Abstract
Objective
To determine whether serial measurements of cervical length can improve the prediction of preterm birth in asymptomatic women with twin gestations compared with a single measurement of cervical length at mid-gestation.
Materials and Methods
This was a retrospective cohort study of women with twin pregnancies followed in a tertiary medical center between 2012 and 2014. All participants underwent routine measurement of cervical length at mid-gestation and every 2–3 weeks thereafter until 28–32 weeks. For each patient, cervical length was determined at the following time periods: 18+0 to 21+6 weeks (Period 1, routine exam), 22+0 to 24+6 weeks (Period 2), 25+0 to 27+6 weeks (Period 3) and 28+0 to 32+0 weeks (Period 4). Measurements of cervical length at Periods 2–4 were analyzed in the form of either absolute length (in mm) or percent shortening relative to cervical length at Period 1. The performance of a stepwise algorithm that incorporates serial measurements of cervical length for the prediction of preterm birth was compared to that achieved with a single measurement of cervical length at Period 1.
Results
Overall 441 women with twin pregnancies who were eligible for the study underwent a total of 2,374 cervical length measurements. The association of a short cervix (<10th percentile) with preterm birth at <32 weeks persisted in each of the four periods of gestation [Odds ratio (95%-confidence interval): 7.2 (3.1–16.5), 15.3 (6.4–36.7), 10.3 (4.4–24.3) and 23.1(8.3–64.1), respectively]. Compared with a single measurement of cervical length at mid-gestation (Period 1), a stepwise algorithm integrating serial cervical length measurements from all four successive gestational age periods resulted in a significant increase in the area under the ROC curve (0.917 vs. 0.613, p<0.001). Similarly, when a target false positive rate of 5% was used, the same stepwise algorithm was associated with a higher detection rate (69% vs. 28%, p<0.001), higher positive likelihood ratio (14.54 vs. 5.12) and a lower negative likelihood ratio (0.32 vs. 0.76) for preterm birth at <32 weeks compared with a single measurement of cervical length at Period 1.
Conclusions
Integration of serial measurements of cervical length using a stepwise algorithm in asymptomatic women with twin gestations can improve the detection of women at risk of preterm birth. Prospective studies are needed to validate these findings, and to investigate whether improved risk assessment performance is sufficient to offset the additional costs associated with serial cervical length measurements.
Keywords: cervical shortening, cervix, labor, prematurity, screening, prediction, preterm labor, cervical ripening, biomarker, longitudinal study, pregnancy, ultrasound
Condensation
Serial measurements of sonographic cervical length improve the predictive accuracy for preterm birth in twin gestations.
INTRODUCTION
Preterm birth (PTB) is the main cause of neonatal mortality and morbidity in twin pregnancies.1–16 Although current interventions for the prevention of PTB in women with twins are of limited success,17–36 early prediction of PTB in these pregnancies can assist care providers in identifying those women with twin gestation who are at the highest risk of PTB and who may benefit from closer monitoring and administration of antenatal corticosteroids for fetal lung maturation.29, 37–47 Furthermore, identification of patients at risk is a prerequisite for testing interventions to reduce the rate of preterm birth.
Cervical length (CL) at midtrimester, as measured by transvaginal ultrasound, is a powerful predictor of PTB in both singleton 23, 48–68 and twin gestations.37, 42, 69–92 However, given the fact that, in some cases, cervical shortening may become evident only later during the second trimester, it seems reasonable that serial monitoring of CL may improve the overall detection of women who are at high risk of PTB.93–97 In an effort to improve the predictive accuracy of CL, measurement of CL is often repeated during the second and early third trimester.78, 81, 98–106 However, evidence that serial measurements of CL improve the prediction of PTB is limited, and the results of available studies are conflicting. 107–109 In a recent systematic review and meta-analysis on the predictive accuracy of changes in sonographic CL over time in singleton and twin pregnancies, Conde-Agudelo and Romero found that the shortening of cervical length over time had only a low to moderate predictive accuracy for preterm birth.110 The contradictory results may be related to the limitations of some of the studies which have been subject to systematic review and meta-analysis. For example, some studies have a small sample size, and there is variability in the timing of CL measurement, the definition of cervical shortening, and the cutoff of CL used to define a short cervix. In addition, in most of these studies, the analysis of the predictive value of serial measurements of CL was limited to data derived from only two sequential measurements of CL.
The aim of the current study was to determine whether the integration of information obtained from serial measurements of CL can improve the prediction of PTB in asymptomatic women with twin gestations compared to a single measurement of cervical length at 18+0 to 21+6 weeks of gestation.
MATERIALS AND METHODS
Study population
This was a retrospective study of all women with twin gestations who were followed in the Twins Clinic in a single referral center between January 2012 and December 2014. Pregnancies complicated by any of the following conditions were excluded: less than 3 measurements of cervical length during gestation, cervical cerclage, uncertain pregnancy dating, indicated preterm delivery at <34 weeks for maternal or fetal indications, birth weight of either twin <500g, gestational age at delivery <24 weeks, stillbirth of one or both fetuses, monoamniotic twins, monochorionic twins complicated by twin-to-twin transfusion syndrome (TTTS), or genetic or structural anomalies. The rationale for the exclusion of women who gave birth before 24 weeks is that in most cases these women would not have multiple measurements of cervical length (which is the focus of the current study). In addition, these women most likely represent a different subgroup of pregnancies resulting in late miscarriage or pre-viable preterm birth for whom the cervical changes will be seen very early and will be very evident, and so the contribution of serial monitoring of cervical length in these cases is expected to me minor. The study was approved by the Sunnybrook Health Sciences Center Research Ethics Board.
Monitoring of cervical length
All women were followed by a single physician in the Twins Clinic of the Sunnybrook Health Sciences Center during the study period. All women who are being followed in this clinic undergo serial transvaginal measurement of CL every 2–3 weeks between 14–18 weeks and 28–32 weeks of gestation. Progesterone was not used in the management of women with twins and a short cervix given the lack of solid evidence regarding the benefit of progesterone in this context. Cervical cerclage was performed in selected cases of women with suspected mechanical cervical insufficiency (N=18), and these cases were excluded from the analysis. Women who were noted to have a short cervix were recommended to decrease the level of activity, although it was advised to avoid strict bed rest. There was no standardized protocol with respect to activity restriction. All sonographic measurements included in the study involved only asymptomatic women. Women with preterm labor and women receiving tocolysis for premature contractions were not included in the analysis. The obstetrician in the Twins Clinic was not blinded to the results of CL measurement.
All sonographic examinations were performed by experienced sonographers. Sonographic CL measurement was performed transvaginally with an empty bladder, according to a standard technique.111 Briefly, the measurement of cervical length was performed in a sagittal plane, visualizing the full length of the cervical canal from the internal os to the external cervical os while exerting as little pressure with the transducer as possible. Unless there was evidence of severe cervical shortening, the measurement of cervical length is repeat following fundal pressure or the Valsalva maneuver. At least three measurements were obtained and the shortest measurement was recorded.
Data collection
Data were extracted from the medical charts and included demographic and obstetrical characteristics, chorionicity, validation of gestational age by first trimester ultrasound, pregnancy complications, presence of cervical cerclage, and neonatal outcome. The reports of all ultrasound examinations performed during pregnancy were reviewed in detail for information on CL.
Predictive value of cervical length at each period
For each patient, CL was determined at 4 time periods along gestation: 18+0 to 21+6 weeks (Period 1), 22+0 to 24+6 weeks (Period 2), 25+0 to 27+6 weeks (Period 3) and 28+0 to 32+0 weeks (Period 4). When more than one measurement of CL was available within any of these periods, the value that was recorded for that period was the average of all CL measurements performed for the individual patient within this time period. The degree of cervical shortening between two given time periods was calculated as follows: ([cervical length in later period – cervical length in earlier period] / cervical length in earlier period) x100. Thus, lower (more negative) values reflect greater cervical shortening.
To determine whether CL has a predictive role at each of the 4 periods, the following measures of accuracy were determined for CL and percent shortening of CL at each of the 4 periods: 1) The association of short cervix (<10th percentile within each specific period) with delivery <32 weeks using logistic regression analysis; 2) The correlation between CL and gestational age at birth (using Pearson’s correlation coefficient); and 3) The area under the ROC curve (AUC) for delivery <32 weeks.
Integration of serial measurements of cervical length
To determine whether integration of serial measurements of CL can improve the predictive accuracy for PTB compared with a single measurement of CL at Period 1, we compared the performance of 4 models/algorithm that integrated increasing numbers of CL measurements using a stepwise approach (Supplemental Figure 1):
CLPeriod1 < threshold1mm (Supplemental Figure 1A);
CLPeriod1 < threshold1mm OR CLPeriod2 < threshold2mm (Supplemental Figure 1B);
CLPeriod1 < threshold1mm, CLPeriod2 < threshold2mm, OR CLPeriod3 < threshold3mm (Supplemental Figure 1C);
CLPeriod1 < threshold1mm, CLPeriod2 < threshold2mm, CLPeriod3 < threshold3mm, OR CLPeriod4 < threshold4mm (Supplemental Figure 1D).
These models/algorithms were designed using a pragmatic stepwise approach that can be easily applied to clinical practice. Once CL at any given period was below the threshold for that specific period, that result of the model was classified as POSITIVE, and no further CL measurements were required. If all CL measurements included in the model were equal or above their corresponding thresholds, the result of the model was classified as NEGATIVE. Women who had missing CL measurements in the later periods because they gave birth prematurely (e.g., women who gave birth at 27 weeks of gestation and therefore could not have a CL measurement in Period 4) were classified as either TRUE POSITIVE (if any of the previous CL measurements were below their corresponding threshold) or FALSE NEGATIVE (if all of the previous CL measurements prior to the premature birth were equal or above their corresponding threshold) (Supplemental Figure 1). Women for whom information on CL in one or more periods was missing for reasons other than preterm birth, were not included in the calculation of the performance of models that were based on CL measurements in these periods. For this reason, the number of women used for the calculation of the performance of the four models was highest for model 1 and lowest for model 4.
To facilitate comparison of the detection rate of the different models, the predictive accuracy of each of the 4 models was determined for the same target false positive rate (2% and 5%). Practically, the only way on to maintain such a fixed false positive rate for models that integrate serial measurements of CL was through the use of lower CL threshold values for the early periods (i.e., Periods 1 and 2) in these models compared with the threshold values used during the same periods for models that integrate only one or two measurements of CL (i.e., models 1 and 2). Thus, for example, the threshold of CL used in Period 1 was highest in the case of model 1 and lowest in the case of model 4. An iteration process was used to identify the set of thresholds of CL at each of the periods that yields the highest detection rate for the target false positive rate for each of the four models.
The following measures of predictive accuracy for PTB were calculated for each of the models: 1) area under the ROC curve (AUC), 2) Detection rate (DR, or sensitivity), 3) False-positive rate (FPR, or 1-specificity), 4) Positive and negative predictive values (PPV and NPV, respectively), and 5) Positive and negative likelihood ratios (LR+ and LR−, respectively). A LR+ >10 and a LR− <0.1 were considered to provide strong predictive evidence; LR+ of 5–10 and LR− of 0.1–0.2 were considered to reflect moderate predictive value; and LR+ <5 and LR− >0.2 reflect only low predictive value.
The same process described above was performed for another set of 4 models that similarly integrated increasing number of CL measurements but analyzed the percent shortening of CL rather than absolute CL:
CLPeriod1 < threshold1mm;
CLPeriod1 < threshold1mm OR percent shortening Periods 1→2 < threshold2%;
CLPeriod1 < threshold1mm, percent shortening Periods 1→2 <threshold2% OR percent shortening Periods 1→ 3 < threshold3%;
CLPeriod1 < threshold1mm, percent shortening Periods 1→2 < threshold2%, percent shortening Periods 1→3 < threshold3% OR percent shortening Periods 1→ 4 < threshold4%.
Statistical analysis
Student’s t-test was used for comparison of mean cervical length at each of the four periods in women who did or did not delivery at <32 weeks. The McNemar’s test for correlated proportions was used to compare the detection rate of the different models using the model that is based on a single measurement of CL at Period 1 (model 1) as reference. The AUC of the different models were compared based on the method of Hanley 112 and using the AUC of the model that is based on a single measurement of CL at Period 1 (model 1) as reference.
Statistical analysis was performed with SPSS v21.0 software (Armonk, NY: IBM Corp). Results were considered significant when the P-value was less than 0.05.
RESULTS
Characteristics of study population
A total of 586 women with twin pregnancies were identified during the study period, of whom 441 (75.3%) were eligible for the study and contributed 2,374 measurements of CL (Table 1). The rate of PTB at less than 34 and 32 weeks was 15.9% and 7.9%, respectively (Table 1).
Table 1.
Characteristics of the study group
| Characteristic | Value |
|---|---|
| N | 441 |
| Maternal age (years) | 32.9 ± 4.1 |
| Chorionicity | |
| DC/DA | 358 (81.2) |
| MC/DA | 83 (18.8) |
| Number of Cervical length measurements | |
| Total | 2,374 |
| Period 1: 18+0 to 21+6 | 688 |
| Period 2: 22+0 to 24+6 | 605 |
| Period 3: 25+0 to 27+6 | 620 |
| Period 4: 28+0 to 32+0 | 461 |
| GA at delivery (weeks) | 35.4 ± 2.4 |
| <34 weeks | 70 (15.9) |
| <32 weeks | 35 (7.9) |
| Birthweight (gr) | |
| Twin A | 2389 ± 557 |
| Twin B | 2333 ± 567 |
DC, di-chorionic; DA, di-amniotic; MC, mono-chorionic
Values are presented as N (%) or mean ± SD unless stated otherwise.
Relationship between cervical length at different periods of gestation and risk of preterm birth
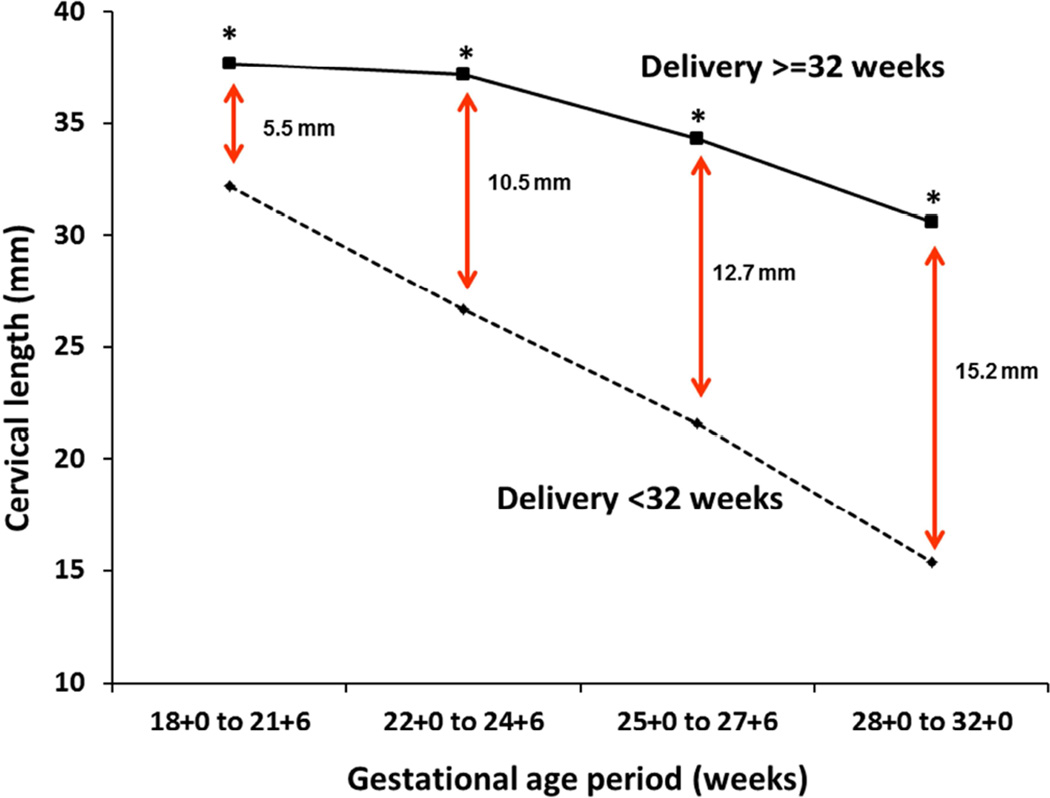
The first question that we addressed was whether measurement of CL at different periods of gestation can identify women who will give birth prematurely. Figure 1 presents the mean CL at each of the four periods of gestation in women who did or did not give birth prior to 32 weeks of gestation. The mean CL was significantly lower in women who gave birth prior to 32 weeks at each of the four gestational age periods and these differences increased with gestational age (Figure 1). The fact that measurement of CL at any of the 4 periods is predictive of PTB is also reflected by the observation that a short cervix (<10th percentile) at each of the 4 periods was significantly associated with PTB <32 weeks, by the significant correlation between CL at each of the 4 periods and gestational age at birth, and by the AUC for PTB<32 weeks at each of the 4 periods (Table 2).
Figure 1. Cervical length at each period of gestation in women who did or did not deliver at <32 weeks.
*p<0.001 (Student’s t-test).
Data reflect the mean cervical length at each gestational age period.
Similar findings were observed for women who did or did not deliver at <34 weeks (data not shown).
Table 2.
Risk of preterm delivery in women with short cervix (<10th percentile) diagnosed at different periods of gestation
| Gestational age period |
Mean ± SD (mm) |
10th percentile (mm) |
Association of CL <10th percentile with delivery < 32 weeks OR (95%-CI) |
Correlation with GA at delivery |
AUC for delivery <32 weeks (95%-CI) |
|---|---|---|---|---|---|
|
Period 1: 18+0 to 21+6 |
37.3 ± 7.1 | 30 | 7.2 (3.1–16.5) | 0.166 * | 0.72 (0.55–0.88) |
|
Period 2: 22+0 to 24+6 |
36.4 ± 8.7 | 25 | 15.3 (6.4–36.7) | 0.286 * | 0.85 (0.73–0.98) |
|
Period 3: 25+0 to 27+6 |
33.5 ± 10.4 | 19 | 10.3 (4.4–24.3) | 0.290 * | 0.85 (0.71–0.98) |
|
Period 4: 28+0 to 32+0 |
29.7 ± 11.4 | 13 | 23.1 (8.3–64.1) | 0.350 * | 0.89 (0.78–1.00) |
CL, cervical length; GA, gestational age; AUC, area under the receiver operating characteristics (ROC) curve
Pearson’s correlation coefficient , p-value <0.01
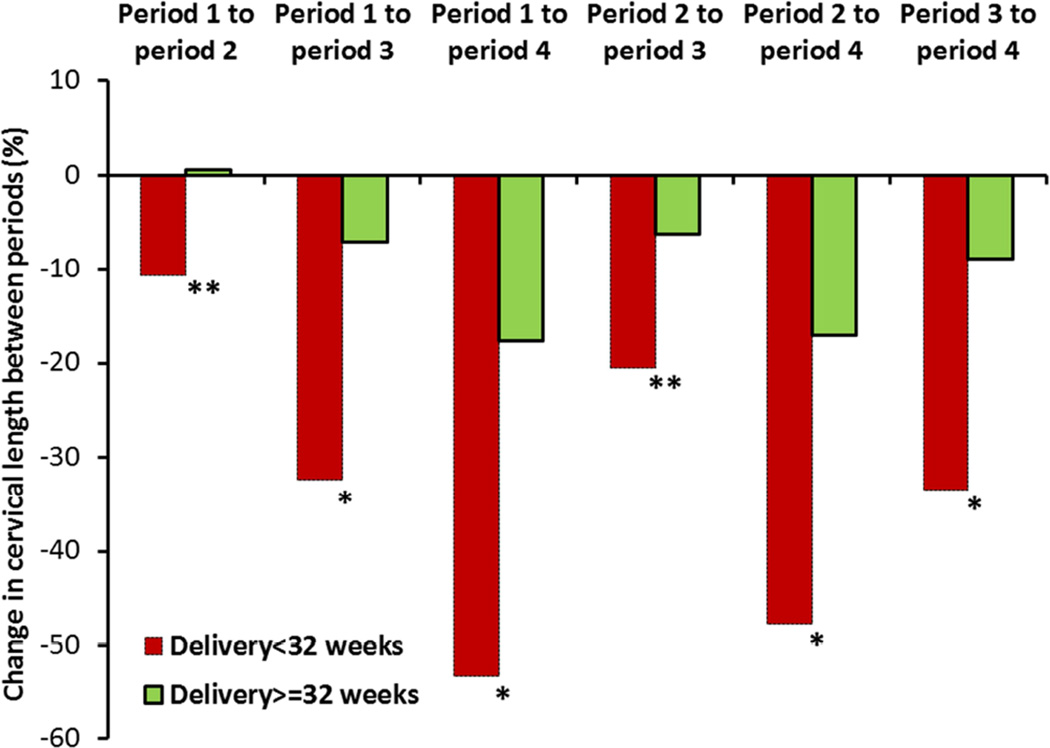
Relationship between cervical shortening and the risk of preterm birth
The value of serial CL measurements can also be analyzed by means of the relative degree of cervical shortening between successive periods rather than the absolute CL at each period. Similar to the absolute measurements of CL at each period of gestation, the degree of cervical shortening between successive periods (expressed as percent shortening) discriminated between women who did or did not give birth prematurely (Figure 2). The fact that the degree of cervical shortening is predictive of PTB is also reflected by the significant association of a high degree of cervical shortening (<10th percentile) with PTB <32 weeks, by the significant correlation between percent shortening at each of the 4 periods and gestational age at birth, and by the AUC for PTB<32 weeks at each of the 4 periods (Table 3).
Figure 2. Change in cervical length between successive periods of gestation in women who did or did not deliver at <32 weeks.
*p<0.001
**p<0.01
Data reflect the change in cervical length between different period of gestation. The change in cervical length was calculated as follows: ([cervical length in later period - cervical length in earlier period] / cervical length in earlier period) x100. Thus, lower (more negative) values reflect greater cervical shortening.
Similar findings were observed for women who did or did not deliver at <34 weeks (data not shown).
Table 3.
Risk of preterm delivery in women with cervical shortening between different periods of gestation
| Gestational age periods † |
Mean ± SD (%) |
10th percentile (%)‡ |
Association of percentage shortening <10th percentile with delivery < 32 weeks ‡ OR (95%-CI) |
Correlation with GA at delivery |
AUC for delivery <32 weeks (95%-CI) |
|---|---|---|---|---|---|
|
Change in CL between periods 1 → 2 |
−0.2% ± 20.8% | −26.8% | 7.1 (3.0–17.1) | 0.178 ** | 0.76 (0.59–0.94) |
|
Change in CL between periods 1 → 3 |
−8.6% ± 26.0% | −43.3% | 7.5 (3.0–18.9) | 0.215 ** | 0.74 (0.55–0.92) |
|
Change in CL between periods 1 → 4 |
−19.8% ± 27.9% | −57.2% | 17.0 (6.0–48.0) | 0.301** | 0.85 (0.71–0.99) |
|
Change in CL between periods 2 → 3 |
−7.0% ± 21.9% | −31.6% | 6.7 (2.7–17.6) | 0.132* | 0.62 (0.44–0.80) |
|
Change in CL between periods 2 → 4 |
−18.5% ± 23.2% | −50.0% | 10.5 (3.2–34.1) | 0.258 ** | 0.85 (0.73–0.97) |
|
Change in CL between periods 3 → 4 |
−10.3% ± 25.6% | −41.0% | 8.1 (2.8–23.3) | 0.124 ** | 0.74 (0.57–0.91) |
CL, cervical length; GA, gestational age; AUC, area under the receiver operating characteristics (ROC) curve
p-value <0.05,
Pearson’s correlation coefficient, p-value <0.01
Calculated as follows: ([cervical length in later period – cervical length in earlier period] / cervical length in earlier period) x100. Thus, lower (more negative) values reflect greater cervical shortening.
Values below the 10th percentile reflect cases with the greatest (most negative) cervical shortening.
Does the integration of serial measurements of cervical length improve the predictive accuracy for preterm birth?
Finally, given that measurement of CL at each of the 4 periods was found to be predictive of PTB, we questioned whether integration of serial measurements of CL at each of these periods using a stepwise algorithm can improve the prediction of PTB (Supplemental Figure 1).
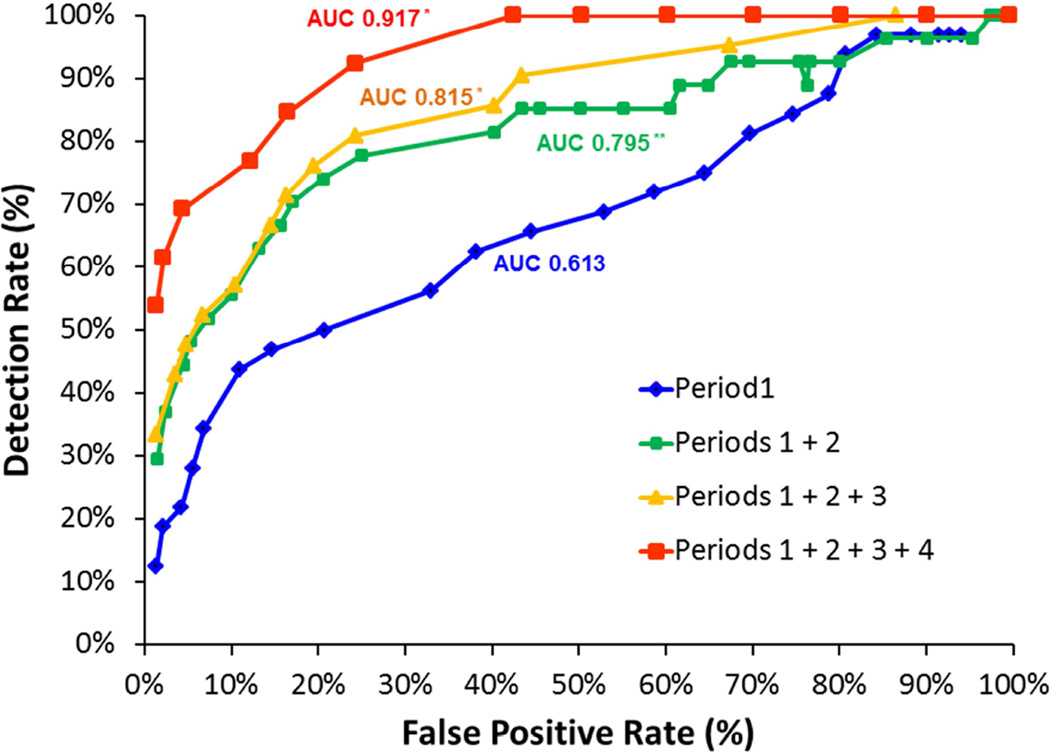
We first addressed this question by comparing the predictive accuracy of models that integrated two (Periods 1 and 2), three (Periods 1, 2 and 3) and four (Periods 1, 2, 3 and 4) successive measurements of CL with that achieved by a single measurement of CL at mid gestation (Period 1). The AUC for the prediction of PTB <32 weeks increased with the number of CL measurements included in the model (0.795, 0.815 and 0.917 for integration of two, three and four measurements, respectively) and was significantly higher than that achieved with a single measurement of CL at Period 1 (0.613, p<0.005) (Figure 3). Similarly, for a given target false positive rate (2% or 5%), integration of each additional measurement of CL increased the detection rate and the positive likelihood ratio, and decreased the negative likelihood ratio for PTB <32 weeks (Table 4). The detection rate achieved with a model that integrated all 4 measurements of CL was significantly higher than that achieved with a single measurement of CL at period 1 (for false positive rate 2%: 62% vs. 19%, p=0.025; for false positive rate 5%: 69% vs. 28%, p=0.019) (Table 4). Measurement of CL at Period 2 and Period 4 had the largest relative contribution to the overall detection rate (Table 4).
Figure 3. Effect of serial measurements of cervical length (interpreted as absolute length in mm) on the ROC curve for the prediction of preterm birth <32 weeks.
ROC, Receiver operating characteristic; AUC, area under the ROC curve; CL cervical length ROC curves are presented for: single measurement of CL in Period 1 (blue line), combination of CL in periods 1 and 2 (green line), combination of CL in periods 1, 2 and 3 (orange line), and combination of CL in periods 1, 2, 3 and 4 (red line).
*p<0.001 compared with AUC of single measurement of cervical length in period 1
**p=0.004 compared with AUC of single measurement of cervical length in period 1
Table 4.
Effect of serial measurements of cervical length (interpreted as absolute length in mm) on the predictive accuracy for preterm birth <32 weeks
| Model | Thresholds * (mm) |
Predictive accuracy | Relative contribution to DR | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | TP (n) |
FP (n) |
FN (n) |
TN (n) |
DR (%) |
FPR (%) |
PPV (%) |
NPV (%) |
LR+ | LR− | P-value | P1 | P2 | P3 | P4 | |
| For FPR of 2% | |||||||||||||||||||
| CL on Period 1 < threshold | 26 | 6 | 8 | 26 | 374 | 19% | 2% | 43% | 94% | 8.95 | 0.83 | Ref | 19% | ||||||
| CL on Periods 1 OR 2 < thresholds | 23 | 20 | 10 | 8 | 17 | 333 | 37% | 2% | 56% | 95% | 15.79 | 0.64 | 0.13 | 13% | 37% | ||||
| CL on Periods 1, 2 OR 3 < thresholds | 20 | 15 | 10 | 7 | 5 | 14 | 313 | 33% | 2% | 58% | 96% | 21.20 | 0.68 | 0.24 | 6% | 22% | 33% | ||
| CL on Periods 1, 2, 3 OR 4 < thresholds | 15 | 14 | 12 | 10 | 8 | 5 | 5 | 226 | 62% | 2% | 62% | 98% | 28.43 | 0.39 | 0.025 | 6% | 19% | 32% | 62% |
| For FPR of 5% | |||||||||||||||||||
| CL on Period 1 < threshold | 29 | 9 | 21 | 23 | 361 | 28% | 5% | 30% | 94% | 5.12 | 0.76 | Ref | 28% | ||||||
| CL on Periods 1 OR 2 < thresholds | 26 | 25 | 13 | 18 | 14 | 323 | 48% | 5% | 42% | 96% | 9.12 | 0.55 | 0.22 | 19% | 48% | ||||
| CL on Periods 1, 2 OR 3 < thresholds | 25 | 23 | 17 | 10 | 15 | 11 | 303 | 48% | 5% | 40% | 96% | 10.10 | 0.55 | 0.22 | 13% | 44% | 48% | ||
| CL on Periods 1, 2, 3 OR 4 < thresholds | 25 | 20 | 15 | 10 | 9 | 11 | 4 | 220 | 69% | 5% | 45% | 98% | 14.54 | 0.32 | 0.019 | 13% | 37% | 40% | 69% |
CL, cervical length; TP, true positive; FP, false positive; FN, false negative; TN, true negative; DR, detection rate (=sensitivity); FPR, false positive rate (=1-specificity); PPV, positive predictive value; NPV negative predictive value; LR+, positive likelihood ratio; LR−, negative likelihood ratio; P1, Period 1 (18+0 to 21+6 weeks); P2, Period 2 (22+0 to 24+6 weeks); P3, Period 3 (25+0 to 27+6 weeks); P4, Period 4 (28+0 to 32+0 weeks).
The predictive accuracy of serial measurements of cervical length (interpreted as absolute length in mm) for preterm birth <32 weeks was calculated for 4 combinations of serial measurements of cervical length: Period 1 only, Periods 1+2, Periods 1+2+3, and Periods 1+2+3+4.
For each of the 4 combinations, the predictive accuracy was calculated using different sets of thresholds. These thresholds were determined using an iteration process that identified the set of thresholds that is associated with the highest detection rate for each of the following target levels of false positive rate: 2% and 5%.
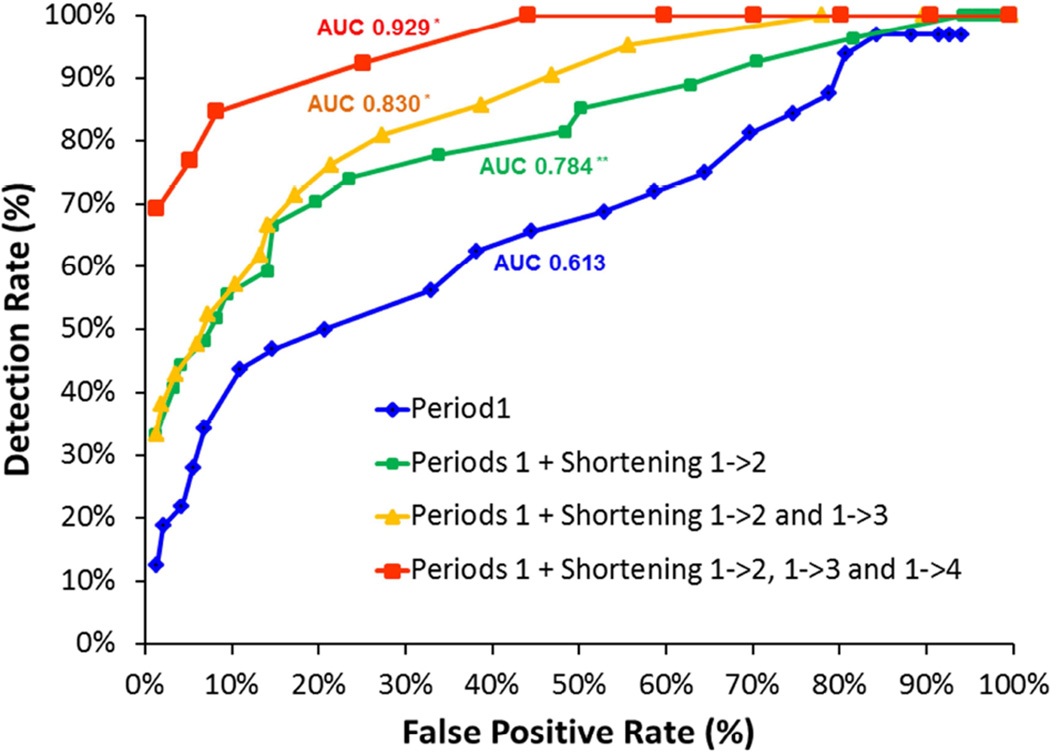
Findings were similar when the serial measurements of CL were analyzed as percent shortening (relative to CL at period 1) rather than absolute length (Figure 4 and Table 5). The AUC for the prediction of PTB <32 weeks increased with the number of CL measurements (analyzed as percent shortening relative to CL at Period 1) included in the model (0.784, 0.830 and 0.929 for integration of two, three and four measurements, respectively) and was significantly higher than that achieved with a single measurement of CL at Period 1 (0.613, p<0.007) (Figure 4). Similarly, for a given target false positive rate (2% or 5%), integration of each additional measurement of CL increased the detection rate and the positive likelihood ratio, and decreased the negative likelihood ratio for PTB <32 weeks (Table 5). The detection rate achieved with a model that integrated all 4 measurements of CL was significantly higher than that achieved with a single measurement of CL at period 1 (for false positive rate 2%: 69% vs. 19%, p=0.016; for false positive rate 5%: 77% vs. 28%, p=0.016) (Table 5).
Figure 4. Effect of serial measurements of cervical length (interpreted as percentage shortening compared with period 1) on the ROC curve for the prediction of preterm birth <32 weeks.
ROC, Receiver operating characteristic; AUC, area under the ROC curve; CL cervical length ROC curves are presented for: single measurement of CL in Period 1 (blue line), combination of CL in period1 and percentage shortening between period 1→2 (green line), combination of CL in period1 and percentage shortening between periods 1→2 and 1→3 (orange line), and combination of CL in period1 and percentage shortening between periods 1→2, 1→3 and 1→4 (red line).
*p<0.001 compared with AUC of single measurement of cervical length in period 1 **p=0.007 compared with AUC of single measurement of cervical length in period 1
Table 5.
Effect of serial measurements of cervical length (interpreted as percentage shortening compared with period 1) on the predictive accuracy for preterm birth <32 weeks
| Model | Thresholds * | Predictive accuracy | Relative contribution to DR | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 (mm) |
P2 (%) |
P3 (%) |
P4 (%) |
TP (n) |
FP (n) |
FN (n) |
TN (n) |
DR (%) |
FPR (%) |
PPV (%) |
NPV (%) |
LR+ | LR− | P- value |
P1 | P2 | P3 | P4 | |
| For FPR of 2% | |||||||||||||||||||
| CL on Period 1 < threshold | 26mm | 6 | 8 | 26 | 374 | 19% | 2% | 43% | 94% | 8.95 | 0.83 | Ref | 19% | ||||||
| CL on Periods 1 < threshold OR percentage shortening between Periods 1→2 < threshold |
20mm | −40% | 9 | 6 | 18 | 335 | 33% | 2% | 60% | 95% | 18.94 | 0.68 | 0.15 | 6% | 33% | ||||
| CL on Periods 1 < threshold OR percentage shortening between Periods 1→2 or 1→3 < threshold |
15mm | −40% | −60% | 8 | 6 | 13 | 312 | 38% | 2% | 57% | 96% | 20.20 | 0.63 | 0.063 | 6% | 33% | 38% | ||
| CL on Periods 1 < threshold OR percentage shortening between Periods 1→2, 1→3 or 1→4 < threshold |
20mm | −60% | −70% | −72% | 9 | 5 | 4 | 226 | 69% | 2% | 64% | 98% | 31.98 | 0.31 | 0.016 | 6% | 7% | 25% | 69% |
| For FPR of 5% | |||||||||||||||||||
| CL on Period 1 < threshold | 29mm | 9 | 21 | 23 | 361 | 28% | 5% | 30% | 94% | 5.12 | 0.76 | Ref | 28% | ||||||
| CL on Periods 1 < threshold OR percentage shortening between Periods 1→2 < threshold |
25mm | −32% | 12 | 17 | 15 | 324 | 44% | 5% | 41% | 96% | 8.92 | 0.58 | 0.17 | 13% | 44% | ||||
| CL on Periods 1 < threshold OR percentage shortening between Periods 1→2 or 1→3 < threshold |
25mm | −34% | −60% | 9 | 16 | 12 | 302 | 43% | 5% | 36% | 96% | 8.52 | 0.60 | 0.16 | 13% | 42% | 43% | ||
| CL on Periods 1 < threshold OR percentage shortening between Periods 1→2, 1→3 or 1→4 < threshold |
15mm | −34% | −60% | −74% | 10 | 12 | 3 | 219 | 77% | 5% | 45% | 99% | 14.81 | 0.24 | 0.016 | 6% | 41% | 45% | 77% |
CL, cervical length; TP, true positive; FP, false positive; FN, false negative; TN, true negative; DR, detection rate; FPR, false positive rate; PPV, positive predictive value; NPV negative predictive value; LR+, positive likelihood ratio; LR−, negative likelihood ratio; P1, Period 1 (18+0 to 21+6 weeks); P2, Period 2 (22+0 to 24+6 weeks); P3, Period 3 (25+0 to 27+6 weeks); P4, Period 4 (28+0 to 32+0 weeks).
The predictive accuracy of serial measurements of cervical length (interpreted as percentage shortening compared with period 1) for preterm birth <32 weeks was calculated for 4 combinations of serial measurements: Period 1 only, Period 1 + %shortening between period 1→2, Period 1 + %shortening between periods 1→2 and 1→3, and Period 1 + %shortening between periods 1→2, 1→3 and 1→4.
For each of the 4 combinations, the predictive accuracy was calculated using different sets of thresholds. These thresholds were determined using an iteration process that identified the set of thresholds that is associated with the highest detection rate for each of the following target levels of false positive rate: 2% and 5%.
COMMENTS
Principal findings of the study
1) Cervical length measured in asymptomatic women with twin gestations is associated with PTB at < 32 weeks of gestation, even when measurements are performed during the late second or early third trimester; 2) Integration of the information obtained from serial measurements of CL in asymptomatic women with twins, either in the form of absolute values of CL or relative degree of cervical shortening, can improve the prediction of PTB at <32 weeks (i.e., increase the detection rate without increasing the false positive rate) compared with a single measurement of CL at mid-gestation.
Results of the study in the context of other observations
CL was predictive of PTB when measured during late 2nd trimester or early 3rd trimester. This finding is in agreement with previous studies.81, 100, 104 For example, Levêque et al., in a study of 120 asymptomatic women with twin pregnancies found that measurement of CL at either 22 weeks or 27 weeks was associated with the risk of PTB at <34 weeks.104
Since cervical shortening may only become evident for the first time late in the second trimester, it is reasonable to assume that serial measurement of CL will detect more women who are at risk of PTB that were not identified as such based on CL measurement at mid-gestation. A potential concern, however, is that repeated measurements of CL would also increase the overall false positive rate for PTB compared with a single measurement of CL at mid-gestation. However, we have demonstrated that integration of the information obtained from serial measurements of CL improves the detection of women at risk of PTB (compared with a single measurement of CL at mid-gestation) without increasing the overall false positive rate. The reason for this is that when serial measurements of CL are employed, lower thresholds of CL can be used to define the test as positive when CL is measured during the second trimester (e.g., Periods 1 and 2), thereby decreasing the overall false positive rate.
Although the use of such lower thresholds would decrease the detection rate of each individual measurement of CL, the overall cumulative detection rate achieved with the multiple measurements of CL is higher than that achieved with a single measurement of CL at Period 1 that uses a higher threshold. This is demonstrated clearly in Tables 4 and 5 where in models that integrated more than one measurement of CL, the thresholds used for CL at Period 1 were lower than that used by the model that included only a single measurement of CL at Period 1.
In contrast to our findings, previous studies, including a recent meta-analysis, concluded that information on cervical shortening between successive measurements of CL in twins is not beneficial and provides only little added benefit over a single measurement of CL.100–102, 104, 110 However, in addition to the small number of available studies, the interpretation of these data is limited by small sample size and variation in the number and timing of CL measurement, the definition of cervical shortening (e.g., mm/week vs. percent shortening), and definition of the outcome measures (e.g., some studies used delivery at less than 35 or 36 weeks as the primary outcome measure which is of questionable clinical significance).78, 81 Most importantly, in all of these studies, the analysis of cervical shortening was limited to the change in CL between only two time points, so that data regarding the integration of multiple (more than two) serial measurements of CL in twins, as was done in the current study, are lacking.
Strengths and Limitations
This is the largest study on serial measurements of CL in asymptomatic women with twins, and the first to assess the effect of integration of multiple (>2) measurements of CL on the predictive accuracy for PTB. Another advantage is that all women were followed in a single clinic dedicated to twin pregnancies according to a standardized protocol, and all sonographic measurements of CL were performed by experienced sonographers.
The main limitations of the study include its retrospective design, those related to missing information, unknown generalizability to broader populations, and that care providers were not blinded to cervical length measurements. Although we included only asymptomatic women, it cannot be ruled out that, given the retrospective design, a small number of women with some degree of uterine activity were included in the study either because they did not report it to their care provider or because the care provider did not mention it in the chart. Another limitation relates to fact that the number of women used for the calculation of the performance of the models was not identical for all four models and decreased with the number of measurements included in the model (i.e., the number of women was highest for model 1 and lowest for model 4). This was the result of the fact that for some women information of CL was not available for all four gestational age periods, mainly due to missed visits. The variation in the size of the study groups used to assess the performance of the four models may complicate the direct comparison between the models and may result in a detection rate that might be optimistically high. In addition, the use of lower thresholds of CL in the earlier periods of gestation (such as in the case of models that integrate 3 or 4 measurements of CL) may delay the detection of some women who are at risk of early PTB.
Conclusion and clinical implications
Integration of serial CL measurements can improve the detection rate for PTB in asymptomatic women with twin gestations, without increasing the fraction of women who receive false positive risk determinations. Better detection of women at risk of PTB is important for patient counseling and can guide decisions regarding patient transfer, admission, frequency of monitoring, and administration of corticosteroids for fetal lung maturation. Indeed, in a recent study, serial monitoring of CL in twins was associated with improved rates of exposure to corticosteroids in women who delivered preterm,46 although data regarding the beneficial effects of antenatal corticosteroids in twins pregnancies are conflicting.113–123 In addition, in a recent individual-patient meta-analysis, Romero et al. found that the use of progesterone in asymptomatic women with twin pregnancy and a short cervix decreased the rate of PTB at <33 weeks by 30% and the rate of neonatal morbidity by approximately 50%.66 Similarly, in a recent randomized controlled trial of women with twin pregnancy and a short cervix, it was found that administration of progesterone decreased the likelihood of preterm birth and the rate of neonatal morbidity.124 Finally, there is recent evidence that cervical pessary may decrease the likelihood of preterm birth in women with twin pregnancies. 36, 125–129 Thus, the improved prediction of PTB using serial measurement of CL may facilitate treatment with progesterone if more evidence becomes available to support the use of progesterone in that context. 22, 26, 66, 124, 130, 131 Still, it should be emphasized that there is currently no level-I evidence that routine monitoring of cervical length in women with twin pregnancies improves pregnancy outcome,132 and therefore current recommendations do not support such a practice.133
Prospective studies are required to confirm our findings, and to determine whether the enhanced risk assessment translates into health benefits sufficient to offset the additional costs or burden incurred by incorporating serial cervical length measurement into routine practice.
Supplementary Material
Acknowledgments
Sources of financial support: This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NICHD/NIH); and, in part, with Federal funds from NICHD, NIH under Contract No. HSN27520130000
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: The authors report no conflict of interest.
Presentation: This paper was presented as a poster at the 35th Annual Meeting of the Society for Maternal Fetal Medicine, Feb 2–7th, 2015, San-Diego.
References
- 1.Garite TJ, Clark RH, Elliott JP, Thorp JA. Twins and triplets: the effect of plurality and growth on neonatal outcome compared with singleton infants. American journal of obstetrics and gynecology. 2004;191:700–707. doi: 10.1016/j.ajog.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 2.Shinwell ES, Blickstein I. The risks for very low birth weight infants from multiple pregnancies. Clinics in perinatology. 2007;34:587–597. vi–vii. doi: 10.1016/j.clp.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chauhan SP, Scardo JA, Hayes E, Abuhamad AZ, Berghella V. Twins: prevalence, problems, and preterm births. American journal of obstetrics and gynecology. 2010;203:305–315. doi: 10.1016/j.ajog.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 5.Refuerzo JS, Momirova V, Peaceman AM, et al. Neonatal outcomes in twin pregnancies delivered moderately preterm, late preterm, and term. American journal of perinatology. 2010;27:537–542. doi: 10.1055/s-0030-1248940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogel JP, Torloni MR, Seuc A, et al. Maternal and perinatal outcomes of twin pregnancy in 23 low- and middle-income countries. PloS one. 2013;8:e70549. doi: 10.1371/journal.pone.0070549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin JA, Hamilton BE, Ventura SJ, Osterman MJ, Mathews TJ. Births: final data for 2011. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2013;62:1–69. 72. [PubMed] [Google Scholar]
- 8.Shamshirsaz AA, Ravangard SF, Ozhand A, et al. Short-term neonatal outcomes in diamniotic twin pregnancies delivered after 32 weeks and indications of late preterm deliveries. American journal of perinatology. 2014;31:365–372. doi: 10.1055/s-0033-1334458. [DOI] [PubMed] [Google Scholar]
- 9.Manuck TA, Sheng X, Yoder BA, Varner MW. Correlation between initial neonatal and early childhood outcomes following preterm birth. American journal of obstetrics and gynecology. 2014;210:426, e1–e9. doi: 10.1016/j.ajog.2014.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendez-Figueroa H, Dahlke JD, Viteri OA, et al. Neonatal and infant outcomes in twin gestations with preterm premature rupture of membranes at 24–31 weeks of gestation. Obstetrics and gynecology. 2014;124:323–331. doi: 10.1097/AOG.0000000000000369. [DOI] [PubMed] [Google Scholar]
- 11.Burgess JL, Unal ER, Nietert PJ, Newman RB. Risk of late-preterm stillbirth and neonatal morbidity for monochorionic and dichorionic twins. American journal of obstetrics and gynecology. 2014;210:578, e1–e9. doi: 10.1016/j.ajog.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong LF, Holmgren CM, Silver RM, Varner MW, Manuck TA. Outcomes of expectantly managed pregnancies with multiple gestations and preterm premature rupture of membranes prior to 26 weeks. American journal of obstetrics and gynecology. 2015;212:215, e1–e9. doi: 10.1016/j.ajog.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berezowsky A, Mazkereth R, Ashwal E, et al. Neonatal outcome of late preterm uncomplicated monochorionic twins: what is the optimal time for delivery? The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2015:1–5. doi: 10.3109/14767058.2015.1043262. [DOI] [PubMed] [Google Scholar]
- 14.Gnanendran L, Bajuk B, Oei J, Lui K, Abdel-Latif ME. Neurodevelopmental outcomes of preterm singletons, twins and higher-order gestations: a population-based cohort study. Archives of disease in childhood Fetal and neonatal edition. 2015;100:F106–F114. doi: 10.1136/archdischild-2013-305677. [DOI] [PubMed] [Google Scholar]
- 15.Sparks T, Caughey A, Cheng Y. Abstract No. 559: Periviable twins versus singletons: how do neonatal outcomes compare? American journal of obstetrics and gynecology. 2015;212:S278. [Google Scholar]
- 16.Aduloju OP, Olofinbiyi B, Olagbuji BN, Ade-Ojo IP, Akintayo A. Obstetric outcome of twin gestations in a tertiary hospital South-western Nigeria. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2015;28:900–904. doi: 10.3109/14767058.2014.937690. [DOI] [PubMed] [Google Scholar]
- 17.Rode L, Klein K, Nicolaides KH, Krampl-Bettelheim E, Tabor A. Prevention of preterm delivery in twin gestations (PREDICT): a multicenter, randomized, placebo-controlled trial on the effect of vaginal micronized progesterone. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2011;38:272–280. doi: 10.1002/uog.9093. [DOI] [PubMed] [Google Scholar]
- 18.Klein K, Rode L, Nicolaides KH, Krampl-Bettelheim E, Tabor A. Vaginal micronized progesterone and risk of preterm delivery in high-risk twin pregnancies: secondary analysis of a placebo-controlled randomized trial and meta-analysis. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2011;38:281–287. doi: 10.1002/uog.9092. [DOI] [PubMed] [Google Scholar]
- 19.Combs CA, Garite T, Maurel K, Das A, Porto M. 17-hydroxyprogesterone caproate for twin pregnancy: a double-blind, randomized clinical trial. American journal of obstetrics and gynecology. 2011;204:221, e1–e8. doi: 10.1016/j.ajog.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 20.Cetingoz E, Cam C, Sakalli M, Karateke A, Celik C, Sancak A. Progesterone effects on preterm birth in high-risk pregnancies: a randomized placebo-controlled trial. Archives of gynecology and obstetrics. 2011;283:423–429. doi: 10.1007/s00404-009-1351-2. [DOI] [PubMed] [Google Scholar]
- 21.Wood S, Ross S, Tang S, Miller L, Sauve R, Brant R. Vaginal progesterone to prevent preterm birth in multiple pregnancy: a randomized controlled trial. Journal of perinatal medicine. 2012;40:593–599. doi: 10.1515/jpm-2012-0057. [DOI] [PubMed] [Google Scholar]
- 22.Romero R. Progesterone to prevent preterm birth in twin gestations: what is the next step forward? BJOG : an international journal of obstetrics and gynaecology. 2013;120:1–4. doi: 10.1111/1471-0528.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romero R, Yeo L, Miranda J, Hassan SS, Conde-Agudelo A, Chaiworapongsa T. A blueprint for the prevention of preterm birth: vaginal progesterone in women with a short cervix. Journal of perinatal medicine. 2013;41:27–44. doi: 10.1515/jpm-2012-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Senat MV, Porcher R, Winer N, et al. Prevention of preterm delivery by 17 alpha-hydroxyprogesterone caproate in asymptomatic twin pregnancies with a short cervix: a randomized controlled trial. American journal of obstetrics and gynecology. 2013;208:194, e1–e8. doi: 10.1016/j.ajog.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 25.Rode L, Tabor A. Prevention of preterm delivery in twin pregnancy. Best practice & research Clinical obstetrics & gynaecology. 2014;28:273–283. doi: 10.1016/j.bpobgyn.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Schuit E, Stock S, Rode L, et al. Effectiveness of progestogens to improve perinatal outcome in twin pregnancies: an individual participant data meta-analysis. BJOG : an international journal of obstetrics and gynaecology. 2015;122:27–37. doi: 10.1111/1471-0528.13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNamara HC, Wood R, Chalmers J, et al. STOPPIT Baby Follow-up Study: the effect of prophylactic progesterone in twin pregnancy on childhood outcome. PloS one. 2015;10:e0122341. doi: 10.1371/journal.pone.0122341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brizot ML, Hernandez W, Liao AW, et al. Vaginal progesterone for the prevention of preterm birth in twin gestations: a randomized placebo-controlled double-blind study. American journal of obstetrics and gynecology. 2015;213:82, e1–e9. doi: 10.1016/j.ajog.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 29.Collins A, Shennan A. A clinical opinion on how to manage the risk of preterm birth in twins based on literature review. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2015:1–6. doi: 10.3109/14767058.2015.1037734. [DOI] [PubMed] [Google Scholar]
- 30.Awwad J, Usta IM, Ghazeeri G, et al. A randomised controlled double-blind clinical trial of 17-hydroxyprogesterone caproate for the prevention of preterm birth in twin gestation (PROGESTWIN): evidence for reduced neonatal morbidity. BJOG : an international journal of obstetrics and gynaecology. 2015;122:71–79. doi: 10.1111/1471-0528.13031. [DOI] [PubMed] [Google Scholar]
- 31.Roman AS, Saltzman DH, Fox N, et al. Prophylactic cerclage in the management of twin pregnancies. American journal of perinatology. 2013;30:751–754. doi: 10.1055/s-0032-1332796. [DOI] [PubMed] [Google Scholar]
- 32.Berghella V, Roman A. Cerclage in twins: we can do better! American journal of obstetrics and gynecology. 2014;211:5–6. doi: 10.1016/j.ajog.2014.03.037. [DOI] [PubMed] [Google Scholar]
- 33.Holcomb WL, Amon E, Albert JH. The effect of cerclage in twin gestations with short cervix: a Bayesian evaluation. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2015;28:448–453. doi: 10.3109/14767058.2014.921151. [DOI] [PubMed] [Google Scholar]
- 34.Saccone G, Rust O, Althuisius S, Roman A, Berghella V. Cerclage for short cervix in twin pregnancies: systematic review and meta-analysis of randomized trials using individual patient-level data. Acta obstetricia et gynecologica Scandinavica. 2015;94:352–358. doi: 10.1111/aogs.12600. [DOI] [PubMed] [Google Scholar]
- 35.Hegeman MA, Bekedam DJ, Bloemenkamp KW, et al. Pessaries in multiple pregnancy as a prevention of preterm birth: the ProTwin Trial. BMC pregnancy and childbirth. 2009;9:44. doi: 10.1186/1471-2393-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liem S, Schuit E, Hegeman M, et al. Cervical pessaries for prevention of preterm birth in women with a multiple pregnancy (ProTWIN): a multicentre, open-label randomised controlled trial. Lancet. 2013;382:1341–1349. doi: 10.1016/S0140-6736(13)61408-7. [DOI] [PubMed] [Google Scholar]
- 37.Goldenberg RL, Iams JD, Miodovnik M, et al. The preterm prediction study: risk factors in twin gestations. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. American journal of obstetrics and gynecology. 1996;175:1047–1053. doi: 10.1016/s0002-9378(96)80051-2. [DOI] [PubMed] [Google Scholar]
- 38.Conde-Agudelo A, Romero R. Cervicovaginal fetal fibronectin for the prediction of spontaneous preterm birth in multiple pregnancies: a systematic review and meta-analysis. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2010;23:1365–1376. doi: 10.3109/14767058.2010.499484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adeyemi O, Osoba L. The role of phosphorylated insulin-like growth factor binding protein-1 in predicting pre-term labour in twin pregnancies. Journal of obstetrics and gynaecology : the journal of the Institute of Obstetrics and Gynaecology. 2010;30:571–573. doi: 10.3109/01443615.2010.494203. [DOI] [PubMed] [Google Scholar]
- 40.Brubaker SG, Gyamfi C. Prediction and prevention of spontaneous preterm birth in twin gestations. Seminars in perinatology. 2012;36:190–194. doi: 10.1053/j.semperi.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 41.Giuffre M, Piro E, Corsello G. Prematurity and twinning. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2012;25(Suppl 3):6–10. doi: 10.3109/14767058.2012.712350. [DOI] [PubMed] [Google Scholar]
- 42.Conde-Agudelo A, Romero R. Prediction of preterm birth in twin gestations using biophysical and biochemical tests. American journal of obstetrics and gynecology. 2014;211:583–595. doi: 10.1016/j.ajog.2014.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Combs CA, Garite TJ, Maurel K, Das A. Fetal fibronectin versus cervical length as predictors of preterm birth in twin pregnancy with or without 17-hydroxyprogesterone caproate. American journal of perinatology. 2014;31:1023–1030. doi: 10.1055/s-0034-1370342. [DOI] [PubMed] [Google Scholar]
- 44.Makrydimas G, Sotiriadis A. Prediction of preterm birth in twins. Best practice & research Clinical obstetrics & gynaecology. 2014;28:265–272. doi: 10.1016/j.bpobgyn.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 45.Premru-Srsen T, Verdenik I, Steblovnik L, Ban-Frangez H. Early prediction of spontaneous twin very preterm birth: a population based study 2002–2012. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2014:1–6. doi: 10.3109/14767058.2014.968774. [DOI] [PubMed] [Google Scholar]
- 46.Lifshitz SJ, Razavi A, Bibbo C, et al. Routine cervical length and fetal fibronectin screening in asymptomatic twin pregnancies: is there clinical benefit? The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2014;27:566–570. doi: 10.3109/14767058.2013.831067. [DOI] [PubMed] [Google Scholar]
- 47.Fichera A, Prefumo F, Zanardini C, Stagnati V, Frusca T. Rapid cervical phIGFBP-1 test in asymptomatic twin pregnancies: role in mid-pregnancy prediction of spontaneous preterm delivery. Prenatal diagnosis. 2014;34:450–459. doi: 10.1002/pd.4328. [DOI] [PubMed] [Google Scholar]
- 48.Andersen HF, Nugent CE, Wanty SD, Hayashi RH. Prediction of risk for preterm delivery by ultrasonographic measurement of cervical length. American journal of obstetrics and gynecology. 1990;163:859–867. doi: 10.1016/0002-9378(90)91084-p. [DOI] [PubMed] [Google Scholar]
- 49.Riley L, Frigoletto FD, Jr, Benacerraf BR. The implications of sonographically identified cervical changes in patients not necessarily at risk for preterm birth. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 1992;11:75–79. doi: 10.7863/jum.1992.11.3.75. [DOI] [PubMed] [Google Scholar]
- 50.Okitsu O, Mimura T, Nakayama T, Aono T. Early prediction of preterm delivery by transvaginal ultrasonography. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 1992;2:402–409. doi: 10.1046/j.1469-0705.1992.02060402.x. [DOI] [PubMed] [Google Scholar]
- 51.Iams JD, Johnson FF, Sonek J, Sachs L, Gebauer C, Samuels P. Cervical competence as a continuum: a study of ultrasonographic cervical length and obstetric performance. American journal of obstetrics and gynecology. 1995;172:1097–1103. doi: 10.1016/0002-9378(95)91469-2. discussion 104–6. [DOI] [PubMed] [Google Scholar]
- 52.Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. The New England journal of medicine. 1996;334:567–572. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- 53.Berghella V, Tolosa JE, Kuhlman K, Weiner S, Bolognese RJ, Wapner RJ. Cervical ultrasonography compared with manual examination as a predictor of preterm delivery. American journal of obstetrics and gynecology. 1997;177:723–730. doi: 10.1016/s0002-9378(97)70259-x. [DOI] [PubMed] [Google Scholar]
- 54.Heath VC, Southall TR, Souka AP, Elisseou A, Nicolaides KH. Cervical length at 23 weeks of gestation: prediction of spontaneous preterm delivery. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 1998;12:312–317. doi: 10.1046/j.1469-0705.1998.12050312.x. [DOI] [PubMed] [Google Scholar]
- 55.Taipale P, Hiilesmaa V. Sonographic measurement of uterine cervix at 18–22 weeks’ gestation and the risk of preterm delivery. Obstetrics and gynecology. 1998;92:902–907. doi: 10.1016/s0029-7844(98)00346-9. [DOI] [PubMed] [Google Scholar]
- 56.Leitich H, Brunbauer M, Kaider A, Egarter C, Husslein P. Cervical length and dilatation of the internal cervical os detected by vaginal ultrasonography as markers for preterm delivery: A systematic review. American journal of obstetrics and gynecology. 1999;181:1465–1472. doi: 10.1016/s0002-9378(99)70407-2. [DOI] [PubMed] [Google Scholar]
- 57.Hassan SS, Romero R, Berry SM, et al. Patients with an ultrasonographic cervical length < or =15 mm have nearly a 50% risk of early spontaneous preterm delivery. American journal of obstetrics and gynecology. 2000;182:1458–1467. doi: 10.1067/mob.2000.106851. [DOI] [PubMed] [Google Scholar]
- 58.Owen J, Yost N, Berghella V, et al. Mid-trimester endovaginal sonography in women at high risk for spontaneous preterm birth. Jama. 2001;286:1340–1348. doi: 10.1001/jama.286.11.1340. [DOI] [PubMed] [Google Scholar]
- 59.Honest H, Bachmann LM, Coomarasamy A, Gupta JK, Kleijnen J, Khan KS. Accuracy of cervical transvaginal sonography in predicting preterm birth: a systematic review. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2003;22:305–322. doi: 10.1002/uog.202. [DOI] [PubMed] [Google Scholar]
- 60.de Carvalho MH, Bittar RE, Brizot Mde L, Bicudo C, Zugaib M. Prediction of preterm delivery in the second trimester. Obstetrics and gynecology. 2005;105:532–536. doi: 10.1097/01.AOG.0000154157.22500.1d. [DOI] [PubMed] [Google Scholar]
- 61.To MS, Skentou CA, Royston P, Yu CK, Nicolaides KH. Prediction of patient-specific risk of early preterm delivery using maternal history and sonographic measurement of cervical length: a population-based prospective study. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2006;27:362–367. doi: 10.1002/uog.2773. [DOI] [PubMed] [Google Scholar]
- 62.Celik E, To M, Gajewska K, Smith GC, Nicolaides KH Fetal Medicine Foundation Second Trimester Screening G. Cervical length and obstetric history predict spontaneous preterm birth: development and validation of a model to provide individualized risk assessment. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2008;31:549–554. doi: 10.1002/uog.5333. [DOI] [PubMed] [Google Scholar]
- 63.Crane JM, Hutchens D. Transvaginal sonographic measurement of cervical length to predict preterm birth in asymptomatic women at increased risk: a systematic review. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2008;31:579–587. doi: 10.1002/uog.5323. [DOI] [PubMed] [Google Scholar]
- 64.Honest H, Forbes CA, Duree KH, et al. Screening to prevent spontaneous preterm birth: systematic reviews of accuracy and effectiveness literature with economic modelling. Health technology assessment. 2009;13:1–627. doi: 10.3310/hta13430. [DOI] [PubMed] [Google Scholar]
- 65.Domin CM, Smith EJ, Terplan M. Transvaginal ultrasonographic measurement of cervical length as a predictor of preterm birth: a systematic review with meta-analysis. Ultrasound quarterly. 2010;26:241–248. doi: 10.1097/RUQ.0b013e3181fe0e05. [DOI] [PubMed] [Google Scholar]
- 66.Romero R, Nicolaides K, Conde-Agudelo A, et al. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: a systematic review and metaanalysis of individual patient data. American journal of obstetrics and gynecology. 2012;206:124, e1–e19. doi: 10.1016/j.ajog.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Conde-Agudelo A, Romero R, Nicolaides K, et al. Vaginal progesterone vs. cervical cerclage for the prevention of preterm birth in women with a sonographic short cervix, previous preterm birth, and singleton gestation: a systematic review and indirect comparison metaanalysis. American journal of obstetrics and gynecology. 2013;208:42, e1–e42, e18. doi: 10.1016/j.ajog.2012.10.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barros-Silva J, Pedrosa AC, Matias A. Sonographic measurement of cervical length as a predictor of preterm delivery: a systematic review. Journal of perinatal medicine. 2014;42:281–293. doi: 10.1515/jpm-2013-0115. [DOI] [PubMed] [Google Scholar]
- 69.Crane JM, Van den Hof M, Armson BA, Liston R. Transvaginal ultrasound in the prediction of preterm delivery: singleton and twin gestations. Obstetrics and gynecology. 1997;90:357–363. doi: 10.1016/s0029-7844(97)00277-9. [DOI] [PubMed] [Google Scholar]
- 70.Imseis HM, Albert TA, Iams JD. Identifying twin gestations at low risk for preterm birth with a transvaginal ultrasonographic cervical measurement at 24 to 26 weeks’ gestation. American journal of obstetrics and gynecology. 1997;177:1149–1155. doi: 10.1016/s0002-9378(97)70032-2. [DOI] [PubMed] [Google Scholar]
- 71.Grisaru-Granovsky S, Farine D, Barret J. Is a single ultrasound measurement of cervical length a predictor of the risk of preterm delivery in multiple pregnancy? American journal of obstetrics and gynecology. 1998;178:S191. [Google Scholar]
- 72.Souka AP, Heath V, Flint S, Sevastopoulou I, Nicolaides KH. Cervical length at 23 weeks in twins in predicting spontaneous preterm delivery. Obstetrics and gynecology. 1999;94:450–454. doi: 10.1016/s0029-7844(99)00277-x. [DOI] [PubMed] [Google Scholar]
- 73.Guzman ER, Walters C, O‘Reilly-Green C, et al. Use of cervical ultrasonography in prediction of spontaneous preterm birth in twin gestations. American journal of obstetrics and gynecology. 2000;183:1103–1107. doi: 10.1067/mob.2000.108896. [DOI] [PubMed] [Google Scholar]
- 74.Yang JH, Kuhlman K, Daly S, Berghella V. Prediction of preterm birth by second trimester cervical sonography in twin pregnancies. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2000;15:288–291. doi: 10.1046/j.1469-0705.2000.00087.x. [DOI] [PubMed] [Google Scholar]
- 75.Skentou C, Souka AP, To MS, Liao AW, Nicolaides KH. Prediction of preterm delivery in twins by cervical assessment at 23 weeks. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2001;17:7–10. doi: 10.1046/j.1469-0705.2001.00357.x. [DOI] [PubMed] [Google Scholar]
- 76.Soriano D, Weisz B, Seidman DS, et al. The role of sonographic assessment of cervical length in the prediction of preterm birth in primigravidae with twin gestation conceived after infertility treatment. Acta obstetricia et gynecologica Scandinavica. 2002;81:39–43. doi: 10.1046/j.0001-6349.2001.00466.x. [DOI] [PubMed] [Google Scholar]
- 77.Vayssiere C, Favre R, Audibert F, et al. Cervical length and funneling at 22 and 27 weeks to predict spontaneous birth before 32 weeks in twin pregnancies: a French prospective multicenter study. American journal of obstetrics and gynecology. 2002;187:1596–1604. doi: 10.1067/mob.2002.127380. [DOI] [PubMed] [Google Scholar]
- 78.Gibson JL, Macara LM, Owen P, Young D, Macauley J, Mackenzie F. Prediction of preterm delivery in twin pregnancy: a prospective, observational study of cervical length and fetal fibronectin testing. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2004;23:561–566. doi: 10.1002/uog.1048. [DOI] [PubMed] [Google Scholar]
- 79.Sperling L, Kiil C, Larsen LU, et al. How to identify twins at low risk of spontaneous preterm delivery. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2005;26:138–144. doi: 10.1002/uog.1938. [DOI] [PubMed] [Google Scholar]
- 80.Fait G, Har-Toov J, Gull I, Lessing JB, Jaffa A, Wolman I. Cervical length, multifetal pregnancy reduction, and prediction of preterm birth. Journal of clinical ultrasound : JCU. 2005;33:329–332. doi: 10.1002/jcu.20159. [DOI] [PubMed] [Google Scholar]
- 81.Arabin B, Roos C, Kollen B, van Eyck J. Comparison of transvaginal sonography in recumbent and standing maternal positions to predict spontaneous preterm birth in singleton and twin pregnancies. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2006;27:377–386. doi: 10.1002/uog.2694. [DOI] [PubMed] [Google Scholar]
- 82.To MS, Fonseca EB, Molina FS, Cacho AM, Nicolaides KH. Maternal characteristics and cervical length in the prediction of spontaneous early preterm delivery in twins. American journal of obstetrics and gynecology. 2006;194:1360–1365. doi: 10.1016/j.ajog.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 83.Klein K, Gregor H, Hirtenlehner-Ferber K, et al. Prediction of spontaneous preterm delivery in twin pregnancies by cervical length at mid-gestation. Twin research and human genetics : the official journal of the International Society for Twin Studies. 2008;11:552–557. doi: 10.1375/twin.11.5.552. [DOI] [PubMed] [Google Scholar]
- 84.Fox NS, Saltzman DH, Klauser CK, Peress D, Gutierrez CV, Rebarber A. Prediction of spontaneous preterm birth in asymptomatic twin pregnancies with the use of combined fetal fibronectin and cervical length. American journal of obstetrics and gynecology. 2009;201:313, e1–e5. doi: 10.1016/j.ajog.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 85.Conde-Agudelo A, Romero R, Hassan SS, Yeo L. Transvaginal sonographic cervical length for the prediction of spontaneous preterm birth in twin pregnancies: a systematic review and metaanalysis. American journal of obstetrics and gynecology. 2010;203:128, e1–e12. doi: 10.1016/j.ajog.2010.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lim AC, Hegeman MA, Huis In TVMA, Opmeer BC, Bruinse HW, Mol BW. Cervical length measurement for the prediction of preterm birth in multiple pregnancies: a systematic review and bivariate meta-analysis. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2011;38:10–17. doi: 10.1002/uog.9013. [DOI] [PubMed] [Google Scholar]
- 87.Vayssiere C, Leveque C, Favre R, Audibert F, Chauvet MP, Maillard F. Abstract No.338 Cervical length in asymptomatic twin pregnancies: prospective multicenter comparison of predictive indicators. American journal of obstetrics and gynecology. 2012;206:S159. doi: 10.3109/14767058.2014.900038. [DOI] [PubMed] [Google Scholar]
- 88.Asnafi N, Basirat Z, Hajian-Tilaki K, Dadvar S. Assessment of cervical length by transvaginal ultrasonography to predict preterm delivery in twin pregnancy. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2013;26:1435–1438. doi: 10.3109/14767058.2013.783805. [DOI] [PubMed] [Google Scholar]
- 89.Fox NS, Bergh E, Oppal S, Saltzman DH, Klauser CK, Gupta S. Abstract No.823: The association between a short cervix, fetal fibronectin, and preterm birth in twin pregnancies, analyzed by cause of preterm birth: preterm labor, premature rupture of membranes, and indicated preterm birth. American journal of obstetrics and gynecology. 2014;210:S400. [Google Scholar]
- 90.Kurtzman J, Hezelgrave N, Abbott D, Norman J, Stock S, Shennan A. Abstract No.789: Quantitative fetal fibronectin and cervical length screening at 22–27 6/7 weeks’ GA illuminate the spectrum of risk of preterm birth in asymptomatic twin gestations. American journal of obstetrics and gynecology. 2014;210:S386. [Google Scholar]
- 91.Hermans F, Gyamfi-Bannerman C, Lim A, Liem S, Serra V, Perales A. Abstract No.474: Cervical length and risk of preterm birth in European women with a twin pregnancy. American journal of obstetrics and gynecology. 2015:212. [Google Scholar]
- 92.Purisch S, Schwartz N, Romero J, Elovitz M, Levine L. Abstract No.876: Short cervical length remains a risk factor for preterm birth in multiparous women. American journal of obstetrics and gynecology. 2015;212:S417–S418. [Google Scholar]
- 93.Berghella V, Talucci M, Desai A. Does transvaginal sonographic measurement of cervical length before 14 weeks predict preterm delivery in high-risk pregnancies? Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2003;21:140–144. doi: 10.1002/uog.28. [DOI] [PubMed] [Google Scholar]
- 94.Owen J, Yost N, Berghella V, et al. Can shortened midtrimester cervical length predict very early spontaneous preterm birth? American journal of obstetrics and gynecology. 2004;191:298–303. doi: 10.1016/j.ajog.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 95.Fox NS, Jean-Pierre C, Predanic M, Chasen ST. Short cervix: is a follow-up measurement useful? Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2007;29:44–46. doi: 10.1002/uog.3902. [DOI] [PubMed] [Google Scholar]
- 96.Dilek TU, Yazici G, Gurbuz A, et al. Progressive cervical length changes versus single cervical length measurement by transvaginal ultrasound for prediction of preterm delivery. Gynecologic and obstetric investigation. 2007;64:175–179. doi: 10.1159/000106486. [DOI] [PubMed] [Google Scholar]
- 97.Crane JM, Hutchens D. Follow-up cervical length in asymptomatic high-risk women and the risk of spontaneous preterm birth. Journal of perinatology : official journal of the California Perinatal Association. 2011;31:318–323. doi: 10.1038/jp.2010.149. [DOI] [PubMed] [Google Scholar]
- 98.Bergelin I, Valentin L. Cervical changes in twin pregnancies observed by transvaginal ultrasound during the latter half of pregnancy: a longitudinal, observational study. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2003;21:556–563. doi: 10.1002/uog.150. [DOI] [PubMed] [Google Scholar]
- 99.Fox NS, Rebarber A, Klauser CK, Peress D, Gutierrez CV, Saltzman DH. Prediction of spontaneous preterm birth in asymptomatic twin pregnancies using the change in cervical length over time. American journal of obstetrics and gynecology. 2010;202:155, e1–e4. doi: 10.1016/j.ajog.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 100.Hofmeister C, Brizot Mde L, Liao A, Francisco RP, Zugaib M. Two-stage transvaginal cervical length screening for preterm birth in twin pregnancies. Journal of perinatal medicine. 2010;38:479–484. doi: 10.1515/jpm.2010.088. [DOI] [PubMed] [Google Scholar]
- 101.Oh KJ, Park KH, Jeong EH, Lee SY, Ryu A, Kim SN. The change in cervical length over time as a predictor of preterm delivery in asymptomatic women with twin pregnancies who have a normal mid-trimester cervical length. Twin research and human genetics : the official journal of the International Society for Twin Studies. 2012;15:516–521. doi: 10.1017/thg.2012.27. [DOI] [PubMed] [Google Scholar]
- 102.Khalil MI, Alzahrani MH, Ullah A. The use of cervical length and change in cervical length for prediction of spontaneous preterm birth in asymptomatic twin pregnancies. European journal of obstetrics, gynecology, and reproductive biology. 2013;169:193–196. doi: 10.1016/j.ejogrb.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 103.Iams JD. Cervical length--time to report the rate of change? American journal of obstetrics and gynecology. 2014;211:443. doi: 10.1016/j.ajog.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 104.Leveque C, Vayssiere C, Favre R, et al. Cervical length in asymptomatic twin pregnancies: prospective multicenter comparison of predictive indicators. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2015;28:37–40. doi: 10.3109/14767058.2014.900038. [DOI] [PubMed] [Google Scholar]
- 105.Moroz L, Drassinower D, Ananth C, Zork N, Gyamfi-Bannermer C. Abstract No.183: The rate of sonographic cervical shortening and the risk for spontaneous preterm delivery in twins. American journal of obstetrics and gynecology. 2015;212:S106. [Google Scholar]
- 106.Melamed N, Pittini A, Romero R, Nevo O, Ladhani NN, Cohen H. Abstract No. 596: Serial cervical length determination in twin pregnancies reveals four distinct patterns with prognostic significance of preterm birth. American journal of obstetrics and gynecology. 2015;212:S297. doi: 10.1016/j.ajog.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ong S, Smith A, Smith N, Campbell D, Wilson A. Cervical length assessment in twin pregnancies using transvaginal ultrasound. Acta obstetricia et gynecologica Scandinavica. 2000;79:851–853. [PubMed] [Google Scholar]
- 108.Antsaklis A, Daskalakis G, Papantoniou G, Mesogitis N, Antsaklis S. The role of cervical length change from first to second trimester of pregnancy for the prediction of preterm delivery. Journal of maternal fetal and neonatal medicine. 2012;25:55. [Google Scholar]
- 109.Bastek JA, Hirshberg A, Chandrasekaran S, et al. Biomarkers and cervical length to predict spontaneous preterm birth in asymptomatic high-risk women. Obstetrics and gynecology. 2013;122:283–289. doi: 10.1097/AOG.0b013e31829ab714. [DOI] [PubMed] [Google Scholar]
- 110.Conde-Agudelo A, Romero R. Predictive Accuracy of Changes in Transvaginal Sonographic Cervical Length Over Time for Preterm Birth: A Systematic Review and Meta-Analysis. American journal of obstetrics and gynecology. 2015 doi: 10.1016/j.ajog.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fuchs I, Tsoi E, Henrich W, Dudenhausen JW, Nicolaides KH. Sonographic measurement of cervical length in twin pregnancies in threatened preterm labor. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2004;23:42–45. doi: 10.1002/uog.951. [DOI] [PubMed] [Google Scholar]
- 112.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 113.Spinillo A, Capuzzo E, Ometto A, Stronati M, Baltaro F, Iasci A. Value of antenatal corticosteroid therapy in preterm birth. Early human development. 1995;42:37–47. doi: 10.1016/0378-3782(95)01638-j. [DOI] [PubMed] [Google Scholar]
- 114.Battista L, Winovitch KC, Rumney PJ, Davis E, Hagemann C, Wing DA. A case-control comparison of the effectiveness of betamethasone to prevent neonatal morbidity and mortality in preterm twin and singleton pregnancies. American journal of perinatology. 2008;25:449–453. doi: 10.1055/s-0028-1085070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kuk JY, An JJ, Cha HH, et al. Optimal time interval between a single course of antenatal corticosteroids and delivery for reduction of respiratory distress syndrome in preterm twins. American journal of obstetrics and gynecology. 2013;209:256, e1–e7. doi: 10.1016/j.ajog.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 116.Blickstein I, Shinwell ES, Lusky A, Reichman B, Israel Neonatal N. Plurality-dependent risk of respiratory distress syndrome among very-low-birth-weight infants and antepartum corticosteroid treatment. American journal of obstetrics and gynecology. 2005;192:360–364. doi: 10.1016/j.ajog.2004.10.604. [DOI] [PubMed] [Google Scholar]
- 117.Blickstein I, Reichman B, Lusky A, Shinwell ES, Israel Neonatal N. Plurality-dependent risk of severe intraventricular hemorrhage among very low birth weight infants and antepartum corticosteroid treatment. American journal of obstetrics and gynecology. 2006;194:1329–1333. doi: 10.1016/j.ajog.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 118.Burkett G, Bauer CR, Morrison J, Curet LB. Effect of prenatal dexamethasone administration on prevention of respiratory distress syndrome in twin pregnancies. J Perinatol. 1986;6:304–308. [Google Scholar]
- 119.Silver RK, Vyskocil C, Solomon SL, Ragin A, Neerhof MG, Farrell EE. Randomized trial of antenatal dexamethasone in surfactant-treated infants delivered before 30 weeks’ gestation. Obstetrics and gynecology. 1996;87:683–691. doi: 10.1016/0029-7844(96)00033-6. [DOI] [PubMed] [Google Scholar]
- 120.Turrentine MA, Wilson PD, Wilkins IA. A retrospective analysis of the effect of antenatal steroid administration on the incidence of respiratory distress syndrome in preterm twin pregnancies. American journal of perinatology. 1996;13:351–354. doi: 10.1055/s-2007-994355. [DOI] [PubMed] [Google Scholar]
- 121.Quist-Therson EC, Myhr TL, Ohlsson A. Antenatal steroids to prevent respiratory distress syndrome: multiple gestation as an effect modifier. Acta obstetricia et gynecologica Scandinavica. 1999;78:388–392. [PubMed] [Google Scholar]
- 122.Choi SJ, Song SE, Seo ES, Oh SY, Kim JH, Roh CR. The effect of single or multiple courses of antenatal corticosteroid therapy on neonatal respiratory distress syndrome in singleton versus twin pregnancies. The Australian & New Zealand journal of obstetrics & gynaecology. 2009;49:173–179. doi: 10.1111/j.1479-828X.2009.00970.x. [DOI] [PubMed] [Google Scholar]
- 123.Melamed N, Shah J, Yoon EW, et al. The role of antenatal corticosteroids in twin pregnancies complicated by preterm birth. American journal of obstetrics and gynecology. 2016 doi: 10.1016/j.ajog.2016.05.037. [DOI] [PubMed] [Google Scholar]
- 124.El-Refaie W, Abdelhafez MS, Badawy A. Vaginal progesterone for prevention of preterm labor in asymptomatic twin pregnancies with sonographic short cervix: a randomized clinical trial of efficacy and safety. Archives of gynecology and obstetrics. 2015 doi: 10.1007/s00404-015-3767-1. [DOI] [PubMed] [Google Scholar]
- 125.Liem SM, van Baaren GJ, Delemarre FM, et al. Economic analysis of use of pessary to prevent preterm birth in women with multiple pregnancy (ProTWIN trial) Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2014;44:338–345. doi: 10.1002/uog.13432. [DOI] [PubMed] [Google Scholar]
- 126.Di Tommaso M, Seravalli V, Arduino S, Bossotti C, Sisti G, Todros T. Arabin cervical pessary to prevent preterm birth in twin pregnancies with short cervix. Journal of obstetrics and gynaecology : the journal of the Institute of Obstetrics and Gynaecology. 2016:1–4. doi: 10.3109/01443615.2016.1148127. [DOI] [PubMed] [Google Scholar]
- 127.Fox NS, Gupta S, Lam-Rachlin J, Rebarber A, Klauser CK, Saltzman DH. Cervical Pessary and Vaginal Progesterone in Twin Pregnancies With a Short Cervix. Obstetrics and gynecology. 2016;127:625–630. doi: 10.1097/AOG.0000000000001300. [DOI] [PubMed] [Google Scholar]
- 128.Goya M, de la Calle M, Pratcorona L, et al. Cervical pessary to prevent preterm birth in women with twin gestation and sonographic short cervix: a multicenter randomized controlled trial (PECEP-Twins) American journal of obstetrics and gynecology. 2016;214:145–152. doi: 10.1016/j.ajog.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 129.Nicolaides KH, Syngelaki A, Poon LC, et al. Cervical pessary placement for prevention of preterm birth in unselected twin pregnancies: a randomized controlled trial. American journal of obstetrics and gynecology. 2016;214:3, e1–e9. doi: 10.1016/j.ajog.2015.08.051. [DOI] [PubMed] [Google Scholar]
- 130.Schuit E, Stock S, Groenwold RH, et al. Progestogens to prevent preterm birth in twin pregnancies: an individual participant data meta-analysis of randomized trials. BMC pregnancy and childbirth. 2012;12:13. doi: 10.1186/1471-2393-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schuit E, Stock S, Rouse D, Lim AC, Rode L, Norman J. Abstract No.472: Progesterone in twin pregnancies: an individual participants data meta-analysis of randomized trials. American journal of obstetrics and gynecology. 2012;206:S215. [Google Scholar]
- 132.Gordon MC, McKenna DS, Stewart TL, et al. Transvaginal cervical length scans to prevent prematurity in twins: a randomized controlled trial. American journal of obstetrics and gynecology. 2016;214:277, e1–e7. doi: 10.1016/j.ajog.2015.08.065. [DOI] [PubMed] [Google Scholar]
- 133.American College of O, Gynecologists, Society for Maternal-Fetal M. ACOG Practice Bulletin No. 144: Multifetal gestations: twin, triplet, and higher-order multifetal pregnancies. Obstetrics and gynecology. 2014;123:1118–1132. doi: 10.1097/01.AOG.0000446856.51061.3e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.