Abstract
Background
Fatigue remains a prevalent and debilitating symptom in persons with non-small cell lung cancer (NSCLC). Exercise has been shown to be effective in reducing fatigue; yet interventions are limited for post-surgical NSCLC patients. To date, while surgery is offered as a standard curative treatment for NSCLC, no formal guidelines exist for post-surgical rehabilitation.
Objective
This study focuses on the design and testing of a post-surgical intervention for NSCLC patients to promote perceived self-efficacy for fatigue self-management targeting cancer-related fatigue (CRF) severity and its associated fatigability through exercise.
Interventions/ Methods
A two-arm randomized controlled trial (RCT) was used to examine the impact of a 6-week rehabilitative CRF self-management exercise intervention on 37 NSCLC participants compared with 35 control group participants receiving usual care from diagnosis to 6 weeks post-surgical hospital discharge.
Results
We exceeded goals for recruitment (66%), retention (97%), adherence (93%), and acceptability. Our 6-week exercise intervention demonstrated preliminary efficacy in significantly reducing CRF severity and fatigability as compared to usual care, with mean CRF levels restored to levels lower than pre-surgery. Likewise, the exercise group's functional performance (physical and mental health scores) exceeded usual care. Further, no adverse events were reported; participants had a mean age of 67 and a mean of 8 comorbid conditions.
Conclusions
An exercise intervention for post-surgical NSCLC patients is feasible, safe, and highly acceptable showing positive changes in CRF self-management.
Implications for Practice
To advance practice, testing of the effectiveness of this health-promoting self-management exercise intervention in a larger-scale RCT.
Persons with non-small cell lung cancer (NSCLC) report more unmet supportive care needs than other cancer populations.1 Paradoxically, the NSCLC population is among the most vulnerable and least studied.2 Overcoming fatigue and becoming engaged in exercise are the most prevalent unmet supportive care needs of the NSCLC population that are essential to meet the physical demands of daily living.3 Fatigue remains a prevalent, severe, and debilitating cancer symptom particularly in persons with NSCLC, and fatigue persists after diagnosis and treatment.4,5 To date, while surgery is offered as a standard curative treatment for NSCLC, no formal guidelines exist for post-surgical rehabilitation.6 This study focuses on the design and testing of an intervention that fills this void for post-surgical rehabilitation of persons with NSCLC to promote fatigue self-management targeting fatigue severity and its associated fatigability (the degree of fatigue associated with activity) through exercise.
Cancer-Related Fatigue
Cancer-related fatigue (CRF) is a clinical problem known by its definition as a distressing, persistent subjective sense of tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning.7 Fatigue remains the most common, severe, distressing and unmanaged symptom in persons with cancer.4 In one 2013 study among 3,106 ambulatory patients with breast, prostate, colorectal, or lung cancer with nearly 75% receiving treatment, fatigue was the more prevalent and severe symptom in each group.8 Moderate or severe fatigue was most prevalent in persons with lung cancer than in persons with breast, prostate, or colorectal cancer. In 2014, Wang and colleagues reported that among persons with solid tumors receiving cancer treatment, having lung cancer was one of the most important contributors to moderate to severe fatigue.9 Fatigue is persistent even one year post-diagnosis and after treatment, as shown in a 2011 national population-based study of 4,512 cancer survivors.10 In this study, greater than 25% of the participants reported having high symptom burden more than one year after diagnosis, with persons with lung cancer reporting high symptom burden with fatigue having the greatest impact on health-related quality of life (QOL). In a 2012 study of long-term lung cancer survivors (> 5 years), participants reported significantly worsened symptoms over time, including the most prevalent symptom: fatigue.11 These studies confirm that little has changed with CRF since 2008. At that time, 80% of a cross-sectional national survey of 1,569 persons with cancer undergoing treatment reported having fatigue, with nearly 67% rating fatigue as debilitating (≥6 on a 10-point scale) and 33% rating a reduction in fatigue a 10 (“very important”) on a 10-point scale.12
Another perspective of fatigue, fatigability, relates to the degree of fatigue associated with activity; whereas, fatigue is a subjective sense of tiredness or exhaustion that interferes with usual activity.13 Our research is potentially transformative because it is the first study to target a priority symptom, fatigue and its associated fatigability, in the vulnerable post-surgical NSCLC patient during the transition from hospital to home. Studies have not addressed the critical post-surgical hospital to home transition leaving patients without a plan to address symptoms such as CRF severity and its associated fatigability. Research suggests that light exercise may improve functioning and QOL and may be an alternative to higher intensity exercise programs for deconditioned populations with co-morbid conditions such as the NSCLC population.14-17 Also, no exercise intervention studies for the post-surgical NSCLC population have utilized fatigability to measure the effectiveness of a fatigue intervention. It is critical to understand how effective an exercise intervention is by understanding its effect on fatigue when a patient is subjected to a given level of exertion. If an exercise intervention is to positively affect a person's quality of life, it needs to continue to have a positive effect on fatigue even during exertion. Thus, without a fatigability measure, it is difficult to measure the effectiveness of an intervention for fatigue. Failure to consider the associated activity level may lead to inaccurate conclusions about the efficacy of an intervention for CRF. For example, an exercise intervention that leads to unchanged subjective fatigue but improved activity may seem ineffective if only fatigue is evaluated. However, an exercise intervention that leads to reduced fatigue and also reduced activity may seem effective if only fatigue is evaluated. To further highlight the importance of differentiating between fatigue and fatigability, a person may consider two older persons the same age with the same degree of fatigue. One person may be healthy and physically active and the other may have multiple comorbid conditions and modestly active. Whereas both persons may report the same degree of fatigue, the latter has greater fatigability (same fatigue with less activity).
Building Evidence for a Post-Surgical Rehabilitative Exercise Solution
No guidelines exist for the rehabilitation of the post-surgical NSCLC population and the optimal exercise program for surgical recovery is not known.18-20 Research investigating the effect of exercise in the lung cancer population following surgery is limited to heterogeneity of exercise programs.20,21 Consequently, there is limited evidence on the most essential attributes to making exercise effective (mode, intensity, frequency, duration, timing, subjective aspects such as enjoyment and motivation) in this population. However, while limited in number, studies report on attributes of exercise interventions that present barriers to participation.22 Barriers include travel to facility-based exercise programs, the monotony of using traditional equipment and the onerous nature of fulfilling moderate to vigorous intensity exercise prescriptions. According to Bandura, these barriers need to be removed to set the stage to build perceived self-efficacy (perception of ability; (PSE)) to engage in exercise.23 Moreover, successful adoption of exercise even in healthy populations includes providing knowledge, skills, and abilities to initiate, advance, and maintain an exercise program, especially when facing added barriers such as post-surgical symptoms including CRF.23
The approach in this study was to leverage pilot work from a single-arm (no control group) study where barriers to exercise were removed by incorporating a light-intensity, home-based, self-paced, enjoyable exercise supported by a registered nurse.14,15 This research addresses the National Cancer Institute recommendations for high-priority research focused on overcoming barriers and the implementation of strategies to increase uptake and effect size of interventions such as exercise to promote CRF self-management.24 To date, the eligibility criteria for exercise interventions in post-surgical NSCLC patients have excluded much of the NSCLC post-surgical population by excluding patients with common comorbid conditions.25 As a result, we designed our intervention to ensure that post-surgical NSCLC patients with very common comorbid conditions would be included and able to safely perform the intervention.
Theoretical Framework
Consequently, this study proposes a highly accessible solution to meet the unmet rehabilitative needs via a PSE-enhancing intervention to improve CRF severity, fatigability and functional performance. As shown in Figure 1, the proposed research is based on Principles of the Transitional Care Model, which underpins the approach to the implementation of the proposed intervention.26 A tested theoretical framework from the Theory of Symptom Self-Management (TSSM) guides its design and testing.27,28 The TSSM provides insight into how a person's PSE in his or her ability to self-manage symptoms such as CRF influences performance of those behaviors. Cancer-related fatigue and PSE affect CRF self-management behaviors. The performance of CRF self-management behaviors affects fatigue and functional status. Two dimensions of functional status are functional capacity (one's maximum potential to perform activities) and functional performance (daily activities in the normal course of their lives).13 This study investigates effects of an intervention on CRF severity and fatigability, with fatigability indicated by a person's functional capacity that enables or restricts functional performance.
Figure 1. Theoretical Framework from Hoffman27, 28 and Naylor26.
Methods
Research Design
This study incorporated a two-arm randomized controlled trial design. Participants were persons who were undergoing surgery for NSCLC and randomly assigned to 1 of 2 groups following successful completion of informed consent and baseline assessments: Group 1 was an intervention group (IG) that participated in an exercise and balance program. Group 2 was a control group (CG) receiving usual care, standard medical care from their health providers. A random number table was generated by the team statistician to identify whether the potential participant was assigned to the IG or CG. The random number table included decimal numbers between 0 and 1. The random allocation sequences were determined by the table of random numbers. As participants enrolled in the study, they were assigned a predefined random number; if the number was 0.5 or greater, the participant was assigned to the CG, if the number was less than 0.5 the participant was assigned to the IG. The random allocation was a predefined assigned slot when a patient enrolled in the study. The recruiter used the next available pre-defined slot for allocation. Potential participants were given incomplete disclosure about the intervention as approved by the institutional review board since it may be possible to replicate the exercise intervention. Practitioners were blinded during recruitment; however, it was not possible to blind research staff to the participant's assigned group since the activity in each group was easily discernable. To ensure equal groups at baseline, the randomization scheme included blocking factors to include the patient's age (< or equal to 64 years; 65 to 74 years; > or equal to 75 years) and functional capacity level (those that achieve > or equal to 452 meters as measured by the 6 minute walk test [6MW]). These age group categories accounted for the increasing prevalence of lung cancer by age and served to inform policy recommendations by providing evidence at the 64 to 65 year boundary where many patients transition to Medicare.29 Further, Jones et al.30 found that the 6MWT distance provides important objective prognostic information for persons with NSCLC relative to survival with 452 meters marking the minimum distance achieved for those in the high capacity functioning group who had significantly prolonged survival.
Sample
Participants were recruited from three university teaching hospitals in the state of Michigan. All participants were men and women at least 21 years of age with suspected NSCLC to be confirmed after surgery; had a Karnofsky Performance Status Score of 70% or greater with medically stable co-morbid conditions pre-surgery; had thoracic surgeon pre- and post-surgery approval to participate; had phone access capability and were able to speak and write English, and owned a television. Exclusion criteria included severe impairment in seeing, hearing or speaking; active treatment for malignancy within the past six months (other than non-melanoma skin cancer) and when undergoing long-term hormonal treatment for common cancers such as breast and prostate where disease was stable; presence of metastatic disease; required portable oxygen for activities of daily living; weighed greater than 330 pounds (weight capacity of the Wii board); had a history of photosensitive seizures or dementia; or any condition or disorder that would impede safe participation as directed or unable to fully participate.
The sample size for this study was determined via a priori power analysis for regression using 10 predictor variables, a one-tailed test with a level of significance set at 0.05, power set at 0.80, and an effect size of 0.30 requiring a minimum sample size of 64, using G-Power International Statistical Power Analysis Program.31 We over sampled to account for an expected attrition rate of 30% based on prior cancer exercise studies. The study flow is presented in Figure 2. Of the 206 potential participants screened, 119 persons either did not meet eligibility criteria (n = 75) or were not interested in the study (n = 44). A total of 87 participants entered the study with 47 randomized to the IG and 40 to the CG culminating with 37 participants analyzed in the IG and 35 analyzed in the CG. Note that while participants were screened for eligibility and then assigned to the IG and CG, there was a time gap between assignment and start of the intervention. During this gap, eligibility changed for some patients. Patients could become ineligible prior to starting the study post-surgery after discharge from the hospital to home at baseline data collection. For example, three patients decided not to have surgery and underwent chemotherapy instead. Further, two participants became ineligible before discharge from the hospital because they no longer had a television—one participant's family member took the participant's television away while the participant was in the hospital and a second participant's television was inoperable.
Figure 2. Study Flow.
Abbreviation: NSCLC, non-small cell lung cancer. TV, television
General IG and CG Procedures
Participants scheduled to undergo surgery for NSCLC were identified by the surgeon's office in advance of surgery, recruited, and enrolled by the nurse recruiter with research staff support collecting data after informed consent. These data were collected again by research staff by phone visit within 3 days after hospital discharge post-surgery; and at the end of weeks 1—6 post-surgery baseline. Each research staff member was trained and followed an outlined protocol for quality assurance. The CG received phone visits post-surgery which provided for control for staff interaction and data collection. Both the IG and CG received a pedometer and instructions on how to use the pedometer for recording their total number of steps per day starting after hospital discharge post-surgery for 6 weeks. In addition, a primary registered nurse (nurse) was assigned to each IG participant for the duration of the study which follows the principles of the Transitional Care Model, particularly participant collaboration and continuity of care. The nurse engaged each participant after pre-surgery baseline data were collected via a hospital visit and provided an overview of the exercise intervention while teaching PSE-enhancing CRF self-management education which used the National Comprehensive Cancer Network Guidelines for Cancer-Related Fatigue. Note that should an IG or CG participant experience unmanaged symptoms (> 5 on a scale of 0—10, 10 = most severe) or any clinical problem, participants of both groups were referred to their healthcare provider to ensure safe follow-up and to mitigate variation between groups.
Post-Surgery IG Procedures
Home Screen
The nurse conducted a phone visit with the goal of within 24 hours after hospital discharge to screen the participant's readiness to start the intervention. Participants were deemed ready to start if their usual level of symptom severity for key symptoms of pain, fatigue, vomiting, and dyspnea were rated ≤ 5 on an 11-point scale (10 = most severe). If the participant was ready to start exercise, a home visit was scheduled to start the exercise within 4 days from discharge. When the severity of a key symptom was rated > 5, the nurse would contact the participant's surgeon's office for recommendations. The nurse would repeat this step each day until the participant was ready to start exercising. In this study, the mean home screening was completed 37.9 hours (SD, 24) after hospital discharge with exercise initiated within a mean of 4 days (SD, 2 days) after hospital discharge.
Initial Home Visit with the IG and Nurse
The initial home visit included connecting the Nintendo Wii Fit Plus to the participant's television, teaching the exercise intervention, and reinforcing the CRF self-management education and exercise safety. The nurse followed-up the initial home visit with a phone visit within 24 hours to answer any questions and concerns regarding the intervention.
Follow-up Visits with IG and Nurse
The nurse conducted a home visit at the start of week 2 of the intervention and phone visits at the start of weeks 3—6 with additional visits scheduled on request of the participant for those not yet comfortable with implementing the intervention on their own. The mean number of home visits performed was 2.8 (SD, 1.1), with 54% only needing 2 visits. During these visits, the nurse reviewed the IG participant's Daily Diary and provided PSE enhancing CRF self-management interventions to support the exercise program and adjust the exercise prescription per guidelines. The participant used the Daily Diary to record exercise activity each week.
The Exercise Program
The exercise program was designed to promote regular, light-intensity walking and balance exercise of < 3.0 metabolic equivalents (METs) that correspond to levels of usual activities of daily living.32 Participants performed warm-up exercises designed for this patient population. Walking with the Wii throughout this article implies walking in place with the Wii. Wii walking was self-paced and comfortable with the Wii exercise equipment creating a virtual environment where the participant saw themselves, their friends, and pets walking on a sunny day in a town of happy people providing encouragement to continue. Duration of walking started at 5 minutes each day for 5 days during week 1 and continued to build by 5 minutes per day each week with the goal of walking with the Wii of 30 minutes per day during week 6. At the start of each week, the nurse assessed the participant's readiness to advance the walking prescription. The duration was increased by 5 minutes each week if the participant's PSE for attaining that duration was ≥ 70% on a 0—100% scale, with 100% having the highest PSE.33 The participants also completed balance exercises 5 days a week for weeks 1—6 from a menu of predetermined Wii balance exercises which used a gaming format. Data were recorded in the participant's Daily Diary and confirmed by research staff as recorded in the Wii Fit-Plus.
Exercise Program Safety
Participants were provided a safety checklist to review ensuring safety prior to the start of exercise. In addition, participants were taught to maintain a light-intensity dose of exercise by: 1) monitoring heart rate using a heart rate monitor to ensure their heart rate was ≤ 60% of their heart rate reserve; keeping their exertion level ≤ 3 on a scale of 0 to 10; and completing the Talk Test by being able to talk and sing while exercising.34 On a daily basis, participants documented any symptoms or problems that occurred that were exercise related for review with their nurse. The nurse was accessible by phone and could make a home visit at the request of the participant. The nurse conducted a weekly visit either at the home or by phone and screened for any participant symptoms or clinical problems.
Primary Outcome Measures
Feasibility
Feasibility was determined by analyzing rates of recruitment, adherence, retention and monitoring for adverse events. The recruitment rate was measured as the percentage of the eligible patients that were recruited that enrolled. Adherence was measured as the percentage of those participating in the exercise program that adhered to the recommended exercise. Weekly adherence was calculated by taking the number of times the prescribed exercise was completed divided by the total number of exercises prescribed for the week. Retention was measured as the percentage of those enrolled that completed the program from the first phone visit after surgery to the final data collection at week 6. Adverse events were recorded in the Daily Diary and monitored by the nurse and Principal Investigator. Our goal was to show feasibility by the ability to recruit 50% of eligible participants; and show adherence and retention to the intervention at a rate of 70%.
Acceptability
Acceptability refers to the opinion of the participants on how acceptable our intervention was while using it in the study.14 An Acceptability Questionnaire was assessed at the end of week 6 (post-intervention) and was analogous to other studies with a 15-item questionnaire developed by the research team with a Flesch-Kincaid reading grade level of 6. Our goal was an average positive acceptability score of 4 (scale 0-6, 6 = highest acceptability).
Secondary Outcome Measures—Preliminary Efficacy
CRF severity and impact on daily functioning
was measured using the 9-item Brief Fatigue Inventory (BFI). On an 11-point scale (0–10, 10 = most severe), 3-items measure fatigue severity (now, usual, and worst fatigue) and 6-items measure the impact of fatigue on daily functioning in the past 24 hours. The score from the Brief Fatigue Inventory was calculated by summing each participant's response for 9 items and dividing by 9 for a composite score on an 11-point scale. Substantial evidence supports the psychometrics of the BFI in the cancer population.14,35
PSE for Fatigue Self-Management (PSEFSM)
was measured using the 6-item PSEFSM Instrument. An 11-point scale (0–10, 10 = very certain) measures confidence in performing fatigue self-management behaviors. Content and construct validity through mediation analyses and structural modeling demonstrated sound psychometrics in persons with cancer diagnoses.14, 51
PSE for Walking Duration
was measured using an instrument to assess a person's confidence to complete incremental 5-minute periods of light-intensity pace walking (5 to 30 minutes) on an 11-point scale (0%–100%, 100% = highly confident). Internal consistency and reliability reported that a sedentary, older sample with co-morbid conditions including cancer for pre-and post-exercise had a Cronbach's alpha > 0.95.14,36
Balance confidence
was measured with the Activities-Specific Balance Confidence Scale, a 16-item scale measuring confidence for balance during specific everyday physical activities. On an 11-point scale (0%–100%, 100% = most confident), higher scores indicate greater balance confidence during physical activities such as walking in and outside the home. The scale has demonstrated sound psychometrics tested in populations of older adults.14,37
CRF self-management behaviors (walking and balance)
A weekly diary with a structured format was completed by participants for which participants entered their weekly exercise prescription (provided by the nurse during the weekly home or phone visit) as well as their actual daily exercise performance. This format provided participants with the ability to record CRF self-management behaviors such as the daily number of minutes walked and number and type of balance exercises performed.
Functional status
was measured via two dimensions: functional capacity otherwise known as fatigability, and functional performance. Fatigability (fatigue with activity, indicator of functional capacity) was measured by the 6-minute walk test (6MWT) pre-surgery and post-study, by assessing fatigue reported during the 6MWT and the distance walked in 6 minutes. The 6MWT was implemented as per American Thoracic Society guidelines.14,38 The 6MWT is an assessment of submaximal functional capacity measured in distance walked in meters in 6 minutes and the associated fatigue levels. Fatigue levels were measured using the modified Borg Scale, a scale that has been used successfully in the post-surgery NSCLC population, measuring fatigue severity during the 6MWT on a 0–10 scale (10= most severe). The 6MWT is relevant to daily activities and is an objective measure used successfully in persons before and after lung resection.39 Functional performance was assessed via the Medical Outcomes Study Short Form-36 Version 2 Acute Recall (SF-36). The SF-36 is a widely used instrument that measures eight subscales encompassing the physical and mental health components of functional status. The scores of the subscales are linearly transformed, producing normative scores ranging from 0–100, with higher scores indicating greater functional status. The norm-based scoring focuses on physical and mental health components of the SF-36 scored showing a mean (50) and SD (10) in the general U.S. population, with component scores generally ranging from 20 to 70.17,40
The demographic questionnaire at baseline was completed by the participants and included age, race, gender, ethnicity, marital status, education level, employment status, income level, and smoking status. Medical chart review included surgical procedure, cancer stage, histological type, length of hospital stay, post-surgery inpatient complications, co-morbid conditions, medication history, and adjuvant treatment. In addition, a modified version of the Comorbidity Questionnaire was used to inquire about the presence of 15 chronic health conditions with content validity and test-retest reliability having been established.14,41 Smoking status (never, former, current) was assessed through responses to questions based on the Behavioral Risk Factor Survey regarding tobacco history and current smoking status.42 The Karnofsky Performance Status (KPS) was used as an appraisal of physical functioning. The KPS is commonly used in oncology and is scored from 0%, meaning dead, to 100% meaning no complaints, no special needs with no evidence of disease.14
Outcome Measures Collection Schedule
Outcome measures were collected by trained research staff. Instruments measuring CRF severity, PSEFSM, PSE for Walking Duration, and Balance Confidence were collected by research staff in person pre-surgery, and via telephone post-surgery within 72 hours of discharge from the hospital, and at the end of the exercise program weeks 1–6. Functional performance as indicated by the SF-36 questionnaire, was collected pre- and post-surgery, and at weeks 3, and 6. Functional capacity data were collected via the 6MWT on two occasions: 1) pre-surgery and 2) post-surgery prior to receiving chemotherapy and/or radiation therapy.
Data Analysis
All analyses were conducted with IBM SPSS version 19. Descriptive statistics were calculated to estimate the frequencies, means, and SDs of the study variables. Next, the pattern of change in CRF severity; PSE for fatigue self-management, walking and balance; CRF self-management walking and balance exercise behaviors were graphically displayed from pre- and post-surgery prior to the start of the intervention and at the end of each week of the 6-week study. Likewise, results for functional performance outcomes were graphed pre- and post-surgery, at week 3, and at the end of the intervention (6 weeks). Preliminary efficacy was assessed by comparing results of the IG (N=37) with those of the CG (N=35) on secondary outcomes of CRF severity and fatigability. Effect sizes were calculated using Cohen's d mean difference methodology by calculating the mean difference between intervention group and usual care divided by SD of control group (more conservative).43 All analyses were completed on the randomized groups by intent to treat. The analyses imputed data for participants with at least 50% of their data available, and excluded those with < 50%.44 For this study, except for one item, post-study 6MWT, there were 4 out of 72 participants results estimated. Two participants were not able to attend the post-study 6MWT due severe winter weather driving conditions and two participants refusing to perform the post-study 6MWT test. These values were estimated using the mean value approximation. Statistical significance was preset at p < 0.05.
Results
Baseline Participant Characteristics
Participants mean age was 67 years (range, 32-89 years); most were women (56%); married (74%); and retired (56%) followed by employed outside the home (29%). Most were White (89%) with 7% Black (n = 5) and 4% American Indian/Alaska Native (n = 3). Nineteen percent did not complete high school, 43% completed high school only; 28% had some college or post-high school training, and 10% with college and/or post-graduate education. Participants were assessed pre-surgery at a mean of 94% (SD 9) on the Karnofsky Performance Status Scale (0-100%, 100% = greatest performance status), meaning that they had minor signs and symptoms of disease. Sixteen percent of the participants in the IG and 11% in the CG reported never using tobacco. At the time of learning they required surgery for suspected NSCLC, 8% of the IG and 14% of the CG reported using tobacco. The mean number of comorbid conditions was 8 (SD 4; range 0-17). The mean BMI (in kg/m2) for participants before surgery was 28.6 (SD 5.2; range 18.7-41.6), with 26% participants having a normal BMI ranging from 20-24.9; 42% overweight (BMI 25-29.9), 29% type I and II obesity (BMI 30-39.9), and 3% morbidly obese (BMI > 40).
Baseline Clinical Characteristics
The majority of participants (90%) underwent an open thoracotomy with 10% undergoing video-assisted thoracoscopic surgery (VATS) or robotic lung resection. Surgical procedures for NSCLC included: 69% of patients had a lobectomy; 17% a wedge resection; 5% and 4% underwent pneumonectomy and bilobectomy resections respectively, and 5% had other forms of resections. The majority cell type following resection were adenocarcinoma (63%) and squamous (31%). The majority post-surgical cancer staging were stage I (62%) and stage II (21%), followed by stage III (11%) and stage IV (3%); another 3% were indeterminate. For the 4 participants in the IG and 2 in the CG receiving chemotherapy, chemotherapy was initiated at a mean time of 4 weeks after the start of the study protocol. Chemotherapy protocols used 2 drugs; one drug was a platinum compound with the second drug varying by participant.
The tests for equivalency at baseline between the randomized IG and CG regarding participant and clinical characteristics and outcome variables are presented in Table 1. The baseline independent and dependent variables were balanced except for the 6MWT distance and PSE for Fatigue Self-Management whereby the IG as compared to the CG walked less meters in 6 minutes and reported poorer PSE for fatigue self-management.
Table 1. Comparison of Participant Demographic and Clinical Characteristics at Baseline.
| IG (n = 37) | CG (n = 35) | ||||
|---|---|---|---|---|---|
| Variable | n | % | n | % | p |
| Age, mean (SD), years | 67.4 (9.7) | --- | 65.6 (10.1) | --- | .44 |
| Gender | |||||
| Women | 20 | 54 | 20 | 57 | .79 |
| Men | 17 | 46 | 15 | 43 | |
| Race | |||||
| Caucasian | 34 | 92 | 30 | 86 | .40 |
| Not Caucasian | 3 | 8 | 5 | 14 | |
| Marital status | |||||
| Married | 26 | 70 | 27 | 77 | .51 |
| Not married | 11 | 30 | 8 | 23 | |
| Education | |||||
| Less than high school | 4 | 11 | 10 | 28 | .06 |
| High school graduate | 21 | 57 | 10 | 28 | |
| Some college | 11 | 29 | 9 | 26 | |
| College | --- | --- | 4 | 12 | |
| Post-college graduate | 1 | 3 | 2 | 6 | |
| Employment status | |||||
| Employed | 13 | 35 | 7 | 20 | .15 |
| Retired | 20 | 54 | 20 | 57 | |
| Not employed | 4 | 11 | 8 | 23 | |
| KPS pre-surgery, mean (SD), % | 93 (9.1) | --- | 96.3 (6.9) | --- | .11 |
| 6 Minute Walk Test, mean (SD), meters | 365 (91) | --- | 407 (64) | --- | .03 |
| Co-morbid conditions, mean (SD), # | 8.3 (3.9) | --- | 7.4 (3.7) | --- | .31 |
| BMI, mean (SD), kg/m2 | 29.1 (4.8) | --- | 28.1 (5.6) | --- | .43 |
| Cancer stage | |||||
| Early | 29 | 78 | 31 | 89 | .50 |
| Late | 6 | 16 | 4 | 11 | |
| Indeterminate | 2 | 6 | --- | --- | |
| Surgery type | |||||
| Open thoracotomy | 32 | 86 | 27 | 77 | .30 |
| Minimally invasive | 5 | 14 | 8 | 23 | |
| Extent of surgical resection | |||||
| Wedge | 4 | 11 | 7 | 20 | --- |
| Lobectomy | 25 | 68 | 26 | 74 | |
| Bilobectomy | 2 | 5 | --- | --- | |
| Pneumonectomy | 3 | 8 | --- | --- | |
| Other resections | 3 | 8 | 2 | 6 | |
| Cell type following resection | |||||
| Adenocarcinoma | 24 | 65 | 21 | 60 | --- |
| Squamous | 10 | 27 | 12 | 34 | |
| Oher | 3 | 8 | 2 | 6 | |
| Scale Scores | Mean | SD | Mean | SD | p |
| Brief Fatigue Inventory | 2.2 | 2.0 | 1.96 | 1.95 | .66 |
| Modified Borg Fatigue Scale, during the 6 minute walk test | 2.0 | 1.7 | 1.5 | 1.8 | .17 |
| Medical Outcomes Study Short Form-36 Physical Function Component | 48.2 | 9.3 | 46.1 | 9.2 | .35 |
| Medical Outcomes Study Short Form-36 Mental Function Component | 48.1 | 10.6 | 50.0 | 10.4 | .46 |
| Perceived Self-Efficacy for Fatigue Self-Management | 7.3 | 2.1 | 8.6 | 1.8 | .007 |
| Perceived Self-Efficacy for Walking Duration | 80.1 | 25.0 | 75.8 | 25.8 | .052 |
| Activities-Specific Balance Confidence Scale | 89.2 | 14.1 | 91.1 | 10.0 | .052 |
Abbreviations: CG, control group; IG, intervention group; KP, Karnofsky Performance Status; SD, standard deviation.
Study Aim 1: Feasibility (Recruitment, Retention, Adherence, and Safety) and Acceptability
We recruited 66% of eligible participants, exceeding our goal of 50%. Also, recruiting goals of N=64 were exceeded: 72 participants completed the study, recruited from one hospital site in west Michigan and two hospitals in mid-Michigan. Even though this vulnerable population had a mean of 8 comorbid conditions, the goal of 70% retention was exceeded: 97% of participants completed the intervention. The goal of 70% adherence was exceeded, as the 37 participants adhered to their prescribed exercise at a rate of 93%. Relative to safety, no adverse events were reported. The 37 participants in the IG gave a mean rank of 5.6 (SD, 0.49) (scale 0-6, 6 = highest acceptability) for intervention acceptability finding it “enjoyable”, “convenient to exercise at home” and “easy to use” exceeding our goal of a mean positive acceptability score of 4.
Study Aim 2: Preliminary Efficacy of the Effect of the Intervention on Outcomes
CRF severity
Results in Figure 3 indicate on a scale of 0-10 (10 = most severe), the pre-surgery values (IG, M = 2.2, SD 2.0; CG, M = 2.0, SD 1.9; t (70) = 0.44, p = .66; 95% CI - 0.73 to 1.14) and post-surgery values (IG, M = 4.1, SD 1.9; CG, M = 3.9, SD 2.7; t (61) = .33, p = .74, 95% CI -0.92 to 1.3) for the IG and CG show no significant difference. Each week of the intervention showed improvement in the IG versus the CG culminating at week 6 where a significant difference was found between the IG (M=0.7, SD 0.7) and the CG (M=4.0, SD 2.0) [t (42) = -9.3, p<.001; 95% CI -4.0 to -2.6; d = 1.7]. The IG's week 6 CRF severity level (M=0.7, SD 0.7) not only showed significant recovery from post-surgery (M=4.1, SD1.9) [t (36) = 9.9, p <.001; 95% CI 2.7 to 4.1; d = 1.8], but restored to a CRF severity lower than pre-surgery levels (M=2.2, SD 2.0) [t (36) = 4.4, p<.001; 95% CI 0.8 to 2.1; d = 0.7].
Figure 3. Cancer-Related Fatigue Severity Mean Scores (0-10, 10 = Most Severe).
PSE for CRF Self-Management
As shown in Figure 4, the IG's PSE for CRF self- management improves from post-surgery (M=7.0, SD 2.0) to week 6 (M=9.6, SD 0.7), while the CG's PSE decreased from post-surgery (M=7.6, SD 2.6) to week 6 (M=6.9, SD 2.1) resulting in a significant difference between groups at week 6 [t (41.8) = 7.4, p<.001; 95% CI 2.0 to 3.5; d = 1.3]. This is consistent with what the TSSM would project for the IG in that as CRF severity improved with the use of the exercise intervention, so did the participant's PSE for CRF self-management. Likewise, for the CG, when CRF severity did not improve over time, the CG participants' PSE for CRF self-management did not improve over time.
Figure 4. Perceived Self-Efficacy for Fatigue Self-Management Mean Scores (0-10, 10 = Greatest Self-Efficacy for Fatigue Self-Management).
PSE for Walking
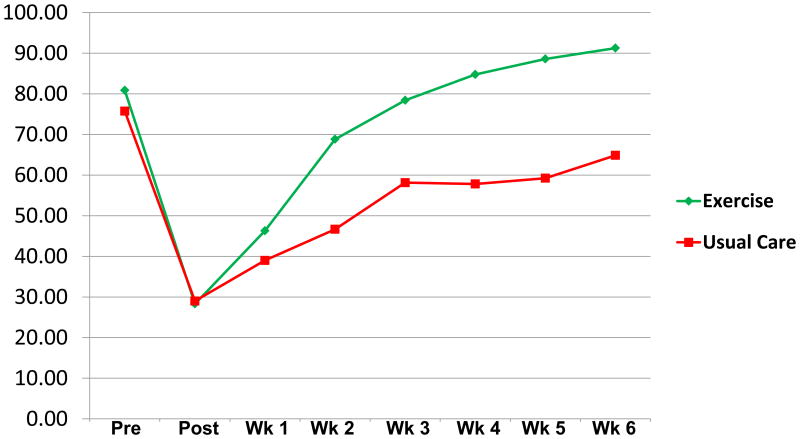
PSE for walking 30 minutes without stopping (Figure 5) improved from a mean of 28% (SD 22) post-surgery to 91% (SD 18) at week 6 for the IG. The CG started at a mean of 29% (SD 27) post-surgery but improved to only 65% (SD 32) at week 6, a significant difference between groups at week 6 [t (52.2) = 4.3; p<.001; 95% CI 14 to 39; d = 0.8].
Figure 5. Perceived Self-Efficacy to Walk 30 Minutes Without Stopping with Mean Scores in % (0-100%, 100% = Greatest Perceived Self-Efficacy for Walking 30 Min. Without Stopping).
PSE for Balance
Post-surgery as seen in Figure 6 started at mean = 64% (SD 23) for the IG and improved to 93% (SD 8) at week 6, while the CG started at mean = 65% (SD 25) post-surgery and improved to only 80% (SD 17) at week 6, a significant difference between groups at week 6 [t (46.9) = 4.1; p<.001; 95% CI 6.6 to 19.5; d = 0.7].
Figure 6. Perceived Self-Efficacy for Balance Mean Scores in % (0-100%, 100% = Greatest Perceived Self-Efficacy for Balance).
CRF Self-management Walking and Balance Exercise Behaviors
The mean minimum prescription for walking increased, with participants walking 4.9 (SD, 0.3) minute/day, 5 days a week for the first week, and incrementally increasing to 22 (SD, 7) minutes/day at week 6, with 19% of participants walking 30 minutes a day. Participants exceeded the minimum walking prescription with a mean walking time of 5.9 (SD, 2.6) minutes/day during the first week of exercise and increased walking time to 22.9 (SD, 11.3) min/d during the last week of the exercise intervention. Participants' mean minimum number of prescribed balance exercises per day was set at 3 initially and remained relatively constant through the 6-week intervention. Participants exceeded the minimum number of prescribed balance exercises per day, averaging 6.9 (SD, 1.9) balance exercises per day in week 1 remaining relatively constant throughout and completing the study averaging 7.6 (SD, 4.9) balance exercises per day in week 6.
Fatigability
The IG's week 6, post-study fatigability decreased from pre-surgery while the CG's increased. The IG's fatigue during 6MWT (0-10, 10= most severe) decreased from pre-surgery (M=2.0, SD 1.7) to post-intervention (M=0.4, SD 0.8) while their distance walked increased by a mean of 15% (SD 15). Conversely, the CG's fatigability increased as their fatigue during the 6MWT increased from pre-surgery (M=1.5, SD 1.8) to post 6 weeks (M=3.1, SD 1.5) while their distance walked decreased by a mean of 21% (SD 19). The difference between the IG and CGs' fatigue during the post-study 6MWT was significant [t (50.9) = -9.3, p<.001; 95% CI -3.3 to -2.1; d = 1.8) as was the percent change in distance walked post-study vs. pre-surgery 6MWT [t (70) = 9.0; p < .001; 95% CI 28 to 44; d = 1.9].
Functional Performance
As seen in Figure 7, the Mental Health component of the IG consistently improved from a mean pre-surgery level of 48.2 to 57.5 at week 6 (exceeding the national norm of 50). Conversely, the CGs' mean Mental Health component scores drop after surgery and stay relatively flat for weeks 3 & 6 (47.4 and 47 respectively). Physical Health component scores drop for both groups post-surgery, with the IG's improving from a post-surgery level of 30.2 to a week 6 level of 45.2. The CG improves at a much slower rate, starting at post-surgery level of 32.7 and improving to 38.8 at week 6 post-study.
Figure 7. Physical and Mental Health Component of Functional Performance.
Discussion
In order for an intervention to be considered a candidate for a standard of care in rehabilitation it needs to be feasible, acceptable and efficacious. In addressing the gap in post-thoracotomy lung cancer rehabilitation, our team had to address how to safely exercise participants whose age ranged from 32 to 89, who just returned home from the hospital after a major surgery, and had an average of 8 co-morbid conditions. Further, we wanted to incorporate safe exercise while making exercise fun and convenient. We addressed the gap by combining walking and balance with gaming technology that could be used at any time by the participant in their own home to self-manage the debilitating effects of CRF.
The study results were very encouraging as the IG participants found the intervention to be highly acceptable, to be “enjoyable”, “convenient to exercise at home”, “easy to use” and safe showing excellent adherence at 93% over a 6 week period. Further, there were no reported safety issues with 37 participants with a mean age of 67 years completing 6 weeks of the intervention. Also, the IG when compared to the CG showed preliminary efficacy in reducing CRF severity on a weekly basis for the 6 weeks of the intervention. Likewise, the IG improved on a weekly basis relative to the CG in PSE for fatigue self-management. This is consistent with what the TSSM would project for the IG in that as CRF severity improved with the use of the exercise intervention, so did the participants' PSE for CRF self-management.
Similarly, for the CG, when CRF severity did not improve over time, the CG participants' PSE for CRF self-management did not improve over time. Comparable to fatigue, the IG improved in self-efficacy for balance and walking 30 minutes without stopping. This is also consistent with what we expected to find as participants continued to increase their walking and balance exercises their self-efficacy for balance and walking distance improved versus the CG.
The 6MWT was used as an objective measure of fatigability (functional capacity) and we found the fatigability in the IG was greatly reduced relative to that of the CG as the walking intervention improved their conditioning versus the CG so they could walk further with less fatigue.
Mental and physical health components of functional performance also showed excellent improvement for the IG versus the CG. With exercise known to have positive effects on mental and physical health, we expected to see some improvement in mental and physical health but the improvements in the IG over the CG were even starker than we had anticipated.
The study results are very encouraging indicating that the walking and balance exercise intervention is feasible, acceptable, and demonstrates preliminary efficacy. Next steps need to further confirm the efficacy of the intervention for a larger patient population. Should a larger study corroborate these results, the stage would be set for this walking and balance exercise intervention to fill the rehabilitation void for post-thoracotomy NSCLC patients.
Strengths and Limitations
This research was completed accomplishing all study objectives and guided by the use of two theories underpinning both the implementation of the study via the Transitional Care Model and the design and testing via the TSSM. In doing so, we were able to apply methodological rigor that has not been previously accomplished in this patient population. Our team was able to successfully implement a two-arm multisite RCT with early intervention to promote self-management of a priority symptom, CRF, post-hospital discharge via light-exercise.
In addition, while the participants were a homogenous group having all undergone thoracotomy for NSCLC and received an intervention targeting CRF, the patients also had their own diverse characteristics demonstrating the applicability of the intervention for most post-thoracotomy NSCLC patients. For example, most participants had multiple comorbid conditions; were undergoing varied thoracotomy procedures from minimally invasive procedures including video assisted thoracoscopic and robotics to a full open procedure for the removal of a portion of the lung to a complete pneumonectomy, Patients in the study covered a wide range of ages from 32 to 89 years with males and females from different geographical areas both urban and rural, living up to 90 minutes from the acute care hospital; and were from varied socio-economic backgrounds.
Since it could be possible to replicate the exercise intervention, the CG was blinded to the exercise intervention. However, it is a limitation of the study that members of the research staff were not blind to the participant's assigned group since the activity in each group was easily discernable. In addition, it was not possible to blind research staff monitoring exercise to the participant assignment since research staff are aware whether a participant is performing exercise and participants were aware whether they were exercising or not.
Most participants were white but this was near reflective of the catchment area for recruitment of minorities and the report by the American Cancer Society states that accumulating evidence has found that when lung cancer is diagnosed at an early stage, African Americans are less likely to undergo surgery as compared to whites.45
Last, while recruitment and retention led to completing this study exceeding sample size requirements accruing 72 participants as compared to the goal of 64, increasing the sample size to fully test the intervention's effect and benefit on fatigue and fatigability is needed to further confirm these positive results.
Implications for Research to Advance Practice
As shown in the findings of this study, CRF is a significant symptom that requires further understanding to improve functional status for post-thoracotomy NSCLC patients. The CG showed an immediate increase and plateau in CRF severity levels through week 6 of the study as compared to the IG who not only showed recovery of CRF severity levels to those of pre-surgery, but also restoration to levels lower than pre-surgery. The CG's CRF severity escalated from a mild fatigue level pre-surgery to a recently termed “nontrivial” fatigue, a moderate level of fatigue noted immediately upon discharge from the hospital that persists for 6 weeks to post-study.9 As in the CG, Wang and colleagues' research illustrate the increased risk of developing nontrivial fatigue (moderate to severe fatigue levels) in persons with lung cancer who are undergoing active treatment for the disease.9
Furthermore, the CG's fatigability increased indicative of deteriorating functional capacity as compared to the IG who showed improvement in their fatigability, decreased fatigue under exertion with increased distance walked during the 6MWT. Jones and colleagues report that functional capacity as measured by the 6MWT distance is a strong predictor of survival in the lung cancer population.30 Specifically, Jones notes in his research that each 50 meter improvement in the 6MWT distance is associated with a 13% reduction in the risk of death. Moreover, in Dr. Jones' study, 452 meters was found to be the minimum distance achieved for those denoted as high capacity functioning with those who walked >452 meters having a significantly prolonged survival relative to patients walking <452m. In the current study, most participants in both the CG and IG fell well below this high functioning threshold pre-surgery with mean 6MWT distances of 407 (SD 64) and 365 (SD 91) meters respectively. Post-study after 6 weeks of usual care without exercise, the CG detrimentally alters their survival prognosis with a 21% decrease in their mean distance walked with increased fatigue scores at a moderate level of fatigue under exertion. Conversely, the IG with 6 weeks of light-intensity, gradually building, self-paced walking and balance exercise and while in less physically optimal shape pre-surgery improved their mean distance walked by 15%, over 50 meters improved from pre-surgery enhancing survivability. Moreover, the IG improved their fatigue levels under exertion to < 1, on a scale of 0 to 10 (10 = severe fatigue).
While research on exercise to improve physical and mental health components of functional status in the NSCLC population pre- and immediately post-surgery is limited; the positive findings in this study parallels prior research in the post-surgical NSCLC14,18,19 and general cancer populations.20,46 These findings suggest that exercise may potentially improve both components of physical and mental functional status but these findings require further study. One study that specifically highlights the importance of the relationship between the components of physical and mental functional status in the post-surgical NSCLC population showed a 10% decrease in physical and mental functional status during the first six months after surgery and was associated with an 18% and 13% increased risk of death respectively.47 Similarly, Handy et al. report significant persistent decline in physical and mental functional status from pre-surgical to six month post-surgical scores.48
The Cochrane review of non-invasive interventions for improving well-being and QOL in lung cancer patients recommends the development of interventions focused on symptom self-management to promote functional ability.49 In addition, the latest Cochrane reviews on exercise interventions on health-related QOL, including fatigue during/after active cancer treatment, recommend research investigating how to sustain positive effects of exercise over time.50 The IG participants in this study incurred a drastically reduced physical health functional performance score well below the population mean of 50 and incurred a new nontrivial CRF score upon hospital discharge. However, the IG improved to near pre-surgical physical health functional performance. The IG's improvement in symptom and functional status outcomes was unlike the CG and further study to understand the central attributes of the exercise prescription to achieve optimal benefits is needed. Consequently, this study clearly demonstrates the potential clinical value of early rehabilitation for a vulnerable population, post-surgical NSCLC patients, focusing on enhancing PSE for fatigue self-management with exercise to reduce fatigue and fatigability.
Short-and mid-term recovery and well-being is very important, but another indicator of success is sustainability of an exercise intervention for long-term well-being and survival. Our current results have allowed us to plan future research directions to conduct a study to promote the continued use/effectiveness of exercise that is practical, portable, and low-cost to reduce CRF severity and fatigability that is enjoyable and applicable to nearly all post-thoracotomy lung cancer patients to ensure the survivorship trajectory for the long run.
Acknowledgments
Source of Funding: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number R21CA164515. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We wish to acknowledge the expertise and support from the following members of our research team: Dr. Lawrence Patzelt, MD, and fellow physician colleagues and staff at West Michigan Cardiothoracic Surgeons, Grand Rapids, MI; Dr. Divyakant Gandhi, MD, and Becky Loomis, BSN, RN, McLaren Health Care, Lansing, MI; Bree Peters, BSN, RN, Sylvia L. Kirgis, ACNP-BC, MSN, Dr. Norbert Baumgartner, MD, and Dr. Alonso Collar, MD, from Sparrow Hospital, Lansing, MI.
Footnotes
Conflict of Interest: The authors have no conflicts of interest to disclose.
Statement of Authorship: Each person listed on this manuscript has participated in the study to a significant extent, and has adhered to ethical standards of research conduct.
Contributor Information
Amy J. Hoffman, College of Nursing, Michigan State University, East Lansing, Michigan.
Ruth Ann Brintnall, Kirkhof College of Nursing, Grand Valley State University, Grand Rapids, Michigan.
Barbara A. Given, College of Nursing, Michigan State University, East Lansing, Michigan.
Alexander von Eye, Psychology Department, Michigan State University, East Lansing, Michigan.
Lee W. Jones, Department of Medicine, Memorial Sloan Kettering Cancer Center, NY, NY.
Jean K. Brown, School of Nursing, University at Buffalo, the State University of New York, Buffalo, New York.
References
- 1.Brown N, Lui C, Robinson P, Boyle F. Supportive care needs and preferences of lung cancer patients: A semi-structured qualitative interview study. Support Care Cancer. 2015;23:1533–1539. doi: 10.1007/s00520-014-2508-5. [DOI] [PubMed] [Google Scholar]
- 2.Maguire R, Papadopoulou C, Kotronoulas G, Simpson M, McPhelim J, Irvine L. A systematic review of supportive care needs of people living with lung cancer. Eur J Oncol Nurs. 2013;17:449–454. doi: 10.1016/j.ejon.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Sanders S, Bantum E, Owen J, Thornton A, Stanton A. Supportive care needs in patients with lung cancer. Psychooncology. 2010;10(5):480–489. doi: 10.1002/pon.1577. [DOI] [PubMed] [Google Scholar]
- 4.Bower J, Bak K, Berger A, et al. Screening, assessment, and management of fatigue in adult survivors of cancer: An American Society of Clinical oncology clinical practice guideline adapatation. J Clin Oncol. 2014;32:1840–1850. doi: 10.1200/JCO.2013.53.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger A, Mitchell S, Jacobsen P, Pirl W. Screening, evaluation, and management of cancer-related fatigue: Ready for implementation to practice? CA: Cancer J Clin. 2015 Mar 11; doi: 10.3322/caac.21268. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 6.Stubblefield M, Schmitz K, Ness K. Physical functioning and rehabilitation for the cancer survivor. Semin Oncol. 2013;40:784–795. doi: 10.1053/j.seminoncol.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell S, Hoffman A, Clark J, et al. Putting evidence into practice: An update of evidence-based interventions for cancer-related fatigue during and following treatment. Clin J Oncol Nurs. 2014;18(s6):38–58. doi: 10.1188/14.CJON.S3.38-58. [DOI] [PubMed] [Google Scholar]
- 8.Cleeland C, Fengmin Z, Chang V, et al. The symptom burden of cancer: Evidence for a core set of cancer-related and treatment-related symptoms from the Eastern Cooperative Oncology Group Symptom Outcomes and Practice Patterns study. Cancer. 2013;119:4333–4340. doi: 10.1002/cncr.28376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Zhao F, Fisch M, et al. Prevalence and characteristics of moderate to severe fatigue. Cancer. 2014;120(3):425–432. doi: 10.1002/cncr.28434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi Q, Smith T, Michonski J, Stein K, Kaw C, Cleeland C. Symptom burden in cancer survivors 1 year after diagnosis: A report form the American Cancer Society's Studies of Cancer Survivors. Cancer. 2011;117:2779–2790. doi: 10.1002/cncr.26146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang P, Cheville A, Wampfler J, et al. Quality of life and symptom burden among long-term lung cancer survivors. J Thorac Oncol. 2012;7:64–70. doi: 10.1097/JTO.0b013e3182397b3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henry D, Viswanathan H, Elkin E, Traina S, Wade S, Cella D. Symptoms and treatment burden associated with cancer treatment: Results from a cross-sectional national survey in the U.S. Support Care Cancer. 2008;16:791–801. doi: 10.1007/s00520-007-0380-2. [DOI] [PubMed] [Google Scholar]
- 13.Leidy N. Functional status and the forward progress of merry-go-rounds: Toward a coherent analytical framework. Nurs Res. 1994;43:196–202. [PubMed] [Google Scholar]
- 14.Hoffman A, Brintnall R, Brown J, et al. Too sick NOT to exercise: Using a 6-week, home-based exercise intervention for cancer-related fatigue self-management for post-surgical non-small cell lung cancer. Cancer Nurs. 2013;36:175–188. doi: 10.1097/NCC.0b013e31826c7763. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman AJ, Brintnall RA, Brown JK, et al. Virtual reality bringing a new reality to postthoracotomy lung cancer patients via a home-based exercise intervention targeting fatigue while undergoing adjuvant treatment. Cancer Nurs. 2014;37:23–33. doi: 10.1097/NCC.0b013e318278d52f. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman A, Brintnall R, Von Eye A, et al. A rehabilitation program for lung cancer patients during postthoracotomy chemotherapy. Onco Targets Ther. 2014;7:415–423. doi: 10.2147/OTT.S57262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffman A, Brintnall R, von Eye A, et al. Home-based exercise: promising rehabilitation for symptom relief, improved functional status and quality of life for post-surgical lung cancer patients. J Thorac Dis. 2014;6:632–640. doi: 10.3978/j.issn.2072-1439.2014.06.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crandall K, Maguire R, Campbell A, Kearney N. Exercise intervention for patients surgically treated for Non-Small Cell Lung Cancer (NSCLC): A systematic review. Surg Oncol. 2014;23:17–30. doi: 10.1016/j.suronc.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Cavalheri V, Tahirah F, Nonoyama M, Jenkins S, Hill K. Exercise training for people following lung resection for non-small cell lung cancer: A Cochrane systematic review. Cancer Treatment Rev. 2014;40:585–594. doi: 10.1016/j.ctrv.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Mishra S, Scherer R, Snyder C, Geigle P, Berlanstein D, Topaloglu O. Exercise interventions on health-related quality of life for people with cancer during active treatment (Review) Cochrane Database of Syst Rev. 2012;8 doi: 10.1002/14651858.CD008465.pub2. CD008465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mishra S, Scherer R, Geigle P, et al. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst Rev. 2012;8 doi: 10.1002/14651858.CD007566.pub2. CD007566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poghosyan H, Sheldon L, Leveille S, Cooley M. Health-related quality of life after surgical treatment in patients with non-small cell lung cancer: a systematic review. Lung Cancer. 2013;81(1):11–26. doi: 10.1016/j.lungcan.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 23.Bandura A. Self-efficacy: The Exercise of Control. NY, NY: W.H. Freeman and Company; 1997. [Google Scholar]
- 24.Barsevick A, Irwin M, Hinds P, et al. Recommendations for high-priority research on cancer-related fatigue in children and adults. J Natl Cancer Inst. 2013;105:1432–1440. doi: 10.1093/jnci/djt242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brocki B, Andreasen J, Nielsen L, Nekrasas V, Gorst-Rasmussen A, Westerdahl E. Short and long-term effects of supervised versus unsupervised exercise training on health-related quality of life and functional outcomes following lung cancer surgery-A randomized controlled trial. Lung Cancer. 2014;83:102–108. doi: 10.1016/j.lungcan.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 26.Naylor M, Bowles K, McCauley K, Maccoy MM, Maislin G, Pauly M, Krakauer R. High-value transitional care: Translation of research into practice. J Eval Clin Pract. 2013;19:727–733. doi: 10.1111/j.1365-2753.2011.01659.x. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman A. Enhancing self-efficacy for optimized patient outcomes through the Theory of Symptom Self-Management. Cancer Nurs. 2013;36:E16–E26. doi: 10.1097/NCC.0b013e31824a730a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffman A, Von Eye A, Gift A, Given B, Given C, Rothert M. Testing a theoretical model of perceived self-efficacy for cancer-related fatigue self-management and optimal physical functional status. Nurs Res. 2009;58:32–41. doi: 10.1097/NNR.0b013e3181903d7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.American Cancer Society. [May 5, 2015];What are the key statistics about lung cancer? 2015 http://www.cancer.org/cancer/lungcancer-non-smallcell/detailedguide/non-small-cell-lung-cancer-key-statistics.
- 30.Jones L, WE H, Goetzinger A, et al. Prognostic significance of functional capacity and exercise behavior in patients with metastatic non-small cell lung cancer. Lung Cancer. 2012;76(2):248–252. doi: 10.1016/j.lungcan.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erdfelder E, Faul F, Buchner A. G-Power: A general power analysis program. Behav Res Methods. 1996;28:1–11. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 32.Ainsworth B, Haskell W, Whitt M, et al. Compendium of physical activities: An update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 33.Jonas S, Phillips E. ACSM's Exercise is Medicine. Philadelphia, PA: Lippincott Williams & Wilkins; 2009. [Google Scholar]
- 34.Pescatello L, editor. American College of Sports Medicine's Guidelines for Exercise Testing and Prescription. 9th. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins Health; 2014. [Google Scholar]
- 35.Mendoza T, Wang S, Cleeland C, et al. The rapid assessment of fatigue severity in cancer patients: Use of the brief fatigue inventory. Cancer. 1999;85:1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 36.Rudolph D, McAuley E. Self-efficacy and perceptions of effort: a reciprocal relationship. J Sport Exerc Psychol. 1996;18:216–233. [Google Scholar]
- 37.Powell L, Myers A. The Activities-Specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci. 1995;50A:M28–34. doi: 10.1093/gerona/50a.1.m28. [DOI] [PubMed] [Google Scholar]
- 38.American Thoracic Society. ATS statement: guidelins for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 39.Steele B. Timed walking tests of exercise capacity in chronic cardiopulmonary illness. Journal of Cardiopulmonary Rehabilitation. 1996;16:25–33. doi: 10.1097/00008483-199601000-00003. [DOI] [PubMed] [Google Scholar]
- 40.McHorney C, Ware J, Raczek A. The MOS 36-Item Short-Form Survey (SF-36): psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Katz J, Chang L, Sangha O, Fossel A, Bates D. Can co-morbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Centers for Disease Control and Prevention. Smoking status. 2011 http://dhds.cdc.gov/guides/healthtopics/indicator?i=SmokingStatus.
- 43.Cohen J. A power primer. Psychological Bulletin. 1992;112(1):155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 44.Little R, Rubin D. Statistical analysis with missing data. Hoboken, NJ: Wiley; 2002. [Google Scholar]
- 45.American Cancer Society. American Cancer Society. Atlanta: 2013. Cancer facts & figures for African Americans 2013-2014. [Google Scholar]
- 46.Cramp F, Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. 2012 doi: 10.1002/14651858.CD006145.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moller A, Sartipy U. Associations between changes in quality of life and survival after lung cancer surgery. J Thorac Oncol. 2012;7(1):183–187. doi: 10.1097/JTO.0b013e3182340abb. [DOI] [PubMed] [Google Scholar]
- 48.Handy J, Asaph J, Skokan L, et al. What happens to patients undergoing lung cancer surgery? Outcomes and quality of life before and after surgery. CHEST. 2002;122(1):21–30. doi: 10.1378/chest.122.1.21. [DOI] [PubMed] [Google Scholar]
- 49.Rueda J, Sola I, Pascual A, Subirana Casacuberta M. Non-invasive interventions for improving well-being and quality of life in patients with lung cancer. Cochrane Database Syst Rev. 2011;9 doi: 10.1002/14651858.CD004282.pub3. Art No. CD004282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mishra S, Scherer R, Snyder C, Geigle P, Gotay C. The effectiveness of exercise interventions for improving health-related quality of life from diagnosis through active treatment. Oncology Nursing Forum. 2015;42:E33–53. doi: 10.1188/15.ONF.E33-E53. [DOI] [PubMed] [Google Scholar]
- 51.Hoffman A, von Eye A, Gift A, Given B, Given C, Rothert M. The development and testing of an instrument for perceived self-efficacy for fatigue self-management. Cancer Nurs. 2011;34:167–175. doi: 10.1097/NCC.0b013e31820f4ed1. [DOI] [PMC free article] [PubMed] [Google Scholar]