Summary
Mechanisms to vary the phenotypic characteristics of fungi are diverse and can be important for their life cycle. This review summarizes phenotypic variability in fungi and divides this phenomenon into three topics: (i) morphological transitions, which are environmentally induced and involve the entire fungal population, (ii) reversible phenotypic switching between different colony morphologies, which is restricted to a small fraction of the population, and (iii) antigenic variation of surface antigens, which can be immuno-dominant epitopes happens in individual fungal cells.
Introduction
Phenotypic variation in microorganisms allows rapid adaptation to a constantly changing environment. Most pathogenic fungi are environmental microbes that accidentally invade the host. The exceptions are Candida albicans and Pneumocystis spp., which reside predominantly in their hosts. Variation of the microbial cell surface can impact host–pathogen interaction, and facilitate evasion of an evolving host immune response. Virulence traits often constitute important survival mechanisms in the environment and are selected in the host.
Phenotypic changes are common in fungi and induced by many different mechanisms. For clarity we will differentiate three kinds of phenotypic changes, albeit this division has many overlaps. First, morphological transitions (MT) are described in most fungi and induced by environmental signals like starvation, temperature, pH change and mating associated factors. Examples include sporulation-associated MTs, bud-hyphal MTs, capsule induction and phase variation in dimorphic fungi. These MTs are easily distinguishable, highly controlled and involve the entire population. Second, complex phenotypic changes can be achieved through reversible phenotypic switching (PS). PS occurs in a small fraction of the population, is random, reversible and represents an epigenetic state that is not necessarily induced by external signals. Third, antigenic variation (AV) involves alternating the expression of surface proteins (or carbohydrates), which happens on cellular level in the entire population but can underlie strong selection pressure. Overlaps are common, e.g. MTs can involve AVs and PS can affect the percentage of MTs in a colony.
MT in fungi
As part of their life cycle most fungi manifest many phenotypic morphologies that involve remodelling of the outer surface. In particular hyphae, biofilm and spherule formation as well as capsule induction contribute to virulence and evasion of the host response. Expression of morphology-specific genes, many of which are GPI-anchored, are controlled by diverse signal transduction pathways, including MAP kinase, HOG, calcineurin, cyclic AMP pathway, that respond to environmental signals. Although these pathways are generally conserved, significant differences in these regulatory networks occur even in closely related species.
Phase variation in dimorphic fungi
The dimorphic fungi can cause diverse diseases after inhalation. At ambient temperatures, they grow as filamentous saprophobic molds in the environment. Elongating hyphae asexually produce propagules in the form of conidia or arthroconidia, which can be inhaled. Spores from Coccidioides spp. undergo isotropic growth and convert into multinucleate round cells (spherules) in the lungs. The mycelial forms are adapted to climate and soils, which determines the geographical restriction. The yeast morphology is exclusively associated with the pathogenic life phase in the mammalian host. Thus genes that are differentially regulated in the yeast versus the hyphal forms are often important for virulence. Examples include CBP, α-(1,3)-glucan in Histoplasma capsulatum (Kugler et al., 2000), BAD1 in Blastomyces dermatitidis, and SOWgp and Mep1 in Coccidioides immitis and C. posadasii (Rappleye and Goldman, 2006).
Hyphal formation in pathogenic yeasts and molds
Candida albicans is both a commensal and a pathogen, which can exhibit a yeast, hyphal or pseudohyphal morphology. The yeast form is associated with dissemination, and the hyphal form with adhesion, tissue invasion and proteolytic activity (Whiteway and Bachewich, 2007). Accordingly genes involved in these functions (ALS3, SAP4-6, HWP1, HYR1, ECE1) are differentially expressed (Birse et al., 1993; Bailey et al., 1996; Sanglard et al., 1997; Hoyer et al., 1998; Staab and Sundstrom, 1998; Martchenko et al., 2004). The MAP kinase, cAMP and the pH-sensing Rim101 signal transduction pathways (Whiteway and Bachewich, 2007) regulate cellular morphology and expression of hyphal associated genes. These MTs may enhance colonization at different anatomical sites, or invasion and is also seen in other Candida species.
Aspergillus fumigatus also undergoes MT to cause invasive aspergillosis. Inhaled conidia must complete germination, which involves isotropic growth, and emergence of the initial germ tube. The germling then elongates by apical extension to tube-like invasive hyphae. Genes involved in MT include pkaR, the regulatory subunit of the cAMP-dependent protein kinase, and rasB, a ras family subhomologue (Fortwendel et al., 2005; Zhao et al., 2006). Mutants of the calcineurin pathway also exhibit defects in conidial germination and polarized hyphal growth (Cramer et al., 2008).
Capsule induction
Cryptococcus neoformans var. neoformans (C. neoformans) and var. grubii (C. grubii), and Cryptococcus var. gattii (C. gattii) are ubiquitous encapsulated yeasts that cause chronic meningoencephalitis, pneumonia and disseminated disease in susceptible individuals. In vivo, they induce their polysaccharide capsule in response to low iron, higher pH and CO2 levels, and starvation (reviewed in Zaragoza et al., 2009). Capsule induction is required for virulence as it affects phagocytosis and migration across the blood brain barrier (Garcia-Hermoso et al., 2004; Charlier et al., 2005).
Biofilm formation
Surface-associated fungi can grow embedded in extracellular matrix (ECM) that is composed of carbohydrates and proteins. Other Candida spp. (Iraqui et al., 2005) and Cryptococcus spp. (Martinez and Casadevall, 2005) and even A. fumigatus (Mowat et al., 2009) form biofilms with ECM. Biofilm-associated gene regulation is predominantly studied in C. albicans (for review, Blankenship and Mitchell, 2006), where hyphal formation is important for robust biofilm formation (BF). Two transcription factors, Tec1 and Bcr1, are important and regulate hypha-specific genes, and genes downstream of hyphal differentiation (Schweizer et al., 2000; Nobile et al., 2006). Candida genes that control adherence, attachment hyphal formation and quorum sensing molecules also regulate BF. Biofilms are resistant to antifungal drugs and thus greatly contribute to fungal pathogenesis.
Phenotypic switching
Colony switching is mainly described in yeasts because it is defined as the spontaneous emergence of colony variants, a phenomenon harder to distinguish in molds.
Candida albicans
High frequency PS was first described in Candida strains 3153A and WO-1 (Slutsky et al., 1985; 1987; also Soll, 1992). The model strain WO-1 switches between two colony morphologies, whereas 3153A generates many unstable colony phenotypes (Slutsky et al., 1985; 1987) White-to-opaque switching (WOS) occurs reversibly at a frequency of 1 in 104–105 (Fig. 1, upper panel). White-phase cells are round whereas large opaque-phase cells are elongated (Anderson and Soll, 1987; Slutsky et al., 1987; Rikkerink et al., 1988). Opaque-phase cells are mating-competent whereas white-phase cells survive better within the mammalian host, yet can switch to mating-competent cells when needed (Miller and Johnson, 2002). Physiological CO2 levels induce WOS at 37°C (Huang et al., 2009). Clinical isolates can undergo WOS if they are homozygous (a/a or α/α) whereas heterozygous (a/α) strains cannot switch (Lockhart et al., 2002; Legrand et al., 2004).
Fig. 1.
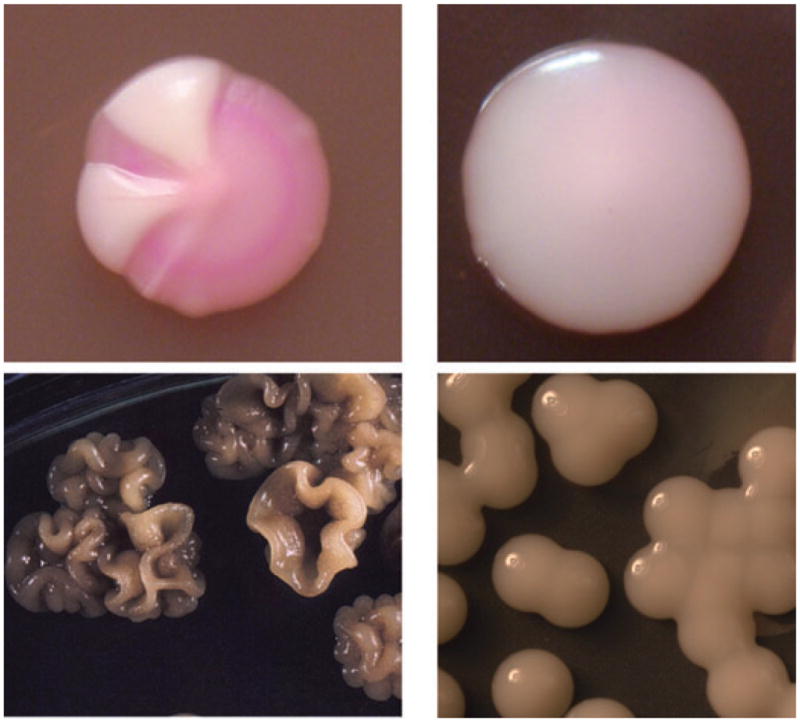
Upper panel shows sectored and wt WO-1 C. albicans strain phenotype on Phloxine B containing agar. Sectors denote the different switch types. Lower panel shows a wrinkled melanized and a mucoid switch variant of 24067 C. neoformans strain; both variants exhibit enhanced virulence in vivo.
Phase-specific genes are regulated by phase-specific trans-acting factors (Srikantha et al., 1995; Lockhart et al., 1998; Sonneborn et al., 1999) and implicated in virulence (Morrow et al., 1992; Hube et al., 1994; Srikantha et al., 1995; Balan et al., 1997). White-phase cells are more virulent in intravenous infection (Kvaal et al., 1999) and opaque-phase cells colonize skin better (Lachke et al., 2003). WOS also affects other virulence traits, including the bud-hyphal transition (Anderson et al., 1990), sensitivity to neutrophils and oxidants (Kolotila and Diamond, 1990), antigenicity (Anderson et al., 1990), adhesion (Kennedy et al., 1988), secretion of proteinase (Morrow et al., 1992; Vargas et al., 2000), drug susceptibility and phagocytosis by macrophages (Lohse and Johnson, 2008). All of these altered traits can potentially affect survival in the mammalian host.
More than 373 genes are differentially expressed in WO variants (Lan et al., 2002). These genes represent diverse functions including metabolism, adhesion, cell surface composition, stress response, signalling, mating type and virulence. Metabolic specialization of switch phenotypes may enhance selection at different anatomical sites. Consistent with that hypothesis, opaque cells expressed genes associated with oxidative metabolism whereas genes in white cells were associated with fermentative metabolism. Wor1 has been identified as a master regulator of WOS, as its deletion blocks opaque cell formation (Huang et al., 2006). Interlocking feedback loop networks maintain the epigenetic state of switch variants through cell divisions. MTLa1, MTLα2, WOR1, CZF1, WOR2 and EFG1 constitute a circuit that regulates WOS. This circuit is not present in closely related fungi and could be a recent adaptation in the mammalian host (Zordan et al., 2006; 2007).
Candida glabrata
Candida glabrata, another pathogenic yeast, undergoes ‘core switching’ (Lachke et al., 2002) on agar containing CuSO4. Core switching occurs in the majority of clinical strains and results in white (W), light brown (LB), dark brown (DB), very dark brown (vDB) and irregular wrinkle (IWr) colonies, which results from graded conversions of Cu2+ to Cu1+ and reduction of SO42− to S1−. Transcriptional profiling showed that most upregulated genes in DB were involved in CuSO4 assimilation and stress responses (Srikantha et al., 2005). PS may play a fundamental role in virulence because DB predominates among natural isolates and in mice has a colonization advantage over other colony types (Srikantha et al., 2008).
Candida lusitaniae
Candida lusitaniae, a rare human pathogen (Merz, 1984), frequently develops resistance to amphotericin B (AMB) during infection (Young et al., 2003). C. lusitaniae can switch between LB, DB and W colonies on CuSO4-supplemented agar. High AMB resistance is associated with W, whereas low AMB resistance and filamentation is associated with LB and DB colonies. PS happens at high frequency (1 in 102–104) and may confer a selective advantage in a host that is treated with AMB.
Cryptococcus spp
Phenotypic switching occurs in C. grubii (SB4, J32) (Goldman et al., 1998), C. neoformans (24067A, RC-2) (Fries et al., 2001) and C. gattii (NP-1) (Jain et al., 2006). Colony variants arise at a frequency of about 1 in 104–105. Smooth colonies (S and SM) of SB4, 24067 and NP-1 have a smooth dome, and mucoid (M and MC) colonies have a shiny and mucoid appearing colony surface. Wrinkled (WR), serrated (C) and pseudohyphal (PH) colonies exhibit an irregular dome surface with or without serrated margins and are rarely observed in clinical isolates (Fig. 1, upper panel). Serotype D strain RC-2 has stable switching rates and is used for research.
RC-2-MC has a larger capsule than RC2-SM and produces a viscous exo-polysaccharide. The predominant capsular polysaccharide Glucuronoxylomannan (GXM) is composed of linear α-D-mannopyranan chain with β-D-xylopyranosyl (Xylp) and β-D-glucupyranosyluronic acid (GlcpA) side-residues. Six (M1–M6) structural reporter groups (SRG) are defined by amount and position of linked Xylp, and GlcpA residues. In strains SB4 and 24067A PS results in changes of SRGs (Fries et al., 1999). GXMs of SB4-C are composed of M2 and M3 SRGs whereas SB4-SM exhibits only M2. The addition of Xylp at the 4-0 position in M3 most likely required activation of different enzymes, which are traditionally thought to be used only by C. gattii. In RC-2, PS alters biophysical properties of GXM (McFadden et al., 2007). Although not proven this may result from changes in the spacing of charged GlcpA along the Mannose backbone, which are not apparent by NMR.
Phenotypic switching to RC-2-MC results in enhanced virulence in mice or rats. Similar effects are seen for 24067A and SB4. For C. gattii strain NP1, NP1-MC was more virulent in the pulmonary model; however, only NP1-SM could elicit CNS infection. PS to NP1-SM promoted crossing of the blood brain barrier. Infection with RC-2-MC but not RC-2 SM caused elevated intracranial pressure in rats (Fries et al., 2005a). RC-2-MC was selected in vivo in the setting of antifungal drug therapy and antibody (Ab) administration (Fries et al., 2005b). Therefore it is conceivable that PS may contribute to treatment failures.
The molecular mechanisms mediating PS in C. neoformans are not understood. PS was associated with down-regulation of genes in RC2-MC relative to RC2-SM among them ALL1 whose function is unclear but associated with capsule formation. Loss of ALL1 function mimics the hypervirulence of the MC variant including the over-stimulated host response (Jain et al., 2009).
Antigenic variation
Antigenic variation can be achieved by varying expression of surface proteins that are controlled by subtelomeric silencing. In addition intragenic tandem repeats can play an important role because they can be varied and affect the expression and possibly the function of important surface molecules.
Pneumocystis spp
Pneumocystis is a genus containing many species, which infect different mammalian hosts. The human pathogen Pneumocystis jiroveci causes a severe pneumonia (PCP) in immunocompromised patients (Stringer et al., 2002). Pneumocystis carinii infects rats and is the model species used to study AV because Pneumocystis spp. do not grow in vitro. Expression cloning from infected rat tissue identified a gene family called ‘Major Surface Glycoprotein’ (MSG) encoding a surface antigen that is also called gpA. This gene family has been identified in several Pneumocystis spp. (Haidaris et al., 1992; Kovacs et al., 1993; Wada et al., 1993; Garbe and Stringer, 1994; Kitada et al., 1994; Linke et al., 1994; Wright et al., 1995). Less is known about MSR1 and PRT1, two other families not present in all species. In P. carinii 85 distinct MSG genes are organized in clusters (Sunkin et al., 1994). They vary in structure, and are composed of one or more PRT–MSR–MSG clusters. The number of MSG genes between the last MSR gene and the subtelomere varies from 1 to 3 (Keely et al., 2005). The current model proposes the existence of only one fixed expression site that includes an Upstream Conserved Sequence (UCS) that lies upstream of a constitutively active transcriptional promoter. Gene conversion appears to be the primary mechanism of MSG sequence change. Multiple PRT genes and MSR genes are expressed in populations of P. carinii dominated by a single MSG gene at the expression site (Keely et al., 2003; Ambrose et al., 2004). The MSR gene family may also contribute to surface variation. Three different types of MSR genes (Keely et al., 2005) that differ in size are described. One MSR type contains a long tract of G:C base-pairs in the middle. Variation of that G:C tract can occur by several mechanisms and cause frameshifts during translation that produce altered Msr proteins similar to other microorganisms.
The current hypothesis is that AV allows Pneumocystis to persist long enough in a healthy host to assure transmission to the next host. Consistent with these data, drug resistance alleles are found in patients never treated with sulfonamides (Iliades et al., 2004; Meneau et al., 2004). Patients with multiple-independent episodes of PCP are infected by distinct genetic strains (Keely et al., 1996). Hence, AV may be a survival strategy in this host-dependent fungus to avoid eradication by the host.
C. glabrata
Epa1 mediates adherence of C. glabrata to mammalian epithelial cells. EPA1 is a GPI-anchored cell wall protein. In C. glabrata strain BG2 23 paralogues of EPA1 are characterized, all encoding proteins highly related to Epa1. Most of these EPA genes are located in subtelomeric position, where they are transcriptionally silenced (Castano et al., 2005). Transcription of some subtelomeric EPA genes can be de-repressed by limitation of NAD+ precursors (Domergue et al., 2005). Null mutations in SIR3, SIR4 and RIF1 and deletion of the C-terminal 28 amino acids of Rap1 lead to expression of many EPA genes, resulting in a hyper-adherent phenotype when cells are grown under standard laboratory conditions (De Las Penas et al., 2003; Castano et al., 2005; Iraqui et al., 2005). While SIR proteins are required for silencing of EPA genes, the Rif1 and the Ku proteins regulate silencing only in a subset of telomeres (Rosas-Hernandez et al., 2008).
C. posadasii and C. immitis
Coccidioides spp. can cause pneumonia, meningitis and disseminated disease in immuno-suppressed and -competent individuals. SOWgp is an immuno-dominant antigen of the spherule outer wall, which elicits both antibody- and cell-mediated responses in patients. This glycoprotein consists of a signal peptide and propeptide, with a tandem repeat motif, and a GPI anchor signal consensus sequence. The repeat domain contains three to six copies of proline- and aspartic acid-rich sequences (strain-dependent), which is recognized by Abs of infected individuals. A metalloproteinase (Mep1) produced during the endosporulation event digests SOWgp. Thus, Mep1 contributes to Coccidioides virulence by ensuring endospores are devoid of SOWgp, thereby allowing them to evade host detection. It is conceivable that this mechanism could contribute to AV in vivo (Hung et al., 2000; 2002; 2005; Johannesson et al., 2005).
C. neoformans
Antibody staining with capsule specific Abs has demonstrated that C. neoformans cells manifest AV in the polysaccharide capsule during murine infection (Garcia-Hermoso et al., 2004; Charlier et al., 2005). This variation is generated by infinite combination of polysaccharide triads. Evidence that selection occurs during cloning passage exists (McFadden et al., 2007).
Summary and future perspective
Understanding the dynamic qualities of genomes that allow pathogens to survive, adapt and escape immune responses is vital to our knowledge about host–pathogen interactions. Future studies should focus on studying phenotypic variation in vivo with a combination of proteomic and genomic approaches. Determining variation resulting from post-translation modifications of proteins remains a challenge. Understanding the relevance and the selection of phenotypic changes in vivo will be of considerable importance for vaccine development.
Acknowledgments
This work was support by Grant AI059681-05 to B.C.F., by pilot funds from a CFAR grant (AI 051519).
References
- Ambrose HE, Keely SP, Aliouat EM, Dei-Cas E, Wakefield AE, Miller RF, Stringer JR. Expression and complexity of the PRT1 multigene family of Pneumocystis carinii. Microbiology. 2004;150:293–300. doi: 10.1099/mic.0.26539-0. [DOI] [PubMed] [Google Scholar]
- Anderson J, Mihalik R, Soll DR. Ultrastructure and antigenicity of the unique cell wall pimple of the Candida opaque phenotype. J Bacteriol. 1990;172:224–235. doi: 10.1128/jb.172.1.224-235.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JM, Soll DR. Unique phenotype of opaque cells in the white-opaque transition of Candida albicans. J Bacteriol. 1987;169:5579–5588. doi: 10.1128/jb.169.12.5579-5588.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DA, Feldmann PJ, Bovey M, Gow NA, Brown AJ. The Candida albicans HYR1 gene, which is activated in response to hyphal development, belongs to a gene family encoding yeast cell wall proteins. J Bacteriol. 1996;178:5353–5360. doi: 10.1128/jb.178.18.5353-5360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balan I, Alarco AM, Raymond M. The Candida albicans CDR3 gene codes for an opaque-phase ABC transporter. J Bacteriol. 1997;179:7210–7218. doi: 10.1128/jb.179.23.7210-7218.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birse CE, Irwin MY, Fonzi WA, Sypherd PS. Cloning and characterization of ECE1, a gene expressed in association with cell elongation of the dimorphic pathogen Candida albicans. Infect Immun. 1993;61:3648–3655. doi: 10.1128/iai.61.9.3648-3655.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship JR, Mitchell AP. How to build a biofilm: a fungal perspective. Curr Opin Microbiol. 2006;9:588–594. doi: 10.1016/j.mib.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Castano I, Pan SJ, Zupancic M, Hennequin C, Dujon B, Cormack BP. Telomere length control and transcriptional regulation of subtelomeric adhesins in Candida glabrata. Mol Microbiol. 2005;55:1246–1258. doi: 10.1111/j.1365-2958.2004.04465.x. [DOI] [PubMed] [Google Scholar]
- Charlier C, Chretien F, Baudrimont M, Mordelet E, Lortholary O, Dromer F. Capsule structure changes associated with Cryptococcus neoformans crossing of the blood–brain barrier. Am J Pathol. 2005;166:421–432. doi: 10.1016/S0002-9440(10)62265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer RA, Jr, Perfect BZ, Pinchai N, Park S, Perlin DS, Asfaw YG, et al. Calcineurin target CrzA regulates conidial germination, hyphal growth, and pathogenesis of Aspergillus fumigatus. Eukaryot Cell. 2008;7:1085–1097. doi: 10.1128/EC.00086-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Las Penas A, Pan SJ, Castano I, Alder J, Cregg R, Cormack BP. Virulence-related surface glycoproteins in the yeast pathogen Candida glabrata are encoded in subtelomeric clusters and subject to RAP1- and SIR-dependent transcriptional silencing. Genes Dev. 2003;17:2245–2258. doi: 10.1101/gad.1121003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domergue R, Castano I, De Las Penas A, Zupancic M, Lockatell V, Hebel JR, et al. Nicotinic acid limitation regulates silencing of Candida adhesins during UTI. Science. 2005;308:866–870. doi: 10.1126/science.1108640. [DOI] [PubMed] [Google Scholar]
- Fortwendel JR, Zhao W, Bhabhra R, Park S, Perlin DS, Askew DS, Rhodes JC. A fungus-specific ras homolog contributes to the hyphal growth and virulence of Aspergillus fumigatus. Eukaryot Cell. 2005;4:1982–1989. doi: 10.1128/EC.4.12.1982-1989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries BC, Goldman DL, Cherniak R, Ju R, Casadevall A. Phenotypic switching in Cryptococcus neoformans results in changes in cellular morphology and glucuronoxylomannan structure. Infect Immun. 1999;67:6076–6083. doi: 10.1128/iai.67.11.6076-6083.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries BC, Taborda CP, Serfass E, Casadevall A. Phenotypic switching of Cryptococcus neoformans occurs in vivo and influences the outcome of infection. J Clin Invest. 2001;108:1639–1648. doi: 10.1172/JCI13407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries BC, Lee SC, Kennan R, Zhao W, Casadevall A, Goldman DL. Phenotypic switching of Cryptococcus neoformans can produce variants that elicit increased intracranial pressure in a rat model of cryptococcal meningoencephalitis. Infect Immun. 2005a;73:1779–1787. doi: 10.1128/IAI.73.3.1779-1787.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries BC, Cook E, Wang X, Casadevall A. Effects of antifungal interventions on the outcome of experimental infections with phenotypic switch variants of Cryptococcus neoformans. Antimicrob Agents Chemother. 2005b;49:350–357. doi: 10.1128/AAC.49.1.350-357.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbe TR, Stringer JR. Molecular characterization of clustered variants of genes encoding major surface antigens of human Pneumocystis carinii. Infect Immun. 1994;62:3092–3101. doi: 10.1128/iai.62.8.3092-3101.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Hermoso D, Dromer F, Janbon G. Cryptococcus neoformans capsule structure evolution in vitro and during murine infection. Infect Immun. 2004;72:3359–3365. doi: 10.1128/IAI.72.6.3359-3365.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman DL, Fries BC, Franzot SP, Montella L, Casadevall A. Phenotypic switching in the human pathogenic fungus Cryptococcus neoformans is associated with changes in virulence and pulmonary inflammatory response in rodents. Proc Natl Acad Sci USA. 1998;95:14967–14972. doi: 10.1073/pnas.95.25.14967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidaris PJ, Wright TW, Gigliotti F, Haidaris CG. Expression and characterization of a cDNA clone encoding an immunodominant surface glycoprotein of Pneumocystis carinii. J Infect Dis. 1992;166:1113–1123. doi: 10.1093/infdis/166.5.1113. [DOI] [PubMed] [Google Scholar]
- Hoyer LL, Payne TL, Bell M, Myers AM, Scherer S. Candida albicans ALS3 and insights into the nature of the ALS gene family. Curr Genet. 1998;33:451–459. doi: 10.1007/s002940050359. [DOI] [PubMed] [Google Scholar]
- Huang G, Wang H, Chou S, Nie X, Chen J, Liu H. Bistable expression of WOR1, a master regulator of white-opaque switching in Candida albicans. Proc Natl Acad Sci USA. 2006;103:12813–12818. doi: 10.1073/pnas.0605270103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Srikantha T, Sahni N, Yi S, Soll DR. CO(2) regulates white-to-opaque switching in Candida albicans. Curr Biol. 2009;19:330–334. doi: 10.1016/j.cub.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hube B, Monod M, Schofield DA, Brown AJ, Gow NA. Expression of seven members of the gene family encoding secretory aspartyl proteinases in Candida albicans. Mol Microbiol. 1994;14:87–99. doi: 10.1111/j.1365-2958.1994.tb01269.x. [DOI] [PubMed] [Google Scholar]
- Hung CY, Ampel NM, Christian L, Seshan KR, Cole GT. A major cell surface antigen of Coccidioides immitis which elicits both humoral and cellular immune responses. Infect Immun. 2000;68:584–593. doi: 10.1128/iai.68.2.584-593.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung CY, Yu JJ, Seshan KR, Reichard U, Cole GT. A parasitic phase-specific adhesin of Coccidioides immitis contributes to the virulence of this respiratory fungal pathogen. Infect Immun. 2002;70:3443–3456. doi: 10.1128/IAI.70.7.3443-3456.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung CY, Seshan KR, Yu JJ, Schaller R, Xue J, Basrur V, et al. A metalloproteinase of Coccidioides posadasii contributes to evasion of host detection. Infect Immun. 2005;73:6689–6703. doi: 10.1128/IAI.73.10.6689-6703.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliades P, Meshnick SR, Macreadie IG. Dihydropteroate synthase mutations in Pneumocystis jiroveci can affect sulfamethoxazole resistance in a Saccharomyces cerevisiae model. Antimicrob Agents Chemother. 2004;48:2617–2623. doi: 10.1128/AAC.48.7.2617-2623.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iraqui I, Garcia-Sanchez S, Aubert S, Dromer F, Ghigo JM, d’Enfert C, Janbon G. The Yak1p kinase controls expression of adhesins and biofilm formation in Candida glabrata in a Sir4p-dependent pathway. Mol Microbiol. 2005;55:1259–1271. doi: 10.1111/j.1365-2958.2004.04475.x. [DOI] [PubMed] [Google Scholar]
- Jain N, Li L, McFadden DC, Banarjee U, Wang X, Cook E, Fries BC. Phenotypic switching in a Cryptococcus neoformans variety gattii strain is associated with changes in virulence and promotes dissemination to the central nervous system. Infect Immun. 2006;74:896–903. doi: 10.1128/IAI.74.2.896-903.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain N, Li L, Hsueh YP, Guerrero A, Heitman J, Goldman DL, Fries BC. Loss of allergen 1 confers a hypervirulent phenotype that resembles mucoid switch variants of Cryptococcus neoformans. Infect Immun. 2009;77:128–140. doi: 10.1128/IAI.01079-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannesson H, Townsend JP, Hung CY, Cole GT, Taylor JW. Concerted evolution in the repeats of an immunomodulating cell surface protein, SOWgp, of the human pathogenic fungi Coccidioides immitis and C. posadasii. Genetics. 2005;171:109–117. doi: 10.1534/genetics.105.040923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keely SP, Baughman RP, Smulian AG, Dohn MN, Stringer JR. Source of Pneumocystis carinii in recurrent episodes of pneumonia in AIDS patients. Aids. 1996;10:881–888. doi: 10.1097/00002030-199607000-00011. [DOI] [PubMed] [Google Scholar]
- Keely SP, Cushion MT, Stringer JR. Diversity at the locus associated with transcription of a variable surface antigen of Pneumocystis carinii as an index of population structure and dynamics in infected rats. Infect Immun. 2003;71:47–60. doi: 10.1128/IAI.71.1.47-60.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keely SP, Renauld H, Wakefield AE, Cushion MT, Smulian AG, Fosker N, et al. Gene arrays at Pneumocystis carinii telomeres. Genetics. 2005;170:1589–1600. doi: 10.1534/genetics.105.040733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MJ, Rogers AL, Hanselmen LR, Soll DR, Yancey RJ., Jr Variation in adhesion and cell surface hydrophobicity in Candida albicans white and opaque phenotypes. Mycopathologia. 1988;102:149–156. doi: 10.1007/BF00437397. [DOI] [PubMed] [Google Scholar]
- Kitada K, Wada M, Nakamura Y. Multi-gene family of major surface glycoproteins of Pneumocystis carinii: full-size cDNA cloning and expression. DNA Res. 1994;1:57–66. doi: 10.1093/dnares/1.2.57. [DOI] [PubMed] [Google Scholar]
- Kolotila MP, Diamond RD. Effects of neutrophils and in vitro oxidants on survival and phenotypic switching of Candida albicans WO-1. Infect Immun. 1990;58:1174–1179. doi: 10.1128/iai.58.5.1174-1179.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs JA, Powell F, Edman JC, Lundgren B, Martinez A, Drew B, Angus CW. Multiple genes encode the major surface glycoprotein of Pneumocystis carinii. J Biol Chem. 1993;268:6034–6040. [PubMed] [Google Scholar]
- Kugler S, Schurtz Sebghati T, Groppe Eissenberg L, Goldman WE. Phenotypic variation and intracellular parasitism by Histoplasma capsulatum. Proc Natl Acad Sci USA. 2000;97:8794–8798. doi: 10.1073/pnas.97.16.8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvaal C, Lachke SA, Srikantha T, Daniels K, McCoy J, Soll DR. Misexpression of the opaque-phase-specific gene PEP1 (SAP1) in the white phase of Candida albicans confers increased virulence in a mouse model of cutaneous infection. Infect Immun. 1999;67:6652–6662. doi: 10.1128/iai.67.12.6652-6662.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachke SA, Joly S, Daniels K, Soll DR. Phenotypic switching and filamentation in Candida glabrata. Microbiology. 2002;148:2661–2674. doi: 10.1099/00221287-148-9-2661. [DOI] [PubMed] [Google Scholar]
- Lachke SA, Lockhart SR, Daniels KJ, Soll DR. Skin facilitates Candida albicans mating. Infect Immun. 2003;71:4970–4976. doi: 10.1128/IAI.71.9.4970-4976.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan CY, Newport G, Murillo LA, Jones T, Scherer S, Davis RW, Agabian N. Metabolic specialization associated with phenotypic switching in Candidaalbicans. Proc Natl Acad Sci USA. 2002;99:14907–14912. doi: 10.1073/pnas.232566499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand M, Lephart P, Forche A, Mueller FM, Walsh T, Magee PT, Magee BB. Homozygosity at the MTL locus in clinical strains of Candida albicans: karyotypic rearrangements and tetraploid formation. Mol Microbiol. 2004;52:1451–1462. doi: 10.1111/j.1365-2958.2004.04068.x. [DOI] [PubMed] [Google Scholar]
- Linke MJ, Smulian AG, Stringer JR, Walzer PD. Characterization of multiple unique cDNAs encoding the major surface glycoprotein of rat-derived Pneumocystis carinii. Parasitol Res. 1994;80:478–486. doi: 10.1007/BF00932694. [DOI] [PubMed] [Google Scholar]
- Lockhart SR, Nguyen M, Srikantha T, Soll DR. A MADS box protein consensus binding site is necessary and sufficient for activation of the opaque-phase-specific gene OP4 of Candida albicans. J Bacteriol. 1998;180:6607–6616. doi: 10.1128/jb.180.24.6607-6616.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart SR, Pujol C, Daniels KJ, Miller MG, Johnson AD, Pfaller MA, Soll DR. In Candida albicans, white-opaque switchers are homozygous for mating type. Genetics. 2002;162:737–745. doi: 10.1093/genetics/162.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse MB, Johnson AD. Differential phagocytosis of white versus opaque Candida albicans by drosophila and mouse phagocytes. PLoS ONE. 2008;3:e1473. doi: 10.1371/journal.pone.0001473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden DC, Fries BC, Wang F, Casadevall A. Capsule structural heterogeneity and antigenic variation in Cryptococcus neoformans. Eukaryot Cell. 2007;6:1464–1473. doi: 10.1128/EC.00162-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martchenko M, Alarco AM, Harcus D, Whiteway M. Superoxide dismutases in Candida albicans: transcriptional regulation and functional characterization of the hyphal-induced SOD5 gene. Mol Biol Cell. 2004;15:456–467. doi: 10.1091/mbc.E03-03-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LR, Casadevall A. Specific antibody can prevent fungal biofilm formation and this effect correlates with protective efficacy. Infect Immun. 2005;73:6350–6362. doi: 10.1128/IAI.73.10.6350-6362.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneau I, Sanglard D, Bille J, Hauser PM. Pneumocystis jiroveci dihydropteroate synthase polymorphisms confer resistance to sulfadoxine and sulfanilamide in Saccharomyces cerevisiae. Antimicrob Agents Chemother. 2004;48:2610–2616. doi: 10.1128/AAC.48.7.2610-2616.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz WG. Candida lusitaniae: frequency of recovery, colonization, infection, and amphotericin B resistance. J Clin Microbiol. 1984;20:1194–1195. doi: 10.1128/jcm.20.6.1194-1195.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MG, Johnson AD. White-opaque switching in Candida albicans is controlled by mating-type locus homeodomain proteins and allows efficient mating. Cell. 2002;110:293–302. doi: 10.1016/s0092-8674(02)00837-1. [DOI] [PubMed] [Google Scholar]
- Morrow B, Srikantha T, Soll DR. Transcription of the gene for a pepsinogen, PEP1, is regulated by white-opaque switching in Candida albicans. Mol Cell Biol. 1992;12:2997–3005. doi: 10.1128/mcb.12.7.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat E, Williams C, Jones B, McChlery S, Ramage G. The characteristics of Aspergillus fumigatus mycetoma development: is this a biofilm? Med Mycol. 2009;47(Suppl 1):S120–S126. doi: 10.1080/13693780802238834. [DOI] [PubMed] [Google Scholar]
- Nobile CJ, Andes DR, Nett JE, Smith FJ, Yue F, Phan QT, et al. Critical role of Bcr1-dependent adhesins in C. albicans biofilm formation in vitro and in vivo. PLoS Pathog. 2006;2:e63. doi: 10.1371/journal.ppat.0020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappleye CA, Goldman WE. Defining virulence genes in the dimorphic fungi. Annu Rev Microbiol. 2006;60:281–303. doi: 10.1146/annurev.micro.59.030804.121055. [DOI] [PubMed] [Google Scholar]
- Rikkerink EH, Magee BB, Magee PT. Opaque-white phenotype transition: a programmed morphological transition in Candida albicans. J Bacteriol. 1988;170:895–899. doi: 10.1128/jb.170.2.895-899.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas-Hernandez LL, Juarez-Reyes A, Arroyo-Helguera OE, De Las Penas A, Pan SJ, Cormack BP, Castano I. yKu70/yKu80 and Rif1 regulate silencing differentially at telomeres in Candida glabrata. Eukaryot Cell. 2008;7:2168–2178. doi: 10.1128/EC.00228-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanglard D, Hube B, Monod M, Odds FC, Gow NA. A triple deletion of the secreted aspartyl proteinase genes SAP4, SAP5, and SAP6 of Candida albicans causes attenuated virulence. Infect Immun. 1997;65:3539–3546. doi: 10.1128/iai.65.9.3539-3546.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer A, Rupp S, Taylor BN, Rollinghoff M, Schroppel K. The TEA/ATTS transcription factor CaTec1p regulates hyphal development and virulence in Candida albicans. Mol Microbiol. 2000;38:435–445. doi: 10.1046/j.1365-2958.2000.02132.x. [DOI] [PubMed] [Google Scholar]
- Slutsky B, Buffo J, Soll DR. High-frequency switching of colony morphology in Candida albicans. Science. 1985;230:666–669. doi: 10.1126/science.3901258. [DOI] [PubMed] [Google Scholar]
- Slutsky B, Staebell M, Anderson J, Risen L, Pfaller M, Soll DR. ‘White-opaque transition’: a second high-frequency switching system in Candida albicans. J Bacteriol. 1987;169:189–197. doi: 10.1128/jb.169.1.189-197.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll DR. High-frequency switching in Candida albicans. Clin Microbiol Rev. 1992;5:183–203. doi: 10.1128/cmr.5.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonneborn A, Tebarth B, Ernst JF. Control of white-opaque phenotypic switching in Candida albicans by the Efg1p morphogenetic regulator. Infect Immun. 1999;67:4655–4660. doi: 10.1128/iai.67.9.4655-4660.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikantha T, Chandrasekhar A, Soll DR. Functional analysis of the promoter of the phase-specific WH11 gene of Candida albicans. Mol Cell Biol. 1995;15:1797–1805. doi: 10.1128/mcb.15.3.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikantha T, Zhao R, Daniels K, Radke J, Soll DR. Phenotypic switching in Candida glabrata accompanied by changes in expression of genes with deduced functions in copper detoxification and stress. Eukaryot Cell. 2005;4:1434–1445. doi: 10.1128/EC.4.8.1434-1445.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikantha T, Daniels KJ, Wu W, Lockhart SR, Yi S, Sahni N, et al. Dark brown is the more virulent of the switch phenotypes of Candida glabrata. Microbiology. 2008;154:3309–3318. doi: 10.1099/mic.0.2008/020578-0. [DOI] [PubMed] [Google Scholar]
- Staab JF, Sundstrom P. Genetic organization and sequence analysis of the hypha-specific cell wall protein gene HWP1 of Candida albicans. Yeast. 1998;14:681–686. doi: 10.1002/(SICI)1097-0061(199805)14:7<681::AID-YEA256>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Stringer JR, Beard CB, Miller RF, Wakefield AE. A new name (Pneumocystis jiroveci) for Pneumocystis from humans. Emerg Infect Dis. 2002;8:891–896. doi: 10.3201/eid0809.020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkin SM, Stringer SL, Stringer JR. A tandem repeat of rat-derived Pneumocystis carinii genes encoding the major surface glycoprotein. J Eukaryot Microbiol. 1994;41:292–300. doi: 10.1111/j.1550-7408.1994.tb01509.x. [DOI] [PubMed] [Google Scholar]
- Vargas K, Messer SA, Pfaller M, Lockhart SR, Stapleton JT, Hellstein J, Soll DR. Elevated phenotypic switching and drug resistance of Candida albicans from human immunodeficiency virus-positive individuals prior to first thrush episode. J Clin Microbiol. 2000;38:3595–3607. doi: 10.1128/jcm.38.10.3595-3607.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada M, Kitada K, Saito M, Egawa K, Nakamura Y. cDNA sequence diversity and genomic clusters of major surface glycoprotein genes of Pneumocystis carinii. J Infect Dis. 1993;168:979–985. doi: 10.1093/infdis/168.4.979. [DOI] [PubMed] [Google Scholar]
- Whiteway M, Bachewich C. Morphogenesis in Candida albicans. Annu Rev Microbiol. 2007;61:529–553. doi: 10.1146/annurev.micro.61.080706.093341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright TW, Gigliotti F, Haidaris CG, Simpson-Haidaris PJ. Cloning and characterization of a conserved region of human and rhesus macaque Pneumocystis carinii gpA. Gene. 1995;167:185–189. doi: 10.1016/0378-1119(95)00704-0. [DOI] [PubMed] [Google Scholar]
- Young LY, Hull CM, Heitman J. Disruption of ergosterol biosynthesis confers resistance to amphotericin B in Candida lusitaniae. Antimicrob Agents Chemother. 2003;47:2717–2724. doi: 10.1128/AAC.47.9.2717-2724.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaragoza O, Rodrigues ML, De Jesus M, Frases S, Dadachova E, Casadevall A. The capsule of the fungal pathogen Cryptococcus neoformans. Adv Appl Microbiol. 2009;68:133–216. doi: 10.1016/S0065-2164(09)01204-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Panepinto JC, Fortwendel JR, Fox L, Oliver BG, Askew DS, Rhodes JC. Deletion of the regulatory subunit of protein kinase A in Aspergillus fumigatus alters morphology, sensitivity to oxidative damage, and virulence. Infect Immun. 2006;74:4865–4874. doi: 10.1128/IAI.00565-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zordan RE, Galgoczy DJ, Johnson AD. Epigenetic properties of white-opaque switching in Candida albicans are based on a self-sustaining transcriptional feedback loop. Proc Natl Acad Sci USA. 2006;103:12807–12812. doi: 10.1073/pnas.0605138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zordan RE, Miller MG, Galgoczy DJ, Tuch BB, Johnson AD. Interlocking transcriptional feedback loops control white-opaque switching in Candida albicans. PLoS Biol. 2007;5:e256. doi: 10.1371/journal.pbio.0050256. [DOI] [PMC free article] [PubMed] [Google Scholar]


