Abstract
Background
Recent studies have suggested that metabolic surgery reduces cancer risk. This study aims to determine if incident cancer is associated with the extent of weight loss after Roux-en-Y Gastric Bypass (RYGB).
Methods
Patients at a large tertiary bariatric surgery center were retrospectively reviewed to identify patients with no history of cancer at the time of RYGB. Diagnoses in the electronic health record, a tumor registry, and chart review were used to identify post-operative incident solid organ cancer. The overall incidence of organ cancer was estimated using Kaplan-Meier analysis. The percent total body weight loss (%TWL) in the 48 months after surgery but prior to cancer was compared between those that developed organ cancer versus those that did not using repeated measures linear regression.
Results
The 2943 patients had a mean age of 45.6 years (SD=11.1), 81% were female, and a mean baseline body mass index (BMI) of 47.2 kg/m2 (SD=7.9). Median follow-up after surgery was 3.8 years (range= [<1, 12]). Incident organ cancer developed and was verified in 54 of the 2943 patients (1.8%). Kaplan-Meier estimates for cancer at 3, 5, and 10 years post-surgery were 1.3%, 2.5%, and 4.2%. After adjusting for age, BMI, sex, diabetes, hypertension, and dyslipidemia, patients that developed organ cancer achieved less weight loss (-1.5 %TWL, 95% CI=[-2.9%, -0.1%], p=0.034).
Conclusions
Greater weight loss after metabolic surgery may be associated with lower organ cancer risk.
Keywords: metabolic surgery, obesity, cancer risk, cancer incidence
Introduction
Between 1980 and 2008, the prevalence of overweight and obesity in developing countries tripled from 250 million to 904 million. Combined with an increase in the developed world's rates, a total of 1.5 billion overweight/obese adults was reached [1]. In the United States, seven out of ten people are currently overweight or obese [2]. As a consequence of the deleterious effects of obesity, a white male between the ages of 20 to 30 with a BMI of >45 will lose a maximum of 13 years of life as a result of obesity. This translates to a 22% reduction in the life expectancy. Reduced life expectancy related to obesity results from an increased risk of cardiovascular disease, hypertension, type 2 diabetes, stroke, gall bladder disease, sleep apnea, asthma, depression, and certain cancers [3].
The 2007 World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) Diet and Cancer Report documented several common cancers linked to obesity, yet less than one-half of all Americans are aware of this relationship [4]. The International Agency for Research on Cancer (IARC) predicts that by the year 2032, new cancers will increase by about 57% each year; cancer deaths will increase by about 59% [5]. According to the AIRC, about 400,000 cases of the most common cancers in America can potentially be prevented annually by maintaining healthy lifestyles which include maintaining a normal weight [6]. Numerous large cohort studies with long-term follow-up, as well as meta analyses and systematic reviews have demonstrated a consistent association between BMI, mortality, and several cancers including breast, renal, ovarian, esophagus, pancreas, prostate, cervix, hepatobiliary, gall bladder, colorectal, thyroid and melanoma [7-13].
The health restorative power of metabolic surgery has been established by many long-term studies which have demonstrated a benefit in all-cause mortality [14-17], improved cardiovascular health [14-16], diabetes resolution [14-16], and resolution of metabolic syndrome components [14]. More recent analysis of mortality benefits after metabolic surgery suggest that a contributor to increased longevity is a reduced risk of cancer [18-20]. The results to date suggest that successful durable weight loss after metabolic surgery results in lower cancer rates.
The inconsistencies in medical weight management may explain the lack of evidence relating voluntary weight reduction to reduced cancer risk. Metabolic surgery is the only current treatment which offers durable weight loss for extremely obese individuals. While the majority of metabolic surgery patients experience successful post-operative weight loss, the amount of weight loss varies between procedures and between patients having the same procedure [21].
Although one might postulate that if weight loss causes the reduced cancer risk, then the extent of weight loss should also be related to the reduced risk [7]. In at least one study, this has been investigated and no correlation was found [20]. The purpose of this study is to further assess the relationship between the magnitude of the weight loss achievement after metabolic surgery and cancer incidence utilizing a large single-center clinical registry.
Methods and Materials
Metabolic surgery patients were enrolled into an IRB-approved research program on obesity at the Center for Nutrition and Weight Management at Geisinger Clinic. The program includes a standardized pre-operative program and comprehensive clinical data collection before and after surgery [22]. For this study, the parent cohort included patients with primary RYGB surgery completed between 1/1/2002 and 6/30/2013 (n=3087). All patients had a baseline BMI >35 kg/m2 and at least 30 days of follow-up after surgery. A thorough review of electronic medical records and local tumor registries was conducted to identify and exclude those with a history of cancer prior to RYGB (n=107). The remaining 2983 patients were reviewed for post-operative cancer diagnoses.
Both electronic health records and the health system tumor registry were used to identify and confirm patients with post-operative incident solid organ cancer. In both cases, the timing and type of cancers were noted and compared between the two sources. All discrepancies were resolved through subsequent chart review. Patients with benign tumors (n=12), skin cancers (n=17), bone cancers (n=1), or blood-cell derived cancers (n=7) were excluded from the analysis, leaving 2943 patients in the final analysis. The list of post-surgery new solid organ cancer patient cases were categorized according to organ systems including Breast/Genitourinary, Digestive, Endocrine, Respiratory, and Nervous System.
The characteristics of patients that developed solid organ cancers were compared to those that did not using two-sample t-tests and Chi-squared tests. The overall incidence of post-operative solid organ cancer was estimated using Kaplan-Meier analysis. Post-operative weight measures at 6, 12, 24, 36, and 48 months post-surgery were evaluated for all patients. If a patient developed solid organ cancer, only weight measures occurring before the organ cancer diagnoses were included. The weight measures were used to calculate percent of total body weight loss (%TWL) at each time point. %TWL was compared between those that developed organ cancer and those that did not at each time point using two-sample t-test. In multiple regression analysis, a repeated-measures model was used to determine if overall weight loss was lower in the cancer group after adjusting for BMI, age at surgery, sex, and metabolic co-morbidity burden. SAS version 9.3 was used for statistical analysis and p-values <0.05 were considered significant.
Results
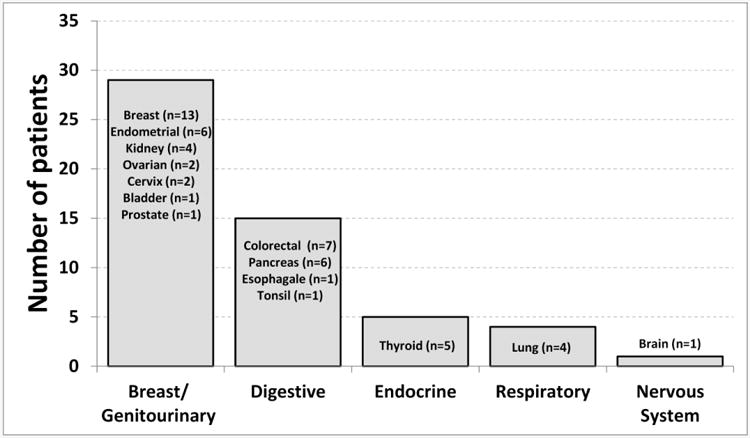
The 2943 patients had a mean age of 45.6 years (SD=11.1), 81% were female, and a mean baseline BMI of 47.2 kg/m2 (SD=7.9). Median follow-up after surgery was 3.8 years (range=[<1, 12]). Incident solid organ cancer was verified in 54 of the 2943 patients (1.8%). The most common organ system with incident cancer was breast/genitourinary (n=29), followed by digestive (n=15), endocrine (n=5), respiratory (n=4), and nervous system (n=1) (Figure 1).
Figure 1. Organ systems with organ cancers after Roux-en-Y gastric bypass.
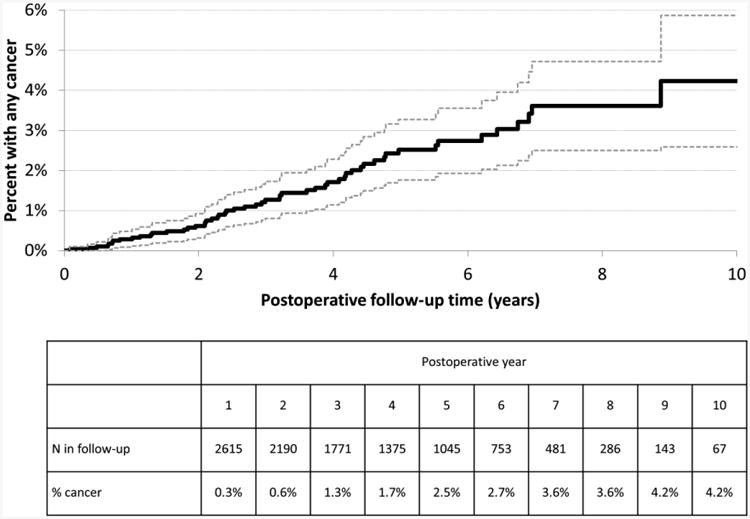
Patients that developed cancer were older at surgery as compared to those that did not develop cancer (53.3 years versus 45.4 years, p<0.0001, Table 1). Mean BMI (p=0.825) and sex distribution (p=0.608) were similar between the groups. Though not significant, the organ cancer group had a higher percentage of patients with diabetes (44% vs 35%, p=0.161) and hypertension (57% vs 49%, p=0.203). The percent with a dyslipidemia diagnosis was 39% in both groups (p=0.940). Kaplan-Meier estimated rates of incident solid organ cancer at 3, 5, and 10 years post-surgery were 1.3%, 2.5%, and 4.2% (Figure 2).
Table 1.
Patient characteristics of the of the study population (n=2943) and compared between the organ cancer (n=54) and no organ cancer (n=2889) groups.
| Overall (n=2943) | Any organ cancer (n=54) | No organ cancer (n=2929) | p-value | ||
|---|---|---|---|---|---|
|
| |||||
| Age | Mean (SD) | 45.6 (11.1) | 53.3 (10.7) | 45.4 (11.1) | <0.00011 |
| Range | [18, 74] | [27, 72] | [18, 74] | ||
|
| |||||
| BMI | Mean (SD) | 47.2 (7.9) | 47.4 (9.2) | 47.2 (7.9) | 0.8251 |
| Range | [35.0, 83.9] | [35.9, 76.7] | [35.0, 83.9] | ||
|
| |||||
| Sex | Male, % (n) | 19% (n=571) | 17% (n=9) | 19% (n=562) | 0.6082 |
| Female, % (n) | 81% (n=2372) | 83% (n=45) | 81% (n=2327) | ||
|
| |||||
| Diabetes | %, (n) | 35% (n=1042) | 44% (n=24) | 35% (n=1018) | 0.1612 |
|
| |||||
| Hypertension | %, (n) | 49% (n=1437) | 57% (n=31) | 49% (n=1406) | 0.2032 |
|
| |||||
| Dyslipidemia | %, (n) | 39% (n=1159) | 39% (n=21) | 39% (n=1138) | 0.9402 |
BMI=Body Mass Index
Two-sample t-test,
Chi-square test
Figure 2. Kaplan-Meier curve for time until incident organ cancer after Roux-en-Y gastric bypass (N=2943).
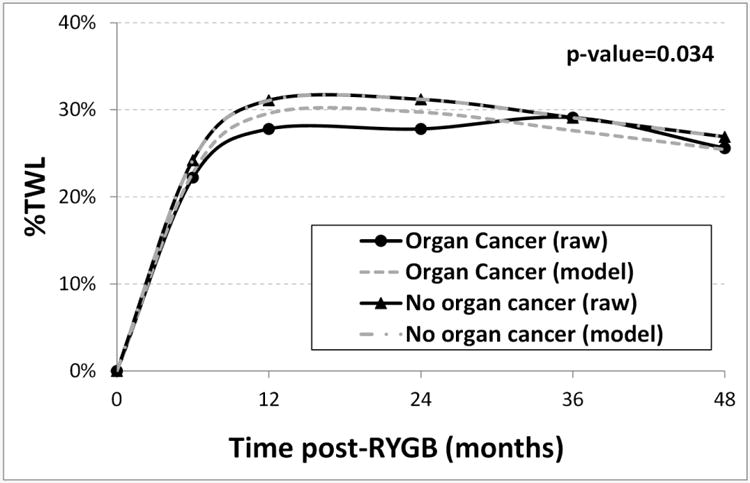
At 6 months and 12 months post-surgery, the mean %TWL in the solid organ cancer group was significantly lower than the %TWL in the no solid organ cancer group (6 months: 22.2% vs 24.2%, p=0.017; 12 months: 27.8% vs 31.1%, p=0.0079; Table 2). Though not significant, the %TWL was 1.3-3.4% lower in the organ cancer group at 24 and 48 months after surgery. In the regression model for %TWL across the 48 months after surgery, the patients that developed a solid organ cancer had a net decrease of 2.3% less TWL (95% CI=[-3.7%, -0.9%], p=0.0012). After adjusting for age, BMI, sex, diabetes, hypertension, and dyslipidemia, patients that developed organ cancer had a net decrease of 1.5% less TWL (95% CI=[-2.9%, -0.1%], p=0.034) (Figure 3).
Table 2.
Mean %TWL in cancer and no cancer groups over time post-surgery.
| Time | Organ cancer (n=54) | No organ cancer (n=2889) | p-value | ||
|---|---|---|---|---|---|
| n | Mean (SD) | N | Mean (SD) | ||
| 6-months | 48 | 22.2% (6.0) | 2661 | 24.2% (5.6) | 0.017 |
| 12-months | 41 | 27.8% (8.9) | 2348 | 31.1% (10.3) | 0.0079 |
| 24-months | 29 | 27.8% (8.9) | 1680 | 31.2% (10.3) | 0.076 |
| 36-months | 19 | 29.1% (11.7) | 1276 | 29.1% (10.6) | 0.982 |
| 48-months | 14 | 25.6% (10.6) | 947 | 26.9% (11.0) | 0.645 |
Two-sample t-test
Figure 3. Mean %EWL (raw means and model estimated means) compared between patients that had post- Roux-en-Y gastric bypass organ cancer (n=54) versus those without organ cancer (n=2889).
Conclusions
Metabolic surgery has emerged as a powerful tool restoring health and quality of life as many of the important obesity-related medical conditions either resolve or improve in association with surgical weight loss. The recent findings suggesting a reduction in cancer risk with surgical weight loss provide additional support for the importance of this treatment.
The association between cancer and obesity is now well established. The IARC reviewed the obesity prevalence in Europe and the current cancer risks in obesity from published studies and found that obesity was associated with significant numbers of cancers of the colon, breast, endometrial, and kidney, and esophagus [23]. In 2003, Calle prospectively studied over 900,000 cancer-free adults. With a follow-up of 16 years, 57,145 cancer fatalities were noted. A higher BMI was associated with increased death rates from solid organ cancers [8]. The AICR and WCRF have reported that there is clear and consistent evidence showing that body fatness increases the risk of esophageal, pancreatic, gall bladder, colorectal, post-menopausal breast, ovarian, kidney, prostatic, endometrial, and liver cancers [24]. In a prospective cohort study of over 1 million people in the United Kingdom, Reeves reported that increasing obesity was associated with an increased incidence of endometrial cancer, esophageal adenocarcinoma, renal cancer, leukemia, multiple myeloma, pancreatic cancer, and non-Hodgkin's lymphoma [10]. In a review of published epidemiological studies of obesity and cancer, Wolin estimated that overweight and obesity is responsible for 20% of cancer cases [9]. These findings are also supported by several systematic reviews of the association of increasing obesity and cancer incidence [7-8, 14]. In addition, recent studies add strong evidence for an association between ovarian, prostate, and post-menopausal breast cancer with increasing obesity [25].
The mechanisms which explain the relationship between obesity and its co-morbid conditions which include diabetes, hypertension, cardiovascular disease, and cancer are felt to be related to physiologic alterations in white adipose tissue (WAT) associated with expansion in the setting of excess calorie intake. The primary function of WAT is energy storage as lipids in order to maintain energy availability in case of caloric deprivation. In addition to its known storage function, adipose tissue is now considered an endocrine organ because of its secretory products called adipokines. Diet induced adipose tissue expansion results in alterations in adipokine secretion resulting in systemic effects, which include metabolic dysfunction, and a chronic state of low-grade inflammation, both of which are felt to contribute to carcinogenesis, tumor progression and tumor spread [26].
The expansion of adipose tissue results in an influx of macrophages which stimulate the release of TNF-α, IL-6, and IL-1β and enhance local inflammation. Alterations in intestinal permeability may also result in translocation of bacterial products which contributes to the chronic inflammatory state [27]. Increased inflammatory activity results contribute to metabolic dysfunction and insulin resistance [26, 28]. Increases in inflammatory mediators and associated metabolic dysfunction result in increased levels of insulin and estrogen which have stimulatory effects on human cancers. Increases in insulin will increase levels of insulin-like growth factor-1 (IGF-1) which also has mitogenic properties [28].
The favorable benefits of metabolic surgery on metabolic dysfunction, adipokine profile, and inflammation likely explain the emerging evidence which suggests that metabolic surgery reduces cancer risk. Christou compared health outcomes in 1035 patients who underwent metabolic surgery compared with 5746 non-surgical obese controls and demonstrated reductions in medical encounters for cardiovascular disease and other conditions including cancer in the surgical cohort [16]. In a similar study with cancer as the primary endpoint, these authors found a significant reduction in breast cancer cases and a trend toward reduction in other cancers in metabolic surgery patients in comparison to non-surgical obese controls [18]. In a similar sequence of studies, Adams demonstrated improved survival in metabolic surgery patients when compared to non-operated controls [15] and, subsequently showed that a contributor to the improved health outcomes associated with metabolic surgery was a reduced cancer incidence in women [19]. Similar findings have been reported by Sjostrom in the Swedish Obesity Study demonstrating both improved survival as well as a reduction in cancer incidence in obese women in longitudinal studies of metabolic surgery outcomes when compared with non-surgical obese controls [17, 20]. Several recent studies with the focus on longer term follow-up have raised questions about the relationship between obesity, bariatric surgery, and colorectal cancer risk with suggestive evidence that the risk of colorectal cancer may be increased after bariatric surgery [29-30]. However, the large majority of evidence as well as several systematic reviews and a meta-analyses suggest that bariatric surgery favorably affects the cancer risk for many solid organ cancers, especially in women [7, 14, 31-32].
This study reflects a single center experience in review of new solid organ cancers identified in metabolic surgery patients and attempts to explore the relationship between weight loss and cancer incidence. We chose to include only those solid organ cancers where there is strong evidence confirming an association between obesity and cancer risk. Tumors such as melanoma, bone cancer, and blood-cell derived cancers where there is controversial or limited evidence regarding an association with obesity were excluded from this study. In sensitivity analysis, we found that the results of the study did not change due to the exclusion of this small subgroup of patients.
Recommendations from the current literature suggest the use of %TWL versus %EWL when comparing different patients or non-randomized groups of bariatric patients [33]. Therefore, our analysis was described in %TWL. However, further calculations were performed and after adjusting for baseline BMI, sex, age, diabetes, hypertension, and dyslipidemia, analysis of the data by %EWL was no different.
In this study, post-operative patients who developed solid organ cancers were identified and confirmed by review of electronic medical records, review of tumor registry data, and confirmatory chart review. Although a single center for this study was employed, the data came from a large, validated cohort of bariatric patients from a consistent source. An additional strength of this study is the long-term follow-up time that included longitudinal post-operative weight measurements.
Limitations of this study relate to the demographics of the study population, derived from a homogenous population that was limited to RYGB surgery in a mostly Caucasian, rural population thereby making it difficult to extrapolate results to other populations. The small number of cases resulted in a lower statistical power, particularly if there was an interest in determining if the association between weight loss and cancer is modified by the type of organ cancer (e. g. breast versus digestive). In addition, the current methodology does not exclude the possibility that some of the solid organ cancers identified in the early post-operative period may have been present at the time of metabolic surgery. Acknowledging the variation of the median follow-up after surgery, we also realize that the surgery effect may be due to age at the incidence.
Our data demonstrates that there may be a relationship between the magnitude of weight loss and the extent of cancer risk reduction following metabolic surgery. This is the first study which demonstrates a possible relationship between the extent of weight loss and cancer risk reduction. Although the actual weight loss differences between those developing cancer after metabolic surgery and the non-solid organ cancer group are statistically significant only at 12 months, the regression model reflecting adjustment for age, BMI, sex, gender, diabetes, dyslipidemia and hypertension demonstrates a statistically significant reduction in weight loss across the 48 months in those who developed a new solid organ cancer. We suspect that the lack of statistical significance at other points in time is a combined result of the diminishing sample size at and after the 24-month time point (e.g. only n=29 remaining in the organ cancer group at 24 months) and the diminishing effect of surgery as time from surgery increases.
It is known that weight loss results in improvement in metabolic dysfunction, systemic inflammation, and the adipokine secretory profile. Thus, it seems intuitive that more weight loss might result in physiologic alterations which would favorably affect cancer risk [34]. Only one of the longitudinal studies addressing cancer occurrence after bariatric surgery has addressed this issue. No association between the extent of weight loss and cancer risk was noted [20]. Hopefully, these preliminary findings will lead to more formal studies of the relationship between the extent of weight loss after metabolic surgery and cancer risk reduction. Furthermore, a positive association between surgical weight loss and cancer risk might prove to be an added incentive for patients to adhere to lifestyle changes that impact their overall survival.
Acknowledgments
This research was supported by research funds from the Geisinger Health System and the National Institutes of Health (Grant nos. DK072488 to C. D. Still and G.C. Wood).
Footnotes
Conflict of Interest Disclosure Statement:
Marie A. Hunsinger RN BSHS: no conflict of interest
G. Craig Wood MS: no conflict of interest
Christopher D. Still DO: no conflict of interest
Anthony T. Petrick MD: no conflict of interest
Joseph A. Blansfield MD: no conflict of interest
Mohsen M. Shabahang MD, PhD: no conflict of interest
Peter N. Benotti MD: no conflict of interest
Ethical Approval: This article does not contain any studies with human participants or animals performed by any of the authors. For this type of study, formal consent is not required.
Informed Consent: Does not apply.
Contributor Information
Marie A Hunsinger, Email: mahunsinger@geisinger.edu, Department of Surgery, Geisinger Clinic, 100 N Academy Ave, Danville, PA 17822.
G Craig Wood, Email: cwood@geisinger.edu, Geisinger Obesity Research Institute, Geisinger Clinic, 100 N Academy Ave, Danville, PA 17822.
Chris Still, Email: cstill@geisinger.edu, Geisinger Obesity Research Institute, Geisinger Clinic, 100 N Academy Ave, Danville, PA 17822; Department of Gastroenterology, Geisinger Clinic, Danville, PA 17822.
Anthony Petrick, Email: atpetrick@geisinger.edu, Department of Surgery, Geisinger Clinic, 100 N Academy Ave, Danville, PA 17822.
Joseph Blansfield, Email: Jblansfield1@geisinger.edu, Department of Surgery, Geisinger Clinic, 100 N Academy Ave, Danville, PA 17822.
Mohsen Shabahang, Email: mmshabahang@geisinger.edu, Department of Surgery, Geisinger Clinic, 100 N Academy Ave, Danville, PA 17822.
Peter Benotti, Email: pnbenotti@geisinger.edu, Geisinger Obesity Research Institute, Geisinger Clinic, 100 N Academy Ave, Danville, PA 17822.
References
- 1.American Institute of Cancer Research. Washington DC: The American Institute of Cancer Research; Internet. c1982-2015 [updated 2014; cited 2015 Jan 5]. Available: http://www.aicr.org/cancer-research-update/2014/january_08/cru-report-billion-overweight-obese.html. [Google Scholar]
- 2.Division of Nutrition, Physical Activity, and Obesity. Georgia: National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention; Internet. c 2009-2015 [updated 2012; cited 2015 Jan 5]. Available: http://www.cdc.gov/nccdphp/dnpa/obesity/economic_consequences.htm. [Google Scholar]
- 3.Fontaine K, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA. 2003;289:187–193. doi: 10.1001/jama.289.2.187. [DOI] [PubMed] [Google Scholar]
- 4.American Institute of Cancer Research. Washington DC: The American Institute of Cancer Research; Internet. c 1982-2015 [updated 2012; cited 2015 Jan 5]. Available: http://www.aicr.org/assets/docs/pdf/education/aicr-cancer-awareness-report-2012.pdf. [Google Scholar]
- 5.International Agency for Research on Cancer. France: International Agency for Research on Cancer; Internet. c1965-2015 [updated 2014; cited 2015 Jan 5]. Available: http://www.iarc.fr/en/media-centre/pr/2014/pdfs/pr224_E.pdf. [Google Scholar]
- 6.American Institute of Cancer Research. Washington DC: The American Institute of Cancer Research; Internet. c 1982-2015 [updated 2012; cited 2015 Jan 5]. Available: http://www.aicr.org/reduce-your-cancer-risk/cancer-prevention/reduce_cancer_by_numbers.html. [Google Scholar]
- 7.Renehan A, Tyson M, Egger M, Heller R, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 8.Calle E, Rodriguez C, Walker-Thurmond K, Thun M. Overweight, obesity and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 9.Wolin KY, Carson K, Colditz GA. Obesity and cancer. Oncologist. 2010;15(6):556–565. doi: 10.1634/theoncologist.2009-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the million women study: cohort study. BMJ. 2007 Dec 1;335(7630):1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DePergola G, Sivestris F. Obesity as a major risk factor for cancer. J Obes. 2013 Jul 25;2013:291546. doi: 10.1155/2013/291546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnold M, Pandeya N, Byrnes G, Renehan A, Stevens G, Ezzati M, et al. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. [cited 2015 Jan 10];Lancet Oncol. 2014 Nov 26;:13. doi: 10.1016/S1470-2045(14)71123-4. Available from: http://press.thelancet.com/BMIcancer.pdf. [DOI] [PMC free article] [PubMed]
- 13.Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, et al. Body-mass index and cause-specific mortality in 900,000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009 Mar;373:083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashrafian H, Ahmed K, Rowland SP. Metabolic Surgery and Cancer. Cancer. 2011;117(9):1788–1799. doi: 10.1002/cncr.25738. [DOI] [PubMed] [Google Scholar]
- 15.Adams TD, Gress MA, Smith SC, et al. Long-term mortality after gastric bypass surgery. NEJM. 2007;357:753–61. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 16.Christou NV, Sampali JS, Liberman M, Look D, Auger S, McLean AP, MacLean LD. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg. 2004 Sep;240(3):416–23. doi: 10.1097/01.sla.0000137343.63376.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357(8):741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 18.Christou N, Lieberman M, Sampalis F, Sampalis J. Bariatric surgery reduces cancer risk in morbidly obese patients. SOARD. 2008 Nov-Dec;4(6):691–5. doi: 10.1016/j.soard.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 19.Adams TD, Stroup AM, Gress RE, Adams KF, Calle EE, Smith SC, et al. Cancer incidence and mortality after gastric bypass surgery. Obesity. 2009 Apr;17(4):796–802. doi: 10.1038/oby.2008.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sjostrom L, Gummesson A, Sjostrom CD, Narbro K, Peltonen M, Wedel H, et al. Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish obese study subjects): a prospective, controlled intervention trial. Lancet. 2009 Jul;10(7):653–62. doi: 10.1016/S1470-2045(09)70159-7. [DOI] [PubMed] [Google Scholar]
- 21.Still CD, Wood GC, Chu X, Manney C, Strodel W, Petrick A, et al. Clinical factors associated with weight loss outcomes after Roux-en-Y gastric bypass surgery. Obesity. 2014 Mar;22(3):888–94. doi: 10.1002/oby.20529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wood GC, Chu X, Manney C, Strodel W, Petrick A, Gabrielsen J, et al. An electronic health record-enabled obesity database. BMC Med Informatics Decis Mak. 2012;12:45. doi: 10.1186/1472-6947-12-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.International Agency for Research on Cancer. France: International Agency for Research on Cancer; Internet. c1965-2015 [cited 2015 Jan 10]. Available: http://www.iarc.fr/en/publications/pdfs-online/prev/handbook6. [Google Scholar]
- 24.American Institute of Cancer Research. Washington DC: The American Institute of Cancer Research; Internet. c 1982-2015 [updated 2007; cited 2015 Jan 10]. Available: http://www.aicr.org/research/research_science_expert_report.html. [Google Scholar]
- 25.Berger NA. Obesity-associated gastrointestinal tract cancer: from beginning to end. Cancer. 2014 Apr 1;120(7):935–939. doi: 10.1002/cncr.28534. [DOI] [PubMed] [Google Scholar]
- 26.Harwood J. The adipocyte as an endocrine organ in the regulation of metabolic homeostasis. Neuropharmacology. 2012 Jul;63(1):57–75. doi: 10.1016/j.neuropharm.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 27.Musso G, Gambino R, Cassader M. Obesity, diabetes, and gut microbiota: the hygiene hypothesis expanded. Diabetes Care. 2010;33:2277–2284. doi: 10.2337/dc10-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iyenegar N, Hudis C, Dannenberg A. Obesity and cancer: local and systemic mechanisms. Annu Rev Med. 2015;66:297–309. doi: 10.1146/annurev-med-050913-022228. [DOI] [PubMed] [Google Scholar]
- 29.Ostlund MP, Lu YL, Lagergren J. Risk of obesity-related cancer after obesity surgery in a population-based cohort study. Ann Surg. 2010 Dec;252(6):972–6. doi: 10.1097/SLA.0b013e3181e33778. [DOI] [PubMed] [Google Scholar]
- 30.Derogar M, Hull MA, Kant P, Ostlund M, Lu Y, Lagergren J. Increased risk of colorectal cancer after obesity surgery. Ann Surg. 2013 Dec;258(6):983–8. doi: 10.1097/SLA.0b013e318288463a. [DOI] [PubMed] [Google Scholar]
- 31.Tee MC, Cao Y, Warnock G, Hu FB, Chavarro JE. Effects of bariatric surgery on oncologic outcomes: a systematic review and meta-analysis. Surg Endosc. 2013 Dec;27(12):4449–56. doi: 10.1007/s00464-013-3127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van de Laar A, de Caluwe L, Dillemans B. Relative outcome measures for bariatric surgery. Evidence against excess weight loss and excess body mass index loss from a series of laparoscopic Roux-en-Y gastric bypass patients. Obes Surg. 2011 Jun;(6):763–7. doi: 10.1007/s11695-010-0347-0. [DOI] [PubMed] [Google Scholar]
- 33.Renehan A. Bariatric surgery, weight reduction, and cancer prevention. Lancet Oncology. 2009 Jul;10(7):640–641. doi: 10.1016/S1470-2045(09)70170-6. [DOI] [PubMed] [Google Scholar]


