Abstract
The purpose of the present study was to investigate the immediate effects of acute exposure to intense sound on spontaneous and stimulus-driven activity in the dorsal cochlear nucleus (DCN). We examined the levels of multi- and single-unit spontaneous activity before and immediately following brief exposure (2 minutes) to tones at levels of either 109 or 85 dB SPL. Exposure frequency was selected to either correspond to the units’ best frequency (BF) or fall within the borders of its inhibitory side band. The results demonstrate that these exposure conditions caused significant alterations in spontaneous activity and responses to BF tones. The induced changes have a fast onset (minutes) and are persistent for durations of at least 20 minutes. The directions of the change were found to depend on the frequency of exposure relative to BF. Transient decreases followed by more sustained increases in spontaneous activity were induced when the exposure frequency was at or near the units’ BF, while sustained decreases of activity resulted when the exposure frequency fell inside the inhibitory side band. Follow-up studies at the single unit level revealed that the observed activity changes were found on unit types having properties which have previously been found to represent fusiform cells. The changes in spontaneous activity occurred despite only minor changes in response thresholds. Noteworthy changes also occurred in the strength of responses to BF tones, although these changes tended to be in the direction opposite those of the spontaneous rate changes. We discuss the possible role of activity-dependent plasticity as a mechanism underlying the rapid emergence of increased spontaneous activity after tone exposure and suggest that these changes may represent a neural correlate of acute noise-induced tinnitus.
Keywords: Dorsal cochlear nucleus, hyperactivity, long-term potentiation, acute noise induced tinnitus
INTRODUCTION
The DCN, one of the first central processing stations in the ascending auditory pathway, receives acoustic input from auditory nerve fibers and multimodal inputs from diverse brain areas through a population of granule cells and their parallel fiber axons. Both auditory nerve and parallel fibers have excitatory synapses onto the principal output neurons of the DCN, the fusiform cells. These cells have been shown to become hyperactive following intense noise exposure (Brozoski et al., 2002; Finlayson and Kaltenbach, 2009; Shore et al., 2008; Dehmel et al., 2012), which is manifest as an increase in spontaneous activity that develops gradually following induction of hearing loss and persists for up to at least 6 months (Kaltenbach et al., 1998; Kaltenbach et al., 2000). The induced hyperactivity is relayed to higher levels of the auditory system, and there is evidence suggesting that it may be an important factor contributing to tinnitus in noise-exposed animals (Brozoski et al., 2002; Kaltenbach et al., 2004; Ma et al., 2006; Shore et al., 2008).
Noise-induced hyperactivity is widely thought to result from a shift in the balance of excitation and inhibition in the central auditory system (see reviews of Roberts et al., 2010). There is evidence that noise exposure and related manipulations that impair peripheral function, cause decreases of inhibitory and increases in excitatory synaptic transmission in the DCN (Asako et al., 2005; Cransac et al., 1998; Muly et al., 2004; Potashner et al., 2000; Shore, 2011; Suneja et al., 1998; Wang et al., 2009; Zeng et al., 2009). Although little is known about the mechanism that triggers such a shift, one hypothesis is that the observed changes in synaptic connectivity are triggered by the loss of normal primary afferent input to neurons in the cochlear nucleus. Loss of hair cell integrity leads to degeneration or weakening of primary afferents, and the deafferented neurons may undergo transneuronal degeneration and/or various forms of plastic alterations, including sprouting of new synapses (Bilak et al., 1997, Kim et al., 1997; 2004; Morest et al., 1997), and/or up- and down-regulations of receptors on existing synapses (Wang et al., 2009; Milbrandt et al., 2000; Dong et al., 2010a; Zeng et al., 2009; Kaltenbach and Zhang, 2006). These changes are widely believed to underlie the chronic form of tinnitus, but they appear to emerge too slowly to underlie all forms of noise-induced tinnitus. The acute form of tinnitus, which is observed following moderate sound exposure conditions that are not permanently damaging to the auditory periphery, develops within seconds or minutes following exposure (Atherley et al., 1968; Loeb and Smith, 1967). This suggests a fast acting mechanism that is independent of deafferentation.
One mechanism that could underlie the acute form of tinnitus and could also set in motion changes that lead to the chronic form of tinnitus is activity-dependent plasticity (Tzounopoulos, 2008). Two forms of activity-dependent plasticity, long term potentiation (LTP) and long term depression (LTD), have been found in DCN fusiform and cartwheel cells in brain slice preparations (Fujino and Oertel, 2003, Tzounopoulos et al., 2004). Intrinsic membrane properties can also be regulated in an activity dependent manner (Turrigiano et al., 1994; Aizenman and Linden, 1999; Desai et al., 1999; Egorov et al., 2002; Smith and Otis, 2003), leading to modulation of neuronal spontaneous rate. Thus far, studies demonstrating induction of hyperactivity in the DCN have not been comparable to those inducing LTP or LTD, since they have employed long exposure conditions (1–4 hours) that cause injury to cochlear receptors and/or auditory nerve fibers, and changes in spontaneous activity have generally been observed in the range of days to months after exposure. In contrast, neither LTP nor LTD are dependent on injury to cochlear receptors, and both are observed within minutes following co-activation of their postsynaptic membranes and inputting parallel fibers (Fujino and Oertel, 2003; Tzounopoulos et al., 2004). The effect of intense tone exposure on spontaneous activity on the time scale of minutes and using conditions that do not damage the peripheral receptor epithelium has not been systematically tested and described.
Here, we investigated the immediate effect of acute sound exposure on spontaneous and stimulus-driven activity in the DCN of anesthetized hamsters. The level and duration of exposure were selected so as to constitute a condition of acoustic overexposure without inducing significant injury to the primary afferents. This allowed us to test the effects of over-activation of primary afferents without the confounding effects of deafferentation and hearing loss. The exposures were also designed to activate inputs to fusiform cells from auditory nerve fibers and parallel fibers and to resemble stimuli that have previously been shown to induce acute tinnitus in human subjects (80–109 dB) (Loeb and Smith, 1967; Atherley et al., 1968). We also examined the effects of these exposure conditions on stimulus-driven activity over a wide range of sound levels. We report that limited exposure to moderate level tones induces an immediate change of spontaneous activity and evoked responses in neurons with the properties of fusiform cells.
MATERIALS AND METHODS
Animal subjects
The animals used in all experiments were adult male Syrian golden hamsters (LVG strain) obtained from Charles River Laboratories. Animals were housed in the animal vivarium of the Cleveland Clinic and were cared for in accordance with NIH Guidelines for the care and use of animals in research. Animals were between 2 and 3 months of age at the time experiments were conducted.
Surgical preparations
Animals were anesthetized by i.m. injections of ketamine (117 mg/kg) and xylazine (18mg/kg). Areflexia was maintained with supplementary injections of anesthetic. Core body temperature was maintained at 37°C using a rectal probe and heating pad. A tracheotomy was performed to maintain normal breathing during anesthesia. The animal’s head was held firmly in a brace, and the left DCN was exposed by parieto-occipital craniotomy followed by aspiration of the overlying portion of the cerebellum.
Electrophysiological recordings
Electrodes were micropipettes filled with 0.3 M NaCl and had tip impedances of either 0.4–0.5 MΩ for multiunit recordings or 2–10 MΩ for single unit recordings. The output of the microelectrode was fed through an amplifier (1000X) and bandpass-filtered (0.3–10 kHz). The electrode was lowered until contact was made with the DCN surface as signaled by the disappearance of 60 cycle noise and the emergence of neural activity. Recordings were conducted before, during and after presentation of an exposure tone (see below). For the multiunit recordings, only a single attempt at tone-induced plasticity was made in each animal to avoid the potential for contamination from previously induced plasticity. For single unit recordings, due to the lower abundance and long term stability of units, two attempts of exposure-induced plasticity were made in each animal with a recovery period of at least 60 minutes between exposures. At the beginning of the pre-exposure period, frequency tuning properties were tested with pure tones (50 ms including 5 ms rise-fall ramps) over a range of frequencies (3–32 kHz) and intensities (6–96 dB SPL). Generally, frequency tuning curves from multiunit clusters were similar to those of single units, except that the multiunit tuning was sometimes slightly broader. However, we found no evidence to suggest that the BFs based on multiunit recordings differed from those of single units taken from the same recording sites. Spontaneous rates were quantified and monitored over an extended duration spanning the periods before and following the exposure tone. The time axis was divided into 5 second time bins (epochs). For multiunits recordings, spontaneous rates were measured by counting in each epoch the number of voltage events exceeding (more negative than) −100 mV. These counts were converted to rates, expressed in events/s, by dividing the counts by 5 (the duration of each epoch). Single unit spontaneous rates were quantified and monitored over a similar time frame, but in each 5 second epoch, rates were measured by counting the number of spikes of similar amplitude and waveform in each bin and dividing by 5. Both multi- and single-unit spontaneous rates and tuning properties were acquired using customized Matlab software. Thresholds and tuning properties were based on frequency-intensity response maps as described previously (Finlayson and Kaltenbach, 2009). These properties were determined for both single- and multi-unit recordings after a pre-exposure baseline was established just before the exposure tone, then again after a 25–30 minute post-exposure recording of spontaneous activity was completed. Any recordings that showed large shifts of tuning curve tip thresholds (≥18 dB) after tone exposure were discarded. PST histograms (PSTHs) with bin resolutions of 1 ms were obtained from responses to 100 presentations of 40 ms tones set at the units’ best frequency (BF) at 70 dB SPL presented at 1/s using Datawave (Sciworks) software. For both tuning curves and PST histograms, sound stimuli were presented monaurally at a rate of 10/s to the left ear through a Beyer Dynamic DT-48 headphone coupled to the external auditory meatus using a plastic conical insert (Finlayson and Kaltenbach, 2009).
Sound exposure
The exposure sound was a moderately intense tone presented during the recording session to the left ear using the same headphone used to test tuning properties and PSTHs. With the earphone coupled to the concha, a tone was delivered to the left ear at levels of 85 or 109 dB SPL and at frequencies selected to match either the BF or the center of the high frequency inhibitory side band of the recorded unit. For each exposure condition, the tone was turned on for 2 minutes.
Data analysis
For the multiunit recordings, the effect induced by each type of exposure condition on spontaneous activity was calculated by the ratio of activity 20 minutes after exposure to baseline activity during the 3 minute period immediately preceding the exposure. Thresholds and frequency response patterns were used to derive BF, BF thresholds and rate-intensity functions. The RIF curves were generated using Lowess fitting, Prism 3.0. The effect of sound exposure on single unit spontaneous activity was determined by calculating the ratio of activity (spikes/s) 10 minutes after exposure (average of spontaneous discharge rate from 8 to 11 minutes postexposure) to the baseline rate (average discharge rate over a 3 minute period before the exposure). Baseline activity was considered to be sufficiently stable if the spontaneous rates, in events/s for multiunit recordings or in spikes/s for single unit recordings, did not vary by more than 15%, and data from units not showing such stability were excluded from further analysis (Gao and Strowbridge, 2009). The significance of the change of spontaneous activity induced by each test condition was tested using the Mann-Whitney U-test, unless noted otherwise. Differences were considered significant if p ≤ 0.05. All statistical calculations were performed using Origin.
RESULTS
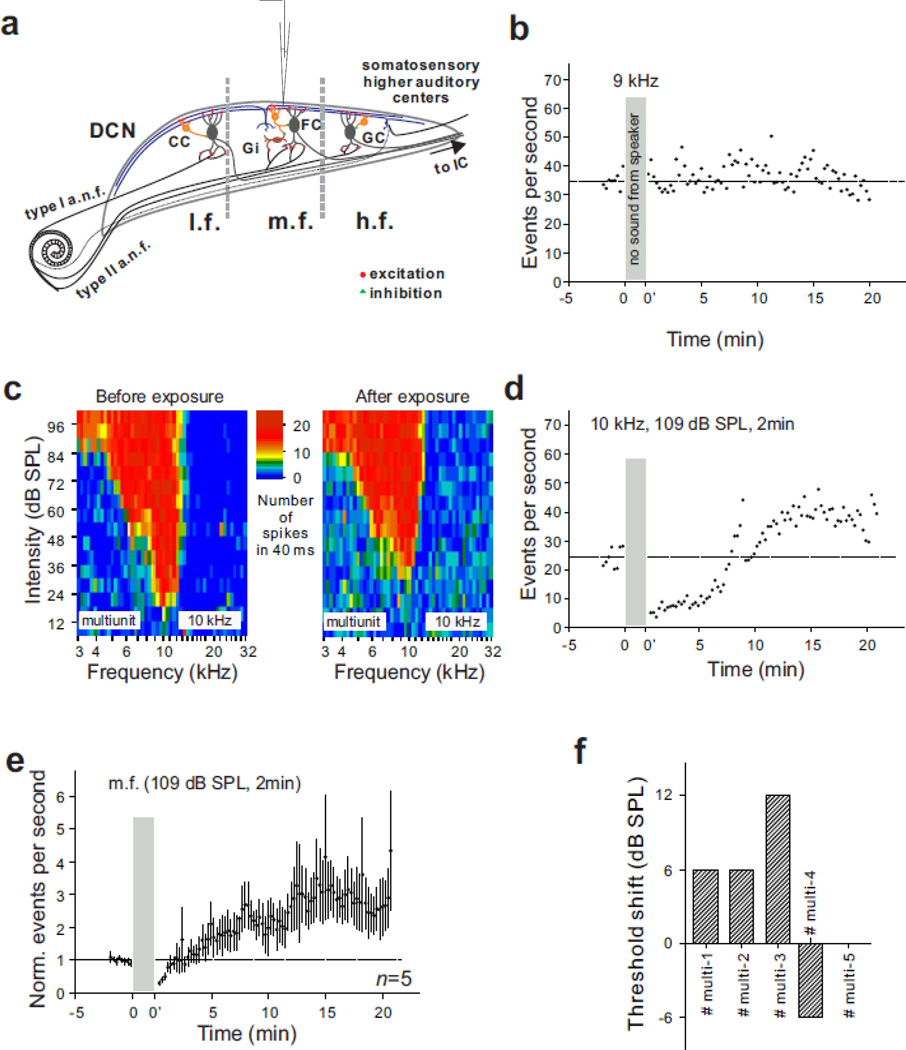
We started with multiunit recordings on the DCN surface (Fig. 1a), where activity could be examined without excluding specific cell classes. Multiunit recordings represent the activity of all nearby neurons, including fusiform cells, giant cells, cartwheel cells, granule cells, stellate cells and vertical cells; however, such recordings are probably dominated by spontaneously active cell types near the DCN surface, which include the fusiform and cartwheel cells (Davis and Young, 1997; Ding and Voigt, 1997; Godfrey et al., 1975; Golding and Oertel, 1997; Hancock and Voigt, 2002; Portfors and Roberts, 2007). Other nearby cell types are either not spontaneously active (vertical and stellate cells) or are too small (granule cells), too distant or too few in number (giant cells) to be recorded with our microelectrodes (Golding and Oertel, 1997; Balakrishnan and Trussell, 2008; Smith et al., 2005).
Figure 1.
Effect of an acute 10 kHz tone exposure on multiunit surface activity in the middle frequency region of the DCN. (a) Transverse view of the DCN showing the location of the recording electrode in the m.f. (mid-frequency: 8–12 kHz) region of the DCN. l.f., low frequency. h.f., high frequency. (b) Spontaneous activity recorded from the 9 kHz locus during an extended period of silence. Dashed line indicates baseline activity. (c–d) Tuning curves and spontaneous activity recorded from the 9 kHz locus of a single animal before and after a 2 minute exposure to a 10 kHz (109 db SPL) tone. (e) Mean spontaneous activity of 5 animals recorded in region m.f. before and after a two minute exposure to a 10 kHz tone at 109 dB SPL. (f) Averaged mean threshold shifts at BF in the five animals that were included in (e).
Multiunit data
Baseline activity
Multiunit activity typically showed enough stability to allow us to compare activity after a brief sound exposure period with pre-exposure baselines. In the example of Fig. 1b, wherein no sound was delivered during the time window to be reserved for acoustic exposure in future recordings, the ratios of activity 10 and 20 minutes after this window to pre-window baseline activity were 1.1 and 0.91, respectively. Neither of these changes was statistically significant (p = 0.067 for 10 minutes, p = 0.095 for 20 minutes).
Activity changes in the mid-frequency region following exposure to a mid-frequency tone. We first tested the effect of a 2 minute exposure to a 10 kHz tone at a level of 109 dB SPL in the mid-frequency region of the DCN (Fig. 1a). The acoustic response in the mid-frequency (8–12 kHz) region of the DCN (Fig. 1c) and the spontaneous activity (Fig. 1d–e) were recorded before and after sound exposure. The post-exposure tuning curve was recorded 25 minutes after exposure to evaluate the level of hearing loss. The BF of the unit cluster at this location was 10 kHz, while the BF threshold was 18 dB SPL before exposure and 30 dB SPL after exposure. Multiunit spontaneous activity was significantly increased after sound exposure following a transient depression of activity (Fig. 1d). Indeed, all five animals showed a statistically significant enhancement of spontaneous activity 20 minutes after sound exposure when they were analyzed individually. The mean ratio of post-exposure to pre-exposure activity was 0.68 at 2 minutes (p < 0.0006), 2.23 at 10 minutes (n = 5, p < 0.00001) and 2.74 at 20 minutes after sound exposure (n = 5, p < 0.0001). The average upward-shift of thresholds in all five animals for Fig. 1e was 3.6 dB (Fig. 1f). The data in Figure 1 thus demonstrate that a long-lasting increase of DCN multiunit spontaneous activity can be induced by intense sound, in vivo, with only minimal impairment of tuning curve thresholds.
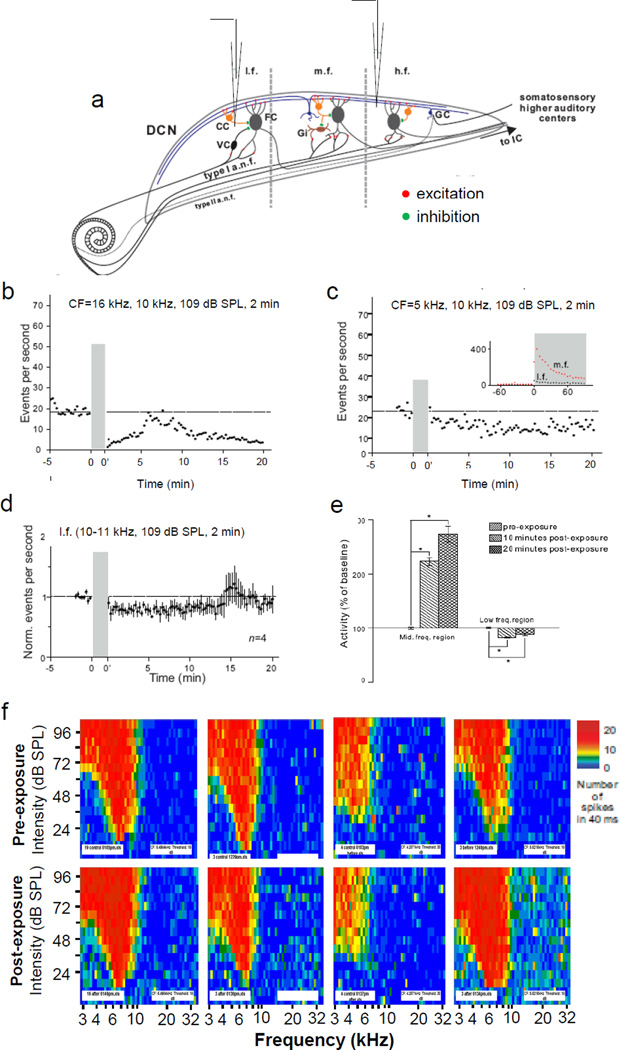
Activity changes in the high and low frequency regions following exposure to a mid-frequency tone. The tonotopic organization of the DCN in hamsters is characterized by a mediolateral gradient in which higher BFs are located medially and lower BFs laterally (Kaltenbach and Lazor, 1991). Therefore, fusiform cells in the low (< 7 kHz) or high frequency (> 15 kHz) regions (Fig. 2a) should only be weakly activated by primary auditory nerve fibers when a mid-frequency (10 kHz) tone is presented; however, the activation of cartwheel cells and their inhibitory input to fusiform cells are less likely to show a similar location dependence because cartwheel cells are driven by excitatory inputs from parallel fibers, which extend across isofrequency bands (Blackstad et al., 1984) (Fig. 2a). We then asked the question how the same sound exposure protocol that significantly increased DCN spontaneous activity in the middle frequency region, affects spontaneous activity at low and high frequency loci. The same exposure condition that increased spontaneous activity in the mid-frequency region (Fig. 1) also caused a statistically significant decrease of spontaneous activity in the low and high frequency regions of the DCN (Fig. 2b–c). The ratio of post-exposure to pre-exposure activity at the 16 kHz locus was 0.20 at 2 minutes (p < 0.00001), 0.57 at 10 minutes (p < 0.00001) and 0.25 at 20 minutes after sound exposure (p < 0.00001) (Fig. 2b). A decrease of spontaneous activity induced by the same sound exposure was also observed at the low frequency locus with a BF around 5 kHz (Fig. 2c). In this region, the mean ratio was 0.79 at 2 minutes (p < 0.0004), 0.83 at 10 minutes (n = 4, p < 0.00001) and 0.88 at 20 minutes after exposure (n = 4, p < 0.005). Two of four animals showed a statistically significant decrease, one showed a significant increase, and one had no significant change at 20 minutes after exposure when they were analyzed individually. The thresholds of tuning curves measured in these four animals were shifted only negligibly (Fig. 2f). Based on the data reported in Figs. 1 and 2, we conclude that a long lasting change in spontaneous activity is inducible across the tonotopic range, but the polarity of the change varies, depending on the relationship between the BF of the unit cluster and the frequency of the exposure tone (Fig. 2e): Increases occur when the exposure frequency is centered on the BF of the unit cluster, and decreases occur when there is significant gap between the exposure frequency and the cluster’s BF.
Figure 2.
Effect of an acute 10 kHz tone exposure on multiunit surface activity in the low (3–8 kHz) and high (12–30 kHz) frequency regions of the DCN. (a) Transverse view of the DCN showing the locations of the three frequency regions (l.f., m.f., h.f.) and the differences in their underlying circuit connections with the cochlea. (b–c) Spontaneous activity recorded at the 16 kHz and 5 kHz loci, respectively. Inset in (c), comparison between DCN surface activity recorded at l.f. and m.f. in the same animal. (d) Normalized mean spontaneous activity of 4 animals recorded at l.f. before and after exposure to a 10 kHz tone (109 dB SPL). (e) Comparison of changes in mean multiunit spontaneous activity in the mid- and low-frequency regions of the DCN. Exposure tone was the same as that used for Fig. 1 data (10 kHz, 109 dB SPL, 2 minutes). (f) Tuning curves measured in the same 4 animals represented in (d) before (upper row) and after exposure (lower row). CC, cartwheel cell; FC, fusiform cell; GC, granule cell; Gi, giant cell; VC, vertical cell.
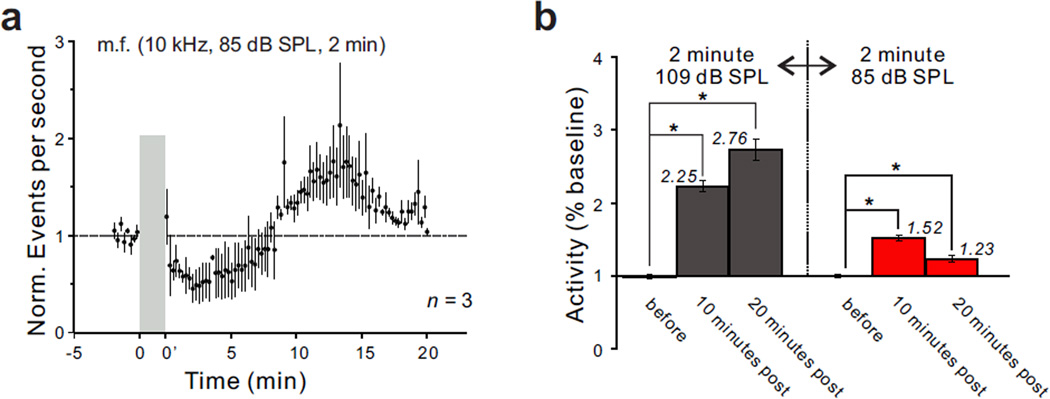
Effect of decreasing exposure tone intensity
The next question we asked was whether the high intensity of the sound was critical to the induction of a long-lasting enhancement of DCN activity, or whether a similar increase might be inducible using a lower level of sound. To address this question, we decreased the intensity of exposure from 109 dB to 85 dB SPL. The level of spontaneous activity was still significantly elevated following the 2 minute sound exposure (Fig. 3a). However, this enhancement was weaker than that induced by the 109 dB SPL exposure tone. The ratio of post-exposure to pre-exposure activity was 0.70 at 2 minutes (p < 0.00008), 1.52 at 10 minutes (p < 0.00001) and 1.23 at 20 minutes after sound exposure (n = 3, p < 0.0003). All three animals showed a significant increase when they were analyzed individually. This result indicates that the intensity of sound exposure was a critical factor in determining the degree to which DCN activity increased (Fig. 3b).
Figure 3.
Intensity dependence of the long-lasting enhancement of multiunit spontaneous activity recorded in the m.f. region of the DCN following a 2 minute exposure to a 10 kHz tone. (a) Time course of activity changes recorded before and following tone exposure at a level of 85 dB SPL. Dashed line indicates baseline activity before sound exposure. (b) Comparison of the ratios of post-exposure to pre-exposure activity obtained following exposure to a tone at 109 and 85 dB SPL. Asterisks indicate p < 0.05, Mann-Whitney U test.
Single unit data
Identification of unit type
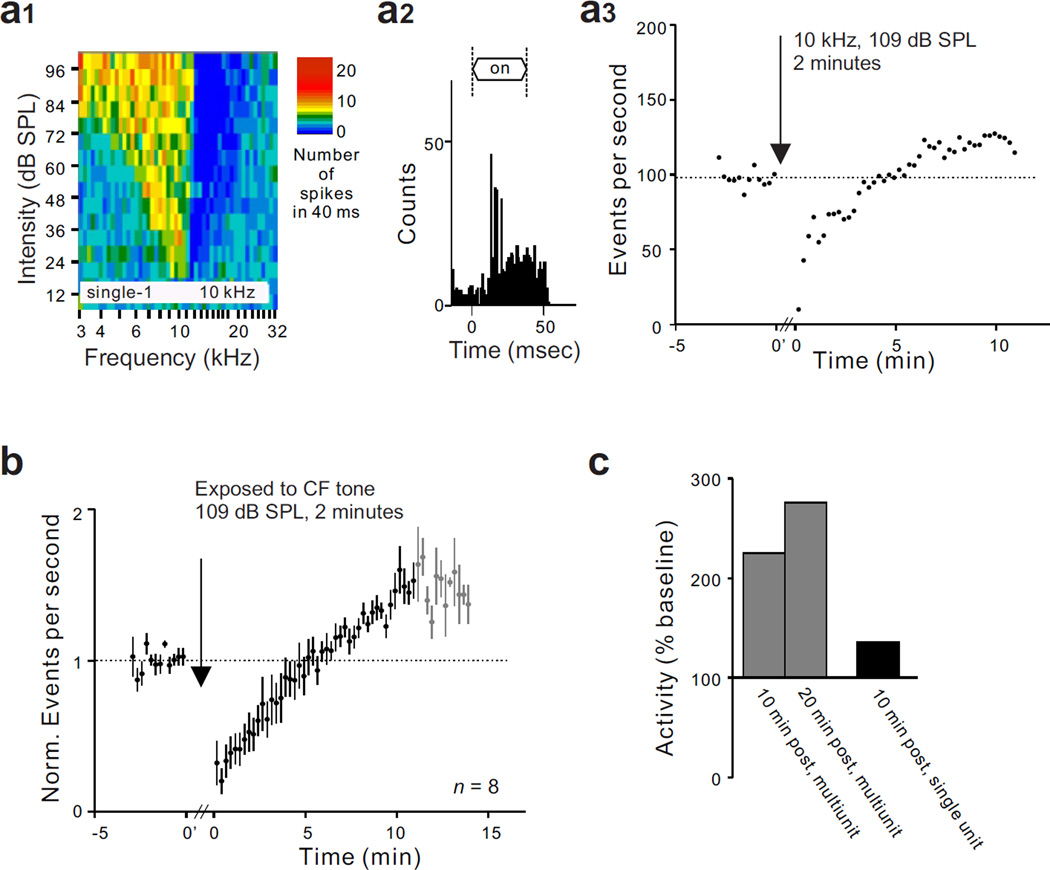
To determine the identity of neurons showing the aforementioned activity changes, we next performed single unit recordings in the fusiform cell layer of the DCN, which lies between 100 and 300 µm below its surface (Finlayson and Kaltenbach, 2009). We tested the effect of sound exposure on cells with properties that are cell-type specific. Units were selected for study if they showed the properties of fusiform cells: simple spike waveforms, the presence of moderate levels of spontaneous activity, frequency response maps characterized by vigorous responses to tones and having low threshold excitatory regions flanked by inhibitory side bands (type III) (Fig. 4a1) and non-monotonic responses to BF tone bursts (Young and Brownell, 1976; Ding et al., 1999; Parsons et al., 2001). Further characterization of units was obtained by collecting PST histograms (Fig. 4a2). We excluded a subtype of fusiform cells with low spontaneous firing rates (SFR) (< 2.5 spikes/s, n = 2) (Hancock and Voigt, 2002), both of which interestingly did not show a persistent enhancement of SFR following an exposure to BF tone (p > 0.05, data not shown).
Figure 4.
Effect of tone exposure on single unit spontaneous activity when the frequency of the exposure tone (10 kHz) was at or near the unit’s BF (9 kHz). (a) Demonstration of a persistent increase of SFR in a neuron with the properties of fusiform cells. The unit displayed a typical type III frequency response map (a1) and a chopper PST histogram pattern (a2). In panel a3, it can be seen that spontaneous activity in the same cell represented in a1 and a2 increased after a 2 minute exposure to the BF tone (109 dB SPL). (b) Normalized mean spontaneous activity recorded in 8 type III units, showing the persistent increase of SFR following exposure to a two minute BF tone (109 dB SPL). Black dots represent the normalized mean spontaneous activity from 8 units and grey dots represent points where data was available for only 3 of the 8 units. (c) Comparison of long-term activity changes observed in multiunit and single unit recordings. Dashed line in a3 and b indicates baseline activity before sound exposure.
Activity change when exposure frequency matched unit BF. After a cell with the properties of a fusiform cell was identified, the acute effect of a 2 minute exposure to a BF tone set at a level of 109 dB SPL was tested. Units with these properties showed a strong transient post-stimulus suppression of spontaneous activity immediately after the exposure stimulus was turned off, but following this initial suppression, activity recovered and eventually increased above the pre-exposure baseline (Fig. 4a3). When analyzed individually, all eight units tested showed a statistically significant increase of SFR following exposure to a tone that matched their BFs (p < 0.001). The mean time course of activity for the 8 units is shown in Fig. 4b. The mean ratio of post-exposure activity to pre-exposure baseline activity was 1.39 at 10 minutes after exposure (n = 8, p = 0.0078) (Fig. 4c). This ratio is considerably lower than that measured by multiunit recordings; however, it is only slightly lower than that observed in vitro by others following induction of LTP of fusiform cells (Fig. 4c)(Fujino and Oertel, 2003; Tzounopoulos et al., 2004; Kim et al., 2004).
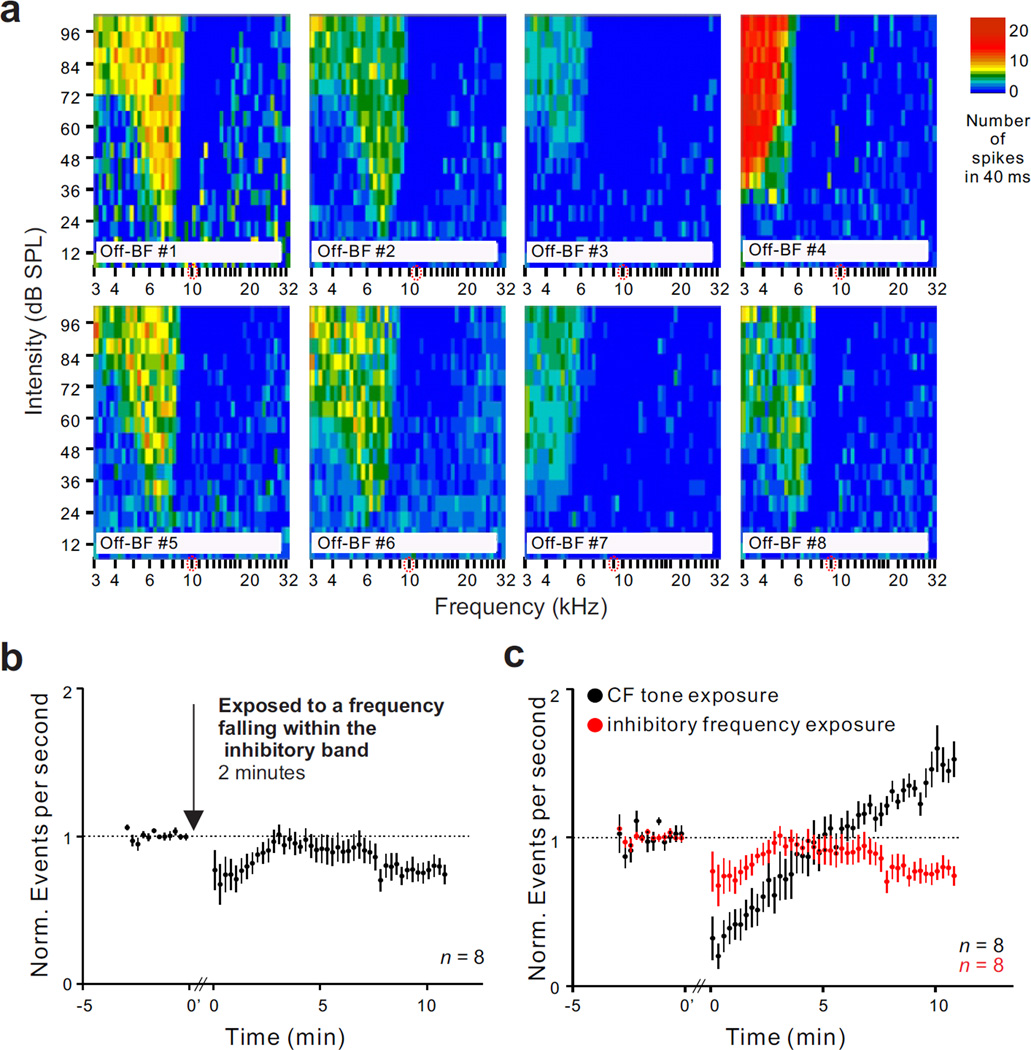
Activity change when exposure frequency fell inside the inhibitory side band. We continued to test, using single unit recordings, the effect of acute sound exposure but this time, with the recording electrode in the lower frequency region. The eight units tested in this way had BFs between 4 and 7 kHz, and the exposure frequency fell well within the borders of the inhibitory side bands which are shown in Fig. 5a. Exposing animals to a 10 kHz tone at 109 dB SPL for 2 minutes resulted in a long-lasting reduction of spontaneous activity (Fig. 5b). The mean ratio of activity at 10 minutes post-exposure to pre-exposure baseline was 0.77 (n = 8, p = 0.0078). The decreases in spontaneous activity for six of the eight units tested were statistically significant. The results of Figs. 4 and 5 indicate that fusiform cells are major generators of hyperactivity following acute BF tone exposure but become hypoactive following exposure tones that activate the inhibitory side band.
Figure 5.
Effect of tone exposure on single unit spontaneous activity when the frequency of the exposure tone (10 kHz) fell within the unit’s inhibitory side band. (a) Response maps for each of 8 type III units, showing the tuned excitatory areas and their flanking inhibitory side bands (areas in which spontaneous activity was absent). (b) Normalized time course of mean spontaneous activity change averaged across the 8 type III units shown in panel a. Tone exposure duration and intensity were two minutes and 109 dB SPL, respectively. c) Differences of the long-term activity changes following the tone exposure to different frequencies. Dashed lines in b and c indicate baseline activity before and after sound exposure.
Only a single example was tested in the high frequency region (16 kHz), but it was consistent with the multiunit results from the same location and with results from the low frequency region in showing a decrease of spontaneous activity following the 10 kHz exposure tone (data not shown).
Effect on stimulus-driven activity
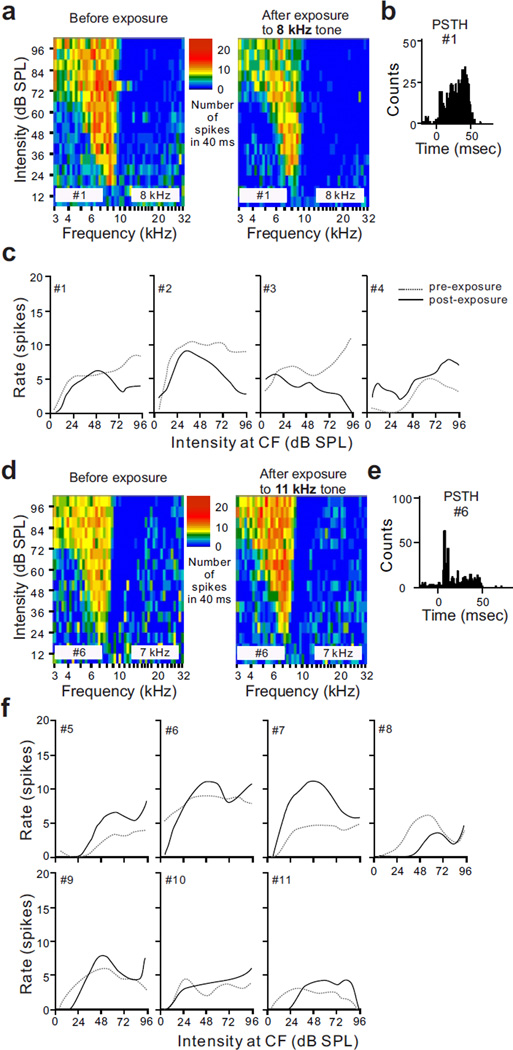
The last question we asked was whether the acute tone exposure that caused enhancement of spontaneous activity also caused increases in the strength of tone-elicited responses. To answer this question, we generated rate-intensity functions (RIFs) at BF from the previously derived tuning curves for each unit that was tested with a 2 minute, 109 dB SPL tone exposure (Fig. 6). First, we examined the change of RIFs at BF in the fusiform cells after an exposure to a BF tone. The data in Fig. 6 suggests that a brief exposure not only changed SFR in DCN fusiform cells, but also shifted the level of peak activity in the RIFs. Three out of four units that were exposed to a BF tone showed a decrease in peak activity in the RIF in response to a subsequent BF tone (Fig. 6c); six out of seven cells that were exposed to a non-BF tone (tone frequency that activated the inhibitory side band), showed enhanced RIFs at BF (Fig. 6f). Thus, the plasticity observed in recordings of spontaneous activity was associated with plasticity of stimulus-driven activity, although the polarity of these changes was in the direction opposite to that observed in the changes in spontaneous activity.
Figure 6.
Effect of tone exposure on stimulus-elicited responses of type III units based on comparisons of RIFs collected at BF before and after tone exposure. (a) Tuning curves measured from a unit (#1) before and after exposure to a BF tone (8 kHz, 109 dB SPL, 2 minutes). (b) PSTH collected in the same unit as that in (a). (c) RIFs at BF in 4 type III units (#1–4). The post-exposure RIF curves showed lower maxima than the pre-exposure curves except that collected in unit #4. (d) Response maps from a unit with a type III response (#6) before and after exposure to an 11 kHz tone (109 dB SPL, 2 minute), which fell into the unit’s inhibitory side band (BF = 7 kHz). (e) PST histogram collected from the same unit as in (d). (f) RIFs collected at BF from 7 units with type III response maps (#5–11). The post-exposure RIF curve showed higher maxima than the pre-exposure curve except that collected from unit #8.
DISCUSSION
The results demonstrate that multi- and single-unit spontaneous activity can be altered by acute tone exposure (85 dB SPL and 109 dB SPL), despite negligible changes in neural response thresholds. The changes in spontaneous activity are associated with significant changes in responses to moderate to high level BF tones, although the direction of these latter changes were in the direction opposite those of the spontaneous rate changes. We now discuss these results in light of previous studies and consider some underlying mechanisms of the observed changes and some of their implications.
Tonotopic difference in SFR changes
An intriguing aspect of our results were the differences in the changes in spontaneous activity observed when the recordings were performed in different tonotopic regions relative to the exposure tone. When the recordings were performed in tonotopic locations (9–10 kHz) that corresponded closely to the frequency of the exposure tone (10 kHz, 109 dB SPL), the effect was a transient decrease in activity that lasted from 3–7 minutes and which was followed by a gradual and longer lasting increase of spontaneous activity that did not return to normal baseline over the 20 minute period of observation; a similar bimodality in response polarity was observed when the exposure tone was presented at a level of 85 dB SPL, although the increase in spontaneous activity was correspondingly weaker. The early decrement of activity that preceded the longer lasting increase in spontaneous activity most likely resulted from synaptic fatique or short term depression and was of secondary interest in this study. In contrast, when recordings were performed on units with BFs substantially below the 10 kHz exposure frequency (BF < 7 kHz), such that the exposure frequency fell well within the boundaries of the high frequency inhibitory side band of the unit, the initial suppression of spontaneous activity immediately after exposure tone offset was followed by a long lasting decrement in spontaneous activity. Although not as intensively studied, both multiunit and single unit data suggested that a similar decrement of spontaneous activity occurred when BF was substantially higher (16 kHz) than the 10 kHz exposure frequency. These results suggest that the balance of excitation/inhibition shifted towards the side of increased excitation when the exposure frequency matched BF, but favored an increase in inhibition when the exposure frequency substantially differed from BF.
These results differ substantially from those reported for the auditory nerve by Lonsbury-Martin and Meikle (1978), which showed no changes in resting ‘spontaneous’ activity after a 1 minute exposure to a moderate level tone either at CF or ½ octave below CF (85–90 dB SPL). In the auditory nerve, spontaneous discharge rates recorded during periods of silence alternating with periods of tone burst stimulation following the exposure sound remained normal and stable from 1 minute after exposure and beyond. Assuming that this result extends to other species, such as the hamster, this would seem to rule out a peripheral origin of the increased spontaneous activity observed in the present study of the DCN. Our results also differ from those observed in the ventral cochlear nucleus (VCN) by Lonsbury-Martin and Martin (1981). They examined the effects of moderate tone exposure (100 dB SPL, 1 minute) on ventral cochlear nucleus neurons of unanesthetized Rhesus monkeys. As in our study, response thresholds had recovered to normal or near normal levels within 20 minutes following exposure (Lonsbury-Martin and Martin, 1981). One third of their units showed no changes in spontaneous activity immediately following exposure, but the other two thirds showed either transient increases or transient decreases of spontaneous activity which recovered to pre-exposure baseline levels within 3–4 minutes. No evidence of a persistent change in spontaneous activity beyond this 3–4 minute period was observed. Our results therefore may indicate that the mechanisms controlling the balance of excitation and inhibition in the DCN are more complex and operate over more extended time frames than those present in the VCN. The possibility that such differences might also be related to the small differences in exposure duration or even differences in species and/or anesthetic state cannot be ruled out.
Relationship between noise-induced changes in spontaneous and stimulus-driven activity
Our results also showed that acute tone exposure caused changes in the responses of fusiform cells to BF tone bursts. The direction of this change did not correspond to the direction of changes in their levels of spontaneous activity. Exposure to a tone with the frequency equal to the unit’s BF resulted in an increased SFR but a decrease in maximal firing rate in response to BF tones. Conversely, when the frequency of the exposure tone fell within the borders of the inhibitory side band, SFR was decreased, but the maximal discharge rate in response to a BF tone was increased. The fact that the responses to sound were shifted in directions opposite those for spontaneous activity indicates that the mechanisms controlling the levels of spontaneous activity are separate from and independent of those controlling responses to sound. This conclusion is also supported by our previous findings that the emergence of increased spontaneous activity after intense tone exposure follows a different time course from that of response threshold shift (Kaltenbach et al., 1998). Additional work showing that drugs affecting spontaneous activity may have little or no effect on response thresholds (Caspary et al., 1987) is also consistent with this inference.
Possible mechanisms underlying the observed changes in spontaneous activity
The fact that increases in spontaneous activity were induced by exposure conditions that caused little or no changes in response thresholds suggests that the underlying inducing mechanism is an activity-dependent process, rather than one that requires degeneration or damage to cochlear receptors. This mechanism is therefore different from that which has been invoked previously to explain most cases of noise-induced hyperactivity such as homeostatic scaling (Schaette and Kempter, 2006), adjustments in anatomical connectivity (synaptic sprouting) (Bilak et al., 1997, Kim et al., 1997; 2004; Morest et al., 1997; Illing et al., 2005; Meidinger et al., 2006), changes in functional connectivity (reorganization)(Robertson and Irvine, 1989; Norena and Eggermont, 2006) and changes in receptor expression (Wang et al., 2009; Milbrandt et al., 1997; Whiting et al., 2009; Abbott et al., 1999; Dong et al., 2010a, 2010b; Rubio, 2006; Caspary et al., 1995; Jin and Godfrey, 2006; Jin et al., 2005, 2006; Caspary et al., 1999, 2008; Asako et al., 2005), all of which are phenomena triggered by hearing loss and/or degeneration or injury to peripheral receptors.
One type of activity-dependent processes that has been suggested as a basis of hyperactivity in the DCN is long term potentiation (LTP) (Tzounopoulos, 2008). This enhancement of synaptic efficacy often results when there is co-activation of pre- and post-synaptic membranes (Hebbian LTP). In the DCN, LTP can be induced in fusiform cells when activation of presynaptic inputs from parallel fiber coincides with depolarization of the post-synaptic membrane (Fujino and Oertel, 2003, Tzounopoulos et al., 2004). Cartwheel cells of the DCN also show LTP inducibility, although whether these cells become potentiated by stimulus conditions identical to (Fujino and Oertel, 2003) or different from (Tzounopoulos et al., 2004, Tzounopoulos, 2008) those that cause fusiform cells to become potentiated is not yet clear. An objective of future studies should be to determine whether the tone exposure conditions used here result in co-activation of parallel fibers and fusiform cells. Recent studies demonstrating that stimuli formerly shown to activate fusiform cells can also activate granule cells (Yang et al., 2005; Carretta et al., 1999), the parent cell type of parallel fiber inputs to fusiform cells, open up the possibility that an intense sound exposure, such as that used here, might increase the probability of pre- and post-synaptic activation of fusiform cells needed for LTP induction. One concern that might raise question about the role of LTP in the present results is that it normally involves activation of NMDA receptors (Zhao and Tzounopoulos, 2011), which would have been blocked by the use of ketamine anesthesia. On the other hand, ketamine may not have completely blocked NMDA receptors in the present study because it was used in combination with xylazine, which enhances ketamine’s anesthesia-inducing effects (Naccarato and Hunter, 1979). Perhaps the enhancing effect of xylazine might have allowed induction of anesthesia with ketamine without completely saturating the NMDA receptors. Thus, NMDA receptors could have been at least partially available for LTP induction. Even if NMDA receptors were completely blocked, LTP could have still been induced through non-NMDA receptor dependent mechanisms that have yet to be characterized in the DCN.
An alternative activity-dependent mechanism might be alterations in the intrinsic membrane properties of neurons which have been implicated as a basis of neural plasticity in a number of neurological diseases (Devor, 2006; Beck and Yaari, 2008). At the cellular level, the change of intrinsic excitability is usually due to an active modulation of ion channel conductance by constitutive phosphorylation. One possible modulating pathway is through the nitric oxide-cGMP signaling cascade, which is known to be responsible for the persistent change of cerebellar Purkinje cells’ SFR in an activity-dependent manner (Smith and Otis, 2003). Given the presence of nitric oxide synthase in DCN neurons (Rodrigo et al., 1994; Zheng et al., 2006) and the similarity in structure between cerebellum and DCN, the possibility that nitric oxide synthase expression in DCN is enhanced after acoustic trauma must also be considered. Activation of protein kinases through other signal pathways may also occur in DCN neurons, as that found in hippocampal neurons, dorsal root ganglion and vestibular nucleus neurons (Smith et al., 2002, Gerevich et al., 2004, Misonou et al., 2004). A thoughtful study of active modulation of ion channel conductance in fusiform and cartwheel cells following noise exposure will extend our understanding of mechanisms underlying this noise-induced change of fusiform cells SFR.
Possible mechanisms underlying the observed changes in stimulus-driven activity
Previous work has shown that the amplitude of the suprathreshold response, which determines the peak amplitude of the rate-intensity function, is highly controlled by inhibitory side bands, which are thought to be derived from glycinergic inputs from nearby vertical (tuberculoventral) cells (Voigt and Young, 1990; Davis and Young, 2000). For example, in cat DCN, responses of fusiform cells to suprathreshold levels of stimulation can be increased by iontophoretic application of strychnine, suggesting a glycinergic, inhibitory source (Davis and Young, 2000). Cross correlation studies have shown that DCN fusiform cells receive direct inhibitory input from vertical (tuberculoventral) cells (Voigt and Young, 1980), and a number of studies suggest that vertical cells help shape the slope and maximal firing rate in fusiform cell rate/intensity functions in response to BF tones (Davis and Young, 2000; Voigt and Young, 1980; Nelken and Young, 1994, Anderson and Young, 2004). In the present study, when the exposure tone over-activated the inhibitory side band of the recorded unit, the inhibition from vertical cells may have been weakened by the exposure, causing the fusiform cell response to a BF tone burst to be augmented at high levels without changing the BF thresholds. But when the exposure frequency matched the unit’s BF, the inhibitory input from vertical cells was less affected, but the cells excitatory input at high levels might have been slightly weakened, causing a reduction in the strength of the suprathreshold response to BF tone bursts. These findings parallel those found in the VCN (Boettcher and Salvi, 1993).
Implications
One implication of these results is that, if LTP is involved in the induction of hyperactivity, the co-presence of fusiform and cartwheel cell LTP may help to balance one another to maintain a stable response gain (stable sound response threshold as seen in Fig. 1): fusiform cell LTP leads to an increased EPSP which broadens the integration window; while cartwheel cell LTP leads to an increased IPSP which narrows the integration window (Doiron et al., 2011). Overstimulation from noise exposure may upset this balance and thereby disrupt the modulation of response gain. If this is true, an upward shift of thresholds could occur allowing for increased susceptibility to injury to cartwheel cells.
A second implication of our results concerns its clinical relevance to tinnitus. Thus far, the DCN’s role in tinnitus has been discussed in the literature only in terms of its relationship with chronic noise-induced tinnitus. These studies differ from our study in that they have employed high sound exposure levels that are damaging to the cochlea (Zhang and Kaltenbach, 1998; Kaltenbach and Afman, 2000; Brozoski et al., 2002), and assessments of activity changes were obtained days to months after exposure. Although previous studies have demonstrated induction of spike-timing dependent plasticity in DCN fusiform cells in vivo, the induction of this plasticity was achieved using bimodal stimuli (Koehler and Shore, 2013a, 2013b; Stefanescue and Shore, 2015). Thus, our results are the first reporting evidence for induction of activity-dependent plasticity in fusiform cells by noise exposure alone and by conditions of exposure that are similar to those known to cause acute tinnitus in humans (Atherley et al. 1968; Loeb and Smith, 1967).
The acute form of noise-induced tinnitus has been described in several previous reports based on studies in human subjects (Loeb and Smith, 1967; Atherley et al., 1968; George and Kemp, 1989; Chermak and Dengerink., 1987). Some of its key features are its rapid onset (seconds to minutes) and its temporary duration (minutes to hours). The fact that acute tinnitus is temporary suggests that it may involve different mechanisms from those underlying chronic tinnitus. Chronic noise-induced tinnitus usually involves injury to hair cells, which triggers slow changes centrally, presumed to result from gradual shifts in the balance of excitation and inhibition. The increase in activity we observed immediately after intense tone exposure offers a starting point for studying the mechanism of acute noise-induced tinnitus. Knowledge of this mechanism is likely to provide insight into how changes in the balance of excitation and inhibition leading to tinnitus on the time scale of minutes may eventually become permanent.
RESEARCH HIGHLIGHTS.
Brief sound exposure induces long lasting increases in spontaneous activity of DCN fusiform cells.
The increase occurs when the exposure frequency is close to the units’ best frequency.
The increased activity likely involves induction of long term potentiation.
This condition may represent a neural correlate of the acute form of noise-induced tinnitus.
Acknowledgments
The authors wish to thank Frank Licari for his role in development of the Matlab software. This study was supported by NIH DC009097.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- Abbott SD, Hughes LF, Bauer CA, Salvi R, Caspary DM. Detection of glutamate decarboxylase isoforms in rat inferior colliculus following acoustic exposure. Neuroscience. 1999;93:1375–1381. doi: 10.1016/s0306-4522(99)00300-0. [DOI] [PubMed] [Google Scholar]
- Aizenman CD, Linden DJ. Regulation of the rebound depolarization and spontaneous firing patterns of deep nuclear neurons in slices of rat cerebellum. J Neurophysiol. 1999;82:1697–1709. doi: 10.1152/jn.1999.82.4.1697. [DOI] [PubMed] [Google Scholar]
- Anderson MJ, Young ED. Isoflurane/N2O anesthesia suppresses narrowband but not wideband inhibition in dorsal cochlear nucleus. Hear Res. 2004;188:29–41. doi: 10.1016/S0378-5955(03)00348-4. [DOI] [PubMed] [Google Scholar]
- Asako M, Holt AG, Griffith RD, Buras ED, Altschuler RA. Deafness-related decreases in glycine-immunoreactive labelling in the rat cochlear nucleus. J Neurosci Res. 2005;81:102–109. doi: 10.1002/jnr.20542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherley CC, Hempstock TI, Noble WG. Study of tinnitus induced temporarily by noise. J Acoust Soc Am. 1968;44:1503–1506. doi: 10.1121/1.1911288. [DOI] [PubMed] [Google Scholar]
- Balakrishnan V, Trussell LO. Synaptic inputs to granule cells of the dorsal cochlear nucleus. J Neurophysiol. 2008;99:208–219. doi: 10.1152/jn.00971.2007. [DOI] [PubMed] [Google Scholar]
- Beck H, Yaari Y. Plasticity of intrinsic neuronal properties in CNS disorders. Nat Rev Neurosci. 2008;9:357–369. doi: 10.1038/nrn2371. [DOI] [PubMed] [Google Scholar]
- Bilak M, Kim J, Potashner SJ, Bohne BA, Morest DK. New growth of axons in the cochlear nucleus of adult chinchillas after acoustic trauma. Exp Neurol. 1997;147:256–268. doi: 10.1006/exnr.1997.6636. [DOI] [PubMed] [Google Scholar]
- Blackstad TW, Osen KK, Mugnaini E. Pyramidal neurones of the dorsal cochlear nulceus: a Golgi and computer reconstruction study in cat. Neurosci. 1984;13:827–854. doi: 10.1016/0306-4522(84)90099-x. [DOI] [PubMed] [Google Scholar]
- Boettcher FA, Salvi RJ. Functional changes in the ventral cochlear nucleus following acute acoustic overstimulation. J Acoust Soc Am. 1993;94:2123–2134. doi: 10.1121/1.407484. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Bauer CA, Caspary DM. Elevated fusiform cell activity in the dorsal cochlear nucleus of Chinchillas with psychophysical evidence of tinnitus. J Neurosci. 2002;22:2383–2390. doi: 10.1523/JNEUROSCI.22-06-02383.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretta D, Hervé-Minvielle A, Bajo VM, Villa AE, Rouiller EM. Preferential induction of fos-like immunoreactivity in granule cells of the cochlear nucleus by acoustic stimulation in behaving rats. Neurosci Lett. 1999;259:123–126. doi: 10.1016/s0304-3940(98)00852-0. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Pazara KE, Kossl M, Faingold CL. Strychnine alters the fusiform cell output from the dorsal cochlear nucleus. Brain Res. 1987;417:273–282. doi: 10.1016/0006-8993(87)90452-5. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Milbrandt JC, Helfert RH. Central auditory aging: GABA changes in the inferior colliculus. Exp Gerontol. 1995;30:349–360. doi: 10.1016/0531-5565(94)00052-5. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Holder TM, Hughes LF, Milbrandt JC, McKernan RM, Naritoku DK. Age-related changes in GABA(A) receptor subunit composition and function in rat auditory system. Neuroscience. 1999;93:307–312. doi: 10.1016/s0306-4522(99)00121-9. [DOI] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Turner JG, Hughes LF. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. J Exp Biol. 2008;211:1781–1791. doi: 10.1242/jeb.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chermak GD, Dengerink JE. Characteristics of temporary noise-induced tinnitus in male and female subjects. Scand Audiol. 1987;16:67–73. doi: 10.3109/01050398709042158. [DOI] [PubMed] [Google Scholar]
- Cransac H, Cottet-Emard JM, Hellström S, Peyrin L. Specific sound-induced noradrenergic and serotonergic activation in central auditory structures. Hear Res. 1998;118:151–156. doi: 10.1016/s0378-5955(98)00031-8. [DOI] [PubMed] [Google Scholar]
- Davis KA, Young ED. Granule cell activation of complex-spiking neurons in dorsal cochlear nucleus. J Neruosci. 1997;17:6798–6806. doi: 10.1523/JNEUROSCI.17-17-06798.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KA, Young ED. Pharmacological evidence of inhibitory and disinhibitory neuronal circuits in dorsal cochlear nucleus. J Neurophysiol. 2000;83:926–940. doi: 10.1152/jn.2000.83.2.926. [DOI] [PubMed] [Google Scholar]
- Dehmel S, Shashwati P, Koehler S, Bledsoe S, Shore S. Noise overexposure alters long-term somatosensory-auditory processing in the dorsal cochlear nucleus-possible basis for tinnitus-related hyperactivity? J Neurosci. 2012;32:1660–1671. doi: 10.1523/JNEUROSCI.4608-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai NS, Rutherford LC, Turrigiano GG. Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nat Neurosci. 1999;2:515–520. doi: 10.1038/9165. [DOI] [PubMed] [Google Scholar]
- Devor M. Sodium channels and mechanisms of neuropathic pain. J Pain. 2006;7:S3–S12. doi: 10.1016/j.jpain.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Ding J, Voigt HF. Intracellular response properties of units in the dorsal cochlear nucleus of unanesthetized decerebrate gerbil. J Neurophysiol. 1997;77:2549–2572. doi: 10.1152/jn.1997.77.5.2549. [DOI] [PubMed] [Google Scholar]
- Ding J, Benson TE, Voigt HF. Acoustic and current-pulse responses of identified neurons in the dorsal cochlear nucleus of unanesthetized, decerebrate gerbils. J Neurophysiol. 1999;82:3434–3457. doi: 10.1152/jn.1999.82.6.3434. [DOI] [PubMed] [Google Scholar]
- Doiron B, Zhao Y, Tzounopoulos T. Combined LTP and LTD of modulatory inputs controls neuronal processing of primary sensory inputs. J Neruosci. 2011;31:1592–1611. doi: 10.1523/JNEUROSCI.1592-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S, Mulders WH, Rodger J, Woo S, Robertson D. Acoustic trauma evokes hyperactivity and changes in gene expression in guinea-pig auditory brainstem. Eur J Neurosci. 2010a;31:1616–1628. doi: 10.1111/j.1460-9568.2010.07183.x. [DOI] [PubMed] [Google Scholar]
- Dong S, Rodger J, Mulders WH, Robertson D. Tonotopic changes in GABA receptor expression in guinea pig inferior colliculus after partial unilateral hearing loss. Brain Res. 2010b:1342. doi: 10.1016/j.brainres.2010.04.067. [DOI] [PubMed] [Google Scholar]
- Egorov AV, Hamam BN, Fransén E, Hasselmo ME, Alonso AA. Graded persistent activity in entorhinal cortex neurons. Nature. 2002;420:173–178. doi: 10.1038/nature01171. [DOI] [PubMed] [Google Scholar]
- Finlayson PG, Kaltenbach JA. Alterations in the spontaneouse discharge patterns of single units in the dorsal cochlear nucleus following intense sound exposure. Hear Res. 2009;256:104–117. doi: 10.1016/j.heares.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino K, Oertel D. Bidirectional synaptic plasticity in the cerebellum-like mammalian dorsal cochlear nucleus. Proc Natl Acad Sci. 2003;100:265–270. doi: 10.1073/pnas.0135345100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Strowbridge BW. Long-term plasticity of excitatory inputs to granule cells in the rat olfactory bulb. Nat Neurosci. 2009;12:731–733. doi: 10.1038/nn.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George RN, Kemp S. Investigation of tinnitus induced by sound and its relationship to ongoing tinnitus. J Speech Hear Res. 1989;32:366–372. doi: 10.1044/jshr.3202.366. [DOI] [PubMed] [Google Scholar]
- Gerevich Z, Borvendeg SJ, Schröder W, Franke H, Wirkner K, Nörenberg W, Fürst S, Gillen C, Illes P. Inhibition of N-type voltage-activated calcium channels in rat dorsal root ganglion neurons by P2Y receptors is a possible mechanism of ADP-induced analgesia. J Neurosci. 2004;24:797–807. doi: 10.1523/JNEUROSCI.4019-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey DA, Kiang NY, Norris BE. Single unit activity in the dorsal cochlear nucleus of the cat. J Comp Neurol. 1975;162:269–284. doi: 10.1002/cne.901620207. [DOI] [PubMed] [Google Scholar]
- Golding NL, Oertel D. Physiological identification of the targets of cartwheel cells in the dorsal cochlear nucleus. J Neurophysiol. 1997;78:248–260. doi: 10.1152/jn.1997.78.1.248. [DOI] [PubMed] [Google Scholar]
- Hancock KE, Voigt HF. Intracellularly labled fusiform cells in dorsal cochlear nucleus of the Gerbil.II. comparision of physiology and anatomy. J Neurophysiol. 2002;87:2520–2530. doi: 10.1152/jn.2002.87.5.2520. [DOI] [PubMed] [Google Scholar]
- Illing RB, Kraus KS, Meidinger MA. Reconnecting neuronal networks in the auditory brainstem following unilateral deafening. Hear Res. 2005;206:185–199. doi: 10.1016/j.heares.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Jin YM, Godfrey DA, Sun Y. Effects of cochlear ablation on choline acetyl-transferase activity in the rat cochlear nucleus and superior olive. J Neurosci Res. 2005;81:91–101. doi: 10.1002/jnr.20536. [DOI] [PubMed] [Google Scholar]
- Jin YM, Godfrey DA. Effects of cochlear ablation on muscarinic acetylcholine receptor binding in the rat cochlear nucleus. J Neurosci Res. 2006;83:157–166. doi: 10.1002/jnr.20706. [DOI] [PubMed] [Google Scholar]
- Jin YM, Godfrey DA, Wang J, Kaltenbach JA. Effects of intense tone exposure on choline acetyl-transferase activity in the hamster cochlear nucleus. Hear Res. 2006;216–217:168–175. doi: 10.1016/j.heares.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Afman CE. Hyperactivity in the dorsal cochlear nucleus after intense sound exposure and its resemblance to tone-evoked activity: a physiological model for tinnitus. Hear Res. 2000;140:165–172. doi: 10.1016/s0378-5955(99)00197-5. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Lazor J. Tonotopic maps obtained from the surface of the dorsal cochlear nucleus of the hamster and rat. Hear Res. 1991;51:149–160. doi: 10.1016/0378-5955(91)90013-y. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Godfrey DA, Neumann JB, McCaslin DL, Afman CE, Zhang J. Changes in spontaneous neural activity in the dorsal cochlear nucleus following exposure to intense sound: relation to threshold shift. Hear Res. 1998;124:78–84. doi: 10.1016/s0378-5955(98)00119-1. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Zhang J, Afman CE. Plasticity of spontaneous neural activity in the dorsal cochlear nucleus after intense sound exposure. Hear Res. 2000;147:282–292. doi: 10.1016/s0378-5955(00)00138-6. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Zacharek MA, Zhang J, Frederick S. Activity in the dorsal cochlear nucleus of hamsters previously tested for tinnitus following intense tone exposure. Neurosci Lett. 2004;355:121–125. doi: 10.1016/j.neulet.2003.10.038. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Zhang JS. Intense sound-induced plasticity in the dorsal cochlear nucleus of rats: evidence for cholinergic receptor upregulation. Hear Res. 2006;226:232–243. doi: 10.1016/j.heares.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Kim J, Morest DK, Bohne BA. Degeneration of axons in the brainstem of the chinchilla after auditory overstimulation. Hear Res. 1997;103:169–191. doi: 10.1016/s0378-5955(96)00173-6. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Gross J, Potashner SJ, Morest DK. Fine structure of long-term changes in the cochlear nucleus after acoustic overstimulation: chronic degeneration and new growth of synaptic endings. J Neurosci Res Sep. 2004;77:817–828. doi: 10.1002/jnr.20212. [DOI] [PubMed] [Google Scholar]
- Koehler SD, Shore SE. Stimulus-timing dependent multisensory plasticity in the guinea pig dorsal cochlear nucleus. Plos One. 2013;8:e59828. doi: 10.1371/journal.pone.0059828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler SD, Shore SE. Stimulus timing-dependent plasticity in dorsal cochlear nucleus is altered in tinnitus. J Neurosci. 2013;33:19647–19656. doi: 10.1523/JNEUROSCI.2788-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb M, Smith RP. Relation of induced tinnitus to physical characteristics of the inducing stimuli. J Acoust Soc Am. 1967;42:453–455. doi: 10.1121/1.1910600. [DOI] [PubMed] [Google Scholar]
- Lonsbury-Martin BL, Martin GK. Temporary hearing loss from exposure to moderately intense tones in rhesus monkeys. Am J Otolaryngol. 1981;2:321–335. doi: 10.1016/s0196-0709(81)80042-7. [DOI] [PubMed] [Google Scholar]
- Lonsbury-Martin BL, Meikle MB. Neural correlates of auditory fatigue: frequency-dependent changes in activity of single cochlear nerve fibers. J Neurophysiol. 1978;41:987–1006. doi: 10.1152/jn.1978.41.4.987. [DOI] [PubMed] [Google Scholar]
- Ma WL, Hidaka H, May BJ. Spontaneous activity in the inferior colliculus of CBA/J mice after manipulations that induce tinnitus. Hear Res. 2006;212:9–21. doi: 10.1016/j.heares.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Meidinger MA, Hildebrandt-Schoenfeld H, Illing RB. Cochlear damage induces GAP-43 expression in cholinergic synapses of the cochlear nucleus in the adult rat: a light and electron microscopic study. Eur J Neurosci. 2006;23:3187–3199. doi: 10.1111/j.1460-9568.2006.04853.x. [DOI] [PubMed] [Google Scholar]
- Milbrandt JC, Hunter C, Caspary DM. Alterations of GABBA receptor subunit mRNA levels in the aging Fischer 344 rat inferior colliculus. J Comp Neurol. 1997;379:455–465. doi: 10.1002/(sici)1096-9861(19970317)379:3<455::aid-cne10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Milbrandt JC, Holder TM, Wilson MC, Salvi RJ, Caspary DM. GAD levels and muscimol binding in rat inferior colliculus following acoustic trauma. Hear Res. 2000;147:251–60. doi: 10.1016/s0378-5955(00)00135-0. [DOI] [PubMed] [Google Scholar]
- Misonou H, Mohapatra DP, Park EW, Leung V, Zhen D, Misonou K, Anderson AE, Trimmer JS. Regulation of ion channel localization and phosphorylation by neuronal activity. Nat Neurosci. 2004;7:711–718. doi: 10.1038/nn1260. [DOI] [PubMed] [Google Scholar]
- Morest DK, Kim J, Bohne BA. Neuronal and transneuronal degeneration of auditory axons in the brainstem after cochlear lesions in the chinchilla: cochleotopic and non-cochleotopic patterns. Hear Res. 1997;103:151–168. doi: 10.1016/s0378-5955(96)00172-4. [DOI] [PubMed] [Google Scholar]
- Muly SM, Gross JS, Potashner SJ. Noise trauma alters D-[3H]aspartate release and AMPA binding in chinchilla cochlear nucleus. J Neurosci Res. 2004;75:585–596. doi: 10.1002/jnr.20011. [DOI] [PubMed] [Google Scholar]
- Naccarato EF, Hunter WS. Aneasthesia effects of various ratios of ketamine and xylazine in rhesus monkeys (Macacca mulatta) Lab Anim. 1979;13:317–319. doi: 10.1258/002367779780943314. [DOI] [PubMed] [Google Scholar]
- Nelken I, Young ED. Two separate inhibitory mechanisms shape the responses of dorsal cochlear nucleus tyep IV units to narrowband and wideband stimuli. J Neurophysiol. 1994;71:2446–2462. doi: 10.1152/jn.1994.71.6.2446. [DOI] [PubMed] [Google Scholar]
- Nelson AB, Du Lac S. Regulation of firing response gain by calcium-dependent mechanisms in vestibular nucleus neurons. J Neurophysiol. 2002;87:2031–2042. doi: 10.1152/jn.00821.2001. [DOI] [PubMed] [Google Scholar]
- Norena AJ, Eggermont JJ. Enriched acoustic environment after noise trauma abolishes neural signs of tinnitus. Neuroreport. 2006;17:559–563. doi: 10.1097/00001756-200604240-00001. [DOI] [PubMed] [Google Scholar]
- Parsons JE, Lim E, Voigt HF. Type III units in the gerbil dorsal cochlear nucleus may be spectral notch detectors. Ann Biomed Eng. 2001;29:887–896. doi: 10.1114/1.1408924. [DOI] [PubMed] [Google Scholar]
- Portfors CV, Roberts PD. Temporal and frequency characteristics of cartwheel cells in the dorsal cochlear nucleus of the awake mouse. J Neurophysiol. 2007;98:744–756. doi: 10.1152/jn.01356.2006. [DOI] [PubMed] [Google Scholar]
- Potashner SJ, Suneja SK, Benson CG. Altered glycinergic synaptic activities in guinea pig brain stem auditory nuclei after unilateral cochlear ablation. Hear Res. 2000;147:125–136. doi: 10.1016/s0378-5955(00)00126-x. [DOI] [PubMed] [Google Scholar]
- Roberts LE, Eggermont JJ, Caspary DM, Shore SE, Melcher JR, Kaltenbach JA. Ringing ears: the neuroscience of tinnitus. J Neurosci. 2010;30:14972–14979. doi: 10.1523/JNEUROSCI.4028-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D, Irvine DR. Plasticity of frequency organization in auditory cortex of guinea pigs with partial unilateral deafness. J Comp Neurol. 1989;282:456–471. doi: 10.1002/cne.902820311. [DOI] [PubMed] [Google Scholar]
- Rodrigo J, Springall DR, Uttenthal O, Bentura ML, Abadia-Molina F, Riveros-Moreno V, Martínez-Murillo R, Polak JM, Moncada S. Localization of nitric oxide synthase in the adult rat brain. Philos Trans R Soc Lond B Biol Sci. 1994;345:175–221. doi: 10.1098/rstb.1994.0096. [DOI] [PubMed] [Google Scholar]
- Rubio ME. Redistribution of synaptic AMPA receptors at glutamatergic synapses in the dorsal cochlear nucleus as an early response to cochlear ablation in rats. Hear Res. 2006;216–217:154–167. doi: 10.1016/j.heares.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Schaette R, Kempter R. Development of tinnitus-related neuronal hyperactivity through homeostatic plasticity after hearing loss: a computational model. Eur J Neurosci. 2006;23:3124–3138. doi: 10.1111/j.1460-9568.2006.04774.x. [DOI] [PubMed] [Google Scholar]
- Shore SE. Plasticity of somatosensory inputs to the cochlear nucleus-implications for tinnitus. Hear Res. 2011;281:38–46. doi: 10.1016/j.heares.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore SE, Koehler S, Oldakowski M, Hughes LF, Syed S. Dorsal cochlear nucleus responses to somatosensory stimulation are enhanced after noise-induced hearing loss. Eur J Neurosci. 2008;27:155–168. doi: 10.1111/j.1460-9568.2007.05983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PH, Massie A, Joris PX. Acoustic stria: anatomy of physiologically characterized cells and their axonal projection patterns. J Comp Neurol. 2005;482:349–371. doi: 10.1002/cne.20407. [DOI] [PubMed] [Google Scholar]
- Smith SL, Otis TS. Persistent changes in spontaneous firing of purkinje neurons triggered by the nitric oxide signaling cascade. J Neruosci. 2003;23:367–372. doi: 10.1523/JNEUROSCI.23-02-00367.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suneja SK, Benson CG, Potashner SJ. Glycine receptors in adult guinea pig brain stem auditory nuclei: regulation after unilateral cochlear ablation. Exp Neurol. 1998;154:473–488. doi: 10.1006/exnr.1998.6946. [DOI] [PubMed] [Google Scholar]
- Stefanescu RA, Koehler SD, Shore SE. Stimulus-timing-dependent modifications of rate-level functions in animals with and without tinnitus. J Neurophysiol. 2015;113:956–970. doi: 10.1152/jn.00457.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano G, Abbott LF, Marder E. Activity-dependent changes in intrinsic properties of cultured neurons. Science. 1994;264:974–977. doi: 10.1126/science.8178157. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T, Kim Y, Oertel D, Trussell LO. Cell-specific, spike timing-dependent plasticity in the dorsal cochlear nucleus. Nat Neurosci. 2004;7:719–725. doi: 10.1038/nn1272. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T. Mechanisms of synaptic plasticity in the dorsal cochlear nucleus: plasticity-induced changes that could underlie tinnitus. Am J Audiol. 2008;17:S170–S175. doi: 10.1044/1059-0889(2008/07-0030). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt HF, Young ED. Evidence of inhibitory interneurons between neurons in dorsal cochlear nucleus. J physiol. 1980;44:76–96. doi: 10.1152/jn.1980.44.1.76. [DOI] [PubMed] [Google Scholar]
- Voigt HF, Young ED. Cross-correlation analysis of inhibitory interactions in dorsal cochlear nucleus. J Neurophysiol. 1990;64:1590–1610. doi: 10.1152/jn.1990.64.5.1590. [DOI] [PubMed] [Google Scholar]
- Wang H, Brozoski TJ, Turner JG, Ling L, Parrish JL, Hughes LF, Caspary DM. Plasticity at glycinergic synapses in dorsal cochlear nucleus of rats with behavioral evidence of tinnitus. neuroscience. 2009;164:747–759. doi: 10.1016/j.neuroscience.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting B, Moiseff A, Rubio ME. Cochlear nucleus neurons redistribute synaptic AMPA and glycine receptors in response to monaural conductive hearing loss. Neuroscience. 2009;163:1264–1276. doi: 10.1016/j.neuroscience.2009.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Saint Marie RL, Oliver DL. Granule cells in the cochlear nucleus sensitive to sound activation detected by Fos protein expression. Neurosci. 2005;136:865–882. doi: 10.1016/j.neuroscience.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Young ED, Brownell WE. Responses to tones and noise of single cells in dorsal cochlear nucleus of unanesthetized cats. J Neurophysiol. 1976;39:282–300. doi: 10.1152/jn.1976.39.2.282. [DOI] [PubMed] [Google Scholar]
- Zeng C, Nannapaneni N, Zhou J, Hughes LF, Shore S. Cochlear damage changes the distribution of vesicular glutamate transporters associated with auditory and non-auditory inputs to the cochlear nucleus. J Neurosci. 2009;29:4210–4217. doi: 10.1523/JNEUROSCI.0208-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JS, Kaltenbach JA. Increases in spontaneous activity in the dorsal cochlear nucleus of the rat following exposure to high-intensity sound. Neurosci Lett. 1998;250:197–200. doi: 10.1016/s0304-3940(98)00482-0. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Seung Lee H, Smith PF, Darlington CL. Neuronal nitric oxide synthase expression in the cochlear nucleus in a salicylate model of tinnitus. Brain Res. 2006;1123:201–206. doi: 10.1016/j.brainres.2006.09.045. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Tzounopoulos T. Physiological activation of cholinergic inputs controls associative synaptic plasticity via modulation of endocannabinoid signaling. J Neurosci. 2011;31:3158–3168. doi: 10.1523/JNEUROSCI.5303-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]