Abstract
In chronic Trypanosoma cruzi infections, parasite burden is controlled by effective, but non-sterilising immune responses. Infected cells are difficult to detect because they are scarce and focally distributed in multiple sites. However, advances in detection technologies have established a link between parasite persistence and the pathogenesis of Chagas heart disease. Long-term persistence likely involves episodic reinvasion as well as continuous infection, to an extent that varies between tissues. The primary reservoir sites in humans are not definitively known, but analysis of murine models has identified the gastrointestinal tract. Here, we highlight that quantitative, spatial and temporal aspects of T. cruzi infection are central to a fuller understanding of the association between persistence, pathogenesis and immunity, and for optimising treatment.
Beyond Parasite Persistence
The majority of people infected with Trypansoma cruzi survive the acute phase and progress to a chronic asymptomatic infection. Chagas cardiomyopathy is then estimated to develop at a rate of ~2% per year [1]. Megasyndromes of the gastrointestinal (GI) tract develop in a smaller proportion of cases, sometimes in combination with cardiac disease [2]. T. cruzi occurs predominantly in the form of intracellular amastigotes, which replicate in the cytosol of infected cells. These cells are scarce and focally distributed in a range of potential target tissues, making them difficult to detect. The apparent absence of parasites from heart tissue in many people affected by chronic chagasic cardiomyopathy [3, 4] contributed to Chagas disease being regarded as principally an autoimmune pathology. Over the past two decades the consensus has resoundingly changed. Few now support a purely autoimmune aetiology; strong evidence suggests that ongoing infection with T. cruzi is necessary to sustain the tissue damage characteristic of the disease. This idea was encapsulated within the parasite persistence hypothesis [5], which stated “the persistence of Trypanosoma cruzi at specific sites in the infected host results in chronic inflammatory reactivity”. The evidence base supporting the hypothesis is built on the detection of parasite-derived biomolecules (DNA, antigen) in chagasic heart tissue [6, 7], the lack of autoimmune reactivity in the absence of concomitant infection [8], and the efficacy of early anti-parasitic chemotherapy [9]. The benefit of treatment is not apparent for those with late stage heart disease [10], most probably because cardiac damage is largely irreversible. The immunopathogenesis of chagasic cardiomyopathy has been comprehensively reviewed [11-14]; here we will focus on the causative agent.
Beyond the requirement that ongoing T. cruzi infection is necessary for the development of cardiomyopathy, little attention has been paid to the possible modes of parasite persistence within chronically infected individuals (Figure 1, Key Figure). Emerging evidence suggests that the intensity of infection can vary substantially between different tissues and over time, and that these dynamics may vary further between infected individuals. Multiple factors are likely to underpin this heterogeneity, including genetic diversity of the host and the infecting parasite strain(s) and environmental factors. The T. cruzi species comprises six major genetic subtypes (TcI–VI) that have suspected, though largely unproven, associations with many aspects of Chagas disease, including modes of transmission, tissue tropism, severity of outcomes and treatment efficacy (reviewed in [15]). It is likely that the cumulative effect of host-parasite interactions played out over many years, in multiple tissues, ultimately determines clinical outcomes. These include chronic cardiomyopathy of varying severity, digestive megasyndromes, acute meningoencephalitis, and most commonly, the long-term absence of recognized symptoms [2]. In this review, we discuss the spatial, temporal and quantitative dynamics of T. cruzi infections. We also explore why a deeper understanding of parasite persistence is necessary to explain the contribution of T. cruzi to Chagas disease pathogenesis and to inform the development of more effective treatment strategies.
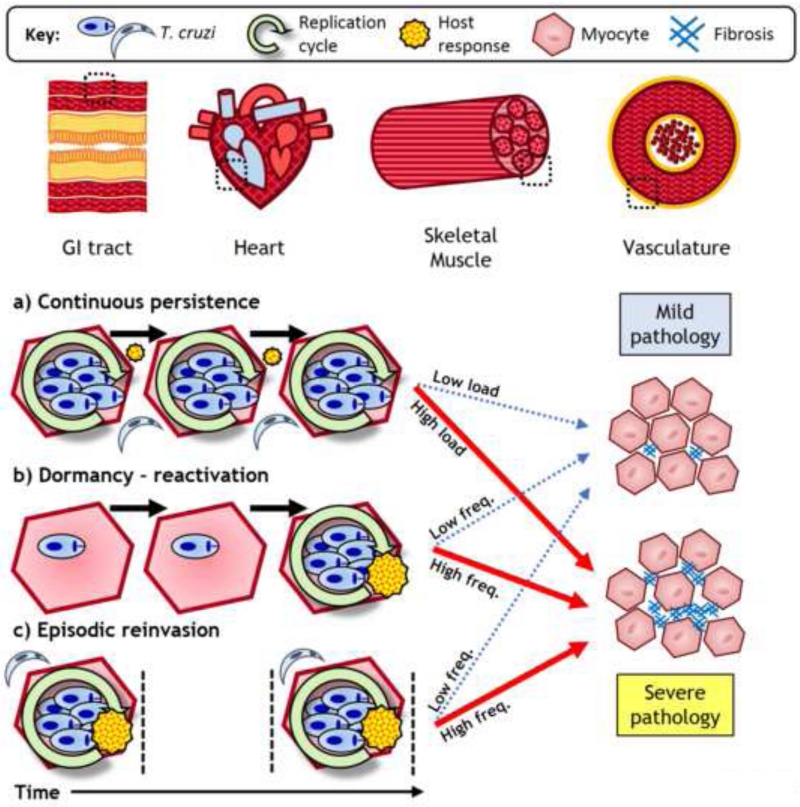
Figure 1. Key Figure. Modes of parasite persistence in long-term Trypanosoma cruzi infections.
In chronic infections T. cruzi predominantly parasitizes myocytes. These infected cells are typically scarce and focally distributed; they can be within cardiac, skeletal or smooth muscle tissues, such as those from the vasculature or the gastrointestinal (GI) tract. The heart is the most common site of pathology. Parasite persistence within an individual host may occur through different modes: a) Continuous persistence describes an ever-present, low abundance parasite load that is sustained as a locally contained equilibrium between intracellular parasite replication and host immune responses; b) Although they have not been proven to exist, dormant forms of T. cruzi may reside within tissues and evade host immunity. As seen for other pathogens, reactivation into typical replication cycles could occur on an intermittent basis; c) Due to the ability of T. cruzi to invade multiple tissues and migrate between them, an organ may be subject to discrete episodes of infection by reinvasion. These three modes are not mutually exclusive and may overlap to different degrees at different times. Over time, the cumulative parasite load is likely to dictate the frequency and intensity of local inflammatory responses, which, depending on their quality, result in differing degrees of pathology. The figure is intentionally simplified and does not convey the molecular and cellular complexity of Chagas disease immunopathogenesis.
Measuring Parasite Loads
The number of parasites and their anatomical location over the course of infection are key parameters. Most importantly, an accurate parasite load measurement, or proxy measure, helps to determine curative outcomes after therapeutic interventions (Box 1). There are a variety of techniques to detect and potentially quantify T. cruzi in tissue samples, each with benefits and drawbacks (Table 1). Detection sensitivity is a marginal concern in analyses of acute or reactivated infections when parasite loads are high, but becomes critical in the chronic phase, when parasite abundance is decreased by several orders of magnitude [16]. For example, extracellular, non-replicating trypomastigotes are routinely detectable in the blood for a period during acute infection and can be observed by light microscopy. However, these forms become rare after the host establishes control of the infection. Acute blood parasitaemia is not necessarily correlated with tissue parasite burdens or predictive of outcomes in the chronic phase. Isolation of live organisms can be achieved from fresh tissues using ex vivo parasite culturing techniques, and this generates enough material for genetic typing [17-19].
Box 1: Cured or not cured?
Identifying whether a patient or experimental animal has been cured of T. cruzi infection is a critical question, whether assessing the effectiveness of the front-line drugs (benznidazole or nifurtimox), or testing new chemotherapeutic agents and immunotherapies [72]. Detection of T. cruzi in humans is usually restricted to blood. Consequently, prediction of parasitological cure is normally based on the presence or absence of parasite-specific immunoglobulins or parasite DNA. Post-cure conversion to a seronegative status may take years to occur. Quantitative PCR can be sufficiently sensitive to detect a single parasite in 5 ml of blood [101]. However, the main utility of PCR is to confirm failure to cure, because even consistently negative results cannot prove that tissue parasites have been completely eliminated [2]. Ongoing investigation of a number of other biomarkers may expand the options available to monitor clinical interventions e.g. [24].
The increased sampling possibilities available for experimental animal models can potentially generate a greater degree of confidence in predicted cure rates [72]. Quantitative PCR is a powerful tool, but suffers from limitations arising from the focal and dynamic nature of chronic infections. Bioluminescence imaging models can overcome this problem, yet are subject to their own detection limits and will have a restricted ability to detect metabolically quiescent parasites. Dormant forms of T. cruzi are currently hypothetical and their role in disease progression would probably be minor. They could still be important for sustaining persistence though, and would be particularly concerning in the context of drug treatment. The use of immunosuppression in tests involving predictive animal models is therefore seen as an essential procedure because it leads to rapid expansion and dissemination of T. cruzi [33, 34, 75, 80, 94]. The most common protocol used in drug efficacy studies involves treatment of mice with cyclophosphamide, which is cytotoxic to many leukocyte populations. It is important to note that cyclophosphamide dosing regimens have never been systematically validated in the context of chronic T. cruzi infection. Nevertheless, consistently negative parasite detection tests conducted on multiple tissues, even after cyclophosphamide-induced immunosuppression, is currently the most convincing criterion of cure in murine models.
Table 1. Common methods for detection of Trypanosoma cruzi in mammalian hosts.
| Method | Target | Main Advantages | Main Drawbacks |
Example Refs |
|---|---|---|---|---|
|
Serology |
Anti-parasite antibodies |
Simple, low cost Diagnostic gold standard |
Indirect, qualitative Long time to seronegativisation in cured subjects |
[9, 10, 25, 58, 79, 97] |
| Fresh blood microscopy |
Live trypomastigotes |
Definitive Fast result |
Mainly restricted to acute phase Blood poor proxy for tissue |
[98] |
| Haemoculture | Live parasites | Low cost Definitive |
Long time until result Low sensitivity |
[18] |
| Xenodiagnosis | Live parasites | Low cost Definitive |
Long time until result Requires triatomine colony |
[111] |
|
Histology |
Intact fixed organisms |
Parasites seen directly in situ Morphological and pathological context available |
Low sensitivity Sampling bias a Snapshot data |
[3, 4, 19, 20, 41, 59, 61, 73, 98] |
|
Immunohistochemistry |
Parasite antigen |
More sensitive than routine histology Semi-quantitative Morphological and pathological context available |
Careful optimisation of signal and background needed Sampling bias a Snapshot data |
[21, 61, 66, 69, 97] |
|
PCR |
Parasite DNA |
High sensitivity Lowest limit of detection Can be quantitative Some targets allow discrimination of T. cruzi subtypes |
Sampling bias a Snapshot data Risk of contamination or non-specific amplification generating false positive results |
[6, 16, 17, 19, 22, 23, 52, 57, 61, 66-68, 75- 77, 84, 101] |
|
Bioluminescence / Fluorescence Imaging |
Transgene expression in live parasites |
Allows serial evaluation Can be highly sensitive Minimal tissue sampling bias Only live parasites detected |
Animal models only High cost Requires optimisation of transgene expression |
[26-29, 32-34] |
Owing to the variability in distribution of parasite foci over time and between tissue sites.
Clusters of T. cruzi amastigotes can be readily observed in tissue by histological staining. This allows visualisation of infected cells in situ and in the context of local pathology. In the chronic phase, such infected cells are typically scarce, which limits the scope of histology for parasite load estimation. Sensitivity can be improved in experimental studies through the use of parasites expressing reporter genes such as lacZ β-galactosidase [20]. Immunohistochemistry has frequently been used to detect T. cruzi antigens in tissue sections. In some cases, this eases the difficulty of visualising intact trypomastigotes and lone amastigotes [21], but more often it reveals antigen in the absence of intact parasites [22]. The presence of these antigen deposits is presumed to indicate the remnants of recently destroyed organisms.
PCR-based methodology is a mainstay of parasite detection in both diagnostic and experimental settings. Two loci in particular, the nuclear 195 bp satellite and kinetoplast DNA minicircles, allow very high sensitivity because there are thousands of copies per genome. Quantitative PCR protocols allow estimates of parasite burdens and comparison across tissues; however, they may overestimate the number of viable parasites. The presence of organisms in blood can confound interpretations of tissue residence and repeated PCR assays can be necessary to ensure accurate results [6]. T. cruzi subtypes can be ascertained using PCR-based methods, but this generally requires analysis of lower copy-number genes [23]. The resulting reduction in sensitivity means that direct identification of parasite genotypes in chronic infections remains challenging. New detection tools continue to be developed. For example, nucleic acid aptamers to identify T. cruzi secreted antigens in serum may surpass the sensitivity of PCR [24], and lineage-specific serology has the potential to define historical exposure to different parasite subtypes [25].
Experimental studies with predictive animal models can generate insights into T. cruzi infection dynamics that are not possible in humans. The techniques mentioned above can be applied to animal samples at specific times post-infection, in combination with defined experimental parameters, e.g. genotypes, inocula or treatments. Broad tissue-type sampling can be easily conducted for groups of identically-treated animals, although in practice most animal studies concentrate on a limited set of tissues. Real-time imaging methods have also been developed that allow serial analysis of T. cruzi infections in individual mice [26-29]. These methods employ transgenic parasites expressing luciferases or fluorescent proteins, such that light signals emitted by parasites can be quantified and pinpointed to anatomical locations. The intensity of light emitted from tissue samples ex vivo can also be used as a proxy for parasite loads in specific organs. A key development was the introduction of a firefly luciferase gene engineered to emit longer wavelength light, which has enhanced tissue penetrating capacity [30, 31]. The use of T. cruzi expressing this red-shifted luciferase enabled highly sensitive imaging of chronically infected mice [16, 32-34]. Drawbacks include the high associated costs and, potentially, a reduced ability to detect parasites that are metabolically quiescent. Interpretation of imaging data also requires careful consideration of the light absorbing and scattering properties of different tissues. For example, peripheral infection foci in sites such as the skin will have a lower associated limit of detection than those in visceral organs.
In summary, there are now multiple sensitive approaches to detect and estimate numbers of T. cruzi in diverse tissue types. Emerging technologies could soon increase sensitivity further and generate new insights into the parasite’s biology in vivo. For example, aptamers [35] or spliced leader trapping [36] can potentially enrich samples for rare parasites or parasite-derived mRNAs, while ribosomal profiling [37], fluorescence dilution [38] and isotope labelling [39] approaches could help differentiate between dormant and active parasites. In the following sections we review current understanding of T. cruzi infection dynamics and explore why the utility of the parasite detection techniques described above depends on appropriate tissue sampling strategies.
Acute Infection and Tropisms
Upon primary infection from the insect vector, metacyclic trypomastigotes invade various cell types local to the site of inoculation and transform into amastigotes, which then undergo multiple rounds of mitotic replication. This proceeds for approximately one week and is followed by differentiation into bloodstream trypomastigotes and host cell rupture. The release of motile trypomastigotes into the haemolymphatics permits systemic dissemination and the acute phase of infection continues until the immune response brings parasite loads under control several weeks later. T. cruzi has the ability to invade and replicate inside almost any type of nucleated mammalian cell in vitro. The broad range of infected organs was noted in the earliest autopsy studies of fatal acute T. cruzi infection [40]. Analyses of acutely infected mice have shown that diverse parasite strains indeed infect a huge array of cell types in virtually any tissue [16, 20-22, 26, 33, 41-47]. However, the relative abundance of parasites in different cell or tissue types varies greatly. Sites reported to harbour the highest acute infection intensities include skeletal, smooth and cardiac muscle, mononuclear phagocytes and adipose tissues. Conversely, T. cruzi is comparatively rare where the blood/oxygen supply is poor, for example in osteocytes and chondrocytes [42], cartilage [41] and in immune-privileged sites including ovaries and testes [43]. It is worth making a distinction between cell and tissue types, which is possible with microscopic detection of parasites, but not with methods that analyse homogenized tissue samples or macro-scale imaging. For example, bioluminescence imaging readily identifies the GI tract and lung as sites of infection [16, 26], but not the infected cell types. Using histological analysis, Guarner et al. [21] also described lung infections and found that amastigotes were only localised to the muscular stratum of pulmonary blood vessels. Similarly, within the GI tract, amastigotes can be more easily found in the smooth myocytes or within the myenteric plexus, than in the submucosa [45].
Heterogeneity in site-specific infection intensities with different parasite strains resulted in the concept of tropism being applied to T. cruzi [45, 48, 49]. However, the presence or absence of T. cruzi in particular cells and tissues may also be influenced by factors other than innate parasite preferences. For example, the route of inoculation, the dose, and the occurrence of other infections could all act to determine which of the viable niches the parasite occupies in vivo. Notwithstanding these caveats, all T. cruzi strains appear to share the ability to replicate within muscle cells (myocytes), and “myotropic” is something of a default term. It has been suggested that the preference for muscle might be an adaptation to access myoglobin as a source of heme [50]. Myocytes may also be invaded preferentially due to their highly active plasma membrane repair pathway, which T. cruzi can hijack to facilitate invasion [51]. Variability has also been described between different muscles, for example, the Brazil strain (TcI) is associated with higher parasite burdens in skeletal than in cardiac muscle, for unknown reasons [52].
A subset of strains, e.g. Y (TcII), have been described as reticulotropic because, in addition to infecting muscle, they have a greater capacity to parasitize both resident and inflammatory mononuclear phagocytes compared to other strains, at least in acutely infected mice [45, 48, 53]. Targeting these cell types allows these strains to infect a wider variety of tissues and may be related to increased virulence.
In some circumstances, T. cruzi is able to cross the blood-brain barrier, potentially leading to fatal meningoencephalitis. A subset of TcI strains, ~20%, have been associated with this phenotype in mice [44, 54, 55]. Nevertheless, central nervous system (CNS) infections in humans are rare; they tend to follow immunosuppression [56] and can be caused by other lineages [57, 58]. The data available are not sufficient to conclude whether CNS involvement is a consequence of parasite-intrinsic virulence factors, a result of increased host susceptibility, or a combination.
Elusively Reclusive: T. cruzi in the Chronic Phase
The heart
Evidence for parasite tropisms is based almost exclusively on acute infections. The long-term dynamics of T. cruzi infection have remained vague because of the difficulty in detecting rare parasite foci during the chronic phase. This has limited progress in understanding factors that influence chronic parasite loads in the heart and their connection to pathogenesis. Histological studies typically identified T. cruzi amastigotes in fewer than 30% of chagasic human hearts [3, 4, 59-64]. Higher sensitivity molecular detection methods indicated the presence of T. cruzi DNA or antigen at frequencies of 50 – 95% [6, 61, 65-70]. The presence of T. cruzi or derived material frequently co-occurs with myocarditis [65, 67, 69, 70], but quantitative correlation has not been demonstrated. Importantly, inflammation is only one of several pathological processes that contributes to the development of chagasic heart disease. Limited data suggest that an association between infection dynamics and fibrosis or tissue re-modelling is absent [65, 71], and evidence is lacking for links to denervation and conduction or microvascular abnormalities. Comparative analyses of human cases tend to rest on the presence or absence of parasites in samples from small cohorts of patients who had died or required a heart transplant. Causation is therefore uncertain because parasite loads at these times may not accurately reflect the preceding asymptomatic and early symptomatic period.
Experimental studies have also generated valuable data. Non-human primates, dogs, rabbits, rats and guinea pigs are all useful to study chronic cardiac infection [72], but for practical and ethical reasons mice are by far the most commonly used models. Drawing firm conclusions from the existing literature is complicated by variations in experimental design. This includes widespread use of different strains of parasites and mice, inoculum sizes, routes of inoculation, end-points, methods of parasite detection and parameters for pathology. Only a few mouse models of chronic T. cruzi infection have been described that involve heart parasite burdens high enough for consistent detection using histology [73, 74]. Similar to some human data, Zhang and Tarleton [22] found that the presence of T. cruzi kDNA was qualitatively associated with co-localized inflammatory infiltrates, indicative of ongoing immune responses against parasites persisting within the hearts of chronically infected mice. Estimates of cardiac parasite loads made using qPCR range from below the limit of detection [16, 33], 2 – 80 per 50 ng of host DNA [52, 75], 20 per mg of tissue [76] to 1 per 200 host cells [77]. However, direct comparisons of qPCR loads with pathology at the level of individual animals have not been reported.
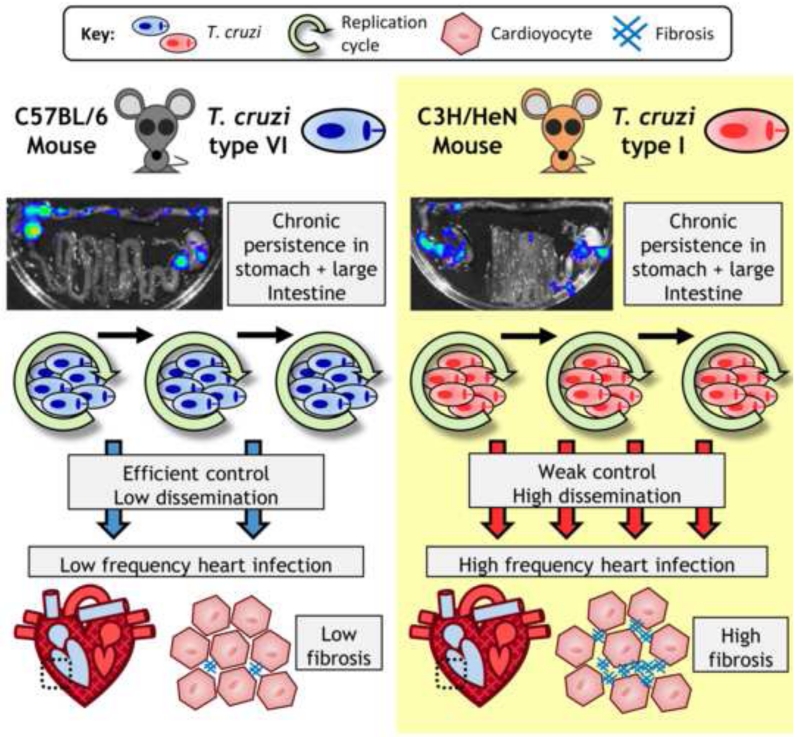
The recent application of real-time bioluminescence imaging to quantify tissue-specific parasite burdens in multiple mouse models has generated unexpected insights into the dynamics of chronic infections. For the CL Brener strain (TcVI), cardiac-localised parasite bioluminescence was not detected in C57BL/6, and only ~10% of BALB/c or C3H mice, but frequencies of 40%, 50% and 88% respectively, were detected for a TcI strain (JRcl4) [16, 34]. Cardiac fibrosis was consistently observed, but there was no correlation with parasite loads in individual animals. The host-parasite genotype combinations used in these studies had differing frequencies of infection in multiple organs other than the heart (see below). Furthermore, models exhibiting the most broadly disseminated infections had significantly higher levels of cardiac fibrosis. This correlation, together with additional lines of evidence, implied that infection of the heart is likely to be sporadic and repetitive, rather than continuous, and to occur at a frequency determined by the overall systemic distribution of parasites [34] (Figure 2). Further work is required to validate this hypothesis and to determine whether it can help to explain the relationship between cardiac parasitism and pathogenesis.
Figure 2. Model for a link between host-parasite genetics, infection dynamics and chagasic cardiac pathology.
In experimental murine models of chronic Trypanosoma cruzi infection, varying host and parasite genotype combinations can generate different severities of cardiac pathology. Real-time in vivo imaging studies of tissue-specific infection dynamics [16, 34] have suggested that an important factor is the extent of parasite dissemination. Regions of the gastrointestinal (GI) tract serve as permanent reservoirs of infection, especially the proximal large intestine and stomach, regardless of the host-parasite genotype combination. The photos show ex vivo imaged GI tracts overlaid with pseudocolour heat-maps of bioluminescence intensity, which is used as a proxy for parasite numbers. Other tissue sites, including the heart, are actively infected more sporadically. Some models, such as C57BL/6 mice infected with a T. cruzi type VI strain (CLBR) have low parasite burdens outside the gut, heart infection is relatively infrequent and cardiac fibrosis is relatively mild. Other models, particularly C3H/HeN mice with chronic T. cruzi type I infections, have more broadly disseminated infections, frequent infection foci localized to the heart and more severe cardiac fibrosis.
Other Sites
In humans with established infections, T. cruzi is clearly not restricted to cardiac tissue. For example, approximately half of Chagas disease patients that receive heart transplants develop symptomatic reactivation of T. cruzi infection [78]. Transmission via infected blood transfusions and the practice of xenodiagnosis demonstrate that parasites can be present in the blood after the acute phase [2]. Parasitemia clearly fluctuates because PCR analysis is not consistently positive or negative in individuals over time [10]. T. cruzi can also cross the placenta leading to congenital transmission in ~5% of cases, with some geographic variability linked to T. cruzi genotype [79]. As outlined below, parasites have been detected in a wide of range of other organs, but whether any of these sites serve as genuine long-term reservoirs, or simply become transiently infected is far from clear.
Several cases of transmission caused by transplantation of livers or kidneys from seropositive donors have been recorded, although the risk is lower than for hearts [60]. Parasites can be frequently detected in skeletal muscle in some chronic mouse infection models [20, 52, 75, 80, 81]. This site is poorly documented in humans – some physiological abnormalities have been described, but not the presence of parasites or inflammation [82]. The smooth muscles of blood vessels have also been identified as a site of chronic infection. For example, parasites were detected in the central vein of the adrenal gland in several small autopsy studies at frequencies of 5% [6], 30% [4] and 50% [83]. Progressive multi-organ vasculitis has been reported in mice infected with the high virulence Colombiana (TcI) strain [84].
Adipose tissue has also been suggested as a site of persistence. T. cruzi readily parasitizes adipocytes in vitro and lipid rich tissues can have high parasite loads in acutely infected mice [16, 41, 77, 85]. Indeed, adipose tissue was a major site of T. cruzi persistence associated with posaconazole treatment failure in acutely infected mice [33], and panniculitis (inflammation of the fatty subcutaneous tissue) is a common symptom of reactivated Chagas disease [78]. The majority of C3H mice with long-term TcI infections have parasites in adipose tissue [34, 77], but this is less frequent for other genotype combinations [34]. A small study that analysed subcutaneous fat samples from ten seropositive patients found that three of them contained T. cruzi kDNA [86]. It is not known which cell type(s) within adipose tissue harbour T. cruzi in chronically infected subjects, nor whether infection is continuous or sporadic.
T. cruzi might parasitize the peripheral, enteric and central nervous systems in settings other than acute or reactivated infection, but direct evidence from humans is scarce. Amastigotes have been found in the sciatic nerve and lumbar spinal cord of C3H mice 8-10 months post-infection [20, 87]. Neuronal damage and loss within the affected organs are important features of both cardiac and digestive Chagas disease, but this is more likely to be collateral to active inflammation in adjacent tissue compartments [88].
Owing to the focal pleiotropism of T. cruzi, studies on persistence have frequently been associated with tissue sampling biases. The development of sensitive real-time imaging has largely overcome this issue, at least for mouse models. Recent work in our laboratory revealed that the GI tract, specifically the large intestine and stomach, is the predominant site of parasite persistence for multiple mouse-parasite genotype combinations [16, 34]. One reason for this may be a local trade-off between incentives for the host to limit parasite numbers and to avoid bacterial translocation, which can occur in an inflamed gut [89]. Particular features of the intestinal microenvironment that might contribute to T. cruzi persistence include macrophage populations that are refractory to activation, as well as high levels of IL-10 and abundant regulatory T cells, which dampen inflammatory responses [90, 91]. These data raise the possibility that ongoing GI infection continuously contributes to the development of digestive forms of Chagas disease. A lack of data on chronic GI infections in humans means this will remain an open question (Box 2). Nevertheless, in the context of drug development strategies, it appears prudent to consider the ability of compounds to reach effective concentrations in GI tissues as an important parameter.
Box 2: Chagas enteropathy, a most neglected disease?
Digestive forms of Chagas disease are characterised by progressive dilation of sections of the digestive tract, usually but not exclusively the oesophagus or colon. Cases are most common in Bolivia, Chile, northern Argentina and southern Brazil; and despite fragmentary data, the overall incidence is estimated at 10-15% of infected people [2]. Understanding of the molecular and cellular basis of pathogenesis lags far behind the advances made for chagasic cardiomyopathy. Dilation is associated with loss of neurons, dysperistalsis and hypertrophy, inflammation and fibrosis of the smooth muscle layers [102, 103]. The most common symptoms include difficult or painful swallowing, abdominal pain, constipation and faecaloma. Treatment options are limited to palliative, dietary and surgical interventions [2].
Anti-parasitic chemotherapy has not been considered justifiable for seropositive individuals with digestive symptoms, but normal heart function [104]. This is primarily because clinical trials have not addressed the efficacy of treatment in the context of digestive outcomes. It is also influenced by a prevailing view that megasyndromes result from irreversible enteric denervation during the acute phase [102, 105], in which anti-parasitic inflammatory responses are thought to cause iNOS-dependent collateral damage to neurons [88]. Further age-related denervation is posited to gradually unmask the parasite-driven losses, leading to progressive organ dysfunction [105]. The finding that the colon and stomach are the primary reservoirs of T. cruzi infection in mice [16, 34] raises the possibility that local infection may in fact continue to influence the development of digestive forms of Chagas disease into the chronic phase. Indeed, histological analyses have identified persistence of parasites in GI samples in 20-50% of megaesophagus cases [106, 107] and, using PCR-based strategies, other authors have found T. cruzi DNA in 100% of such samples [108]. Long-term infection in the dog is considered a useful animal model of chagasic megasyndromes [109] and some features of nascent enteropathy can also be observed in experimentally infected mice [110]. These studies, amongst others, now form a framework for further experimental investigation, not only of the role of T. cruzi in digestive pathogenesis, but also of the ability of specific chemotherapy targeting the parasite to treat this type of Chagas disease.
These imaging studies also identified T. cruzi in a variety of other sites, including the heart, lung, skeletal muscle, skin and visceral fat. Unlike the GI tract, these infection foci were only ever detected in a subset of animals. Different combinations of parasite and mouse strains were associated with varying levels of disseminated infection foci outside the GI tract, but without any evidence of differential tissue tropism. For example, C57BL/6 mice infected with a TcVI strain rarely had dissemination outside the gut, whereas C3H mice infected with a TcI strain typically had multiple systemic parasite foci. Importantly, models with a higher degree of dissemination had significantly more severe cardiac fibrosis - a key marker of Chagas cardiomyopathy [34] (Figure 2). These findings highlight the importance of understanding both tissue-specific interactions between host and parasite, as well as the potential interconnectedness of infection between sites over time.
Re-Invasion as a Route to Parasite-Driven Cardiopathogenesis
Adaptive immune responses, particularly those mediated by CD8+ T cells, are critical to maintain a stable long-term host-parasite equilibrium [92]. Humans with reduced immune function (e.g. HIV co-infection or immunosuppressive treatment),typically have lowered ability to control T. cruzi and often experience pathology in atypical sites (CNS, skin, GI tract) [56-58, 93]. Antibody-mediated depletion of T cells leads to exacerbated heart parasite loads and myocarditis in mice [94], and chronically infected mice treated with the immunosuppressant cyclophosphamide experience rapid systemic expansion of parasite loads, which resembles the acute phase pattern of infection [34]. Therefore, the niches available for T. cruzi within the chronically infected host are primarily defined by host responses rather than parasite tropism.
The dynamic nature of chronic phase parasite distribution means it is important to distinguish between repeated short-term infection and long-term persistence within defined tissues, because alternative modes of infection can be expected to provoke different types of immune response. Data from animal imaging models has led us to propose a model of continual parasitism of the GI tract that is tolerated by the host, combined with repeated, sporadic reinvasions of the heart and other sites, which provoke effective host responses. There is good evidence that chronically infected mice can efficiently control systemic parasites. C57BL/6 mice generate plentiful T. cruzi-specific CD8+ T cells with a cytotoxic, non-exhausted phenotype [80]. The number of interferon- γ (IFN-γ) producing T cells increases after high-dose intravenous secondary infection, concomitant with rapid clearance of parasites from the re-infected spleen, lung and liver [19]. Similarly, neither homologous nor heterologous superinfections of chronically infected C3H mice had a lasting impact on muscle tissue burdens [52]. Immunohistochemical detection of T. cruzi in chronic phase hearts typically reveals debris-like antigen deposits, and not intact organisms [21]. In terms of pathogenesis, the reinvasion model (Figure 1, Key Figure) could explain the lack of consistent correlation between tissue fibrosis (a cumulative, largely irreversible pathology) and ‘snapshot in time’ measurements, such as inflammation and local parasite load [34]. It may also lead to a better understanding of why the majority of Chagas disease deaths are attributed to sudden arrhythmic events or embolism, rather than chronic heart failure [95].
The logical next questions concern the source and mechanism of active (re-)infection foci and the consequent inflammatory responses in particular sites. Addressing this question is likely to be difficult given the requirement to track the movement of individual parasites between distant sites in vivo. It is not known how trypomastigotes transit between tissues and the circulation. The trypomastigote surface glycoprotein gp85 does have high avidity for vascular endothelial cells [96] and vasculitis can be a feature of chronic infections [84]. Nevertheless, superinfection experiments indicate that the majority of parasites entering the blood of chronically infected hosts are expected to be rapidly opsonized and cleared, mainly in the liver by Kupffer cells [19]. This would be particularly true for parasites exiting the GI tract into the portal venous system. Trafficking via the lymphatics could allow T. cruzi to circumvent the liver, promoting both dissemination and transmission. Pertinently, parasites or infected cells exiting the GI tract by a lymphatic route would drain into the subclavian vein and then directly encounter the right atrium. Imaging shows that mesenteric tissue is a relatively frequent site of infection in mice [34], but it remains unclear whether live parasites can evade the firewall function of the mesenteric lymph nodes. Besides reinvasion by free trypomastigotes, several studies provide circumstantial evidence that trafficking of parasitized myeloid cells into organs could be important. T. cruzi antigens often localize to interstitial dendritic cells in the heart [97]. Peripheral infection foci typically appear and disappear over the course of hours, consistent with trafficking of infected host cells [16]. Furthermore, in a fatal acute infection model, parasite burdens in the heart depended on the ability of T. cruzi to replicate specifically in myeloid cells expressing Slamf1 [98], a surface receptor with a pro-migratory function [99, 100]. Lastly, it is important to acknowledge the possibility that reinvasion may occur alongside reactivation of (quasi)dormant parasite foci. Indeed, evidence for in vivo metabolic heterogeneity in other intracellular pathogens [38, 39] suggests this could be a vital, but largely unexplored aspect of T. cruzi biology.
Concluding Remarks
T. cruzi is a fascinatingly versatile microorganism. It parasitizes diverse cell types in multiple tissues, in hundreds of different mammal hosts, and is transmitted by dozens of triatomine vector species. This promiscuity makes T. cruzi infections challenging to study in both clinical and experimental settings (see Outstanding Questions). Nevertheless, increasingly sophisticated parasite detection technologies are leading to a better appreciation of the dynamic nature of chronic infections and how this intersects with Chagas disease pathogenesis. Host immune responses clearly enforce a dramatic restriction of the niches within which T. cruzi can persist. However, the mechanism(s) of long-term immune evasion within individual hosts remains largely unknown. Different modes of persistence may occur within and between organs, including continual low-level infection, dormancy-reactivation and episodic re-invasion. Distinguishing the contribution of these processes to long-term tissue-specific infection dynamics will require careful experimental investigation. An even greater challenge will be to analyse them in humans and define their contribution to the development of both cardiac and digestive Chagas disease.
Outstanding Questions.
Why is T. cruzi preferentially able to parasitize myocytes in chronic phase infections?
What are the mechanisms of long-term immune evasion?
Is there a metabolically quiescent or dormant form of T. cruzi that may have a role in long-term persistence and perhaps treatment failure?
Which modes of persistence occur in humans and how closely do they match the mouse model e.g. does the GI tract serve as a ‘safe haven’ for parasites and is heart infection episodic or continuous?
What is the source of parasites when reactivation of Chagas disease occurs under immunosuppression? For example, are cutaneous and CNS pathologies due to local expansion of underlying infection or to re-invasion from other sites?
What is the mechanism that drives the development of digestive megasyndromes and is it dependent on chronic persistence of T. cruzi within the GI tract?
What is the identity and phenotype of the cells in the GI tract that act as reservoirs of infection?
Will elimination of parasites from the gut reservoir lead to a sterile cure of chronic infections?
Is immunotherapy an option for inducing immune-mediated killing of parasites in the sites of persistence?
Trends Box.
Advances in the sensitivity and accuracy of molecular and imaging technologies are leading to a better understanding of quantitative, spatial and temporal variation in Trypanosoma cruzi infections.
T. cruzi is pan-tropic in acute phase infections, with some strain-specific heterogeneity in parasite loads between cell and tissue types.
The gastrointestinal tract serves as the main parasite reservoir in mice during chronic infections; there is currently insufficient evidence to define long-term reservoirs in humans.
Targeting myeloid cells for infection may allow T. cruzi to evade adaptive immune responses, re-invade tissues and achieve transmission.
A model of repeated reinvasion of the heart has the potential to better explain chagasic cardiac pathology than one of continual local persistence.
Acknowledgements
The authors are grateful for support from the Wellcome Trust (Grant 084175), the British Heart Foundation (Grant PG/13/88/30556), the Drugs for Neglected Diseases Initiative and the European Commission Marie Curie Fellowships program. This work was supported in part by the Division of Intramural Research, NIAID, NIH. We thank Martin Taylor and Amanda Francisco for insightful discussions and comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sabino EC, et al. Ten-Year Incidence of Chagas Cardiomyopathy Among Asymptomatic Trypanosoma cruzi–Seropositive Former Blood Donors. Circulation. 2013;127:1105–1115. doi: 10.1161/CIRCULATIONAHA.112.123612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rassi A, Jr, et al. Chagas disease. Lancet. 2010;375:1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- 3.Laranja FS, et al. Chagas’ Disease: A Clinical, Epidemiologic, and Pathologic Study. Circulation. 1956;14:1035–1060. doi: 10.1161/01.cir.14.6.1035. [DOI] [PubMed] [Google Scholar]
- 4.Barbosa A.d.A., Jr., Andrade ZA. Identificação do Trypanosoma cruzi nos tecidos extracardíacos de portadores de miocardite crônica chagásica. Revista da Sociedade Brasileira de Medicina Tropical. 1984;17:123–126. [Google Scholar]
- 5.Tarleton RL. Parasite persistence in the aetiology of Chagas disease. International Journal for Parasitology. 2001;31:549–553. doi: 10.1016/s0020-7519(01)00158-8. [DOI] [PubMed] [Google Scholar]
- 6.Jones EM, et al. Amplification of a Trypanosoma cruzi DNA Sequence from Inflammatory Lesions in Human Chagasic Cardiomyopathy. Am J Trop Med Hyg. 1993;48:348–357. doi: 10.4269/ajtmh.1993.48.348. [DOI] [PubMed] [Google Scholar]
- 7.Higuchi M.d.L., et al. Correlation between Trypanosoma cruzi parasitism and myocardial inflammatory infiltrate in human chronic chagasic myocarditis: light microscopy and immunohistochemical findings. Cardiovascular Pathology. 1993;2:101–106. doi: 10.1016/1054-8807(93)90021-S. [DOI] [PubMed] [Google Scholar]
- 8.Hyland KV, et al. Modulation of Autoimmunity by Treatment of an Infectious Disease. Infection and Immunity. 2007;75:3641–3650. doi: 10.1128/IAI.00423-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Andrade A, et al. Randomised trial of efficacy of benznidazole in treatment of early Trypanosoma cruzi infection. The Lancet. 1996;348:1407–1413. doi: 10.1016/s0140-6736(96)04128-1. [DOI] [PubMed] [Google Scholar]
- 10.Morillo CA, et al. Randomized Trial of Benznidazole for Chronic Chagas’ Cardiomyopathy. New England Journal of Medicine. 2015;373:1295–1306. doi: 10.1056/NEJMoa1507574. [DOI] [PubMed] [Google Scholar]
- 11.Bonney KM, Engman DM. Autoimmune Pathogenesis of Chagas Heart Disease: Looking Back, Looking Ahead. The American Journal of Pathology. 2015;185:1537–1547. doi: 10.1016/j.ajpath.2014.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marin-Neto JA, et al. Pathogenesis of chronic Chagas heart disease. Circulation. 2007;115:1109–1123. doi: 10.1161/CIRCULATIONAHA.106.624296. [DOI] [PubMed] [Google Scholar]
- 13.Gutierrez FRS, et al. The role of parasite persistence in pathogenesis of Chagas heart disease. Parasite Immunol. 2009;31:673–685. doi: 10.1111/j.1365-3024.2009.01108.x. [DOI] [PubMed] [Google Scholar]
- 14.Machado FS, et al. Current understanding of immunity to Trypanosoma cruzi infection and pathogenesis of Chagas disease. Seminars in Immunopathology. 2012;34:753–770. doi: 10.1007/s00281-012-0351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Messenger LA, et al. Between a bug and a hard place: Trypanosoma cruzi genetic diversity and the clinical outcomes of Chagas disease. Expert Review of Anti-infective Therapy. 2015;13:995–1029. doi: 10.1586/14787210.2015.1056158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis MD, et al. Bioluminescence imaging of chronic Trypanosoma cruzi infections reveals tissue-specific parasite dynamics and heart disease in the absence of locally persistent infection. Cellular Microbiology. 2014;16:1285–1300. doi: 10.1111/cmi.12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Ávila DA, et al. Probing Population Dynamics of Trypanosoma cruzi during Progression of the Chronic Phase in Chagasic Patients. Journal of Clinical Microbiology. 2009;47:1718–1725. doi: 10.1128/JCM.01658-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.da Rocha Siriano L, et al. Chagas Disease: Increased Parasitemia during Pregnancy Detected by Hemoculture. The American Journal of Tropical Medicine and Hygiene. 2011;84:569–574. doi: 10.4269/ajtmh.2011.10-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sardinha LR, et al. The Liver Plays a Major Role in Clearance and Destruction of Blood Trypomastigotes in Trypanosoma cruzi Chronically Infected Mice. PLoS Negl Trop Dis. 2010;4:e578. doi: 10.1371/journal.pntd.0000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buckner FS, et al. Detection of Live Trypanosoma cruzi in Tissues of Infected Mice by Using Histochemical Stain for β-Galactosidase. Infection and Immunity. 1999;67:403–409. doi: 10.1128/iai.67.1.403-409.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guarner J, et al. Mouse model for Chagas disease: immunohistochemical distribution of different stages of Trypanosoma cruzi in tissues throughout infection. The American Journal of Tropical Medicine and Hygiene. 2001;65:152–158. doi: 10.4269/ajtmh.2001.65.152. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Tarleton RL. Parasite Persistence Correlates with Disease Severity and Localization in Chronic Chagas’ Disease. J Infect Dis. 1999;180:480–486. doi: 10.1086/314889. [DOI] [PubMed] [Google Scholar]
- 23.Cura CI, et al. Multiplex Real-Time PCR Assay Using TaqMan Probes for the Identification of Trypanosoma cruzi DTUs in Biological and Clinical Samples. PLoS Negl Trop Dis. 2015;9:e0003765. doi: 10.1371/journal.pntd.0003765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Araujo FF, et al. Aptamer-Based Detection of Disease Biomarkers in Mouse Models for Chagas Drug Discovery. PLoS Negl Trop Dis. 2015;9:e3451. doi: 10.1371/journal.pntd.0003451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhattacharyya T, et al. Development of peptide-based lineage-specific serology for chronic Chagas disease: geographical and clinical distribution of epitope recognition. PLoS Negl Trop Dis. 2014;8:e2892. doi: 10.1371/journal.pntd.0002892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hyland KV, et al. Bioluminescent imaging of Trypanosoma cruzi infection. Int J Parasitol. 2008;38:1391–1400. doi: 10.1016/j.ijpara.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andriani G, et al. Activity In Vivo of Anti-Trypanosoma cruzi Compounds Selected from a High Throughput Screening. PLoS Negl Trop Dis. 2011;5:e1298. doi: 10.1371/journal.pntd.0001298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Canavaci AMC, et al. In Vitro and In Vivo High-Throughput Assays for the Testing of Anti-Trypanosoma cruzi Compounds. PLoS Negl Trop Dis. 2010;4:e740. doi: 10.1371/journal.pntd.0000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henriques C, et al. In vivo imaging of mice infected with bioluminescent Trypanosoma cruzi unveils novel sites of infection. Parasites & Vectors. 2014;7:1–15. doi: 10.1186/1756-3305-7-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Branchini BR, et al. Red-emitting luciferases for bioluminescence reporter and imaging applications. Anal Biochem. 2010;396:290–297. doi: 10.1016/j.ab.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 31.McLatchie AP, et al. Highly Sensitive In Vivo Imaging of Trypanosoma brucei Expressing “Red-Shifted” Luciferase. PLoS Negl Trop Dis. 2013;7:e2571. doi: 10.1371/journal.pntd.0002571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor MC, et al. The Trypanosoma cruzi Vitamin C Dependent Peroxidase Confers Protection against Oxidative Stress but Is Not a Determinant of Virulence. PLoS Negl Trop Dis. 2015;9:e0003707. doi: 10.1371/journal.pntd.0003707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Francisco AF, et al. The limited ability of posaconazole to cure both acute and chronic Trypanosoma cruzi infections revealed by highly sensitive in vivo imaging. Antimicrobial Agents and Chemotherapy. 2015;59:4653–4661. doi: 10.1128/AAC.00520-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewis MD, et al. Host and parasite genetics shape a link between Trypanosoma cruzi infection dynamics and chronic cardiomyopathy. Cellular Microbiology. 2016 doi: 10.1111/cmi.12584. in press. doi:10.1111/cmi.12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagarkatti R, et al. Development of an Aptamer-Based Concentration Method for the Detection of Trypanosoma cruzi in Blood. PLoS ONE. 2012;7:e43533. doi: 10.1371/journal.pone.0043533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nilsson D, et al. Spliced Leader Trapping Reveals Widespread Alternative Splicing Patterns in the Highly Dynamic Transcriptome of Trypanosoma brucei. PLoS Pathog. 2010;6:e1001037. doi: 10.1371/journal.ppat.1001037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smircich P, et al. Ribosome profiling reveals translation control as a key mechanism generating differential gene expression in Trypanosoma cruzi. BMC Genomics. 2015;16:1–14. doi: 10.1186/s12864-015-1563-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Helaine S, et al. Internalization of Salmonella by macrophages induces formation of nonreplicating persisters. Science. 2014;343:204–208. doi: 10.1126/science.1244705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kloehn J, et al. Characterization of metabolically quiescent Leishmania parasites in murine lesions using heavy water labeling. PLoS Pathog. 2015;11:e1004683. doi: 10.1371/journal.ppat.1004683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chagas C. Tripanosomiase americana: forma aguda da molestia. Memórias do Instituto Oswaldo Cruz. 1916;8:37–60. [Google Scholar]
- 41.Lenzi HL, et al. Trypanosoma cruzi: Paninfectivity of CL Strain during Murine Acute Infection. Exp Parasitol. 1996;84:16–27. doi: 10.1006/expr.1996.0086. [DOI] [PubMed] [Google Scholar]
- 42.Morocoima A, et al. Trypanosoma cruzi: experimental parasitism of bone and cartilage. Parasitology Research. 2006;99:663–668. doi: 10.1007/s00436-006-0211-2. [DOI] [PubMed] [Google Scholar]
- 43.Calabrese KS, et al. Trypanosoma cruzi: histopathology of endocrine system in immunocompromised mice. Int J Exp Pathol. 1994;75:453–462. [PMC free article] [PubMed] [Google Scholar]
- 44.Postan M, et al. Studies of Trypanosoma cruzi Clones in Inbred Mice: I. A Comparison of the Course of Infection of C3H/HEN- Mice with Two Clones Isolated from a Common Source. Am J Trop Med Hyg. 1983;32:497–506. doi: 10.4269/ajtmh.1983.32.497. [DOI] [PubMed] [Google Scholar]
- 45.Melo R, Brener Z. Tissue tropism of different Trypanosoma cruzi strains. J Parasitol. 1978;64:475–482. [PubMed] [Google Scholar]
- 46.Camandaroba E, et al. Trypanosoma cruzi: clones isolated from the Colombian strain, reproduce the parental strain characteristics, with ubiquitous histotropism. International Journal of Experimental Pathology. 2006;87:209–217. doi: 10.1111/j.1365-2613.2006.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gómez-Hernández C, et al. Molecular characterization of Trypanosoma cruzi Mexican strains and their behavior in the mouse experimental model. Revista da Sociedade Brasileira de Medicina Tropical. 2011;44:684–690. doi: 10.1590/s0037-86822011005000058. [DOI] [PubMed] [Google Scholar]
- 48.Andrade SG, Magalhães JB. Biodemes and zymodemes of Trypanosoma cruzi strains: correlations with clinical data and experimental pathology. Rev Soc Bras Med Tro. 1997;30:27–35. doi: 10.1590/s0037-86821997000100006. [DOI] [PubMed] [Google Scholar]
- 49.Macedo AM, et al. Trypanosoma cruzi: genetic structure of populations and relevance of genetic variability to the pathogenesis of chagas disease. Memórias do Instituto Oswaldo Cruz. 2004;99:1–12. doi: 10.1590/s0074-02762004000100001. [DOI] [PubMed] [Google Scholar]
- 50.Taylor MC, Kelly JM. Iron metabolism in trypanosomatids, and its crucial role in infection. Parasitology. 2010;137:899–917. doi: 10.1017/S0031182009991880. [DOI] [PubMed] [Google Scholar]
- 51.Fernandes MC, et al. Trypanosoma cruzi subverts the sphingomyelinase-mediated plasma membrane repair pathway for cell invasion. The Journal of Experimental Medicine. 2011;208:909–921. doi: 10.1084/jem.20102518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cummings KL, Tarleton RL. Rapid quantitation of Trypanosoma cruzi in host tissue by real-time PCR. Mol Biochem Parasitol. 2003;129:53–59. doi: 10.1016/s0166-6851(03)00093-8. [DOI] [PubMed] [Google Scholar]
- 53.Taliaferro WH, Tulo P. Connective Tissue Reactions in Normal and Immunized Mice to a Reticulotropic Strain of Trypanosoma cruzi. Journal of Infectious Diseases. 1955;96:199–226. doi: 10.1093/infdis/96.3.199. [DOI] [PubMed] [Google Scholar]
- 54.Marinho CRF, et al. IFN-γ, But Not Nitric Oxide or Specific IgG, is Essential for the In vivo Control of Low-virulence Sylvio X10/4 Trypanosoma cruzi Parasites. Scandinavian Journal of Immunology. 2007;66:297–308. doi: 10.1111/j.1365-3083.2007.01958.x. [DOI] [PubMed] [Google Scholar]
- 55.Zeledón R, Ponce C. Neurotropism in Costa Rican strains of Trypanosoma cruzi. J Parasitol. 1972;58:180–181. [PubMed] [Google Scholar]
- 56.Pinazo M-J, et al. Immunosuppression and Chagas Disease: A Management Challenge. PLoS Negl Trop Dis. 2013;7:e1965. doi: 10.1371/journal.pntd.0001965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burgos JM, et al. Molecular Identification of Trypanosoma cruzi I Tropism for Central Nervous System in Chagas Reactivation Due to AIDS. Am J Trop Med Hyg. 2008;78:294–297. [PubMed] [Google Scholar]
- 58.Bisio M, et al. Benznidazole treatment of chagasic encephalitis in pregnant woman with AIDS. Emerg Infect Dis. 2013;19:1490–1492. doi: 10.3201/eid1909.130667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mott KE, Hagstrom JWC. The Pathologic Lesions of the Cardiac Autonomic Nervous System in Chronic Chagas’ Myocarditis. Circulation. 1965;31:273–286. doi: 10.1161/01.cir.31.2.273. [DOI] [PubMed] [Google Scholar]
- 60.Kransdorf EP, et al. Heart Transplantation for Chagas Cardiomyopathy in the United States. American Journal of Transplantation. 2013;13:3262–3268. doi: 10.1111/ajt.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Benvenuti LA, et al. Trypanosoma cruzi persistence in the native heart is associated with high-grade myocarditis, but not with Chagas’ disease reactivation after heart transplantation. J Heart Lung Transplant. 2014;33:698–703. doi: 10.1016/j.healun.2014.01.920. [DOI] [PubMed] [Google Scholar]
- 62.Anselmi A, et al. Myocardiopathy in Chagas’ disease. American Heart Journal. 1966;72:469–481. doi: 10.1016/0002-8703(66)90104-9. [DOI] [PubMed] [Google Scholar]
- 63.Palacios-Prü E, et al. Ultrastructural Characteristics of Different Stages of Human Chagasic Myocarditis. The American Journal of Tropical Medicine and Hygiene. 1989;41:29–40. [PubMed] [Google Scholar]
- 64.Bestetti RB, et al. Chronic Chagas’ heart disease in the elderly: a clinicopathologic study. Cardiology. 1987;74:344–351. doi: 10.1159/000174221. [DOI] [PubMed] [Google Scholar]
- 65.Higuchi M.d.L., et al. Immunohistochemical characterization of infiltrating cells in human chronic chagasic myocarditis: Comparison with myocardial rejection process. Virchows Archiv. 1993;423:157–160. doi: 10.1007/BF01614765. [DOI] [PubMed] [Google Scholar]
- 66.Añez N, et al. Myocardial parasite persistence in chronic chagasic patients. Am J Trop Med Hyg. 1999;60:726–732. doi: 10.4269/ajtmh.1999.60.726. [DOI] [PubMed] [Google Scholar]
- 67.Schijman AG, et al. Trypanosoma cruzi DNA in cardiac lesions of Argentinean patients with end-stage chronic chagas heart disease. The American Journal of Tropical Medicine and Hygiene. 2004;70:210–220. [PubMed] [Google Scholar]
- 68.Burgos JM, et al. Molecular identification of Trypanosoma cruzi discrete typing units in end-stage chronic Chagas heart diseaseand reactivation after heart transplantation. Clin Infect Dis. 2010;51 doi: 10.1086/655680. [DOI] [PubMed] [Google Scholar]
- 69.Belloti G, et al. In vivo detection of Trypanosoma cruzi antigens in hearts of patients with chronic Chagas’ heart disease. American Heart Journal. 1996;131:301–307. doi: 10.1016/s0002-8703(96)90358-0. [DOI] [PubMed] [Google Scholar]
- 70.Olivares-Villagómez D, et al. Polymerase chain reaction amplification of three different Trypanosoma cruzi DNA sequences from human chagasic cardiac tissue. The American Journal of Tropical Medicine and Hygiene. 1998;59:563–570. doi: 10.4269/ajtmh.1998.59.563. [DOI] [PubMed] [Google Scholar]
- 71.Marcon GEB, et al. Trypanosoma cruzi: parasite persistence in tissues in chronic chagasic Brazilian patients. Memórias do Instituto Oswaldo Cruz. 2011;106:85–91. doi: 10.1590/s0074-02762011000100014. [DOI] [PubMed] [Google Scholar]
- 72.Chatelain E, Konar N. Translational challenges of animal models in Chagas disease drug development: a review. Drug design, development and therapy. 2015;9:4807. doi: 10.2147/DDDT.S90208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pereira IR, et al. A Human Type 5 Adenovirus-Based Trypanosoma cruzi Therapeutic Vaccine Re-programs Immune Response and Reverses Chronic Cardiomyopathy. PLoS Pathog. 2015;11:e1004594. doi: 10.1371/journal.ppat.1004594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Federici EE, et al. Chronic and Progressive Myocarditis and Myositis in C3H Mice Infected with Trypanosoma cruzi. The American Journal of Tropical Medicine and Hygiene. 1964;13:272–280. doi: 10.4269/ajtmh.1964.13.272. [DOI] [PubMed] [Google Scholar]
- 75.Cencig S, et al. Parasitic Loads in Tissues of Mice Infected with Trypanosoma cruzi and Treated with AmBisome. PLoS Negl Trop Dis. 2011;5:e1216. doi: 10.1371/journal.pntd.0001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rodriguez HO, et al. Trypanosoma cruzi strains cause different myocarditis patterns in infected mice. Acta Tropica. 2014;139:57–66. doi: 10.1016/j.actatropica.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 77.Combs TP, et al. The adipocyte as an important target cell for Trypanosoma cruzi infection. J Biol Chem. 2005;280 doi: 10.1074/jbc.M412802200. [DOI] [PubMed] [Google Scholar]
- 78.Bestetti RB, Theodoropoulos TAD. A Systematic Review of Studies on Heart Transplantation for Patients With End-Stage Chagas Heart Disease. Journal of Cardiac Failure. 2009;15:249–255. doi: 10.1016/j.cardfail.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 79.Luquetti AO, et al. Congenital transmission of Trypanosoma cruzi in central Brazil. A study of 1,211 individuals born to infected mothers. Memórias do Instituto Oswaldo Cruz. 2015;110:369–376. doi: 10.1590/0074-02760140410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bustamante JM, et al. Drug-induced cure drives conversion to a stable and protective CD8+ T central memory response in chronic Chagas disease. Nat Med. 2008;14:542–550. doi: 10.1038/nm1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lieke T, et al. NK Cells Contribute to the Control of Trypanosoma cruzi Infection by Killing Free Parasites by Perforin-Independent Mechanisms. Infection and Immunity. 2004;72:6817–6825. doi: 10.1128/IAI.72.12.6817-6825.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Laguens R, et al. Immunopathologic and morphologic studies of skeletal muscle in Chagas’ disease. The American journal of pathology. 1975;80:153. [PMC free article] [PubMed] [Google Scholar]
- 83.Teixeira VDPA, et al. Correlation between Adrenal Central Vein Parasitism and Heart Fibrosis in Chronic Chagasic Myocarditis. The American Journal of Tropical Medicine and Hygiene. 1997;56:177–180. doi: 10.4269/ajtmh.1997.56.177. [DOI] [PubMed] [Google Scholar]
- 84.Roffê E, et al. Trypanosoma cruzi Causes Paralyzing Systemic Necrotizing Vasculitis Driven by Pathogen-Specific Type I Immunity in Mice. Infection and Immunity. 2016;84:1123–1136. doi: 10.1128/IAI.01497-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nagajyothi F, et al. Response of adipose tissue to early infection with Trypanosoma cruzi (Brazil strain) Journal of Infectious Diseases. 2012;205:830–840. doi: 10.1093/infdis/jir840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Matos Ferreira AV, et al. Evidence for Trypanosoma cruzi in adipose tissue in human chronic Chagas disease. Microbes Infect. 2011;13:1002–1005. doi: 10.1016/j.micinf.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Molina HA, et al. The neuromuscular pathology of experimental Chagas’ disease. Journal of the neurological sciences. 1987;81:287–300. doi: 10.1016/0022-510x(87)90104-3. [DOI] [PubMed] [Google Scholar]
- 88.Arantes RME, et al. Interferon-γ-Induced Nitric Oxide Causes Intrinsic Intestinal Denervation in Trypanosoma cruzi-Infected Mice. Am J Pathol. 2004;164:1361–1368. doi: 10.1016/s0002-9440(10)63222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nature Reviews Immunology. 2010;10:159–169. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 90.Smythies LE, et al. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005;115:66–75. doi: 10.1172/JCI19229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shouval DS, et al. Interleukin 10 Receptor Signaling: Master Regulator of Intestinal Mucosal Homeostasis in Mice and Humans. In: Frederick WA, editor. Advances in Immunology. Academic Press; 2014. pp. 177–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tarleton RL. Seminars in immunopathology. Springer; 2015. CD8+ T cells in Trypanosoma cruzi infection; pp. 233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bern C. Chagas disease in the immunosuppressed host. Current opinion in infectious diseases. 2012;25:450–457. doi: 10.1097/QCO.0b013e328354f179. [DOI] [PubMed] [Google Scholar]
- 94.Tarleton RL, et al. Depletion of T-cell subpopulations results in exacerbation of myocarditis and parasitism in experimental Chagas’ disease. Infection and immunity. 1994;62:1820–1829. doi: 10.1128/iai.62.5.1820-1829.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rassi A, Jr, et al. Sudden Death in Chagas’ Disease. Arq Bras Cardiol. 2001;76:86–96. doi: 10.1590/s0066-782x2001000100008. [DOI] [PubMed] [Google Scholar]
- 96.Tonelli RR, et al. Role of the gp85/trans-sialidases in Trypanosoma cruzi tissue tropism: preferential binding of a conserved peptide motif to the vasculature in vivo. PLoS Negl Trop Dis. 2010;4:e864. doi: 10.1371/journal.pntd.0000864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Portella RS, Andrade SG. Trypanosoma cruzi: parasite antigens sequestered in heart interstitial dendritic cells are related to persisting myocarditis in benznidazole-treated mice. Memórias do Instituto Oswaldo Cruz. 2009;104:1023–1030. doi: 10.1590/s0074-02762009000700015. [DOI] [PubMed] [Google Scholar]
- 98.Calderón J, et al. The Receptor Slamf1 on the Surface of Myeloid Lineage Cells Controls Susceptibility to Infection by Trypanosoma cruzi. PLoS Pathog. 2012;8:e1002799. doi: 10.1371/journal.ppat.1002799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van Driel B, et al. Signaling Lymphocyte Activation Molecule Regulates Development of Colitis in Mice. Gastroenterology. 2012;143:1544–1554. e1547. doi: 10.1053/j.gastro.2012.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang G, et al. Migration of Myeloid Cells during Inflammation Is Differentially Regulated by the Cell Surface Receptors Slamf1 and Slamf8. PLoS ONE. 2015;10:e0121968. doi: 10.1371/journal.pone.0121968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ramírez JC, et al. Analytical Validation of Quantitative Real-Time PCR Methods for Quantification of Trypanosoma cruzi DNA in Blood Samples from Chagas Disease Patients. The Journal of Molecular Diagnostics. 2015;17:605–615. doi: 10.1016/j.jmoldx.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.de Oliveira RB, et al. Gastrointestinal manifestations of Chagas’ disease. Am J Gastroenterol. 1998;93:884–889. doi: 10.1111/j.1572-0241.1998.270_r.x. [DOI] [PubMed] [Google Scholar]
- 103.Iantorno G, et al. The enteric nervous system in chagasic and idiopathic megacolon. The American journal of surgical pathology. 2007;31:460–468. doi: 10.1097/01.pas.0000213371.79300.a8. [DOI] [PubMed] [Google Scholar]
- 104.Bern C. Antitrypanosomal Therapy for Chronic Chagas’ Disease. New Engl J Med. 2011;364:2527–2534. doi: 10.1056/NEJMct1014204. [DOI] [PubMed] [Google Scholar]
- 105.Köberle F. Chagas’ disease and Chagas’ syndromes: the pathology of American trypanosomiasis. Advances in Parasitology. 1968;6:63–116. doi: 10.1016/s0065-308x(08)60472-8. [DOI] [PubMed] [Google Scholar]
- 106.de Castro Côbo E, et al. Research on Trypanosoma cruzi and Analysis of Inflammatory Infiltrate in Esophagus and Colon from Chronic Chagasic Patients with and without Mega. J Trop Med. 2012:232646. doi: 10.1155/2012/232646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Adad SJ, et al. Contribuição ao estudo da anatomia patológica do megaesôfago chagásico. Rev I Med Trop. 1991;33:443–450. [PubMed] [Google Scholar]
- 108.da Silveira ABM, et al. Comparative study of the presence of Trypanosoma cruzi kDNA, inflammation and denervation in chagasic patients with and without megaesophagus. Parasitology. 2005;131:627–634. doi: 10.1017/S0031182005008061. [DOI] [PubMed] [Google Scholar]
- 109.Nogueira-Paiva NC, et al. Myenteric plexus is differentially affected by infection with distinct Trypanosoma cruzi strains in Beagle dogs. Memórias do Instituto Oswaldo Cruz. 2014;109:51–60. doi: 10.1590/0074-0276130216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Campos CF, et al. Enteric Neuronal Damage, Intramuscular Denervation and Smooth Muscle Phenotype Changes as Mechanisms of Chagasic Megacolon: Evidence from a Long-Term Murine Model of Trypanosoma cruzi Infection. PLoS ONE. 2016;11:e0153038. doi: 10.1371/journal.pone.0153038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Saavedra M, et al. Quantification by real-time PCR of Trypanosoma cruzi DNA in samples of Triatoma infestans used in xenodiagnosis of chronic Chagas disease patients. Parasites & Vectors. 2016;9:382. doi: 10.1186/s13071-016-1664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]