Abstract
Objective
Phthalates, which are commonly used to render plastics into soft and flexible materials, have also been determined as developmental and reproductive toxicants in human and animals. The purpose of this study was to evaluate the effect of mono-(2- ethylhexyl) phthalate (MEHP) and di-(2-ethylhexyl) phthalate (DEHP) oral administrations on maturation of mouse oocytes, apoptosis and gene transcription levels.
Materials and Methods
In this experimental study, immature oocytes recovered from Naval Medical Research Institute (NMRI) mouse strain (6-8 weeks), were divided into seven different experimental and control groups. Control group oocytes were retrieved from mice that received only normal saline. The experimental groups I, II or III oocytes were retrieved from mice treated with 50, 100 or 200 µl DEHP (2.56 µM) solution, respectively. The experimental groups IV, V or VI oocytes were retrieved from mouse exposed to 50, 100 or 200 µl MEHP (2.56 µM) solution, respectively. Fertilization and embryonic development were carried out in OMM and T6 medium. Apoptosis was assessed by annexin V-FITC/Dead Cell Apoptosis Kit, with PI staining. In addition, the mRNA levels of Pou5f1, Ccna1 and Asah1 were examined in oocytes. Finally, mouse embryo at early blastocyst stage was stained with acridine-orange (AO) and ethidium-bromide (EB), in order to access their viability.
Results
The proportion of oocytes that progressed up to metaphase II (MII) and 2-cells embryo formation stage was significantly decreased by exposure to MEHP or DEHP, in a dose-dependent manner. Annexin V and PI positive oocytes showed greater quantity in the treated mice than control. Quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) revealed that expression levels of Pou5f1, Asah1 and Ccna1 were significantly lower in the treated mouse oocytes than control. The total cell count for blastocyst developed from the treated mouse oocytes was lower than the controls.
Conclusion
These results indicate that oral administration of MEHP and DEHP could negatively affect mouse oocyte meiotic maturation and development in vivo, suggesting that phthalates could be risk factors for mammalians’ reproductive health. Additionally, phthalate-induced changes in Pou5f1, Asah1 and Ccna1 transcription level could explain in part, the reduced developmental ability of mouse-treated oocytes.
Keywords: Oocyte Maturation, Apoptosis, Gene Expression
Introduction
Decline in human and animal fertility, mainly caused by environmental chemicals, desires more attentions of scientific communities as well as general public (1). Meanwhile, phthalates, as a group of artificial organic chemicals, are generally used to give softness and flexibility to plastics. Regarding the massive applications in laboratory and medical products, slow release into the nature, inability for entering into the environmental cycles and exposing to human body (2, 3), phthalates raise the concern on the potential health side-effects.
The di-(2-ethylhexyl) phthalate (DEHP) is a high production volume (HPV) chemical, leaching of which in environment causes adverse effects on reproduction and development. In several species, DEHP are hydrolyzed into specific hydrolytic monoesters in mouth, skin, stomach, intestine or blood, called mono-(2-ethylhexyl) phthalate (MEHP). It is reported that MEHP could be an active compound, reliably demonstrating many effects of DEHP in vivo (4).
Investigations indicated that exposing to phthalates could lead to birth defects, asthma, neuro-developmental problems in newborns as well as obesity or infertility (5). It has been demonstrated the critical adverse effects of phthalates on male or female reproductive systems by interfering with production of testosterone or estradiol, respectively (6). Thus far, very few studies have examined the potential effect of phthalates on ovarian follicles in vivo, as a target site of this chemical agent.
It has been shown that DEHP exposure in animal and culture models could decrease estradiol level due to reduced aromatase expression, prolonged estrous cycles, ovulation defect and ovarian deterioration (7). In 2003, Anas et al. (8) revealed that exposing to MEHP in vitro inhibited meiotic maturation of bovine oocytes in a dose-dependent manner. This finding was subsequently validated on mouse oocytes, where Shahverdi and colleagues showed that oral MEHP administration prohibited meiotic maturation in the mouse embryos and oocytes (9). In addition, investigations on zebrafish exhibited deleterious effects of DEHP on the particular molecular biomarkers of oogenesis and fetal development (10). In that respect, findings revealed that both DEHP and MEHP inhibited follicle growth in mice by reducing estradiol production in antral follicles (4).
It has currently been proved that fully mature mammalian oocyte, preserving maternal genetic information, is transcriptionally silent and use transcripts which is only synthesized and accumulated through early development (11). Therefore, determining the gene expression profiles that occur during the oocyte development and progression of a fertilized egg is necessary (12). In line with that, mRNA expression level of particular genes was investigated in bovine oocytes exposed to phthalates. Findings of this study showed reduced mRNA expression levels of Pou5f1 (pluripotency factor), Asah1 (an anti-apoptotic maker) and Ccna2 (involved in cell cycle control) due to the exposure of MEHP into mature oocytes (13).
In current study, we determined the effect of DEHP or MEHP oral administration on oocyte meiotic maturation and apoptosis. We also estimated the Pou5f1, Ccna1 and Asah1 transcription levels by real-time polymerase chain reaction (RT-PCR) in metaphase II (MII) stage of oogenesis. In addition, blastocyst quality was evaluated by acridine-orange (AO) and ethidium-bromide (EB) staining.
Materials and Methods
Immature oocyte retrieval
In this experimental study, 210 healthy adult female Naval Medical Research Institute (NMRI) mice (6-8 weeks old, 20-30 g) were obtained from Jundishapur University Experimental Research Center. The animals were housed under standard laboratory conditions with 12 hours dark and 12 hours light cycles, relative humidity of 50 ± 5% and temperature of 22 ± 3˚C. The mice were divided into six groups and orally administrated 50, 100 or 200 μl of 2.56 μM DEHP or MEHP solution (1 μl DEHP or 0.7 μl MEHP dissolved in Dimethyl Sulfoxide (DMSO, 0.1%, respectively) (9) for 15 days. Subsequently, the mice were sacrificed by spinal dislocation, followed by dissection under sterile conditions and collection of ovary tissues. Next, these tissues were preserved in 500 μl drops of Minimum Essential Medium Eagle-Alpha (MEM-α) culture media, containing 5% fetal calf serum (FCS). Cumulus oocyte complexes (COCs) were aspirated from follicles using insulin syringe. COCs with at least three layers of cumulus surrounding a homogeneous cytoplasm were selected for each group. All procedures were ethically performed in accordance with Jundishapur University (Ahvaz, Iran) animal scientific procedure acts.
In vitro maturation
COCs, were washed three times in phosphate-buffered saline (PBS) and transferred to 50 μl droplets of ovarian maturation medium (OMM) overlaying with mineral oil. The COC-containing droplets were incubated in humidified air condition with 5% CO2 for 22 hours at 38.5˚C. This experiment was evaluated on the control (contains 131 normal saline treated oocytes) and six experimental groups. The experimental groups I, II and III were respectively composed of 101, 112 and 119 oocytes treated with 50, 100 and 200 μl of DEHP (2.56 μM, Sigma-Aldrich Laborchemikalien GmbH, Germany). In contrast, the experimental groups IV, V and VI were respectively comprised of 107, 114 and 124 oocytes exposed to 50, 100 and 200 μl of MEHP (2.56 μM, Wako Chemical GmbH, USA). In vitro maturation procedures were evaluated by inverted microscope after denuding the cumulus cells in hyaluronidase (1.000 U/ml, Sigma-Aldrich, USA) by gentle vortexing. In this part, oocytes without any change in their nuclei were considered as germinal vesicle (GV) or immature; those with nuclear breakdown were evaluated as GV breakdown (GVBD), while the oocytes with meiotic sings and polar bodies were deemed as mature or MII oocytes.
In vitro fertilization and development of matured oocytes
The epididymis tails of sacrificed male NMRI mice were dissected and placed into 500 μl drops of T6 media containing 5 mg/ml bovine serum albumin (BSA, Gibco, USA). After incubation at 37˚C temperature and 5% CO2 concentration for 1.5 hours, the active and normal sperms were collected and transferred along with oocytes into drops of T6 media, containing 16 mg/ml BSA. Following 4-6 hours incubation, MII oocytes were transferred into a new medium condition, containing T6 with 5 mg/ml BSA. Ultimately, the percentage of oocytes cleaved to twoor four-cells stage was assessed at 42-44 hours post-fertilization. In addition, the average number of developed embryos up to the blastocyst stage was assessed on days 7-8 of the post-fertilization by inverted microscope (Olympus, Japan).
Annexin V staining
Apoptosis was determined using Annexin V-FITC/propidium iodide (PI) apoptosis detection kit (Beyotime Institute of Biotechnology, China). As a phospholipid-binding protein, Annexin V-FITC has a strong affinity to phosphatidyl serine (PS) on the membrane of early-apoptotic cells. On the other hand, PI can permeate the membrane of late-apoptotic and necrotic cells and stain the nuclei. In this experiment, the mouse oocytes that exposed with 50, 100 or 200 μl of MEHP or DEHP were cultured for 22 hours in OMM, followed by denudation in 1,000 U/ml of hyaluronidase. They were subsequently washed twice with PBS and suspended in 200 μl binding buffer (Beyotime Institute of Biotechnology, China). Afterwards, 5 μl Annexin V-FITC and 10 μl PI were added to 100 μl resultant cell suspensions and incubated for 15 minutes at room temperature protecting from light exposure. Next, 400 μl PBS was added to those cells and the proportion of apoptotic cells were analyzed by FC500 flow-cytometer (Beckman coulter, USA). This experiment was performed for 114 oocytes in control group, as well as 98, 102, 110, 120, 101 and 117 oocytes for experimental groups I-VI, respectively.
Reverse transcription polymerase chain reaction quantification
In this study, we relatively compared the mRNA expression level of three genes (Pou5f1, Ccna1 and Asah1) to a housekeeping gene (Gapdh), using quantitative RT-PCR (qRT-PCR). To do so, putative arrested MII stage mouse oocytes, exposed to 100 μl MEHP (n=112) or DEHP (n=123), were collected after 22 hours of in vitro maturation and cumulus cells were denuded in hyaluronidase (1,000 U/ml) by gentle vortexing. The experiments were performed in at least three biological repeats. All collected samples were washed in PBS, snap frozen in liquid nitrogen and stored at -80˚C until RNA extraction.
Using the RNX-PlusTM (Fermentase, Germany), RNA was extracted from samples according to the manufacturer’s instruction. RNA concentration was then determined by UV spectrophotometry (Eppendorff, Germany). To eliminate genomic contamination, RNA was treated with DNase I kit (EN0521, Fermentase, Germany). The cDNA was produced from RNA samples using RevertAidTM first strand cDNA synthesis kit (Fermentas, Germany) based on the manufacturer’s instruction. Primers were designed by using the NCBI website (Table 1) and synthesized by Cinnagen Company (Iran). PCR reactions were performed using SYBER Green master mix (Applied Biosystems, USA), cDNA samples and individual primer sets. QRT-PCR program was started with an initial melting cycle for 5 minutes at 95˚C to activate the polymerase, followed by 40 cycles of melting (10 seconds at 95˚C), annealing (15 seconds at 60˚C) and extension (30 seconds at 72˚C). The quality of the qRT-PCR reactions were confirmed by melting curve analysis. The efficiency of individual gene mRNA expression was determined by using the standard curve. Relative quantification levels were identified using 2-ΔΔCt method.
Table 1.
List of the primers utilized for qRT-PCR experiment
| Gene | Primer sequences (5ˊ-3ˊ) |
|---|---|
| Pou5f1 | F: AGAGGGAACCTCCTCTGAGC |
| R: CCAAGGTGATCCTCTTCTGC | |
| Ccna1 | F: CGCACAGAGACCCTGTACTT |
| R: TTGGAACGGTCAGATCAAAT | |
| Asah1 | F: TAACCGCAGAACACCGGCC |
| R: TTGACCTTTGGT | |
| Gapdh | F: TGCAGTGCCAGCCTCGTG |
| R: TTGATGGCAACAATCTCCACTT | |
QRT-PCR; Quantitative reverse transcription polymerase chain reaction.
Acridine-orange and ethidium-bromide staining of blastocyst
AO and EB staining are generally appropriate candidates for evaluation of cell viability. Using this method, the live cells show a green fluorescence color due to the AO staining, while dead cells represent an orange fluorescence upon EB staining. In this experiment, a stock solution was prepared by dissolving 50 mg EB and 15 mg AO (both purchased from Sigma-Aldrich, USA) in 50 ml of ethanol 2% (1 ml ethanol 95% diluted in 49 ml distilled water). The stock solution was divided into 1 ml aliquots and preserved at -20˚C temperature. Each aliquot was later diluted in 1x PBS, and preserved in dark glass tubes at 4˚C. For embryo staining, 25 μl of the prepared master-mix was added to each sample spread on the slide. The slides were ultimately observed by fluorescence microscope (Olympus, Japan) with a 515 nm filter .
Statistical analysis
All data were analyzed using one-way repeated measure analysis of variance (ANOVA) followed by Tukey’s post hoc test with a significance threshold of P<0.05. The statistics software package SPSS Version 16.0 was used to perform the calculations. The error-bars were represented as mean ± SD.
Results
Mono-(2-ethylhexyl) phthalate and di-(2-ethylhexyl) phthalate influences on oocyte development
The effects of DEHP or MEHP exposure on oocyte development were assessed in vitro by comparing several doses of these phthalates with control group (Table 2). Findings showed that exposing to DEHP or MEHP, in dose-dependent manner, led to significantly reduced frequency of the oocytes progressing into MII stage. In addition, both, DEHP and MEHP, had a deleterious carrying-over effect on the oocyte developmental competence, reflected by decrease in the percentage of developing blastocysts in the treated groups, compared to the control (P<0.05). Further analyses revealed that oocytes development percentage at the maturation MII stage was significantly decreased with exposure to 50, 100 or 200 μl DEHP (42.8, 36.2 or 26.5%, respectively) in the treated mice compared to the controls (67.37%). This frequency was significantly lower in 200 μl DEHP treatment compared to the other concentrations. In contrast to the control (67.37%), exposure of 50, 100 or 200 μl MEHP (2.56 μm) for 22 hours in vitro demonstrated respectively a frequency of 37.8, 27.6 or 17.3% for the MII stage oocytes (P<0.05). Similar to DEHP, the lowest rate of MII stage oocytes proportion was observed in 200 μl MEHP exposed cells, compared to the other treatments (P<0.05). In addition, the rate of oocytes developing 2-4 cells stage embryo and blastocyst stage was lower in all experimental groups than control. The oral administration of DEHP or MEHP effectively decreased the percentage of embryo formation in comparison to the control. Comparing MEHP and DEHP experimental groups showed deleterious effects of MEHP on developmental competence of oocyte and embryo up to blastocyst stage.
Table 2.
Analysis of in vitro maturation (IVM) and fertilization (IVF), after oocyte culturing in experimental and control groups
| Stages of development | Groups | Stages of development | Groups | ||||
|---|---|---|---|---|---|---|---|
| 96 hours after IVF | MII | 48 hours after IVF | 96 hours after IVF | MII | 48 hours after IVF | ||
| 21.6 ± 1 | 37.6 ± 1.1 | 67.3 ± 2 | Control (n=131) | 21.62 ± 1 | 37.6 ± 1.1 | 67.3 ± 2 | Control (n=131) |
| 5.5 ± 0.3a, b | 18.37 ± 0.4a | 37.87 ± 0.9a | Exp IV 50 μL MEHP (n=107) | 8.6 ± 0.3a | 20.37 ± 0.7a | 41.87 ± 1a | Exp I 50 μL DEHP (n=101) |
| 2.5 ± 0.3a, c, e | 10.6 ± 0.4a, c, e | 27.62 ± 0.9a, c, e | Exp V 100 μL MEHP (n=114) | 5.2 ± 0.3a, b | 14.12 ± 0.3a, b | 36.22 ± 1a | Exp II 100 μL DEHP (n=112) |
| 0.8 ± 0.2a, d, e | 6.7 ± 0.3a, e, f | 19 ± 0.01a, e, f | Exp VI 200 μL MEHP (n=124) | 1.3 ± 0.3a, b, c | 8.6 ± 0.5a, b, c | 26.5 ± 0.8a, b, c | Exp III 200 μL DEHP (n=119) |
Exp; Experiment, a; Significant differences with control group, P<0.05, b; Significant differences with experimental group I in the same column and row, P<0.05, c; Significant difference with experimental group II in the same column and row, P<0.05, d; Significant difference with experimental group III in the same column and row, P<0.05, e; Significant difference with experimental group IV in the same column and row, P<0.05, f; Significant difference with experimental group V in the same column and row, P<0.05, MEHP; Mono-(2-ethylhexyl) phthalate and DEHP; Di-(2-ethylhexyl) phthalat.
Effects of mono-(2-ethylhexyl) phthalate and di-(2-ethylhexyl) phthalate on oocyte apoptosis/necrosis/health
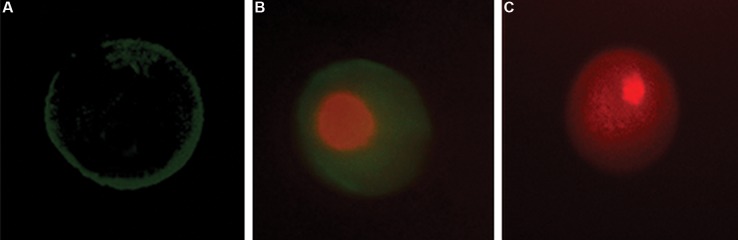
Following the Annexin V-FITC staining, denuded oocytes were classified into four groups: i. Necrotic cells with PI-positive red nuclei and cytoplasm, ii. Early apoptotic cells with homogeneous Annexin V-positive signals in membrane, iii. Eate apoptotic cells with PI-positive nuclei, and iv. Normal cells which is not stained by Annexin V-FITC or PI (Fig .1).
Fig.1.
The Representative image of the oocyte staining with propidium iodide (PI) and Annexin V-FITC. A. Early apoptotic, B. Late apoptotic, and C. Necrotic mouse oocytes using PI and Annexin V-FITC staining.
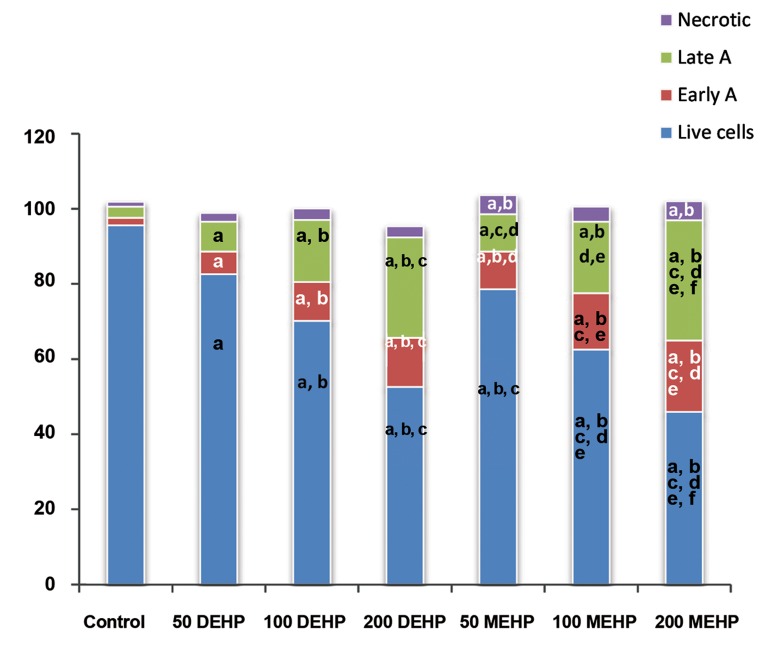
Analysis of the oocyte groups (Fig .2), exposed to DEHP, showed a dose-dependent (from low to high concentration) increase in early and late apoptosis, 14, 26.9 and 39.8%, respectively (P<0.05). In contrast, findings showed significantly lower normal oocytes in experimental groups treated with DEHP, compared to the control. No difference was observed between different groups, for necrotic cell staining (Fig .2). In comparison with DEHP, the effect of MEHP on the oocytes was less reversible with no dose-dependency manner. Early apoptosis in mouse oocytes exposed to 50 or 100 μl MEHP was significantly increased in contrast to mouse oocytes exposed to similar concentration of DEHP (Fig .2). Whereas, oral administration of the MEHP (200 μl) can affect early and late apoptotic oocytes compared to 200 μl DEHP treated mice. Necrotic oocytes in all exposed MEHP groups showed a significant difference rather than control (5, 4 and 5 vs. 1.3%, respectively).
Fig.2.
Image represents frequency of the live, early apoptotic, late apoptotic and necrotic oocytes (percentages) in experimental and control groups. Early A; Early apoptosis, Late A; Late apoptosis, MEHP; Mono-(2-ethylhexyl) phthalate, DEHP; Di-(2-ethylhexyl) phthalate, a; Significant difference with control group, b; Significant difference with experimental group-I (50 μl DEHP), c; Significant difference with experimental group-II (100 μl DEHP), d; Significant difference with experimental group-III (200 μl DEHP), e; Significant difference with experimental group-IV (50 μl MEHP), and f; Significant difference with experimental group-V (100 μl MEHP).
The effect of mono-(2-ethylhexyl) phthalate and di-(2-ethylhexyl) phthalate on maternal mRNA expression level
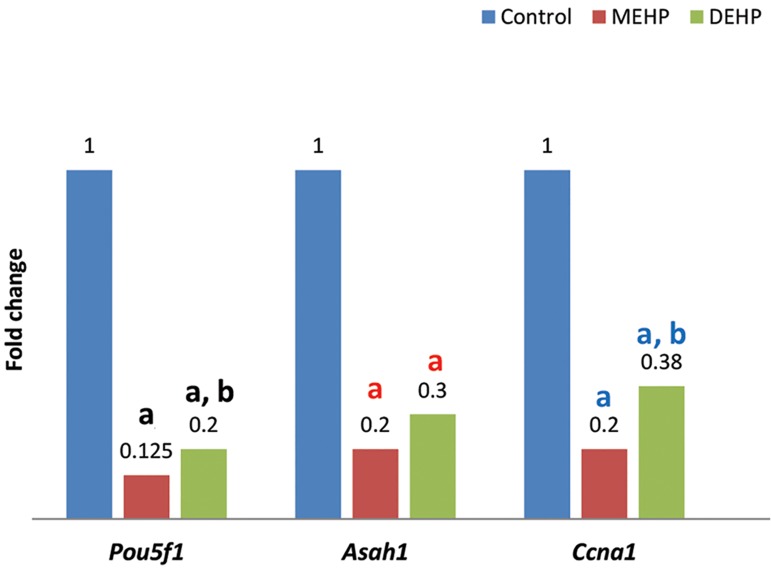
Real-time PCR analysis revealed significantly reduced level of Ccna1, Pou5f1, and Asah1 mRNA expression in the mouse oocytes, exposed to 100 μl MEHP or 100 μl DEHP (2.56 μM) compared to the control group (Fig .3). Further analyses demonstrated that mRNA level of Ccna1 and Pou5f1 genes were significantly lower in the mouse oocytes exposed by 100 μl MEHP rather than 100 μl DEHP. Although lower Asah1 mRNA expression was also observed in oocytes exposed by MEHP, no significant difference was observed in contrast to DEHP.
Fig.3.
mRNA expression level analysis of Pou5f1, Asah1 and Ccna1 in control group and oocytes exposed to 100 μl of MEHP or DEHP. MEHP; Mono-(2-ethylhexyl) phthalate, DEHP; di-(2-ethylhexyl) phthalate, a; Significant difference of control group and DEHP, P<0.05 and b; Significant difference of MEHP group and DEHP, P<0.05.
Evaluation of the survived and dead blastomeres in developing blastocysts
Ultimately, out of three repeats, one or two blastocyst(s) was selected from each experimental and control group to analyze the rate of dead and survived blastomers by using AO and EB staining (Fig .4). Findings showed that the rate of living blastomeres per embryo was significantly lower while the oocytes were exposed to MEHP or DEHP (47.9 ± 5.01, 56.66 ± 5.4) compared to the control group (84.1 ± 7.06, Table 3). The proportion of dead cells per embryo tended to be higher in the MEHPor DEHP-treated embryos relative to controls (P<0.05, Table 3). In addition, analyses of the blastomeres presented significantly different proportions of survived and dead cells in the MEHP-treated cells compared to DEHP.
Fig.4.
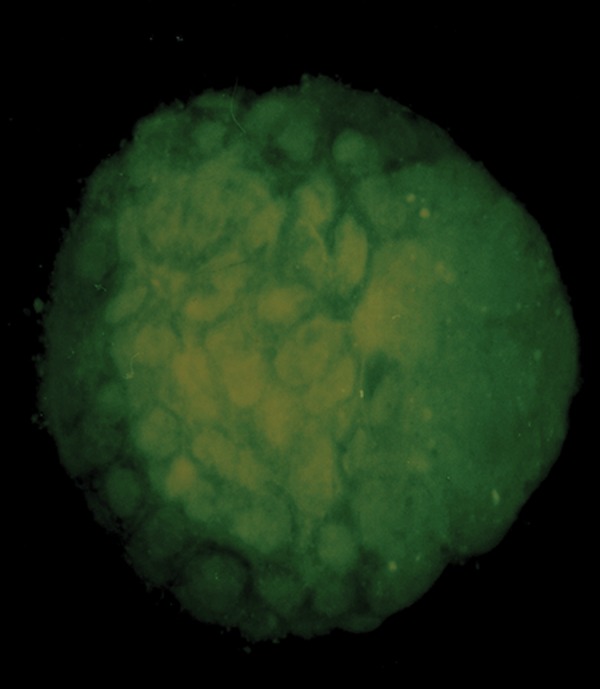
Representative image of the blastocyst staining with AO and EB. AO; Acridine-orange and EB; Ethidium-bromide.
Table 3.
Comparison of live and dead blastomeres stained in control group and the cells exposed to 100 μl MEHP or 100 μl DEHP
| Groups | Number of staining blastocysts | Mean of Live blastomeres ± SD | Dead cells |
|---|---|---|---|
| Control | 4 | 84.1 ± 7.06 | 0 |
| MEHP | 3 | 47.9 ± 5.01a | 3.4 ± 0.55a |
| DEHP | 3 | 56.66 ± 5.4a, b | 2.67 ± 1.37a, b |
MEHP; Mono-(2-ethylhexyl) phthalate, DEHP; Di-(2-ethylhexyl) phthalate, a; Significant difference of control and MEHP-treated groups, P<0.05, and b; Significant difference of DEHP and MEHP-treated groups, P<0.05.
Discussion
Releasing the environmental toxicants -such as phthalatesinto the air, soil and surface water has led to serious health hazards for human and animals. Thus these factors could affect embryonic development and reproductive organs (1, 14, 15). The current study provides evidences that oral administration of phthalates can impairs the meiotic and developmental competence of mouse oocytes.
This experiment showed that DEHP or MEHP induction suppressed oocyte development at all examined concentrations in a dose-dependent manner, after gavage administration. It has been demonstrated that DEHP, as peroxisome proliferator, has specific effects on estradiol production by inhibiting follicle stimulating hormone (FSH)- stimulated cAMP accumulation in rat granulosa cells (16, 17). Mechanistically, previous studies revealed that DEHP, through its metabolite MEHP, aberrantly regulates peroxisome proliferator activated receptor (PPAR)-mediated signaling pathways, leading to suppressing transcription of aromatase (P450Arom) as well as estradiol production, independent of cAMP stimulation in the ovary (18, 19).
PPARs play essential roles in the management of cellular differentiation, development, metabolism and tumorigenesis at higher organisms. It has been suggested that PPARs (20) could interrupt the growth time and follicular differentiation by inducing enzymatic free radical and oxidative stress pathways (21). Regarding these data, conversion of DEHP to the MEHD is proposed as a crucial point for PPAR activation and toxicological effects (18). In this experiment, we showed that MEHP bears more toxic effect rather than DEHP. In competence with this finding, evidences showed that MEHP cytotoxicity was 10-fold more than its parent compound (DEHP), due to the activity of MEHP (22, 23). It was suggested that MEHP, compared to DEHP, induces oxidative stress by suppressing different antioxidant enzymes (24). Therefore, it is most likely important to develop an approach to inhibit the ability of body in converting DEHP into MEHP. In addition, structural differences between DEHP and MEHP may lead to their distinct effects on antioxidant enzymes. DEHP, through lipophilic feature caused by two 2-ethylhexanol branched chains, can activate intracellular signal cascades while cross the lipid membrane. In contrast, MEHP has only one 2-ethylhexanol branched chain and consequently less lipophilic feature than DEHP. It has been demonstrated that MEHP affects the signaling molecules located on membrane, instead of activating intracellular molecules (20).
Our findings, based on Annexin V/PI staining, implicated that exposure to MEHP or DEHP might cause oocyte death by induction of apoptotic signaling pathway. However, further investigations are required to determine mechanisms involved in apoptosis, due to oral phthalate esters induction.
On the other hand, with regards to the study indicating that regulation of maternal mRNA expression in oocytes could direct maturation procedure (28), we evaluated the phthalate effects on this procedure via analysis of mRNA expression level. In particular, we showed reduced level of Pou5f1, Asah1 and Ccna1 mRNA expressions in the experimental groups, suggesting that phthalate might influence the quality of developed embryo by regulating particular gene transcriptions. Pou5f1 is one of the maternal regulated genes, playing critical role in defining totipotency and inducing pluripotency of embryonic cells (29). Upor down-regulation of Pou5f1 can alter cell signaling, epigenetic fate, transcriptional and post-transcriptional regulation, cell cycle behavior and apoptosis during early embryonic development (30-32). In current study, reduced level of Pou5f1 expression could be a likely cause of decreased developmental competence in treated oocyte groups.
It has been shown that Pou5f1 activity could cause blastocyst developmental arrest through mitochondrial dysfunction and induction of apoptosis. In current experiment, AO and EB staining of blastocysts, derived from treated mouse oocytes, indicated decreased embryo quality and increased death incidence in embryonic cells.
Consistent to this, Gendelman and Roth (33) showed that thermal stress reduced transcription of Pou5f1 before further expression of embryonic genome activation at the blastocyst stage. It is proposed that impairment of Pou5f1, led by exposure to MEHP or DEHP, could be intensified in later developmental stages, subsequently culminating in reduced frequency of developed embryos into the blastocyst stage.
It is has been approved that genetic programs, contributing to cell division control, play critical role on transformation of meiotic into mitotic cell cycle upon fertilization. Meanwhile, cell cycle progression is regulated by various cyclin activities (33). Ccna1 encodes cyclin A1 which is involved in DNA double strand break repair and regulation of metaphase in meiotic cell cycles (34, 35). Previous studies have reported expression of cyclin A1 in testis (36-39). In addition, there are evidences implicating on the expression of this cyclin in ovary/oocyte (36). Fuchimoto et al. (39) showed that cyclin A1 was expressed in oocytes as well as single cell embryos. Interestingly, our findings indicated that cyclin A1 was expressed in mature oocytes. It was suggested that expression of cyclin A1 in oocytes is part of normal role of this gene (34). Thus, reduced Ccna1 mRNA expression level in MEHP or DEHP treated oocytes seems to explain the reduced normal function of this gene, through oogenesis stages. Down-regulation of these gene expressions, by exposing to the other endocrine disruptors like 4-nonylphenol, could lead to defect of cyclin A and cyclin B1 during maturation (39, 40).
Asah1 mRNA encodes acid ceramidase (AC), an enzyme required for early embryo survival, by maintaining a balance between ceramide (a pro-apoptotic lipid) and an anti-apoptotic lipid named sphingosine-1-phosphate (S1P). It has been shown that AC has important role in ceramide-related changes, leading to cell cycle arrest and/or death (41). Eliyahu et al. (42) revealed that AC depletion, during follicular transition from secondary to antral stages, led to apoptosis of oocytes. Here, we reported phthalate-induced reduction in Asah1 transcription levels in MII-stage oocytes. This finding might associate with an increased number of annexin V-positive oocytes, presumably due to an increase in ceramide levels. Moreover, the reduced transcription level of Asah1 mRNA might explain the decreased percentage of cleaved embryos, leading to further development towards blastocyst stage as well as increased proportion of cell death in the blastocysts. Investigations proposed that exposure to DEHP causes apoptosis through activation of PPARs pathway (43). Moreover, it has been suggested that PPARs are the mediators of phthalate-induced alterations in the male and female reproductive tract (44); therefore, PPAR activation could be considered as a critical process at this experiment. Recent reports have also shown that S1P, a downstream product of AC activity, can prevent apoptosis in the ovaries of several species (42). All of these findings are in agreement with our hypothesis suggesting that AC has a major protective function during oocyte and follicle development.
Conclusion
Ultimately, our investigations on the models in vivo indicated that phthalates had a deleterious carrying-over effect on oocyte developmental competence, reflected by a reduced proportion of oocytes undergoing maturation, fertilization, cleavage, and further development towards the blastocyst stage. The reduced developmental competence of MEHP-treated oocytes was strongly associated with alterations in the levels of Ccna1, Asah1, and Pou5f1 mRNA expression. This study revealed that exposure to the environmental concentrations of phthalate affected the ovarian pool of oocytes and these effects persisted into the later stage. However, phthalate-induced alterations in oocyte maternal mRNA may highlight the risk association with exposure of animals to environmental contaminations and their potential to compromise fertility.
Acknowledgments
We thank Cellular and Molecular Research Center of Ahvaz Jundishapur University for supporting this study under grant agreement number CMRC-109, in 2014. The authors have no conflicts of interest to declare.
References
- 1.Bernanke J, Köhler HR. The impact of environmental chemicals on wildlife vertebrates. Rev Environ Contam Toxicol. 2009;198:1–47. doi: 10.1007/978-0-387-09647-6_1. [DOI] [PubMed] [Google Scholar]
- 2.Koch HM, Calafat AM. Human body burdens of chemicals used in plastic manufacture. Philos Trans R Soc Lond B Biol Sci. 2009;364(1526):2063–2078. doi: 10.1098/rstb.2008.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kay VR, Chambers C, Foster WG. Reproductive and developmental effects of phthalate diesters in females. Crit Rev Toxicol. 2013;43(3):200–219. doi: 10.3109/10408444.2013.766149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang W, Craig ZR, Basavarajappa MS, Gupta RK, Flaws JA. Di (2ethylhexyl) phthalate inhibits growth of mouse ovarian antral follicle through an oxidative stress pathway. Toxicol Appl Pharmacol. 2012;258(2):288–295. doi: 10.1016/j.taap.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kavlock R, Barr D, Boekelheide K, Breslin W, Breysse P, Chapin R, et al. NTP-CERHR expert panel update on the reproductive and developmental toxicity of di (2-ethylhexyl) phthalate. Reprod Toxicol. 2006;22(3):291–399. doi: 10.1016/j.reprotox.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Fabjan E, Hulzebos E, Mennes W, Piersma AH. A category approach for reproductive effects of phthalates. Crit Rev Toxicol. 2006;36(9):695–726. doi: 10.1080/10408440600894914. [DOI] [PubMed] [Google Scholar]
- 7.Lovekamp TM, Davis BJ. Mono-(2-ethylhexyl) phthalate suppresses aromatase transcript levels and estradiol production in cultured rat granulosa cells. Toxicol Appl Pharmacol. 2001;172(3):217–224. doi: 10.1006/taap.2001.9156. [DOI] [PubMed] [Google Scholar]
- 8.Anas M, Suzuki C, Yoshioka K, Iwamura S. Effect of mono-(2-ethylhexyl) phthalate on bovine oocyte maturation in vitro. Reprod Toxicol. 2003;17(3):305–310. doi: 10.1016/s0890-6238(03)00014-5. [DOI] [PubMed] [Google Scholar]
- 9.Dalman A, Eimani H, Sepehri H, Ashtiani SK, Valojerdi MR, Eftekhari Yazdi P, et al. Effect of mono-(2-ethylhexyl) phthalate (MEHP) on resumption of meiosis, in vitro maturation and embryo development of immature mouse oocytes.Biofactors. Biofactors; 2008. pp. 149–155. [DOI] [PubMed] [Google Scholar]
- 10.Carnevali O, Tusti L, Speciale C, Peng C, Zhu Y, Maradona F. DEHP impairs zebrafish reproduction by affecting critical factors in oogenesis. PLoS One. 2010;5(4):e10201–e10201. doi: 10.1371/journal.pone.0010201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Susor A, Jansova D, Cerna R, Danylevska A, Anger M, Toralova T, et al. Temporal and spatial regulation of translation in the mammalian oocyte via the mTOR-eIF4F pathway. Nat Commun. 2015;6:6078–6078. doi: 10.1038/ncomms7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan H, Oberien MJ, Wigglesworth K, Eppig JJ, Schultz RM. Transcript profiling during mouse oocyte development and the effects of gonadotropin priming and development in vitro. Dev Biol. 2005;286(2):493–506. doi: 10.1016/j.ydbio.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 13.Grossman D, Kalo D, Gendelman M, Roth Z. Effect of di-(2-ethylhexyl) phthalate and mono-(2-ethylhexyl) phthalate on in vitro developmental competence of bovine oocytes. Cell Biol Toxicol. 2012;28(6):383–396. doi: 10.1007/s10565-012-9230-1. [DOI] [PubMed] [Google Scholar]
- 14.Heudorf U, Mersch-Sundermann V, Angerer J. Phthalates: toxicology and exposure. Int J Hyg Environ Health. 2007;210(5):623–634. doi: 10.1016/j.ijheh.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Lyche JL, Gutleb AC, Bergman A, Eriksen GS, Murk AJ, Ropstad E, et al. Reproductive and developmental toxicity of phthalates. J Toxicol Environ Health B Crit Rev. 2009;12(4):225–249. doi: 10.1080/10937400903094091. [DOI] [PubMed] [Google Scholar]
- 16.Ito Y, Yamanoshita O, Asaeda N, Tagawa Y, Lee CH, Aoyama T, et al. Di(2-ethylhexyl)phthalate induces hepatic tumorigenesis through a peroxisome proliferator-activated receptor alpha-independent pathway. J Occup Health. 2007;49(3):172–182. doi: 10.1539/joh.49.172. [DOI] [PubMed] [Google Scholar]
- 17.Davis BJ, Weaver R, Gaines LJ, Heindel JJ. Mono-(2ethylhexyl) phthalate suppresses estradiol production independent of fsh-camp stimulation in rat granulosa cells. Toxicol Appl Pharmacol. 1994;128(2):224–228. doi: 10.1006/taap.1994.1201. [DOI] [PubMed] [Google Scholar]
- 18.Lovekamp-Swan T, Davis BJ. Mechanisms of phthalate ester toxicity in the female reproductive system. Environ Health Perspect. 2003;111(2):139–145. doi: 10.1289/ehp.5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurst CH, Waxman DJ. Activation of PPARalpha and PPARgamma by environmental phthalate monoesters. Toxicol Sci. 2003;74(2):297–308. doi: 10.1093/toxsci/kfg145. [DOI] [PubMed] [Google Scholar]
- 20.Gazouli M, Yao ZX, Boujrad N, Corton JC, Culty M, Papadopoulos V. Effect of peroxisome proliferators on Leydig cell peripheral-type benzodiazepine receptor gene expression, hormone-stimulated cholesterol transport, and steroidogenesis: role of the peroxisome proliferatoractivator receptor alpha. Endocrinology. 2002;143(7):2571–2583. doi: 10.1210/endo.143.7.8895. [DOI] [PubMed] [Google Scholar]
- 21.O'Brien MC, Champion H, D'Agostino RB, Martin BA, McCoy TP, Wolfson M, et al. How ready are colleges for an environmental approach that utilizes campus/community coalitions? Int Q Community Health Educ. 2005-2006;25(3):295–305. doi: 10.2190/G542-W332-GG21-056U. [DOI] [PubMed] [Google Scholar]
- 22.Yang G, Zhang W, Qin Q, Wang J, Zheng H, Xiong W, et al. Mono(2-ethylhexyl) phthalate induces apoptosis in p53-silenced L02 cells via activation of both mitochondrial and death receptor pathways. Environ Toxicol. 2015;30(10):1178–1191. doi: 10.1002/tox.21990. [DOI] [PubMed] [Google Scholar]
- 23.Dees JH, Gazouli M, Papadopoulos V. Effect of monoethylhexyl phthalate on MA-10 Leydig tumor cells. Reprod Toxicol. 2001;15(2):171–187. doi: 10.1016/s0890-6238(01)00110-1. [DOI] [PubMed] [Google Scholar]
- 24.Wang W, Craig ZR, Basavarajappa MS, Hafner KS, Flaws JA. Mono-(2-Ethylhexyl) phthalate induces oxidative stress and inhibits growth of mouse ovarian antral follicles. Biol Reprod. 2012;87(6):152–152. doi: 10.1095/biolreprod.112.102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu Y, He CJ, Ji PY, Zhuo ZY, Tian XZ, Wang F, et al. Effects of melatonin on the proliferation and apoptosis of sheep granulosa cells under thermal stress. Int J Mol Sci. 2014;15(11):21090–21104. doi: 10.3390/ijms151121090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rogers R, Ouellet G, Brown C, Moyer B, Rasoulpour T, Hixon M. Cross-talk between the akt and nf-κb signaling pathways inhibits mehp-induced germ cell apoptosis. Toxicol Sci. 2008;106(2):497–508. doi: 10.1093/toxsci/kfn186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen J, Torcia S, Xie F, Lin CH, Cakmak H, Franciosi F, et al. Somatic cells regulate maternal mRNA translation and developmental competence of mouse oocytes. Nat Cell Biol. 2013;15(12):1415–1423. doi: 10.1038/ncb2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu G, Schöler HR. Role of Oct4 in the early embryo development. Cell Regen(Lond) 2014;3(1):7–7. doi: 10.1186/2045-9769-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niwa H, Masui SH, Chambers I, Smith AG, Miyazaki J. Phenotypic complementation establishes requirements for specific pou domain and generic transactivation function of oct-3/4 in embryonic stem cells. Mol Cell Biol. 2002;22(5):1526–1536. doi: 10.1128/mcb.22.5.1526-1536.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foygel K, Choi B, Jun S, Leong DE, Lee A, Wong CC, et al. A novel and critical role for Oct4 as a regulator of the maternal-embryonic transition. PLoS One. 2008;3(12):e4109–e4109. doi: 10.1371/journal.pone.0004109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edson MA, Nagaraja AK, Matzuk MM. The mammalian ovary from genesis to revelation. Endocr Rev. 2009;30(6):624–712. doi: 10.1210/er.2009-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gendelman M, Roth Z. Seasonal effect on germinal vesiclestage bovine oocytes is further expressed by alterations in transcript levels in the developing embryos associated with reduced developmental competence. Biol Reprod. 2012;86(1):1–9. doi: 10.1095/biolreprod.111.092882. [DOI] [PubMed] [Google Scholar]
- 34.Wei H, Li Y, Zhao CH, Jiang W, Chen H, Lang MF, et al. Cyclin A1 Is Expressed in Mouse Ovary. Int J Med Sci. 2014;11(7):754–757. doi: 10.7150/ijms.8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolgemuth DJ. Function of cyclins in regulating the mitotic and meiotic cell cycles in male germ cells. Cell Cycle. 2008;7(22):3509–3513. doi: 10.4161/cc.7.22.6978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu D, Matzuk MM, Sung WK, Guo Q, Wang P, Wolgemuth DJ. Cyclin A1 is required for meiosis in the male mouse. Nat Genet. 1998;20(4):377–380. doi: 10.1038/3855. [DOI] [PubMed] [Google Scholar]
- 37.Yang R, Morosetti R, Koeffler HP. Characterization of a second human cyclin a that is highly expressed in testis and in several leukemic cell lines. Cancer Res. 1997;57(5):913–920. [PubMed] [Google Scholar]
- 38.Persson JL, Zhang Q, Wang XY, Ravnik SE, Muhlrad S, Wolgemuth DJ. Distinct roles for the mammalian A-type cyclins during oogenesis. Reproduction. 2005;130(4):411–422. doi: 10.1530/rep.1.00719. [DOI] [PubMed] [Google Scholar]
- 39.Fuchimoto D, Mizukoshi A, Schultz RM, Sakai S, Aoki F. Posttranscriptional regulation of cyclin A1 and cyclin A2 during mouse oocyte meiotic maturation and preimplantation development. Biol Reprod. 2001;65(4):986–993. doi: 10.1095/biolreprod65.4.986. [DOI] [PubMed] [Google Scholar]
- 40.Kudo C, Wada K, Masuda T, Yonemura T, Shibuya A, Fujimoto Y, et al. Nonylphenol induces the death of neural stem cells due to activation of the caspase cascade and regulation of the cell cycle. J Neurochem. 2004;88(6):1416–1423. doi: 10.1046/j.1471-4159.2003.02270.x. [DOI] [PubMed] [Google Scholar]
- 41.Haimovitz-Friedman A, Cordon-Cardo C, Bayoumy S, Garzotto M, McLoughlin M, Gallily R, et al. Lipopolysaccharide induces disseminated endothelial apoptosis requiring ceramide generation. J Exp Med. 1997;186(11):1831–1841. doi: 10.1084/jem.186.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eliyahu E, Shtraizent N, Shalgi R, Schuchman ER. Construction of conditional acid ceramidase knockout mice and in vivo effects on oocyte development and fertility. Cell Physiol Biochem. 2012;30(3):735–748. doi: 10.1159/000341453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ryu JY, Whang J, Park H, Im JY, Kim J, Ahn MY, et al. Di(2ethylhexyl) phthalate induces apoptosis through peroxisome proliferators-activated receptor-gamma and ERK 1/ 2 activation in testis of Sprague-Dawley rats.J Toxicol. Environ Health A. 2007;70(15-16):1296–1303. doi: 10.1080/15287390701432160. [DOI] [PubMed] [Google Scholar]
- 44.Latini G, Scoditti E, Verrotti A, De Felice C, Massaro M. Peroxisome proliferator-activated receptors as mediators of phthalate-induced effects in the male and female reproductive tract: epidemiological and experimental evidence. PPAR Res. 2008;2008:359267–359267. doi: 10.1155/2008/359267. [DOI] [PMC free article] [PubMed] [Google Scholar]