Abstract
Background
Clinical trial data suggest amphetamine treatment is most efficacious in moderate to high frequency cocaine users. However, preclinical studies have examined amphetamine treatment effects under relatively limited cocaine access conditions with low to moderate cocaine intakes. This study determined d-amphetamine treatment effects on cocaine self-administration in rhesus monkeys under cocaine access conditions allowing for high daily cocaine intake. For comparison and as a negative control, treatment effects with the antipsychotic risperidone were also examined.
Methods
Continuous 21-day treatments with ramping doses of d-amphetamine (days 1–7: 0.032 mg/kg/h; days 8–21: 0.1 mg/kg/h, i.v.) or risperidone (days 1–7: 0.001 mg/kg/h; days 8–14: 0.0032 mg/kg/h; days 15–21: 0.0056 mg/kg/h, i.v.) were administered to rhesus monkeys (n = 4) with daily access to two types of cocaine self-administration sessions: (1) a 2-h ‘choice’ session with concurrent availability of 1-g food pellets and intravenous cocaine injections (0–0.1 mg/kg per injection) and (2) a 20-h ‘extended-access’ session with 0.1 mg/kg per injection cocaine availability.
Results
Total daily cocaine intake increased >6-fold during extended cocaine access. d-Amphetamine significantly decreased total cocaine intake, but not cocaine vs food choice. In contrast, risperidone did not significantly alter either total cocaine intake or cocaine vs. food choice.
Conclusions
These results confirm and extend previous results supporting treatment effectiveness for monoamine releasers, but not dopamine antagonists, to reduce cocaine self-administration. Moreover, these results suggest amphetamine treatment efficacy to decrease preclinical cocaine vs. food choice may depend upon cocaine access conditions.
Keywords: Cocaine, Choice, Amphetamine, Risperidone, Rhesus monkey
1. Introduction
Cocaine addiction is a significant and global public health problem, and an estimated 0.4% of the global population has used cocaine at least once (UNODC, 2015). In addition, approximately 6.9% of all substance abuse treatment admissions report that cocaine is the primary abused substance (SAMHSA, 2015). Currently, there is no Food and Drug Administration-approved pharmacotherapy for cocaine addiction (Acri and Skolnick, 2013; Czoty et al., 2016). Taken together, the persistence of cocaine addiction and the absence of effective treatments support continued preclinical research in the development of effective therapeutic strategies.
Over the past decade, “agonist” candidate medications, such as the dopamine and norepinephrine releaser d-amphetamine, have emerged as the most promising pharmacotherapeutics for cocaine addiction (Banks et al., 2015b; Negus and Henningfield, 2015; Nuijten et al., 2016; Pérez-Mañá et al., 2011). In contrast, “antagonist” candidate medications, such as the dopamine antagonists flupenthixol or risperidone, have consistently demonstrated poor efficacy to attenuate the abuse-related effects of cocaine in both preclinical (John et al., 2015; Negus, 2003; Woolverton and Balster, 1981) and human laboratory drug self-administration studies (Haney et al., 2001), and clinical trials (for review, see Indave et al., 2016). Overall, this literature supports preclinical research aimed towards improving our understanding of the environmental conditions under which amphetamine treatment decreases cocaine self-administration.
One important environmental determinant of medication treatment efficacy may be cocaine access conditions and corresponding cocaine intake. For example, a recent clinical trial suggested that amphetamine in combination with topiramate treatment efficacy varied according to baseline cocaine use frequency, such that amphetamine treatment was most effective in patients who reported the most frequent cocaine use (Mariani et al., 2012). Consistent with this clinical trial, preclinical studies have also suggested differential treatment sensitivity to acute pharmacological manipulations as a consequence of baseline cocaine self-administration rates (Wee et al., 2009). Previous studies from our laboratory (Banks et al., 2013b, 2015a; Negus, 2003) and others (Thomsen et al., 2013) have demonstrated d-amphetamine treatment efficacy to attenuate cocaine vs food choice under 2-h cocaine access conditions that allow for limited (~ 1.2 mg/kg) cocaine intake. Whether d-amphetamine treatment retains efficacy to decrease preclinical cocaine vs food choice under extended cocaine access conditions allowing for high daily cocaine intake remains to be empirically determined.
Accordingly, the present study aim was to determine d-amphetamine treatment effects on cocaine vs. food choice under cocaine access conditions allowing for high daily cocaine intake. Specifically, 21-day d-amphetamine treatment effects were examined under conditions that allowed cocaine access 22 h/day during two types of cocaine self-administration sessions: (1) a 2-h cocaine vs. food choice sessions to assess pharmacological treatment efficacy to reallocate behavior away from cocaine choice and towards food choice, and (2) a 20-h extended cocaine access session to assess treatment efficacy during high daily cocaine intakes (Fig. 1). For comparison, and as a negative control, we also determined risperidone treatment effects under the same experimental conditions. Although risperidone has been extensively evaluated as a candidate medication for cocaine addiction in clinical trials (Grabowski et al., 2000; Loebl et al., 2008; Smelson et al., 2004), risperidone treatment on cocaine self-administration has not been determined in preclinical studies. We hypothesized that d-amphetamine treatment would attenuate both cocaine vs food choice and extended cocaine access self-administration. Furthermore, we hypothesized risperidone treatment would fail to attenuate both cocaine vs. food choice and extended cocaine access self-administration consistent with previous human laboratory and clinical trial results.
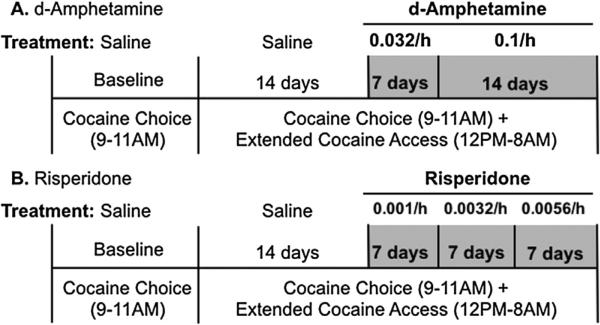
Fig. 1.
Experimental timeline. Following a baseline period during which cocaine self-administration was only available during daily 2-h cocaine vs food choice sessions (0900–1100 h), monkeys were subsequently provided access to cocaine during both choice sessions and daily 20-h extended access sessions (1200–0800 h). During extended-access sessions, 0.1 mg/kg/injection cocaine was available under a FR 10/time out 15-min schedule of reinforcement. (A) After 14 days of extended cocaine access, 0.032 mg/kg/h d-amphetamine was continuously infused for 7 days, and then the dose was increased to 0.1 mg/kg/h d-amphetamine for 14 days for a total of 21 consecutive d-amphetamine treatment days. (B) After 14 days of extended cocaine access, 0.001 mg/kg/h risperidone was continuously infused for 7 days, then 0.0032 mg/kg/h for 7 days, and then 0.0056 mg/kg/h for 7 days for a total of 21 consecutive risperidone treatment days.
2. Methods
2.1. Animals
A total of six adult male rhesus monkeys (Macaca mulatta) of either Indian or Chinese origin served as subjects and were surgically implanted with a double-lumen catheter (Reiss Manufacturing, Blackstone, VA or STI Flow, Raleigh, NC) inserted into a major vein (femoral or jugular). All subjects had prior cocaine self-administration histories (Banks et al., 2013a; Hutsell et al., 2016). Monkeys weighed 9–13 kg and were maintained on a diet of fresh fruit and food biscuits (Lab Diet High Protein Monkey Biscuits no. 5045; PMI Nutrition, St. Louis, MO) provided after daily choice sessions. Water was continuously available via an automatic watering system. A 12-h light-dark cycle was in effect (lights on from 0600 to 1800 h).
Animal research and maintenance were conducted according to the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011) and the ARRIVE guidelines (Kilkenny et al., 2010). Animal facilities were licensed by the United States Department of Agriculture and accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. The Institutional Animal Care and Use Committee approved the research and enrichment protocols. Monkeys had visual, auditory, and olfactory contact with other monkeys throughout the study. Operant procedures and foraging devices were provided for environmental manipulation and enrichment. Videos were played daily in animal housing rooms to provide additional environmental enrichment.
2.2. Apparatus and catheter maintenance
Monkeys were housed individually in well-ventilated, stainless steel chambers that also served as experimental chambers. Each chamber was equipped with a custom operant panel mounted on the front wall. Three square translucent response keys were arranged in a horizontal row, and only the left and right keys were used in the present study. Each chamber was also equipped with a pellet dispenser (Model ENV-203-1000; Med Associates, St Albans, VT) and two syringe pumps (Model PHM-108; Med Associates), one for each lumen of the double lumen catheter. One syringe pump (the “self-administration” pump) delivered response-contingent cocaine injections. The second syringe pump (the “treatment” pump) delivered noncontingent saline, d-amphetamine, or risperidone injections through the second lumen of the catheter at a programmed rate of 0.1-ml infusions every 20 min from 1200 to 1100. The intravenous catheter was protected by a tether and jacket system (Lomir Biomedical, Malone, NY) that allowed the monkeys to move freely. Catheter patency was periodically evaluated by intravenous (i.v.) ketamine (3 mg/kg) administration through the catheter lumen, and after any treatment that decreased cocaine vs food choice. The catheter was considered patent if ketamine produced a loss of muscle tone within 10 s.
2.3. Behavioral procedures
Initially, monkeys responded in daily 2-h choice sessions (0900–1100 h) that consisted of a five-component concurrent schedule of food pellet and i.v. cocaine availability as previously described (Negus, 2003). During each component, responses on the left key were reinforced with food (1-g banana-flavored pellet; Test Diets, Richmond, IN) according to a fixed-ratio (FR) 100 schedule, and responses on the right key were reinforced with i.v. cocaine (0–0.1 mg/kg/injection) according to an FR 10 schedule. A response on one key reset the ratio requirement on the alternative key. Each reinforcer delivery was followed by a 3-s timeout during which all stimulus lights were extinguished, and responding had no programmed consequences. During each component, the food key was transilluminated red. The cocaine key was transilluminated green and flashed on and off in 3 s cycles. Longer flashes were associated with larger cocaine doses. Across choice procedure components, response requirement completion on the food key always produced delivery of a single food pellet, whereas response requirement completion on the cocaine key produced the available cocaine dose (0, 0.0032, 0.01, 0.032, and 0.1 mg/kg/injection during components 1–5, respectively) by manipulating the injection volume (0, 0.01, 0.03, 0.1, and 0.3 ml/injection, respectively). Each component was in effect until 10 total reinforcers were earned or 20 min elapsed, whichever occurred first. If 10 reinforcers were delivered in less than 20 min, then stimulus lights were turned off, and responding had no scheduled consequences for the remainder of that component. Response allocation was deemed stable when the smallest unit-cocaine dose maintaining at least 80% preference for cocaine varied by ≤ 0.5 log units for 3 consecutive days. Choice session parameters used in this study were based on extensive parametric manipulations reported previously (Banks et al., 2013c; Negus, 2003). Accordingly, these parameters engendered cocaine choice ED50 values in the middle of the cocaine dose range (between 0.01 and 0.032 mg/kg/injection), and therefore permitted detection of both leftward and rightward shifts in the cocaine vs. food choice dose–effect function.
Once cocaine vs food choice was stable, a simple FR10 schedule of cocaine reinforcement was initiated to provide extended-cocaine access for 20 h each day (1200 to 0800) in addition to the 2-h choice session (Banks et al., 2013a; Banks and Negus, 2010). During the extended cocaine access session, the cocaine key was transilluminated green, and response requirement (FR10) completion extinguished the green light, activated the self-administration pump to deliver 0.1 mg/kg per injection of cocaine, and initiated a 15-min timeout. These two daily cocaine self-administration sessions were implemented for 14 consecutive days prior to d-amphetamine or risperidone treatment (Fig. 1A-B).
Subsequently, a 21-day test period was initiated during which a test solution was administered via the “treatment pump” (Fig. 1A-B). The test solutions and doses examined in this study were d-amphetamine (days 1–7: 0.032 mg/kg/h; days 8–21: 0.1 mg/kg/h) and risperidone (days 1–7: 0.001 mg/kg/h; days 8–14: 0.0032 mg/kg/h; days 15–21: 0.0056 mg/kg/h). Drug doses were evaluated in ascending order to model one aspect of clinical trials using escalating candidate medication dosing regimens (Grabowski et al., 2001, 2004). Each drug was tested in a group of 4 monkeys, and at the conclusion of each experiment, saline treatment was reinstated, extended-access components were terminated, and daily choice sessions continued until responding recovered to baseline levels.
2.4. Data analysis
The primary dependent measures for choice components were “percent cocaine choice per component,” defined as ((number of completed ratios on the cocaine-associated key during a given component ÷ total completed ratios in that component) × 100), and the numbers of cocaine and food reinforcers earned per session. The primary dependent measure for extended cocaine access sessions was the number of cocaine injections earned during each 4-h bin of the 20-h session. Daily cocaine intake (mg/kg/day) was also used as a summary measure, and the maximum possible cocaine intake was 9.45 mg/kg/day. Data for each of these variables were first averaged within a monkey for the last 3 days of each 7d treatment block, and then across monkeys.
Cocaine choice results were analyzed using two-way repeated-measures (RM) ANOVA with treatment dose and cocaine dose as the fixed effects. In the presence of a significant main effect of treatment dose or an interaction, a Holm-Sidak post-hoc test was performed. However, because d-amphetamine treatment eliminated responding in some choice components for some monkeys, two-way RM ANOVA was not performed, as this model does not appropriately accommodate missing data points. Thus, for both d-amphetamine and risperidone treatments, group mean cocaine choice dose-effect functions were analyzed using linear mixed effects (LME) modelling with the continuous variables cocaine and treatment dose as fixed effects and the nominal variable “subject” as a random effect. The numbers of cocaine and food reinforcers earned were analyzed using linear regression with experimental manipulation as the predictor variable. Model comparison was used to determine whether manipulations had an effect on the regression coefficients (β1) by an extra sum-of-squares F-test (Motulsky and Christopoulous, 2003). Daily cocaine intake was analyzed using one-way RM ANOVA. A significant ANOVA was followed by the Holm-Sidak multiple comparisons post-hoc test. The criterion for statistical significance was set at the 95% confidence level (P < 0.05). Analyses were conducted using Prism 6.0 for Mac (GraphPad Software, La Jolla, CA) or JMP Pro 11.1 for Mac (SAS Institute, Cary, NC).
2.5. Drugs
(−)-Cocaine HCl was provided by the National Institute on Drug Abuse (Bethesda, MD) Drug Supply Program. d-Amphetamine hemisulfate and risperidone base were purchased from Sigma Aldrich (St. Louis, MO). Cocaine and d-amphetamine were dissolved in sterile saline. Risperidone was dissolved in 2% lactic acid (Sigma Aldrich) and the pH adjusted to between 5 and 7 by the addition of NaOH. All solutions were passed through a sterile 0.22-μm Millipore filter (Millipore Corp, Billerica, MA) before intravenous delivery. Drug doses were calculated using the salt or base forms listed above.
3. Results
3.1. Baseline choice between cocaine and food and effects of extended cocaine access
Under baseline conditions during which cocaine was only available during the cocaine vs food choice session, monkeys chose almost exclusively food when no or small cocaine doses were available (0–0.01 mg/kg/injection) and almost exclusively chose cocaine when large cocaine doses were available (0.032–0.1 mg/kg/injection) (Supplemental Fig. 1A-B). Furthermore, introduction of an extended cocaine access session had no significant effect on cocaine vs food choice or the number of total, food, or cocaine choices completed during the choice session (Supplemental Fig. 1A-D). Total daily cocaine intake averaged 1.25 ± 0.02 mg/kg during baseline cocaine vs food choice; total daily intake increased to 8.33 ± 0.32 mg/kg at the end of 14 days of extended cocaine access.
3.2. Effects of d-amphetamine and risperidone on cocaine self-administration
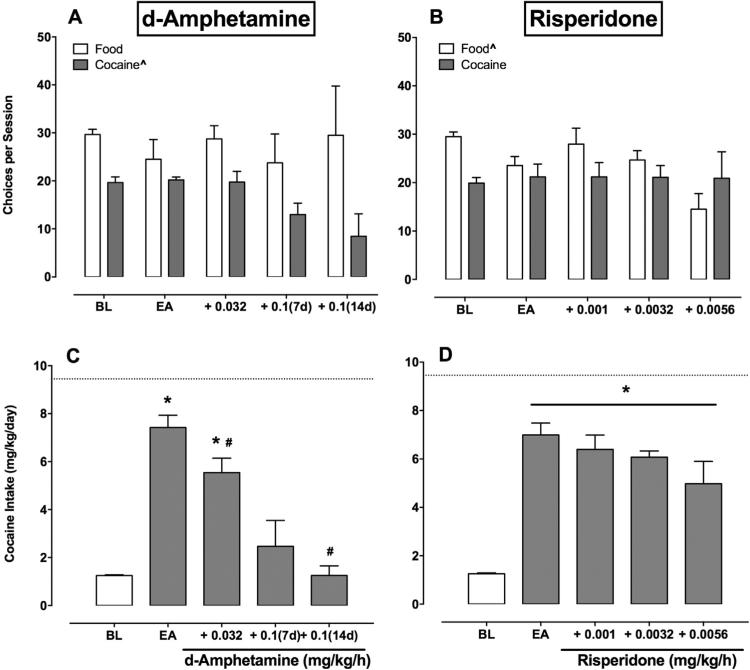
Fig. 2 shows group mean food and cocaine choices during the last 3 days of each 7-day d-amphetamine (2A) and risperidone (2B) treatment period. d-Amphetamine did not alter food choices, but significantly decreased cocaine choices (F(2,36) = 9.38, P = 0.0005). The regression coefficient for food choices did not differ from zero (β1 = −0.11, 95% CI ± 3.62), while the regression coefficient was negative for cocaine choices (β1 = −2.95, 95% CI ± 1.74). In contrast, risperidone significantly decreased food choices, but did not alter cocaine choices (F(2,36) = 4.37, P = 0.02). The regression coefficient for food choices was negative (β1 = −2.89, 95% CI ± 1.86), while the regression coefficient for cocaine choices did not differ from zero (β1 = 0.19, 95% CI ± 1.97). Fig. 2C-D shows group mean total daily cocaine intakes during the last 3 days of each 7-day d-amphetamine (F(1.43,4.28) = 23.67, P = 0.0057) and risperidone (F(2.03,6.09) = 53.23, P = 0.0001) treatment period. For both treatments, extended cocaine access increased cocaine intake compared to baseline (P < 0.05). d-Amphetamine treatment (0.032, 0.1(14d)) significantly decreased cocaine intake compared to extended cocaine access, and by the end of 21 treatment days, total daily cocaine intake did not differ from baseline conditions during which cocaine availability was only during the 2-h choice session (P > 0.05).
Fig. 2.
Continuous 21-day d-amphetamine (A, C) or risperidone (B, D) treatment effects on food and cocaine choices and total daily cocaine intakes in rhesus monkeys (n = 4). (A-B) Ordinates: food and cocaine choices completed per daily choice session. Abscissae: experimental condition. (C-D) Ordinates: total daily cocaine intake (mg/kg/day). Abscissae: experimental condition. Symbols indicate: (significant regression coefficient), *(significantly different from baseline (BL)), and #(significantly different from extended access (EA)).
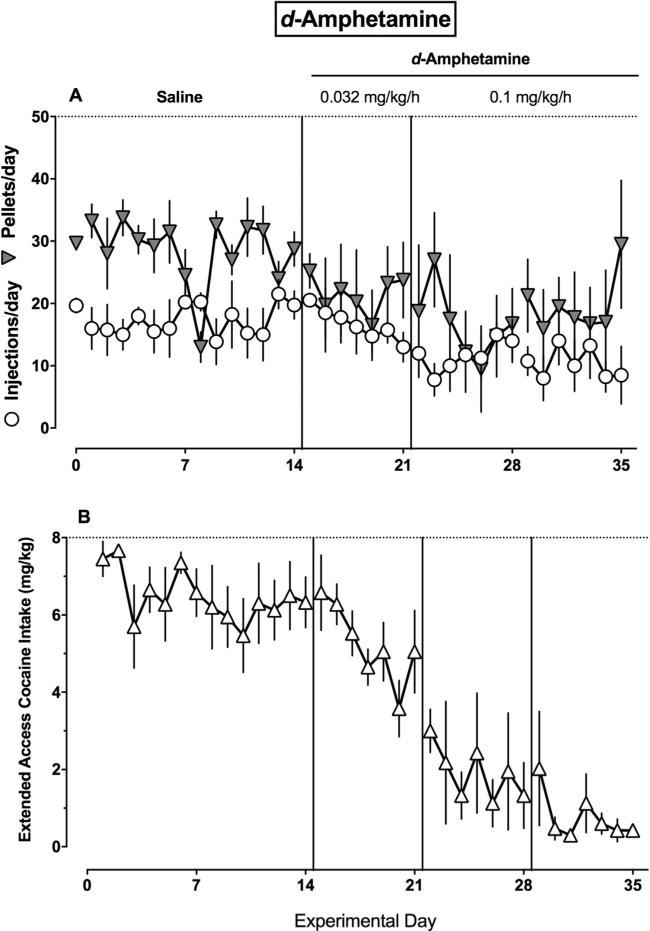
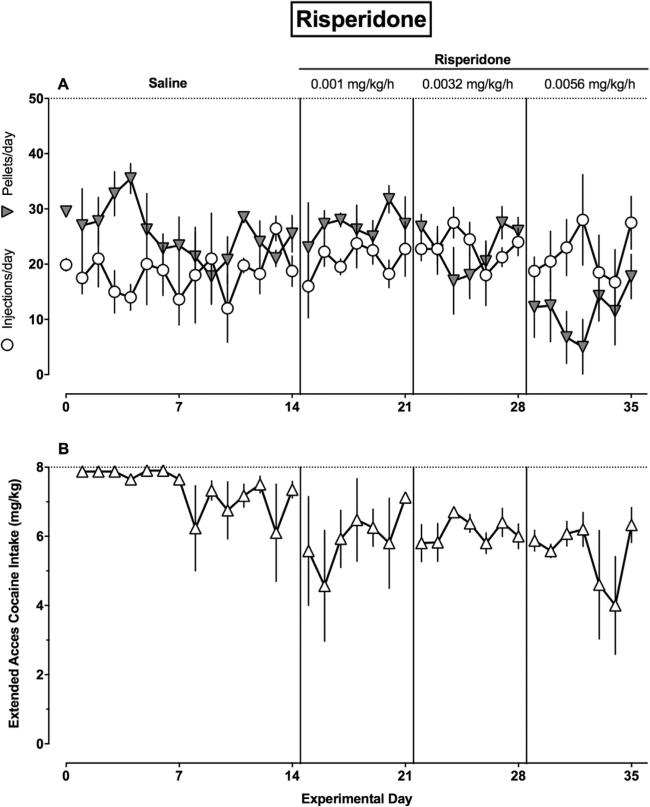
The time courses of d-amphetamine and risperidone treatment effects during daily cocaine vs food choice sessions (Panel A) and extended cocaine access sessions (Panel B) are shown in Figs. 3 and 4, respectively. d-Amphetamine treatment did not significantly decrease the number of pellets earned per day, but did significantly decrease the number of cocaine injections earned per day during choice sessions (F(2172) = 9.1, P = 0.0002). The regression coefficient for food pellets earned did not differ from zero (β1 = −0.23, 95% CI ± 0.35), while the regression coefficient was negative for cocaine injections earned during the choice session (β1 = −0.43, 95% CI ± 0.18). Furthermore, the regression coefficient for extended access cocaine intake also differed from zero (β1 = −0.33, 95% CI ± 0.06). In contrast, risperidone significantly decreased the number of pellets per day, but did not alter the number of cocaine injections per day during the choice session (F(2172) = 10.5, P < 0.0001). The regression coefficient for food pellets was negative (β1 = −0.84, 95% CI ± 0.37), while the regression coefficient for cocaine injections did not differ from zero (β1 = 0.09, 95% CI ± 0.05). Furthermore, the regression coefficient for extended access cocaine intake also did not differ from zero (β1 = −0.03, 95% CI ± 0.06).
Fig. 3.
Time course of 21-day d-amphetamine treatment effects in rhesus monkeys (n = 4). (A) Ordinate: the number of food pellets earned per day (filled downward triangle) and number of cocaine injections earned per day (open circles) during the 2-h choice component. Abscissa: experimental day. (B) Ordinate: extended access cocaine intake (mg/kg). Abscissa: experimental day. Vertical lines denote experimental treatment changes as described in Fig. 1. Horizontal dashed lines denote maximum number of reinforcers available during the choice session (A) and maximum extended access cocaine intake (B).
Fig. 4.
Time course of 21-day risperidone treatment effects in rhesus monkeys (n = 4). (A) Ordinate: the number of food pellets earned per day (filled downward triangle) and number of cocaine injections earned per day (open circles) during the 2-h choice component. Abscissa: experimental day. (B) Ordinate: extended access cocaine intake (mg/kg). Abscissa: experimental day. Vertical lines denote experimental treatment changes as described in Fig. 1. Horizontal dashed lines denote maximum number of reinforcers available during the choice session (A) and maximum extended access cocaine intake (B).
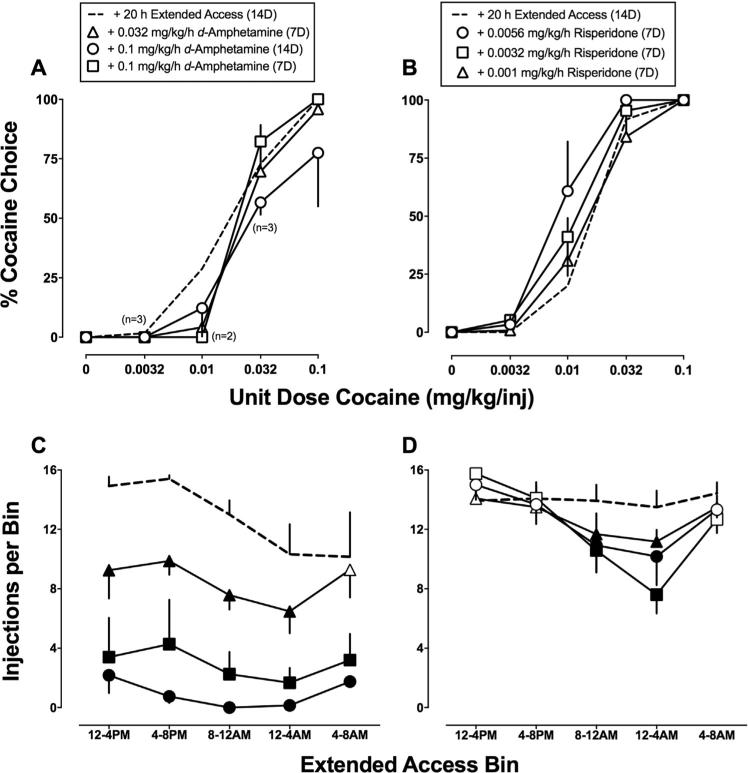
Fig. 5 shows d-amphetamine (A) and risperidone (B) treatment effects on cocaine vs food choice during the last three days of each 7-day treatment period. There was a main effect of cocaine dose [d-amphetamine: F(1,69.1) = 55.0, P < 0.001, risperidone: F(1,73 = 40.1, P < 0.001)], but no significant main effect of d-amphetamine or risperidone treatment and no significant interaction. Fig. 5 also shows d-amphetamine (C) and risperidone (D) treatment effects per 4-h bin of the 20-h extended cocaine access session. Both d-amphetamine [d-amphetamine: F(3,9) = 25.0, P = 0.0001; bin: F(4,12) = 6.5, P = 0.0051] and risperidone [bin: F(4,12) = 15.2, P = 0.0001; interaction: F(12,36) = 3.0, P = 0.0054] significantly decreased the number of cocaine injections earned per 4-h bin. d-Amphetamine decreased the number of cocaine injections in all but one 4-h bin of the extended access component (all Ps < 0.05). Risperidone decreased the number of cocaine injections during 4-h bins that corresponded primarily with the dark cycle (all Ps < 0.05). Supplemental Fig. 2 shows d-amphetamine and risperidone treatment effects on cocaine vs. food choice in individual monkeys. d-Amphetamine treatment decreased cocaine vs. food choice in one monkey (M1505), produced no effect in two monkeys (M1475 and M1504), and increased cocaine choice in one monkey (M1489). In contrast, risperidone treatment increased cocaine vs food choice in two monkeys (M1474 and M1475) and did not shift cocaine choice in two monkeys (M1473 and M1489). Larger risperidone doses were not evaluated in all monkeys due to the emergence of cataleptic-like behavior within a few days after 0.01 mg/kg/h risperidone initiation in a single monkey.
Fig. 5.
Continuous 21-day d-amphetamine and risperidone treatment effects on (A-B) cocaine vs food choice and (C-D) extended access cocaine self-administration in rhesus monkeys (n = 4). (A-B) Ordinates: percent cocaine choice. Abscissae: unit dose cocaine in mg/kg/injection. (C-D) Ordinates: cocaine injections per bin. Abscissae: 4-h bins of the extended access cocaine self-administration session. All data points represent means during the last three days of each 7-day treatment period. Numbers in parentheses indicate the number of subjects contributing to that data point if fewer than the total number of subjects (4) tested during treatment with 0.1 mg/kg/h d-amphetamine. This number indicates d-amphetamine treatment eliminated responding in one or more subjects during that component of the choice procedure. Filled symbols indicate statistical significance (P < 0.05) compared with extended access condition.
4. Discussion
The present study aim was to determine d-amphetamine treatment efficacy to decrease cocaine vs food choice under experimental conditions that engendered high daily cocaine intake. There were three main findings. First, introduction of a 20-h extended cocaine self-administration session increased total daily cocaine intake > 6-fold. Second, continuous 21-day d-amphetamine treatment significantly decreased daily cocaine intake 6-fold and total cocaine choices during the choice session without altering food choices. In addition, the measure of cocaine vs food choice during individual components of daily choice sessions was attenuated in one out of four monkeys. In contrast, continuous 21-day risperidone treatment failed to attenuate daily cocaine intake and significantly decreased food choices during the choice session without altering cocaine choices. In addition, cocaine vs. food choice was enhanced in two out of four monkeys. Overall, the present results confirm and extend previous research supporting monoamine releaser, but not dopamine antagonist, treatment efficacy to attenuate cocaine self-administration. Moreover, the present results suggest d-amphetamine treatment efficacy may be influenced by cocaine self-administration access conditions and consequent daily cocaine intake.
4.1. Baseline cocaine choice and effects of extended cocaine access
During cocaine vs food choice sessions that preceded extended cocaine access, cocaine maintained a dose-dependent increase in choice vs an alternative, nondrug reinforcer. These results are consistent with the extant literature involving humans (Hart et al., 2000; Stoops et al., 2012), nonhuman primates (John et al., 2015; Woolverton and Balster, 1981), and rodents (Kerstetter et al., 2012; Thomsen et al., 2013) as research subjects. Extended cocaine access introduction increased daily cocaine intake approximately 6-fold and resulted in a small but nonsignificant rightward shift in the cocaine choice dose-effect function. These extended cocaine access effects on cocaine vs. food choice are consistent with previous studies in nonhuman primates (Banks et al., 2013a; Banks and Negus, 2010; Hutsell et al., 2016) and rats (Cantin et al., 2010). Overall, the consistency of these baseline behavioral dependent measures provides the empirical foundation for determining d-amphetamine and risperidone treatment effects.
4.2. Statistical analyses in preclinical addiction medication research
Pharmacological treatment effects on cocaine self-administration were evaluated using a ramping, subchronic dosing regimen to model dosing regimens used in clinical trials (Grabowski et al., 2001, 2004). During d-amphetamine treatment, one or more monkeys failed to complete a single response requirement during at least one component of the choice session, and this outcome prevented a balanced statistical analysis. As a consequence of d-amphetamine's reinforcement-independent rate-altering effects, mixed-effects regression modelling was utilized to appropriately analyze the incomplete data set (Everitt, 1998). Furthermore, mixed-effects regression analyses may contribute to preclinical drug addiction medication research for at least two other reasons. First, while mixed-effect regression analyses may be underutilized in the preclinical literature, it is a common technique used in double blind, placebo controlled clinical trials where participant attrition is expected (e.g., Grabowski et al., 2001, 2004; Mariani et al., 2012). Second, mixed-effect regression models provide an empirical framework for repeated-measures experimental designs that can accommodate missing data while also providing treatment effect estimates at both the group and individual-subject level (Gueorguieva and Krystal, 2004; Young et al., 2009). Overall, the present results support the utility of mixed-effects regression analyses in preclinical drug addiction medication research (Goldberg et al., 1996).
4.3. d-Amphetamine treatment effects on cocaine self-administration
d-Amphetamine treatment significantly decreased extended access cocaine self-administration rates to levels similar in magnitude to substituting saline for cocaine (Banks et al., 2013a). Furthermore, the present d-amphetamine treatment effects on extended access cocaine self-administration were similar in magnitude to effects under other schedules of cocaine reinforcement that do not include concurrent availability of an alternative, nondrug reinforcer (Czoty et al., 2011; Negus and Mello, 2003a, 2003b). However, because these cocaine self-administration procedures rely on rate-based dependent measures that reflect not only the reinforcing effects of cocaine but also reinforcement-independent rate-altering effects of the treatment (Banks and Negus, 2012), a second behavioral “choice” session was utilized in the present study to assess pharmacological treatment efficacy using a behavioral allocation dependent measure.
In the present study, d-amphetamine treatment significantly decreased total cocaine choices without affecting total food choices indicating a modest, but selective, decrease in behavioral allocation between cocaine injections and food pellets. However, analysis of percent cocaine choice during individual components of daily choice sessions did not reveal a significant d-amphetamine treatment effect. At this group analysis effect level, the present d-amphetamine treatment effects on cocaine vs food choice are weaker than previously reported under conditions where cocaine availability was limited to the 2-h choice session (Banks et al., 2013b, 2015a; Negus, 2003). At least three factors contribute to this apparent discrepancy. First, the cocaine vs food choice dose-effect function was slightly, albeit nonsignificantly, right-shifted during extended cocaine access, and this shifted baseline may have reduced sensitivity to subsequent d-amphetamine treatment effects. Second, with regard to dopamine transmission, cocaine blocks dopamine transporters to reduce uptake not only of dopamine, but also of drugs like d-amphetamine that are dopamine transporter substrates (Cameron et al., 2013). Thus, high levels of cocaine intake during initial d-amphetamine exposure may have attenuated and/or delayed d-amphetamine effects on cocaine vs. food choice. Consistent with this hypothesis, combined treatment with the dopamine transporter inhibitor modafanil and d-amphetamine failed to attenuate cocaine use compared to d-amphetamine treatment alone in a clinical trial (Schmitz et al., 2012). Lastly, the failure of d-amphetamine to produce a significant decrease in group data for cocaine vs. food choice reflects individual differences in d-amphetamine effects. Although cocaine vs. food choice was significantly reduced in only 1 of 4 monkeys, clinical trials have shown a similar rate of treatment response to amphetamine maintenance (Grabowski et al., 2001; Mariani et al., 2012; Nuijten et al., 2016; Pérez-Mañá et al., 2011; Schmitz et al., 2012). Together, these results may highlight the importance of examining individual subject data for individualized or ‘precision’ addiction medicine (Nader, 2016).
4.4. Risperidone treatment effects on cocaine self-administration
In contrast to d-amphetamine, continuous risperidone treatment failed to decrease either cocaine vs food choice or daily cocaine intake. Risperidone was selected as a reverse-translation negative control because it has been extensively evaluated as a candidate medication for cocaine addiction and consistently failed in clinical trials (Grabowski et al., 2004; Loebl et al., 2008; Smelson et al., 2004), but has not been evaluated in any preclinical cocaine self-administration procedure. The absence of risperidone treatment efficacy in this study is in agreement with previous preclinical (John et al., 2015; Negus, 2003; Woolverton and Balster, 1981) and human laboratory (Haney et al., 2001) cocaine choice studies examining other dopamine antagonist treatments, such as flupenthixol, haloperidol, buspirone, and ecopipam. Overall, these risperidone treatment results provide further support for the translational concordance between preclinical cocaine vs food choice studies and human studies.
4.5. Implications for preclinical cocaine addiction candidate medication evaluation
In summary, the present results highlight two implications for the preclinical evaluation of candidate medications for cocaine addiction. First, the schedule of reinforcement is an important determinant of pharmacological treatment efficacy. The multiple-schedule, within-subjects experimental design of the present study revealed differential d-amphetamine treatment effects on cocaine self-administration, such that effects were most robust under a simple FR10 schedule of cocaine reinforcement during the extended access session and less effective under a concurrent schedule of cocaine and food pellet availability. Risperidone treatment also attenuated cocaine self-administration during the extended access session, though to a lesser extent than d-amphetamine, and risperidone failed to attenuate cocaine vs food choice. Thus, extended access cocaine self-administration was more sensitive than cocaine vs. food choice to effects of both pharmacological manipulations. Second, these results also highlight that cocaine self-administration access conditions and consequent cocaine intake may be important determinants of pharmacological treatment efficacy. In particular, these results suggest that high levels of ongoing cocaine intake may attenuate or delay d-amphetamine effects of cocaine vs. food choice.
Supplementary Material
Acknowledgements
We acknowledge the technical assistance of Jennifer Gough and Kevin Costa for writing the original version of the behavioral programs.
Role of funding source
Research reported in this publication was supported by the National Institute on Drug Abuse (NIDA) of the National Institutes of Health under Award Numbers R01 DA026946 and T32 DA007027. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Contributors
Banks was responsible for study concept and design. Hutsell performed the data analysis. Hutsell and Banks drafted the manuscript. Negus assisted with result interpretations and provided critical manuscript revision for intellectual content. All authors critically reviewed the content and approved the final version for publication.
Conflicts of interest
None of the authors have any conflict of interest to declare.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.drugalcdep.2016.08.637.
References
- Acri J, Skolnick P. Pharmacotherapy of substance use disorders. In: Charney D, Buxbaum J, Sklar P, Nestler EJ, editors. Neurobiology Of Mental Illness. Oxford University Press; London: 2013. pp. 761–771. [Google Scholar]
- Banks ML, Negus SS. Effects of extended cocaine access and cocaine withdrawal on choice between cocaine and food in rhesus monkeys. Neuropsychopharmacology. 2010;35:493–504. doi: 10.1038/npp.2009.154. http://dx.doi.org/10.1038/npp.2009. 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Negus SS. Preclinical determinants of drug choice under concurrent schedules of drug self-administration. Adv. Pharmacol. Sci. 2012:281768. doi: 10.1155/2012/281768. http://dx.doi.org/10.1155/2012/281768. [DOI] [PMC free article] [PubMed]
- Banks ML, Blough BE, Fennell TR, Snyder RW, Negus SS. Effects of phendimetrazine treatment on cocaine vs food choice and extended-access cocaine consumption in rhesus monkeys. Neuropsychopharmacology. 2013a;38:2698–2707. doi: 10.1038/npp.2013.180. http://dx.doi.org/10.1038/npp.2013.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Negus SS. Effects of 14-day treatment with the schedule III anorectic phendimetrazine on choice between cocaine and food in rhesus monkeys. Drug Alcohol Depend. 2013b;131:204–213. doi: 10.1016/j.drugalcdep.2013.05.005. http://dx.doi.org/10.1016/j.drugalcdep.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Stevens Negus S. Interaction between behavioral and pharmacological treatment strategies to decrease cocaine choice in rhesus monkeys. Neuropsychopharmacology. 2013c;38:395–404. doi: 10.1038/npp.2012.193. http://www.nature.com/npp/journal/v38/n3/suppinfo/npp2012193s1.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Hutsell BA, Blough BE, Poklis JL, Negus SS. Preclinical assessment of lisdexamfetamine as an agonist medication candidate for cocaine addiction: effects in rhesus monkeys trained to discriminate cocaine or to self-administer cocaine in a cocaine versus food choice procedure. Int. J. Neuropsychopharmacol. 2015a;18:pyv009. doi: 10.1093/ijnp/pyv009. http://dx.doi.org/10.1093/ijnp/pyv009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Hutsell BA, Schwienteck KL, Negus SS. Use of preclinical drug vs. food choice procedures to evaluate candidate medications for cocaine addiction. Curr. Treat. Options Psychiatry. 2015b;2:136–150. doi: 10.1007/s40501-015-0042-9. http://dx.doi.org/10.1007/s40501-015-0042-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron KN, Kolanos R, Solis E, Glennon RA, De Felice LJ. Bath salts components mephedrone and methylenedioxypyrovalerone (MDPV) act synergistically at the human dopamine transporter. Br. J. Pharmacol. 2013;168:1750–1757. doi: 10.1111/bph.12061. http://dx.doi.org/10.1111/bph.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantin L, Lenoir M, Augier E, Vanhille N, Dubreucq S, Serre F, Vouillac C, Ahmed SH. Cocaine is low on the value ladder of rats: possible evidence for resilience to addiction. PLoS One. 2010;5:e11592. doi: 10.1371/journal.pone.0011592. http://dx.doi.org/10.1371/journal.pone.0011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Gould RW, Martelle JL, Nader MA. Prolonged attenuation of the reinforcing strength of cocaine by chronic d-amphetamine in rhesus monkeys. Neuropsychopharmacology. 2011;36:539–547. doi: 10.1038/npp.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Stoops WW, Rush CR. Evaluation of the Pipeline for development of medications for cocaine use disorder: a review of translational preclinical, human laboratory, and clinical trial research. Pharmacol. Rev. 2016;68:533–562. doi: 10.1124/pr.115.011668. http://dx.doi.org/10.1124/pr.115.011668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BS. Analysis of longitudinal data. beyond MANOVA. Br. J. Psychiatry. 1998;172:7–10. doi: 10.1192/bjp.172.1.7. [DOI] [PubMed] [Google Scholar]
- Goldberg AM, Zurlo J, Rudacille D. The three Rs and biomedical research. Science. 1996;272:1403–1403. doi: 10.1126/science.272.5267.1403. http://dx.doi.org/10.1126/science.272.5267.1403. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Silverman P, Schmitz JM, Stotts A, Creson D, Bailey R. Risperidone for the treatment of cocaine dependence: randomized, double-blind trial. J. Clin. Psychopharmacol. 2000;20:305–310. doi: 10.1097/00004714-200006000-00003. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Schmitz J, Stotts A, Daruzska LA, Creson D, Moeller FG. Dextroamphetamine for cocaine-dependence treatment: a double-blind randomized clinical trial. J. Clin. Psychopharmacol. 2001;21:522–526. doi: 10.1097/00004714-200110000-00010. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Stotts A, Cowan K, Kopecky C, Dougherty A, Moeller FG, Hassan S, Schmitz J. Agonist-like or antagonist-like treatment for cocaine dependence with methadone for heroin dependence: two double-blind randomized clinical trials. Neuropsychopharmacology. 2004;29:969–981. doi: 10.1038/sj.npp.1300392. [DOI] [PubMed] [Google Scholar]
- Gueorguieva R, Krystal JH. Move over anova: progress in analyzing repeated-measures data andits reflection in papers published in the archives of general psychiatry. Arch. Gen. Psychiatry. 2004;61:310–317. doi: 10.1001/archpsyc.61.3.310. http://dx.doi.org/10.1001/archpsyc.61.3.310. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward A, Foltin R, Fischman M. Effects of ecopipam, a selective dopamine D1 antagonist, on smoked cocaine self-administration by humans. Psychopharmacology (Berl) 2001;155:330–337. doi: 10.1007/s002130100725. http://dx.doi.org/10.1007/s002130100725. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Foltin RW, Fischman MW. Alternative reinforcers differentially modify cocaine self-administration by humans. Behav. Pharmacol. 2000;11:87–91. doi: 10.1097/00008877-200002000-00010. [DOI] [PubMed] [Google Scholar]
- Hutsell BA, Cheng K, Rice KC, Negus SS, Banks ML. Effects of the kappa opioid receptor antagonist nor-binaltorphimine (nor-BNI) on cocaine versus food choice and extended-access cocaine intake in rhesus monkeys. Addict. Biol. 2016;21:360–373. doi: 10.1111/adb.12206. http://dx.doi.org/10.1111/adb.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indave BI, Minozzi S, Pani PP, Amato L. Antipsychotic medications for cocaine dependence. Cochrane Database Syst. Rev. 3. 2016:CD006306. doi: 10.1002/14651858.CD006306.pub3. http://dx.doi.org/10.1002/14651858.cd006306.pub3. [DOI] [PMC free article] [PubMed]
- John WS, Banala AK, Newman AH, Nader MA. Effects of buspirone and the dopamine D3 receptor compound PG619 on cocaine and methamphetamine self-administration in rhesus monkeys using a food-drug choice paradigm. Psychopharmacology (Berl) 2015;232:1279–1289. doi: 10.1007/s00213-014-3760-6. http://dx.doi.org/10.1007/s00213-014-3760-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerstetter KA, Ballis MA, Duffin-Lutgen S, Carr AE, Behrens AM, Kippin TE. Sex differences in selecting between food and cocaine reinforcement are mediated by estrogen. Neuropsychopharmacology. 2012;37:2605–2614. doi: 10.1038/npp.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. http://dx.doi.org/10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loebl T, Angarita GA, Pachas GN, Huang KL, Lee SH, Nino J, Logvinenko T, Culhane MA, Evins AE. A randomized, double-blind, placebo-controlled trial of long-acting risperidone in cocaine-dependent men. J. Clin. Psychiatry. 2008;69:480–486. doi: 10.4088/jcp.v69n0321. [DOI] [PubMed] [Google Scholar]
- Mariani JJ, Pavlicova M, Bisaga A, Nunes EV, Brooks DJ, Levin FR. Extended-release mixed amphetamine salts and topiramate for cocaine dependence: a randomized controlled trial. Biol. Psychiatry. 2012;72:950–956. doi: 10.1016/j.biopsych.2012.05.032. http://dx.doi.org/10.1016/j.biopsych.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motulsky H, Christopoulous A. A Practical Guide To Curve Fitting. GraphPad Software; San Diego: 2003. Fitting models to biological data using linear and nonlinear regression. [Google Scholar]
- Nader MA. Chapter 1 - animal models for addiction medicine: from vulnerable phenotypes to addicted individuals. Prog. Brain Res. 2016;224:3–24. doi: 10.1016/bs.pbr.2015.07.012. [DOI] [PubMed] [Google Scholar]
- National Research Council . Guide For The Care And Use Of Laboratory Animals. National Academies Press; Washington DC.: 2011. [Google Scholar]
- Negus SS, Henningfield J. Agonist medications for the treatment of cocaine use disorder. Neuropsychopharmacology. 2015;40:1815–1825. doi: 10.1038/npp.2014.322. http://dx.doi.org/10.1038/npp.2014.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a progressive-ratio schedule in rhesus monkeys. Psychopharmacology (Berl) 2003a;167:324–332. doi: 10.1007/s00213-003-1409-y. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a second-order schedule in rhesus monkeys. Drug Alcohol Depend. 2003b;70:39–52. doi: 10.1016/s0376-8716(02)00339-3. [DOI] [PubMed] [Google Scholar]
- Negus SS. Rapid assessment of choice between cocaine and food in rhesus monkeys: effects of environmental manipulations and treatment with d-amphetamine and flupenthixol. Neuropsychopharmacology. 2003;28:919–931. doi: 10.1038/sj.npp.1300096. [DOI] [PubMed] [Google Scholar]
- Nuijten M, Blanken P, van de Wetering B, Nuijen B, van den Brink W, Hendriks VM. Sustained-release dexamfetamine in the treatment of chronic cocaine-dependent patients on heroin-assisted treatment: a randomised, double-blind, placebo-controlled trial. Lancet. 2016;387:2226–2234. doi: 10.1016/S0140-6736(16)00205-1. http://dx.doi.org/10.1016/S0140-6736(16)00205-1. [DOI] [PubMed] [Google Scholar]
- Pérez-Mañás C, Castells X, Vidal X, Casas M, Capellà D. Efficacy of indirect dopamine agonists for psychostimulant dependence: a systematic review and meta-analysis of randomized controlled trials. J. Subst. Abuse Treat. 2011;40:109–122. doi: 10.1016/j.jsat.2010.08.012. http://dx.doi.org/10.1016/j.jsat.2010.08.012. [DOI] [PubMed] [Google Scholar]
- SAMHSA . Results from the 2014 National Survey on Drug Use and Health: Detailed Tables. SAMHSA; Rockville, MD.: 2015. [Google Scholar]
- Schmitz JM, Rathnayaka N, Green C, Moeller FG, Dougherty AE, Grabowski J. Combination of modafinil and d-amphetamine for the treatment of cocaine dependence: a preliminary investigation. Front. Psychiatry. 2012;3 doi: 10.3389/fpsyt.2012.00077. http://dx.doi.org/10.3389/fpsyt.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smelson DA, Williams J, Ziedonis D, Sussner BD, Losonczy MF, Engelhart C, Kaune M. A double-blind placebo-controlled pilot study of risperidone for decreasing cue-elicited craving in recently withdrawn cocaine dependent patients. J. Subst. Abus. Treat. 2004;27:45–49. doi: 10.1016/j.jsat.2004.03.009. http://dx.doi.org/10.1016/j.jsat.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Glaser PE, Hays LR, Rush CR. Alternative reinforcer response cost impacts cocaine choice in humans. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2012;36:189–193. doi: 10.1016/j.pnpbp.2011.10.003. http://dx.doi.org/10.1016/j.pnpbp.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Barrett AC, Negus SS, Caine SB. Cocaine versus food choice procedure in rats: environmental manipulations and effects of amphetamine. J. Exp. Anal. Behav. 2013;99:211–233. doi: 10.1002/jeab.15. http://dx.doi.org/10.1002/jeab.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNODC . World Drug Report 2015. United Nations Office on Drugs and Crime; Vienna: 2015. [Google Scholar]
- Wee S, Orio L, Ghirmai S, Cashman J, Koob G. Inhibition of kappa opioid receptors attenuated increased cocaine intake in rats with extended access to cocaine. Psychopharmacology (Berl) 2009;205:565–575. doi: 10.1007/s00213-009-1563-y. http://dx.doi.org/10.1007/s00213-009-1563-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL, Balster RL. Effects of antipsychotic compounds in rhesus monkeys given a choice between cocaine and food. Drug Alcohol Depend. 1981;8:69–78. doi: 10.1016/0376-8716(81)90088-0. [DOI] [PubMed] [Google Scholar]
- Young M, Clark M, Goffus A, Hoane M. Mixed effects modeling of morris water maze data: advantages and cautionary notes. Learn. Motiv. 2009;40:160–177. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.