle 8°; matrix 256 × 256 × 192; FOV, 256 × 256 mm; slice thickness 1 mm; acquisition time, 7 min and 24 s. For the remaining 13 subjects, images were acquired with parameters: TR, 8 ms; TE, 3.0 ms; flip angle 12°; matrix 256 × 256 × 192; FOV, 240 × 240 mm; slice thickness 1 mm; acquisition time, 7 min and 4 s.
Preprocessing on structural images was conducted using FreeSurfer v5.3 software (http://surfer.nmr.mgh.harvard.edu; (Fischl et al., 2002). Estimated total intracranial volume (ICV) was calculated using FreeSurfer’s automatic quantification, which included registration of the image to standard space. All data were visually inspected for quality control. FreeSurfer’s automated processing was found adequate for most images; however, the skull-stripping step removed portions of the pituitary gland in 3 images (1 AE and 2 CON). These images were manually edited to restore the missing voxels and reprocessed.
Pituitary Tracing
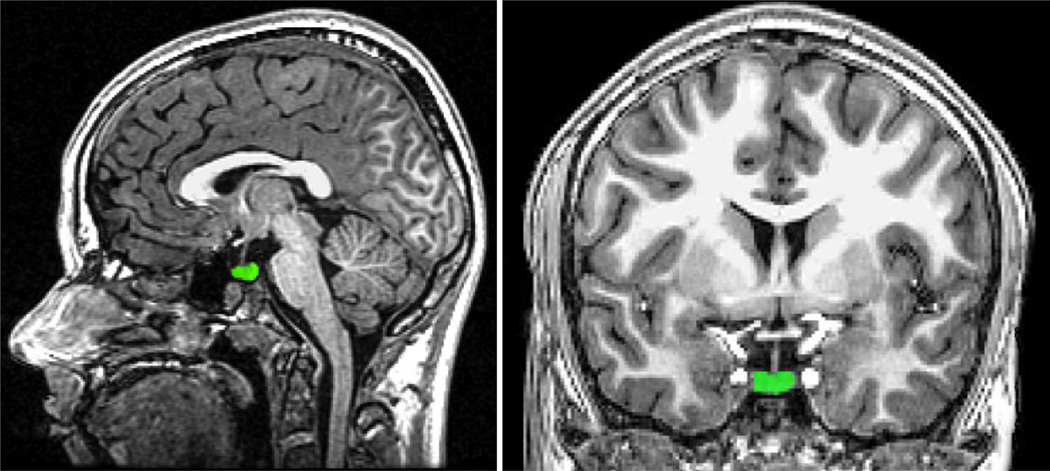
Figure 1 displays a representative pituitary tracing. Using FreeSurfer’s tkmedit tool, the pituitary gland was labeled. In the coronal view, the pituitary was identified and the gland was traced in all slices where it could be visualized. The infundibular stalk was not included in tracings. Labels were inspected and edited in the sagittal and horizontal planes ensuring that the final pituitary label conformed to the expected pituitary shape. A single analyst blinded to group assignment traced the pituitary. To assess intra-rater reliability a random sample of 10 images were selected and the pituitary was retraced. Pearson correlations demonstrated excellent intra-rater (r=0.93, p<0.001) reliability. Total pituitary volume was extracted for use in a between group analysis. We were also interested in examining signal intensity of the posterior pituitary, which is delineated from the anterior pituitary based on the signal intensity. However, upon visual inspection it was clear that there was substantial variation between subjects on this variable. We worried that a systematic bias would be introduced in delineating the borders of the posterior pituitary if the average signal intensity were different between AE and CON. Thus, we chose to trace the pituitary gland as a whole and extract the single voxel with the greatest signal intensity to determine if there may be group differences. Visual inspection revealed that the voxel used for the signal intensity analysis was located posterior to the infundibular stalk in all cases, providing an approximation of the posterior pituitary signal intensity.
Figure 1.
Sample pituitary tracing in the sagittal (left) and coronal (right) planes.
Statistical Analyses
Statistical analyses were conducted using SPSS 22. Results were considered significant at p<0.05.
Subject Characteristics
Differences in age, SES, and FSIQ were evaluated with separate 2 × 2 (exposure × sex) analysis of variance (ANOVA) analyses. Separate chi-squared analyses were conducted within group and sex to evaluate differences in race, ethnicity, handedness, and rate of psychopathology.
Evaluation of Potential Covariates
Given the reported pituitary growth spurt during adolescence, age was evaluated as a potential covariate for analysis of variance (ANOVA) tests. Age did not interact with the independent variables for either volume or signal intensity, indicating homogeneity of regression assumptions were met. However, age was not significantly associated with either pituitary volume (R2=.038, p=.160) or signal intensity (R2=.028, p=.228); therefore, it was not retained as a covariate in subsequent analyses.
To increase our sample sizes we used anatomical images that were acquired as part of two separate neuroimaging projects. Thus, this data is comprised of MRI scans that were acquired using two different scan parameters. To account for this additional source of variance, we dummy coded for scan parameters and evaluated this as a potential covariate. Scanning parameters significantly predicted signal intensity (R2=.124, p=.010) but not volume (R2=.053, p=.097). Further, there were no interactions between scanning parameter and either exposure group or sex, indicating the homogeneity of regression assumption was met. Therefore, scan parameter was retained as a covariate for the signal intensity analysis.
Pituitary Analyses
The independent variables in each analysis were exposure history (AE vs. CON) and sex (male vs. female). A 2 × 2 analysis of variance (ANOVA) evaluated pituitary volume. A 2 × 2 analysis of covariance (ANCOVA) examined signal intensity while accounting for the variance associated with the two scanning parameters used to acquire the data. As individuals with prenatal alcohol exposure may have microcephaly and ICV is known to differ between males and females, additional 2 × 2 ANOVAs were planned to examine both ICV and pituitary volume as a proportion of ICV (adjusted-PV). Simple effects pairwise comparisons were used to follow up significant interactions.
Results
Demographics
Groups were similar across demographic variables, excepting FSIQ and presence of psychopathology. Compared to controls, the AE group had a lower FSIQ [F(1,49)=19.8, p<.001] and higher rates of psychopathology [χ2(2, 53)=23.5, p<.001]. Overall, 13 psychiatric disorders were represented in our sample. There was a tendency for the AE group to have higher rates than controls for most disorders. The AE group demonstrated significantly higher rates of attention-deficit/hyperactivity disorder (7.4% CON vs. 61.5% AE, p<.001), oppositional defiant disorder (7.4% CON vs. 30.8% AE, p=.039), transient or chronic tic disorders (0.0% CON vs. 23.1% AE, p=.010), and a trend towards a higher rate of specific phobia (7.4% CON vs. 26.9% AE, p=.076). The CON group did not have a higher rate of any psychiatric disorder compared to the AE group. Demographics are presented in Table 1.
Table 1.
Demographic data from the alcohol-exposed (AE) and control (CON) groups.
| Male | Female | |||
|---|---|---|---|---|
| Variable | CON n=16 |
AE n=15 |
CON n=11 |
AE n=11 |
| Age [M (SD)] | 15.2 (1.43) | 15.2 (1.71) | 14.2 (1.19) | 15.0 (1.40) |
| SES [M (SD)] | 47.8 (11.67) | 42.7 (12.74) | 47.8 (12.90) | 48.9 (11.87) |
| FSIQ [M (SD)]* | 99.3 (15.00) | 88.6 (13.78) | 107.3 (9.75) | 82.6 (17.32) |
| Race [n (%) White] | 9 (56.3) | 8 (53.3) | 8 (72.7) | 8 (72.7) |
| Ethnicity [n (%) Hispanic] | 4 (25.0) | 4 (26.7) | 5 (45.5) | 3 (27.3) |
| Handedness [n (%) Right] | 15 (93.8) | 13 (86.7) | 10 (90.9) | 8 (72.7) |
| Psychopathology [n (%) +Dx]* | 3 (18.8) | 14 (93.3) | 3 (27.3) | 9 (81.8) |
| FAS [n (%) FAS] | -- | 4 (26.7) | -- | 5 (45.5) |
Note:
Asterisk (*p<0.001) indicates significant difference between exposure groups. The AE group had a lower FSIQ on average as well as higher rates of psychopathology (defined as meeting full criteria for any diagnosis on the DISC) as compared to controls.
Pituitary and ICV
Table 2 reports group differences in the pituitary and ICV.
Table 2.
Average pituitary volume (PV), signal intensity (PSI), and intracranial volume (ICV) for the alcohol-exposed (AE) and control (CON) groups
| Male | Female | |||
|---|---|---|---|---|
| Variable | CON n=16 |
AE n=15 |
CON n=11 |
AE n=11 |
| Volume [M (SD)]+ | 524.2 (99.35) | 546.7 (100.41) | 629.8 (82.75) | 529.7 (113.99) |
| Intensity [M (SD)]* | 159.6 (22.14) | 135.9 (17.83) | 179.1 (19.50) | 169.0 (18.71) |
| ICV [M (SD)]* | 1,597,057.3 (124,860.90) |
1,439,811.7 (126,725.76) |
1,377,361.6 (120,925.46) |
1,328,902.9 (115,129.02) |
Note: Pituitary and intracranial volume units are expressed in mm3; signal intensity (i.e., brightness) is expressed as T1 MR. Asterisk indicates main effect of exposure group (AE<CON):
p<0.05; cross indicates exposure × sex interaction (AE females < CON females > CON males):
p<0.05.
For pituitary volume, there was a significant exposure × sex interaction [F(1,49)=4.9, p=.032, η2=.090]. Follow-up simple effects pairwise comparisons revealed that CON females had significantly greater volume than CON males (p=.009, η2=.130) but this sex effect was not seen in the AE subjects (p=.671, η2=.004). Additionally, CON females displayed greater volume than AE females (p=.023, η2=.102) but there was no difference between CON and AE males (p=.534, η2=.008). There was no significant main effect of sex (p=.117, η2=.049) or exposure (p=.169, η2=.038).
For signal intensity, the exposure × sex interaction was not significant (p=.226, η2=.030). However, significant main effects of exposure [CON>AE; F(1,48)=8.2, p=.006, η2=.146] and sex [females>males; F(1,48)=24.8, p<.001, η2=.341] were detected after accounting for signal intensity differences associated with scanning parameters [F(1,48)=7.5, p=.009, η2=.135).
For ICV, there was no interaction of exposure and sex (p=.118, η2=.049). There were significant main effects of exposure [CON>AE; F(1,49)=9.0, p=.004, η2=.156] and sex [males>females; F(1,49)=23.4, p<.001, η2=.323].
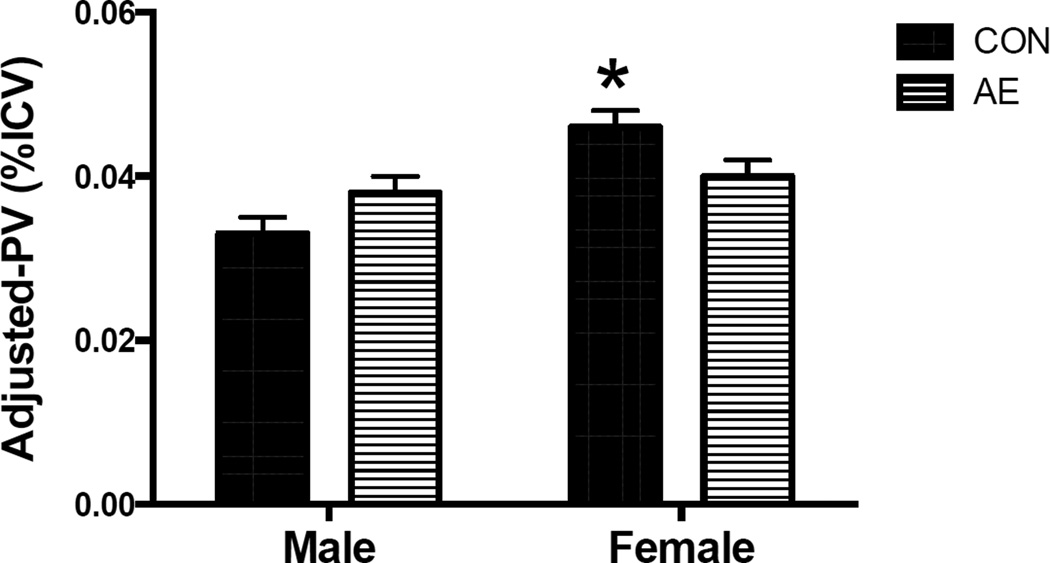
The analysis of pituitary volume adjusted for ICV (adjusted-PV, Figure 2) revealed a significant exposure × sex interaction [F(1,49)= 7.9, p=.007, η2=.139]. Follow-up simple effects pairwise comparisons revealed that the adjusted-PV was significantly greater in CON females compared to CON males (p<.001, η2=.296) but this sex effect was not observed in the AE group (p=.60, η2=.006). Additionally, CON females displayed greater adjusted-PV volume than AE females (p=.048, η2=.078) and there was a trend for AE males to have a larger adjusted-PV compared to CON males (p=.056, η2=.072). A significant main effect of sex was also detected [females>males; F(1,49)=12.7, p=.001, η2=.205] but there was no main effect of exposure (p=.770, η2=.002).
Figure 2.
While there is no effect of exposure history on pituitary volume in males, AE females have significantly smaller pituitary volumes than do CON females. Pituitary volumes do not differ between male and female adolescents with prenatal alcohol exposure histories. However, CON females display significantly greater pituitary volumes than do CON males. *Indicates CON males < CON females > AE females, ps<.05.
Discussion
We examined whether having a history of heavy prenatal alcohol exposure impacts volume or signal intensity of the pituitary gland in adolescents. Control females had larger pituitary volumes than control males, consistent with the expected sexual-dimorphism for this age (Doraiswamy et al., 1992; MacMaster et al., 2007; Peyster et al., 1986; Peyster et al., 1983; Suzuki et al., 1990; Tsunoda et al., 1997). However, we found that alcohol-exposed females had pituitary volumes that were more similar in volume to that of males, thus lacking the expected sexual dimorphism. The atypical pituitary gland volume among alcohol-exposed females could indicate disruption of any number of endocrine functions. One possibility is that the lack of sexual dimorphism may be related to alterations in the hypothalamic-pituitary-gonadal axis. In animal models, following prenatal alcohol exposure, higher prolactin and lower luteinizing hormone levels have been reported in periadolescent females (Esquifino et al., 1986), while lower levels of both hormones have been noted in adult females (Hannigan et al., 1997). In humans, prenatal alcohol exposure is associated with lower testosterone levels in both sexes (Carter et al., 2014). The observed lack of sexual dimorphism in alcohol-exposed adolescents could also reflect a delay in puberty. Female rats with prenatal alcohol exposure showed delayed vaginal opening (McGivern and Yellon, 1992). In male alcohol-exposed rats delayed onset of spermatogenesis occurred during pubertal development (Lan et al., 2013). However, prenatal alcohol exposure did not impact age at menarche in human girls and was unrelated to self-reported Tanner stage of pubertal development in either sex (Carter et al., 2014). In human boys, binge alcohol exposure during gestation may delay the first voice break and nocturnal emission (non-significant trends, Håkonsen et al., 2014).
We found that the maximum signal intensity in alcohol-exposed adolescents was lower than in controls. Visual inspection revealed that the voxel containing the maximum signal intensity value was located posteriorly to the infundibular stalk in all cases. Signal intensity is typically hyperintense in the posterior lobe of the pituitary on T1-weighted MRI. Dehydration or infusion of hypertonic saline solution causes an attenuation of this signal intensity, which negatively corresponds to blood plasma levels of vasopressin (Lee et al., 2001; Teshima et al., 2008). That the alcohol-exposed youth had lower maximum signal intensity in their posterior pituitary may suggest that they had less vasopressin stored in their neurohypophysis at the time of scanning. Animal studies indicate that prenatal alcohol exposure produce changes in vasopressin release and fluid homeostasis (Dow-Edwards et al., 1989). Prenatal alcohol exposed rats show increased water consumption and urinary output along with changes to osmotic- or hemorrhage- stimulated vasopressin release (McGivern et al., 1998; Knee et al., 2004; Bird et al., 2006). Both the brain and systemic responses appear to be impacted by prenatal alcohol exposure to alter these homeostatic responses (Godino et al., 2015). Importantly, pituitary vasopressin content was reduced in rats with prenatal alcohol exposure (Knee et al., 2004; Bird et al., 2006), which is consistent with our observations of reduced signal intensity in the pituitary. Vasopressin is involved in hormone secretion from the anterior pituitary, water reabsorption in the kidneys, and cardiovascular homeostasis (Koshimizu et al., 2012). However, it is important to note that vasopressin also has modulatory actions on a number of behaviors, including aggression, social bonding, and anxiety (Mavani et al., 2015). The reduced signal intensity in our alcohol-exposed subjects may have important implications for any of these processes.
Our findings should be considered in light of several limitations. While examining both alcohol-exposure history and sex strengthens our study, we do have a small sample size and narrow age-range preventing our ability to adequately examine the relation between age and pituitary variables. Our results should be replicated in a larger sample, across several time points, and verified with additional measures, such as body fluid samples for hormonal evaluation, assessment of pubertal stage, and determination of stress levels or reactivity.
Postnatal environmental factors may contribute to pituitary variables. For example, a trend for reduced pituitary volume has been noted in patients with borderline personality disorder who experienced childhood trauma (Garner et al. 2007). Prior studies have shown that age at the time of first placement and past traumatic experiences can impact development of children with FASD, where early age at placement outside of biological family decreases and traumatic experiences increases socio-emotional problems (Koponen et al. 2009; 2013). The majority of alcohol-exposed subjects in this study were no longer living with their biological mothers, having been placed with adoptive or foster families, but we did not evaluate past traumatic experiences.
Additionally, the majority of individuals with prenatal alcohol exposure meet diagnostic criteria for a psychiatric disorder (O’Connor et al., 2002; Fryer et al., 2007) and many are treated with medications. In our sample, 88% of the alcohol-exposed group met criteria for a psychiatric diagnosis versus only 22% in the control group. Hypothalamic-pituitary-adrenal axis dysfunction is implicated in a number of psychiatric disorders, highlighting the possibility that psychiatric comorbidity may impact the pituitary in those with FASD. For example, smaller pituitary volumes have been noted in patients with obsessive-compulsive disorder (Atmaca et al. 2009; Jung et al. 2009), but larger volumes have been found in patients with first episode psychosis (Pariante et al. 2004; Büschlen et al. 2011). Furthermore, medications can impact pituitary structure (Jung et al. 2009; MacMaster et al. 2007; Pariante et al. 2005). Caregivers reported that 73% of the alcohol-exposed subjects were using medication (e.g., psychostimulants, antidepressants, antipsychotics, anticonvulsants, or asthma/allergy treatments), supplementation (e.g., melatonin, GABA), or hormonal treatments (e.g., growth hormone, letrozole) versus only 15% of the control subjects (psychostimulants or asthma/allergy treatments).
An evaluation of environmental factors, psychiatric symptoms, medication use, and their relation to pituitary structure in those with FASD could be an interesting and potentially important future study. Despite these limitations, our study suggests that prenatal alcohol exposure is associated with altered pituitary structure in females and reduced pituitary signal intensity in both sexes. This work supports animal research indicating that pituitary development is disrupted by prenatal alcohol exposure. These pituitary abnormalities may have important consequences for endocrine functioning in individuals with prenatal alcohol exposure but additional research is needed to examine hormonal imbalances, such as vasopressin.
Highlights.
Reduced pituitary volume in adolescent females with prenatal alcohol exposure.
Reduced posterior pituitary T1 signal intensity in alcohol-exposed youth.
Endocrine function should be evaluated in youth with prenatal alcohol exposure.
Acknowledgments
This work was supported by the National Institute on Alcohol Abuse and Alcoholism (NIAAA): R01 AA019605, U24 AA014811, U01 AA014834, F31 AA022033, T32 AA013525, K99 AA022661; an American Fellowship from AAUW; and a National Science Foundation (NSF) Graduate Research Fellowship: DGE-1247398. We thank Monica Manibusan, Benjamin Deweese, Amy Flink, and Heather Holden for their support in data collection. We also thank the study participants and their families who generously supply their time and support our research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- Atmaca M, Yildirim H, Ozler S, Koc M, Kara B, Sec S. Smaller pituitary volume in adult patients with obsessive-compulsive disorder. Psychiatry and clinical neurosciences. 2009;63:516–520. doi: 10.1111/j.1440-1819.2009.01981.x. [DOI] [PubMed] [Google Scholar]
- Bird DN, Sato AK, Knee DS, Uyehara CFT, Person DA, Claybaugh JR. Effects of prenatal alcohol exposure and sex on the arginine vasopressin response to hemorrhage in the rat. American journal of physiology: regulative, integrative, and comparative physiology. 2006;291:R77–R82. doi: 10.1152/ajpregu.00740.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büschlen J, Berger GE, Borgwardt SJ, Aston J, Gschwandtner U, Pflueger MO, Kuster P, Radü EW, Steiglitz R-D, Riecher-Rössler A. Pituitary volume increase during emerging psychosis. Schizophrenia research. 2011;125:41–48. doi: 10.1016/j.schres.2010.09.022. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) Alcohol use and binge drinking among women of childbearing age - United States, 2006–2010. In: C.f.D.C.a., editor. Prevention. 2012. pp. 534–538. [Google Scholar]
- Carter RC, Jacobson JL, Dodge NC, Granger DA, Jacobson SW. Effects of prenatal alcohol exposure on testosterone and pubertal development. Alcoholism: clinical and experimental research. 2014;38:1671–1679. doi: 10.1111/acer.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter CL, Leech RW, Schaefer GB, Scheithauer BW, Brumback RA. Midline cerebral dysgenesis, dysfunction of the hypothalamic-pituitary axis, and fetal alcohol effects. Archives of neurology. 1993;50:771–775. doi: 10.1001/archneur.1993.00540070083022. [DOI] [PubMed] [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society: JINS. 2009;15:331–343. doi: 10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinç H, Esen F, Demirci A, Sari A, Resit Gumele H. Pituitary dimensions and volume measurements in pregnancy and post partum. MR assessment. Acta radiologica (Stockholm, Sweden: 1987) 1998;39:64–69. doi: 10.1080/02841859809172152. [DOI] [PubMed] [Google Scholar]
- Doraiswamy PM, Potts JM, Axelson DA, Husain MM, Lurie SN, Na C, Escalona PR, McDonald WM, Figiel GS, Ellinwood EH, Jr, et al. MR assessment of pituitary gland morphology in healthy volunteers: age- and gender-related differences. AJNR. American journal of neuroradiology. 1992;13:1295–1299. [PMC free article] [PubMed] [Google Scholar]
- Dow-Edwards DL, Trachtman H, Riley EP, Freed LA, Milhorat TH. Arginine vasopressin and body fluid homeostasis in the fetal alcohol exposed rat. Alcohol. 1989;6:193–198. doi: 10.1016/0741-8329(89)90018-9. [DOI] [PubMed] [Google Scholar]
- Elster AD, Chen MY, Williams DW, 3rd, Key LL. Pituitary gland: MR imaging of physiologic hypertrophy in adolescence. Radiology. 1990;174:681–685. doi: 10.1148/radiology.174.3.2305049. [DOI] [PubMed] [Google Scholar]
- Esquifino AI, Sanchis R, Guerri C. Effect of prenatal alcohol exposure on sexual maturation of female rat offspring. Neuroendocrinology. 1986;44:483–487. doi: 10.1159/000124690. [DOI] [PubMed] [Google Scholar]
- Faravelli C, Lo Sauro C, Godini L, Lelli L, Benni L, Pietrini F, Lazzeretti L, Talamba GA, Fioravanti G, Ricca V. Childhood stressful events, HPA axis and anxiety disorders. World journal of psychiatry. 2012;2:13–25. doi: 10.5498/wjp.v2.i1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fryer SL, McGee CL, Matt GE, Riley EP, Mattson SN. Evaluation of psychopathological conditions in children with heavy prenatal alcohol exposure. Pediatrics. 2007;119:e733–e741. doi: 10.1542/peds.2006-1606. [DOI] [PubMed] [Google Scholar]
- Garner B, Chanen AM, Phillips L, Velakoulis D, Wood SJ, Jackson HJ, Pantelis C, McGorry PD. Pituitary volume in teenagers with first-presentation borderline personality disorder. Psychiatry research: neuroimaging. 2007;156:257–261. doi: 10.1016/j.pscychresns.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Godin EA, O'Leary-Moore SK, Khan AA, Parnell SE, Ament JJ, Dehart DB, Johnson BW, Allan Johnson G, Styner MA, Sulik KK. Magnetic resonance microscopy defines ethanol-induced brain abnormalities in prenatal mice: effects of acute insult on gestational day 7. Alcoholism: clinical and experimental research. 2010;34:98–111. doi: 10.1111/j.1530-0277.2009.01071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godino A, Abate P, Amigone JL, Vivas L, Molina JC. Prenatal binge-like alcohol exposure alters brain and systemic responses to reach sodium and water balance. Neuroscience. 2015 doi: 10.1016/j.neuroscience.2015.10.004. in press. [DOI] [PubMed] [Google Scholar]
- Håkonsen LB, Brath-Lund ML, Hounsgaard ML, Olsen J, Ernst A, Thulstrup AM, Bech BH, Ramlau-Hansen CH. In utero exposure to alcohol and puberty in boys: a pregnancy cohort study. BMJ open. 2014;4:e004467. doi: 10.1136/bmjopen-2013-004467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley DW, Handmaker NS, Lowe J. Infant stress reactivity and prenatal alcohol exposure. Alcoholism: clinical and experimental research. 2006;30:2055–2064. doi: 10.1111/j.1530-0277.2006.00251.x. [DOI] [PubMed] [Google Scholar]
- Hannigan JH, Hackett JA, Tilak J, Subramanian MG. Sulpiride-induced increases in serum prolactin levels in female rats exposed prenatally to alcohol. Alcohol. 1997;14:585–592. doi: 10.1016/s0741-8329(97)00053-0. [DOI] [PubMed] [Google Scholar]
- Hellemans KG, Sliwowska JH, Verma P, Weinberg J. Prenatal alcohol exposure: fetal programming and later life vulnerability to stress, depression and anxiety disorders. Neuroscience and biobehavioral reviews. 2010a;34:791–807. doi: 10.1016/j.neubiorev.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans KG, Verma P, Yoon E, Yu WK, Young AH, Weinberg J. Prenatal alcohol exposure and chronic mild stress differentially alter depressive- and anxiety-like behaviors in male and female offspring. Alcoholism: clinical and experimental research. 2010b;34:633–645. doi: 10.1111/j.1530-0277.2009.01132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. 1975 Unpublished working paper. [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N, Viljoen DL, Jones KL, Robinson LK. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 institute of medicine criteria. Pediatrics. 2005;115:39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Bihun JT, Chiodo LM. Effects of prenatal alcohol and cocaine exposure on infant cortisol levels. Development and psychopathology. 1999;11:195–208. doi: 10.1017/s0954579499002011. [DOI] [PubMed] [Google Scholar]
- Johnson VP, Swayze VW, II, Sato Y, Andreasen NC. Fetal alcohol syndrome: craniofacial and central nervous system manifestations. Am J Med Genet. 1996;61:329–339. doi: 10.1002/(SICI)1096-8628(19960202)61:4<329::AID-AJMG6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Jung MH, Huh MJ, Kang D-H, Choi J-S, Jung WH, Jang JH, Park J-Y, Han JY, Choi C-H, Kwon JS. Volumetric differences in the pituitary between drug-naïve and medicated patients with obsessive-compulsive disorder. Progress in neuropsychopharmacology & biological psychiatry. 2009;33:605–609. doi: 10.1016/j.pnpbp.2009.02.017. [DOI] [PubMed] [Google Scholar]
- Kato K, Saeki N, Yamaura A. Morphological changes on MR imaging of the normal pituitary gland related to age and sex: main emphasis on pubescent females. Journal of clinical neuroscience: official journal of the Neurosurgical Society of Australasia. 2002;9:53–56. doi: 10.1054/jocn.2001.0973. [DOI] [PubMed] [Google Scholar]
- Keiver K, Bertram CP, Orr AP, Clarren S. Salivary cortisol levels are elevated in the afternoon and at bedtime in children with prenatal alcohol exposure. Alcohol. 2015;49:79–87. doi: 10.1016/j.alcohol.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Knee DS, Sato AK, Uyehara CFT, Claybaugh JR. Prenatal exposure to ethanol causes partial diabetes insipidus in adult rats. American journal of physiology: regulative, integrative, and comparative physiology. 2004;287:R277–R283. doi: 10.1152/ajpregu.00223.2003. [DOI] [PubMed] [Google Scholar]
- Kopenen AM, Kalland M, Autti-Rämö I. Caregiving environment and socio-emotional development of foster-placed FASD-children. Children and youth services review. 2009;31:1049–1056. [Google Scholar]
- Koponen AM, Kalland M, Autti-Rämö I, Laamanen R, Suominen S. Socio-emotional development of children with foetal alcohol spectrum disorders in long-term foster family care: a qualitative study. Nordic Social Work Research. 2013;3:38–58. [Google Scholar]
- Kormos V, Gaszner B. Role of neuropeptides in anxiety, stress, and depression: from animals to humans. Neuropeptides. 2013;47:401–419. doi: 10.1016/j.npep.2013.10.014. [DOI] [PubMed] [Google Scholar]
- Koshimizu TA, Nakamura K, Egashira N, Hiroyama M, Nonoguchi H, Tanoue A. Vasopressin V1a and V1b receptors: from molecules to physiological systems. Physiological reviews. 2012;92:1813–1864. doi: 10.1152/physrev.00035.2011. [DOI] [PubMed] [Google Scholar]
- Kotch LE, Sulik KK. Patterns of ethanol-induced cell death in the developing nervous system of mice; neural fold states through the time of anterior neural tube closure. International journal of developmental neuroscience: the official journal of the International Society for Developmental Neuroscience. 1992;10:273–279. doi: 10.1016/0736-5748(92)90016-s. [DOI] [PubMed] [Google Scholar]
- Lan N, Vogl AW, Weinberg J. Prenatal ethanol exposure delays the onset of spermatogenesis in the rat. Alcoholism, clinical and experimental research. 2013;37:1074–1081. doi: 10.1111/acer.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, Choi HY, Sung YA, Lee JK. High signal intensity of the posterior pituitary gland on T1-weighted MR images. Correlation with plasma vasopressin concentration to water deprivation. Acta radiologica (Stockholm, Sweden: 1987) 2001;42:129–134. doi: 10.1034/j.1600-0455.2001.042002129.x. [DOI] [PubMed] [Google Scholar]
- MacMaster FP, El-Sheikh R, Upadhyaya AR, Nutche J, Rosenberg DR, Keshavan M. Effect of antipsychotics on pituitary gland volume in treatment-naive first-episode schizophrenia: a pilot study. Schizophrenia research. 2007;92:207–210. doi: 10.1016/j.schres.2007.01.022. [DOI] [PubMed] [Google Scholar]
- MacMaster FP, Keshavan M, Mirza Y, Carrey N, Upadhyaya AR, El-Sheikh R, Buhagiar CJ, Taormina SP, Boyd C, Lynch M, Rose M, Ivey J, Moore GJ, Rosenberg DR. Development and sexual dimorphism of the pituitary gland. Life sciences. 2007;80:940–944. doi: 10.1016/j.lfs.2006.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMaster FP, Russell A, Mirza Y, Keshavan MS, Taormina SP, Bhandari R, Boyd C, Lynch M, Rose M, Ivey J, Moore GJ, Rosenberg DR. Pituitary volume in treatment-naive pediatric major depressive disorder. Biological psychiatry. 2006;60:862–866. doi: 10.1016/j.biopsych.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Foroud T, Sowell ER, Jones KL, Coles CD, Fagerlund A, Autti-Rämö I, May PA, Adnams CM, Konovalova V, Wetherill L, Arenson AD, Barnett WK, Riley EP the CIFASD. Collaborative initiative on fetal alcohol spectrum disorders: methodology of clinical projects. Alcohol. 2010;44:635–641. doi: 10.1016/j.alcohol.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavani GP, DeVita MV, Michelis MF. A review of the nonpressor and nonantidiuretic actions of the hormone vasopressin. Frontiers in medicine. 2015;2:19. doi: 10.3389/fmed.2015.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGivern RF, Ervin MG, McGeary J, Somes C, Handa RJ. Prenatal ethanol exposure induces a sexually dimorphic effect on daily water consumption in prepubertal and adult rats. Alcoholism: clinical and experimental research. 1998;22:868–875. [PubMed] [Google Scholar]
- McGivern RF, Yellon SM. Delayed onset of puberty and subtle alterations in GnRH neuronal morphology in female rats exposed prenatally to ethanol. Alcohol. 1992;9:335–340. doi: 10.1016/0741-8329(92)90077-n. [DOI] [PubMed] [Google Scholar]
- O’Connor MJ, Shah B, Whaley S, Cronin P, Gunderson B, Graham J. Psychiatric illness in a clinical sample of children with prenatal alcohol exposure. The American Journal of Drug and Alcohol Abuse. 2002;28:743–754. doi: 10.1081/ada-120015880. [DOI] [PubMed] [Google Scholar]
- O'Leary-Moore SK, Parnell SE, Godin EA, Dehart DB, Ament JJ, Khan AA, Johnson GA, Styner MA, Sulik KK. Magnetic resonance microscopy-based analyses of the brains of normal and ethanol-exposed fetal mice. Birth defects research. Part A, Clinical and molecular teratology. 2010;88:953–964. doi: 10.1002/bdra.20719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante CM, Dazzan P, Danese A, Morgan KD, Brudaglio F, Morgan C, Fearon P, Orr K, Hutchinson G, Pantelis C, Velakoulis D. Increased pituitary volume in antipsychotic-free and antipsychotic-treated patients of the AEsop first-onset psychosis study. Neuropsychopharmacology. 2005;30:1923–1931. doi: 10.1038/sj.npp.1300766. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Vassilopoulou K, Velakoulis D, Phillips L, Soulsby B, Wood SJ, Brewer W, Smith DJ, Dazzan P, Yung AR, Zervas IM, Christodoulou GN, Murray R, McGorry PD, Pantelis C. Pituitary volume in psychosis. British journal of psychiatry. 2004;185:5–10. doi: 10.1192/bjp.185.1.5. [DOI] [PubMed] [Google Scholar]
- Parnell SE, O'Leary-Moore SK, Godin EA, Dehart DB, Johnson BW, Allan Johnson G, Styner MA, Sulik KK. Magnetic resonance microscopy defines ethanol-induced brain abnormalities in prenatal mice: effects of acute insult on gestational day 8. Alcoholism: clinical and experimental research. 2009;33:1001–1011. doi: 10.1111/j.1530-0277.2009.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper JS, Brouwer RM, van Leeuwen M, Schnack HG, Boomsma DI, Kahn RS, Hulshoff Pol HE. HPG-axis hormones during puberty: a study on the association with hypothalamic and pituitary volumes. Psychoneuroendocrinology. 2010;35:133–140. doi: 10.1016/j.psyneuen.2009.05.025. [DOI] [PubMed] [Google Scholar]
- Peyster RG, Adler LP, Viscarello RR, Hoover ED, Skarzynski J. CT of the normal pituitary gland. Neuroradiology. 1986;28:161–165. doi: 10.1007/BF00327890. [DOI] [PubMed] [Google Scholar]
- Peyster RG, Hoover ED, Viscarello RR, Moshang T, Haskin ME. CT appearance of the adolescent and preadolescent pituitary gland. AJNR. American journal of neuroradiology. 1983;4:411–414. [PMC free article] [PubMed] [Google Scholar]
- Ramsay DS, Bendersky MI, Lewis M. Effect of prenatal alcohol and cigarette exposure on two- and six-month-old infants' adrenocortical reactivity to stress. Journal of pediatric psychology. 1996;21:833–840. doi: 10.1093/jpepsy/21.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versios, and reliability of some common diagnoses. Journal of the american academy of child and adolescent psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Smith SM, Garic A, Flentke GR, Berres ME. Neural crest development in fetal alcohol syndrome. Birth defects research. Part C, Embryo today: reviews. 2014;102:210–220. doi: 10.1002/bdrc.21078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulik KK, Johnston MC, Daft PA, Russell WE, Dehart DB. Fetal alcohol syndrome and DiGeorge anomaly: critical ethanol exposure periods for craniofacial malformations as illustrated in an animal model. American journal of medical genetics: supplement. 1986;2:97–112. doi: 10.1002/ajmg.1320250614. [DOI] [PubMed] [Google Scholar]
- Sulik KK, Lauder JM, Dehart DB. Brain malformations in prenatal mice following acute maternal ethanol administration. International journal of developmental neuroscience: the official journal of the international society for developmental neuroscience. 1984;2:203–214. doi: 10.1016/0736-5748(84)90014-5. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Takashima T, Kadoya M, Konishi H, Kameyama T, Yoshikawa J, Gabata T, Arai K, Tamura S, Yamamoto T, et al. Height of normal pituitary gland on MR imaging: age and sex differentiation. Journal of computer assisted tomography. 1990;14:36–39. doi: 10.1097/00004728-199001000-00006. [DOI] [PubMed] [Google Scholar]
- Teshima T, Hara Y, Masuda H, Taoda T, Nezu Y, Harada Y, Yogo T, Hasegawa D, Orima H, Osamura RY, Tagawa M. Relationship between arginine vasopressin and high signal intensity in the pituitary posterior lobe on T1-weighted MR images in dogs. The Journal of veterinary medical science / the Japanese Society of Veterinary Science. 2008;70:693–699. doi: 10.1292/jvms.70.693. [DOI] [PubMed] [Google Scholar]
- Tsunoda A, Okuda O, Sato K. MR height of the pituitary gland as a function of age and sex: especially physiological hypertrophy in adolescence and in climacterium. AJNR. American journal of neuroradiology. 1997;18:551–554. [PMC free article] [PubMed] [Google Scholar]
- Weinberg J, Sliwowska JH, Lan N, Hellemans KG. Prenatal alcohol exposure: foetal programming, the hypothalamic-pituitary-adrenal axis and sex differences in outcome. Journal of neuroendocrinology. 2008;20:470–488. doi: 10.1111/j.1365-2826.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children-third edition manual. 3rd. San Antonio: The Psychological Corporation; 1991. [Google Scholar]
- Wechsler D. Manual for the Wechsler Intelligence Scale for Children-fourth edition integrated (4th ed.) San Antonio: Pearson. 2004 [Google Scholar]