Abstract
BACKGROUND
Data from studies in nonhuman primates suggest that the triple monoclonal antibody cocktail ZMapp is a promising immune-based treatment for Ebola virus disease (EVD).
METHODS
Beginning in March 2015, we conducted a randomized, controlled trial of ZMapp plus the current standard of care as compared with the current standard of care alone in patients with EVD that was diagnosed in West Africa by polymerase-chain-reaction (PCR) assay. Eligible patients of any age were randomly assigned in a 1:1 ratio to receive either the current standard of care or the current standard of care plus three intravenous infusions of ZMapp (50 mg per kilogram of body weight, administered every third day). Patients were stratified according to baseline PCR cycle-threshold value for the virus (≤22 vs. >22) and country of enrollment. Oral favipiravir was part of the current standard of care in Guinea. The primary end point was mortality at 28 days.
RESULTS
A total of 72 patients were enrolled at sites in Liberia, Sierra Leone, Guinea, and the United States. Of the 71 patients who could be evaluated, 21 died, representing an overall case fatality rate of 30%. Death occurred in 13 of 35 patients (37%) who received the current standard of care alone and in 8 of 36 patients (22%) who received the current standard of care plus ZMapp. The observed posterior probability that ZMapp plus the current standard of care was superior to the current standard of care alone was 91.2%, falling short of the prespecified threshold of 97.5%. Frequentist analyses yielded similar results (absolute difference in mortality with ZMapp, −15 percentage points; 95% confidence interval, −36 to 7). Baseline viral load was strongly predictive of both mortality and duration of hospitalization in all age groups.
CONCLUSIONS
In this randomized, controlled trial of a putative therapeutic agent for EVD, although the estimated effect of ZMapp appeared to be beneficial, the result did not meet the prespecified statistical threshold for efficacy. (Funded by the National Institute of Allergy and Infectious Diseases and others; PREVAIL II ClinicalTrials.gov number, NCT02363322.)
The 2014–2016 Ebola outbreak in West Africa was unprecedented in sheer scope, duration, and number of human casualties.1,2 The outbreak resulted in more than 28,000 suspected or confirmed cases of Ebola virus disease (EVD) and more than 11,000 deaths.3 Fragile health care infrastructures that were often already severely compromised by past years of civil strife played a substantial role in the propagation of the outbreak. Although the final postmortem analysis of the global response has yet to be written, there can be little doubt that the lack of therapeutic agents and vaccines with proven efficacy against EVD further contributed to the ultimate magnitude of the epidemic.
From the outset, the most appropriate means of testing the small number of drugs that showed favorable preclinical activity against EVD for safety and possible efficacy has been debated.4,5 Varied opinions on prioritization of agents and the most effective means of testing them in the midst of a public health crisis persist to this day.6,7 The Partnership for Research on Ebola Virus in Liberia II (PREVAIL II) study was a randomized, controlled trial of one of the most promising of the available treatments, ZMapp, in patients who had received a diagnosis of acute Zaire ebolavirus (EBOV) infection.8 ZMapp is a mixture of three monoclonal antibodies directed against the surface glycoprotein of EBOV.
METHODS
TRIAL OVERSIGHT
The PREVAIL II trial was designed by the National Institute of Allergy and Infectious Diseases (NIAID) and implemented in partnership with INSERM, the Republic of Sierra Leone Armed Forces, and the Ministries of Health in Liberia, Sierra Leone, and Guinea. The members of the writing group were responsible for the collection and analysis of the data and for the preparation of the manuscript. Patients were enrolled from March 2015 through November 2015. Patients of any age who had positive test results for EBOV infection on a polymerase-chain-reaction (PCR) assay and from whom written informed consent could be obtained were eligible. Ebola treatment units capable of providing the current standard of care were selected as trial sites by regional partners. Minimum requirements for the current standard of care included hemodynamic monitoring, the provision of intravenous fluids, laboratory testing, and the ability to deliver concomitant medications. The trial was approved by the NIAID institutional review board and applicable ethics boards in each site or country. Full details of trial design, conduct, and analyses can be found in the protocol and statistical analysis plan, available with the full text of this article at NEJM.org.
TRIAL DESIGN
ZMapp (Mapp Biopharmaceutical) was selected for an adaptive randomized, controlled trial design.9–11 A feature of this adaptive design was that an investigational agent that was subsequently shown to have activity against EVD could then be incorporated into an evolving standard of care, which was provided as the backbone of therapy in each trial group and against which newer agents could be tested. The primary objective was to determine whether the combination of ZMapp plus the current standard of care was both safe and superior to the current standard of care alone in managing EVD. Before launching the trial in Guinea, the Ministry of Health determined that the results of the JIKI trial were sufficiently compelling to warrant adding oral favipiravir therapy to the definition of the current standard of care for patients enrolled in that country.12
Randomization was stratified according to baseline PCR cycle-threshold value (≤22 cycles vs. >22 cycles) and location (Liberia and Sierra Leone vs. Guinea vs. the United States), resulting in six strata. ZMapp treatment was generally begun within 12 to 24 hours after randomization and consisted of three intravenous infusions of ZMapp (50 mg per kilogram of body weight), administered every third day.
Baseline symptoms were assessed as close to the time of randomization as possible, although with varying practices at Ebola treatment units, initial interventional measures (e.g., intravenous fluids and antipyretic agents) may in some cases have been instituted overnight before these assessments. Clinical status was recorded daily up to day 28, and vital status was reaffirmed on day 58 (data on serious adverse events were captured on both days).
An independent data and safety monitoring board convened seven times during the trial. In late 2015, the board endorsed a plan for stopping the trial if the already low incidence of EVD did not increase. On January 29, 2016, after Liberia, Sierra Leone, and Guinea had been declared nearly Ebola-free, the trial was closed and the data unblinded.
STATISTICAL ANALYSIS
The primary end point was mortality at day 28. We calculated that 100 patients per group would need to be enrolled for the trial to have 88% power to detect a 50% relative difference in mortality between the two groups, assuming that the 28-day mortality in the group receiving the current standard of care alone was 40%.
Interim and final analyses were performed with the use of a Bayesian approach, as described previously.10,11 In brief, a skeptical “prior” distribution was formulated for the treatment effect: smaller effects were more likely than larger ones, and results were equally likely to favor either group. This prior opinion was then revised on the basis of trial data, leading to a “posterior” probability distribution for treatment effect for computation of the probability that ZMapp plus the current standard of care results in lower 28-day mortality than the current standard of care alone. A probability of 97.5% or more (akin to a one-sided type I error rate of 2.5%) was required to establish efficacy. Key analyses were summarized with both posterior probabilities and 95% credible intervals. Frequentist (conventional non-Bayesian) analyses using Fisher’s exact test and Barnard’s test and the calculation of confidence intervals were also performed.
Kaplan–Meier survival curves were used to compare the two treatment groups. A stratified rank test was used to compare the two groups with respect to the time to viral clearance, which was defined as the time to a first negative PCR assay. For this analysis, patients who died within 28 days were given a worse rank than survivors, with earlier deaths given worse ranks than later deaths. To address concerns about whether some patients were too sick to receive a treatment benefit, we conducted a prespecified principal stratification analysis using logistic regression on baseline variables to develop a risk score for death and then to stratify according to low risk (≤50% probability) versus high risk (>50% probability). Statistical analyses were performed with the use of SAS software, version 9.3 (SAS Institute), and R software, version 3.2.3.13
RESULTS
PATIENT CHARACTERISTICS
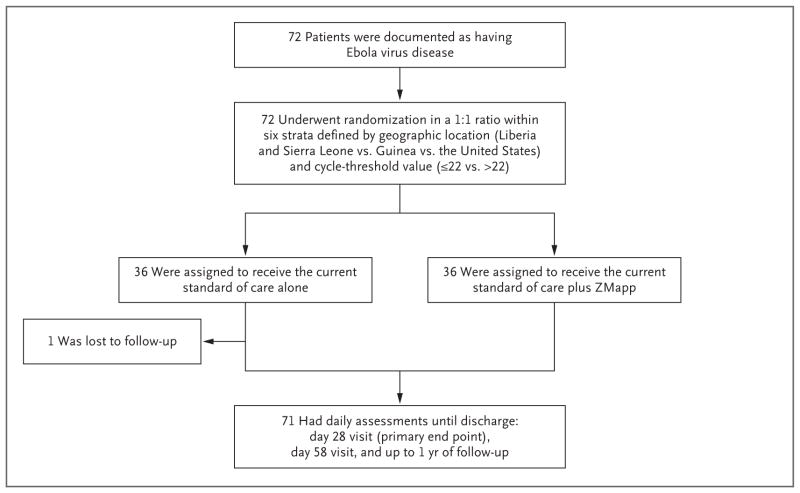
The first patient enrolled was an American health care worker who was medically evacuated from Sierra Leone to the United States in March 2015. The last patient was enrolled in November 2015 in Liberia during a brief resurgence of EVD in that country. A total of 72 patients (36 per group) (Fig. 1) were enrolled at two sites in Liberia, seven sites in Sierra Leone, one site in Guinea, and one site in the United States (Table S1 in the Supplementary Appendix, available at NEJM.org).
Figure 1. Randomization and Follow-up.
One patient in the group assigned to the current standard of care alone was lost to follow-up after day 1, could not be evaluated, and was not included in the primary analysis.
The two treatment groups were generally well balanced, although higher percentages of children and of women were enrolled in the ZMapp group than in the group that received the standard of care alone (children, 42% vs. 22%; and women, 64% vs. 47%) (Table 1). In the cohort as a whole, the mean (±SD) time from the onset of clinical symptoms to randomization was 4.2±2.7 days. The mean PCR cycle-threshold value for EBOV at trial entry was 23.9±5.3 cycles, with 42% of the patients having cycle-threshold values of 22 or less.
Table 1.
Baseline Demographic and Clinical Characteristics of the Trial Population.*
| Characteristic | All Patients (N = 72) | Current Standard of Care Alone (N = 36) | Current Standard of Care plus ZMapp (N = 36) |
|---|---|---|---|
| Age — yr | 26.1±17.4 | 27.9±16.4 | 24.3±18.3 |
| Age <18 yr — no. (%) | 23 (32) | 8 (22) | 15 (42) |
| Female sex — no. (%) | 40 (56) | 17 (47) | 23 (64) |
| Race — no./total no. (%)† | |||
| Black | 60/61 (98) | 31/32 (97) | 29/29 (100) |
| White | 1/61 (2) | 1/32 (3) | 0/29 |
| Enrolled in West Africa — no. (%) | 71 (99) | 35 (97) | 36 (100) |
| Country of birth — no. (%) | |||
| Sierra Leone | 54 (75) | 28 (78) | 26 (72) |
| Guinea | 12 (17) | 5 (14) | 7 (19) |
| Liberia | 5 (7) | 2 (6) | 3 (8) |
| United States | 1 (1) | 1 (3) | 0 |
| Work involving contact with persons with EVD — no. (%) | 5 (7) | 5 (14) | 0 |
| Current illness | |||
| Days since first onset of symptoms | 4.2±2.7 | 4.4±2.9 | 3.9±2.5 |
| Days since first seen by clinician | 1.8±1.6 | 1.9±1.6 | 1.8±1.5 |
| RT-PCR cycle-threshold value | 23.9±5.3 | 23.8±5.4 | 24.1±5.3 |
| RT-PCR cycle-threshold value ≤22 — no. (%) | 30 (42) | 15 (42) | 15 (42) |
| Favipiravir use — no.‡ | 12 | 5 | 7 |
| Body-mass index§ | 18.8±3.6 | 18.9±4.5 | 18.7±3.4 |
| Vital signs | |||
| Blood pressure — mm Hg | |||
| Systolic | 113.5±19.4 | 115.4±19.0 | 111.1±20.0 |
| Diastolic | 71.4±15.7 | 72.6±14.8 | 69.9±16.9 |
| Pulse — beats/min | 92.9±19.9 | 94.4±18.6 | 91.4±21.2 |
| Body temperature — °C | 37.9 ±1.3 | 37.9±1.4 | 37.8±1.3 |
| Respiratory rate — breaths/min | 25.4±6.7 | 25.2±7.0 | 25.6±6.5 |
| Oxygen saturation — % | 95.7±4.8 | 96.1±2.0 | 95.4±6.2 |
| Positive result on pregnancy test — no. | 0 | 0 | 0 |
| Serum chemical values | |||
| Creatinine — mg/dl | 1.3±1.3 | 1.5±1.1 | 1.2±1.5 |
| Potassium — mmol/liter | 4.5±1.8 | 5.0±2.2 | 4.1±1.3 |
Plus–minus values are means ±SD. Between-group differences were not assessed for statistical significance. To convert the values for creatinine to micromoles per liter, multiply by 88.4. To convert the values for potassium to milligrams per deciliter, divide by 0.2558. EVD denotes Ebola virus disease, and RT-PCR reverse-transcriptase polymerase chain reaction.
Race was self-reported.
Favipiravir was prescribed as part of the current standard of care in Guinea.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
At enrollment, patients were asked to report any subjective symptoms of EVD that they had had within the previous 24 hours. The symptoms reported by at least half the participants at trial entry were loss of appetite (72%), weakness (69%), fever (68%), fatigue (59%), and abdominal pain (50%) (Table S2 in the Supplementary Appendix).
SUPPORTIVE CARE AND ZMAPP INFUSIONS
The supportive measures that were provided as the current standard of care during days 1 through 14 of the trial were similar in the two groups (Table S3 in the Supplementary Appendix). Before ZMapp infusions, patients were often given antihistamines (94% in cycle 1) and antipyretic agents (65% in cycle 1) to ameliorate side effects (Table S4 in the Supplementary Appendix).
MORTALITY
Of the 71 patients who could be evaluated, 21 died, representing an overall case fatality rate of 30%. Death occurred in 13 of 35 patients who received the current standard of care alone and in 8 of 36 patients who also received ZMapp, leading to crude estimates of 28-day mortality of 37% and 22%, respectively (Table 2). The Bayesian estimate of the absolute difference in mortality between the ZMapp group and the group that received the standard of care alone was −14 percentage points, and the relative difference was −38%. These mortality differences gave a 91.2% posterior probability that ZMapp plus the current standard of care was superior to the current standard of care alone; this value was below the prespecified probability threshold (≥97.5%) for declaring superiority of the investigational treatment. The 95% credible interval for the absolute difference in mortality was −34 to 6 percentage points, and the 95% credible interval for the relative risk of death was 0.29 to 1.24. Frequentist results gave a mortality difference of −15 percentage points (95% confidence interval [CI], −36 to 7) and a relative risk of 0.60 (95% CI, 0.25 to 1.27). A sensitivity analysis that included the single patient who was lost to follow-up resulted in posterior probabilities of the superiority of ZMapp of 89.8% under the assumption that the patient survived and 93.5% under the assumption that the patient died.
Table 2.
Comparison of 28-Day Mortality According to Treatment Group.*
| Variable | Current Standard of Care Alone | Current Standard of Care plus ZMapp | Bayesian Estimate of Absolute Difference | Bayesian Estimate of Relative Risk | Posterior Probability That ZMapp Was Superior |
|---|---|---|---|---|---|
| percentage points (95% CI) | value (95% CI) | % | |||
| No. of patients alive | 22 | 28 | |||
|
| |||||
| No. of patients who died | 13 | 8 | |||
|
| |||||
| No. of patients lost to follow-up | 1 | 0 | |||
|
| |||||
| 28-Day mortality — % | 37† | 22† | −14 (−34 to 6) | 0.62 (0.29 to 1.24) | 91.2 |
CI denotes credible interval.
These are crude non-Bayesian estimates.
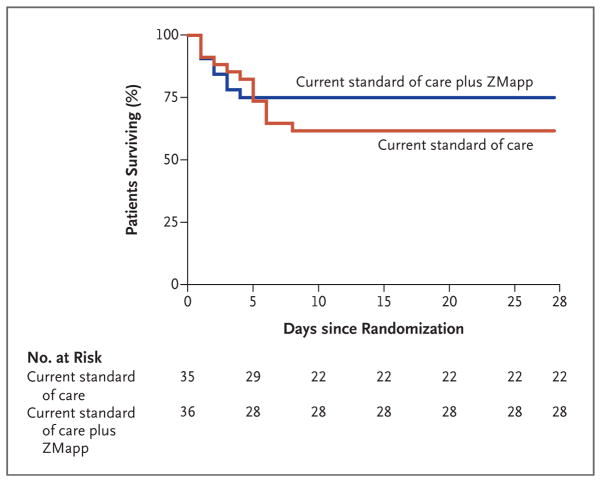
Seven of the eight deaths recorded in ZMapp recipients occurred before day 4 — that is, before the second of three planned infusions of ZMapp (Fig. 2). The exception was one patient who received a second infusion on day 4 and died later that day. In the group that received the current standard of care alone, all 13 deaths occurred during the first 8 days of follow-up (Table S6 in the Supplementary Appendix).
Figure 2. Kaplan–Meier Plot of Survival, According to the Two Assigned Treatment Groups.
There were no deaths in either group after day 8 of the trial.
SECONDARY EFFICACY OUTCOMES
We evaluated the influence of both treatment assignment and baseline cycle-threshold values on the median number of days to viral load clearance and to discharge from the Ebola treatment unit (Fig. S1 in the Supplementary Appendix). Among patients with cycle-threshold values above 22 at trial entry, those who received the current standard of care plus ZMapp had significantly shorter stays in Ebola treatment units than did recipients of the current standard of care alone.
The percentage of patients who had various clinical symptoms of EVD (Fig. S2 in the Supplementary Appendix) and the total number of symptoms reported daily (Fig. S3 in the Supplementary Appendix) during the first 2 weeks were examined, with adjustment for the number of reports collected at each time point. Although there was a suggestion that the symptoms in the ZMapp recipients cleared more readily, these observations were confounded by the differential mortality in the two groups (i.e., patients who died no longer contributed to daily reports) and therefore a potential for bias, even though this effect should have favored the group that received the current standard of care alone.
SAFETY
The percentage of patients with serious adverse events was similar in the two groups: 37% in the group that received the current standard of care alone and 31% in the group that also received ZMapp (P = 0.62). Only one serious adverse event (hypertension) in ZMapp recipients was judged to be related to the infusion itself (Table S5 in the Supplementary Appendix).
Adverse events related to ZMapp infusions were reported most commonly during the first infusion (25%) and decreased with subsequent infusions (11% with infusion 3). Fever (14% with infusion 1) and hypotension (11% with infusion 1) were the most common adverse events reported (Table S4 in the Supplementary Appendix). Mitigation actions consisted primarily of either infusing the drug more slowly during a particular administration or stopping the infusions plus administering antipyretic agents or other medications. A total of 8 of 93 infusions (9%) were stopped owing to adverse events, whereas 9 of 93 infusions (10%) were slowed in order to ameliorate side effects.
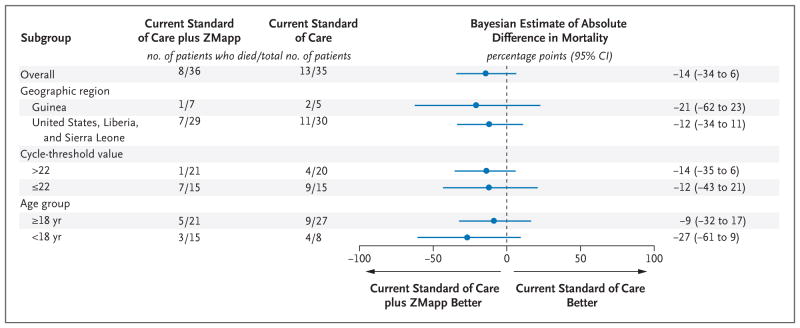
SUBGROUP ANALYSES OF MORTALITY
We performed analyses in subgroups defined according to age, geographic location of enrollment, and baseline cycle-threshold value. Across each of these subgroups, mortality differences favored ZMapp recipients (Fig. 3). Principal stratification analysis showed that among patients with a lower risk of death (≤50% probability), those who received ZMapp had significantly lower mortality than those who received the current standard of care alone (0 of 24 patients vs. 4 of 20 patients), with a Bayesian estimate of an absolute difference in risk of −18 percentage points (95% credible interval, −39 to −2); the posterior probability that ZMapp plus the current standard of care was superior to the current standard of care alone was 98.5%. Frequentist analyses supported this conclusion (see the Statistical Supplement in the Supplementary Appendix).
Figure 3.
Forest Plot of Absolute Difference between Groups in 28-Day Mortality, Overall and According to Subgroup.
DISCUSSION
In this randomized, controlled trial of a new treatment for acute EVD, the posterior probability that ZMapp plus the current standard of care was superior to the current standard of care alone was 91.2%. The overall 28-day crude mortality was 15 percentage points lower among those assigned to ZMapp plus the current standard of care than among those assigned to the current standard of care alone (22% vs. 37%), which corresponds to a 40% lower relative risk of death with ZMapp. If sustained, a mortality difference of 15 percentage points would translate to approximately 15 lives saved for every 100 patients treated. However, this outcome fell short of the prespecified 97.5% probability for superiority.
Although the trial was launched within weeks after the trial drug became available, the notable successes of numerous public health measures in reducing and aiming to extinguish EVD in the affected countries resulted in our not being able to attain the desired enrollment of 100 participants per group. As a consequence of early termination, the 95% credible intervals for the absolute difference in mortality and for the relative risk of death are wide: −34 to 6 percentage points and 0.29 to 1.24, respectively. In addition, although mortality differences according to the age of enrollees (<18 vs. ≥18 years of age), location of enrollment (country), and baseline viral load (cycle-threshold value) all favored the ZMapp group, credible intervals were even wider for these subgroups.
If ZMapp did indeed confer some degree of therapeutic benefit, at least two main factors may have limited the magnitude of the mortality trend that we observed. Although trial patients were clinically symptomatic for only a few days before randomization, at enrollment they were probably 1 week or longer past their date of actual infection. This delay in initiating therapy exceeded the 5-day window within which ZMapp had been shown to provide 90% or greater survival in the nonhuman primate lethal-challenge model.8 In addition, seven of the eight deaths recorded in ZMapp recipients occurred before day 4, which was before administration of the second of three planned infusions. Thus, theoretically, if the full potential efficacy of ZMapp is realized only after the completion of multiple infusions, most of the patients dying from EVD in that group would have died before full dosing was achieved.
Stratification according to country was included because of the concern that access to the types of supportive measures generally available in North America might be limited in West Africa or unevenly distributed among the three involved West African countries. In this regard, certain, but not all, Ebola treatment units (e.g., the Emergency Ebola Treatment Unit in Sierra Leone) were able to provide care at the level of an intensive care unit. Too few patients were enrolled in Guinea to determine whether inclusion of favipiravir further enhanced survival in that country.
Stratification according to baseline cycle-threshold value was instituted because of the perception that patients with very high viral loads at presentation might die despite the use of generally effective medical countermeasures. This was one of the conclusions drawn from the JIKI trial of favipiravir in Guinea, for example, and was raised by several other studies as well.14–20
No major safety concerns were identified with the use of ZMapp. Despite the drawback of an intravenous infusion that had to be given three times over the course of a week, the full course of ZMapp was successfully administered 91% of the time to recipients who survived that first week of the trial.
In considering both the choice of investigational drugs and the most appropriate trial design in which to study them, each group testing clinical research interventions during the 2014–2016 epidemic faced the unenviable task of having to weigh numerous exigencies accompanying the study of potential therapeutic interventions for a highly lethal infection, their own proper sense of the moral imperatives imposed on trial design by a humanitarian crisis, and the evolving cultural milieu in which those considerations arose. In the case of this trial, which was launched during the second half of the epidemic, we believed that a randomized, controlled design would be the most expedient and definitive means of establishing the absence of a harmful effect and of determining whether the very favorable preclinical data in support of ZMapp might actually translate directly into lives saved. The advantages of randomized studies have been discussed extensively by others.5,21–23 True confidence in the findings of studies of treatments with potentially small-to-moderate effects on mortality is often enhanced by well-performed randomized trials; in their absence, there is a greater risk that such treatment effects may be masked by selection bias and confounding. Although a major strength of the PREVAIL II trial was its randomized design, its weaknesses include an open-label as opposed to double-blind design (i.e., potentially influencing observational bias at the bedside) and the early termination owing to the dramatic decline in the number of infected patients.
The laudable and rapid decline in eligible new cases of EVD was a factor that no trial design could anticipate, and it affected our ability to reach definitive conclusions. Despite the concerted efforts of many dedicated researchers domestically and internationally who participated in this and other trials, the outbreak appears to have ended with no incontrovertible evidence that any single treatment intervention, or combination of interventions, was unequivocally superior to the types of supportive medical care typically provided.24
In another sense, however, the trial did succeed in establishing that it is indeed feasible to conduct a randomized, controlled trial in the context of a major public health emergency despite the challenges involved. Furthermore, with a 91.2% probability favoring a treatment effect for ZMapp, arguably this inconclusive but suggestive outcome has altered the sense of equipoise that accompanied this particular product at the start of the trial. How far from neutral this equipoise has shifted may be a matter of judgment. However, in the event of another outbreak, that experimental niche should probably be filled by one of a small number of other promising, but unproven, treatments that have emerged since the beginning of the recent crisis.25–27 As new epidemics emerge, undoubtedly coupled with their own set of challenges, it is important that any experimental interventions be evaluated in as rigorous a manner as possible so that their success or failure can be declared with the confidence that public health policy demands.
Supplementary Material
Acknowledgments
Financial or logistic support for the trial was provided by the National Institute of Allergy and Infectious Diseases (NIAID), INSERM, the Republic of Sierra Leone Armed Forces, the Ministries of Health and the U.S. Embassy staff in the countries of Liberia, Sierra Leone, and Guinea, the Centers for Disease Control and Prevention (CDC) and the CDC Foundation, the Biomedical Advanced Research and Development Authority, and the Defense Threat Reduction Agency.
We thank the PREVAIL II trial participants without whom this trial would not have been possible, the members of the data and safety monitoring board (chair, Lisa Cooper) for advice and assistance in guiding the progress of the trial, the many health care workers who provided care and research support in participating Ebola treatment units, the support and administrative staff affiliated with those units, the leadership and technical staff of the several government and nongovernment mobile laboratories that performed blood chemical measurements and viral-load determinations, members of the Division of Clinical Research and the Integrated Research Facility within the NIAID, and personnel at Leidos Biomedical Research, the Office of the Assistant Secretary for Preparedness and Response, the U.S. Public Health Service, and the numerous other governmental and nongovernmental agencies that assisted in the conduct of the trial in the affected regions.
APPENDIX
The affiliations of the writing-group members are as follows: the National Institute of Allergy and Infectious Diseases, Bethesda (R.T.D., L.D., M.A.P., J.T., H.C.L., A.S.F.), and Leidos Biomedical Research, Frederick (J.B.) — both in Maryland; University of Minnesota, Minneapolis (J.N., J.N.N., J.S.K.); Liberian Ministry of Health, Monrovia (M.B.F.M.); Republic of Sierra Leone Armed Forces, Freetown (F.S.); and INSERM, University of Bordeaux, Bordeaux, France (D.M.).
Footnotes
Disclosure forms provided by the members of the writing group are available with the full text of this article at NEJM.org.
References
- 1.International Commission. Ebola haemorrhagic fever in Zaire, 1976. Bull World Health Organ. 1978;56:271–93. [PMC free article] [PubMed] [Google Scholar]
- 2.Piot P, Muyembe JJ, Edmunds WJ. Ebola in west Africa: from disease outbreak to humanitarian crisis. Lancet Infect Dis. 2014;14:1034–5. doi: 10.1016/S1473-3099(14)70956-9. [DOI] [PubMed] [Google Scholar]
- 3.Ebola data and statistics: situation summary. Geneva: World Health Organization; Jan 20, 2016. http://apps.who.int/gho/data/view.ebola-sitrep.ebola-summary-20160120?lang=en. [Google Scholar]
- 4.Whitehead J, Olliaro P, Lang T, Horby P. Trial design for evaluating novel treatments during an outbreak of an infectious disease. Clin Trials. 2016;13:31–8. doi: 10.1177/1740774515617740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox E, Borio L, Temple R. Evaluating Ebola therapies — the case for RCTs. N Engl J Med. 2014;371:2350–1. doi: 10.1056/NEJMp1414145. [DOI] [PubMed] [Google Scholar]
- 6.van Griensven J, Edwards T, de Lamballerie X, et al. Evaluation of convalescent plasma for Ebola virus disease in Guinea. N Engl J Med. 2016;374:33–42. doi: 10.1056/NEJMoa1511812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yazdanpanah Y, Horby P, van Griensven J, et al. Drug assessment in the Ebola virus disease epidemic in west Africa. Lancet Infect Dis. 2015;15:1258. doi: 10.1016/S1473-3099(15)00344-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qiu X, Wong G, Audet J, et al. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature. 2014;514:47–53. doi: 10.1038/nature13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jahrling PB, Hensley LE, Barrett K, Lane HC, Davey RT. State-of-the-art workshops on medical countermeasures potentially available for human use following accidental exposures to Ebola virus. J Infect Dis. 2015;212(Suppl 2):S84–90. doi: 10.1093/infdis/jiv115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dodd LE, Proschan MA, Neuhaus J, et al. Design of a randomized controlled trial for Ebola virus disease medical countermeasures: PREVAIL II, the Ebola MCM Study. J Infect Dis. 2016;213:1906–13. doi: 10.1093/infdis/jiw061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Proschan MA, Dodd LE, Price D. Statistical considerations for a trial of Ebola virus disease therapeutics. Clin Trials. 2016;13:39–48. doi: 10.1177/1740774515620145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sissoko D, Laouenan C, Folkesson E, et al. Experimental treatment with favipiravir for Ebola virus disease (the JIKI trial): a historically controlled, single-arm proof-of-concept trial in Guinea. PLoS Med. 2016;13(3):e1001967. doi: 10.1371/journal.pmed.1001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2015. https://cran.r-project.org/bin/windows/base/old/3.2.3/ [Google Scholar]
- 14.Crowe SJ, Maenner MJ, Kuah S, et al. Prognostic indicators for Ebola patient survival. Emerg Infect Dis. 2016;22:217–23. doi: 10.3201/eid2202.151250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de La Vega MA, Caleo G, Audet J, et al. Ebola viral load at diagnosis associates with patient outcome and outbreak evolution. J Clin Invest. 2015;125:4421–8. doi: 10.1172/JCI83162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faye O, Andronico A, Faye O, et al. Use of viremia to evaluate the baseline case fatality ratio of Ebola virus disease and inform treatment studies: a retrospective cohort study. PLoS Med. 2015;12(12):e1001908. doi: 10.1371/journal.pmed.1001908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fitzpatrick G, Vogt F, Moi Gbabai OB, et al. The contribution of Ebola viral load at admission and other patient characteristics to mortality in a Médecins Sans Frontières Ebola case management centre, Kailahun, Sierra Leone, June–October 2014. J Infect Dis. 2015;212:1752–8. doi: 10.1093/infdis/jiv304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunt L, Gupta-Wright A, Simms V, et al. Clinical presentation, biochemical, and haematological parameters and their association with outcome in patients with Ebola virus disease: an observational cohort study. Lancet Infect Dis. 2015;15:1292–9. doi: 10.1016/S1473-3099(15)00144-9. [DOI] [PubMed] [Google Scholar]
- 19.Lanini S, Portella G, Vairo F, et al. Blood kinetics of Ebola virus in survivors and nonsurvivors. J Clin Invest. 2015;125:4692–8. doi: 10.1172/JCI83111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Duan HJ, Chen HY, et al. Age and Ebola viral load correlate with mortality and survival time in 288 Ebola virus disease patients. Int J Infect Dis. 2016;42:34–9. doi: 10.1016/j.ijid.2015.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins R, MacMahon S. Reliable assessment of the effects of treatment on mortality and major morbidity. I. Clinical trials. Lancet. 2001;357:373–80. doi: 10.1016/S0140-6736(00)03651-5. [DOI] [PubMed] [Google Scholar]
- 22.Fleming TR, Ellenberg SS. Evaluating interventions for Ebola: the need for randomized trials. Clin Trials. 2016;13:6–9. doi: 10.1177/1740774515616944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanini S, Zumla A, Ioannidis JPA, et al. Are adaptive randomised trials or non-randomised studies the best way to address the Ebola outbreak in west Africa? Lancet Infect Dis. 2015;15:738–45. doi: 10.1016/S1473-3099(15)70106-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen J, Enserink M. As Ebola epidemic draws to a close, a thin scientific harvest. Science. 2016;351:12–3. doi: 10.1126/science.351.6268.12. [DOI] [PubMed] [Google Scholar]
- 25.Warren TK, Jordan R, Lo MK, et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531:381–5. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warren TK, Wells J, Panchal RG, et al. Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature. 2014;508:402–5. doi: 10.1038/nature13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Clercq E. Ebola virus (EBOV) infection: therapeutic strategies. Biochem Pharmacol. 2015;93:1–10. doi: 10.1016/j.bcp.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.