Summary
Bats are an important reservoir for emerging zoonotic pathogens. Close human–bat interactions, including the sharing of living spaces and hunting and butchering of bats for food and medicines, may lead to spillover of zoonotic disease into human populations. We used bat exposure and environmental data gathered from 207 Bangladeshi villages to characterize bat exposures and hunting in Bangladesh. Eleven percent of households reported having a bat roost near their homes, 65% reported seeing bats flying over their households at dusk, and 31% reported seeing bats inside their compounds or courtyard areas. Twenty percent of households reported that members had at least daily exposure to bats. Bat hunting occurred in 49% of the villages surveyed and was more likely to occur in households that reported nearby bat roosts (adjusted prevalence ratio [aPR] 2.3, 95% CI 1.1–4.9) and villages located in north‐west (aPR 7.5, 95% CI 2.5–23.0) and south‐west (aPR 6.8, 95% CI 2.1–21.6) regions. Our results suggest high exposure to bats and widespread hunting throughout Bangladesh. This has implications for both zoonotic disease spillover and bat conservation.
Keywords: bats, hunting, human–bat interactions, zoonotic disease, conservation, Pteropus giganteus
Introduction
Bats are important reservoirs for zoonotic pathogens that can both cause severe disease in humans and potentially cause pandemics. Viruses that have been transmitted either directly or indirectly from bats to humans include Ebola virus, the henipaviruses Hendra and Nipah virus and severe acute respiratory syndrome coronavirus (SARS CoV) (Luis et al., 2013; Smith and Wang, 2013; Plowright et al., 2015). The ubiquitous nature, large population sizes, gregarious behaviour and high species diversity of bats combined with their close interactions with livestock, farms and humans likely facilitate their role as a source of zoonotic disease (Olival et al., 2012).
Spillover events from bats to humans require interaction between the infected bat and human host. Close human–bat interactions, including the sharing of living spaces, consumption of shared food resources and hunting and butchering of bats for food and medicines, may lead to human exposure to zoonotic pathogens (Smith and Wang, 2013). Despite the risk to human health, human–bat interactions continue to occur and are largely driven by complex environmental, social and economic factors (Wood et al., 2012).
Bangladesh is home to three species of frugivorous bats that are abundant and known to roost and forage near human settlements: Pteropus giganteus, Cynopterus sphinx and Roussettus leschenaultia (Bates and Harrison, 1997). Pteropus giganteus, or the Indian flying fox, is the largest bodied frugivorous species in Bangladesh and is of key interest as a zoonotic disease reservoir as it is both the natural reservoir for Nipah virus in South Asia and has also been associated with more than 55 other recently identified viruses, some of which may have the potential to cause disease in other animal or human hosts (Anthony et al., 2013). In this paper, we use data gathered in Bangladesh villages to better characterize bat hunting and other causes of exposure to bats in Bangladesh, focusing on interactions with Pteropus giganteus.
Methods
The data used in this analysis were collected as part of a large epidemiological field survey conducted in the winter months of November to February from 2011 to 2013 investigating risk factors for Nipah virus infection in Bangladeshi villages. Field teams from the International Center for Diarrhoeal Disease Research, Bangladesh (ICDDR,B), collected data from all 60 villages reporting primary Nipah cases between 2001 and 2011 located within the spillover region known as the Nipah Belt (Hahn et al., 2014), 73 randomly selected villages within the Nipah Belt with no known Nipah virus cases and 74 villages randomly selected outside of the Nipah Belt area.
In rural Bangladesh, families typically share a common courtyard and live together in a compound (bari). In each village, a structured survey was administered at 25 randomly selected compounds. Within each village, the field team used a random number table to choose which cardinal direction to start surveying compounds, after which compounds were selected at regular intervals to ensure even coverage.
The survey teams addressed questions to the adult head of each compound (referred to as ‘household head’ in this work). Household heads were asked to provide consent before taking part in the study, and the research was approved by the institutional review board at icddr,b. The majority of questions referred specifically to P. giganteus (using the Bangla term ‘badur’). Data collected on human bat exposures included the presence of large‐bodied fruit bat roosts near the household compound, sighting large‐bodied fruit bats in fruit trees and flying overhead in the past month, finding large‐bodied fruit bats within the household compound and courtyards in the past month and finding dead fruit bats of any size in the past year. Household heads were asked about the hunting of large fruit bats by family members and knowledge of bat hunters within their villages. Household heads were also asked about exposure to bat urine, faeces and the placentas of large fruit bats over any time period. Finally, household heads were asked to report the number and variety of all fruit trees present on their land and whether or not bats fed from each type of tree.
Survey teams recorded the village centre latitude and longitude and locations of Pteropus bat roosts within 5 kilometres of each village. The teams visited each roost site, the bats were counted, and these data were used to calculate the number of Pteropus bats per square kilometre and per person for each surveyed village site.
We characterized villagers’ interactions with bats using basic descriptive statistics. We constructed mixed‐effects logistic regression models to study the association between environmental factors and bat hunting activity at the household level. To build models, we first framed a causal diagram to identify associations between variables of interest and to identify confounders (Greenland et al., 1999). We constructed mixed‐effects models consisting of a single independent variable controlling for village clustering as a random effect. The presence of bat hunting at the household level was used as the dependent variable and the presence of bat roosts on the landowner's property, the presence of bats within the household complex and courtyards, the total number of fruit trees on the property, the dominance of trees which were highly attractive to fruit bats around the household, the number of Pteropus bats per square kilometre and population in the village, and two measures of household wealth were all identified as independent variables. To create a variable representing the attractiveness of fruit tress surrounding a household to fruit bats, we used tree counts and the report of bat visits to specific tree varieties provided by household heads. We designated fruit tree varieties with 90% or more of tree owners reporting bat visits as highly attractive to fruit bats and we designated households dominated by these trees at two levels (making up 50% and 80% of all trees planted on the property) in the analysis. These cut‐offs were selected as they represented an increasing gradient and had large enough populations in each group to allow for analysis. As proxies for household wealth (Kumar, 1989), we used two non‐colinear measures of household per capita wealth: the total number of fruit trees and the total number of livestock (which included cattle, buffalo, sheep, pigs, goats and horses) per number of people living in the compound. We then built a multivariable mixed‐effects logistic regression model, using information‐theoretic model selection to generate all possible combinations of the selected independent variables and selected the model with the lowest corrected Akaike information criterion (Burnham and Anderson, 2002).
We studied how bat hunting varied across Bangladesh by constructing a logistic regression model of all villages included in the survey. The presence or absence of bat hunting in each village was used as the dependent variable and was classified as true if either a household head reported hunting within their compound or a household head reported knowing bat hunters within the village. A single report of hunting was sufficient to classify a village as having bat hunters. The location of the village, the number of bats per square kilometre and the number of fruit trees per square kilometre were used as independent variables. Village locations were grouped into north‐west, north‐east, south‐west and south‐east quadrants based on their coordinates. We first built univariate models and then combined all independent variables into a single model. We tested for interactions between independent variables and used k‐fold cross‐validation to select the best multivariable model (Arlot and Celisse, 2010).
Prevalence ratios were calculated using the delta method (Santos et al., 2008). We assessed multicollinearity between independent variables in multivariable models using variance inflation factors. Analysis was conducted in R (R Core Team, 2015) utilizing the lme4 package (Bates et al., 2015) for logistic mixed‐effects models and the MuMIn package for information‐theoretic model selection (Bartoń, 2015).
Results
In the month preceding the survey, 65% of household heads (3287/5056) reported seeing large‐bodied fruit bats flying over their households at dusk, 50% (2510/5056) reported seeing large‐bodied fruit bats in their fruit trees at night, and 31% (1551/5056) reported seeing large‐bodied fruit bats inside their compounds or courtyard areas. In the month prior to completing the survey, 20% (1021/5056) of household heads reported at least daily observations of large‐bodied fruit bats, with sightings of these bats on their property, flying overhead, or in their trees at least daily. Bats often had roost sites near household compounds, with 11% (567/5061) of household heads reporting having a large‐bodied fruit bat roost on their property.
Of the household heads surveyed, 14% (721/5,061) reported finding dead fruit bats in the year preceding the survey. Of these, 38% (276/721) reported finding dead fruit bats tangled in electrical wires, 30% (214/721) reported finding fruit bat carcasses on the ground, 26% (188/721) in trees and 2% (12/721) in date palm sap collection containers. In 25% (184/721) of cases, household heads reported that the bodies of dead fruit bats were disposed of in local bodies of water. A much smaller number of household heads reported household members having physical contact with bat urine (63/5061; 1%), faeces (91/5061; 2%) or large‐bodied fruit bat placentas (15/5061; 0.3%) in the previous year.
Fruit tree ownership and bat visits to trees were common (Table 1). Mango trees were the most commonly owned fruit tree variety (83% of all households), followed by jackfruit (67%), banana (63%) and guava (62%). Fruit bat visits to trees varied widely for different tree varieties, with tree owners most likely to report bats eating fruit from their mango (94% of mango tree owners) and guava trees (92% of guava tree owners).
Table 1.
Common fruit trees owned by households and the presence of bats eating fruit as reported by owners of each tree variety
| Fruit tree variety | Households owning tree % (num) | Households owning tree and reporting bats eating fruit % (num) |
|---|---|---|
| Mango | 83% (4190) | 94% (3930) |
| Guava | 62% (3137) | 92% (2877) |
| Sofeda | 10% (516) | 89% (463) |
| Boroi | 46% (2317) | 89% (2053) |
| Banana | 63% (3196) | 88% (2829) |
| Lychee | 19% (992) | 84% (833) |
| Betel nut | 46% (2361) | 71% (1671) |
| Palmyra | 18% (928) | 68% (630) |
| Blackberry | 16% (816) | 64% (523) |
| Date palm | 27% (1395) | 63% (876) |
| Jackfruit | 67% (3384) | 60% (2036) |
| Custard apple | 14% (705) | 60% (421) |
| Indian olive | 15% (774) | 60% (462) |
| Papaya | 40% (2040) | 59% (1210) |
| Star fruit | 15% (746) | 59% (438) |
| Coconut palm | 57% (2895) | 8% (246) |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Of the household heads surveyed, 63 (1%) reported that members of their own households engaged in bat hunting and 408 (8%) reported knowing bat hunters who were not living in their household but resided in their village. Bat hunting, either within surveyed households or by villagers known to the interviewed household heads, occurred in 49% (101/204) of all villages (Fig. 1). The majority of bat hunting villages (61% of all hunting villages) were classified as actively hunting based on at least one household head knowing bat hunters residing in the village (Table 2). Of the 63 household heads who reported family members hunting bats, 62% (39/63) reported using bats for some combination of medicine and food: 14% (9/63) used bats for both medicine and food, 17% (11/63) for medicine but not food and 30% (19/63) for food but not medicine.
Figure 1.
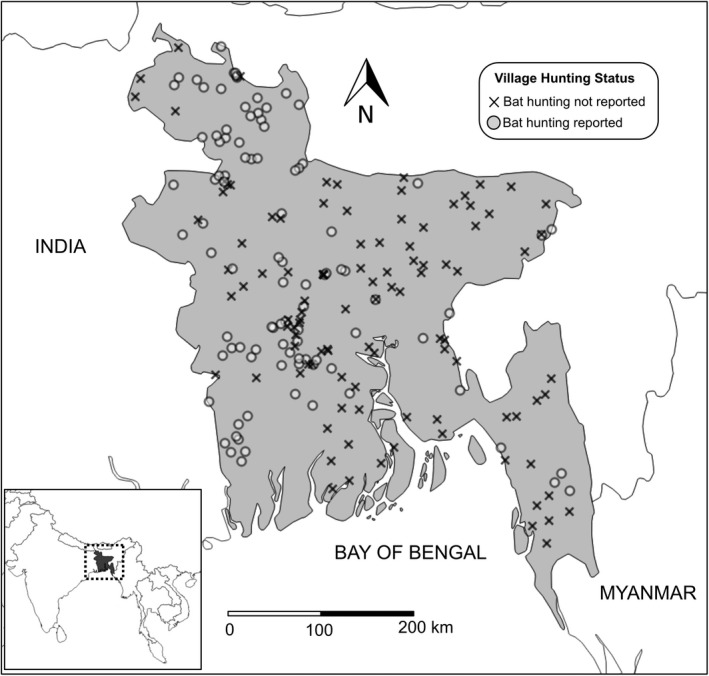
Location of villages included in the survey and bat hunting status. In villages where bat hunting is reported, household heads either reported their family members hunting and/or knowledge of another villager hunting.
Table 2.
Number and percentage of villages by hunting classification
| Hunters within household | Known hunters within village but outside household | Villages (num) | % of all villages | % of hunting villages |
|---|---|---|---|---|
| Yes | Yes | 36 | 18% | 36% |
| Yes | No | 3 | 2% | 3% |
| No | Yes | 62 | 30% | 61% |
| No | No | 103 | 50% | – |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
In unadjusted mixed‐effects models (Table 3), household heads who had a bat roost on their property were more likely to report household members hunting bats (prevalence ratio [PR] 2.3, 95% confidence interval [CI] 1.1–4.9). A higher percentage of households who kept livestock and reported 80% or more of fruit trees on the property being mangos and guavas hunted bats, but in both cases the confidence interval crossed one. Reported bat hunting by households did not increase with increasing bat density per square kilometre (PR 0.7, 95% CI 0.2–2.1), with increasing bats per village population (PR 1.1, 95% CI 0.7–1.7) or with increasing number of fruit trees (PR 1.0, 95% CI 0.9–1.2). Neither per capita tree wealth nor per capita livestock wealth had any bearing on the likelihood that households reported bat hunting (PR 1.1, 95% CI 0.9–1.2 and PR 1.1, 95% CI 0.9–1.4, respectively). In the best‐fit multivariable mixed‐effects logistic regression model (Table 3), which included the presence of a nearby roost, the presence of livestock and the presence of 80% or more of all fruit trees being mangos and/or guavas, bat hunting was also more likely to occur if household heads reported the presence of a bat roost (adjusted PR [aPR] 2.3, 95% CI 1.1–4.9).
Table 3.
Results of mixed‐effects models of environmental variables affecting likelihood of household heads to report bat hunting
| Characteristic | Total households | % (num) Households reporting hunting | Unadjusted + village clustering (PR, 95% CI) | Adjusted + village clustering (aPR, 95% CI) |
|---|---|---|---|---|
| Bat roost on property | ||||
| No | 4494 | 1.1% (51) | Ref | Ref |
| Yes | 567 | 2.1% (12) | 2.3 (1.1–4.9)a | 2.3 (1.1–4.9)a |
| Bats inside courtyards and buildings in month preceding survey | ||||
| No | 3465 | 1.1% (40) | Ref | – |
| Yes | 1528 | 0.7% (23) | 1.1 (0.5–2.1) | – |
| ≥50% fruit trees mango and/or guava | ||||
| No | 4554 | 1.2% (56) | Ref | – |
| Yes | 444 | 1.6% (7) | 1.5 (0.6–3.7) | – |
| ≥80% fruit trees mango and/or guava | ||||
| No | 4891 | 1.2% (60) | Ref | Ref |
| Yes | 107 | 2.7% (3) | 2.9 (0.8–10.6) | 3.0 (0.8–11.1) |
| Livestock ownership | ||||
| No | 1412 | 0.6% (8) | Ref | Ref |
| Yes | 3649 | 1.5% (55) | 2.1 (0.9–4.6) | 2.0 (0.9–4.6) |
| Per capita fruit tree wealth | – | – | 1.1 (0.9–1.2) | – |
| Per capita livestock wealth | – | – | 1.1 (0.9–1.4) | – |
| Total number of trees owned | – | – | 1.0 (0.9–1.2) | – |
| Bats per sq km | – | – | 0.7 (0.2–2.1) | – |
| Bats per village population | – | – | 1.1 (0.7–1.7) | – |
PR, prevalence ratio; aPR, adjusted prevalence ratio; CI, confidence interval.
P < 0.05.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
In univariate analysis on the village level (Table 4), villages located in both the south‐west (PR 5.7, 95% CI 1.9–16.9) and north‐west (PR 6.8, 95% CI 2.3–20.1) regions of Bangladesh were more likely to have bat hunting activity. These geographic associations remained consistent even when controlling for both bat and tree density per square mile (south‐west region: aPR 6.8 95% CI 2.1–21.6; north‐west region: aPR 7.5, 95% CI 2.5–23.0).
Table 4.
Results of univariate and multivariate logistic regression models of village‐level variables affecting likelihood of bat hunting within the village
| Characteristic | Total villages | Villages reporting hunting | % villages reporting hunting | Unadjusted PR, 95% CI | Adjusted PRb, 95% CI |
|---|---|---|---|---|---|
| Village location | |||||
| North‐east | 30 | 3 | 10% | Ref | Ref |
| South‐east | 36 | 9 | 25% | 2.5 (0.7–8.4) | 2.9 (0.8–10.0) |
| North‐west | 75 | 51 | 68% | 6.8 (2.3–20.1)a | 7.5 (2.5–23.0)a |
| South‐west | 60 | 34 | 57% | 5.7 (1.9–16.9)a | 6.8 (2.1–21.6)a |
| Bats per sq km | – | – | – | 0.9 (0.7–1.1) | 0.8 (0.4–1.7) |
| Trees per sq km | – | – | – | 1.1 (0.9–1.2) | 0.9 (0.6–1.4) |
PR, prevalence ratio; CI, confidence interval.
P < 0.05.
Multivariate model includes village location, bats per square kilometre, trees per square kilometre and an interaction term between bat and tree density.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Discussion
Our results suggest that villagers in rural Bangladesh frequently observe large‐bodied frugivorous bats, which are most likely Pteropus giganteus, an important zoonotic disease reservoir. While a smaller proportion live near roosting sites and report large‐bodied fruit bats within home complexes and courtyards, a large proportion observed bats flying overhead and in their fruit trees.
Bat hunting is common, with hunting reported in nearly half of all surveyed villages. Bat hunters report using bats both as a food source and for medicinal purposes. In our analysis, households reporting bat roosts on their property were slightly more likely to report bat hunting. This result suggests that at least some hunting is opportunistic, with villagers more likely to kill bats because they are roosting nearby and represent easy targets or pests. Qualitative work done in some of these same villages has shown that date palm sap harvesters do view bats as pests (Nahar et al., 2010).
In addition, there was a trend towards more bat hunting in households that owned livestock and had orchards dominated by mango and guava trees. These trends may also suggest that household heads kill bats for pest control. Household heads that own livestock may be more likely to see the bats as pests that get into animal enclosures. Because fruit trees provide food for both animals and people, villagers may take steps to protect their fruit trees against frugivorous bats.
Despite these trends suggesting increased hunting when large‐bodied bats roost in close proximity to villagers or threaten fruit and livestock resources, none of these variables were strong predictors of hunting activity. Bat hunting did not increase with greater bat density per square kilometre, suggesting bat hunters are also seeking out and actively targeting the animals, even in areas where bats may be less abundant. Villagers use bats as both food and medicine, suggesting the utility of bats as a commodity. These uses might hint at bat hunting as a profitable enterprise in Bangladesh, although we did not specifically ask about this practice in this study.
Bat hunting was not associated with proxy measures of wealth, suggesting that hunting is not more or less common in different socio‐economic groups. In addition, bat hunting is much more common in Western regions of Bangladesh, even when controlling for bat population densities. This may suggest that the propensity to hunt bats in Bangladesh has social influences that vary across the country and cluster geographically.
Our analysis does have limitations. While our study does suggest bat hunting is widespread, we interviewed relatively few bat hunting households. Because of this, there are limitations to our statistical inference. For example, we may be underappreciating trends, such as increased hunting with livestock ownership and with increasing ownership of mango and guava trees. Additionally, the data used in this analysis were not collected primarily for the purpose of studying bat hunting. A dedicated survey focused on bat hunting and exposures utilizing open‐ended questions would provide a more thorough explication of bat–human interactions. The number of bats killed and other reasons for killing bats besides consumption and medicinal uses are missing from our data set. Questions regarding the frequency of bats hunted and killed for sport or for pest control are also notably missing from our survey. While we know that bat hunting is widespread, we do not know how many individuals participate in hunting. Nor do we have an understanding of the financial rewards of hunting or extent of markets where hunted bats might be sold. Finally, while our survey focused on large‐bodied fruit bats, with the intention of specifically studying interactions with Pteropus giganteus, we cannot confirm that villagers did not report interactions or the presence of other bat species.
Although bat hunting has not been clearly described or quantified in Bangladesh, the hunting of similarly large‐bodied species across Asia, the Pacific and Indian Ocean Islands and Africa is well known (Mickleburgh et al., 2009; Harrison et al., 2011; Kamins et al., 2011). Hunting brings with it the handling, butchering and preparing of bats, and these activities all increase the chances of handlers being bitten or exposed to bat tissues and bodily fluids. These exposures all likely increase the risk of zoonotic disease spillover. Butchering of bats has been linked to spillover of a Nipah‐like henipavirus in Cameroon (Pernet et al., 2014) and the bat bush meat exchange has been directly tied to at least one outbreak of Ebola (Leroy et al., 2009). The presence of bat hunting in Bangladesh is concerning given that Nipah virus infection is enzootic in Pteropus bats, and exposure to the pathogen through hunting or bat bush meat processing or consumption might be a potential spillover pathway. However, at this time, no association between bat hunting and Nipah virus infection in humans has been uncovered in investigations of Nipah outbreaks in Bangladesh (Hossain et al., 2008; Rahman et al., 2012; Hegde et al., 2013).
Bat hunting represents a risk to large‐bodied frugivorous bat populations that play an important role in the tropical ecosystem. Pteropus bats are recognized as a key species in pollination and seed dispersal of economically important trees throughout South Asia and their excrement and dropped fruit spats below roosting sites play an important role as natural organic manures (Fujita and Tuttle, 1991; Goveas et al., 2006). The low reproductive and high natural mortality rates of Pteropus species make them vulnerable to over‐hunting (McIlwee and Martin, 2002). Local management of fruit bat hunting has been suggested to avoid over‐hunting (Epstein et al., 2009). A survey of bat species in Bangladesh suggests that Pteropus bats are threatened due to destruction of roosting sites and exploitation of natural resources (Khan, 2001). While it is unclear from our study how many bats are killed by hunters in Bangladesh, it is clear that this behaviour is widespread, and further studies of the impact of hunting would help determine whether this activity threatens ecologically important Pteropus populations.
Despite limitations, this exploratory study suggests widespread hunting of large‐bodied frugivorous bats in Bangladesh and high exposures between bats and humans. The geographical nature of bat hunting suggests that interventions to reduce pathogen spillover from hunting and butchering could be targeted to specific locations. Studies further detailing the reasons for bat hunting, the social factors involved and the numbers of bats killed are warranted and could potentially help to limit the spillover of zoonotic disease and ensure the conservation of ecologically important large‐bodied fruit bats.
Acknowledgements
This research protocol collecting the data used in this analysis was funded by a National Science Foundation (NSF) and a National Institutes of Health (NIH) grant for Ecology and Evolution of Infectious Diseases, grant number 2R01‐TW005869. ICDDR,B acknowledges with gratitude the commitment of NSF and NIH to its research efforts. ICDDR,B is thankful to the Governments of Bangladesh, Canada, Sweden and the UK for providing core/unrestricted support.
References
- Anthony, S. J. , Epstein J. H., Murray K. A., Navarrete‐Macias I., Zambrana‐Torrelio C. M., Solovyov A., Ojeda‐Flores R., Arrigo N. C., Islam A., Ali Khan S., Hosseini P., Bogich T. L., Olival K. J., Sanchez‐Leon M. D., Karesh W. B., Goldstein T., Luby S. P., Morse S. S., Mazet J. A. K., Daszak P., and Lipkin W. I., 2013: A strategy to estimate unknown viral diversity in mammals. MBio, 4, e00598–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlot, S. , and Celisse A., 2010: A survey of cross‐validation procedures for model selection. Stat. Surv. 4, 40–79. [Google Scholar]
- Bartoń, K. , 2015: MuMIn: Multi‐Model Inference. R package version 1.15.1. http://CRAN.R-project.org/packageMuMIn
- Bates, P. J. , and Harrison D. L., 1997: Bats of the Indian Subcontinent. Harrison Zoological Museum Publications, Kent, England. [Google Scholar]
- Bates, D. , Maechler M., Bolker B., and Walker S., 2015: lme4: Linear mixed‐effects models using Eigen and S4. R package version 1. 1‐9, https://CRAN.R-project.orzrg/package=lme4.
- Burnham, K. P. , and Anderson D. R., 2002: Model Selection and Multimodel Inference: a Practical Information‐Theoretic Approach. 2nd edn Springer‐Verlag, New York. [Google Scholar]
- Epstein, J. H. , Olival K. J., Pulliam J. R. C., Smith C., Westrum J., Hughes T., Dobson A. P., Zubaid A., Rahman S. A., Basir M. M., Field H. E., and Daszak P., 2009: Pteropus vampyrus, a hunted migratory species with a multinational home‐range and a need for regional management. J. Appl. Ecol. 46, 991–1002. [Google Scholar]
- Fujita, M. , and Tuttle M., 1991: Flying Foxes (Chiroptera: Pteropodidae): threatened Animals of Key Ecological and Economic Importance. Conserv. Biol. 5, 455–463. [Google Scholar]
- Goveas, S. , Miranda E., Seena S., and Sridhar K., 2006: Observations on guano and bolus of Indian flying fox, Pteropus Giganteus . Curr. Sci. 90, 160–162. [Google Scholar]
- Greenland, S. , Pearl J., and Robins J. M., 1999: Causal diagrams for epidemiologic research. Epidemiology (Cambridge, Mass. ) 10, 37–48. [PubMed] [Google Scholar]
- Hahn, M. B. , Epstein J. H., Gurley E. S., Islam M. S., Luby S. P., Daszak P., and Patz J. A., 2014: Roosting behaviour and habitat selection of Pteropus giganteusreveal potential links to Nipah virus epidemiology. J. Appl. Ecol. 51, 376–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, M. E. , Cheyne S. M., Darma F., and Ribowo D. A., 2011: Hunting of flying foxes and perception of disease risk in Indonesian Borneo. Biol. Conserv. 144, 1324–1365. [Google Scholar]
- Hegde, S. , Sazzad H. M. S., Hossain M. J., Kenah E., Daszak P., Rahman M., Luby S. P., and Gurley E. S., 2013: Risk factor analysis for Nipah infection in Bangladesh, 2004 to 2012. Am. J. Trop. Med. Hyg. 89, 279. [Google Scholar]
- Hossain, M. J. , Gurley E. S., Montgomery J. M., Bell M., Carroll D. S., Hsu V. P., Formenty P., Croisier A., Bertherat E., Faiz M. A., Azad A. K., Islam R., Molla M. A. R., Ksiazek T. G., Rota P. A., Comer J. A., Rollin P. E., Luby S. P., and Breiman R. F., 2008: Clinical presentation of Nipah virus infection in Bangladesh. Clin. Infect. Dis. 46, 977–984. [DOI] [PubMed] [Google Scholar]
- Kamins, A. O. , Restif O., Ntiamoa‐Baidu Y., Suu‐Ire R., Hayman D. T. S., Cunningham A. A., Wood J. L. N., and Rowcliffe J. M., 2011: Uncovering the fruit bat bushmeat commodity chain and the true extent of fruit bat hunting in Ghana, West Africa. Biol. Conserv. 144, 3000–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, M. A. R. , 2001: Status and distribution of bats in Bangladesh with notes on their ecology. Zoo's Print J 16, 479–483. [Google Scholar]
- Kumar, K. , 1989: Indicators for Measuring Changes in Income, Food Availability and Consumption, and the Natural Resource Base. US Agency for International Development, Washington DC. [Google Scholar]
- Leroy, E. M. , Epelboin A., Mondonge V., Pourrut X., Gonzalez J.‐P., Muyembe Tamfum J.‐J., and Formenty P., 2009: Human ebola outbreak resulting from direct exposure to fruit bats in Luebo, Democratic Republic of Congo, 2007. Vector Borne Zoonotic Dis. 9, 723–728. [DOI] [PubMed] [Google Scholar]
- Luis, A. D. , Hayman D. T. S., O'Shea T. J., Cryan P. M., Gilbert A. T., Pulliam J. R. C., Mills J. N., Timonin M. E., Willis C. K. R., Cunningham A. A., Fooks A. R., Rupprecht C. E., Wood J. L. N., and Webb C. T., 2013: A comparison of bats and rodents as reservoirs of zoonotic viruses: are bats special? Proc. R. Soc. B Biol. Sci. 280, 20122753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwee, A. P. , and Martin L., 2002: On the intrinsic capacity for increases of Australian flying‐foxes (Pteropus spp, Megachiroptera). Aust. Zool. 32, 76–100. [Google Scholar]
- Mickleburgh, S. , Waylen K., and Racey P., 2009: Bats as bushmeat: a global review. Oryx 43, 217. [Google Scholar]
- Nahar, N. , Sultana R., Gurley E., Hossain M. J., and Luby S., 2010: Date palm sap collection: exploring opportunities to prevent Nipah transmission. Ecohealth 7, 196–203. [DOI] [PubMed] [Google Scholar]
- Olival, K. J. , Epstein J. H., Wang L. F., and Field H. E., 2012: Are bats unique viral reservoirs? In: Aguirre A. A., Ostfeld R. S. and Daszak P. (eds), New Directions in Conservation Medicine: applied Cases of Ecological Health, pp. 195–212. Oxford University Press, New York. [Google Scholar]
- Pernet, O. , Schneider B. S., Beaty S. M., Lebreton M., Yun T. E., Park A., Zachariah T. T., Bowden T. A., Hitchens P., Ramirez C. M., Daszak P., Mazet J., Freiberg A. N., Wolfe N. D., and Lee B., 2014: Evidence for henipavirus spillover into human populations in Africa. Nat. Commun. 5, 5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowright, R. K. , Eby P., Hudson P. J., Smith I. L., Westcott D., Bryden W. L., Middleton D., Reid P. A., McFarlane R. A., Martin G., Tabor G. M., Skerratt L. F., Anderson D. L., Crameri G., Quammen D., Jordan D., Freeman P., Wang L.‐F., Epstein J. H., Marsh G. A., Kung N. Y., and McCallum H., 2015: Ecological dynamics of emerging bat virus spillover. Proc. R. Soc. B Biol. Sci. 282, 20142124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team , 2015: R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria: https://www.R-project.org/. [Google Scholar]
- Rahman, M. A. , Hossain M. J., Sultana S., Homaira N., Khan S. U., Rahman M., Gurley E. S., Rollin P. E., Lo M. K., Comer J. A., Lowe L., Rota P. A., Ksiazek T. G., Kenah E., Sharker Y., and Luby S. P., 2012: Date palm sap linked to Nipah virus outbreak in Bangladesh, 2008. Vector Borne Zoonotic Dis. 12, 65–72. [DOI] [PubMed] [Google Scholar]
- Santos, C. , Fiaccone R. L., Oliveira N. F., Cunha S., Barreto M. L., do Carmo M., Moncayo A.‐L., Rodrigues L. C., Cooper P. J., and Amorim L. D., 2008: Estimating adjusted prevalence ratio in clustered cross‐sectional epidemiological data. BMC Med. Res. Methodol. 8, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, I. , and Wang L. F., 2013: Bats and their virome: an important source of emerging viruses capable of infecting humans. Curr. Opin. Virol. 3, 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, J. L. N. , Leach M., Waldman L., Macgregor H., Fooks A. R., Jones K. E., Restif O., Dechmann D., Hayman D. T. S., Baker K. S., Peel A. J., Kamins A. O., Fahr J., Ntiamoa‐Baidu Y., Suu‐Ire R., Breiman R. F., Epstein J. H., Field H. E., and Cunningham A. A., 2012: A framework for the study of zoonotic disease emergence and its drivers: spillover of bat pathogens as a case study. Philos. Trans. R. Soc. Lond. B Biol. Sci., 367, 2881–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]


