Abstract
Mice are frequently used as animal models for human hearing research, yet their auditory capabilities have not been fully explored. Previous studies have established auditory threshold sensitivities for pure tone stimuli in CBA/CaJ mice using ABR and behavioral methodologies. Little is known about how they perceive their own ultrasonic vocalizations (USVs), and nothing is known about how aging influences this perception. The aim of the present study is to establish auditory threshold sensitivity for several USV types, as well as to track these thresholds across the mouse’s lifespan. In order to determine how well mice detect these complex communication stimuli, several CBA/CaJ mice were trained and tested at various ages on a detection task using operant conditioning procedures. Results showed that mice were able to detect USVs into old age. Not surprisingly, thresholds differed for the different USV types. Male mice suffered greater hearing loss than females for all calls but not for 42 kHz tones. In conclusion, the results highlight the importance of studying complex signals across the lifespan.
Keywords: Aging, CBA/CaJ mice, ultrasonic vocalizations, USVs, psychoacoustics, operant conditioning
1. Introduction
Age-related hearing loss (ARHL), or presbycusis, is a universal feature of mammalian aging (Yamasoba et al., 2013). This condition is characterized by the progressive decrease in hearing sensitivity from high to low frequencies, as well as a decrease in complex auditory signal comprehension, such as speech (Huang and Tang, 2010); it is the most common etiology among elderly humans. Further, the percentage of elderly people experiencing problems with speech comprehension increases progressively with age. Deterioration of speech perception in humans could be due to limited explicit processing in working memory (Rönnberg et al., 2008), but Van Rooij and Plomp (1990) have argued that the deterioration of speech perception during aging can be largely accounted for by progressive high-frequency hearing loss.
Mice (Mus musculus) are frequently used as an animal model for human hearing research, due to the mouse cochlea being anatomically similar to the human cochlea (Zheng et al., 1999). One common strain for auditory research is the CBA/CaJ mouse. Radziwon et al. (2009) discovered that the CBA/CaJ strain showed peak sensitivity, as measured by operant conditioning for pure tones, in the 8 to 24 kHz range, with higher thresholds for tones outside of this range. This pattern resembles auditory brainstem response (ABR) thresholds for CBA/CaJ mice (Henry, 2004; Zheng et al., 2009). However, ABR recordings yield higher overall thresholds across all frequencies, limiting their comparisons to auditory acuity in awake, behaving mice (Radziwon et al., 2009).
Other studies have used the CBA/CaJ mouse strain to study the physiological basis of hearing loss. Ohlemiller, Dahl, and Gagnon (2010) explored hearing abilities of the CBA/CaJ strain at different ages using compound action potential (CAP) recordings. The authors observed that pure tone thresholds remained stable in this strain well into their first year of life, until middle adulthood, at which point CAP thresholds increase rapidly at high frequencies. Endocochlear potential decline and loss of strial marginal cells could explain this age related threshold increase (Ohlemiller et al., 2010). Similar patterns are thought to be the basis of hearing loss in humans as well as other animals (Ohlemiller et al., 2006; Pauler et al., 1988). In another study on the effects of aging on the auditory abilities of CBA/CaJ mice, Zheng and colleagues (1999) used ABR recordings to trace correlates of age related hearing loss. They found that this mouse strain exhibited stable thresholds for 8, 16, and 32 kHz tones up to 39 weeks of age; however, thresholds increased at all frequencies by 47 weeks of age.
In humans there are known sex differences in ARHL, with males losing high frequency hearing earlier and becoming progressively less sensitive as they age (Corso, 1963; Gates and Cooper, 1991; Tambs et al., 2003). Longitudinal studies showed that hearing loss occurs twice as fast in men as it does in women in similar noise environments (Pearson et al., 1995). A similar pattern has also been observed in the CBA/CaJ strain of mouse. Henry (2004) examined hearing loss sex differences in the CBA/CaJ mouse model using ABRs, discovering that CBA/CaJ males exhibit poor high frequency thresholds earlier than females. Additionally, male mice had higher ABR thresholds for the high frequency hearing range up to middle adulthood. This pattern mimics human hearing loss and suggests that the CBA/CaJ mouse strain is an excellent animal model for studying ARHL.
As reviewed above, researchers have primarily measured the perception of pure tone stimuli in mice (Ohlemiller and Gagnon, 2007; Ohlemiller et al., 2010; Prosen et al., 2003); however tones do not accurately represent the sounds heard in a mouse’s auditory environment. Mice of both sexes and at most ages produce spectrally and temporally complex vocalizations spanning from low to high frequencies (Ehret and Haack, 1982; Lahvis et al., 2011; Portfors, 2007). Low frequency vocalizations by adult animals are associated with aggressive or defensive behaviors (Lahvis et al., 2011; Portfors, 2007). Furthermore, pups emit wriggling calls during mother pup separation. Ultrasonic vocalizations (USVs) are emitted by all adult animals, and are thought to be important for acoustic communication, with possible functions such as mate attraction, courtship, and other social interactions (Grimsley et al., 2011; Portfors, 2007; reviewed by Willott, 2001). Male and female mice emit a diverse repertoire of USVs with many different call types, or categories (Ehret, 1992; Ehret and Haack, 1982; Portfors, 2007).
The production of USVs by male mice has been extensively studied. Males generate calling bouts that are composed of several types of USVs arranged in non-random fashion (Chabout et al., 2015; Holy and Guo, 2005; Portfors, 2007). A bout is an organized set of USVs that ranges from 0.5 to 30 seconds in duration (review by Nyby, 2001). Bouts are thought to be important for mating. Previous research showed that male mice will produce bouts during social interactions with females and males, as well as to female urine, and to anesthetized females and males (Chabout et al., 2015; Hammerschmidt et al., 2009). Chabout et al. (2015) examined how male mice modify their USVs based on social context, finding that they will alter the syllable type, loudness of bouts, and frequency of syllables based on the presence or absence of a female or even just female urine. Therefore, it can be concluded that male mice will adjust the use of their USVs under different situations, suggesting their importance for communication.
USVs vary based on a variety of spectrotemporal parameters, including frequency, amplitude, and duration. Previous researchers have used statistical analyses of spectrograms in order to separate USVs into several categories (Grimsley et al., 2011; Grimsley et al., 2012; Portfors, 2007). However, it is important to note that the number of categories defined in those studies varies widely, and those categories are not defined by the mouse, but rather by the researchers. Holmstrom et al. (2010) examined whether different USV categories and manipulations of the USV signal elicited different neuronal responses. Inferior colliculus (IC) neurons showed a distinct response for separate USV categories, as well as for altered USVs. Furthermore, Roberts and Portfors (2014) proposed that IC neurons use frequency distortion products to encode the ultrasonic vocalizations. These findings suggest that mice have a distinct neural representation of USVs, possibly not even for the actual frequencies of the vocalizations. Neilans et al. (2014) showed that the CBA/CaJ mouse strain’s behavioral ability to discriminate between various vocalizations is based on the spectrotemporal similarity of the calls, where increased similarity is associated with a decrease in discriminability. Nonetheless, little is known about how mice detect their own USVs, with the effect of aging on the perception of USVs being a particularly understudied phenomenon.
While it is clear that hearing loss in the CBA/CaJ strain resembles human ARHL using pure tones, little is known about how this strain perceives their own vocalizations and even less is known about how aging effects that perception. For the present study, our aim was to establish auditory threshold sensitivity for several USV types, as well as to track these thresholds across the mouse lifespan. The stimuli we used were USVs from six categories outlined in Portfors (2007) and Holmstrom et al. (2010), which included down sweep, up sweep, 30 kHz harmonic, chevron, complex, and male sweep calls, as well as a 42 kHz pure tone. We hypothesized that thresholds would differ across USVs due to differences in spectrotemporal complexity, with the detection of USVs extending into a later age range than pure tones. Further, we expected to see sex differences in USV thresholds, with males having higher thresholds than females, especially at the oldest ages.
2. Methods
2.1 Subjects
The animals used in this experiment were twenty two adult CBA/CaJ strain mice. Eight subjects (4 males and 4 females) were only used in this experiment. The rest of the subjects (7 males and 7 females) entered the experiment after participating in pure tone behavioral discrimination studies in our laboratory. Training began when the mice were approximately 2–3 months old and the experiments lasted up to 33 months of age (see results). The original breeding pairs were acquired from The Jackson Laboratory. Our subjects were bred at the University at Buffalo, SUNY and all procedures were approved by the University at Buffalo, SUNY’s Institutional Animal Care and Use Committee. All mice were housed separately and kept on a reverse day/night cycle (lights off at 6 am and lights on at 6 pm). Accordingly, the mice were tested during the dark portion of their cycle, 1 hour at a time, between 8:00 am and 4:00 pm. All animals were trained and tested at different hours of the dark portion to establish the hour of best productivity for each individual. Once the schedule was established, mice maintained it throughout their lifetime. They were water restricted and kept at approximately 85% of their free-drinking weights during the course of the experiment. The animals had unrestricted access to food, except while they were participating in the experiments.
2.2. Apparatus
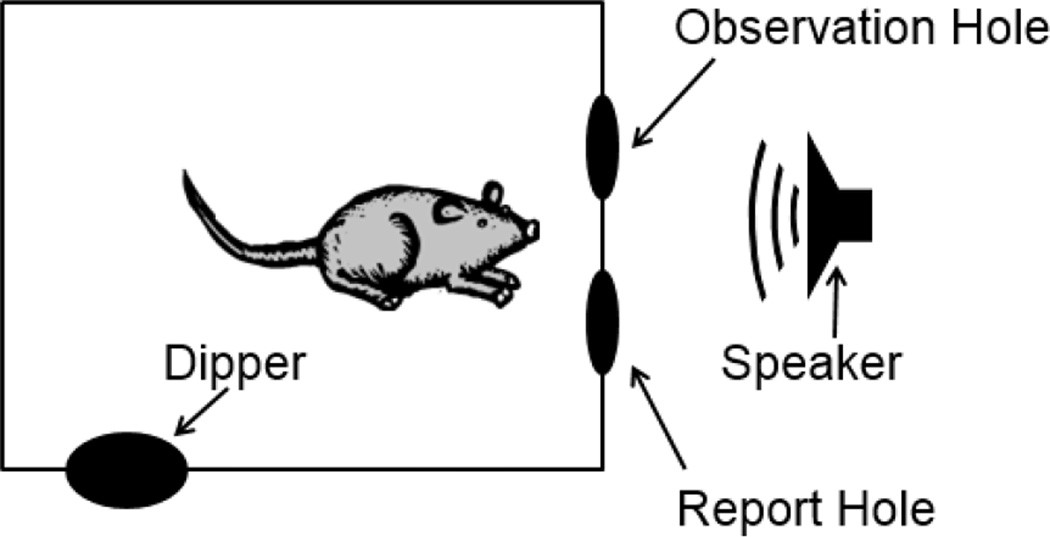
The mice were tested in a wire cage (28 × 56 × 30.5 cm in one of the two setups, and 23 × 39 × 15.5 cm in the other, see Figure 1) placed in a sound-attenuated chamber (53.5 × 54.5 × 57 cm) lined with 4-cm thick Sonex sound attenuating foam (Illbruck Inc., Minneapolis, MN). The chamber was illuminated at all times by a small lamp with an 8-W white light bulb and the behavior of the animals during test sessions was monitored by an overhead web camera (Logitech QuickCam Pro, Model 4000). The test cage consisted of an electrostatic speaker (Tucker-Davis Technologies (TDT), Gainesville, FL, Model ES1), a response dipper (Med Associated Model ENV-302M–UP), and two nose poke holes surrounded by infrared sensors (Med Associates Model ENV-254).
Figure 1.
Schematic of the experimental setup used in this study. Mice began a trial by nose poking to the observation hole. When they detected a stimulus presented from the speaker, they poked to the report hole. If correct, the mice received Ensure® from the dipper.
The experiments were controlled by Dell Optiplex 580 computers operating TDT modules and software. Stimuli were sent through an RP2 signal processor, an SA1 power amplifier, a PA5 programmable attenuator, an ED1 electrostatic speaker driver, and finally to the speaker. Inputs to and outputs from the testing cages were controlled via RP2 and RX6 processors. Power supplies were used to drive the dippers (Elenco Precision, Wheeling, IL, Model XP-603) and infrared sensors (Elenco Precision, Model XP-650). Custom Matlab and TDT RPvds software programs were used to control the hardware.
2.3 Stimuli
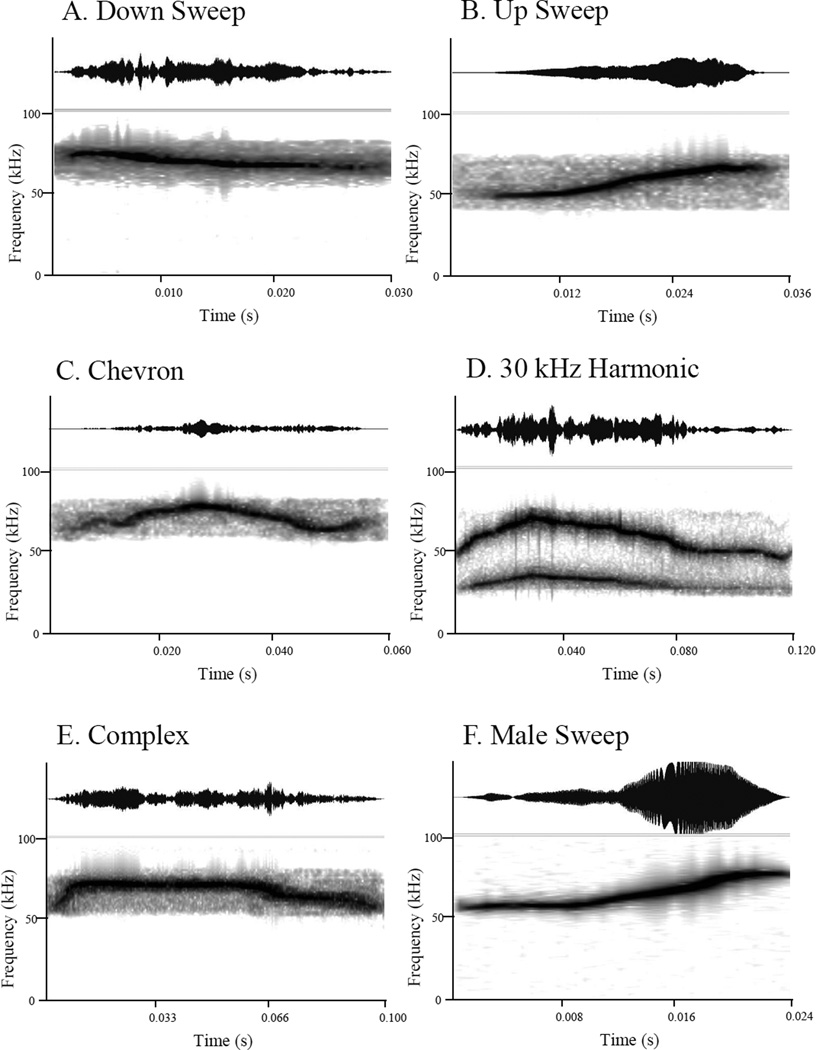
The stimuli consisted of six USVs (see Figure 2) and a 42 kHz pure tone (0.1 s in duration with 0.01 s rise/fall cosine ramps). The down sweep, up sweep, 30 kHz harmonic, complex, and male sweep calls were obtained from Christine Portfors (Washington State University). These USVs were male vocalizations recorded during social adult mouse interactions (see Portfors, 2007), and were previously used in studies by Holmstrom et al. (2010), as well as Neilans et al. (2014). Only ultrasonic vocalizations were used in this study for comparison to the wealth of data on acoustic communication in mice. The chevron ultrasonic vocalization was recorded in our laboratory from an adult male CBA/CaJ mouse (not a subject in this experiment) responding to female urine in dirty bedding, using a condenser microphone (UltraSoundGate CM16/CMPA, flat frequency response (±6 dB) between 25 and 140 kHz) attached to a laptop computer (HP Pavilion dv5) using an Avisoft recorder (UltraSoundGate 416H) at a 300 kHz sampling rate with a 16-bit format. All USV stimuli were typical exemplars of categories specified in Portfors (2007). Stimuli were edited using a band-pass filter (25 to 90 kHz) Adobe Audition (v. 1.5) to eliminate noise. The 42 kHz pure tone was generated in Adobe Audition. The 42 kHz tone was used as a control condition to establish mouse ultrasonic hearing threshold for pure tones across the lifespan and provided a valuable point of reference for prior pure tone hearing mouse studies (Henry, 2004; Ohlemiller et al., 2010; Radziwon et al., 2009). Sound pressure levels for USVs and the 42 kHz tone were calibrated using peak to peak measurements. An ultrasound recording system (Avisoft, Model USG 116–200) and a custom Matlab script, with the microphone (Avisoft Bioacoustics Ultra Sound Gate CM116) were placed at the approximate location where the animal’s head would be during testing. Although the ES1 TDT speaker has a high frequency roll off above 85 kHz, our stimuli were below that range. Calibrations were conducted weekly, and did not vary by more than 5 dB throughout the course of the experiments. The USVs had diverse spectrotemporal characteristics. The down sweep had a frequency range of 69 to 81 kHz and a duration of 0.029 s (Figure 2A). The up sweep call ranged from 52 to 74 kHz and had a duration of 0.028 s (Figure 2B). The chevron call ranged from 66 to 84 kHz and was 0.056 s in duration (Figure 2C). The 30 kHz harmonic call’s frequency ranged from 30 to 75 kHz and the call was 0.120 s in duration (Figure 2D). The complex call ranged from 62 to 78 kHz and was 0.095 s in duration (Figure 2E). The male sweep had a frequency range of 57 to 80 kHz and duration of 0.024 s (Figure 2F). All stimuli ranged from 0 to 50 dB in intensity within the call.
Figure 2.
Time waveforms and spectrograms of the six USV stimuli used in this study.
2.4 Procedure
Mice were trained using a go/no-go operant conditioning procedure on a detection task. They were trained by shaping their behavior to poke to the left hole twice to initiate the trial, wait for the stimulus, and then poke to the right hole once when the stimulus was detected. Training began when the animals were at least 2–3 months of age. The first stage in the training process was to shape the mice to nose poke to the observation hole and then approach the dipper for the chocolate Ensure® reinforcement. Ensure® is a type of nutritional supplement with a consistency like chocolate milk, and it was used in an attempt to maximize the number of trials from the mice. The animals were then trained to repeatedly poke to the observation hole until they heard a stimulus (either a USV or a pure tone), after which they would nose poke to the report hole for the reinforcement. Next, catch trials were phased into the training and the waiting interval was systematically increased. Animals that entered the experiment after completing other studies easily transferred from the discrimination to the detection task type.
The mice were tested in two 30-minute sessions or one 60-minute session, 5 to 6 days per week. The mice typically ran between 100 and 400 trials per session. During testing, the mouse began a trial by nose poking through an observation nose-poke hole, which initiated a variable waiting interval ranging from 1 to 4 s. In the “go” condition, one of the stimuli was played at one intensity. If the mouse detected the stimulus, it was required to nose poke through the report nose-poke hole within 2 s of the onset of the target. In this trial type, a “hit” was recorded if the mouse correctly responded within the response window and the animal received 0.01 ml of Ensure® as reinforcement. A “miss” was recorded if the mouse failed to nose poke through the report nose-poke hole during the waiting interval, the trial was aborted, and the mouse received a 3 to 5 s timeout, during which no trial could be initiated. Sessions with hit rates of at least 80% were included in data analysis. Animals obtained a hit rate of less than 80% approximately 1% of the time, thus very few sessions were excluded for this reason.
Approximately 30% of all trials were “no go”, or sham trials. No stimulus was played during the sham trials. These trials were required to measure the false alarm rate and calculate the animal’s response bias. If the subject nose poked to the report hole during a catch trial, a “false alarm” was recorded and the mouse was punished with a 3 to 5 s timeout interval. However, if the mouse continued to nose poke to the observation hole, a “correct rejection” was recorded and the next trial would begin immediately. In either case, no reinforcement was given. Chance performance was represented by the animal’s false alarm rate. Testing sessions with false alarm rates greater than 20% were excluded from final data analysis, and approximately 1% of all sessions were thrown out for this reason.
Once the animals finished training, they were tested on the seven stimulus conditions (Figure 2) in a randomized order. A different random order was used for each subject. If the mouse completed testing on all seven stimuli, a new random order was generated and they were tested on the stimuli again. Only four animals that participated in this study were able to go through three to four separate random orders, completing between 21 to 28 conditions each. Three other animals were able to complete two repetitions of random orders, 14 conditions each. The rest of the participants completed a variable number of conditions, between 1 and 13 each.
Only one stimulus type was presented per session, at varying sound levels. Initially, all of the stimuli were presented to the subjects in randomized blocks of 10 (7 targets and 3 shams per block), at intensities ranging between 50 and 65 dB SPL. Once performance stabilized, two out of the seven “go” targets were attenuated further in steps of 5 dB, until performance consistently dropped below 50% for one or both of those quieter stimuli. Then 200 more trials were completed and thresholds were calculated from those final 200 trials. Once a threshold was calculated for that stimulus, subjects were moved on to the next stimulus type. Since thresholds were calculated for each condition after this extensive training and testing, we did not find differences between the mice that had previously completed other experiments and the mice that only participated in this experiment.
2.5 Data analysis
Signal detection analysis was performed to factor out the animals’ motivational biases since bias is independent of sensitivity (Steckler, 2001). Mean hit and false alarm rates were used to calculate thresholds using signal detection theory with a threshold criterion of d’= 1.5. Although any threshold criterion is chosen arbitrarily, we set our threshold criterion at 1.5 for two main reasons. For one, this d’ value is comparable to the values used by other rodent researchers (e.g., Klink et al., 2006; Wagner et al., 2003; Radziwon et al., 2009). Also, a d’ of 1.5 is a relatively conservative criterion corresponding to low false alarm rates, which ensures that the animal is responding primarily to the target stimuli and not randomly (Klump and Maier, 1989). Once all thresholds were collected, the data were sorted by stimulus type, yielding seven individual stimulus categories (down sweep, up sweep, chevron, 30 kHz harmonic, complex, male sweep, and 42 kHz tone), and all calls together. All data were further sorted by sex (male and female) for every category. Given the longitudinal design of the current study, we were faced with a data set that contained an unequal number of observations from every animal. Further, some animals contributed multiple points to the same stimulus type, while other animals contributed either one or none. In other words, every stimulus data set for one sex had a variable number of observations, variable subject participation, and variable ages for each threshold data point. Thus, we used a linear regression analysis strategy. A linear regression is an appropriate analysis when the goal of research is to assess the extent of a relationship between two variables. A simple linear regression analysis was performed to determine how age could predict hearing thresholds for different stimulus categories for males and females. F tests were used to assess the significance levels for every regression line. We then used a regression coefficient (r2) measure to determine the amount of variance that aging accounted for in our thresholds.
In order to examine if there were sex differences between males and females for every stimulus type, we tested the difference between two independent correlation coefficients (r) (Preacher, 2002). This comparison operates by standardizing correlation coefficients into z-scores, and allowed us to examine whether two regression lines are different at p < 0. 05. This data analysis was also employed to determine if there were significant within sex differences for stimulus detection. Furthermore, we were interested in examining predicted thresholds at certain ages for all USVs, individual USVs, as well as for a 42 kHz pure tone in order to visualize how hearing changes for these stimuli, and how hearing loss compares to previous studies. We used the trend analysis function in Microsoft Excel (2014) to calculate the predicted thresholds at 200, 400, 600 and 800 days of age for males and females. Such descriptive analyses allowed easy visualization of sex differences across aging.
Finally, in order to assess whether threshold change across lifetime could be explained by different spectrotemporal properties of the different USVs, we performed correlational analyses for variability in our thresholds as predicted by bandwidth, duration, and peak frequency. A trend analysis was performed for every stimulus type at 200 and 800 days of age for males and females. A simple linear regression and regression coefficient (r2) calculations were performed in order to determine the amount of variance that bandwidth, duration and peak frequency accounted for in thresholds.
3. Results
We found that that all mice were able to detect all vocalizations and 42 kHz tones well into old age (Figure 3 A–F; Figure 4 A–B). Thresholds ranged from 4 to 42 dB across calls for females between the ages of 214 to 942 days old, and from 7 to 48 dB across calls between the ages of 262 and 929 days old for males. 42 kHz tone thresholds varied from 13 to 34 dB for females between the ages of 374 and 998 days old, and from 12 to 42 dB for males between the ages of 176 days old and 996 days old.
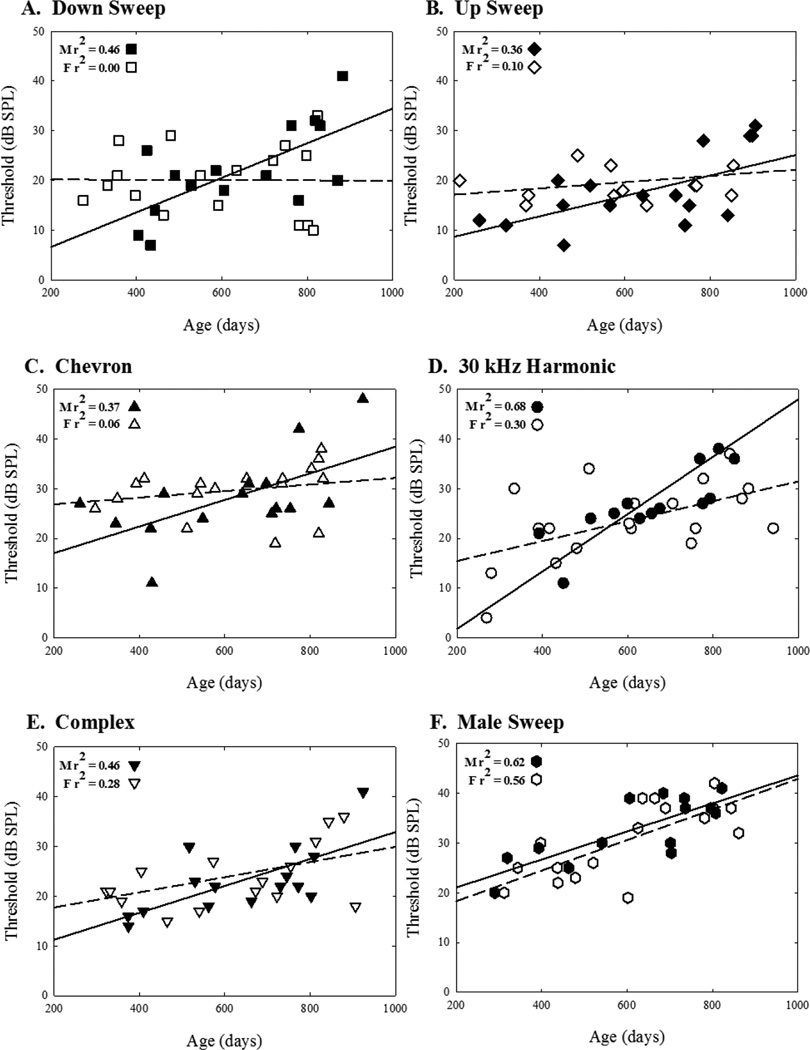
Figure 3.
Regression plots for individual stimuli (A–F). Each plot depicts thresholds from multiple mice across lifetime (males = solid symbols, females = open symbols). Lines represent the best data fit of hearing loss across age (males = solid lines, females = dashed lines). The amount of variability explained by aging is expressed in the form of r2 for males and females separately.
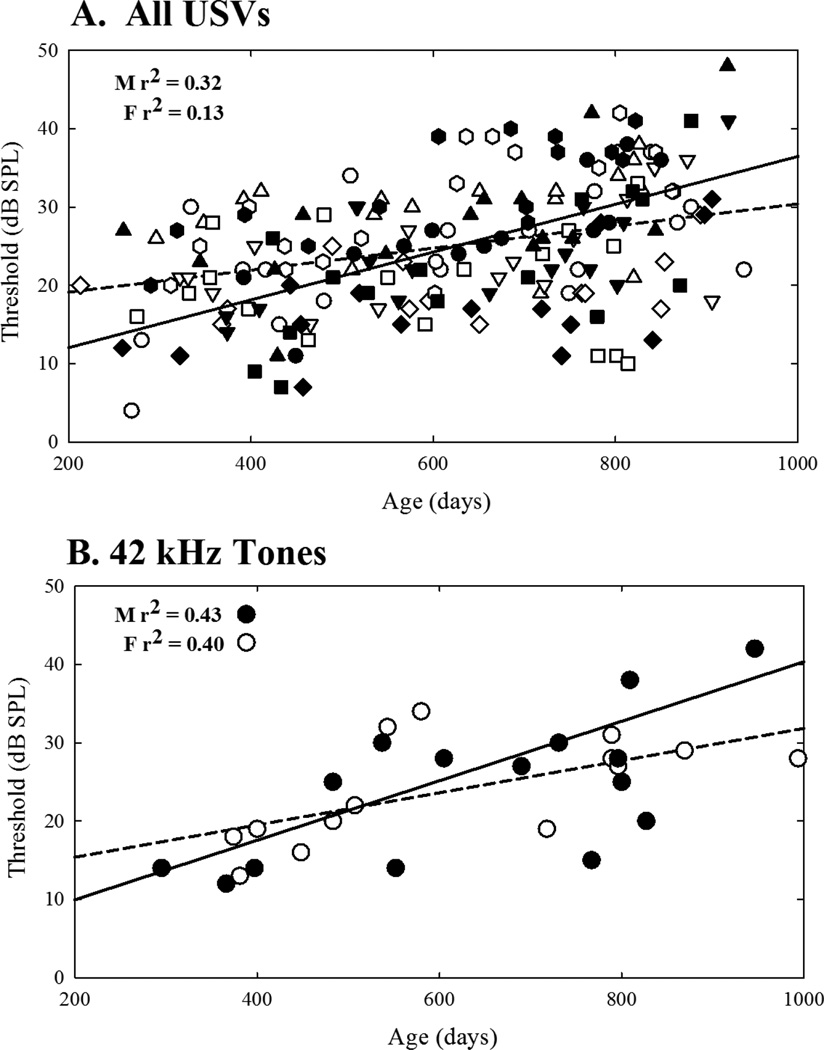
Figure 4.
Regression plots across all ultrasonic vocalizations (A) and the 42 kHz tones (B). Each plot depicts thresholds from multiple mice across their lifetimes. Lines represent the best data fit of hearing loss across age (males = solid lines, females = dashed lines). The amount of variability in data explained by aging is expressed in the form of r2 for males and females separately.
Simple linear regression analyses revealed that, in general, age is a predictor for an increase in thresholds for 42 kHz tones, each USV individually, and all USVs combined, for males (Table 1). Female results revealed a slightly different pattern. For females, aging could predict changes in thresholds across 42 kHz tones, as well as the 30 kHz harmonic, the male sweep USV, and for all USVs combined. The linear regression was not significant for down sweep, upsweep, chevron, and complex USV detection (Table 1). Aging explained between 32 and 62% of variability in thresholds across stimuli for males as measured by the regression coefficients. Only 0 to 52% of the variability across thresholds could be explained by aging for females.
Table 1.
Regression analysis and significance testing for all stimulus types between male and female mice.
| Stimulus | Male | Female | M vs. F | ||
|---|---|---|---|---|---|
| r2 | F test | r2 | F test | ||
| Down Sweep | 0.46 |
F(1,13)=11.00 p<0.01 |
0.00 |
F(1,15)=0.00 n.s. |
p<0.05 |
| Up Sweep | 0.36 |
F(1,13)=7.29 p<0.05 |
0.10 |
F(1,11)=1.26 n.s. |
n.s. |
| 30 kHz Harmonic | 0.36 |
F(1,13)=7.29 p<0.05 |
0.30 |
F(1,17)=7.19 p<0.02 |
n.s. |
| Chevron | 0.37 |
F(1,13)=7.67 p<0.05 |
0.06 |
F(1,15)=0.92 n.s. |
n.s. |
| Complex | 0.46 |
F(1,13)=11.17 p<0.01 |
0.22 |
F(1,14)=3.93 n.s. |
n.s. |
| Male Sweep | 0.62 |
F(1,12)=19.80 p<0.001 |
0.52 |
F(1,16)=17.00 p<0.001 |
n.s. |
| Mean of All Calls | 0.32 |
F(1,86)=41.10 p<0.001 |
0.13 |
F(1,98)=14.35 p<0.001 |
p<0.05 |
| 42 kHz Tone | 0.39 |
F(1,16)=10.35 p<0.01 |
0.40 |
F(1,17)=7.19 p<0.02 |
n.s. |
The sex differences analysis of the correlation coefficients showed that aging influenced stimulus detection differently for males and females (p < 0.05). Aging influenced down sweep call detection differently for males and females (p < 0.05). The up sweep, 30 kHz harmonic, chevron, complex, and male sweep calls, as well as the 42 kHz tone, and all calls were not significantly different between males and females (p > 0.05). Further data analysis revealed that regression lines did not differ across stimulus types for males (p > 0.05). Females showed different aging patterns for detection of the down sweep call and 30 kHz harmonic, the down sweep call and the 42 kHz tone, as well as between the chevron and male sweep calls (all p < 0.05). It is worthy to note that the r comparison is a conservative statistical method and the overall pattern of results should be considered. Furthermore, increasing the number of observations could lead to more pronounced differences.
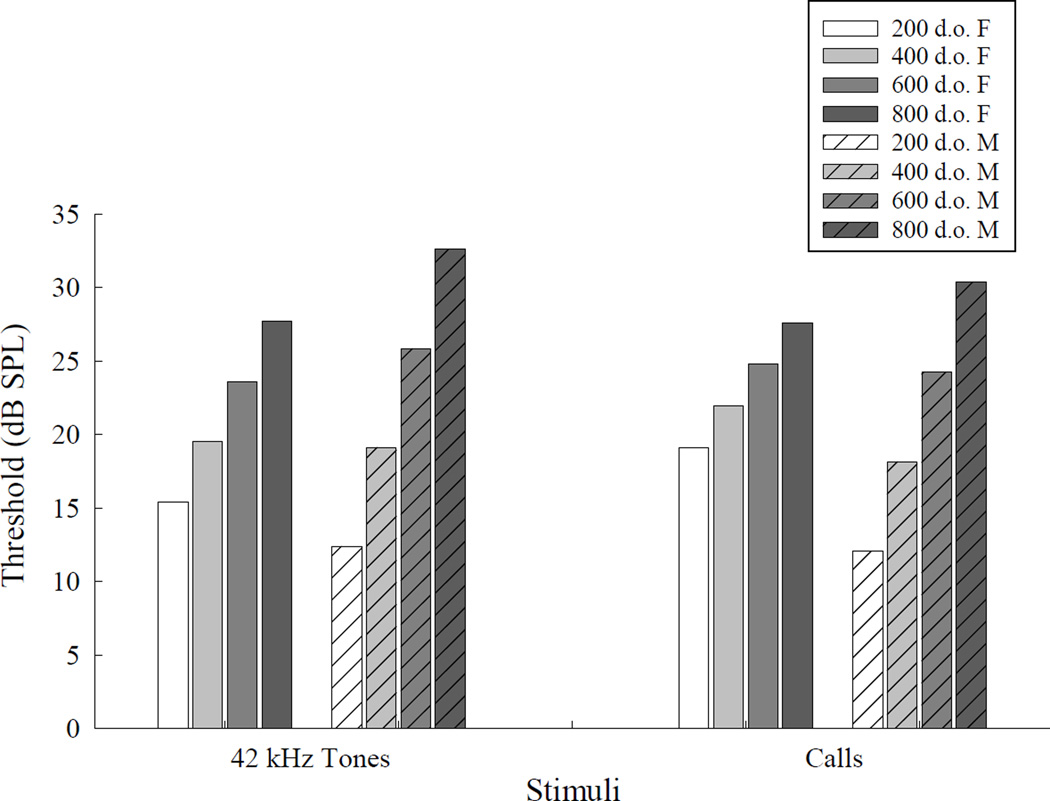
The trend analysis showed that females start out with higher thresholds but lose hearing at a slower rate compared to males for USVs and the 42 kHz tones (Figure 5). Overall this descriptive statistical method revealed that male thresholds for ultrasonic vocalizations increase from 12 to 30 dB over the course of 200 to 800 days of age. The change in 42 kHz tone thresholds resembled the male’s USV threshold pattern, increasing from 12 dB to 33 dB. Females overall exhibited slower hearing loss for ultrasonic vocalizations, showing an increase from 19 to 28 dB over the course from 200 to 800 day of age. Similarly, female hearing loss increased from 15 dB to 28 dB for the 42 kHz tone.
Figure 5.
Predicted hearing thresholds for 42 kHz tones and ultrasonic calls at 200, 400, 600, and 800 days of age for female and male mice.
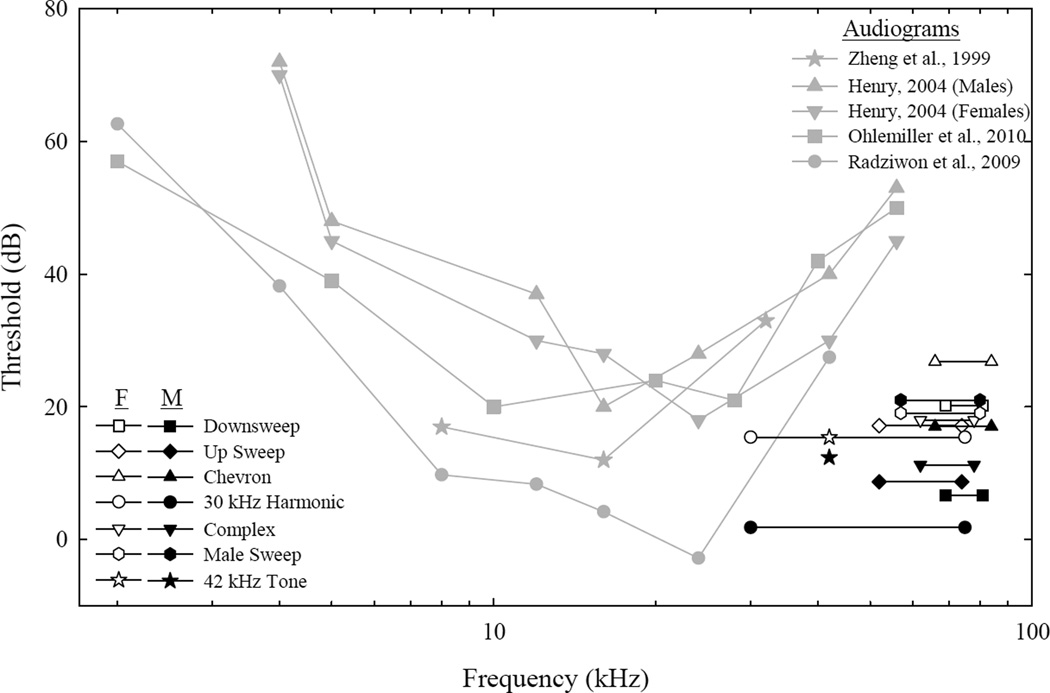
Figure 6 shows 200-day thresholds from males and females on each call type and pure tones, displaying frequency ranges for each of the stimuli. Thresholds from our mice were lower than high frequency pure tone behavioral, ABR, and CAP thresholds from previous studies (Henry, 2004; Ohlemiller et al., 2010; Zheng et al., 1999). Looking at predicted thresholds for individual USVs for 200 to 800 day old male mice revealed that the down sweep increased from 7 to 27 dB, up sweep: 9 to 21 dB, chevron: 17 to 33 dB, 30 kHz harmonic: 2 to 37 dB, complex: 11 to 28 dB, and male sweep 21 to 38 dB. Down sweep thresholds for females did not change across the lifetime. The rest of the female USV thresholds increased as follows: up sweep from 17 to 21 dB, chevron: 27 to 31 dB, 30 kHz harmonic: 15 to 28 dB, complex: 18 to 27 dB, and male sweep: 19 to 36 dB.
Figure 6.
Comparison figure of 200-day old animal detection thresholds in the current study (black lines) to pure tone ABR, CAP, and behavioral audiograms (grey lines and symbols). The thresholds in the present study are expressed as straight lines denoting the frequency band of a stimulus (males = black, females = white). Zheng et al., 1999 (grey star, 329 day old) and Henry, 2004 (grey triangle = males, 350 day old; inverted grey triangle = females, 350 days old) measured pure tone auditory thresholds using ABRs. Ohlemiller et al., 2010 (grey square, 330 to 390 days old) measured auditory thresholds using CAP recordings. Radziwon et al., 2009 (grey circle, 365–500 days old) measured auditory thresholds for pure tones using operant conditioning procedures.
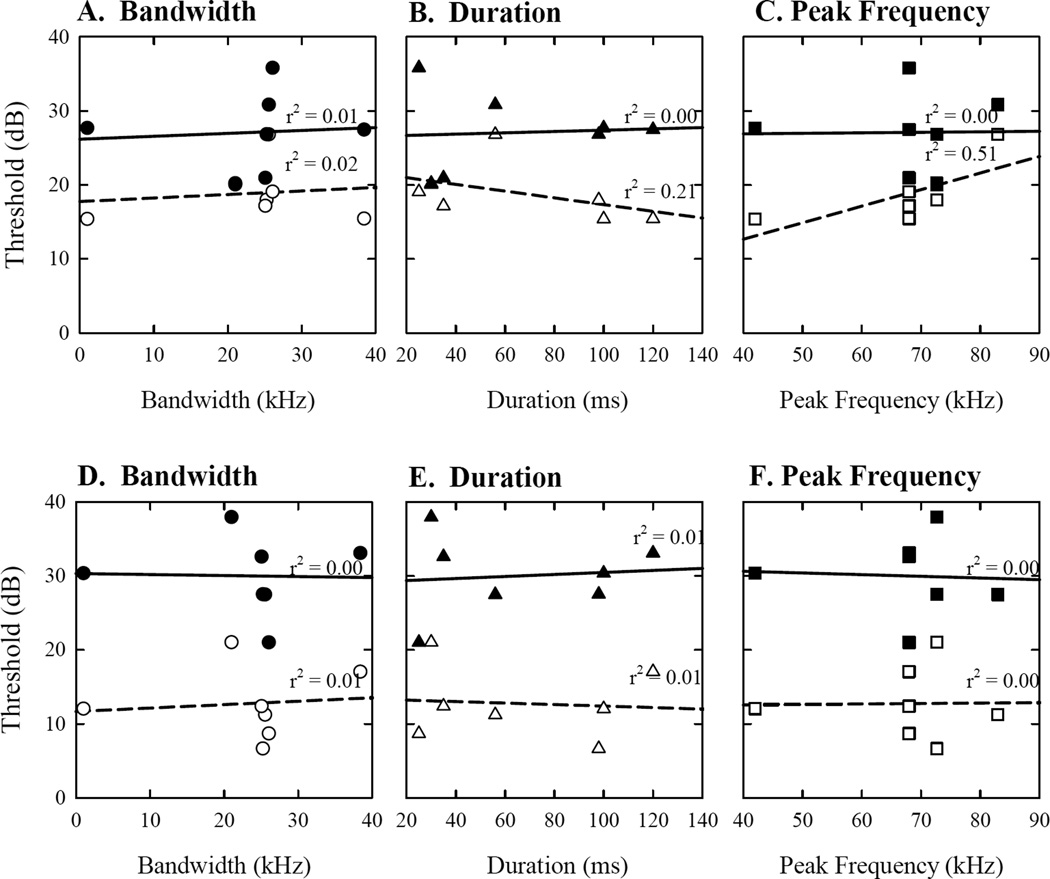
The bandwidth, duration and peak frequency correlation analyses revealed that duration and peak frequency, but not bandwidth, play a role in stimulus detection for 200 day old female mice (Figure 7 A–C), but not for male mice of any age (Figure 7 D–F), or for females at 800 days. The increase in duration was responsible for 21% of variability across thresholds in 200 day old female mice. Peak frequency explained 51% of variability for thresholds in young females.
Figure 7.
Regression plots across all stimuli as predicted by bandwidth (A, D), duration (B, E) and peak frequency (C, F). Each plot depicts individual predicted thresholds for every stimulus type at 200 (white) and 800 (black) days of age for females (A–C) and males (D–F). The lines represent the best data fit for thresholds as predicted by individual spectrotemporal characteristics. The amount of variability in data explained by bandwidth, duration, and peak frequency is expressed in the form of r2 for 200 (dashed line) and 800 (solid line) day old mice separately.
4. Discussion
The goal of the present study was to test the ability of CBA/CaJ mice to detect ultrasonic vocalizations across their life using operant conditioning procedures. This is the first study to our knowledge to show that the CBA/CaJ strain can reliably detect USVs throughout their life. It also showed behavioral age related hearing loss for both USVs and 42 kHz tones. Young animal thresholds for USVs were lower than pure tone thresholds from previous behavioral and physiological studies (Figure 6). Not surprisingly, thresholds differed across the spectrally and temporally diverse stimuli. Changes in thresholds across the lifetime, however, were not associated with bandwidth, duration, or peak frequency of the stimuli (Figure 7). Males lost hearing at a significantly faster rate than females for USVs, but not 42 kHz tones. While we do not yet know if there is functional significance to USVs, our study suggests that these signals could be perceptually relevant throughout a mouse’s life.
The CBA/CaJ mouse strain exhibited behavioral differences in detection thresholds across stimulus types. Most previous auditory studies have examined pure tone stimulus responses using behavioral and physiological measures (e.g., Henry, 2004; Ohlemiller et al., 2010; Radziwon et al., 2009). However, we know that male and female mice utilize the ultrasonic range for acoustic communication by producing signals that vary in bandwidth, duration, peak frequency, and other elements (Ehret, 1992; Ehret and Haack, 1982; Portfors, 2007), and that for communication to be effective, these signals must be easily detected by the mice. Ultrasonic vocalizations have been subdivided into statistical categories in a number of studies by a number of research groups (see Introduction). Several previous studies highlighted the importance of spectrotemporal characteristics in neuronal USV detection (Holmstrom et al., 2010; Roberts and Portfors, 2014), behavioral discrimination of USVs (Neilans et al., 2014), as well as discrimination of whole vs. partial USVs (Holfoth et al., 2014). Thus, it was not surprising that thresholds were different across USV types in our study. It is important to note, in this study and others, that the USV categories that we used were imposed by humans. We do not yet know if those categories are meaningful to mice. Furthermore, the current study employed only one exemplar USV from each category, limiting the generalization of these results across all calls produced by mice. In the future, research on within-category differences in detection and discrimination should be examined.
The CBA/CaJ mouse strain exhibits sex differences in ARHL, mimicking the human sex dimorphism in hearing loss. As previously discussed, human men lose hearing earlier than women (Pearson et al., 1995). In mice, aging affected detection of the down sweep call and overall USVs at significantly different rates for males and females. Male mice had lower thresholds for USVs early in life, but lost hearing for these stimuli at a faster rate than female mice. Females, who started out with higher overall thresholds, exhibited slower hearing loss than males for all USVs, and did not show significant hearing loss for several vocalizations (down sweep, up sweep, chevron, and complex). Such findings could stem from functional differences in USV perception and usage. Males vocalize and alter USV bouts depending on a social (probably sexual) context (Chabout et al., 2015). Male mice have also been shown to modify syllable type, loudness, and frequency based on presence of female urine, as well as whether or not the female is anesthetized or active (Chabout et al., 2015; Hammerschmidt et al., 2009). Female mice, on the other hand, may use USVs for social interactions other than mating, including for female-female communication. In fact, females decrease USV production during sexually receptive states, with more calls being emitted during non-estrous periods (Moles et al., 2007).
Sexual dimorphism in hearing loss cannot be attributed to the bandwidth, duration, or peak frequency of the stimuli (Figure 7). Interestingly, young but not old CBA/CaJ female mice could detect USVs that have low peak frequencies easier than USVs with high peak frequencies. Furthermore, as duration increases, USV detection thresholds decreased for young females. Male mice of any age and old female mice do not appear to benefit from variations in bandwidth, duration, and peak frequency in the USVs. It is likely that the peak frequency and duration of vocalizations communicate some important social and reproductive information to females. In fact, Chabout et al. (2014) showed that male mice produce longer and louder syllables for fresh female urine or awake female mice. Thus, hearing differences across call types for young females could be tied to the functional differences in the use of USVs by the two sexes. Old mice, however, are not reproductively active, and are likely using vocalizations for other social functions.
It appears that females lose hearing for USVs at a slower rate compared to male mice across their lifetimes. Females start out with higher thresholds for USVs than males, but male and female hearing trajectories converge by 1000 days of age. One possible explanation for slower hearing loss in females is hormonal sex differences. Stenberg and colleagues (1999) linked estrogen receptors to the inner ear and auditory pathways in female mice and rats, suggesting that estrogen may have a possible protective effect on hearing (Frisina, 2012). Some evidence suggests that outer hair cells are healthier in middle-aged female mice as compared to males, leading to lower ABR thresholds. This difference disappears in post-menopausal female mice (Guimaraes et al., 2004).
Menopause alone, however, cannot explain the continuous threshold increase and rate of hearing loss in female CBA/CaJ mice, primarily because menopause occurs between 12 and 14 month of age in this strain (Silver, 1995). Other studies link anatomical variability in stria vascularis cell density across several mouse strains to different ARHL rates (Ohlemiller et al., 2010). The low marginal cell density in the CBA/CaJ strain could explain poor pure tone thresholds later in life as measured by CAP recordings. Surprisingly, cell differences in stria vascularis were not linked to the sexually-dimorphic effects of ARHL. Still, female mice exhibited greater loss of cochlear neurons. The authors proposed that hormones, in combination with other sex-related differences, may be responsible for the dimorphism seen in ARHL in the CBA/CaJ mouse strain. Thus, both functional and anatomical changes may be responsible for the sex-dimorphic effects of ARHL in this study.
Surprisingly, behavioral data revealed that male and female CBA/CaJ mice lose hearing at similar rates for the 42 kHz tones. This finding is in conflict with previously established age related sex differences in ABR (Henry, 2004) and CAP thresholds (Ohlemiller et al., 2010). Henry found that high frequency thresholds decrease more rapidly in aging male mice as compared to females. In contrast, Ohlemiller et al. (2010) discovered that females have poorer pure tone thresholds between 15 and 24 months of age using CAP recordings. These conflicting results may be rooted in methodological differences. Radziwon et al. (2009) argued that ABR recordings yield higher overall thresholds than behavioral thresholds across all frequencies, limiting applicability of such data to awake, behaving mice. Furthermore, Dallos and colleagues (1978) reflect that detectable action potential recordings may produce elevated thresholds for high frequency stimuli, leading to general overestimation of thresholds. Methodological limitations in combination with anatomical and hormonal differences between our animals and those from previous studies, make it hard to generalize our results to previous findings.
Lastly, there are some limitations to our findings. While behavioral audiograms contribute to our knowledge of mouse hearing, it is not clear how our results relate to ABR and other physiological measures of pure tone hearing. Particularly, the gap in literature lacks information for dimorphic characteristics of aging mouse hearing in the CBA/CaJ strain. It is difficult to assess whether 42 kHz tone thresholds are representative of USV hearing, and if the comparison is meaningful. In the future, higher frequency behavioral and physiological thresholds need to be established. Further, it is also difficult to assess which spectrotemporal characteristics are responsible for the observed thresholds across animals, particularly at old age. Previous IC recordings by Roberts and Portfors (2014) suggest that mice may be using distortion products, not actual frequencies of the vocalizations, to detect USVs. Thus, the conservation of call detection in old age may not be linked to the high frequencies in those vocalizations but instead on the differences in the frequencies of the vocalizations within each call. This will need to be examined in the future. Arguing against this hypothesis, a recent behavioral study showed that mice are unable to discriminate frequency modulated sweeps when those sweeps occupy the same bandwidth, but would presumably have different distortion products (Screven and Dent, in press). The behavioral relevance of distortion products needs to be examined in greater detail, both in young and old mice, to better understand the mechanics of hearing and hearing loss in mice.
5. Conclusion
In conclusion, the CBA/CaJ mouse strain reliably detects ultrasonic vocalizations and 42 kHz tones using operant conditioning procedures. The mice show similar rates of ARHL for the two stimulus types, but maintain detection well into old age. This strain also exhibits sex differences in ARHL for the vocalizations, making it an excellent animal model for human hearing and hearing loss. The lack of sex differences in detecting the 42 kHz tones in old age using these behavioral measures suggests a future direction for comparing physiological and behavioral studies. Finally, it is important to study complex as well as simple auditory stimuli in order to understand the full range of hearing abilities of any animal.
HIGHLIGHTS.
Call and USV detection were measured in CBA/CaJ mice
Mice maintained signal detection well into old age
Thresholds differed across signal types
Males suffered greater hearing loss for calls than females, but not tones
Acknowledgments
This work was supported by NIDCD grant R01-DC012302 to MLD. We would like to thank numerous undergraduate and graduate assistants in the Dent Lab for their help with data collection. Special thanks to Dr. Chris McNorgan and Whitney Fosco for their help and advice in data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chabout J, Sarkar A, Dunson DB, Jarvis ED. Male mice song syntax depends on social contexts and influences female preferences. Front. Behav. Neurosci. 2015:9. doi: 10.3389/fnbeh.2015.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corso JF. Age and sex differences in pure-tone thresholds: Survey of hearing levels from 18 to 65 years. Arch. Ololaryngol. Head. Neck. Surg. 1963;77:385. doi: 10.1001/archotol.1963.00750010399008. [DOI] [PubMed] [Google Scholar]
- Dallos P, Harris D, Özdamar Ö, Ryan A. Behavioral, compound action potential, and single unit thresholds: Relationship in normal and abnormal ears. J. Acoust. Soc. Am. 1978;64:151–157. doi: 10.1121/1.381980. [DOI] [PubMed] [Google Scholar]
- Ehret G. Categorical perception of mouse-pup ultrasounds in the temporal domain. Anim. Behav. 1992;43:409–416. [Google Scholar]
- Ehret G, Bernecker C. Low-frequency sound communication by mouse pups (Mus musculus): Wriggling calls release maternal behaviour. Anim. Behav. 1986;34:821–830. [Google Scholar]
- Ehret G, Haack B. Ultrasonic recognition in house mice: Key-stimulus configuration and recognition mechanism. J. Comp. Physiol. A. 1982;148:245–251. [Google Scholar]
- Frisina RD. Hormones and hearing: Too much or too little of a good thing can be ototoxic. Semin. Hear. 2012;33:231. [Google Scholar]
- Gates GA, Cooper JC. Incidence of hearing decline in the elderly. Acta Otolaryngol. 1991;111:240–248. doi: 10.3109/00016489109137382. [DOI] [PubMed] [Google Scholar]
- Grimsley JM, Monaghan JJ, Wenstrup JJ. Development of social vocalizations in mice. PLoS ONE. 2011:6. doi: 10.1371/journal.pone.0017460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsley JM, Gadziola MA, Wenstrup JJ. Automated classification of mouse pup isolation syllables: From cluster analysis to an Excel-based “mouse pup syllable classification calculator”. Front. Behav. Neurosci. 2012:6. doi: 10.3389/fnbeh.2012.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimaraes P, Zhu X, Cannon T, Kim S, Frisina RD. Sex differences in distortion product otoacoustic emissions as a function of age in CBA mice. Hear. Res. 2004;192:83–89z. doi: 10.1016/j.heares.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt K, Radyushkin K, Ehrenreich H, Fischer J. Female mice respond to male ultrasonic ‘songs’ with approach behaviour. Biol. Lett. 2009;5:589–592. doi: 10.1098/rsbl.2009.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry KR. Males lose hearing earlier in mouse models of late-onset age-related hearing loss; females lose hearing earlier in mouse models of early-onset hearing loss. Hear. Res. 2004;190:141–148. doi: 10.1016/S0378-5955(03)00401-5. [DOI] [PubMed] [Google Scholar]
- Holfoth DP, Neilans EG, Dent ML. Discrimination of partial from whole ultrasonic vocalizations using a go/no-go task in mice. J. Acoust. Soc. Am. 2014;136:3401–3409. doi: 10.1121/1.4900564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmstrom LA, Eeuwes LB, Roberts PD, Portfors CV. Efficient encoding of vocalizations in the auditory midbrain. J. Neurosci. 2010;30:802–819. doi: 10.1523/JNEUROSCI.1964-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holy TE, Guo Z. Ultrasonic songs of male mice. PLoS Biology. 2005:3. doi: 10.1371/journal.pbio.0030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Tang J. Age-related hearing loss or presbycusis. Eur. Arch. Otorhinolaryngol. 2010;267:1179–1191. doi: 10.1007/s00405-010-1270-7. [DOI] [PubMed] [Google Scholar]
- Klink KB, Bendig G, Klump GM. Operant methods for mouse psychoacoustics. Behav. Res. Methods. 2006;38:1–7. doi: 10.3758/bf03192744. [DOI] [PubMed] [Google Scholar]
- Klump GM, Maier EH. Gap detection in the starling (Sturnus vulgaris) J. Comp. Physiol. A. 1989;164:531–538. [Google Scholar]
- Lahvis GP, Alleva E, Scattoni ML. Translating mouse vocalizations: Prosody and frequency modulation. Genes Brain Behav. 2011;10:4–16. doi: 10.1111/j.1601-183X.2010.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moles A, Costantini F, Garbugino L, Zanettini C, D’Amato FR. Ultrasonic vocalizations emitted during dyadic interactions in female mice: a possible index of sociability? Behav. Brain Res. 2007;182:223–230. doi: 10.1016/j.bbr.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Neilans EG, Holfoth DP, Radziwon KE, Portfors CV, Dent ML. Discrimination of ultrasonic vocalizations by CBA/CaJ mice (Mus musculus) is related to spectrotemporal dissimilarity of vocalizations. PLoS ONE. 2014;9:e85405. doi: 10.1371/journal.pone.0085405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyby JG. Auditory communication among adults. In: Willott JF, editor. Handbook of mouse auditory research: From behavior to molecular biology. Boca Raton, FL: CRC Press; 2001. pp. 3–18. [Google Scholar]
- Ohlemiller KK, Dahl AR, Gagnon PM. Divergent aging characteristics in CBA/J and CBA/CaJ mouse cochleae. J. Assoc. Res. Otolaryngol. 2010;11:605–623. doi: 10.1007/s10162-010-0228-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK, Gagnon PM. Genetic dependence of cochlear cells and structures injured by noise. Hear. Res. 2007;224:34–50. doi: 10.1016/j.heares.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK, Lett JM, Gagnon PM. Cellular correlates of age-related endocochlear potential reduction in a mouse model. Hear. Res. 2006;220:10–26. doi: 10.1016/j.heares.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Pauler M, Schuknecht HF, White JA. Atrophy of the stria vascularis as a cause of sensorineural hearing loss. Laryngoscope. 1988;98:754–759. doi: 10.1288/00005537-198807000-00014. [DOI] [PubMed] [Google Scholar]
- Pearson JD, Morrell CH, Gordon-Salant S, Brant LJ, Metter EJ, Klein LL, Fozard JL. Gender differences in a longitudinal study of age-associated hearing loss. J. Acoust. Soc. Am. 1995;97:1196–1205. doi: 10.1121/1.412231. [DOI] [PubMed] [Google Scholar]
- Portfors CV. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J. Am. Assoc. Lab Anim. Sci. 2007;46:28–34. [PubMed] [Google Scholar]
- Prosen CA, Dore DJ, May BJ. The functional age of hearing loss in a mouse model of presbycusis. I. Behavioral assessments. Hear. Res. 2003;183:44–56. doi: 10.1016/s0378-5955(03)00211-9. [DOI] [PubMed] [Google Scholar]
- Preacher KJ. Calculation for the test of the difference between two independent correlation coefficients [Computer software] 2002 Retrieved from: http://quantpsy.org.
- Radziwon KE, June KM, Stolzberg DJ, Xu-Friedman MA, Salvi RJ, Dent ML. Behaviorally measured audiograms and gap detection thresholds in CBA/CaJ mice. J. Com. Psysiol. A. 2009;195:961–969. doi: 10.1007/s00359-009-0472-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts PD, Portfors CV. Responses to social vocalizations in the dorsal cochlear nucleus of mice. Front. Syst. Neurosci. 2015:9. doi: 10.3389/fnsys.2015.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönnberg J, Rudner M, Foo C, Lunner T. Cognition counts: A working memory system for ease of language understanding (ELU) Int. J. Audiol. 2008;47:99–105. doi: 10.1080/14992020802301167. [DOI] [PubMed] [Google Scholar]
- Screven LA, Dent ML. Discrimination of frequency modulated sweeps by mice. J. Acoust. Soc. Am. doi: 10.1121/1.4962223. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver LM. Mouse genetics: Concepts and applications. New York: Oxford University Press; 1995. [Google Scholar]
- Stenberg AE, Wang H, Sahlin L, Hultcrantz M. Mapping of estrogen receptors α and β in the inner ear of mouse and rat. Hear. Res. 1999;136:29–34. doi: 10.1016/s0378-5955(99)00098-2. [DOI] [PubMed] [Google Scholar]
- Steckler T. Using signal detection methods for analysis of operant performance in mice. Behav. Brain Res. 2001;125:237–248. doi: 10.1016/s0166-4328(01)00305-9. [DOI] [PubMed] [Google Scholar]
- Tambs K, Hoffman HJ, Borchgrevink HM, Holmen J, Samuelsen SO. Hearing loss induced by noise, ear infections, and head injuries: Results from the Nord-Trøndelag hearing loss study. Int. J. Audiol. 2003;42:89–105. doi: 10.3109/14992020309078340. [DOI] [PubMed] [Google Scholar]
- Van Rooij JC, Plomp R. Auditive and cognitive factors in speech perception by elderly listeners. J. Acoust. Soc. Am. 1990;87:25–26. doi: 10.1121/1.399981. [DOI] [PubMed] [Google Scholar]
- Wagner E, Klump GM, Hamann I. Gap detection in Mongolian gerbils (Meriones unguiculatus) Hear. Res. 2003;176:11–16. doi: 10.1016/s0378-5955(02)00643-3. [DOI] [PubMed] [Google Scholar]
- Willott JF. Handbook of mouse auditory research: From behavior to molecular biology. Boca Rato, FL: CRC Press; 2001. pp. 3–18. [Google Scholar]
- Yamasoba T, Lin FR, Someya S, Kashio A, Sakamoto T, Kondo K. Current concepts in age-related hearing loss: Epidemiology and mechanistic pathways. Hear. Res. 2013;303:30–38. doi: 10.1016/j.heares.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng QY, Ding D, Yu H, Salvi RJ, Johnson KR. A locus on distal chromosome 10 (ahl4) affecting age-related hearing loss in A/J mice. Neurobiol. Aging. 2009;30:1693–1705. doi: 10.1016/j.neurobiolaging.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear. Res. 1999;130:94–107. doi: 10.1016/s0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]