Abstract
BACKGROUND
Postpartum hysterectomy is an obstetric procedure that carries significant maternal risk that is not well characterized by hospital volume.
OBJECTIVE
The objective of this study was to determine risk for peripartum hysterectomy for women at low and moderate risk for the procedure.
STUDY DESIGN
This population-based study used data from the Nationwide Inpatient Sample to characterize risk for peripartum hysterectomy. Women with a diagnosis of placenta accreta or prior cesarean and placenta previa were excluded. Obstetrical risk factors along with demographic and hospital factors were evaluated. Multivariable mixed-effects log-linear regression models were developed to determine adjusted risk. Based on these models receiver operating characteristic curves were plotted, and the area under the curve was determined to assess discrimination.
RESULTS
Cesarean hysterectomy occurred in 1 in 1913 deliveries. Risk factors associated with significant risk for hysterectomy included mode of delivery, stillbirth, placental abruption, fibroids, and antepartum hemorrhage. These factors retained their significance in adjusted models: The risk ratio for stillbirth was 3.44 (95% confidence interval (CI) 2.94–4.02), abruption 2.98 (95% CI 2.52–3.20), fibroids 3.63 (95% CI 3.22–4.08), and antepartum hemorrhage 7.15 (95% CI 6.16–8.32). The area under the curve (AUC) for the model was 0.833.
CONCLUSION
Cesarean hysterectomy is a relatively common event that hospitals providing routine obstetric care should be prepared to manage. That specific risk factors are highly associated with risk for hysterectomy supports routine use of hemorrhage risk-assessment tools. However, given that a significant proportion of hysterectomies will be unpredictable, the availability of rapid transfusion protocols may be necessary for hospitals to safely manage these cases.
Keywords: Postpartum hemorrhage, hysterectomy, atony
INTRODUCTION
Postpartum hemorrhage is a leading cause of severe maternal morbidity and mortality worldwide.1 For patients where other medical and surgical treatment options have failed, peripartum hysterectomy may represent a life-saving intervention.2,3 The procedure involves frequent morbidity4 and risk of death ranging from 1 to 6%.5–7 To minimize risk and optimize delivery planning, expert opinion supports referral of women at extremely high risk for peripartum hysterectomy – primarily those with suspected placenta accreta/percreta – to tertiary centers.2,8 Research evidence supports that maternal outcomes may be improved at centers that perform peripartum hysterectomy more frequently9 and use a multidisciplinary approach in planning and delivery.10
While referral may benefit these highest risk patients, a significant proportion of peripartum hysterectomies may occur amongst women without placenta accreta/percreta.11 For these low-risk women, it is unclear how well hemorrhage leading to peripartum hysterectomy can be anticipated based on risk factors. If peripartum hysterectomy is demonstrated to be (i) relatively common on a population basis for low-risk women, and (ii) only partially accounted for by risk factors, effective hospital-level safety planning would require both routine risk assessment of obstetrical patients as well as general preparedness for catastrophic hemorrhage, an approach supported by major maternal safety initiatives, including hemorrhage bundle recommendations from the National Partnership for Maternal Safety.12
Given that unanticipated peripartum hysterectomy may be an important consideration in designing effective hemorrhage safety policies, particularly in low-volume hospitals, the purpose of this study was to characterize hysterectomy risk during delivery hospitalizations among women deemed to be at low and moderate risk for this procedure.
METHODS
Data from the Nationwide Inpatient Sample (NIS) from the Agency for Healthcare Research and Quality were used for the analysis. The NIS is the largest publicly available, allpayer inpatient database in the United States. NIS datasets contain a random sample of approximately 20% of all hospitals within the United States, and through 2011, all discharge claims in a given hospital are included. The sampling frame for the NIS includes nonfederal, general, and specialty-specific hospitals throughout the United States. Sampled hospitals include both academic and community facilities. The NIS included approximately 8 million hospital stays from 45 states in 2010.13 Sampling weights that are included in the NIS can be applied to provide national estimates and were used in this analysis. Due to the de-identified nature of the data set, institutional review board exemption was obtained from Columbia University to perform this study.
We analyzed women from 16 to 49 years of age hospitalized for a delivery between 1998 and 2011. Patients were included if International Classification of Diseases, ninth revision, (ICD-9) billing codes v27.x and 650 were present; these codes identify a large majority of delivery hospitalizations.14 Since the goal of the study was to evaluate the risk of peripartum hysterectomy in low and moderate risk women, those at particularly high risk for peripartum hysterectomy were excluded. Patients with the diagnosis of both placenta previa (641.0x, 641.1x) and prior cesarean delivery (654.2x) were excluded as these patients are at high risk for invasive placentation, as were patients diagnosed with either placenta accreta (667.0x, 667.1x) or vasa previa (663.5x). Women with a diagnosis of ovarian or cervical cancer (180.x and 183.x) were similarly excluded given that they may have been undergoing planned hysterectomy.
The primary outcome for the analysis was peripartum hysterectomy, defined as hysterectomy (68.3x, 68.4x, 68.9) occurring during the delivery hospitalization. In addition to analyzing the low to moderate risk delivery hospitalizations described above, we performed a sensitivity analysis restricted to even lower risk patients. To create the low-risk cohort, we further excluded women with either placenta previa or prior cesarean delivery. Results are presented for both groups: low/moderate-risk patients and low-risk patients.
For each hospital, we calculated the total number of delivery hospitalizations and divided this by the number of years in which a hospital had at least one delivery. Hospitals were categorized as low (≤1000 deliveries per year), medium (1001 to 2000 deliveries per year), and high volume (>2000 deliveries per year). In addition to annualized delivery volume, hospital characteristics included location (urban versus rural), teaching status (teaching versus nonteaching), hospital bed size, and region (Eastern, Midwest, South, or West).
Risk factors for peripartum hysterectomy were determined by a review of the literature and were included in the model.11,15–19 These included mode of delivery (vaginal operative or non-operative delivery, cesarean delivery), induction of labor, multiple gestation, stillbirth, placental abruption, fibroids, antepartum hemorrhage, chorioamnionitis, polyhydramnios, and preeclampsia/eclampsia. The following patient demographic characteristics were also individually evaluated: maternal age, race (white, black, Hispanic), year of discharge, median income for the ZIP code where the patient resides, and payer status.
Prevalence rates of hysterectomy per 10,000 deliveries were calculated. Risk ratios for peripartum hysterectomy were derived from fitting weighted log-linear mixed-effects regression models based on the Poisson distribution. These models used the GENMOD procedure in SAS (SAS Institute, Cary, NC.) to account for clustering of subjects within hospitals; in these models, hospitals formed the random intercepts. All analyses were weighted by the sampling weights that were included in the data set. To determine how well the mixed-effects log-linear models accounted for patient risk for hysterectomy, receiver operating characteristic (ROC) curves were created and the area under the curve (AUC) was determined. Sensitivity analyses for the ROC curves based on fixed effects log-linear Poisson models were performed to test the discriminatory ability of the models apart from center clustering effects.
RESULTS
Of 55,214,208 low and moderate risk deliveries included in the cohort, there were 28,862 (5.2 per 10,000, or 1 in 1913, deliveries) cases of peripartum hysterectomy. To arrive at this cohort, 384,899 deliveries were excluded. When risk factors were present, the hysterectomy frequency was much higher for women with abruption, fibroids, placenta previa (in the absence of a prior cesarean delivery), antepartum hemorrhage, or stillbirth. The rates of hysterectomy for these conditions were 31.9, 57.3, 146.7, 72.0, and 24.2 per 10,000 deliveries respectively (Table 1). Risk was higher during cesarean compared to vaginal delivery and among older women with hysterectomy rates of 12.0, 20.6 and 59.7 per 10,000 deliveries at maternal ages of 35–39, 40–44, and 45–49 respectively. When the cohort was restricted to low risk women (cases of repeat cesarean delivery and placenta previa were excluded), risk of hysterectomy was lower; of 46,834,460 deliveries in the low-risk cohort there were 15,444 cases of hysterectomy, occurring at a frequency of 3.3 per 10,000. Obstetric risk factors and increasing maternal age were similarly associated with increased risk amongst the low risk cohort. Comparing risk at the beginning and end of she study period, hysterectomy rates increased 25.7% in the low risk and 25.8% in the moderate risk group between 1998–9 and 2010–11 (both p<0.001).
Table 1.
Demographic, obstetrical, medical characteristics
| Low risk patients | Low and moderate risk patients | |||
|---|---|---|---|---|
|
| ||||
| Total n (%) |
Hysterectomy rate per 10,000 deliveries n (%) |
Total n (%) |
Hysterectomy rate per 10,000 deliveries n (%) |
|
| All deliveries | 46,834,460 | 3.3 (15,444) | 55,214,208 | 5.2 (28,862) |
|
| ||||
| Obstetric factors | ||||
|
| ||||
| Mode of delivery | ||||
| Primary cesarean | 9,010,656 (19.2) | 9.8 (8,855) | 9,240,373 (16.7) | 12.9 (11,936) |
| Repeat cesarean | NA | NA | 6,700,366 (12.1) | 14.0 (9,389) |
| Operative vaginal | 3,606,723 (7.7) | 3.0 (1,093) | 3,778,882 (6.8) | 3.4 (1,288) |
| Non-operative vaginal | 34,217,081 (73.1) | 1.6 (5,497) | 35,494,587 (64.3) | 1.8 (6,249) |
| Labor induction | 9,508,646 (20.3) | 3.3 (3,170) | 9,850,070 (17.8) | 3.6 (3,517) |
| Multiple gestation | 812,216 (1.7) | 15.3 (1,239) | 948,799 (1.7) | 18.8 (1,782) |
| Stillbirth | 323,929 (0.7) | 16.9 (549) | 368,170 (0.7) | 24.2 (890) |
| Placental abruption | 484,026 (1.0) | 23.9 (1,156) | 573,723 (1.0) | 31.9 (1,831) |
| Fibroids | 296,360 (0.6) | 44.6 (1,322) | 398,707 (0.7) | 57.3 (2,285) |
| Antepartum hemorrhage | 116,424 (0.5) | 48.9 (569) | 140,432 (0.3) | 72.0 (1,011) |
| Polyhydramnios | 280,679 (0.6) | 9.2 (258) | 350,458 (0.6) | 11.1 (388) |
| Preeclampsia/Eclampsia | 1,790,595 (3.8) | 7.4 (1,320) | 2,054,445 (3.7) | 9.2 (1,893) |
| Chorioamnionitis | 873,730 (1.9) | 9.1 (797) | 952,974 (1.7) | 11.5 (1,101) |
| Placenta previa | NA | NA | 217,333 (0.4) | 146.7 (3,189) |
|
| ||||
| Demographic factors | ||||
|
| ||||
| Age (years) | ||||
| 16–17 | 1,374,088 (2.9) | 0.6 (78) | 1,693,219 (3.1) | 0.6 (97) |
| 18–20 | 3,723,756 (8.0) | 0.9 (320) | 3,889,511 (7.0) | 1.1 (411) |
| 20–24 | 12,168,549 (26.0) | 1.5 (1,795) | 13,592,877 (24.6) | 2.1 (2,908) |
| 25–29 | 12,996,344 (27.7) | 2.5 (3,244) | 15,189,048 (27.5) | 3.9 (5,963) |
| 30–34 | 10,583,766 (22.6) | 4.2 (4,449) | 13,036,606 (23.6) | 6.6 (8,634) |
| ≥35 | 5,978,469 (12.8) | 9.3 (5,558) | 7,812,810 (14.2) | 13.9 (10,849) |
| Discharge year | ||||
| 1998 | 3,079,390 (6.5) | 2.6 (807) | 3,505,578 (6.3) | 4.5 (1,580) |
| 1999 | 3,207,632 (6.8) | 3.1 (1,010) | 3,656,664 (6.6) | 4.9 (1,805) |
| 2000 | 3,400,919 (7.3) | 2.9 (971) | 3,884,785 (7.0) | 4.4 (1,720) |
| 2001 | 3,318,450 (7.1) | 3.2 (1,052) | 3,815,910 (6.9) | 4.9 (1,873) |
| 2002 | 3,454,729 (7.4) | 2.7 (939) | 3,988,487 (7.2) | 4.9 (1,945) |
| 2003 | 3,380,353 (7.2) | 3.3 (1,111) | 3,927,782 (7.1) | 5.0 (1,966) |
| 2004 | 3,484,192 (7.4) | 3.2 (1,102) | 4,070,598 (7.4) | 5.1 (2,087) |
| 2005 | 3,486,450 (7.4) | 3.3 (1,153) | 4,096,636 (7.4) | 5.2 (2,115) |
| 2006 | 3,508,993 (7.5) | 3.3 (1,163) | 4,140,424 (7.5) | 5.1 (2,096) |
| 2007 | 3,720,572 (7.9) | 3.3 (1,246) | 4,418,172 (8.0) | 5.1 (2,253) |
| 2008 | 3,463,935 (7.4) | 3.6 (1,260) | 4,126,560 (7.5) | 5.9 (2,441) |
| 2009 | 3,257,933 (7.0) | 4.4 (1,423) | 4,044,407 (7.3) | 6.2 (2,499) |
| 2010 | 3,049,541 (6.5) | 3.1 (960) | 3,786,824 (6.9) | 5.2 (1,986) |
| 2011 | 3,021,372 (6.5) | 4.1 (1,247) | 3,751,381 (6.8) | 6.7 (2,496) |
| Household income | ||||
| Lowest quartile | 9,021,900 (19.3) | 3.3 (2,970) | 10,849,296 (19.6) | 5.5 (5,914) |
| Second quartile | 11,429,017 (24.4) | 3.4 (3,896) | 13,432,555 (24.3) | 5.3 (7,178) |
| Third quartile | 11,854,223 (25.3) | 3.1 (3,712) | 13,873,606 (25.1) | 5.0 (6,869) |
| Highest quartile | 13,690,449 (29.2) | 3.3 (4,554) | 16,066,390 (29.1) | 5.2 (8,340) |
| Unknown | 838,872 (1.8) | 3.7 (313) | 992,362 (1.8) | 5.6 (561) |
| Insurance status | ||||
| Medicare | 218,116 (0.5) | 6.1 (133) | 268,846 (0.5) | 7.9 (212) |
| Medicaid | 18,402,957 (39.3) | 2.9 (5,325) | 21,779,950 | (39.4) 4.9 (10,697) |
| Private | 25,220,421 (53.9) | 3.6 (9,002) | 29,663,361 (53.7) | 5.5 (16,176) |
| Self pay | 1,601,392 (3.4) | 3.3 (529) | 1,875,371 (3.4) | 5.1 (962) |
| Other | 1,287,255 (2.7) | 3.3 (419) | 1,504,706 (2.7) | 5.0 (753) |
| Unknown | 104,319 (0.2) | 3.6 (37) | 121,973 (0.2) | 5.1 (62) |
| Race | ||||
| White | 19,336,275 (41.3) | 3.0 (5,782) | 22,689,016 (41.1) | 4.8 (10,782) |
| Black | 4,731,018 (10.1) | 4.4 (2,102) | 5,673,336 (10.3) | 6.9 (3,908) |
| Hispanic | 8,034,011 (17.2) | 3.4 (2,696) | 9,741,891 (17.6) | 6.0 (5,820) |
| Other | 3,699,890 (7.9) | 4.6 (1,684) | 4,326,777 (7.8) | 6.7 (2,892) |
| Unknown | 11,033,266 (23.6) | 2.9 (3,181) | 12,784,189 (23.2) | 4.3 (5,459) |
|
| ||||
| Hospital Factors | ||||
|
| ||||
| Hospital bed size | ||||
| Small | 5,363,930 (11.5) | 2.5 (1,347) | 6,279,308 (11.4) | 4.0 (2,481) |
| Medium | 12,684,770 (27.1) | 3.0 (3,821) | 14,926,948 (27.0) | 4.8 (7,100) |
| Large | 28,648,663 (61.2) | 3.6 (10,227) | 33,844,564 (61.3) | 5.7 (19,206) |
| Unknown | 137,097 (0.3) | 3.6 (50) | 163,388 (0.3) | 4.7 (76) |
| Hospital Location | ||||
| Rural | 6,165,046 (13.2) | 2.7 (1,685) | 7,237,070 (13.1) | 4.0 (2,890) |
| Urban | 40,532,317 (86.5) | 3.4 (13,710) | 47,813,750 (86.6) | 5.4 (25,896) |
| Unknown | 137,097 (0.3) | 3.6 (50) | 163,388 (0.3) | 4.7 (76) |
| Hospital Region | ||||
| Northeast | 7,847,267 (16.8) | 2.9 (2,289) | 9,216,075 (16.7) | 5.1 (4,677) |
| Midwest | 10,219,284 (21.8) | 2.7 (2,740) | 11,912,669 (21.6) | 4.2 (5,045) |
| South | 17,182,078 (36.7) | 3.6 (6,269) | 20,465,868 (37.1) | 5.7 (11,588) |
| West | 11,585,832 (24.7) | 3.6 (4,146) | 13,619,596 (24.7) | 5.5 (7,552) |
| Annualized delivery volume | ||||
| ≤1000 | 9,603,149 (20.5) | 2.6 (2,516) | 11,230,340 (20.3) | 3.9 (4,375) |
| 1001–2000 | 11,565,167 (24.7) | 2.9 (3,376) | 13,616,401 (24.6) | 4.7 (6,386) |
| >2000 | 25,555,144 (54.8) | 3.7 (9,552) | 30,367,467 (55.0) | 6.0 (18,102) |
| Hospital Teaching | ||||
| Non-teaching | 25,320,180 (54.1) | 2.9 (7,400) | 29,899,914 (54.2) | 4.3 (12.962) |
| Teaching | 21,377,183 (45.6) | 3.7 (7,995) | 25,150,907 (45.6) | 6.3 (15,824) |
| Unknown | 137,097 (0.3) | 3.6 (50) | 163,388 (0.3) | 4.7 (76) |
Discharge weights were used to produce national estimates at the population level, and weighted frequency was rounded to integers. All comparisons were significant with P <0.001, with the exception of labor induction in the low risk model.
In the adjusted analysis, primary and repeat cesarean deliveries were associated with increased risks of hysterectomy (risk ratio [RR] 4.24, 95% confidence interval [CI] 3.90–4.61) and RR 5.93, 95% CI 5.44–6.44 respectively) with non-operative vaginal delivery as the reference (Table 2). Stillbirth (RR 3.44, 95% CI 2.94–4.02), placental abruption (RR 2.84, 95% CI 2.52–3.20), placenta previa (RR 13.02, 95% CI 11.82–14.34), antepartum hemorrhage (7.15, 95% CI 6.16–8.32), and fibroids (RR 3.63, 95% CI 3.22–4.08) were all associated with increased risk for hysterectomy. While increasing maternal age was associated with increased risk for hysterectomy, other demographic factors were not major predictors of the procedure. For the low risk cohort restricted to women without previa or prior cesarean, results from the adjusted analysis were similar. In the sensitivity analysis, obstetric factors and increasing maternal age were also associated with the largest magnitude of risk for hysterectomy. Of note, for both the low-moderate risk cohort and the low-risk cohort, hospital delivery volume was not a statistically significant predictor for hysterectomy.
Table 2.
Adjusted log-linear regression models
| Low risk patients | Low and moderate risk patients | |||
|---|---|---|---|---|
|
| ||||
| Adjusted risk ratio | 95% Confidence interval | Adjusted risk ratio | 95% Confidence interval | |
| Obstetric factors | ||||
|
| ||||
| Mode of delivery | ||||
| Primary cesarean | 4.31 | (3.92–4.74) | 4.24 | (3.90–4.61) |
| Repeat cesarean | NA | NA | 5.93 | (5.44–6.46) |
| Operative vaginal | 1.95 | (1.68–2.26) | 2.02 | (1.77–2.31) |
| Non-operative vaginal | 1.00 | Reference | 1.00 | Reference |
| Labor induction | 1.11 | (1.01–1.22) | 1.05 | (0.97–1.15) |
| Multiple gestation | 1.96 | (1.69–2.28) | 1.79 | (1.59–2.00) |
| Stillbirth | 3.35 | (2.74–4.10) | 3.44 | (2.94–4.02) |
| Placental abruption | 3.38 | (2.92–3.90) | 2.84 | (2.52–3.20) |
| Fibroids | 4.04 | (3.49–4.67) | 3.63 | (3.22–4.08) |
| Antepartum hemorrhage | 7.45 | (6.08–9.14) | 7.15 | (6.16–8.32) |
| Polyhydramnios | 1.48 | (1.13–1.95) | 1.26 | (1.01–1.57) |
| Preeclampsia/Eclampsia | 1.23 | (1.07–1.41) | 1.14 | (1.02–1.28) |
| Chorioamnionitis | 1.70 | (1.45–1.99) | 1.67 | (1.46–1.92) |
| Placenta previa | NA | NA | 13.02 | (11.82–14.34) |
|
| ||||
| Demographic factors | ||||
|
| ||||
| Age (years) | ||||
| 16–17 | 1.00 | Reference | 1.00 | Reference |
| 18–20 | 1.45 | (0.89–2.48) | 1.74 | (1.12–2.72) |
| 20–24 | 2.72 | (1.70–4.34) | 3.33 | (2.22–4.99) |
| 25–29 | 4.90 | (3.08–7.77) | 6.02 | (4.03–9.00) |
| 30–34 | 8.07 | (5.11–12.76) | 9.34 | (6.27–13.91) |
| ≥35 | 15.12 | (9.56–23.92) | 15.91 | (10.67–23.72) |
| Discharge year | ||||
| 1998 | 1.00 | Reference | 1.00 | Reference |
| 1999 | 1.19 | (0.97–1.46) | 1.08 | (0.93–1.27) |
| 2000 | 1.06 | (0.84–1.34) | 0.93 | (0.78–1.11) |
| 2001 | 1.15 | (0.92–1.43) | 1.01 | (0.86–1.19) |
| 2002 | 0.95 | (0.76–1.18) | 0.95 | (0.81–1.12) |
| 2003 | 1.04 | (0.83–1.30) | 0.85 | (0.71–1.00) |
| 2004 | 0.97 | (0.77–1.21) | 0.86 | (0.73–1.02) |
| 2005 | 1.02 | (0.83–1.26) | 0.87 | (0.73–1.02) |
| 2006 | 1.03 | (0.82–1.29) | 0.86 | (0.72–1.02) |
| 2007 | 1.00 | (0.80–1.24) | 0.83 | (0.70–0.98) |
| 2008 | 1.07 | (0.86–1.33) | 0.92 | (0.78–1.09) |
| 2009 | 1.23 | (0.99–1.52) | 0.96 | (0.81–1.14) |
| 2010 | 0.88 | (0.70–1.12) | 0.80 | (0.67–0.96) |
| 2011 | 1.12 | (0.89–1.42) | 0.98 | (0.82–1.16) |
| Household income | ||||
| Lowest quartile | 1.00 | Reference | 1.00 | Reference |
| Second quartile | 1.09 | (0.97–1.21) | 1.04 | (0.96–1.12) |
| Third quartile | 0.91 | (0.80–1.02) | 0.89 | (0.81–0.96) |
| Highest quartile | 0.82 | (0.71–0.94) | 0.80 | (0.73–0.88) |
| Unknown | 1.01 | (0.73–1.39) | 0.91 | (0.72–1.15) |
| Insurance status | ||||
| Medicare | 1.00 | Reference | 1.00 | Reference |
| Medicaid | 0.77 | (0.52–1.14) | 0.94 | (0.69–1.27) |
| Private | 0.62 | (0.42–0.91) | 0.73 | (0.54–0.99) |
| Self pay | 0.73 | (0.48–1.11) | 0.80 | (0.57–1.13) |
| Other | 0.71 | (0.46–1.09) | 0.83 | (0.59–1.17) |
| Unknown | 0.81 | (0.31–2.11) | 0.87 | (0.45–1.68) |
| Race | ||||
| White | 1.00 | Reference | 1.00 | Reference |
| Black | 1.35 | (1.19–1.54) | 1.27 | (1.15–1.41) |
| Hispanic | 1.19 | (1.05–1.35) | 1.23 | (1.12–1.35) |
| Other | 1.34 | (1.18–1.53) | 1.20 | (1.08–1.33) |
| Unknown | 1.36 | (0.79–2.34) | 0.97 | (0.88–1.07) |
|
| ||||
| Hospital Factors | ||||
|
| ||||
| Hospital bed size | ||||
| Small | 1.00 | Reference | 1.00 | Reference |
| Medium | 1.12 | (0.95–1.32) | 1.20 | (1.04–1.37) |
| Large | 1.26 | (1.08–1.48) | 1.35 | (1.18–1.54) |
| Unknown | 1.36 | (0.79–2.34) | 1.01 | (0.62–1.65) |
| Annualized delivery volume | ||||
| ≤1000 | 1.10 | (0.94–1.28) | 1.13 | (0.99–1.28) |
| 1000–2000 | 0.98 | (0.87–1.11) | 1.02 | (0.90–1.15) |
| >2000 | 1.00 | Reference | 1.00 | Reference |
| Hospital Location | ||||
| Rural | 1.02 | (0.86–1.20) | 0.98 | (0.85–1.13) |
| Urban | 1.00 | Reference | 1.00 | Reference |
| Hospital Region | ||||
| Northeast | 1.00 | Reference | 1.00 | Reference |
| Midwest | 1.16 | (0.99–1.37) | 1.09 | (0.96–1.24) |
| South | 1.41 | (1.21–1.65) | 1.30 | (1.13–1.48) |
| West | 1.42 | (1.21–1.67) | 1.25 | (1.09–1.44) |
| Hospital Teaching | ||||
| Non-teaching | 1.00 | Reference | 1.00 | Reference |
| Teaching | 0.84 | (0.76–0.93) | 0.69 | (0.62–0.76) |
The ROC curve for low and moderate risk patients is demonstrated in Figure 1. The AUC for the model is 0.833 (95% CI 0.827–0.838) suggesting good discrimination. For low-risk patients (excluding those with prior cesarean or placenta previa) the AUC was 0.811 (95% CI 0.804–0.819) (figure not shown). Restricted to fixed-effects, the discriminatory effects of the ROC curve models were similar. For low and moderate risk patients, the log-linear model without random effects was 0.823 (95% CI 0.818–0.825).
Figure 1. Receiver operating characteristic curve for log linear regression model including moderate and low risk patients.
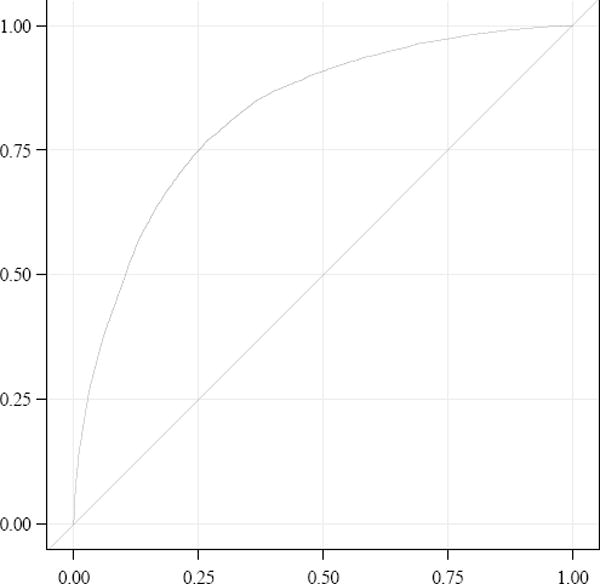
The area under the curve for the model is 0.833 (95% confidence interval 0.827–0.838).
DISCUSSION
This analysis of a nationally representative sample of delivery hospitalizations demonstrated several clinically important findings. First, peripartum hysterectomy is a relatively common clinical scenario. A moderate volume hospital that performs 2,000 deliveries per year and refers high-risk cases of suspected accreta to a tertiary center could still expect to perform on average one unanticipated peripartum hysterectomy each year. Even accounting for the additional exclusion of patients with prior cesarean delivery or placenta previa, peripartum hysterectomy occurred in approximately 1 of 3,000 low-risk deliveries with risk across low, moderate, and high volume hospitals not being statistically different in the adjusted model. Second, several readily identifiable obstetrical factors were associated with increased risk for hysterectomy. Abruption, stillbirth, fibroids, antepartum hemorrhage, and cesarean delivery, after adjustment, were all associated with particularly high risk. The increased risk associated with these factors supports the practice of universal screening of obstetrical patients for hemorrhage risk advocated by the National Partnership for Maternal Safety and other leadership organizations as a “hedge” to reduce risk in the setting of severe hemorrhage by making blood products available, notifying consultants and support personnel, and tailoring a patient-specific management plan.12 In this context, the “low” and “moderate” risk groups, while excluding patients at high risk for morbidly adherent placenta and thus the highest probability for hysterectomy, included patients at relatively high risk for hysterectomy based on other risk factors.
Third, it should be noted that, in comparison to suspected placenta accreta/percreta, many risk factors identified in this analysis are (i) common at the population level; and (ii) associated with a modest absolute increase in risk for hysterectomy. Furthermore, as demonstrated by the ROC curve, a sizeable proportion of hysterectomy cases are not identified by the risk factors in our model. Given the general unpredictability of the need for peripartum hysterectomy, aside from patients with suspected accreta/percreta, routine transfer of patients at modestly increased risk would be infeasible and of limited benefit. Other measures to improve hemorrhage-related outcomes such as instituting up-to-date trauma-based massive transfusion protocols may be critical to improving cesarean hysterectomy outcomes.2,12
In considering the results of this study, there are important limitations that deserve attention. First, is the inability to perform complete case mix adjustment.20,21 The degree to which secondary ICD-9 codes are included may be driven by reimbursement policies.22 Secondary diagnosis coding may be more likely if a diagnosis was of major clinical significance in a complication or required for billing for more complex care and/or procedures in the setting of a complication. For these reasons, our model may be biased towards overestimating the magnitude of risk associated obstetric factors. A second important limitation associated with this analysis is that the NIS offers a relatively limited set of variables characterizing individual hospitals. While we evaluated obstetric volume and such factors as geographic region and teaching status, it is possible that other unmeasured structural, administrative, and staffing factors could be strongly associated risk for hysterectomy, much less outcomes. Third, in terms of mode of delivery, it is unclear to what degree cesareans in the Nationwide Inpatient Sample can be differentiated as planned or unplanned based on secondary diagnosis codes. For that reason, our analysis cannot include whether cesarean was a risk factor that clinicians were aware of prior to labor or presentation for delivery. Regardless, awareness of increased risk associated with cesarean may be helpful in reducing risk by making blood products and consulting with anesthesia even during unplanned cesarean delivery. Fourth, given that the NIS does not allow individual patients to be followed throughout multiple hospitalizations across different hospitals, the analysis is not able to account for individual patients contributing more than one delivery. Fifth, given the large sample, statistically significant association may not be representative of clinically meaningful differences. Finally, for many at-risk patients, the need for hysterectomy may be affected by clinical management and drug and device use prior to the procedure, also factors that we were not able to assess with this database. Strengths of this study are the long temporal period, the diversity of clinical settings included, and a national picture of patient risk and outcomes.
In summary, analysis of this large, nationally representative sample of delivery hospitalizations found that peripartum hysterectomy is relatively common and occurs across a range of delivery volume settings. While certain obstetric factors are associated with increased risk, the need for hysterectomy is unpredictable and all centers providing obstetric care should be prepared to handle this clinical scenario
Acknowledgments
Dr. Friedman is supported by a career development award (1K08HD082287-01A1) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006;367:1066–74. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 2.Clark SL, Hankins GD. Preventing maternal death: 10 clinical diamonds. Obstet Gynecol. 2012;119:360–4. doi: 10.1097/AOG.0b013e3182411907. [DOI] [PubMed] [Google Scholar]
- 3.Clark SL, Yeh SY, Phelan JP, Bruce S, Paul RH. Emergency hysterectomy for obstetric hemorrhage. Obstet Gynecol. 1984;64:376–80. [PubMed] [Google Scholar]
- 4.Wright JD, Devine P, Shah M, et al. Morbidity and mortality of peripartum hysterectomy. Obstet Gynecol. 2010;115:1187–93. doi: 10.1097/AOG.0b013e3181df94fb. [DOI] [PubMed] [Google Scholar]
- 5.Yucel O, Ozdemir I, Yucel N, Somunkiran A. Emergency peripartum hysterectomy: a 9-year review. Arch Gynecol Obstet. 2006;274:84–7. doi: 10.1007/s00404-006-0124-4. [DOI] [PubMed] [Google Scholar]
- 6.Kwee A, Bots ML, Visser GH, Bruinse HW. Emergency peripartum hysterectomy: A prospective study in The Netherlands. Eur J Obstet Gynecol Reprod Biol. 2006;124:187–92. doi: 10.1016/j.ejogrb.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Knight M, Ukoss Peripartum hysterectomy in the UK: management and outcomes of the associated haemorrhage. BJOG. 2007;114:1380–7. doi: 10.1111/j.1471-0528.2007.01507.x. [DOI] [PubMed] [Google Scholar]
- 8.Silver RM, Fox KA, Barton JR, et al. Center of excellence for placenta accreta. Am J Obstet Gynecol. 2015;212:561–8. doi: 10.1016/j.ajog.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 9.Wright JD, Herzog TJ, Shah M, et al. Regionalization of care for obstetric hemorrhage and its effect on maternal mortality. Obstet Gynecol. 2010;115:1194–200. doi: 10.1097/AOG.0b013e3181df94e8. [DOI] [PubMed] [Google Scholar]
- 10.Shamshirsaz AA, Fox KA, Salmanian B, et al. Maternal morbidity in patients with morbidly adherent placenta treated with and without a standardized multidisciplinary approach. Am J Obstet Gynecol. 2015;212:218, e1–9. doi: 10.1016/j.ajog.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 11.Bateman BT, Mhyre JM, Callaghan WM, Kuklina EV. Peripartum hysterectomy in the United States: nationwide 14 year experience. Am J Obstet Gynecol. 2012;206:63, e1–8. doi: 10.1016/j.ajog.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 12.Main EK, Goffman D, Scavone BM, et al. National Partnership for Maternal Safety: Consensus Bundle on Obstetric Hemorrhage. Obstet Gynecol. 2015;126:155–62. doi: 10.1097/AOG.0000000000000869. [DOI] [PubMed] [Google Scholar]
- 13.Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122:233–41. doi: 10.1097/AOG.0b013e318299a6cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuklina EV, Whiteman MK, Hillis SD, et al. An enhanced method for identifying obstetric deliveries: implications for estimating maternal morbidity. Maternal and child health journal. 2008;12:469–77. doi: 10.1007/s10995-007-0256-6. [DOI] [PubMed] [Google Scholar]
- 15.Spiliopoulos M, Kareti A, Jain NJ, Kruse LK, Hanlon A, Dandolu V. Risk of peripartum hysterectomy by mode of delivery and prior obstetric history: data from a population-based study. Arch Gynecol Obstet. 2011;283:1261–8. doi: 10.1007/s00404-010-1554-6. [DOI] [PubMed] [Google Scholar]
- 16.Stivanello E, Knight M, Dallolio L, Frammartino B, Rizzo N, Fantini MP. Peripartum hysterectomy and cesarean delivery: a population-based study. Acta Obstet Gynecol Scand. 2010;89:321–7. doi: 10.3109/00016340903508627. [DOI] [PubMed] [Google Scholar]
- 17.Whiteman MK, Kuklina E, Hillis SD, et al. Incidence and determinants of peripartum hysterectomy. Obstet Gynecol. 2006;108:1486–92. doi: 10.1097/01.AOG.0000245445.36116.c6. [DOI] [PubMed] [Google Scholar]
- 18.Knight M, Kurinczuk JJ, Spark P, Brocklehurst P United Kingdom Obstetric Surveillance System Steering C. Cesarean delivery and peripartum hysterectomy. Obstet Gynecol. 2008;111:97–105. doi: 10.1097/01.AOG.0000296658.83240.6d. [DOI] [PubMed] [Google Scholar]
- 19.Bodelon C, Bernabe-Ortiz A, Schiff MA, Reed SD. Factors associated with peripartum hysterectomy. Obstet Gynecol. 2009;114:115–23. doi: 10.1097/AOG.0b013e3181a81cdd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deeks JJ, Dinnes J, D’Amico R, et al. Evaluating non-randomised intervention studies. Health Technol Assess. 2003;7:iii–x. 1–173. doi: 10.3310/hta7270. [DOI] [PubMed] [Google Scholar]
- 21.Zhan C, Miller MR. Administrative data based patient safety research: a critical review. Qual Saf Health Care. 2003;12(Suppl 2):ii58–63. doi: 10.1136/qhc.12.suppl_2.ii58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarrazin MS, Rosenthal GE. Finding pure and simple truths with administrative data. JAMA. 2012;307:1433–5. doi: 10.1001/jama.2012.404. [DOI] [PubMed] [Google Scholar]


