Abstract
Cataract is a major cause of visual dysfunction and the leading cause of blindness. Elevated levels of cadmium and lead have been found in the lenses of cataract patients, suggesting these metals may play a role in cataract risk. This study aimed to examine the associations of blood lead, blood cadmium and urinary cadmium with cataract risk. We identified 9,763 individuals aged 50 years and older with blood lead and cadmium levels, and a randomly selected subgroup of 3,175 individuals with available urinary cadmium levels, from the National Health and Nutrition Examination Surveys (NHANES) from 1999 to 2008 (mean age=63 years). Participants were considered to have cataract if they self-reported prior cataract surgery in NHANES’s vision examination. Odds ratios (ORs) and 95% confidence intervals (CIs) were computed using survey logistic regression models. We identified 1737 cataract surgery cases (the weighted prevalence=14.1%). With adjustment for age, race/ethnicity, gender, education, diabetes mellitus, body mass index, cigarette smoking (serum cotinine and pack-years) and urine hydration, every 2-fold increase in urinary cadmium was associated with a 23% higher risk of cataract surgery (OR=1.23, 95% CI: 1.04, 1.46, p =0.021). We found no associations of cataract surgery with blood cadmium (OR=0.97, 95% CI: 0.89, 1.07) and blood lead (OR=0.97, 95% CI: 0.88, 1.06). Mediation analysis showed that for the smoking-cadmium-cataract pathway, the ratio of smoking’s indirect effect to the total effect through cadmium was more than 50%. These results suggest that cumulative cadmium exposure may be an important under-recognized risk factor for cataract. However, these findings should be interpreted with a caution because of inconsistent results between urinary cadmium and blood cadmium.
Keywords: Cataract, cadmium, smoking, mediation analysis, NHANES
Introduction
Cataract is one of the major causes of visual impairment and the leading cause of blindness. Approximately 10.8 million people worldwide had cataract-induced vision loss in 2010 (Bourne et al., 2013). The prevalence of cataract in the United States in 2010 was 17% in people aged 40 years or older and more than 50% in people aged 70 years or older (Friedman et al., 2012). The physiopathology of cataract is not fully understood (Bobrow et al., 2015). It is believed that oxidative stress may initiate cataract formation through modification of lens epithelium: the excessive generation of reactive molecules can cause oxidative damage to functional DNA, proteins and lipids in lens cells, overwhelm the antioxidant defense system (such as superoxide dismutase, catalase, lipid peroxidases), impair DNA repair mechanisms, enhance apoptosis of lens epithelial cells, and induces protein and lipid aggregation in lens (Spector, 1995; Truscott, 2005; Thiagarajan and Manikandan, 2013; Manayi et al., 2015; Phaniendra et al., 2015; Babizhayev, 2011; Bobrow et al., 2015). Previous studies reported that concentrations of serum anti-oxidative enzymes and products of oxidative stress were relevant to the risk of cataract, although the association might vary across different subtypes (Beebe et al., 2010; Chang et al., 2013; Cui et al., 2013; Thiagarajan and Manikandan, 2013; Zoric et al., 2008).
Exposure to heavy metals, such as lead and cadmium, can lead to oxidative stress through depletion of glutathione and thiol pool and inhibition of the antioxidant defense systems (Ercal et al., 2001; Jomova and Valko, 2011; Valko et al., 2016). Several studies found elevated levels of lens lead and cadmium in patients who suffered cataract, especially among smokers (Cekic, 1998; Harding, 1995; Mosad et al., 2010; Rácz and Erdöhelyi, 1988; Ramakrishnan et al., 1995). One study reported cadmium might induce cell death in human lens epithelial cells (Kalariya et al., 2010). Few epidemiologic studies have examined the association of cataract with lead or cadmium. Only one study reported an association between cumulative lead exposure (measured by bone lead levels) and cataract in a subset of 642 male participants from the Normative Aging Study (NAS) (Schaumberg et al., 2004). Potential associations between heavy metals and other age-related eye diseases such as age-related macular degeneration (AMD) have also been reported (Erie et al., 2009). Recently, several studies using national survey data from the United States (Wu et al., 2014) and Korea (Hwang et al., 2015; Kim et al., 2014; Park et al., 2015) reported a positive association between blood or urinary cadmium and blood lead and AMD. Another small scale case-control study involving 12 patients also showed a positive association between cadmium levels in aqueous humor and AMD (Jünemann et al., 2013). However, we are unaware of previous epidemiologic studies evaluating the association between cadmium exposure and cataract and could find no reference to it on PubMed, although previous studies have suggested that cigarette smoking, one of the major sources of cadmium exposure, is strongly associated with cataract (Kelly et al., 2005; Ye et al., 2012).
In this study, our primary goal is to assess the associations of lead and cadmium exposure with cataract among adults aged 50 years and older using data from the 1999–2008 National Health and Nutrition Examination Survey (NHANES). Furthermore, we evaluated the potential mediation effect of cadmium on the smoking-cataract pathway.
Methods
Study Population
This study was based on NHANES, a national program of cross-sectional studies designed to analyze the physical status of the U.S. general population. Each cycle of NHANES is a cross-sectional survey. Each cycle is independent and includes different US representative samples. Survey protocols were approved by the National Center for Health Statistics Research Ethics Review Board, and all participants have provided written informed consent. Since cataract usually occurs in older population (young-onset cataracts are more likely to be non-age-related), we limited our study to 12781 participants aged 50 and older, from cycle 1999–2000 to cycle 2007–2008. Among these subjects we excluded 1522 individuals with missing value in blood lead or blood cadmium, and 667 subjects lacking cataract surgery information. After additional exclusion of subjects missing important covariates such as educational level, body mass index (BMI), history of diabetes, pack-years of cigarette smoking, and serum cotinine, 9763 individuals remained in our final dataset for analysis of blood lead and blood cadmium.
By design, urinary cadmium was only assessed in one-third of the NHANES sample, randomly selected from all eligible participants. We identified 3175 individuals aged 50 and older as the study population for analysis of urinary cadmium. Population characteristics are almost identical between two study groups.
Cataract Identification
Participants aged 12 years and older were asked whether they have had eye surgery for cataracts, before undertaking detailed vision examination, according to the NHANES’ Vision Procedures Manual (US Dept of Health and Human Services, 2005). Because an increasing rate and lower threshold of cataract surgery in U.S.,(Bobrow et al., 2015; Lundström et al., 2015) self-reported cataract surgery may roughly represent the existence of clinically significant cataract. This method was used in a previous study (Zhang et al., 2012). Participants who answered “yes” were considered as cataract cases. Those who were completely blind or had severe eye infection were excluded.
Blood Lead, Blood Cadmium and Urinary Cadmium Measurements
Blood lead and blood cadmium were measured at the Division of Laboratory Sciences, National Center for Environmental Health, the Centers for Disease Control and Prevention by a simultaneous multi-element atomic absorption spectrometer (SIMAA 6000; PerkinElmer, Norwalk, CT) with Zeeman background correction in NHANES 1999–2002 and by an inductive coupled plasma mass spectrometry (ICP-MS) (ELAN 6100; PerkinElmer, Shelton, CT) in NHANES 2003–2008. Urinary cadmium was measured by ICP-MS (ELAN 6100; PerkinElmer, Shelton, CT) in all NHANES cycles. A detailed protocol can be found in the NHANES Laboratory/Medical Technologist Procedures Manual (LPM) (US Dept of Health and Human Services; Hyattsville, MD, 2005). The lower detection limit for blood cadmium was 0.20 μg/L and for urinary cadmium was 0.04 μg/L in NHANES 1999–2008. The lower detection limits for blood lead were 0.30 μg/dL in NHANES 1999–2004, 0.25 μg/dL and 0.30 μg/dL in NAHNES 2005–06 and 0.25 μg/dL in NHANES 2007–08. Values below the detection limits were divided by the square root of two. Among the total 9763 observations with blood cadmium and lead levels, 644 (6.60%) and 7 (0.07%) were below the detection limits; among the 3175 subset with urinary cadmium levels, 59 (1.86%) were below the detection limits.
Urinary lead data was available in the NHANES cycles used in the present study but was not included in our analysis. Urinary lead is not considered a reliable biomarker for total body burden: it mainly secondarily reflects lead level in blood and soft tissues and bone, and even such reflection is still unreliable due to a large variation within and between individuals (Sommar et al., 2014).
Other Covariates
Socio-demographic variables such as age (year), gender (male/female), race/ethnicity (Non-Hispanic White, Mexican American, Non-Hispanic Black and other) and educational level (<high school, high school, some college and above) were obtained using computer-assisted personal interview methods. BMI was calculated as weight in kilograms divided by height in meters squared (kg/m2). Diabetes status (self-reported diagnosis or have taken insulin/diabetic pills, yes/no) was included since previous studies reported association between diabetes mellitus and cataracts (Thompson and Lakhani, 2015). Since cataract was highly related to smoking habits (Kelly et al., 2005; Ye et al., 2012), we included two smoking variables: pack-years of cigarettes and serum cotinine levels (ng/dL); the former indicates direct smoking amounts, whereas the latter indicates amounts of both direct and second-hand smoking (Lindsay et al., 2014). Urinary creatinine (mg/dL) was added to adjust for urine dilution in the urinary cadmium models. Since previous literature also suggested an association between cataract and antioxidant vitamin intakes (Cui et al., 2013), which may be associated with heavy metal body burdens (Mosad et al., 2010), we further included daily intake of vitamin A (mg), vitamin C (mg) and vitamin E (mg) by calculating the sum of dietary and supplement intakes in a sensitivity analysis.
Statistical Analysis
We used SAS 9.2 (SAS Institute Inc., Cary, NC. USA) and R 3.2.2 (https://www.r-project.org/) for all statistical analyses. All p-values for significance were 2-tailed (P<0.05). We combined 5 two-year cycles of NHANES dataset (1999–2008) for analysis. According to NHANES Analytic and Reporting Guidelines (Johnson et al., 2013), we calculated new sample weights for all 9763 observations to reduce sampling bias, by using the mobile examination center (MEC) exam weight (WTMEC2YR) for blood lead and blood cadmium. We also computed new sample weights for 3175 observations in the urinary cadmium subsample, by using the Heavy Metal Subsample 2 Year MEC Weight (WTSHM2YR, used in 1999–2002) and MEC weights of subsample A (WTSA2YR, used in 2003–2008). We compared characteristics of study participants by cataract status using survey t-tests for continuous variables and Rao-Scott chi-square tests for categorical variables. Because pack-years of cigarettes, urinary creatinine and metal concentrations were highly skewed, we computed median and interquartile range (IQR) or survey-weighted geometric means and 95% confidence intervals (CIs).
We used survey-weighted logistic regression models to examine the association between heavy metal exposures and the risk of cataract surgery. Models controlling for different confounders were fit based on the diagram (Fig 1): Model A controlled for age, gender, race/ethnicity, educational level (C1); Model B further controlled for diabetes status and BMI (C2); Model C further controlled for smoking variables (pack-years and serum cotinine). In order to correct for the dilution rate on the measurement of urinary cadmium, we applied two methods: covariate-adjustment method (CA) which adjusts for confounding causal pathways involving creatinine and disease (Barr et al., 2005; Schisterman et al., 2005); and a new method called “covariate-adjusted standardization plus covariate adjustment (CAS+CA)” which further adjusts for potential measurement error by hydration that is independent of covariates (O’Brien et al., 2016). In addition to adding creatinine as a covariate (CA method), we fitted a model using risk factors (age, gender, race/ethnicity, BMI and diabetes) to get predicted urinary creatinine level , then divided the observed urinary cadmium level by the ratio of observed and fitted creatinine to get a standardized exposure value (O’Brien et al., 2016). Compared with traditional CA method, the CAS+CA method provided a better estimation on the association between urinary chemicals and health outcomes (O’Brien et al., 2016). Since blood lead, blood cadmium and urinary cadmium levels are highly right skewed, we log-transformed all three exposures and computed odds ratios (ORs) and 95% CIs per 2-fold increase in each metal variable. We tested for effect modification by population characteristics (age, gender, race/ethnicity, and pack-years of cigarette) by adding an interaction term between continuous metal variable and each covariate into Model C.
Figure 1.
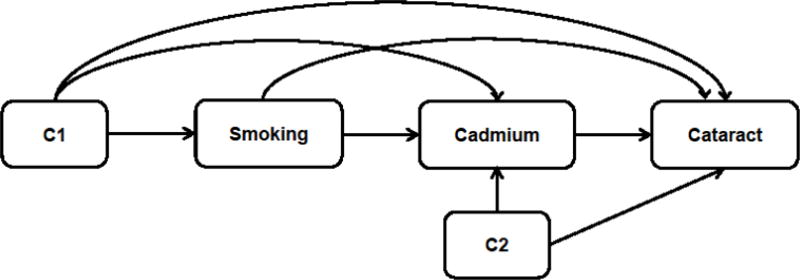
Diagram of smoking-cadmium-cataract pathway. C1: exposure-outcome confounders; C2: mediator-outcome confounders. C1 includes age, gender, race/ethnicity, educational level; C2 includes BMI and diabetes status.
For the mediation analysis of cadmium’s effect on the smoking-cataract pathway, we used categorized pack-years of cigarettes as exposure, log-transformed urinary cadmium as continuous mediator, and cataract surgery as binary outcome (Fig 1). Since there are several confounders and potential interactions in the model, we adopted formulas from Valeri and VanderWeele’s causal inference approach (Valeri and Vanderweele, 2013) to analyze the mediation effect of cadmium (Appendix). Valeri and VanderWeele’s causal inference approach for mediation analysis adapts counterfactual framework, allows clear separation of direct and indirect effects, regardless of interactions or nonlinearities, and is applicable even when confounders are present (Valeri and Vanderweele, 2013). We computed ORs of natural direct effect, natural indirect effect and total effect, and the proportion mediated values.
Results
The descriptive characteristics were similar between two study groups of blood lead/cadmium and urinary cadmium (Table 1 and Supplement Table 1). The mean age of participants was 63.2 (SE=0.2) years in the blood biomarker sample; individuals who had cataract surgery were older than those without cataract surgery (74.1 vs. 61.4 years). The urinary subsample had almost identical age distribution (mean age=63.3 (SE=0.2) years; mean age of the ever had cataract surgery vs. never: 74.2 years vs. 61.4 years). Of all 9763 participants, 4887 (53.3%) were females, 5650 (80.3%) were non-Hispanic white, 2358 (26.7%) had high school diploma and 4043 (51.4%) had at least some college degree, and 1774 (14.0%) had history of diabetes (Table 1). The mean BMI was 28.8 (SE = 0.1) kg/m2. Proportions of non-smokers (pack-years=0) and heavy smokers (pack-years≥20) were 48.2 and 27.0, respectively. The geometric mean of serum cotinine was 0.24 ng/dL (95% CI: 0.21, 0.28). The geometric means of blood lead, blood cadmium and urinary cadmium were 1.94 μg/dL (95% CI: 1.90, 1.99), 0.45 μg/L (95% CI: 0.43, 0.46), and 0.34 μg/L (95% CI: 0.32, 0.36), respectively. The survey weighted prevalence of cataract surgery was 14.1% (1,737 out of 9,763). Participants who had cataract surgery were statistically significantly older, non-Hispanic whites, female, less educated, more likely to have diabetes, smoked more, and had lower BMI. They had higher levels of blood lead, blood cadmium and urinary cadmium. Distributions of blood lead and cadmium by population characteristics were similar between the entire sample and the urinary cadmium subsample (data not shown). Levels of blood lead, blood cadmium, and urinary cadmium were significantly higher in those who were older, and less educated, and who smoked more (data not shown). There were moderate correlations among blood lead, blood cadmium and urinary cadmium (Spearman’s correlation coefficients: 0.22–0.46, P<0.001).
Table 1.
Survey-weighted characteristics of study participants by cataract status, NHANES, 1999–2008.
| Characteristics | total samples | Ever had cataract operation
|
P-valueb | |
|---|---|---|---|---|
| Yes | No | |||
| Total sample, N (%) | 9763 (100) | 1737 (14.1) | 8026 (85.9) | |
| Age, years | 63.2 ± 0.2 | 74.1 ± 0.3 | 61.4 ± 0.2 | <.001 |
| Female, n (%) | 4887 (53.3) | 947 (61.7) | 3940 (51.9) | <.001 |
| Race/ethnicity, n (%) | ||||
| Non-Hispanic White | 5640 (80.3) | 1221 (85.8) | 4419 (79.3) | <.001 |
| Mexican American | 1675 (3.9) | 196 (2.3) | 1479 (4.1) | |
| Non-Hispanic Black | 1667 (8.1) | 203 (5.9) | 1464 (8.5) | |
| Other | 781 (7.8) | 117 (6.0) | 664 (8.0) | |
| Education, n (%) | ||||
| <high school | 3362 (21.9) | 690 (31.0) | 2672 (20.4) | <.001 |
| High school | 2358 (26.7) | 420 (27.5) | 1938 (26.5) | |
| Some college+ | 4043 (51.4) | 627 (41.5) | 3416 (53.1) | |
| Diabetes, n (%) | 1774 (14.0) | 421 (22.1) | 1353 (12.7) | <.001 |
| Body mass index, kg/m2 | 28.8 ± 0.1 | 28.1 ± 0.2 | 28.9 ± 0.1 | <.001 |
| Pack-years, n (%) | ||||
| 0 | 4758 (48.2) | 843 (47.6) | 3915 (48.3) | 0.006 |
| >0 and <20 | 2481 (24.7) | 376 (21.7) | 2105 (25.2) | |
| ≥20 | 2524 (27.0) | 518 (30.7) | 2006 (26.4) | |
| Smoking status, n (%) | ||||
| Non-smoker | 4694 (47.5) | 826 (46.7) | 3868 (47.7) | <.001 |
| Former smoker | 3605 (36.5) | 746 (43.7) | 2859 (35.3) | |
| Current smoker | 1464 (16.0) | 165 (9.6) | 1299 (17.0) | |
| Serum cotinine, ng/dLa | 0.24 (0.21, 0.28) | 0.12 (0.10, 0.14) | 0.27 (0.22, 0.32) | <.001 |
| Blood lead, μg/dLa | 1.94 (1.90, 1.99) | 2.05 (1.98, 2.13) | 1.93 (1.88, 1.97) | <.001 |
| Blood cadmium, μg/Lac | 0.45 (0.43, 0.46) | 0.48 (0.46, 0.49) | 0.44 (0.43, 0.46) | <.001 |
|
| ||||
| Urinary sub-sample, N (%) | 3175 (100) | 587 (14.6) | 2588 (85.4) | |
| Blood lead, μg/dLa | 1.97 (1.92, 2.03) | 2.10 (1.96, 2.24) | 1.95 (1.90, 2.01) | 0.041 |
| Blood cadmium, μg/La | 0.45 (0.43, 0.46) | 0.51 (0.48, 0.54) | 0.44 (0.42, 0.45) | <.001 |
| Urinary cadmium, μg/La | 0.34 (0.32, 0.36) | 0.40 (0.36, 0.43) | 0.33 (0.31, 0.35) | <.001 |
| Urinary creatinine, mg/dLa | 81.3 (78.4, 84.4) | 75.2 (69.6, 81.2) | 82.5 (79.1, 86.0) | <.001 |
Geometric mean (95% confidence interval) is presented because of skewness
P-value based on t-tests for continuous variables and Rao-Scott Chi-squared tests for categorical variables.
SI conversion factors: To convert blood cadmium to nmol/L, multiply values by 8.896.
In logistic regression analyses, only urinary cadmium was associated with the risk of cataract surgery (Table 2). In the fully-adjusted model with adjustment for age, race/ethnicity, gender, education, diabetes status, BMI, and cigarette smoking (Model C), when using a traditional CA method for dilution adjustment, the OR per 2-fold increase in urinary cadmium was 1.26 (95% CI: 1.04, 1.53, p-value=0.021)); CAS+CA method did provided similar result (OR: 1.23, 95% CI: 1.04, 1.46, p-value=0.021). Further adjustment for antioxidant vitamins did not change the association (data not shown). We found no associations of cataract surgery with blood cadmium (OR=0.97, 95% CI: 0.89, 1.07) and blood lead (OR=0.97, 95% CI: 0.88, 1.06). Smoking, age, race/ethnicity and gender did not modify the association between urinary cadmium and cataract surgery; neither did blood lead and blood cadmium (data not shown).
Table 2.
Odds Ratios (95% Confidence Intervals) of Cataract Operation History by blood lead, blood cadmium and urinary cadmium concentrations
| Blood lead (n = 9763) | # cases/all | Model Aa | Model Bb | Model Cc |
|---|---|---|---|---|
| OR per 2-fold change in exposure | 1737/9763 | 0.95 (0.87, 1.04) | 1.00 (0.92, 1.09) | 0.97 (0.88, 1.06) |
| P-value | 0.30 | 0.99 | 0.47 | |
|
| ||||
| Blood cadmium (n = 9763) | ||||
|
| ||||
| OR per 2-fold change in exposure | 1737/9763 | 1.02 (0.95, 1.10) | 1.06 (0.99, 1.15) | 0.97 (0.89, 1.07) |
| P-value | 0.52 | 0.11 | .56 | |
|
| ||||
|
Urinary cadmium (n = 3175) UCr-unadjusted d |
||||
|
| ||||
| OR per 2-fold change in exposure | 587/3175 | 1.18 (1.05, 1.32) | 1.18 (1.05, 1.33) | 1.14 (0.99, 1.30) |
| P-value | 0.008 | 0.006 | 0.068 | |
|
| ||||
| Covariate-adjusted e | ||||
|
| ||||
| OR per 2-fold change in exposure | 587/3175 | 1.31 (1.13, 1.51) | 1.33 (1.14, 1.55) | 1.26 (1.04, 1.53) |
| P-value | <.001 | <.001 | 0.021 | |
|
| ||||
| Covariate-adjusted standardization plus covariate adjustment f | ||||
|
| ||||
| OR per 2-fold change in exposure | 587/3175 | 1.29 (1.13, 1.47) | 1.31 (1.15, 1.49) | 1.23 (1.04, 1.46) |
| P-value | <.001 | <.001 | 0.021 | |
Model A: adjusted for age, race, gender, educational level.
Model B: further adjusted for DM, BMI.
Model C: further adjusted for serum cotinine, categories of pack-year.
Urinary cadmium (UCd) models without adjusted for creatinine: logit[Pr(cataract)]=α + β × UCd + δ × W. W is the above covariates adjusted in different models.
CA methods: logit[Pr(cataract)]=α + β × UCd + λ × UCr + δ × W.
CAS+CA methods: .
In the mediation analysis, for the total effect among those with <20 pack-years of cigarette smoking (OR=1.28), the natural direct and natural indirect effects (ORs) were 1.13 and 1.14, and the proportion mediated through cadmium was 54% (Table 3). For the total effect among those with ≥20 pack-years (OR=1.65), the natural direct and indirect effects were 1.28 and 1.29, and the proportion mediated through cadmium was 57%.
Table 3.
Mediation analysis of cadmium effect on the smoking-cataract pathway by using causal inference approacha.
| Smoking Status | ORNDE | ORNIE | Total Effect | Proportion Mediated |
|---|---|---|---|---|
| <20 pack-years vs. non-smokers | 1.13 | 1.14 | 1.28 | 0.54 |
| ≥20 pack-years vs. non-smokers | 1.28 | 1.29 | 1.65 | 0.57 |
| Ever smoker vs. non-smoker | 1.20 | 1.21 | 1.45 | 0.55 |
NDE: Natural Direct Effect; NIE: Natural Indirect Effect; Total Effect: combination of Natural Direct and Indirect Effect; Proportion Mediated: the Proportion of NIE in Total Effect (see Appendix for details). Calculation based on Model C using CAS+CA method.
Discussion
Our study shows a possible link between long-term cadmium exposure and the risk of cataract surgery. In this large cross-sectional data with 5 cycles of NHANES, urinary cadmium was significantly associated with prevalent cataract surgery. However, there were no significant associations of blood cadmium and blood lead with cataract surgery. The mediation analysis suggested that in the smoking-cadmium-cataract association, more than 50% of the total effect of smoking on the risk of cataract is indirectly affected through cadmium.
A significant association was observed with urinary but not blood cadmium. One possible reason is that urinary cadmium is more relevant to cumulative cadmium exposure. Although the etiology is still unclear, age-related cataract may be more strongly related to long-term exposures. Since approximately 50% of the accumulated cadmium is stored in the kidney, urinary cadmium is widely used as a surrogate for cumulative cadmium exposure, which reflects the body cadmium load (Adams and Newcomb, 2014; Faroon et al., 2012; Järup, 2002; Järup and Akesson, 2009; Johri et al., 2010). However, a recent commentary suggests that low-level urinary cadmium is largely affected by factors other than cumulative cadmium exposure, such as recent cadmium intake and kidney function; the assumption that urinary cadmium is an indicator of cumulative cadmium life-burden is now being doubted (Bernard, 2016). To reduce such an impact, we conducted not only a conventional approach that includes urinary creatinine as a covariate in the models but also a CAS+CA(O’Brien et al., 2016), and the results were robust. We also conducted a sensitivity analysis excluding participants with chronic kidney disease but the results were consistent (data not shown).
On the other hand, although both blood cadmium and urinary cadmium may serve as biomarkers of cumulative exposure in the general population with low-level exposure (Järup et al., 1998), a general understanding is that urinary cadmium reflects long-term exposure whereas blood cadmium reflects more recent exposure (Järup and Akesson, 2009; Lauwerys et al., 1994). A recent study examining NHANES data reported that blood cadmium explained about 30% of the variation in urinary cadmium (Adams and Newcomb, 2014). The authors suggested that urinary cadmium is a more appropriate biomarker if chronic diseases with long latency are examined. Several previous studies have also reported inconsistent associations between blood cadmium and urinary cadmium (Ali et al., 2014; Buser et al., 2016). Given that cataract is a chronic disease with long latency, weaker associations found with blood cadmium in our study may be due to non-differential measurement error which may lead the observed associations toward the null (Tellez-Plaza et al., 2012).
In our study, we considered two smoking variables: pack-years of cigarettes as an indicator of cumulative smoking, and serum cotinine as an indicator of short-term first- and second-hand smoke. Interestingly, individuals with cataract surgery had lower serum cotinine concentrations than those without cataract surgery (Table 1). This seems to be because individuals who had cataract surgery are more likely to have stopped smoking in the past (the proportions of former and current smokers between individuals with and without cataract surgery: 43.7% and 9.6% vs. 35.3% and 17.0%, respectively. Table 1). We also found a significant positive marginal association of cataract surgery with pack-years but not with serum cotinine (data not shown). This also suggests that short-term biomarkers such as blood cadmium and serum cotinine may not be sensitive enough to capture risk of chronic diseases including cataract.
Histologically, the human lens is formed by a single layer of epithelial cells and layers of lens cells which compact centrally; new lens cells are formed externally to the older cells, then gradually stretch and lose organelles and move centrally (Thompson and Lakhani, 2015). One potential biological mechanism of cadmium-induced cataract is that toxic free cadmium ion (Cd2+) may be accumulated in the mitochondria of lens cells, disrupt the respiratory chain, and generate reactive oxygen species (ROS) (Wang et al., 2004); intracellular Cd2+ may also accelerate the degeneration of organelles, which could theoretically lead to the lens cells becoming more susceptible to photo-oxidative effects (Thompson and Lakhani, 2015). Such oxidative damage could result in cataract. Cd2+ could directly affect DNA structure and gene expression (Johri et al., 2010), and interrupt the production of cellular proteins, which may reduce the transparency of lens cells. Through long-term cumulative cadmium exposure, the intracellular structure of lens cells could be damaged, consequently resulting in cataract formation.
Our study calculated natural direct and indirect effect of cadmium on smoking-cataract relationship by Valeri and VanderWeele’s causal inference approach. The finding suggests that cadmium could be a mediator on the causal pathway between smoking and cataract. In the smoking-cadmium-cataract pathway, the ratio of smoking’s natural indirect effect to the total effect was more than 50%. Previous studies reported that one who smokes 20 cigarettes per day may absorb roughly 1 μg cadmium daily by lung (Järup and Akesson, 2009). Although other pathogenic components of cigarettes may also cause cataract, cadmium could be a major inducer of cataract in cigarette smokers. A previous mediation analysis reported that cadmium plays a major role in the smoking-telomere-length association, which is consistent with our result, and taken together implies that cadmium might be a major toxin in cigarettes (Zota et al., 2015).
The present study found no association between blood lead and cataract surgery. Blood lead reflects recent exposure rather than cumulative exposure since its half-life is only about 1 month (Hu et al., 2007). Bone lead, which is the majority of lead body burden and has a half-life of decades, might be a better biomarker of cumulative lead exposure (Hu et al., 2007). Schaumberg et al. found a significant positive association of cataract with bone lead level (especially tibia lead level) among men aged 60 and older in a subset of the Normative Aging Study, but no association with blood lead (Schaumberg et al., 2004). Although our results showed no association between blood lead and cataract surgery, it does not rule out that cumulative low-dose lead exposure has an effect on cataract. Future studies with cumulative lead assessed by bone lead levels should be warranted to determine the role of lead in cataract risk.
Although our study benefits from a large sample size and nationally representative study population, a number of limitations deserve consideration. A primary limitation relates to the cross-sectional nature of the study data and the corresponding limitations as regards the temporality of exposure and cataract development. The methodology of NHANES’ Vision Examination also fails to identify those with prevalent cataract but no cataract surgery, which may underestimate the prevalence. Additionally, NHANES’ Vision Examination did not provide either the age at onset or the subtype of cataract (nuclear, cortical, posterior subcapsular), and the etiology and risk factors for each subtype may differ (Beebe et al., 2010; Zoric et al., 2008). Given that ROS and related oxidative stress is a primary underlying biological mechanism behind cadmium-related cataract risk and oxidative stress is more relevant to the etiology of nuclear cataract (Beebe et al., 2010; Zoric et al., 2008), subtype-specific analyses may provide more accurate cadmium effects.
We did not consider radiation exposure in our analysis because the radiation exposure information was not available in NHANES. Radiation exposure (especially ultraviolet B (UVB) through sunlight) is an important risk factor for cataract (Asbell et al., 2005; Bobrow et al., 2015). However, if radiation exposure is not related to lead or cadmium exposure, it is not a confounder of the association between lead/cadmium and cataract surgery and therefore, it is not necessarily adjusted for in regression models. In the general population examined in our study, we believe that outdoor sunlight exposure which depends on individual behaviors and climate conditions may not be related to environmental exposure to lead and cadmium. There might be some participants whose occupations are related to both lead or cadmium and radiation exposures but the number of such participants might be very small, and therefore the likelihood that the observed findings are due to confounding by radiation is low. However, we cannot rule out that the observed association between urinary cadmium and cataract surgery is due to unmeasured confounding.
According to Bourne et al.’s review (2013), cataract has caused 33% of blindness globally, which represents more than 10 million people (Bourne et al., 2013). Since cataract is highly associated with aging (Thompson and Lakhani, 2015), with life expectancy increasing, higher numbers of people with cataract will continue to pressure the healthcare infrastructure: not only does it decrease individuals’ life quality, but also brings huge burden for society by creating a larger sight-disabled population, especially in developing countries. Reduction or delay in cataract formation by even 10 years was once estimated to cut the burden of need for surgery by nearly ½ (Vision research, 1983). In order to prevent the disease, relevant research on etiology of cataract is needed. If modifiable risk factors for cataract can be identified and exposure to them reduced, it may be possible to impact the societal burden that cataract imposes. Our findings suggest that cadmium exposure could be one such risk factor. The main source of non-occupational cadmium exposure is tobacco smoking and food (Satarug et al., 2010; Satarug and Moore, 2004). Although the evidence is sufficient, the public awareness of smoking-related ocular risks is relatively low compared with other diseases (Bidwell et al., 2005; Handa et al., 2011; Ng et al., 2010); education of such knowledge could help tobacco control (Asfar et al., 2015). Previous studies reported that the “smoking causes blindness” label on cigarette packages used in Australian anti-smoking campaign did increase the smoker’s awareness of risk of blindness, and prompt them to quit smoking (Kennedy et al., 2012; Li et al., 2012). Continued efforts at reduction of cigarette smoking seem warranted. Dietary source of cadmium exposure, such as cadmium-aggregated animals and plants, and cadmium-contaminated water are also of concern (Järup, 2002).
In summary, the present study identified a significant increase in risk of cataract surgery associated with higher urinary cadmium exposure, and further analyses suggested that over 50% of the increased risk of cataract surgery from cigarette smoking may be due to increased cadmium exposure. However, with the discrepancy between blood cadmium and urinary cadmium, our study finding should be interpreted with a caution and further investigations are still required. Taken together with the Surgeon General’s finding that smoking causes cataract (Office of the Surgeon General (US) and Office on Smoking and Health (US), 2004), we think the current findings provide an impetus for renewed public health efforts to achieve reductions in cigarette smoking, which could have a major impact on the enormous global economic burden imposed by cataract.
Supplementary Material
Acknowledgments
This study was supported by the National Institute of Environmental Health Sciences (NIEHS) grant K01-ES016587 and P30-ES017885, and by the Center for Disease Control and Prevention (CDC)/National Institute for Occupational Safety and Health (NIOSH) grant T42-OH008455.
Appendix
Adapting Valeri and VanderWeele’s causal inference approach for mediation analysis(Valeri and Vanderweele, 2013), when the outcome cataract status is binary and mediator urinary cadmium is continuous, the outcome can be modeled by logistic regression (1), and the mediator can be modeled via linear regression (2). Odds Ratios representing Natural Direct Effect (NDE) and Natural Indirect Effect (NIE) can be calculated using parameters derived from regression (1) and (2). Since we found no significant interaction in our study, θ3 for the mediated interaction term was 0. (a: pack-year category for comparison; a*: reference pack-year = 0; m: urinary cadmium level; c: confounders; σ2: variance of the error term in regression for the mediator).
| (1) |
| (2) |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: None reported.
Contributor Information
Weiye Wang, Email: weiyew@umich.edu.
Debra A. Schaumberg, Email: dschaumberg0@shire.com.
Sung Kyun Park, Email: sungkyun@umich.edu.
References
- Adams SV, Newcomb PA. Cadmium blood and urine concentrations as measures of exposure: NHANES 1999–2010. J Expo Sci Environ Epidemiol. 2014;24:163–170. doi: 10.1038/jes.2013.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali I, Engström A, Vahter M, Skerfving S, Lundh T, Lidfeldt J, Samsioe G, Halldin K, Åkesson A. Associations between cadmium exposure and circulating levels of sex hormones in postmenopausal women. Environ Res. 2014;134:265–269. doi: 10.1016/j.envres.2014.08.009. [DOI] [PubMed] [Google Scholar]
- Asbell PA, Dualan I, Mindel J, Brocks D, Ahmad M, Epstein S. Age-related cataract. Lancet Lond Engl. 2005;365:599–609. doi: 10.1016/S0140-6736(05)17911-2. [DOI] [PubMed] [Google Scholar]
- Asfar T, Lam BL, Lee DJ. Smoking Causes Blindness: Time for Eye Care Professionals to Join the Fight Against Tobacco. Invest Ophthalmol Vis Sci. 2015;56:1120–1121. doi: 10.1167/iovs.15-16479. [DOI] [PubMed] [Google Scholar]
- Babizhayev MA. Mitochondria induce oxidative stress, generation of reactive oxygen species and redox state unbalance of the eye lens leading to human cataract formation: disruption of redox lens organization by phospholipid hydroperoxides as a common basis for cataract disease. Cell Biochem Funct. 2011;29:183–206. doi: 10.1002/cbf.1737. [DOI] [PubMed] [Google Scholar]
- Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113:192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe DC, Holekamp NM, Shui YB. Oxidative damage and the prevention of age-related cataracts. Ophthalmic Res. 2010;44:155–165. doi: 10.1159/000316481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard A. Confusion about Cadmium Risks: The Unrecognized Limitations of an Extrapolated Paradigm. Environ Health Perspect. 2016;124:1–5. doi: 10.1289/ehp.1509691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwell G, Sahu A, Edwards R, Harrison RA, Thornton J, Kelly SP. Perceptions of blindness related to smoking: a hospital-based cross-sectional study. Eye Lond Engl. 2005;19:945–948. doi: 10.1038/sj.eye.6701955. [DOI] [PubMed] [Google Scholar]
- Bobrow JC, Beardsley TL, Jick SL, Rsenberg LF, Wiggins MN, Reich J, Isbey E. 2015–2016 Basic and Clinical Science Course, Section 11. American Academy of Ophthalmology; 2015. Lens and Cataract. [Google Scholar]
- Bourne RRA, Stevens GA, White RA, Smith JL, Flaxman SR, Price H, Jonas JB, Keeffe J, Leasher J, Naidoo K, Pesudovs K, Resnikoff S, Taylor HR, Vision Loss Expert Group Causes of vision loss worldwide, 1990–2010: a systematic analysis. Lancet Glob Health. 2013;1:e339–349. doi: 10.1016/S2214-109X(13)70113-X. [DOI] [PubMed] [Google Scholar]
- Buser MC, Ingber SZ, Raines N, Fowler DA, Scinicariello F. Urinary and blood cadmium and lead and kidney function: NHANES 2007–2012. Int J Hyg Environ Health. 2016;219:261–267. doi: 10.1016/j.ijheh.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cekic O. Effect of cigarette smoking on copper, lead, and cadmium accumulation in human lens. Br J Ophthalmol. 1998;82:186–188. doi: 10.1136/bjo.82.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D, Zhang X, Rong S, Sha Q, Liu P, Han T, Pan H. Serum antioxidative enzymes levels and oxidative stress products in age-related cataract patients. Oxid Med Cell Longev. 2013;2013:587826. doi: 10.1155/2013/587826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui YH, Jing CX, Pan HW. Association of blood antioxidants and vitamins with risk of age-related cataract: a meta-analysis of observational studies. Am J Clin Nutr. 2013;98:778–786. doi: 10.3945/ajcn.112.053835. [DOI] [PubMed] [Google Scholar]
- Ercal N, Gurer-Orhan H, Aykin-Burns N. Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem. 2001;1:529–539. doi: 10.2174/1568026013394831. [DOI] [PubMed] [Google Scholar]
- Erie JC, Good JA, Butz JA. Excess lead in the neural retina in age-related macular degeneration. Am J Ophthalmol. 2009;148:890–894. doi: 10.1016/j.ajo.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Faroon O, Ashizawa A, Wright S, Tucker P, Jenkins K, Ingerman L, Rudisill C. Toxicological Profile for Cadmium, Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profiles. Agency for Toxic Substances and Disease Registry (US); Atlanta (GA): 2012. [PubMed] [Google Scholar]
- Friedman DS, O’Colmain BJ, Mestril I. Cataract Prevalence by Age, 2012 Fifth Edition of Vision Problems in the US. 2012 [WWW Document]. URL http://www.visionproblemsus.org/cataract/cataract-by-age.html (accessed 10.4.15)
- Handa S, Woo JH, Wagle AM, Htoon HM, Au Eong KG. Awareness of blindness and other smoking-related diseases and its impact on motivation for smoking cessation in eye patients. Eye Lond Engl. 2011;25:1170–1176. doi: 10.1038/eye.2011.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding JJ. Cigarettes and cataract: cadmium or a lack of vitamin C? Br J Ophthalmol. 1995;79:199–200. doi: 10.1136/bjo.79.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Shih R, Rothenberg S, Schwartz BS. The epidemiology of lead toxicity in adults: measuring dose and consideration of other methodologic issues. Environ Health Perspect. 2007;115:455–462. doi: 10.1289/ehp.9783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang HS, Lee SB, Jee D. Association between Blood Lead Levels and Age-Related Macular Degeneration. PloS One. 2015;10:e0134338. doi: 10.1371/journal.pone.0134338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järup L. Cadmium overload and toxicity. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2002;17(Suppl 2):35–39. doi: 10.1093/ndt/17.suppl_2.35. [DOI] [PubMed] [Google Scholar]
- Järup L, Akesson A. Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol. 2009;238:201–208. doi: 10.1016/j.taap.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Järup L, Berglund M, Elinder CG, Nordberg G, Vahter M. Health effects of cadmium exposure–a review of the literature and a risk estimate. Scand J Work Environ Health. 1998;24(Suppl 1):1–51. [PubMed] [Google Scholar]
- Johnson C, Paulose-Ram R, Ogden C. National Health and Nutrition Examination Survey: Analytic guidelines, 1999–2010. National Center for Health Statistics. Vital Health Stat 2. 2013 [PubMed] [Google Scholar]
- Johri N, Jacquillet G, Unwin R. Heavy metal poisoning: the effects of cadmium on the kidney. Biometals Int J Role Met Ions Biol Biochem Med. 2010;23:783–792. doi: 10.1007/s10534-010-9328-y. [DOI] [PubMed] [Google Scholar]
- Jomova K, Valko M. Advances in metal-induced oxidative stress and human disease. Toxicology. 2011;283:65–87. doi: 10.1016/j.tox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Jünemann AGM, Stopa P, Michalke B, Chaudhri A, Reulbach U, Huchzermeyer C, Schlötzer-Schrehardt U, Kruse FE, Zrenner E, Rejdak R. Levels of Aqueous Humor Trace Elements in Patients with Non-Exsudative Age-related Macular Degeneration: A Case-control Study. PLoS ONE. 2013;8:e56734. doi: 10.1371/journal.pone.0056734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalariya NM, Nair B, Kalariya DK, Wills NK, van Kuijk FJGM. Cadmium-induced induction of cell death in human lens epithelial cells: implications to smoking associated cataractogenesis. Toxicol Lett. 2010;198:56–62. doi: 10.1016/j.toxlet.2010.04.021. [DOI] [PubMed] [Google Scholar]
- Kelly SP, Thornton J, Edwards R, Sahu A, Harrison R. Smoking and cataract: review of causal association. J Cataract Refract Surg. 2005;31:2395–2404. doi: 10.1016/j.jcrs.2005.06.039. [DOI] [PubMed] [Google Scholar]
- Kennedy RD, Spafford MM, Behm I, Hammond D, Fong GT, Borland R. Positive impact of Australian “blindness” tobacco warning labels: findings from the ITC four country survey. Clin Exp Optom. 2012;95:590–598. doi: 10.1111/j.1444-0938.2012.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EC, Cho E, Jee D. Association between blood cadmium level and age-related macular degeneration in a representative Korean population. Invest Ophthalmol Vis Sci. 2014;55:5702–5710. doi: 10.1167/iovs.14-14774. [DOI] [PubMed] [Google Scholar]
- Lauwerys RR, Bernard AM, Roels HA, Buchet JP. Cadmium: exposure markers as predictors of nephrotoxic effects. Clin Chem. 1994;40:1391–1394. [PubMed] [Google Scholar]
- Li L, Borland R, Yong HH, Hitchman SC, Wakefield MA, Kasza KA, Fong GT. The association between exposure to point-of-sale anti-smoking warnings and smokers’ interest in quitting and quit attempts: findings from the International Tobacco Control Four Country Survey. Addict Abingdon Engl. 2012;107:425–433. doi: 10.1111/j.1360-0443.2011.03668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay RP, Tsoh JY, Sung HY, Max W. Secondhand smoke exposure and serum cotinine levels among current smokers in the USA. Tob Control. 2014 doi: 10.1136/tobaccocontrol-2014-051782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundström M, Goh PP, Henry Y, Salowi MA, Barry P, Manning S, Rosen P, Stenevi U. The changing pattern of cataract surgery indications: a 5-year study of 2 cataract surgery databases. Ophthalmology. 2015;122:31–38. doi: 10.1016/j.ophtha.2014.07.047. [DOI] [PubMed] [Google Scholar]
- Manayi A, Abdollahi M, Raman T, Nabavi SF, Habtemariam S, Daglia M, Nabavi SM. Lutein and cataract: from bench to bedside. Crit Rev Biotechnol. 2015:1–11. doi: 10.3109/07388551.2015.1049510. [DOI] [PubMed] [Google Scholar]
- Mosad SM, Ghanem AA, El-Fallal HM, El-Kannishy AM, El Baiomy AA, Al-Diasty AM, Arafa LF. Lens cadmium, lead, and serum vitamins C, E, and beta carotene in cataractous smoking patients. Curr Eye Res. 2010;35:23–30. doi: 10.3109/02713680903362880. [DOI] [PubMed] [Google Scholar]
- Ng DHL, Roxburgh STD, Sanjay S, Au Eong KG. Awareness of smoking risks and attitudes towards graphic health warning labels on cigarette packs: a cross-cultural study of two populations in Singapore and Scotland. Eye. 2010;24:864–868. doi: 10.1038/eye.2009.208. [DOI] [PubMed] [Google Scholar]
- O’Brien KM, Upson K, Cook NR, Weinberg CR. Environmental Chemicals in Urine and Blood: Improving Methods for Creatinine and Lipid Adjustment. Environ Health Perspect. 2016;124:220–227. doi: 10.1289/ehp.1509693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of the Surgeon General (US), Office on Smoking and Health (US) The Health Consequences of Smoking: A Report of the Surgeon General, Reports of the Surgeon General. Centers for Disease Control and Prevention (US); Atlanta (GA): 2004. [Google Scholar]
- Park SJ, Lee JH, Woo SJ, Kang SW, Park KH, Epidemiologic Survey Committee of Korean Ophthalmologic Society Five heavy metallic elements and age-related macular degeneration: Korean National Health and Nutrition Examination Survey, 2008–2011. Ophthalmology. 2015;122:129–137. doi: 10.1016/j.ophtha.2014.07.039. [DOI] [PubMed] [Google Scholar]
- Phaniendra A, Jestadi DB, Periyasamy L. Free radicals: properties, sources, targets, and their implication in various diseases. Indian J Clin Biochem IJCB. 2015;30:11–26. doi: 10.1007/s12291-014-0446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rácz P, Erdöhelyi A. Cadmium, lead and copper concentrations in normal and senile cataractous human lenses. Ophthalmic Res. 1988;20:10–13. doi: 10.1159/000266248. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan S, Sulochana KN, Selvaraj T, Abdul Rahim A, Lakshmi M, Arunagiri K. Smoking of beedies and cataract: cadmium and vitamin C in the lens and blood. Br J Ophthalmol. 1995;79:202–206. doi: 10.1136/bjo.79.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satarug S, Garrett SH, Sens MA, Sens DA. Cadmium, Environmental Exposure, and Health Outcomes. Environ Health Perspect. 2010;118:182–190. doi: 10.1289/ehp.0901234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satarug S, Moore MR. Adverse health effects of chronic exposure to low-level cadmium in foodstuffs and cigarette smoke. Environ Health Perspect. 2004;112:1099–1103. doi: 10.1289/ehp.6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaumberg DA, Mendes F, Balaram M, Dana MR, Sparrow D, Hu H. Accumulated lead exposure and risk of age-related cataract in men. JAMA. 2004;292:2750–2754. doi: 10.1001/jama.292.22.2750. [DOI] [PubMed] [Google Scholar]
- Schisterman EF, Whitcomb BW, Louis GMB, Louis TA. Lipid adjustment in the analysis of environmental contaminants and human health risks. Environ Health Perspect. 2005;113:853–857. doi: 10.1289/ehp.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommar JN, Hedmer M, Lundh T, Nilsson L, Skerfving S, Bergdahl IA. Investigation of lead concentrations in whole blood, plasma and urine as biomarkers for biological monitoring of lead exposure. J Expo Sci Environ Epidemiol. 2014;24:51–57. doi: 10.1038/jes.2013.4. [DOI] [PubMed] [Google Scholar]
- Spector A. Oxidative stress-induced cataract: mechanism of action. FASEB J Off Publ Fed Am Soc Exp Biol. 1995;9:1173–1182. [PubMed] [Google Scholar]
- Tellez-Plaza M, Navas-Acien A, Menke A, Crainiceanu CM, Pastor-Barriuso R, Guallar E. Cadmium exposure and all-cause and cardiovascular mortality in the U.S. general population. Environ Health Perspect. 2012;120:1017–1022. doi: 10.1289/ehp.1104352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagarajan R, Manikandan R. Antioxidants and cataract. Free Radic Res. 2013;47:337–345. doi: 10.3109/10715762.2013.777155. [DOI] [PubMed] [Google Scholar]
- Thompson J, Lakhani N. Cataracts Prim Care. 2015;42:409–423. doi: 10.1016/j.pop.2015.05.012. [DOI] [PubMed] [Google Scholar]
- Truscott RJW. Age-related nuclear cataract-oxidation is the key. Exp Eye Res. 2005;80:709–725. doi: 10.1016/j.exer.2004.12.007. [DOI] [PubMed] [Google Scholar]
- US Dept of Health and Human Services. National Health and Nutrition Examination Survey (NHANES): Vision Procedures Manual [WWW Document] 2005 URL http://www.cdc.gov/nchs/data/nhanes/nhanes_05_06/VI.pdf (accessed 9.6.15)
- US Dept of Health and Human Services. Hyattsville MD. National Health and Nutrition Examination Survey (NHANES) Lab Procedures Manual [WWW Document] 2005 URL http://www.cdc.gov/nchs/data/nhanes/nhanes_05_06/lab.pdf (accessed 9.6.15)
- Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18:137–150. doi: 10.1037/a0031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko M, Jomova K, Rhodes CJ, Kuča K, Musílek K. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch Toxicol. 2016;90:1–37. doi: 10.1007/s00204-015-1579-5. [DOI] [PubMed] [Google Scholar]
- Vision research: a national plan: 1983–1987. U.S. Dept. of Health and Human Services, Public Health Service, National Institutes of Health; 1983. [Google Scholar]
- Wang Y, Fang J, Leonard SS, Rao KMK. Cadmium inhibits the electron transfer chain and induces reactive oxygen species. Free Radic Biol Med. 2004;36:1434–1443. doi: 10.1016/j.freeradbiomed.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Wu EW, Schaumberg DA, Park SK. Environmental cadmium and lead exposures and age-related macular degeneration in U.S. adults: the National Health and Nutrition Examination Survey 2005 to 2008. Environ Res. 2014;133:178–184. doi: 10.1016/j.envres.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, He J, Wang C, Wu H, Shi X, Zhang H, Xie J, Lee SY. Smoking and risk of age-related cataract: a meta-analysis. Invest Ophthalmol Vis Sci. 2012;53:3885–3895. doi: 10.1167/iovs.12-9820. [DOI] [PubMed] [Google Scholar]
- Zhang X, Cotch MF, Ryskulova A, Primo SA, Nair P, Chou CF, Geiss LS, Barker LE, Elliott AF, Crews JE, Saaddine JB. Vision health disparities in the United States by race/ethnicity, education, and economic status: findings from two nationally representative surveys. Am J Ophthalmol. 2012;154:S53–62.e1. doi: 10.1016/j.ajo.2011.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoric L, Elek-Vlajic S, Jovanovic M, Kisic B, Djokic O, Canadanovic V, Cosic V, Jaksic V. Oxidative stress intensity in lens and aqueous depending on age-related cataract type and brunescense. Eur J Ophthalmol. 2008;18:669–674. doi: 10.1177/112067210801800501. [DOI] [PubMed] [Google Scholar]
- Zota AR, Needham BL, Blackburn EH, Lin J, Park SK, Rehkopf DH, Epel ES. Associations of cadmium and lead exposure with leukocyte telomere length: findings from National Health and Nutrition Examination Survey, 1999–2002. Am J Epidemiol. 2015;181:127–136. doi: 10.1093/aje/kwu293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


