Abstract
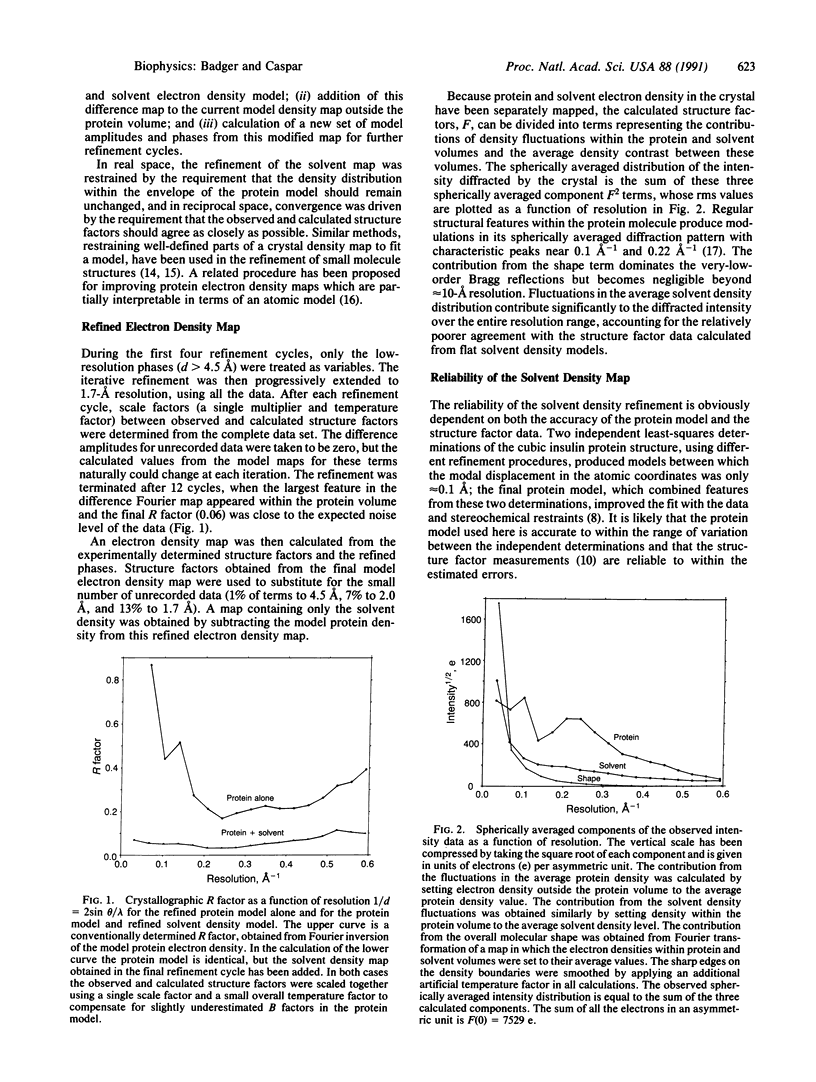
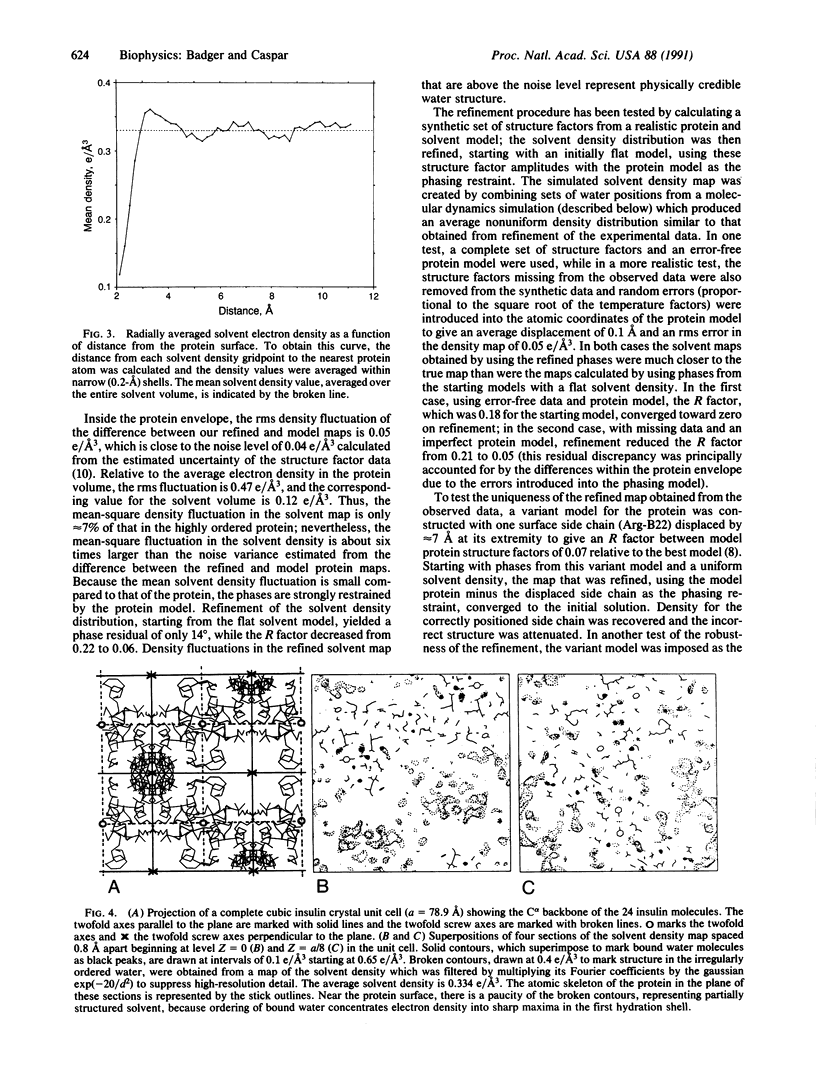
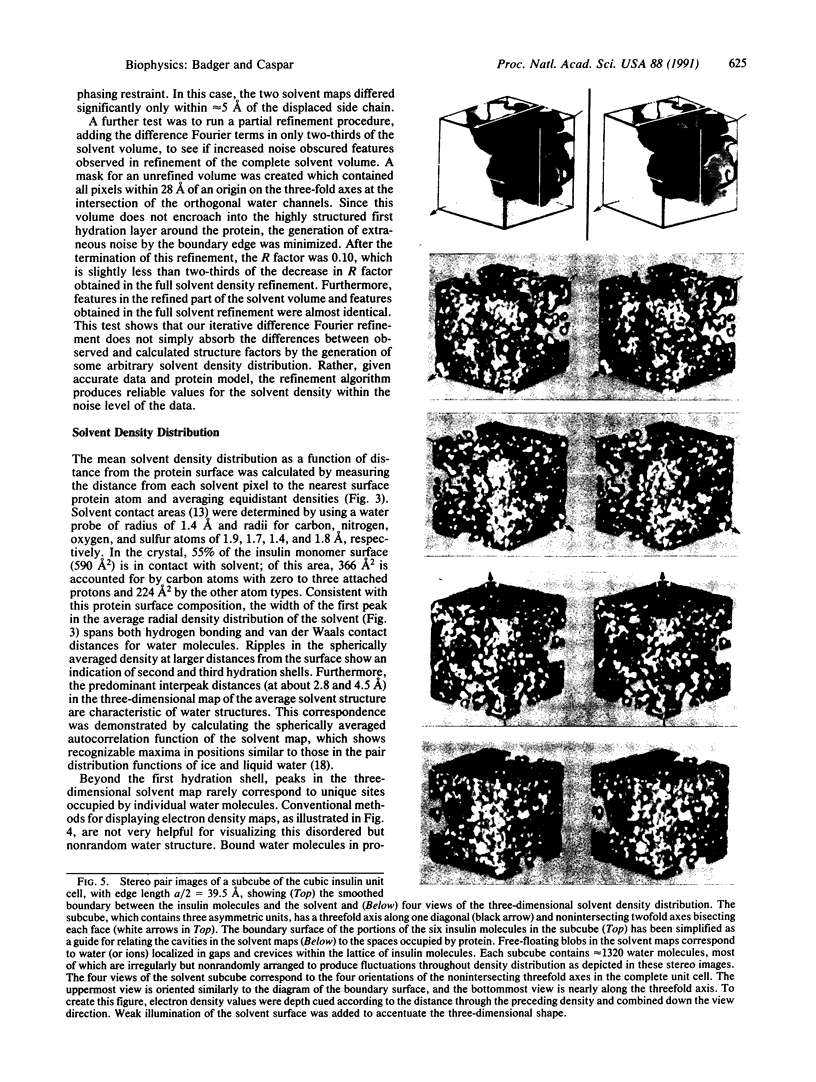
The electron density distribution of the solvent in the cubic insulin crystal structure, which occupies 65% of the volume, has been mapped from 1.7-A resolution diffraction data by an iterative difference Fourier method, using the previously determined protein structure as the refinement restraint. Starting with phases from the protein and a flat solvent model, the difference map calculated from the data was added outside the protein envelope, and the modified map was then used to recalculate phases for the iterative refinement. Tests of the method with model data, with the experimental data and a variant protein model, and by carrying out a partial refinement of the solvent map demonstrate that the refinement algorithm produces reliable values for the solvent density within the noise level of the data. Fluctuations in density are observed throughout the solvent space, demonstrating that nonrandom arrangements of the water molecules extend several layers from the well-ordered hydration shell in contact with the protein surface. Such ordering may account for the hydration force opposing close approach of hydrophilic surfaces and other long-range water-dependent interactions in living structures.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker E. N., Blundell T. L., Cutfield J. F., Cutfield S. M., Dodson E. J., Dodson G. G., Hodgkin D. M., Hubbard R. E., Isaacs N. W., Reynolds C. D. The structure of 2Zn pig insulin crystals at 1.5 A resolution. Philos Trans R Soc Lond B Biol Sci. 1988 Jul 6;319(1195):369–456. doi: 10.1098/rstb.1988.0058. [DOI] [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Blake C. C., Pulford W. C., Artymiuk P. J. X-ray studies of water in crystals of lysozyme. J Mol Biol. 1983 Jul 5;167(3):693–723. doi: 10.1016/s0022-2836(83)80105-3. [DOI] [PubMed] [Google Scholar]
- Brünger A. T., Kuriyan J., Karplus M. Crystallographic R factor refinement by molecular dynamics. Science. 1987 Jan 23;235(4787):458–460. doi: 10.1126/science.235.4787.458. [DOI] [PubMed] [Google Scholar]
- Chothia C. Structural invariants in protein folding. Nature. 1975 Mar 27;254(5498):304–308. doi: 10.1038/254304a0. [DOI] [PubMed] [Google Scholar]
- Dodson E. J., Dodson G. G., Lewitova A., Sabesan M. Zinc-free cubic pig insulin: crystallization and structure determination. J Mol Biol. 1978 Nov 5;125(3):387–396. doi: 10.1016/0022-2836(78)90409-6. [DOI] [PubMed] [Google Scholar]
- Harding M. M., Hodgkin D. C., Kennedy A. F., O'Conor A., Weitzmann P. D. The crystal structure of insulin. II. An investigation of rhombohedral zinc insulin crystals and a report of other crystalline forms. J Mol Biol. 1966 Mar;16(1):212–226. doi: 10.1016/s0022-2836(66)80274-7. [DOI] [PubMed] [Google Scholar]
- Ling G. N. The physical state of water in living cell and model systems. Ann N Y Acad Sci. 1965 Oct 13;125(2):401–417. doi: 10.1111/j.1749-6632.1965.tb45406.x. [DOI] [PubMed] [Google Scholar]
- Matthews B. W. Solvent content of protein crystals. J Mol Biol. 1968 Apr 28;33(2):491–497. doi: 10.1016/0022-2836(68)90205-2. [DOI] [PubMed] [Google Scholar]
- Narten A. H., Levy H. A. Observed diffraction pattern and proposed models of liquid water. Science. 1969 Aug 1;165(3892):447–454. doi: 10.1126/science.165.3892.447. [DOI] [PubMed] [Google Scholar]
- Parsegian V. A., Fuller N., Rand R. P. Measured work of deformation and repulsion of lecithin bilayers. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2750–2754. doi: 10.1073/pnas.76.6.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau D. C., Lee B., Parsegian V. A. Measurement of the repulsive force between polyelectrolyte molecules in ionic solution: hydration forces between parallel DNA double helices. Proc Natl Acad Sci U S A. 1984 May;81(9):2621–2625. doi: 10.1073/pnas.81.9.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards F. M. Areas, volumes, packing and protein structure. Annu Rev Biophys Bioeng. 1977;6:151–176. doi: 10.1146/annurev.bb.06.060177.001055. [DOI] [PubMed] [Google Scholar]
- Schoenborn B. P. Solvent effect in protein crystals. A neutron diffraction analysis of solvent and ion density. J Mol Biol. 1988 Jun 20;201(4):741–749. doi: 10.1016/0022-2836(88)90470-6. [DOI] [PubMed] [Google Scholar]
- Teeter M. M. Water structure of a hydrophobic protein at atomic resolution: Pentagon rings of water molecules in crystals of crambin. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6014–6018. doi: 10.1073/pnas.81.19.6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbitts T. T., Caspar D. L., Phillips W. C., Goodenough D. A. Diffraction diagnosis of protein folding in gap junction connexons. Biophys J. 1990 May;57(5):1025–1036. doi: 10.1016/S0006-3495(90)82621-7. [DOI] [PMC free article] [PubMed] [Google Scholar]