Abstract
Protective postural responses, including stepping, to recover equilibrium are critical for fall prevention and are impaired in people with Parkinson’s disease (PD) with freezing of gait. Improving protective postural responses through training may reduce falls in this population. However, motor learning, the basis of neurorehabilitation, is also impaired in people with PD and, in particular, people with PD who experience freezing. It is unknown whether people with PD who freeze can improve protective postural responses, and whether these improvements are similar to nonfreezers. Our goal was to assess whether people with freezing can improve protective postural responses and retain these improvements similarly to nonfreezers. Twenty eight people with PD (13 freezers, 15 nonfreezers) were enrolled. Improvement in protective postural responses was assessed over the course of 25 forward and 25 backward support surface translations (delivered in pseudo-random order). Postural responses were re-assessed 24 hours later to determine whether improvements were retained. People who freeze did not improve or retain improvement in protective postural responses as well as nonfreezers in our primary outcome variable, center of mass displacement to perturbations (post-hoc across group assessments: freezers- p=0.14 and nonfreezers- p=0.001, respectively). However, other protective stepping outcomes, including margin of stability, step length, and step time, improved similarly across groups. Significant improvements were retained in both groups. In conclusion, people with PD who freeze exhibited reduced ability to improve protective postural responses in some, but not all, outcome variables. Additional training may be necessary to improve protective postural responses in people with PD who freeze.
Keywords: Posture, stepping, freezing of gait, motor learning, Parkinson’s disease
INTRODUCTION
Protective postural responses after perturbations, such as a slip or a trip, may prevent falls (Bloem, et al. 2001; Mansfield, et al. 2015). However, protective postural responses, including protective stepping, are impaired in people with PD (de Kam, et al. 2014; Smulders, et al. 2014). In particular, people with PD who experience freezing of gait (FoG+), often exhibit small (Smulders, et al. 2014), delayed, and multiple (Jacobs, et al. 2009) protective steps compared to those who do not experience freezing (FoG−). Although the mechanism underlying altered protective postural responses in FoG+ is not fully understood, it may relate to an inability to couple postural control and stepping (Jacobs and Horak 2007). Further, midbrain regions play an important role in the storage and release of protective postural responses including protective steps (Honeycutt, et al. 2009; Nonnekes, et al. 2015). This region of the brain is more affected in FoG+ than FoG− (Fling, et al. 2013; Snijders, et al. 2011), possibly contributing to the more severely affected postural responses in this population.
Given the importance of protective postural responses for fall prevention, improving these responses through training could reduce falls. However, learning [defined as the relatively permanent change in performance through practice (Schmidt and Lee 1999)], may be less pronounced in FoG+ than FoG− (Mohammadi, et al. 2015; Smulders, et al. 2014; Vandenbossche, et al. 2013). For example, upper extremity implicit motor learning was stunted in FoG+ compared to FoG− (Vandenbossche, et al. 2013), possibly due to reduced cognitive capacity in FoG+. During splitbelt treadmill training, adaptation and re-adaptation of step asymmetry was slower in FoG+ than FoG− (Mohammadi, et al. 2015). Finally, Smulders and colleagues measured adaptation of protective steps over 8 consecutive support surface translations in FoG+ and FoG−. Protective step performance was improved in FoG− but not in FoG+ (Smulders, et al. 2014); however, authors did not directly compare FoG+ and FoG− performance to determine across group differences. Furthermore, retention of improvements were not assessed in either group.
Understanding whether FoG+ and FoG− improve protective postural responses similarly will inform fall prevention rehabilitation for people with PD. However, despite the importance of protective postural responses and the possible dysfunction of motor learning in FoG+, no studies have directly tested whether FoG+ can improve protective postural responses similarly to FoG−.
The purpose of this study was to identify whether FoG+ improve protective postural responses in response to external perturbations similarly to FoG−. We also determined whether improvements were retained over 24 hours. Given previous results demonstrating reduced learning in FoG+, we hypothesized that FoG+ would exhibit less pronounced improvement in protective postural responses than FoG−.
METHODS
Participants
Twenty eight participants were enrolled. Inclusion criteria were: diagnosis of idiopathic PD, ability to stand without aid for one hour, and currently taking levodopa. Exclusion criteria were: neurological diagnoses other than PD and orthopedic injuries that interfered with gait or balance. Of these participants, 13 were identified as FoG+ based on a score >0 on the New Freezing of Gait Questionnaire (NFoGQ) (Nieuwboer, et al. 2009). Data from the FoG− cohort is currently under review in a manuscript comparing improvement in posture responses in FoG− and healthy adults. FoG+ and FoG− were of similar age, and exhibited similar performance on the Montreal Cognitive Assessment (MoCA), Mini Balance Evaluation Systems Test (MiniBESTest) (Franchignoni, et al. 2010), Unified Parkinson’s Disease Rating Scale (UPDRS). FoG+ and FoG− groups also had similar levodopa dosages (Table 1). Disease duration was significantly longer in FoG+. We also assessed fatigue throughout the course of training via a 10-point scale (1=no fatigue, 10=the most fatigued you’ve ever felt). Average fatigue levels and the change in fatigue from the start of training to the end of training were similar across groups (Table 1). Given the effect of sleep on consolidation of learning (Terpening, et al. 2013), we recorded the number of hours of sleep after training but prior to retention testing. Hours of sleep were similar across groups. Participants provided informed consent, and the research protocol was approved by the OHSU IRB.
Table 1.
Participant characteristics
| FoG− | FoG+ | p-value | |
|---|---|---|---|
| N (female) | 15(4) | 12(5) | 0.43α |
| Age | 66.35(6.02) | 65.4(7.99) | 0.73 |
| UPDRS-III | 24.14(11.78) | 28.88(15.34) | 0.38 |
| NFoG-Q | 0 | 4.4(6.03) | -- |
| Disease Duration | 6.38(4.75) | 11.32(6.76) | 0.04 |
| MiniBEST | 23.20(3.76) | 22.08(3.55) | 0.44 |
| MoCA | 27.47(3.04) | 25.67(3.37) | 0.16 |
| Levodopa | 598.67(228.41) | 545.45(283.24) | 0.6 |
| Fatigue (absolute)‡ | 2.41(1.38) | 2.47(1.59) | 0.92 |
| Fatigue (change)# | 1.21(0.99) | 0.64(1.03) | 0.19 |
| Sleep (hours)** | 6.57(1.53) | 6.44(1.32) | 0.82 |
Fisher’s exact test. All other comparisons are independent sample t-tests.
Fatigue measured on a 1–10 scale;
Change in fatigue over the course of training;
Hours of sleep after the training session.
FoG−: People with PD not experiencing freezing of gait; FoG+: People with PD who experience freezing of gait; MoCA: Montreal Cognitive Assessment; MiniBEST: Mini Balance Evaluation Systems Test; UPDRS-III: Part III (motor score) of the Unified Parkinson’s disease rating scale; NFoGQ: New Freezing of Gait Questionnaire
Protocol
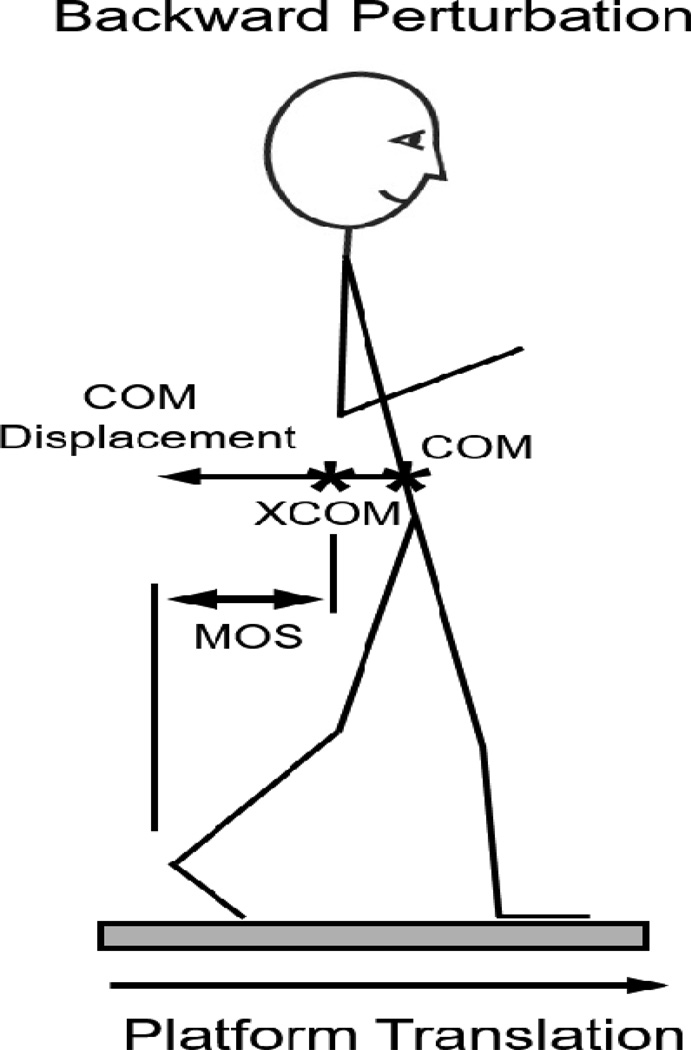
Support surface perturbations were delivered by a custom-built hydraulic platform with 2 force-plates in 4 directions (forward, backward, leftward, rightward) at various speeds (see below). For the purposes of this study, we define postural perturbations by the direction of body center of mass (COM) displacement in response to the perturbations. For example, a “backward” perturbation refers to forward support surface translation with backward displacement of the body COM (Figure 1). Prior to each perturbation, participants stood with their feet together, arms crossed, and eyes open. Participants were instructed to “not anticipate upcoming perturbations and to react naturally to the perturbation when trying to keep balance”. Open-ended instructions were given to avoid altering participants’ natural protective stepping response. A ceiling-mounted harness was worn to protect against falls but did not provide body weight support. Participants were notified that a perturbation would occur, but timing of onset was randomized between 2 and 10 seconds.
Figure 1.
Illustration of primary and secondary outcomes. Backward Perturbation refers to a forward translation of the support surface, with backward displacement of the center of mas (COM). The margin of stability (MOS) represents the distance between the base of support (heel) and the extrapolated CoM (XCOM). Adapted from Peterson et al. 2015; Archives of Physical Medicine & Rehabilitation).
The protocol consisted of two days of testing, 24 hours apart; a training day (Day 1), and a retention day (Day 2). All participants were tested “ON” levodopa, approximately 1 hour after a normal levodopa dose. Upon arrival on Day 1, participants were consented and completed assessments (UPDRS, MiniBESTest, MoCA, NFoGQ). Then, reflective markers were placed on boney landmarks for determination of body COM displacements and step characteristics. Participants then underwent an initial block of perturbations to reduce “first trial startle” effects. The number of perturbations necessary to fully habituate people with PD to multi-directional support surface perturbations is not well understood. However, our goal was to reduce first-trial effects in all directions, which required providing familiarization perturbations in all directions. In addition, people with PD may habituate to perturbations more slowly than healthy adults [up to 5 trials (Nanhoe-Mahabier, et al. 2012)], requiring multiple perturbations to fully remove startling “first-trial reactions”. Therefore, to reduce ‘first trial startle’ effect, participants underwent 14 “familiarization” perturbations (4 forward, 3 backward, 4 leftward, and 3 rightward). These familiarization perturbations started small (forward & backward: 9cm, 19cm/s; left & right: 9cm, 14cm/s) and increased to large amplitude and velocity (forward & backward: 15cm, 56cm/s; left & right: 15cm, 21cm/s). Participants then underwent training of protective stepping training trials that consisted of 25 forward and 25 backward translations (15cm, 56cm/s) delivered in pseudo-random order, separated into blocks of 10 (5 forward & 5 backward translations per block). One participant (FoG+) required smaller and slower perturbation size because large perturbations frequently elicited a lack of step and fall. Data for this participant were excluded, leaving 12 individuals in the FoG+ group for analysis.
Participants returned to the laboratory approximately 24 hours later for day 2 (retention) testing. Medication timing was held consistent across days. Familiarization (14 trials) and lateral (10 trials) perturbations were completed exactly as in day 1. Then, 5 forward and 5 backward (random direction) perturbations were delivered. These were the first 10 perturbations of the 50 trials delivered on day 1.
Data analysis & variables of interest
Our primary variable of interest was the extent of displacement of the whole-body COM in response to perturbations (Figure 1). This variable was chosen as it represents a global measure of disequilibrium after perturbations (Dijkstra, et al. 2015; Nonnekes, et al. 2013; Welch and Ting 2014). Further, minimizing COM kinematics is considered a primary goal of postural responses (Safavynia and Ting 2013). Finally, COM displacement has been shown to be improved by perturbation training in healthy adults (Mansfield, et al. 2015). COM displacement was measured by calculating the weighted sum of the individual limb COM locations via segment kinematics and anthropometric data (Vaughan, et al. 1992). Marker data were captured at 120Hz via a motion analysis system (Motion Analysis Corporation, Santa Rosa, California). Data were filtered with a 4th order Butterworth low pass filter at a frequency of 5Hz. COM displacement was then calculated as the maximum anterior-posterior distance traveled by vertical projection of the whole-body COM to the ground from its position at perturbation onset until the end of the trial.
The effectiveness of the first step after perturbations is critical to prevent a fall. Therefore, secondary variables of interest included the margin of stability at first foot contact, first step length, first step latency, and number of steps. Margin of stability was identified as the distance between the boundary of support (defined as the heel marker for backward stepping, and the toe marker for forward stepping) and the extrapolated COM (XCOM) at the instance of first foot contact (Hof, et al. 2005). XCOM is defined as:
In which x is the position of the vertical projection of the COM to the ground and Vx the AP velocity of the COM. Wo is the eigenfrequency of the inverted pendulum and is defined as:
Where g is gravity (9.81m/s2) and L is the effective pendulum length (trochanteric height times 1.24 (Winter 2009)). The Margin of Stability expresses the effectiveness of the first protective step to reverse the falling COM as the extrapolated COM takes into account the direction and velocity of COM displacement, as well as position, with respect to the base of support (Hof, et al. 2005). Step latency was calculated as the time from perturbation onset to first foot liftoff. Foot liftoff was identified as zero force on a force-plate. Step length was calculated from motion analysis as the distance between feet at the instance of first foot contact. Number of steps was the number of steps to occur between perturbation onset and maximum COM displacement (Dijkstra, et al. 2015; Peterson, et al. 2015a).
Statistical Analysis
The 50 training perturbations on Day 1 were separated into forward and backward trials (25 perturbations each) and averaged into blocks of 5 trials. Thus, we analyzed 5 blocks of 5 trials for both forward and backward perturbations. On Day 2, we averaged the 5 forward and 5 backward perturbations separately to create forward and backward retention blocks. Data were compared between groups (FoG+ and FoG−).
The majority of variables exhibited small or modest skew (P>0.01 on Shapiro-Wilk test), so we used a mixed-design, repeated measures ANOVAs with fixed effects on group (between subjects factor; 2 levels) and block (within subject factor; 5 levels). For two variables, (COM displacement, day 2, backward perturbations, and number of steps, day 1, forward perturbations), moderate skew was observed (P<0.01 on Shapiro-Wilk test), and inverse transformations did not remove skew. Therefore, non-parametric Wilcoxon Signed Rank and Mann-Whitney U tests were used for these 2 variables to assess group, and practice effects. Group by practice interactions were non-parametrically assessed by first taking the difference between the first and last blocks on day 1 (blocks 1 & 5) separately for each group. These “difference” scores were then compared across groups via a Mann-Whitney U test. If group by practice interactions were observed, post-hoc, paired sample, t-tests were used to determine whether improvement was exhibited in FoG+ and FoG− groups separately.
Retention was assessed in variables that showed significant improvement across the course of Day 1 (blocks 1–5). Retention was assessed by comparing performance at the start of Day 1 (block 1) and Day 2 using mixed-design ANOVAs as described above. This comparison determines whether significant improvements remained statistically different with respect to where they started 24 hours before.
RESULTS
Group by practice interaction effects
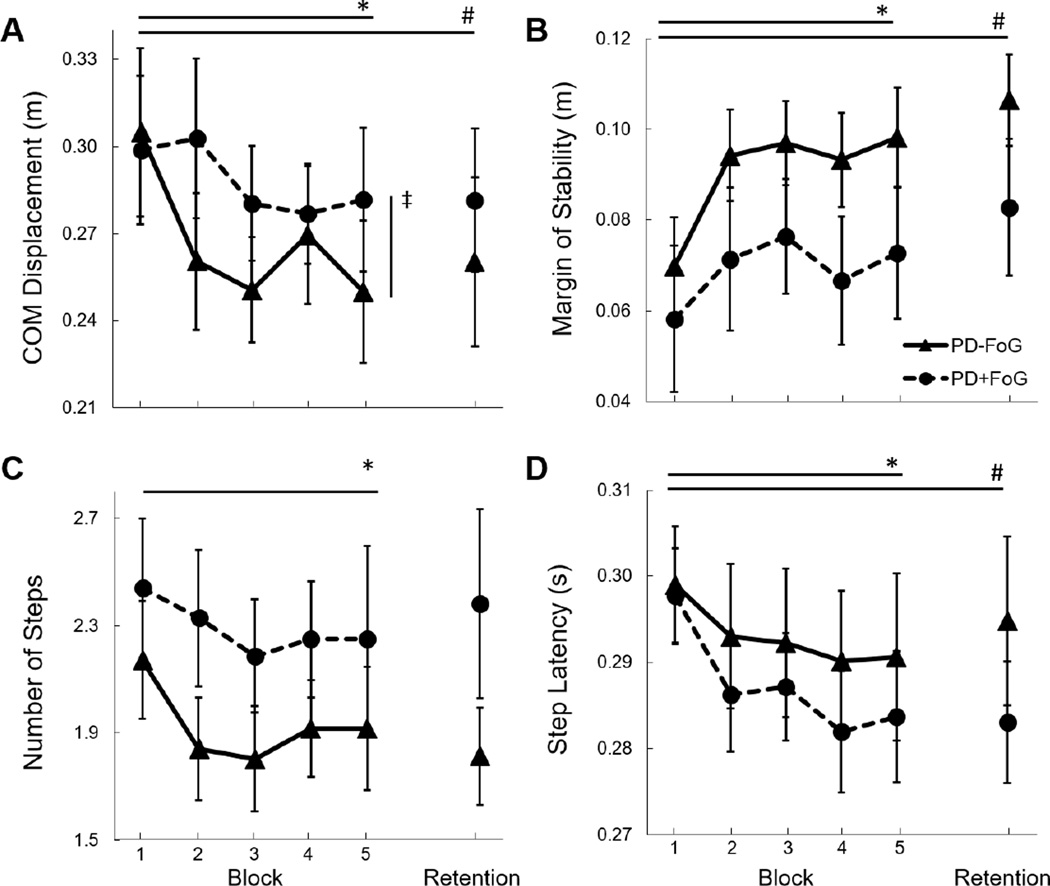
During backward perturbations, a group by training interaction was observed in the primary variable of interest (COM displacement), such that FoG− improved to a larger extent than FoG+ (F4,100=2.9, p=0.025; Figure 2). Within-group post-hoc analyses showed that this interaction was driven by improved COM displacement in the FoG− group (p=0.001), but not in the FoG+ group (p=0.143). No group by practice interactions were observed in any other variables for forward or backward perturbations (p>0.47 for all).
Figure 2.
Group means and standard errors of protective postural responses to backward perturbations across practice blocks (block 1–5), and retention (24 hours later). A. Center of mass (COM) displacement, B. Margin of stability, C. Number of steps, and D. Step latency. *Significant effect of practice across day 1; # Significant retention 24 hours later; ‡Significant group by practice effect.
Practice Effects
During backward perturbations, all variables of interest (COM displacement, margin of stability, step latency, step length, and number of steps) improved over practice trials (p<0.04 for all; Figure 2). During forward perturbations, only number of steps improved (Z=−2.3; p=0.02), represented by a reduction in steps over time.
Group Effects
At baseline (block 1, day 1), no differences were observed between freezers and nonfreezers for any variables of interest (p>0.34 for all). Further, ANOVA results showed no group effects for any variables. That is, when performance was averaged across day 1 training, no differences were observed for the FoG− and FoG+ groups for any variables of interest (p>0.20 for all).
Retention Analyses
Retention analyses were run only for variables that exhibited significant improvement over the course of day 1 (Backward: COM displacement, margin of stability, step latency, step length, number of steps; Forward: number of steps). For backward perturbations, improvements were retained for COM (Z=−2.4; p=0.017), margin of stability (F1,25=16.3; p<0.001), step latency (F1,24=5.9; p=0.023), and step length (F1,25=8.8; p=0.007), but not number of steps (F1,25=3.6; p=0.070). For COM displacement, a group by retention interaction was observed (Z=−2.1; p=0.04), such that retention in the FoG+ group was less pronounced than in the FoG− group. This effect was supported by across time post-hoc assessments, as FoG− (p=0.009), but not FoG+ (p=0.937) demonstrated significant difference between performance on the first block of day 1 and day 2.
For forward perturbations, improvement in number of steps was retained (Z=02.1; p=0.034), however an interaction effect was not observed (Z=−0.03; p=0.981). No group differences were observed for forward or backward perturbations. No group effects were observed for any variable during forward or backward perturbations (p>0.25 for all).
DISCUSSION
People with PD who experience freezing did not improve COM displacements in response to repeated backward perturbations to the same extent as people with PD who do not experience freezing. However, protective stepping performance variables (e.g. margin of stability, step latency, step length) similarly improved in both groups, suggesting that while deficits in learning to improve postural response performance exist, FoG+ are still able to improve some characteristics of their postural responses with training.
The relative lack of improvement in FoG+ in our primary variable, COM displacement, is consistent with our hypothesis. Indeed, previous investigations have suggested that FoG+ exhibit worse motor learning than FoG−. Vandenbossche and colleagues tested implicit motor learning in FoG+ and FoG− via upper extremity sequence learning (Vandenbossche, et al. 2013). While both groups exhibited implicit learning, learning by the FoG+ group was not as pronounced as that of FoG−. Similarly, Mohammadi and colleagues showed that gait adaptation in FoG+, while present, was slower and less pronounced than in FoG− (Mohammadi, et al. 2015). This relative reduction in learning may be related to the impaired cognition observed in FoG+ (Heremans, et al. 2013; Peterson, et al. 2015c). Nevertheless, although we also observed reduced learning (represented by less improvement in COM displacement over training), participants in the FoG− and FoG+ groups exhibited similar MoCA scores. It is however noted that the MoCA is a relatively blunt instrument for characterizing cognitive function.
To our knowledge, no previous studies have directly compared improvement in postural motor learning in FoG+ and FoG−. Smulders and colleagues measured postural performance on 8 consecutive backward perturbations. Results showed that FoG−, but not FoG+ improved performance with this short sequence of repeated exposure (Smulders, et al. 2014); however, no between group analyses were conducted. Our results are consistent with and extend these findings, showing that FoG+ did not improve performance after repeated exposures to external perturbations as well as FoG−. The lack of improvement in postural responses is also consistent with previous reports demonstrating the importance of brainstem regions (e.g. pontomedulary reticular formation; PmRF) in the storage and release of the postural stepping response (Honeycutt, et al. 2009; Nonnekes, et al. 2015). The function and structure of these brainstem regions are often altered in FoG+ compared to FoG− (Pahapill and Lozano 2000; Peterson, et al. 2015b; Snijders, et al. 2011). Furthermore, these brainstem regions have significant connectivity to basal ganglia structures and the cerebellum (Fling, et al. 2013; Pahapill and Lozano 2000), areas associated with learning (Doyon, et al. 2009). Thus, the altered structure and function of brainstem regions may play a direct role in the diminished improvement observed in FoG+.
While FoG+ exhibited less pronounced improvement than FoG− in COM displacement to backward perturbations, other performance measures were not significantly different between groups. For example, step latency, a variable specifically related to falls (Mansfield, et al. 2013), was similar in FoG+ and FoG− and improved at a similar rate in these groups. The cause of the lack of consistency between our primary variable (COM displacement) and secondary variables is not clear. It is possible that subtle group differences in improvement of secondary measures such as margin of stability, step time, and step length collectively resulted in poorer global performance (i.e. COM displacement). Together, these findings show that while changes in learning may exist, FoG+ do exhibit improvement in some stepping performance variables. Therefore, while postural training interventions, including perturbation training, may be less effective for backward perturbations with similar dosage in FoG+, they could still result in measurable improvement in protective stepping performance.
Baseline protective stepping was not different between groups. This is in contrast to previous reports that showed later (Jacobs, et al. 2009), and smaller (Smulders, et al. 2014) protective stepping in FoG+ compared to FoG−. The lack of baseline differences may be related to the relatively mild FoG+ group recruited for the current study. Indeed, participants in the current study were similar with respect to MiniBESTest, MoCA, and UPDRS. We investigated mild FoG+ for two reasons. First, individuals with more severe freezing would likely have difficulty completing the full perturbation battery without substantial fatigue and frequent falls. We sought to avoid fatigue as it may mask improvements in protective stepping. Second, to effectively compare FoG+ and FoG− groups, we matched groups for overall PD severity (via the UPDRS-III), requiring recruitment of mild FoG+.
Practice-related performance improvements in response to forward body perturbations were less frequent in both groups than improvements in response to backward body perturbations. While the rationale for this finding is not entirely clear, backward perturbations are much more challenging for people with PD than forward perturbations (Carpenter, et al. 2004). Thus, the increased difficulty of backward perturbations in people with PD may have given them more room for improvement. Conversely, the lack of improvement in forward perturbations may have been due to a ceiling effect. That is, people with PD did not need to, or were unable to continue to improve protective stepping in the forward direction. Alternatively, it is possible that other, unmeasured physiological or biomechanical measures, such as joint moments or electromyography, may have been altered during forward perturbations. Additional research is necessary to understand whether practice effects exist in these variables.
Limitations
A limitation for generalization to rehabilitation practice is that only one day of training (50 perturbations; 25 forward & 25 backward) plus a day 2 testing block was delivered. Additional perturbations across several weeks should be carried out to understand whether FoG+ can improve and retain improvements in postural stepping responses with further training.
Conclusion
People with PD who experience freezing exhibited less improvement and less retention of improvement in backward postural responses through one day of repeated perturbation exposure than those who do not freeze. However, stepping performance was improved with training, suggesting that while deficits in postural motor learning exist, FoG+ are still able to improve some aspects of performance with training. Additional perturbation exposure or alternative training may be necessary enhance improvements in protective postural responses in FoG+.
Highlights.
-
-
We assessed postural motor learning in people with PD who do and do not freeze
-
-
PD who freeze exhibited less learning in postural responses than nonfreezers
-
-
However, people who freeze did improve performance on some postural variables
Acknowledgments
This work was supported by grants from the United States Department of Veteran’s Affairs Rehabilitation Research and Development Service (Career Development Award-1: #I01BX007080; PI: DP) & VA Merit Award (E1075-R; PI: FH), the National Institutes of Health (R01 AG006457 29 PI: FH), and the Medical Research Foundation of Oregon (Early Investigator Award; PI: DP). The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- Bloem BR, Grimbergen YA, Cramer M, Willemsen M, Zwinderman AH. Prospective assessment of falls in Parkinson's disease. J Neurol. 2001;248:950–958. doi: 10.1007/s004150170047. [DOI] [PubMed] [Google Scholar]
- Carpenter MG, Allum JH, Honegger F, Adkin AL, Bloem BR. Postural abnormalities to multidirectional stance perturbations in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2004;75:1245–1254. doi: 10.1136/jnnp.2003.021147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kam D, Nonnekes J, Oude Nijhuis LB, Geurts AC, Bloem BR, Weerdesteyn V. Dopaminergic medication does not improve stepping responses following backward and forward balance perturbations in patients with Parkinson's disease. J Neurol. 2014;261:2330–2337. doi: 10.1007/s00415-014-7496-3. [DOI] [PubMed] [Google Scholar]
- Dijkstra BW, Horak FB, Kamsma YPT , Peterson DS. Older adults can improve compensatory stepping with repeated postural perturbations. Frontiers in aging neuroscience. 2015;7 doi: 10.3389/fnagi.2015.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon J, Bellec P, Amsel R, Penhune V, Monchi O, Carrier J, Lehericy S, Benali H. Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav Brain Res. 2009;199:61–75. doi: 10.1016/j.bbr.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Fling BW, Cohen RG, Mancini M, Nutt JG, Fair DA, Horak FB. Asymmetric pedunculopontine network connectivity in parkinsonian patients with freezing of gait. Brain. 2013;136:2405–2418. doi: 10.1093/brain/awt172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchignoni F, Horak F, Godi M, Nardone A, Giordano A. Using psychometric techniques to improve the Balance Evaluation Systems Test: the mini-BESTest. J Rehabil Med. 2010;42:323–331. doi: 10.2340/16501977-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heremans E, Nieuwboer A, Spildooren J, Vandenbossche J, Deroost N, Soetens E, Kerckhofs E, Vercruysse S. Cognitive aspects of freezing of gait in Parkinson's disease: a challenge for rehabilitation. J Neural Transm. 2013;120:543–557. doi: 10.1007/s00702-012-0964-y. [DOI] [PubMed] [Google Scholar]
- Hof AL, Gazendam MG, Sinke WE. The condition for dynamic stability. J Biomech. 2005;38:1–8. doi: 10.1016/j.jbiomech.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Honeycutt CF, Gottschall JS, Nichols TR. Electromyographic responses from the hindlimb muscles of the decerebrate cat to horizontal support surface perturbations. J Neurophysiol. 2009;101:2751–2761. doi: 10.1152/jn.91040.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JV, Horak FB. Cortical control of postural responses. J Neural Transm. 2007;114:1339–1348. doi: 10.1007/s00702-007-0657-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JV, Nutt JG, Carlson-Kuhta P, Stephens M, Horak FB. Knee trembling during freezing of gait represents multiple anticipatory postural adjustments. Exp Neurol. 2009;215:334–341. doi: 10.1016/j.expneurol.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield A, Inness EL, Wong JS, Fraser JE, McIlroy WE. Is impaired control of reactive stepping related to falls during inpatient stroke rehabilitation? Neurorehabil Neural Repair. 2013;27:526–533. doi: 10.1177/1545968313478486. [DOI] [PubMed] [Google Scholar]
- Mansfield A, Wong JS, Bryce J, Knorr S, Patterson KK. Does perturbation-based balance training prevent falls? Systematic review and meta-analysis of preliminary randomized controlled trials. Phys Ther. 2015;95:700–709. doi: 10.2522/ptj.20140090. [DOI] [PubMed] [Google Scholar]
- Mohammadi F, Bruijn SM, Vervoort G, van Wegen EE, Kwakkel G, Verschueren S, Nieuwboer A. Motor switching and motor adaptation deficits contribute to freezing of gait in Parkinson's disease. Neurorehabil Neural Repair. 2015;29:132–142. doi: 10.1177/1545968314545175. [DOI] [PubMed] [Google Scholar]
- Nanhoe-Mahabier W, Allum JH, Overeem S, Borm GF, Oude Nijhuis LB, Bloem BR. First trial reactions and habituation rates over successive balance perturbations in Parkinson's disease. Neuroscience. 2012;217:123–129. doi: 10.1016/j.neuroscience.2012.03.064. [DOI] [PubMed] [Google Scholar]
- Nieuwboer A, Rochester L, Herman T, Vandenberghe W, Emil GE, Thomaes T, Giladi N. Reliability of the new freezing of gait questionnaire: agreement between patients with Parkinson's disease and their carers. Gait Posture. 2009;30:459–463. doi: 10.1016/j.gaitpost.2009.07.108. [DOI] [PubMed] [Google Scholar]
- Nonnekes J, Carpenter MG, Inglis JT, Duysens J, Weerdesteyn V. What startles tell us about control of posture and gait. Neurosci Biobehav Rev. 2015;53:131–138. doi: 10.1016/j.neubiorev.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Nonnekes J, Scotti A, Oude Nijhuis LB, Smulders K, Queralt A, Geurts AC, Bloem BR, Weerdesteyn V. Are postural responses to backward and forward perturbations processed by different neural circuits? Neuroscience. 2013;245:109–120. doi: 10.1016/j.neuroscience.2013.04.036. [DOI] [PubMed] [Google Scholar]
- Pahapill PA, Lozano AM. The pedunculopontine nucleus and Parkinson's disease. Brain. 2000;123(Pt 9):1767–1783. doi: 10.1093/brain/123.9.1767. [DOI] [PubMed] [Google Scholar]
- Peterson D, Huisinga JM, Spain R, Horak FB. Characterization of Compensatory Stepping in People with Multiple Sclerosis. Arch Phys Med Rehabil. 2015a;15 doi: 10.1016/j.apmr.2015.10.103. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson DS, Fling BW, Mancini M, Cohen RG, Nutt JG, Horak FB. Dual-task interference and brain structural connectivity in people with Parkinson's disease who freeze. J Neurol Neurosurg Psychiatry. 2015b;86:786–792. doi: 10.1136/jnnp-2014-308840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson DS, King LA, Cohen RG, Horak FB. Cognitive Contributions to Freezing of Gait in Parkinson Disease: Implications for Physical Rehabilitation. Phys Ther. 2015c doi: 10.2522/ptj.20140603. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safavynia SA, Ting LH. Sensorimotor feedback based on task-relevant error robustly predicts temporal recruitment and multidirectional tuning of muscle synergies. J Neurophysiol. 2013;109:31–45. doi: 10.1152/jn.00684.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RA, Lee TD. Motor Control and learning: A behavioral emphasis. Champaign, IL: Human Kinetics; 1999. [Google Scholar]
- Smulders K, Esselink RA, De Swart BJ, Geurts AC, Bloem BR, Weerdesteyn V. Postural inflexibility in PD: does it affect compensatory stepping? Gait Posture. 2014;39:700–706. doi: 10.1016/j.gaitpost.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Snijders AH, Leunissen I, Bakker M, Overeem S, Helmich RC, Bloem BR, Toni I. Gait-related cerebral alterations in patients with Parkinson's disease with freezing of gait. Brain. 2011;134:59–72. doi: 10.1093/brain/awq324. [DOI] [PubMed] [Google Scholar]
- Terpening Z, Naismith S, Melehan K, Gittins C, Bolitho S, Lewis SJ. The contribution of nocturnal sleep to the consolidation of motor skill learning in healthy ageing and Parkinson's disease. Journal of sleep research. 2013;22:398–405. doi: 10.1111/jsr.12028. [DOI] [PubMed] [Google Scholar]
- Vandenbossche J, Deroost N, Soetens E, Coomans D, Spildooren J, Vercruysse S, Nieuwboer A, Kerckhofs E. Impaired implicit sequence learning in Parkinson's disease patients with freezing of gait. Neuropsychology. 2013;27:28–36. doi: 10.1037/a0031278. [DOI] [PubMed] [Google Scholar]
- Vaughan C, Davis B, O'Connor J. Dynamics of Human Gait. Cape Town, SA: Kiboho Publishers; 1992. [Google Scholar]
- Welch TD, Ting LH. Mechanisms of motor adaptation in reactive balance control. PLoS One. 2014;9 doi: 10.1371/journal.pone.0096440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter D. Biomechanics and Motor Control of Human Movement. Hoboken, NJ, USA: John Wiley & Son's; 2009. [Google Scholar]