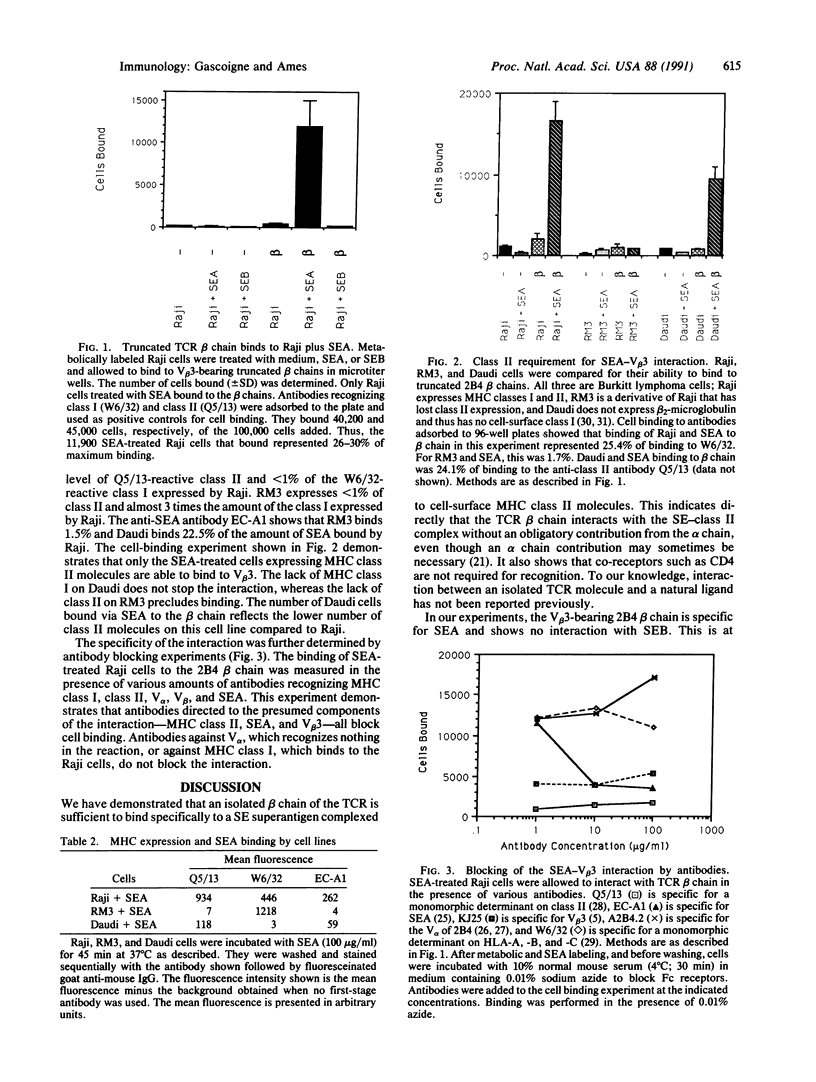
Abstract
The interaction of the T-cell receptor (TCR) with peptide antigen plus major histocompatibility complex (MHC) protein requires both alpha and beta chains of the TCR. The "superantigens" are a group of molecules that are recognized in association with MHC class II but that do not appear to conform to this pattern. Superantigens are defined as such because they cause the activation or thymic deletion of many or all T cells bearing specific TCR beta-chain variable region (V beta) elements. The strong association of particular V beta S with T-cell responses to superantigens suggests that their interaction with the TCR is fundamentally different from that of most antigens. We have directly investigated the involvement of the beta chain in recognition of a superantigen by using a secreted, truncated TCR beta chain and the bacterial superantigen staphylococcal enterotoxin A complexed to cell-surface MHC class II. We demonstrate that this interaction is specific for the enterotoxin and is dependent on MHC class II expression by the cell. The reaction can be inhibited by antibodies against the three components of the reaction: V beta, enterotoxin, and class II. This shows that the TCR beta chain is sufficient to mediate the interaction with a superantigen-class II complex. The TCR alpha chain and co-receptors such as CD4 are not required.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe R., Vacchio M. S., Fox B., Hodes R. J. Preferential expression of the T-cell receptor V beta 3 gene by Mlsc reactive T cells. Nature. 1988 Oct 27;335(6193):827–830. doi: 10.1038/335827a0. [DOI] [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Bill J., Appel V. B., Palmer E. An analysis of T-cell receptor variable region gene expression in major histocompatibility complex disparate mice. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9184–9188. doi: 10.1073/pnas.85.23.9184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan J. E., Herman A., Kappler J. W., Marrack P. Stimulation of B10.BR T cells with superantigenic staphylococcal toxins. J Immunol. 1990 Apr 1;144(7):2473–2479. [PubMed] [Google Scholar]
- Calman A. F., Peterlin B. M. Mutant human B cell lines deficient in class II major histocompatibility complex transcription. J Immunol. 1987 Oct 1;139(7):2489–2495. [PubMed] [Google Scholar]
- Chien Y. H., Gascoigne N. R., Kavaler J., Lee N. E., Davis M. M. Somatic recombination in a murine T-cell receptor gene. Nature. 1984 May 24;309(5966):322–326. doi: 10.1038/309322a0. [DOI] [PubMed] [Google Scholar]
- Choi Y. W., Herman A., DiGiusto D., Wade T., Marrack P., Kappler J. Residues of the variable region of the T-cell-receptor beta-chain that interact with S. aureus toxin superantigens. Nature. 1990 Aug 2;346(6283):471–473. doi: 10.1038/346471a0. [DOI] [PubMed] [Google Scholar]
- Chothia C., Boswell D. R., Lesk A. M. The outline structure of the T-cell alpha beta receptor. EMBO J. 1988 Dec 1;7(12):3745–3755. doi: 10.1002/j.1460-2075.1988.tb03258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claverie J. M., Prochnicka-Chalufour A., Bougueleret L. Implications of a Fab-like structure for the T-cell receptor. Immunol Today. 1989 Jan;10(1):10–14. doi: 10.1016/0167-5699(89)90058-3. [DOI] [PubMed] [Google Scholar]
- Davis M. M., Bjorkman P. J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988 Aug 4;334(6181):395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- Doyle C., Strominger J. L. Interaction between CD4 and class II MHC molecules mediates cell adhesion. Nature. 1987 Nov 19;330(6145):256–259. doi: 10.1038/330256a0. [DOI] [PubMed] [Google Scholar]
- Fischer H., Dohlsten M., Lindvall M., Sjögren H. O., Carlsson R. Binding of staphylococcal enterotoxin A to HLA-DR on B cell lines. J Immunol. 1989 May 1;142(9):3151–3157. [PubMed] [Google Scholar]
- Fleischer B., Schrezenmeier H. T cell stimulation by staphylococcal enterotoxins. Clonally variable response and requirement for major histocompatibility complex class II molecules on accessory or target cells. J Exp Med. 1988 May 1;167(5):1697–1707. doi: 10.1084/jem.167.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser J. D. High-affinity binding of staphylococcal enterotoxins A and B to HLA-DR. Nature. 1989 May 18;339(6221):221–223. doi: 10.1038/339221a0. [DOI] [PubMed] [Google Scholar]
- Fry A. M., Matis L. A. Self-tolerance alters T-cell receptor expression in an antigen-specific MHC restricted immune response. Nature. 1988 Oct 27;335(6193):830–832. doi: 10.1038/335830a0. [DOI] [PubMed] [Google Scholar]
- Gascoigne N. R., Goodnow C. C., Dudzik K. I., Oi V. T., Davis M. M. Secretion of a chimeric T-cell receptor-immunoglobulin protein. Proc Natl Acad Sci U S A. 1987 May;84(9):2936–2940. doi: 10.1073/pnas.84.9.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gascoigne N. R. Transport and secretion of truncated T cell receptor beta-chain occurs in the absence of association with CD3. J Biol Chem. 1990 Jun 5;265(16):9296–9301. [PubMed] [Google Scholar]
- Herrmann T., Maryanski J. L., Romero P., Fleischer B., MacDonald H. R. Activation of MHC class I-restricted CD8+ CTL by microbial T cell mitogens. Dependence upon MHC class II expression of the target cells and V beta usage of the responder T cells. J Immunol. 1990 Feb 15;144(4):1181–1186. [PubMed] [Google Scholar]
- Jameson S. C., Kaye J., Gascoigne N. R. A T cell receptor V alpha region selectively expressed in CD4+ cells. J Immunol. 1990 Sep 1;145(5):1324–1331. [PubMed] [Google Scholar]
- Janeway C. A., Jr, Yagi J., Conrad P. J., Katz M. E., Jones B., Vroegop S., Buxser S. T-cell responses to Mls and to bacterial proteins that mimic its behavior. Immunol Rev. 1989 Feb;107:61–88. doi: 10.1111/j.1600-065x.1989.tb00003.x. [DOI] [PubMed] [Google Scholar]
- Kanagawa O., Ahlem C. Requirement of the T cell antigen receptor occupancy for the target cell lysis by cytolytic T lymphocytes. Int Immunol. 1989;1(6):565–569. doi: 10.1093/intimm/1.6.565. [DOI] [PubMed] [Google Scholar]
- Kubo R. T., Born W., Kappler J. W., Marrack P., Pigeon M. Characterization of a monoclonal antibody which detects all murine alpha beta T cell receptors. J Immunol. 1989 Apr 15;142(8):2736–2742. [PubMed] [Google Scholar]
- Lapeyre C., Kaveri S. V., Janin F., Strosberg A. D. Production and characterization of monoclonal antibodies to staphylococcal enterotoxins: use in immunodetection and immunopurification. Mol Immunol. 1987 Dec;24(12):1243–1254. doi: 10.1016/0161-5890(87)90118-0. [DOI] [PubMed] [Google Scholar]
- Marrack P., Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990 May 11;248(4956):705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- Matis L. A. The molecular basis of T-cell specificity. Annu Rev Immunol. 1990;8:65–82. doi: 10.1146/annurev.iy.08.040190.000433. [DOI] [PubMed] [Google Scholar]
- Mollick J. A., Cook R. G., Rich R. R. Class II MHC molecules are specific receptors for staphylococcus enterotoxin A. Science. 1989 May 19;244(4906):817–820. doi: 10.1126/science.2658055. [DOI] [PubMed] [Google Scholar]
- Norment A. M., Salter R. D., Parham P., Engelhard V. H., Littman D. R. Cell-cell adhesion mediated by CD8 and MHC class I molecules. Nature. 1988 Nov 3;336(6194):79–81. doi: 10.1038/336079a0. [DOI] [PubMed] [Google Scholar]
- Pullen A. M., Marrack P., Kappler J. W. The T-cell repertoire is heavily influenced by tolerance to polymorphic self-antigens. Nature. 1988 Oct 27;335(6193):796–801. doi: 10.1038/335796a0. [DOI] [PubMed] [Google Scholar]
- Pullen A. M., Wade T., Marrack P., Kappler J. W. Identification of the region of T cell receptor beta chain that interacts with the self-superantigen MIs-1a. Cell. 1990 Jun 29;61(7):1365–1374. doi: 10.1016/0092-8674(90)90700-o. [DOI] [PubMed] [Google Scholar]
- Quaranta V., Walker L. E., Pellegrino M. A., Ferrone S. Purification of immunologically functional subsets of human Ia-like antigens on a monoclonal antibody (Q5/13) immunoadsorbent. J Immunol. 1980 Oct;125(4):1421–1425. [PubMed] [Google Scholar]
- Samelson L. E., Germain R. N., Schwartz R. H. Monoclonal antibodies against the antigen receptor on a cloned T-cell hybrid. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6972–6976. doi: 10.1073/pnas.80.22.6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto H., Yoshikai Y., Kishihara K., Matsuzaki G., Kuga H., Otani T., Nomoto K. Stimulation of all T cells bearing V beta 1, V beta 3, V beta 11 and V beta 12 by staphylococcal enterotoxin A. Eur J Immunol. 1990 Mar;20(3):617–621. doi: 10.1002/eji.1830200323. [DOI] [PubMed] [Google Scholar]
- Tomonari K., Lovering E., Fairchild S., Spencer S. Two monoclonal antibodies specific for the T cell receptor V alpha 8. Eur J Immunol. 1989 Jun;19(6):1131–1135. doi: 10.1002/eji.1830190625. [DOI] [PubMed] [Google Scholar]
- White J., Herman A., Pullen A. M., Kubo R., Kappler J. W., Marrack P. The V beta-specific superantigen staphylococcal enterotoxin B: stimulation of mature T cells and clonal deletion in neonatal mice. Cell. 1989 Jan 13;56(1):27–35. doi: 10.1016/0092-8674(89)90980-x. [DOI] [PubMed] [Google Scholar]
- Woodland D., Happ M. P., Bill J., Palmer E. Requirement for cotolerogenic gene products in the clonal deletion of I-E reactive T cells. Science. 1990 Feb 23;247(4945):964–967. doi: 10.1126/science.1968289. [DOI] [PubMed] [Google Scholar]
- de Préval C., Mach B. The absence of beta 2-microglobulin in Daudi cells: active gene but inactive messenger RNA. Immunogenetics. 1983;17(2):133–140. doi: 10.1007/BF00364753. [DOI] [PubMed] [Google Scholar]