Abstract
BACKGROUND
Limited literature suggests sexual dysfunction in women covaries with the metabolic syndrome. This study examined the association of sexual function with metabolic syndrome and cardiovascular disease in healthy older women.
METHODS
376 postmenopausal, community-dwelling women from the Rancho Bernardo Study (mean baseline age = 73) completed a clinic visit during 1999 – 2002 and returned the Female Sexual Function Index (FSFI) questionnaire mailed in 2002.
RESULTS
39% reported being sexually active; 41.5 % met a diagnosis of metabolic syndrome. The number of metabolic syndrome components was strongly associated with decreased sexual activity, desire, and low sexual satisfaction. Waist girth, diabetes, and hypertension were associated with decreased sexual activity. Elevated triglycerides were associated with low desire. Among the cardiovascular endpoints, heart attack, coronary artery bypass, and angina were associated with decreased sexual activity, but not with sexual desire or satisfaction. Past diagnosis of heart failure, poor circulation, and stroke were not associated with sexual function. Sexually active women with metabolic syndrome met criteria for sexual dysfunction in desire, arousal, orgasm, and satisfaction domains. The FSFI Total Score did not differ significantly between sexually active and inactive women.
CONCLUSIONS
Metabolic syndrome was associated with decreased sexual activity, desire, and satisfaction in all women and with sexual dysfunction in most domains in sexually active women. Coronary artery disease was more prevalent in women with low sexual activity.
Keywords: Metabolic syndrome, FSFI Female Sexual Function Index, Sexual dysfunction
INTRODUCTION
Worldwide interest in sexual medicine continues to grow, and the integration of sexual health as a vital sign was proposed1. The etiology of sexual dysfunction--in both sexes-- is not understood. Endothelial dysfunction from disturbances of smooth muscle contraction may explain the association between hypertension and male erectile dysfunction2, which has been described as a marker for cardiovascular disease. While an association of cardiovascular risk factors with female sexual dysfunction has been suggested 3, some have concluded that female sexual dysfunction is more strongly related to psychosocial factors 4, 5., Depression and marital status appear to be independent predictors of female sexual dysfunction 6. Studies report a bidirectional association between anxiety and depression and poor sexual function 7. 8
Studies showing an association between cardiovascular risk factors and sexual dysfunction include a report that successful control of hypertension was related to a lower prevalence of female sexual dysfunction9.
Premenopausal women with metabolic syndrome had lower Female Sexual Function Index (FSFI) total scores compared to those without10; lower sexual desire scores11; and those with hyperlipidemia reported more female sexual dysfunction12 One small case-control study reported more sexual dysfunction in postmenopausal women with metabolic syndrome; however, after correcting for education and relationships, only higher triglyceride levels contributed to the risk of sexual dysfunction13.
Two cross-sectional studies 4, 14 and one case control study15 reported more sexual dysfunction in women with diabetes, however, another study reported similar prevalence16. Among premenopausal women with type 1 diabetes who met a cut point for female sexual dysfunction, BMI, number of children, anxiety, social dysfunction, and depression were significant correlates of sexual dysfunction17. Insulin-treated diabetic women reported the lowest sexual satisfaction followed by non-insulin treated women followed by women without diabetes18. In another study age, metabolic syndrome, and atherogenic dyslipidemia were reported correlates of female sexual dysfunction in addition to depression and marital status6. The association between higher abdominal girth and sexual activity are mixed 17, 19, 20.
Sexual dysfunction was previously over-diagnosed because low sexual desire was classified as dysfunction. The FSFI questionnaire is the most commonly applied instrument to identify sexual problems within six main sexual function domains: desire, arousal, lubrication, orgasm, pain, and satisfaction. Both a domain score and total score can be calculated using prespecified cut points to identify sexual dysfunction. The FSFI Total Score was originally designed for sexually active women; when the index was applied to women regardless of sexual activity, a zero score was inserted for those domains that pertain to sexual activity. Thus a falsely low total score and an incorrect diagnosis of sexual dysfunction can result from sexual inactivity or lack of an intimate partner 21, 22. Lowering FSFI total score cut points for sexual dysfunction has been proposed, because the majority of healthy controls in some populations have been diagnosed with dysfunction using current thresholds. In the most recent edition of the Diagnostic and Statistical Manual of Mental Disorders DSM-523, sexual desire and sexual arousal domains have been combined.
The present study was designed to better describe the association of impaired glucose tolerance, diabetes, hypertension, dyslipidemia, abdominal girth, and metabolic syndrome with sexual activity, desire, and satisfaction in relatively healthy, community-dwelling older women, as well as with the sexual function domains of desire, arousal, lubrication, orgasm, pain, and satisfaction in sexually active women.
MATERIALS AND METHODS
Study Population
The Rancho Bernardo Study (RBS) included 82% of community-dwelling adult residents, mostly couples, who had moved to Rancho Bernardo, a suburb north of San Diego, California. Since the study’s inception in 1972–1974, surviving participants have been followed annually for vital status and morbidity and every other year for specific conditions or behaviors related to cardiovascular disease and healthy aging. The study was approved by the institutional review board of the University of California, San Diego. Mailed questionnaires reminded participants that responses were voluntary and that they did not need to answer any questions they preferred not to answer.
Measures
Between 1999 and 2002, 678 RBS women visited the clinic and provided morning blood samples. Height and weight were recorded with women wearing lightweight clothing without shoes. Waist circumference in centimeters was measured at the minimum waist between the last rib and superior iliac crest; if, due to excessive girth in the abdomen, the individual’s minimum waist was at the iliac crest, the bending point at the umbilicus was measured. Blood pressure was recorded in seated subjects Assays for hemoglobin A1C, HDL, and triglycerides were performed at the Veterans Affairs San Diego Healthcare System hospital laboratory using established commercial assays routinely monitored by external quality-control programs.
In October 2002, 1303 surviving community-dwelling RBS women were mailed a questionnaire about physical and emotional health, menopause, hysterectomy status, current estrogen use, physician diagnosed angina pectoris, myocardial infarction, stroke, transient ischemia attack, and claudication, and the presence or absence of an intimate partner. The FSFI was mailed in the same envelope with the RBS identifier but no personal identifiers.
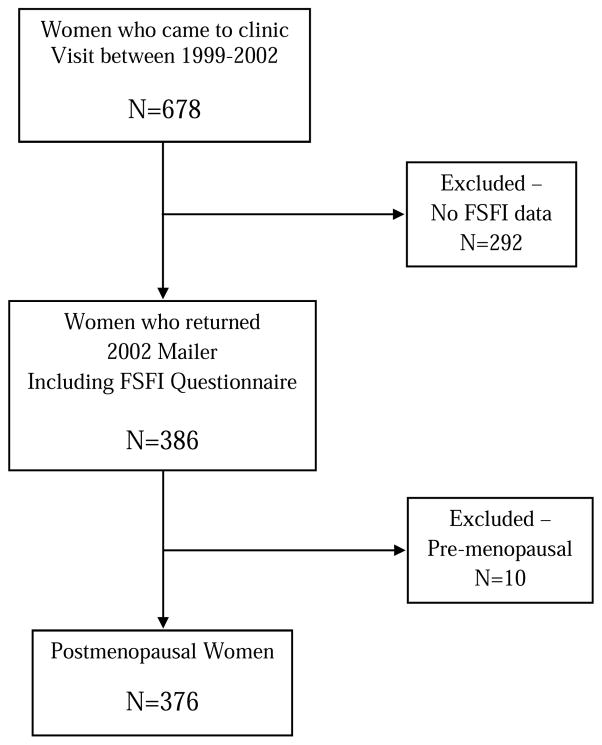
The present analysis included all 376 postmenopausal women ≥ 40 years of age who had visited our clinic between 1999 and 2002 and returned the 2002 FSFI questionnaire and answered the questions about recent sexual activity with or without a partner. Figure 1 provides a flow diagram of the study population.
Figure 1.
Rancho Bernardo Study Flow Diagram
Assessment of Sexual Activity
The Female Sexual Function Index (FSFI) is a 19-item multidimensional measure of female sexual function with each question scored in an ordinal Likert response of 0 to 5 for a maximum total score of 36. FSFI subscale analysis can be calculated using a subscale total in each domain (desire, arousal, lubrication, orgasm, pain, and satisfaction) multiplied by a domain factor for a maximum score of 6 24. The FSFI was initially validated in women with female sexual arousal disorder with demonstrated strong reliability (Cronbach α = .97, range .89 – .96). The cut point for the diagnosis of Female Sexual Dysfunction is an FSFI Total Score ≤ 26.55 25 or an FSFI Domain Score ≤ 4.3 26 We added one additional question (Q3): “Over the past 4 weeks, have you engaged in any sexual activity or intercourse?” yielding a total of 20 questions (Appendix 1).
Sexual desire, activity, and satisfaction were analyzed for all respondents regardless of sexual activity. Arousal, lubrication, orgasm, pain, and the calculation of FSFI Total Score were analyzed only for sexually active women.
Assessment of Metabolic Syndrome
The diagnosis of metabolic syndrome was based on several different guidelines including the World Health Organization, the National Cholesterol Education Program Adult Treatment Panel III (NCEP: ATP III), the International Diabetes Federation, and the American Association of Clinical Endocrinology. Metabolic syndrome was defined as meeting at least 3 of 5 criteria including impaired glucose tolerance; waist circumference > 88 cm; triglycerides ≥ 150 mg/dL; HDL cholesterol < 50 mg/dL; SBP ≥ 130 mm Hg or DBP ≥ 85 mm Hg or a diagnosis of hypertension or current antihypertensive medication use. Impaired glucose tolerance (IGT + DM) was defined as Hgb A1C ≥ 5.7 mg/dL, physician diagnosis of diabetes, or use of diabetes-specific medication--oral or insulin.
Statistical Analysis
Data from 376 postmenopausal women age ≥40 years who returned the questionnaire and participated in RBS clinic visit 9 provides the basis of this report. Sexual activity was defined as a yes answer to Q3 of modified FSFI, sexual desire defined as response 3,4,5 to Q1 (felt desire ≥ half the time), sexual satisfaction defined as 3,4,5 to Q20 (felt satisfaction ≥ half the time). Results are presented as means ± standard deviations of normally distributed continuous variables with t tests used to evaluate significant differences. Non-normally distributed continuous variables are presented as medians (interquartile range) and differences between the groups are assessed using the Wilcoxon-Mann-Whitney test. Categorical variables are shown as numbers and percentages and associations are tested with a Chi square test (or Fisher’s exact test) as appropriate, based on expected cell frequencies. Data are analyzed using SAS (version 9.4; SAS Institute, Inc., Cary, NC) and SPSS (version 17.0; SPSS, Inc., Chicago, IL).
RESULTS
Baseline characteristics of all 376 women are summarized in Table 1. The mean baseline age was 73 years (range 64 – 82), and the mean number of years postmenopause was 26.6. About 60% were living with a spouse or a partner. Almost three-quarters of the women had at least begun college; more than 90% of the (male) heads of household were executives/professionals. More than half had never smoked (n = 196); only 3.5 % (n= 13) were current smokers. Almost 90% of women reported at least good physical/emotional health
Table 1.
Baseline characteristics of all women
| Characteristic | Overall (N=376) |
|---|---|
| Age, y | 72.9 ± 9.4 |
| Years post menopause | 26.6 ± 12.5 |
| Marital Status | |
| Living with spouse/partner | 226 (60.1%) |
| Widowed | 106 (28.2%) |
| Other | 44 (11.7%) |
| Some college | 262 (72.0%) |
| Occupation | |
| Executives/professionals | 283 (90.7%) |
| Skilled/semi-skilled | 26 (8.3%) |
| Other | 3 (1.0%) |
| Smoking | |
| Never | 196 (52.1%) |
| Past | 167 (44.4%) |
| Current | 13 (3.5%) |
| Self-reported physical health | |
| Excellent | 47 (12.8%) |
| Very good | 152 (41.6%) |
| Good | 120 (32.8%) |
| Fair/poor | 47 (12.8%) |
| Self-reported emotional health | |
| Excellent | 78 (21.3%) |
| Very good | 149 (40.7%) |
| Good | 107 (29.2%) |
| Fair/poor | 32 (8.7%) |
| BMI, kg/m2 | 25.8 ± 4.6 |
| Waist, cm | 83.3 ± 11.4 |
| HDL, mg/dL | 67.5 ± 17.2 |
| Triglycerides, mg/dL | 134.0 (93.0) |
| Diabetes | 58 (15.4%) |
| Hypertension | 286 (76.1%) |
| IGT | 226 (60.1%) |
| DM + IGT | 284 (75.5%) |
| Metabolic Syndrome | 156 (41.5%) |
| Number of MetS Components | |
| 0 | 17 (4.5%) |
| 1 | 67 (17.8%) |
| 2 | 136 (36.2%) |
| 3 | 85 (22.6%) |
| 4 | 54 (14.4%) |
| 5 | 17 (4.5%) |
| CVD Endpoints | |
| HA | 18 (5.0%) |
| CABG | 20 (5.6%) |
| HF | 7 (1.9%) |
| Angina | 17 (4.7%) |
| Poor arterial circulation | 28 (7.9%) |
| Stroke | 6 (1.7%) |
| TIA | 23 (6.4%) |
Mean ± standard deviation is presented for normally distributed continuous variables and median (interquartile range) for non-normally distributed variables. Categorical variables are shown as N (%). Definitions: Diabetes => HbA1c ≥ 6.5 or history or meds; Hypertension => SBP ≥ 130 or DBP ≥ 85 or history or meds; IGT => 5.7 ≤ HbA1c < 6.5 and No Diabetes (as defined above); IGT + DM => Diabetes (as defined above) or IGT (as defined above).
The average BMI was 25.8 kg/m2; mean waist circumference was 83.3 cm (32.8 inches). Mean HDL was 67.5 mg/dL, and median triglycerides were 134 mg/dL. Fifteen percent of women had diabetes (n=58), 76% were hypertensive (n = 286), and 60% had impaired glucose tolerance (n=226); three quarters met metabolic syndrome criteria for glucose intolerance (DM + IGT).
Forty two percent (n = 156) met metabolic syndrome criteria; 23% (n = 85) met 3 of 5 criteria, 14% (n = 54) met 4 of 5 criteria, and 5% (n = 17) met 5 criteria.
Clinical and biochemical parameters, number of metabolic syndrome criteria met, diagnosis of metabolic syndrome, and cardiovascular disease endpoints in all women by sexual activity, sexual desire, and sexual satisfaction are presented in Table 2. Sexual activity within the past 4 weeks was reported by about 39% and was associated with older age and living with a spouse or partner, as we previously reported 19; sexual desire was reported by 23.5% (87/370) and sexual satisfaction by 78% (199/255). Older age and years postmenopause were associated with a decrease in sexual activity and sexual desire but not sexual satisfaction 19.
Table 2.
Metabolic syndrome components and cardiovascular disease endpoints in all women
| Sexual Activity | Sexual Desire | Sexual Satisfaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes N=148 |
No N=228 |
P value | Yes N=87 |
No N=283 |
P value | Yes N=199 |
No N=56 |
P value | |
| Age, y | 67.9 ± 8.0 | 76.2 ± 8.8 | <0.0001 | 69.3 ± 8.5 | 74.0 ± 9.4 | <0.0001 | 70.5 ± 8.6 | 69.9 ± 9.6 | 0.6605 |
| Years post menopause | 21.7 ± 11.0 | 29.7 ± 12.4 | <0.0001 | 23.6 ± 11.8 | 27.5 ± 12.6 | 0.0193 | 23.6 ± 10.9 | 22.4 ± 13.3 | 0.5592 |
| Marital Status | <0.0001 | 0.0016 | 0.1348 | ||||||
| Live w/spouse/partner | 117 (79.1%) | 109 (47.8%) | 65 (74.7%) | 156 (55.1%) | 151 (75.9%) | 38 (67.8%) | |||
| Widowed | 17 (11.5%) | 89 (39.0%) | 12 (13.8%) | 93 (32.9%) | 31 (15.6%) | 8 (14.3%) | |||
| Other | 14 (9.4%) | 30 (13.2%) | 10 (11.5%) | 34 (12.0%) | 17 (8.5%) | 10 (17.9%) | |||
| BMI, kg/m2 | 25.6 ± 4.4 | 26.0 ± 4.7 | 0.3958 | 25.9 ± 5.1 | 25.8 ± 4.4 | 0.8761 | 26.0 ± 4.7 | 26.9 ± 5.0 | 0.1746 |
| Waist, cm | 81.5 ± 10.5 | 84.5 ± 11.8 | 0.0125 | 81.5 ± 12.7 | 83.8 ± 11.0 | 0.0946 | 83.0 ± 11.3 | 85.8 ± 11.4 | 0.1032 |
| HDL, mg/dL | 67.6 ± 16.8 | 67.4 ± 17.5 | 0.9047 | 69.2 ± 16.8 | 67.3 ± 17.3 | 0.3527 | 67.8 ± 17.1 | 65.6 ± 17.8 | 0.4071 |
| Triglycerides, mg/dL | 132.0 (94.0) | 135.5 (92.0) | 0.5316 | 121.0 (73.0) | 137.0 (95.0) | 0.0141 | 131.0 (92.0) | 135.5 (105.5) | 0.3242 |
| Diabetes | 13 (8.8%) | 45 (19.7%) | 0.0041 | 10 (11.5%) | 48 (17.0%) | 0.2200 | 29 (14.6%) | 6 (10.7%) | 0.4585 |
| Hypertension | 92 (62.2%) | 194 (85.1%) | <0.0001 | 62 (71.3%) | 218 (77.0%) | 0.2728 | 141 (70.9%) | 42 (75.0%) | 0.5426 |
| IGT | 83 (56.1%) | 143 (62.7%) | 0.1991 | 43 (49.4%) | 179 (63.2%) | 0.0213 | 113 (56.8%) | 35 (62.5%) | 0.4438 |
| DM + IGT | 96 (64.9%) | 188 (82.5%) | 0.0001 | 53 (60.9%) | 227 (80.2%) | 0.0002 | 142 (71.4%) | 41 (73.2%) | 0.7850 |
| Metabolic Syndrome | 46 (31.1%) | 110 (48.3%) | 0.0010 | 22 (25.3%) | 129 (45.6%) | 0.0008 | 74 (37.2%) | 24 (42.9%) | 0.4409 |
| Number of MetS - components | <0.0001 | 0.0002 | 0.0192 | ||||||
| 0 | 12 (8.1%) | 5 (2.2%) | 6 (6.9%) | 11 (3.9%) | 13 (6.5%) | 0 (0.0%) | |||
| 1 | 46 (31.1%) | 21 (9.2%) | 29 (33.3%) | 37(13.1%) | 38 (19.1%) | 16 (28.6%) | |||
| 2 | 44 (29.7%) | 92 (40.3%) | 30 (34.5%) | 106 (37.5%) | 74 (37.2%) | 16 (28.6%) | |||
| 3 | 23 (15.5%) | 62 (27.2%) | 11 (12.6%) | 71 (25.1%) | 43 (21.6%) | 8 (14.3%) | |||
| 4 | 14 (9.5%) | 40 (17.6%) | 7 (8.1%) | 46 (16.3%) | 26 (13.1%) | 11 (19.6%) | |||
| 5 | 9 (6.1%) | 8 (3.5%) | 4 (4.6%) | 12 (4.2%) | 5 (2.5%) | 5 (8.9%) | |||
| CVD Endpoints | |||||||||
| Heart attack | 3 (2.1%) | 15 (6.9%) | 0.0403 | 1 (1.2%) | 16 (5.9%) | 0.0856 | 7 (3.6%) | 2 (3.6%) | 1.0000 |
| CABG | 3 (2.1%) | 17 (7.9%) | 0.0184 | 2 (2.4%) | 17 (6.3%) | 0.2665 | 11 (5.6%) | 0 (0.0%) | 0.1299 |
| Heart failure | 1 (0.7%) | 6 (2.8%) | 0.2502 | 1 (1.2%) | 5 (1.9%) | 1.0000 | 3 (1.6%) | 0 (0.0%) | 1.0000 |
| Angina | 2 (1.4%) | 15 (6.9%) | 0.0149 | 1 (1.2%) | 15 (5.5%) | 0.1317 | 7 (3.6%) | 1 (1.8%) | 0.6879 |
| Poor arterial circulation | 8 (5.6%) | 20 (9.5%) | 0.1742 | 5 (5.9%) | 23 (8.7%) | 0.4035 | 14 (7.2%) | 2 (3.6%) | 0.5353 |
| Stroke | 3 (2.1%) | 3 (1.4%) | 0.6868 | 0 (0.0%) | 5 (1.9%) | 0.3434 | 3 (1.6%) | 0 (0.0%) | 1.0000 |
| TIA | 6 (4.2%) | 17 (7.8%) | 0.1656 | 4 (4.7%) | 18 (6.6%) | 0.5035 | 9 (4.6%) | 3 (5.4%) | 0.7340 |
Mean ± standard deviation is presented for normally distributed continuous variables and associated p values are from t test. Median (interquartile range) for non-normally distributed variables with p values from Wilcoxon-Mann-Whitney test. Categorical variables are shown as N (%) and a chi-square or Fisher’s test was performed. Definitions: Diabetes => HbA1c ≥ 6.5 or history or meds; Hypertension => SBP ≥ 130 or DBP ≥ 85 or history or meds; IGT => 5.7 ≤ HbA1c < 6.5 and No Diabetes (as defined above); IGT + DM => Diabetes (as defined above) or IGT (as defined above).
Compared to sexually active women, non-sexually active women had significantly larger waist circumferences, but similar BMI. Diabetes and hypertension, but not impaired glucose tolerance (A1C >= 5.7 < 6.5), were associated with low sexual activity.
The combination of diabetes and impaired glucose tolerance was associated with sexual desire. Women with no sexual desire had significantly higher triglyceride levels than women who reported sexual desire.
None other clinical and biochemical parameters were associated with sexual satisfaction. The number of metabolic syndrome criteria and the diagnosis of metabolic syndrome were strongly associated with sexual desire and sexual activity. Almost half (48.3%, n = 110) of sexually inactive women met the criteria for the metabolic syndrome diagnosis compared to less than one-third (31.1 %, n = 46) of the sexually active women (p = .001). Close to half (45.6%, n = 129) of the women with no sexual desire met the criteria for MetS compared to about a quarter of the women with sexual desire (p = .0008).
Of the cardiovascular disease endpoints studied, only heart attack, coronary bypass surgery, and angina were associated with sexual activity (Table 2). There was no association of heart failure, poor arterial circulation, or stroke with sexual activity. No association was found between the cardiovascular disease endpoints and sexual desire or satisfaction.
Table 3 shows median and Interquartile Range (IQR), FSFI domain score and FSFI Total Score in sexually active women with and without metabolic syndrome. N (%) refers to the number of women who met or were below the cut point for sexual dysfunction based on individual domain score (≤ 4.3) or FSFI total score (≤ 26.55). FSFI domain, median (IQR), and N (%) meeting the cut point for dysfunction were calculated and compared between sexually active women with and without metabolic syndrome. 87.5% and 48.0% of all sexually active women met the cut point for desire and arousal dysfunction respectively.
Table 3.
Median and Interquartile Range (IQR), FSFI domain score, and FSFI Total Score in sexually active women with and without MetS
| Overall (N=148) | With MetS (N=46) | Without MetS (N=102) | P-value | |
|---|---|---|---|---|
| Desire (N=144) | ||||
| Median (IQR) | 3.6 (1.2) | 3.0 (1.2) | 3.6 (1.2) | 0.0401 |
| N (%) | 126 (87.5%) | 39 (92.9%) | 87 (85.3%) | 0.2123 |
| Arousal (N=146) | ||||
| Median (IQR) | 4.5 (2.1) | 3.8 (2.4) | 4.8 (1.8) | 0.0086 |
| N (%) | 70 (48.0%) | 28 (60.9%) | 42 (42.0%) | 0.0340 |
| Desire + Arousal (N=142) | ||||
| Median (IQR) | 7.8 (3.3) | 6.9 (3.3) | 8.1 (2.4) | 0.0047 |
| N (%) | ||||
| Lubrication (N=133) | ||||
| Median (IQR) | 4.8 (2.4) | 4.4 (2.4) | 5.1 (2.4) | 0.2858 |
| N (%) | 55 (41.4%) | 21 (50.0%) | 34 (37.4%) | 0.1689 |
| Orgasm (N=142) | ||||
| Median (IQR) | 4.8 (2.4) | 4.4 (2.8) | 5.2 (2.0) | 0.0427 |
| N (%) | 50 (35.2%) | 22 (50.0%) | 28 (28.6%) | 0.0134 |
| Pain (N=108) | ||||
| Median (IQR) | 6.0 (1.2) | 6.0 (0.8) | 6.0 (1.2) | 0.3876 |
| N (%) | 17 (15.7%) | 4 (11.8%) | 13 (17.6%) | 0.4419 |
| Satisfaction (N=117) | ||||
| Median (IQR) | 5.6 (1.6) | 4.8 (2.4) | 5.6 (1.2) | 0.1427 |
| N (%) | 26 (22.2%) | 14 (40.0%) | 12 (14.6%) | 0.0025 |
| FSFI Total (N=97) | ||||
| Median (IQR) | 27.6 (8.5) | 26.7 (8.7) | 28.7 (6.6) | 0.1419 |
| N (%) | 38 (39.2%) | 14 (50.0%) | 24 (34.8%) | 0.1641 |
Median (interquartile range) with p values from Wilcoxon-Mann-Whitney test. Categorical variables shown as N (%) and the chi-square or Fisher’s exact test performed as appropriate. N (%) for each domain represents the number categorized as dysfunctional (≤ 4.3). FSFI Total represents the number who fell below the cut point for female sexual dysfunction (≤ 26.55).
Women with metabolic syndrome had significantly lower sexual desire (p=.0401) and lower arousal (p=.0086) scored both individually and combined (p=.0047) compared to women without metabolic syndrome. A lower orgasm frequency was reported by women with metabolic syndrome (p=.0427), with a higher the prevalence of orgasm dysfunction (p=.0134). Satisfaction scores also met a cut point for dysfunction in metabolic syndrome (p=.0025); lubrication and pain domains did not differ between groups for associations with metabolic syndrome.
In this cohort almost 40% of sexually active women met the FSFI criteria for sexual dysfunction with a Total Score ≤ 26.55. The prevalence of sexual dysfunction by FSFI Total Score in women with metabolic syndrome was 50% compared to 34.8% in those without which was not statistically significant.
DISCUSSION
In these healthy community-dwelling older women, the prevalence of low sexual activity and low sexual desire was significantly higher in women who met the diagnostic criteria for metabolic syndrome based on multiple criteria. In addition, we observed a higher prevalence of dysfunction by FSFI domain criteria in desire, arousal, orgasm, and satisfaction, comparing sexually active women with metabolic syndrome to those without. The number of metabolic syndrome criteria was strongly associated with sexual activity, sexual desire, and sexual satisfaction, suggesting a cumulative association of cardiovascular risk factors with sexuality. Prevention of chronic disease and optimization of health may preserve sexual activity and satisfaction.
The prevalence of low sexual activity and low sexual desire was much higher in diabetic women compared to those without diabetes; diabetic women have previously been reported to meet FSFI dysfunction criteria compared to women without diabetes14, 15, 27. High waist circumference was associated with low sexual activity. Elevated triglycerides were associated with low sexual desire. Higher triglycerides have been previously reported to be associated with lower FSFI Total Score and sexual dysfunction in both pre- and post-menopausal women 12, 13. Waning estrogen levels in the perimenopause and an increase in visceral fat have been reported to be associated with regional changes in lipoprotein lipase activity leading to a preferential distribution of visceral over subcutaneous fat28, 29. Perimenopausal lipoprotein lipase changes could also lead to an increase in triglycerides and insulin resistance contributing to cardiovascular risk. Decreasing estrogen levels have also been reported to precede a decrease in SHBG and testosterone 30, which may decrease sexual desire and/or sexual activity. Therefore the decrease in endogenous estrogen during the perimenopause may be linked both to a decrease in sexual function and to an increase in cardiovascular risk.
About 75% of these community-dwelling women were hypertensive comparable to prevalence observed in NHANES31. Partnered sexual activity has previously been reported to be lower in both treated and untreated hypertensive women compared to women without hypertension; “lacking interest” was additionally reported as a sexual problem in nearly half of these women but did not differ significantly between those with hypertension and those without 32. Similarly, in Rancho Bernardo women, sexual activity was much lower in those with hypertension compared to those without; low sexual desire, reported by the majority of women, did not differ between those with and without hypertension in the Rancho Bernardo cohort.
Low HDL, analyzed independently, was not associated with sexual function in this RBS study.
Angina, heart attack, and coronary bypass surgery were associated with low sexual activity; heart failure, poor arterial circulation, and stroke were not. We observed no association between cardiovascular disease and sexual desire or sexual satisfaction. Other studies have reported a decrease in sexual function in women with coronary artery disease 33–35. Older women with heart disease have been reported to engage in sexual activity 36, 37, and targeted sexual counseling efforts may improve health 38. Metabolic syndrome in women may be more closely related to coronary artery disease than other cardiovascular outcomes.
In summary, we have shown that metabolic syndrome is associated with female sexual dysfunction in community-dwelling older women. The high level of reported emotional and physical health and the homogeneity by race, education, and male head of household status limit generalizability to other populations. Classification of dysfunction in this study was made on the basis of FSFI domain analysis and FSFI Total Score. The Female Sexual Distress Scale (FSDS) was not used in this study. Using both indices might have improved diagnostic accuracy.
The strength of this study is the combined analysis of sexual activity, desire, and satisfaction in all women in our cohort, and FSFI Total Score and domain calculations in sexually active women only. Impaired glucose tolerance plus diabetes mellitus were used as the criteria for metabolic syndrome. In addition, we report a separate analysis for impaired glucose tolerance and diabetes (Table 2).
A clear association between sexual function and mood disorders has been reported in recent studies.7, 8 In addition to psychosocial factors, coronary artery disease and sexual function may share other common pathways and pathophysiology. Consistent integration of mental and sexual health with other medical disciplines could improve understanding of the links between cardiovascular disease risk factors with sexual function decline with age.
The present study confirms correlation between metabolic syndrome and sexual function in older women. The prevalence of low sexual activity and low sexual desire was higher in community-dwelling women based on the number of metabolic syndrome components and by the diagnosis of metabolic syndrome. Coronary artery disease endpoints were associated with low sexual activity. Sexually active women with metabolic syndrome had more sexual dysfunction in most domains. Overlapping pathways affecting sexual function in women are complex and still poorly understood; however, both physiological and psychological variables contribute to sexual activity and function.
Low sexual activity was associated with metabolic syndrome diagnosis in all women.
Low sexual desire was associated with metabolic syndrome diagnosis in all women.
Metabolic syndrome was associated with low desire, arousal, orgasm, and satisfaction
Angina, heart attack, and coronary bypass surgery were associated with low sexual activity.
Acknowledgments
The Rancho Bernardo Study has been supported by National Institutes of Health/National Institute on Aging grants AG07181 and AG028507 and the National Institute of Diabetes and Digestive and Kidney Diseases, grant DK31801. This financial support does not represent a conflict of interest; the funding sources had no involvement in study design, collection, analysis, and interpretation of data, writing of the paper, or decision to submit for publication.
Appendix 1. Modified Female Sexual Function Index
How often did you feel sexual desire or interest?
How would you rate your level (degree) of sexual desire or interest?
-
Over the past 4 weeks, have you engaged in any sexual activity or intercourse?
Respondents who answered no were instructed to skip to #19.
How often did you feel sexually aroused during sexual activity or intercourse?
How would you rate your level of sexual arousal during sexual activity or intercourse?
How confident were you about becoming sexually aroused during sexual activity or intercourse?
How often have you been satisfied with your arousal during sexual activity or intercourse?
How often did you become lubricated during sexual activity or intercourse?
How difficult was it to become lubricated during sexual activity or intercourse?
How often did you maintain your lubrication until completion of sexual activity or intercourse?
How difficult was it to maintain your lubrication until completion of sexual activity or intercourse?
When you had sexual stimulation or intercourse, how often did you reach orgasm?
When you had sexual stimulation or intercourse, how difficult was it for you to reach orgasm?
-
How satisfied were you with your ability to reach orgasm during sexual activity or intercourse?
Respondents who do not have a partner were instructed to skip to question #20.
How satisfied have you been with the amount of emotional closeness during sexual activity between you and your partner?
How would you rate your level (degree) of discomfort or pain during or following vaginal penetration?
How often did you experience discomfort or pain during vaginal penetration?
How often did you experience discomfort or pain following vaginal penetration?
How satisfied have you been with your sexual relationship with your partner?
How satisfied have you been with your overall sexual life?
Most responses used a five point Likert scale. Frequency responses – Almost always or always: 5 points, Most times (more than half of the time): 4 points, Sometimes (about half of the time): 3 points, A few times (less than half of the time): 2 points, Almost never or never: 1 point. Level responses – Very high: 5 points, High: 4 points, Moderate: 3 points, Low: 2 points, Very low or none at all: 1 point.
Footnotes
Verification of authorship: All authors had access to the data and had a role in writing the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Susan E. Trompeter, Email: Susan.Trompeter@va.gov.
Ricki Bettencourt, Email: rbettencourt@ucsd.edu.
Elizabeth Barrett-Connor, Email: ebarrettconnor@ucsd.edu.
References
- 1.National Health Care for the Homeless Council Midwest Regional Health and Housing Meeting. Chicago: National LGBT Health and Education Center; 2013. Integrating Sexual Health as a Vital Sign for Improved Patient Health and Patient Engagement. [Google Scholar]
- 2.Nunes KP, Labazi H, Webb RC. New insights into hypertension-associated erectile dysfunction. Curr Opin Nephrol Hypertens. 2012;21:163–170. doi: 10.1097/MNH.0b013e32835021bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miner M, Esposito K, Guay A, Montorsi P, Goldstein I. Cardiometabolic risk and female sexual health: the Princeton III summary. J Sex Med. 2012;9:641–651. doi: 10.1111/j.1743-6109.2012.02649.x. quiz 652. [DOI] [PubMed] [Google Scholar]
- 4.Enzlin P, Rosen R, Wiegel M, et al. Sexual dysfunction in women with type 1 diabetes: long-term findings from the DCCT/EDIC study cohort. Diabetes Care. 2009;32:780–785. doi: 10.2337/dc08-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim YH, Kim SM, Kim JJ, Cho IS, Jeon MJ. Does metabolic syndrome impair sexual function in middle- to old-aged women? J Sex Med. 2011;8:1123–1130. doi: 10.1111/j.1743-6109.2010.02174.x. [DOI] [PubMed] [Google Scholar]
- 6.Esposito K, Maiorino MI, Bellastella G, Giugliano F, Romano M, Giugliano D. Determinants of female sexual dysfunction in type 2 diabetes. Int J Impot Res. 2010;22:179–184. doi: 10.1038/ijir.2010.6. [DOI] [PubMed] [Google Scholar]
- 7.Atlantis E, Sullivan T. Bidirectional association between depression and sexual dysfunction: a systematic review and meta-analysis. J Sex Med. 2012;9:1497–1507. doi: 10.1111/j.1743-6109.2012.02709.x. [DOI] [PubMed] [Google Scholar]
- 8.Kalmbach DA, Kingsberg SA, Ciesla JA. How changes in depression and anxiety symptoms correspond to variations in female sexual response in a nonclinical sample of young women: a daily diary study. J Sex Med. 2014;11:2915–2927. doi: 10.1111/jsm.12692. [DOI] [PubMed] [Google Scholar]
- 9.Doumas M, Tsiodras S, Tsakiris A, et al. Female sexual dysfunction in essential hypertension: a common problem being uncovered. J Hypertens. 2006;24:2387–2392. doi: 10.1097/01.hjh.0000251898.40002.5b. [DOI] [PubMed] [Google Scholar]
- 10.Esposito K, Ciotola M, Marfella R, Di Tommaso D, Cobellis L, Giugliano D. The metabolic syndrome: a cause of sexual dysfunction in women. Int J Impot Res. 2005;17:224–226. doi: 10.1038/sj.ijir.3901310. [DOI] [PubMed] [Google Scholar]
- 11.Ponholzer A, Temml C, Rauchenwald M, Marszalek M, Madersbacher S. Is the metabolic syndrome a risk factor for female sexual dysfunction in sexually active women? Int J Impot Res. 2008;20:100–104. doi: 10.1038/sj.ijir.3901605. [DOI] [PubMed] [Google Scholar]
- 12.Esposito K, Ciotola M, Maiorino MI, et al. Hyperlipidemia and sexual function in premenopausal women. J Sex Med. 2009;6:1696–1703. doi: 10.1111/j.1743-6109.2009.01284.x. [DOI] [PubMed] [Google Scholar]
- 13.Martelli V, Valisella S, Moscatiello S, et al. Prevalence of sexual dysfunction among postmenopausal women with and without metabolic syndrome. J Sex Med. 2012;9:434–441. doi: 10.1111/j.1743-6109.2011.02517.x. [DOI] [PubMed] [Google Scholar]
- 14.Abu Ali RM, Al Hajeri RM, Khader YS, Shegem NS, Ajlouni KM. Sexual dysfunction in Jordanian diabetic women. Diabetes Care. 2008;31:1580–1581. doi: 10.2337/dc08-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yencilek F, Attar R, Erol B, et al. Factors affecting sexual function in premenopausal age women with type 2 diabetes: a comprehensive study. Fertil Steril. 2010;94:1840–1843. doi: 10.1016/j.fertnstert.2009.10.060. [DOI] [PubMed] [Google Scholar]
- 16.Tagliabue M, Gottero C, Zuffranieri M, et al. Sexual function in women with type 1 diabetes matched with a control group: depressive and psychosocial aspects. J Sex Med. 2011;8:1694–1700. doi: 10.1111/j.1743-6109.2011.02262.x. [DOI] [PubMed] [Google Scholar]
- 17.Dimitropoulos K, Bargiota A, Mouzas O, Melekos M, Tzortzis V, Koukoulis G. Sexual functioning and distress among premenopausal women with uncomplicated type 1 diabetes. J Sex Med. 2012;9:1374–1381. doi: 10.1111/j.1743-6109.2012.02664.x. [DOI] [PubMed] [Google Scholar]
- 18.Copeland KL, Brown JS, Creasman JM, et al. Diabetes mellitus and sexual function in middle-aged and older women. Obstet Gynecol. 2012;120:331–340. doi: 10.1097/AOG.0b013e31825ec5fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trompeter S, Bettencourt R, Barrett-Connor E. Sexual Activity and Satisfaction in Healthy Community-dwelling Older Women. American Journal of Medicine. 2012;125:37–45. doi: 10.1016/j.amjmed.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mozafari M, Khajavikhan J, Jaafarpour M, Khani A, Direkvand-Moghadam A, Najafi F. Association of body weight and female sexual dysfunction: a case control study. Iran Red Crescent Med J. 2015;17:e24685. doi: 10.5812/ircmj.24685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer-Bahlburg HF, Dolezal C. The female sexual function index: a methodological critique and suggestions for improvement. J Sex Marital Ther. 2007;33:217–224. doi: 10.1080/00926230701267852. [DOI] [PubMed] [Google Scholar]
- 22.Trompeter SEBR, Barrett-Connor E. Limitations of the Femal Sexual Function Index Total Score in Healthy Community-dwelling Older Women. In: Frederique C, editor. Sexual Dysfunctions: Risk Factors, Psychological Impact and Treatment. New York: Nova Science Pub Inc; 2013. p. 280. [Google Scholar]
- 23.Female Sexual Interest/Arousal Disorder. Diagnostic and Statistical Manual of Mental Disorders Fifth Edition. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 24.Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): A Multidimensional Self-Report Instrument for the Assessment of Female Sexual Function. Journal of Sex and Marital Therapy. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 25.Wiegel M, Meston C, Rosen R. The female sexual function index (FSFI): cross-validation and development of clinical cutoff scores. J Sex Marital Ther. 2005;31:1–20. doi: 10.1080/00926230590475206. [DOI] [PubMed] [Google Scholar]
- 26.Meston CM. Validation of the Female Sexual Function Index (FSFI) in women with female orgasmic disorder and in women with hypoactive sexual desire disorder. J Sex Marital Ther. 2003;29:39–46. doi: 10.1080/713847100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giugliano F, Maiorino MI, Di Palo C, et al. Adherence to Mediterranean diet and sexual function in women with type 2 diabetes. J Sex Med. 2010;7:1883–1890. doi: 10.1111/j.1743-6109.2010.01714.x. [DOI] [PubMed] [Google Scholar]
- 28.Petersen S, Dristensen K, Hermann P, Katzenellenbogen J, Richelsen B. Estrogen controls lipolysis by up-regulating alpha2A-adrenertgic receptors directly in human adipose tissue through the estrognen receptor alpha. Implications for the female fat distribution. J Clin Endocrinol Metab. 2004;89:1869–1878. doi: 10.1210/jc.2003-031327. [DOI] [PubMed] [Google Scholar]
- 29.Lovejoy J, Champagne C, de Jonge L, Xie H, Smith S. Increased visceral fat and decreased energy expenditure during the menopausal transition. In J Obes (Lond) 2008;32:949–958. doi: 10.1038/ijo.2008.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wildman RP, Tepper PG, Crawford S, et al. Do changes in sex steroid hormones precede or follow increases in body weight during the menopause transition? Results from the Study of Women’s Health Across the Nation. J Clin Endocrinol Metab. 2012;97:E1695–1704. doi: 10.1210/jc.2012-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303:2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 32.Spatz ES, Canavan ME, Desai MM, Krumholz HM, Lindau ST. Sexual activity and function among middle-aged and older men and women with hypertension. J Hypertens. 2013;31:1096–1105. doi: 10.1097/HJH.0b013e32835fdefa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaya C, Yilmaz G, Nurkalem Z, Ilktac A, Karaman MI. Sexual function in women with coronary artery disease: a preliminary study. Int J Impot Res. 2007;19:326–329. doi: 10.1038/sj.ijir.3901530. [DOI] [PubMed] [Google Scholar]
- 34.Eyada M, Atwa M. Sexual function in female patients with unstable angina or non-ST-elevation myocardial infarction. J Sex Med. 2007;4:1373–1380. doi: 10.1111/j.1743-6109.2007.00473.x. [DOI] [PubMed] [Google Scholar]
- 35.Drory Y, Kravetz S, Weingarten M. Comparison of sexual activity of women and men after a first acute myocardial infarction. Am J Cardiol. 2000;85:1283–1287. doi: 10.1016/s0002-9149(00)00756-6. [DOI] [PubMed] [Google Scholar]
- 36.Addis IB, Ireland CC, Vittinghoff E, Lin F, Stuenkel CA, Hulley S. Sexual activity and function in postmenopausal women with heart disease. Obstet Gynecol. 2005;106:121–127. doi: 10.1097/01.AOG.0000165276.85777.fb. [DOI] [PubMed] [Google Scholar]
- 37.Lindau ST, Abramsohn E, Gosch K, et al. Patterns and loss of sexual activity in the year following hospitalization for acute myocardial infarction (a United States National Multisite Observational Study) Am J Cardiol. 2012;109:1439–1444. doi: 10.1016/j.amjcard.2012.01.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steinke EE, Jaarsma T, Barnason SA, et al. Sexual counselling for individuals with cardiovascular disease and their partners: a consensus document from the American Heart Association and the ESC Council on Cardiovascular Nursing and Allied Professions (CCNAP) Eur Heart J. 2013;34:3217–3235. doi: 10.1093/eurheartj/eht270. [DOI] [PubMed] [Google Scholar]