Abstract
Epithelial K+ channels are essential for maintaining electrolyte and fluid homeostasis in the kidney. It is recognized that basolateral inward-rectifying K+ (Kir) channels play an important role in the control of resting membrane potential and trans-epithelial voltage, thereby modulating water and electrolyte transport in the distal part of nephron and collecting duct. Monomeric Kir4.1 (encoded by Kcnj10 gene) and heteromeric Kir4.1/Kir5.1 (Kir4.1 together with Kir5.1 (Kcnj16)) channels are abundantly expressed at the basolateral membranes of the distal convoluted tubule and the cortical collecting duct cells. Loss-of-function mutations in KCNJ10 cause EAST/SeSAME tubulopathy in humans associated with salt wasting, hypomagnesemia, metabolic alkalosis, and hypokalemia. In contrast, mice lacking Kir5.1 have severe renal phenotype that, apart from hypokalemia, is the opposite of the phenotype seen in EAST/SeSAME syndrome. Experimental advances using genetic animal models provided critical insights into the physiological role of these channels in electrolyte homeostasis and the control of kidney function. Here, we discuss current knowledge about K+ channels at the basolateral membrane of the distal tubules with specific focus on the homomeric Kir4.1 and heteromeric Kir4.1/Kir5.1 channels. Recently identified molecular mechanisms regulating expression and activity of these channels, such as cell acidification, dopamine, insulin and insulin-like growth factor-1, Src family protein tyrosine kinases etc, as well as the role of these channels in NCC-mediated transport in the distal convoluted tubules, are also described.
Keywords: collecting duct, distal convoluted tubule, Kcnj10, Kcnj16, NCC, resting membrane potential
Introduction
In the kidney, discretionary Na+ reabsorption and K+ secretion in the distal part of the nephron and collecting duct (CD) are responsible for the fine tuning of water and electrolyte homeostasis (Staruschenko, 2012). Inwardly rectifying K+ channels (Kir), specifically Kir4.1 and Kir5.1 (encoded by Kcnj10 and Kcnj16 genes, respectively), are essential for the control of basolateral membrane potential and K+ recycling in the distal convoluted tubules (DCT) and cortical collecting ducts (CCD). This recycling is necessary to maintain a stable source of extracellular K+ in order to perform trans-cellular Na+ reabsorption driven by the Na+/K+-ATPase (Tanemoto, 2007). Thus, the functional expression of K+ channels in these tubule segments is critical for electrolyte homeostasis. In humans, loss-of-function mutations in the KCNJ10 gene have been shown to cause multiple neurological disorders such as epilepsy beginning in infancy, displayed motor impairment with ataxia and sensorineural deafness, and renal salt-losing tubulopathy, all together named SeSAME/EAST syndrome (Bockenhauer et al., 2009, Scholl et al., 2009). Additional analysis revealed that these mutations lead to severe salt wasting, hypomagnesemia, metabolic alkalosis, and hypokalemia, which possibly represent a consequence of defects in the kidney. Moreover, the lack of Kcnj10 resulted in decreased expression of the thiazide-sensitive Na-Cl cotransporter (NCC, encoded by Slc12a3 gene) in DCT (Zhang et al., 2014) and increased expression of the epithelial Na+ channel (ENaC; α-, β-, and γ-subunits encoded by Scnn1a, Scnn1b and Scnn1g, respectively) and water channel aquaporin 2 (Aqp2) in CCD (Su et al., 2016). Targeted disruption of the Kcnj16 gene in mice resulted in hypokalemic, hyperchloremic metabolic acidosis with hypercalciuria (Paulais et al., 2011). Despite these important findings, the role of basolateral K+ channels in the distal parts of nephron and CD has not been extensively studied. The major research focus in this area was devoted to the K+ secretion mediated by the apical renal outer medullary K+ channel (ROMK, Kir1.1; encoded by Kcnj1) and the Ca2+ activated big K+ channels (big K, BK; pore-forming α-subunit is encoded by Kcnma1, there are also several ancillary subunits (Pluznick et al., 2005, Wen et al., 2014, Larsen et al., 2016)). For details please see excellent reviews (Wang and Giebisch, 2009, Welling, 2016) summarizing the role of ROMK and BK channels in the distal renal tubule.
Potassium homeostasis in the kidney and its transport in the distal tubules
K+ is freely filtered by the glomerulus with final K+ adjustments occurring in the distal nephron and CD. K+ secretion begins in the DCT, which can be divided into two functionally distinct portions termed the DCT1 and DCT2 (or the early and late DCTs) (Subramanya and Ellison, 2014, McCormick and Ellison, 2015). The late DCT, connecting tubule (CNT), and CD are often referred to as the aldosterone sensitive distal nephron or ASDN; however, this term is not exactly correct given that the CD morphologically and developmentally is not a part of the nephron. The CD system includes the cortical collecting duct (CCD), the outer medullary CD (OMCD), and the inner medullary CD (IMCD) (Staruschenko, 2012). K+ is secreted in these segments primarily via apical ROMK channels (Boim et al., 1995, Lee and Hebert, 1995). Multiple mechanisms controlling activity of ROMK channels were previously reported, including high K+ intake, Angiotensin II, phosphatidylinositides, various kinases, and many others (Wei et al., 2014, Lin et al., 2015, Liu et al., 2015, Dong et al., 2016). BK channels also play a role in K+ transport under certain conditions, such as shear stress induced increases in intracellular Ca2+ shown in isolated CD tubules ex vivo (Woda et al., 2001) as well as in BK β1- and β2-subunits (Pluznick et al., 2005, Larsen et al., 2016) and α-subunit (Rieg et al., 2007) knock out mice in vivo. In addition to shear stress, there are some other factors reported that contribute to the activity of BK channels, such as nucleotides (Woda et al., 2002) and vasopressin signaling (Rieg et al., 2007). Therefore, secretion in these nephron segments varies according to physiologic requirements, such as dietary manipulations, and is responsible for most of the urinary K+ excretion. However, as it will be discussed below, K+ recycling mediated by Kir4.1/Kir5.1 channels significantly contributes to the maintenance of electrolyte homeostasis and control of kidney function.
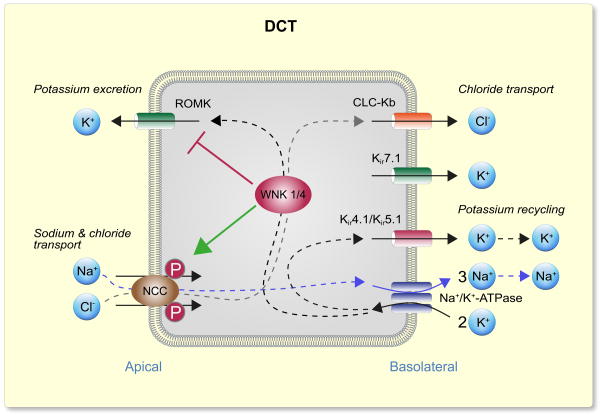
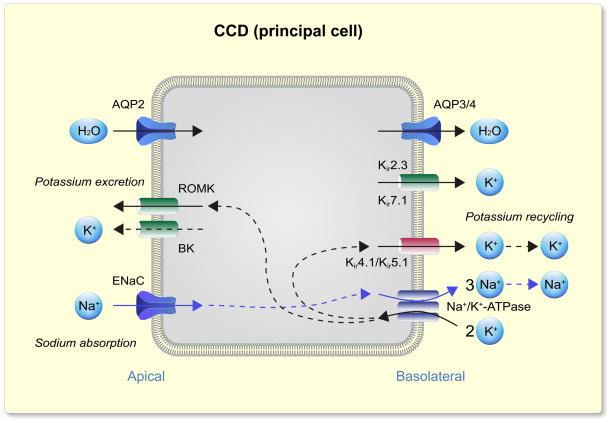
Shown in Figs. 1 and 2 are the current models of electrolyte transport in the DCT and principal cells of the CCD/CNT, respectively. In both nephron segments discussed here (as well as in many other renal epithelial cells), transport is energized by the Na+ gradient generated by the Na+/K+-ATPase localized in the basolateral membrane (McDonough et al., 1990). In DCT, energy of the electrochemical gradient for Na+ is harnessed by the NCC cotransporter in the apical membrane and moves Cl− into the cell against its electrochemical gradient. Cl− then exits across the basolateral membrane passively via Cl− channels (Kieferle et al., 1994, Zaika et al., 2016b). Na+ reabsorption in the principal cells of the CNT and CCDs is mediated by ENaC, which is localized in the apical membranes, and provides a conductive pathway for Na+ entry into the cell (Canessa et al., 1994, Canessa et al., 1993, Staruschenko, 2012). ENaC is also expressed in the late DCT (not shown in the figure), where it also contributes to Na+ reabsorption in this segment, in conjunction with NCC (Ciampolillo et al., 1996, Rubera et al., 2003, González-Núñez et al., 2004). The higher permeability of the luminal membrane for Na+ depolarizes the apical plasma membrane, creating a lumen-negative potential difference (Garcia-Filho et al., 1980), which provides the driving force for secretion of K+ into the lumen.
Figure 1.
Model of electrolyte transport in the distal convoluted tubule (DCT). Active electrolyte transport is powered by a Na+/K+-ATPase (Na+/K+ pump) on the basolateral membrane. In DCT, thiazide-sensitive Na+-Cl− cotransporter (NCC) in the apical membrane utilizes the Na+ electrochemical gradient to drive Cl− into the epithelial cell against its electrochemical gradient. Cl− then exits through the basolateral membrane passively via a CLC-K2 (predominantly kidney-specific Cl− channel). Basolateral Kir4.1/Kir5.1 channels recycles K+ to sustain the Na+/K+ pump, and generate driving force for Cl− and Na+ transport. In the DCT, WNK (with No Lysine [K] Kinase; discussed below) can stimulate, through activation of downstream kinases, NCC phosphorylation and hence activity. At the same time, WNK kinases can inhibit apical inwardly rectifying channel ROMK (Kir1.1) and suppress K+ excretion. The late distal convoluted tubule (DCT2) expresses both apical Na+ transport mechanisms: the NCC and amiloride-sensitive Na+ channel (ENaC, not shown in the figure).
Figure 2.
Model of electrolyte transport in the principal cells of cortical collecting duct (CCD). Basolateral Na+/K+-ATPase (Na+/K+ pump) create the electrochemical gradient which provides a conductive pathway for Na+ entry into the cell down through an epithelial Na+ channel (ENaC) on the apical membrane. The higher permeability of the luminal membrane for Na+ creates a lumen-negative transepithelial potential difference, and provides an important driving force for the secretion of K+ into the lumen via apical K+ channels, apical inwardly rectifying channel ROMK (Kir1.1), and the Ca2+-activated K+ channel (BK). Basolateral membrane K+ channels (heteromeric Kir4.1/Kir5.1 is the main basolateral Kir channel) provide K+ recycling necessary for Na+/K+ pump activity and Na+ transport in principal cells. Apical aquaporin 2 (AQP2) and basolateral aquaporin 3/4 (AQP3/4) water channels carry H2O molecules across cell membranes, and are responsible for water transport in the CD.
Basolateral potassium channels in the distal tubules
The molecular identity of specific channels on the basolateral membrane of the distal tubules is a highly important question investigated by many groups. Initial studies identified several channels with distinct single channel and macroscopic conductance properties (Schlatter et al., 1992, Hirsch and Schlatter, 1993, Wang, 1995, Lu et al., 1997a, Lu et al., 1997b). Most single channel studies identify two types of basolateral K+ channels. Hirsch et al. identified that the conductance of the smaller channel was approximately 67 and 28 pS in cell-attached and excised patches, respectively. The conductance of the larger K+ channel was approximately 148 and 85 pS in cell-attached and excised patches, respectively (Hirsch and Schlatter, 1993). Wang et al. also reported two types of native K+ channels on the basolateral side. Single channel analysis revealed that the conductances of the small-conductance K+ channel was 28 and 30 pS, in an asymmetrical and symmetrical high KCl solutions, respectively, whereas the conductance of the intermediate-conductance channel was approximately 85 pS in symmetrical high KCl solutions (Wang et al., 1994). Analysis of K+ channel currents in the basolateral membrane of rabbit DCT (cell attached configuration) also revealed two different conductances of 49 and 61 pS, and both types of channels were completely blocked by Ba2+ (Taniguchi et al., 1989). Earlier studies of renal K+ channels has been comprehensively reviewed by (Wang et al., 1997). Importantly, while different investigators used various solutions, species, methods of preparations etc., the results of most single channel studies are consistent with two populations of native basolateral channels in both DCT and CD.
New insights into the molecular mechanisms of K+ transport in the distal nephron and CD have been provided by cloning and identification of numerous K+ channels. As described by Hamilton and Devor, there are a number of basolateral K+ channels in the distal tubule segments (Hamilton and Devor, 2012). As an example, Welling described expression, and provided the initial functional analysis of Kir2.3 (Kcnj4) in an immortalized mouse CCD line, M-1 (Welling, 1997). Millar et al. examined the properties of the K+ conductance in the principal cells of freshly isolated mouse CCDs, and reported that whole-cell K+ currents in principal cells show strong inward rectification, high K+ selectivity, and inhibition by Ba2+ in a concentration- and voltage-dependent manner. The authors concluded that the properties of the conductance are consistent with Kir2.3 (Millar et al., 2006). Welling and colleagues further studied molecular and signaling mechanisms mediating expression of Kir2.3. First, they demonstrated, using an epitope-tag approach, that Kir2.3 is exclusively expressed in the basolateral membrane of CCD cells (Le Maout et al., 1997), and that the C-terminus coordinates membrane targeting of this channel (Le Maout et al., 2001). Further studies revealed that polarized expression of Kir2.3 is influenced by the opposing activities of two different PDZ proteins. Mammalian Lin-7 (mLin-7) interacts with Kir2.3, and links the channel with calcium/calmodulin-dependent serine protein kinase (CASK) or related Stardust proteins to coordinate basolateral membrane expression (Olsen et al., 2002, Alewine et al., 2007), whereas the tax interacting protein 1 (TIP-1) competes for interaction with mLin-7 and drives Kir2.3 into the endocytic pathway (Alewine et al., 2006). However, there are some inconsistencies within the published studies since Gray et al. reported that the properties of basolateral K+ conductance in principal cells of rat CCDs are different from previously characterized properties of Kir2.3 (Gray et al., 2005).
Another potential candidate for mediating K+ conductance in the distal tubule is Kir7.1 (encoded by Kcnj13 gene). Using multiple approaches including Western blotting, immunostaining, RNA analysis, and electron microscopic immunocytochemistry, Ookata et al. identified that the Kir7.1 channel is located predominantly in the basolateral membrane of the distal tubules (DCT, CNT and CDs). Importantly, staining along the CD was observed only in principal, but not in intercalated cells. Some staining was also reported in the TAL cells. The mRNA levels and immunoreactivity were decreased under low K+ diet; however it was reversed when diet was supplemented with 4% KCl (Ookata et al., 2000). Interestingly, Derst et al. approximately at the same time reported that tubular fragments of human and guinea pig kidney showed a significant expression of Kir7.1 only in the PT and TAL (RT-PCR analysis defined mRNA levels in both segments and immunocytochemical analysis revealed its expression only in PT) (Derst et al., 2001). Additional studies are required to confirm the specific tubule localization, as well as to determine the functional role of the Kir7.1 channel in the renal epithelia. This channel may also be of significant interest considering the development of novel small-molecule inhibitors specifically targeting Kir7.1 (Bhave et al., 2011, Raphemot et al., 2011).
Kir4.1 and Kir5.1 channels in the distal tubules
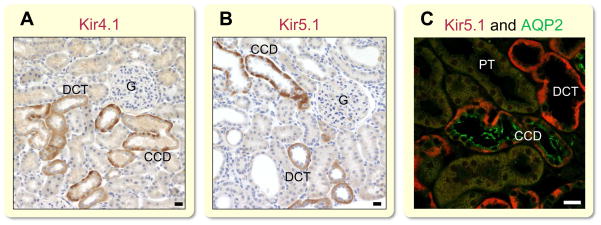
Despite the presence of the aforementioned K+ channels, it is now viewed that Kir4.1 and Kir5.1 are the main Kir channels in the basolateral membrane of DCT and CCD responsible for K+ recycling in these segments. Takumi et al. isolated Kir4.1 about 20 years ago (the authors initially designated this new clone as KAB-2, the second type of inward rectifying K+ channel with an ATP-binding domain). They initially reported that this protein is predominantly expressed in glial cells of the cerebellum and forebrain. In addition to brain, mRNA was also detected in the kidney (Takumi et al., 1995). A subsequent immunohistochemical study revealed that Kir4.1 is strongly expressed in the basolateral membrane of renal distal tubular epithelia (Ito et al., 1996). Figure 3 demonstrates the immunohistochemical analysis of Kir4.1 (Fig. 3A) and Kir5.1 channels (Fig. 3B) in rat kidney cortex as well as colocalization of Kir5.1 and AQP2, specific marker of principal cell (Fig. 3C). It is worth noting that Kir4.1 is also expressed in the cortical part of TAL (cTAL) where this channel may similarly contribute to the basolateral K+ conductance (Reichold et al., 2010, Zhang et al., 2015). However, using Kcnj10 knockout (Kcnj10−/−) mice, the authors demonstrated that the disruption of Kir4.1 has no significant effect on the membrane potential of the cTAL and NKCC2 expression (Zhang et al., 2015). Therefore, additional studies are needed to reveal the physiological role of Kir4.1 in cTAL.
Figure 3.
Immunostaining of Kir4.1 and Kir5.1 basolateral channels in the renal cortex. Dahl salt-sensitive rats kidney cortex sections stained for Kir4.1 (APC-035, Alomone Labs) (A) and for Kir5.1 (SAB4501636, Sigma) (B) shows strong basolateral expression of both proteins. Note absence of protein staining in CD intercalated cells. (C) Double staining with Kir5.1 (red; SAB4501636, Sigma) and AQP2 (green; sc-28629, Santa Cruz) in Dahl salt-sensitive rats. Immunohistochemical experiments were performed as previously published (Pavlov et al., 2013b) and conform with: Good publication practice in physiology (Persson, 2015). G, glomeruli; PT, proximal tubules; DCT, distal convoluted tubule; CCD, cortical collecting duct. Scale bar is 20 μm.
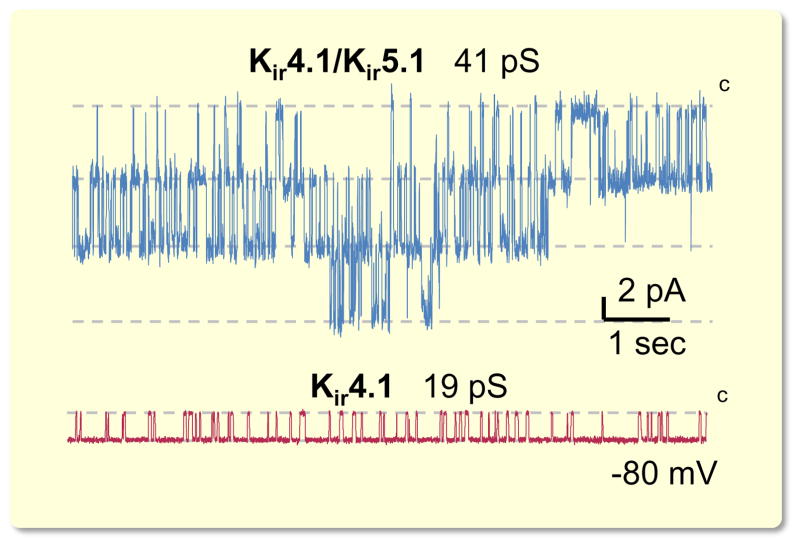
Kir4.1 forms homomeric channels and co-assembles with Kir5.1 to yield Kir4.1/Kir5.1 heteromeric channels (Pessia et al., 1996, Pessia et al., 2001, D’Adamo et al., 2011). Kir4.1/Kir5.1 has unique properties including greater single channel conductance, and much higher sensitivity to pH within the physiologic range when compared to Kir4.1 homomer (Tucker et al., 2000, Pessia et al., 2001, D’Adamo et al., 2011) (see also Fig. 4). We and others reported that the Kir4.1/Kir5.1 heteromer is the predominant basolateral K+ channel in both DCT and CCD (Lourdel et al., 2002, Lachheb et al., 2008, Zaika et al., 2013, Zaika et al., 2016a).
Figure 4.
Single channel recordings of K+ channels localized on the basolateral membrane of CCD. Shown are representative traces demonstrating activity of highly abundant Kir4.1/Kir5.1 heteromeric channel with 41-pS conductance and scarce 19-pS homomeric Kir4.1 channel. Cell attached recordings are performed on freshly isolated mice CCDs. Modified from (Zaika et al., 2013) with permission.
The genetic dissection of renal diseases has identified many components required for normal renal electrolyte homeostasis. Bockenhauer et al. performed linkage analysis in children from two consanguineous families that presenting with epilepsy and abnormal, uncoordinated motor behavior or ataxia, moderate sensorineural hearing loss, and a salt-losing renal tubulopathies. They identified a single significant locus on chromosome 1q23.2, which contained the KCNJ10 gene. Sequencing of this gene, encoding Kir4.1 channel, revealed homozygous missense mutations. Further analysis defined that these mutations cause this autosomal recessive disease, which was called EAST syndrome (epilepsy, ataxia, sensorineural deafness, and tubulopathy) (Bockenhauer et al., 2009). Around the same time, Scholl and colleagues described the same disease, identifying missense or nonsense mutations in Kir4.1, in highly conserved amino acids on both alleles in all affected subjects, but named it SeSAME (seizures, sensorineural deafness, ataxia, mental retardation and electrolyte imbalance) (Scholl et al., 2009). Functional analyses revealed that the disease-causing mutations in the channel led to a defect in K+ conductance. Patients with EAST/SeSAME display hypokalemia, metabolic alkalosis, hypomagnesemia, and elevated levels of renin and aldosterone (Bockenhauer et al., 2009, Scholl et al., 2009). Similarly, Kcnj10−/− mice displayed a phenotype recapitulating the disease including hypokalemia, hypomagnesemia and metabolic alkalosis, and hypocalciuria, salt-wasting and dehydration (Bockenhauer et al., 2009). Additional disease-causing mutations in KCNJ10 were further identified by Zdebik and colleagues (Freudenthal et al., 2011, Parrock et al., 2013).
As described by Méndez González et al., there are over 120 coding-region single nucleotide polymorphisms (SNPs) in the KCNJ10 gene reported in the publicly accessible genome databases (Méndez-González et al., 2016). Electrophysiological analysis revealed that the currents produced by mutant channels carrying disease-associated mutations (R65P, R65P/R199X, G77R, C140R, T164I, and A167V/R297C) were strongly reduced, indicating that the loss of channel function underlies the EAST/SeSAME syndrome (Williams et al., 2010). When mutant Kir4.1 subunits were co-expressed together with Kir5.1 wild type channels, the reduction in function was similar compared to mutant subunits expressed alone. This data indicates that the studied mutations affect both homomeric and heteromeric channels. Importantly, it was revealed that wild type Kir4.1 subunits were unaffected by a reduction of pHi to 6.8, but currents for mutant subunits were essentially abolished at the lower pHi. The authors concluded that this effect likely reflects a shift in pHi sensitivity to more alkaline values, and may contribute to the lower currents observed when these mutants were studied under normal pHi conditions (Williams et al., 2010). Similarly, Reichold et al. studied several other Kir4.1 mutants, specifically the R65P, G77R, R199X, and R175Q. They also observed a decreased current that was mediated by changes in the channel open probabilities (Po) for both homomeric Kir4.1 and heteromeric channels (Reichold et al., 2010). Another group found that a majority of studied Kir4.1 mutations (particularly R297C, C140R, R199X, T164I) caused complete loss of channel function, while two mutations (R65P and A167V) resulted only in partial loss of function (Tang et al., 2010). Sala-Rabanal et al. further applied patch clamp analysis together with radiotracer efflux and identified that all of the studied mutations compromised the channel’s function, but the underlying mechanisms were different. The authors reported that R65P, T164I, and R297C mutations in Kir4.1 caused an alkaline shift in pH sensitivity, indicating that these positions are crucial for pH sensing and pore gating. In R297C, this was due to disruption of the intersubunit salt bridge Glu288-Arg297. C140R breaks the Cys108-Cys140 disulfide bond essential for protein folding and function. A167V did not affect channel properties but may contribute to decreased surface expression in A167V/R297C. In G77R, introduction of a positive charge affected channel structure or gating. R199Stop led to a dramatic decrease in surface expression, but channel activity was restored by co-expression with intact subunits, suggesting remarkable tolerance for truncation of the cytoplasmic domain (Sala-Rabanal et al., 2010). Recent studies further identified G83V, L166Q, and Q212R residues as playing a pivotal role in controlling Kir4.1 channel function. The G83V rendered the channel to be non-functional. The L166Q variant reduced channel function and the Q212R mutant did not reduce overall conductance, but did demonstrate subtle effects on spermine sensitivity (Méndez-González et al., 2016). Tanemoto et al. further revealed that the A167V mutation in Kir4.1 caused compromised trafficking of the mutant channels, and inhibited their expression on the basolateral surface of tubular cells. It was reported that an anchor protein, membrane-associated guanylate kinase with inverted domain structure-1 (MAGI-1), contributes to basolateral K+ recycling. MAGI-1 directly interacts with Kir4.1 and facilitates the basolateral localization of this channel (Tanemoto et al., 2014). All these results provide important insights for the molecular mechanisms that underlie the EAST/SeSAME syndrome.
Recent studies by Slaats et al. further revealed that Kir4.1 along with voltage-gated, kqt-like subfamily, member 1 (Kcnq1); voltage-gated, subfamily F, member 1(Kcnf1) K+ channels; and Cl− channel, voltage-gated 4 (Clcn4) regulate renal ciliogenesis in CD through the periciliary diffusion barrier or the ciliary pocket. A siRNA-based reverse genetics screen identified these channels as potential contributors to ciliopathy pathophysiology. Interestingly, the authors reported that damaging mutations in KCNJ10 as well as in KCNQ1, KCNF1 and CLCN4 cause defects in cilia structure, but that KCNJ10 mutations affecting the ion channel function do not affect the cilia (Slaats et al., 2015). This observation requires further investigation, considering the important role of cilia in epithelial cells.
Identification of mutations within the human gene encoding Kir4.1 causing the SeSAME/EAST syndrome brought a lot of attention to investigation of this particular channel. However, as discussed above, Kir4.1/Kir5.1 heteromeric channels, but not homomeric Kir4.1channels, play the main role in the modulation of the basolateral conductance in the distal nephron and CD. Despite this, our knowledge about the role of Kir5.1 is very limited. Paulais et al. investigated the role of the Kir5.1 subunit in mice with a targeted disruption of the Kir5.1 gene (Kcnj16) (Paulais et al., 2011). They identified the important role that Kir5.1 plays as a pH-sensitive regulator of salt transport in the DCT. It was reported that the Kir5.1−/− mice displayed hypokalemic, hyperchloremic metabolic acidosis with hypercalciuria. To further test the role of Kir5.1 for electrolyte transport in the DCT, they tested the effects of hydrochlorothiazide (HCTZ), a NCC inhibitor. The short-term responses to HCTZ were exaggerated, indicating excessive renal Na+ absorption in this segment. Furthermore, chronic treatment with HCTZ normalized urinary excretion of Na+ and Ca2+, and abolished acidosis in the Kir5.1−/− mice. Interestingly, increased NCC activity is normally associated with hyperkalemia, not hypokalemia, as observed in Kir5.1−/− mice. However, the mechanism of this hypokalemic renal tubular acidosis is not known. In contrast, inhibition of ENaC in CCD with amiloride was not different in the Kir5.1−/− mice compared to wild type littermates, indicating that ENaC-mediated Na+ transport is not altered by deletion of Kir5.1 (Paulais et al., 2011). To further address the role of Kir5.1 in the kidney in the context of a disease state in vivo, we recently generated a Kcnj16−/− rat model in Dahl salt-sensitive (SS) background by using ZFN technology. Our preliminary data revealed that knockout of Kir5.1 in SS rat induces electrolyte imbalance and blood pressure abnormalities (Palygin et al., 2015). However, this model requires further investigation to precisely determine the role of this channel in the development of salt-sensitive hypertension.
Recent studies also identified mutations in KCNJ16 (encoding Kir5.1) in patients with Brugada syndrome. This cardiac disease is potentially fatal with arrhythmia characterized by abnormal rapid heart rhythms that originate in the heart ventricles (Juang et al., 2014). Furthermore, recent Genome-Wide Association Studies (GWAS) of metabolite quantitative traits also identified KCNJ16 as having significant loci with 3-hydroxybutyrate, which links this gene to regulation of intermediary metabolites and possibly to ketosis or ketoacidosis, which results from increased fat metabolism due to a shortage of insulin commonly found in diabetes mellitus (Demirkan et al., 2015). While it is still unclear if these changes play any role in the control of renal function, these observations demonstrate that Kir5.1 (despite its lacking ability to form homomeric channel) might be critical for the development of certain pathologies in humans.
Biophysical properties of Kir channels at the basolateral membrane of the distal tubules
The phenomenon of inward or anomalous rectification, as first described by Katz in 1949, means that membrane conductance increases with hyperpolarization and decreases with depolarization (Katz, 1949). Kir channels are primarily responsible for the resting potential and have been found and described in many tissues. In these channels, the increase of current that follows hyperpolarization has been referred to as activation, so that the reduction of channel current at positive potentials has generally been described as deactivation or rectification (Nichols and Lopatin, 1997).
Renal Kir channels, involved in epithelial transport and K+ homeostasis, include Kir1.1 (ROMK), Kir7.1, Kir4.1 and Kir5.1, which are all expressed in the distal tubules. The above referenced channels belong to the same subgroup of K+ transport proteins and have similar biophysical properties. Functional Kir channels are composed of 4 subunits which are either homo- or heterotetramers. Each subunit has a single pore domain and two transmembrane domains. It was predicted that the pore domain and the second transmembrane domain contributes to permeation pore structure, and the other domain is involved in formation of a selectivity filter (Koster et al., 1998, Sepúlveda et al., 2015). To date no Kir channels have been crystallized.
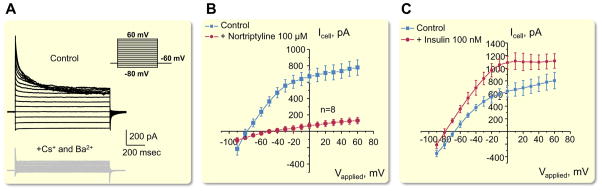
While Kir4.1 is able to form a functional homomeric channel, Kir5.1 lacks this ability as shown when channel subunits were expressed alone in heterologous expression systems (Pessia et al., 1996). Interestingly it was shown in co-transfected HEK-293 cells that homomeric Kir5.1 channel is functional when it forms a complex with PSD-95, a brain anchoring protein (Tanemoto et al., 2002). As it was recently summarized (Sepúlveda et al., 2015), Kir4.1/Kir5.1 channels form heteromeric K+ channel in a specific 4-5-4-5 arrangement that is dependent on external K+ concentration, which relieves the rectification by K+-ion binding at external sites by displacing Mg2+ from sites deeper inside a multi ion pore. Intriguingly, a 4-4-5-5 tetrameric arrangement (in contrast to 4-5-4-5 position of subunits) produces channels with the properties of homomeric Kir4.1 channels, which provides evidence for the importance of subunit position in the properties of heterotetrameric Kir channels (Pessia et al., 1996, Nichols and Lopatin, 1997). Lourdel et al. performed an elegant analysis of the biophysical properties of K+ channels on the basolateral membranes of microdissected DCT using a patch clamp approach (Lourdel et al., 2002). It was shown that Mg2+ considerably reduced the outward conductance of native K+ channels, and that the polycation spermine reduced Po by 50 %. Channel activity was dependent upon the intracellular pH, with acid pH decreasing, and alkaline pH increasing, Po. Internal ATP and Ca2+ had no effect, and channel activity declined irreversibly when the inner side of the patch was exposed to Mg2+ (Lourdel et al., 2002). These channels, similar to other Kir channels, are also inhibited by large cations, such as Cs+ and Ba2+. Shown in Fig. 5A is an example of inhibition of native Kir4.1/Kir5.1 channels in CCD by Cs+ in the presence of extracellular Ba2+ (Zaika et al., 2016a).
Figure 5.
Biophysical properties of basolateral Kir channels in CCD. (A) Macroscopic currents from the basolateral membrane of individual principal cell of CCD isolated from mouse kidney cortex. Equimolar substitution of intracellular K+ with Cs+ in the presence of Ba2+ in the extracellular medium drastically reduces the amplitude of steady-state current indicating its Kir channel-dependent nature (Kir channels except for Kir7.1 are inhibited by Ba2+). (B) Inhibition of K+ macroscopic current with the second-generation tricyclic antidepressant – nortriptyline (100 μM). (C) Insulin (and similarly IGF-1) augmented K+ selective current in principal cells of CCD. Modified from (Zaika et al., 2016a) with permission.
Several studies in heterologous expression systems reported that tricyclic antidepressants nortriptyline and fluoxetine act as reversible inhibitors of the Kir4.1 channel (Furutani et al., 2009, Su et al., 2007, Ohno et al., 2007). We have tested, in isolated CCD, the effect of these drugs on endogenous Kir4.1/Kir5.1 channels, and revealed that fluoxetine had only a minor effect (probably through inhibition of homomeric Kir4.1 channels), while nortriptyline almost completely abolished macroscopic whole cell currents in CCD principal cells (Fig. 5B) (Zaika et al., 2016a).
As it will be discussed below, these channels are also highly modulated by protein kinase C (PKC) phosphorylation, G protein-coupled receptor (GPCR) activation, as well as some other physiological modulators. Figure 5C demonstrates an example of activation of basolateral K+ conductance by insulin (Zaika et al., 2016a).
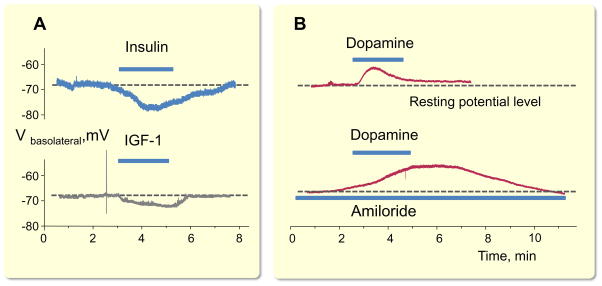
As described above, basolateral K+ channels play a dominant role in determining resting membrane potential and spatial K+ buffering in the distal nephron and CD. Shown in Fig. 6 are examples of acute changes in membrane potential followed by modulation of Kir4.1/Kir5.1 channels after application of diverse hormones. As shown in Fig. 6, addition of insulin, IGF-1, and dopamine, all of which modulate activity of Kir4.1/Kir5.1 channels (Zaika et al., 2013, Zaika et al., 2016a) results in immediate changes in the resting membrane potential level.
Figure 6.
Pharmacological modulation of basolateral Kir channels activity shifts membrane potential in the cortical collecting duct (CCD). (A) Insulin (100 nM) and IGF-1 (500 nM) hyperpolarize basolateral membrane in principal cells by acute increase in open probability of basolateral K+ channels. (B) Dopamine (10 μM) induces acute depolarization of the basolateral membrane via inhibition of Kir4.1/Kir5.1 channels. An even greater degree of depolarization is observed when amiloride (2 μM) inhibits ENaC-mediated apical conductance. A and B modified from (Zaika et al., 2013, Zaika et al., 2016a) with permission.
Signaling mechanisms controlling properties of potassium channels at the basolateral membrane of the distal tubules
A number of earlier studies revealed several important mechanisms controlling activity of native basolateral K+ channels. While these studies do not provide the exact molecular identity of the channels studied, we strongly believe that this information is relevant for understanding signaling pathways controlling basolateral Kir channels. Thus, it was reported that PKC and protein kinase A (PKA) modulate activity of basolateral K+ channels. PKC inhibition reduced channel activity by 90% in cell attached patches (Lu and Wang, 1996a). Addition of the catalytic subunit of PKA increased activity of basolateral K+ channels (Wang, 1995). Moreover, it was shown that nitric oxide (NO) plays an important role in the regulation of the basolateral K+ channels in the CCD (Lu and Wang, 1996b). Inhibition of nitric oxide synthase (NOS) reduced the channels activity. Furthermore, application of a cGMP analogue stimulated basolateral K+ channels, and cGMP also restored the channel activity blocked by NOS inhibitors (Lu and Wang, 1996b). It was further proposed that PKC is involved in the stimulation of the small-conductance basolateral K+ channels by regulation of NOS (Lu and Wang, 1996a).
Zhang et al. provided evidence that the Src family protein tyrosine kinase is involved in the regulation of K+ channels in the basolateral membrane of the mouse DCT1 segment (Zhang et al., 2013). Western blot analysis showed that Kir4.1 was a tyrosine-phosphorylated protein. LC/MS analysis further confirmed that these protein tyrosine kinases phosphorylated Kir4.1 at Tyr8 and Tyr9. Therefore, the modulation of tyrosine phosphorylation of Kir4.1 may play a role in regulating membrane transport function in DCT, similar to its effects on ROMK channels (Lin et al., 2004, Lin et al., 2012). It was further reported that c-Src–induced stimulation of Kir4.1 requires coexpression of caveolin-1. Disruption of this scaffolding protein decreased Kir4.1 activity and depolarized the membrane potential in the DCT at least partially by suppressing the stimulating effects of c-Src on Kir4.1 (Wang et al., 2015).
A potential mechanism involving GPCRs was also reported. It was shown that Kir4.1 interacts with the Ca2+-sensing receptor (CaR) in yeast two-hybrid screens, heterologous expression systems, and in DCT (Huang et al., 2007). CaR inactivated Kir4.1 by reducing its cell surface expression. Mutant, activated Gαq reduced cell surface expression and current density of Kir4.1, and these effects were blocked by regulator of G protein signaling 4 (RGS4), a protein that blocks signaling via Gαi and Gαq. Similar to the study discussed above (Wang et al., 2015), it was shown that caveolin-1 is involved in this pathway. Knockdown of caveolin-1 blocked the effect of Gαq on Kir4.1, whereas knockdown of the clathrin heavy chain had no effect (Cha et al., 2011).
We have reported recently that dopamine, a critical regulator of systemic blood pressure, reversibly decreased the Po of both homomeric Kir4.1 and heteromeric Kir4.1/Kir5.1 channels (Zaika et al., 2013). This effect was mediated by D2-like, but not D1-like dopamine receptors, and PKC blockade abolished the inhibition of these channels by dopamine. Importantly, dopamine induced an acute depolarization of basolateral membrane potential, as directly monitored using current-clamp mode in isolated CCDs (Fig. 6A). We also demonstrated that insulin and insulin-like growth factor-1 (IGF-1) acutely activate single channel Kir4.1/Kir5.1 Po, which results in a respective increase in whole cell K+ currents (Fig. 5C). Inhibition of phosphoinositide 3-kinase eliminates actions of both insulin and IGF-1 on Kir4.1/Kir5.1. Importantly, this regulation is capable of controlling basolateral membrane voltage in the CCD (Fig. 6A) (Zaika et al., 2016a). Therefore, insulin and IGF-1 increase the electrochemical driving force for Na+ reabsorption via its effects on Kir4.1/Kir5.1, which further facilitate ENaC-mediated Na+ absorption as well as Cl− and K+ transports in the CCD (Frindt and Palmer, 2012, Pavlov et al., 2013a, Zaika et al., 2015).
Of special interest is the sensitivity of basolateral K+ channels to inhibition by cell acidification. Even small changes in pHi, resulting in intracellular acidification, could result in decreased basolateral K+ conductance, depolarization of the basolateral membrane, and consecutive changes in Na+ and Cl− transport. Multiple studies reported that the basolateral K+ channel, and specifically Kir4.1 and Kir5.1 are sensitive to changes in cell pH (Lourdel et al., 2002, Paulais et al., 2011). Interestingly, it was shown Kir5.1 also serves as an important determinant of neuronal PCO2/pH sensitivity (D’Adamo et al., 2011, Trapp et al., 2011). Another potential critical regulator of Kir4.1, reported in the brain, is DNA methylation (Nwaobi et al., 2014, Nwaobi and Olsen, 2015). However, no data relevant to DNA methylation of either Kir4.1 or Kir5.1 in the kidney is reported yet.
As it was recently summarized by Ellison et al., there is direct link between the dietary K+ intake and activity of NCC cotransporter in DCT (Ellison et al., 2016). Sorensen et al. reported that potassium intake followed by a significant rise of plasma aldosterone, and enhanced urinary K+ and Na+ excretion, which were accompanied by a rapid and sustained dephosphorylation of NCC and upregulation of proteolytically activated ENaC (Sorensen et al., 2013). Recent data suggest that changes in plasma K+ concentration signals to NCC by altering intracellular Cl− concentrations in the DCT (Terker et al., 2015, Bazúa-Valenti et al., 2015). When plasma K+ concentration is increased, intracellular Cl− concentration is also high, WNK lysine deficient protein kinases (WNKs) are turned off, and NCC is suppressed (see also Fig. 1). In contrast, when dietary potassium intake, and plasma K+ concentration, respectively, are decreased, WNKs phosphorylate the downstream kinases Ste20p-related proline alanine-rich kinase (SPAK), and oxidative stress response 1 kinase (OxSR1) to stimulate activity of NCC (Terker et al., 2015). Therefore, activation of NCC results in reduced delivery of Na+ to the CD, and K+ secretion, respectively. As it was discussed above, one of the primary functions of basolateral Kir4.1/Kir5.1 channels is to recycle K+ across the basolateral membrane for proper function of the Na+-K+-ATPase, among many other functions. Changes in K+ conductance affect membrane potential, and therefore modulate intracellular Cl− concentration. Thus, Kir4.1/Kir5.1 channels play an important role in the described above pathway. In line with this, Zhang et al. reported that Kir4.1 determines the expression of the NCC cotransporter in the DCT. Using the Kir4.1−/− mice, they demonstrated that the disruption of Kir4.1 decreased the basolateral Cl− conductance, and diminished the apical NCC expression in DCT (Zhang et al., 2014). However, it should be taken into account that Kcnj10−/− mice could not survive more than 2 weeks after the birth (Kofuji et al., 2000); therefore, in this manuscript, as well as in some other studies where Kcnj10−/− mice were utilized, experiments were conducted in mice at only a few days postnatal, when the kidney is not fully developed.
Summary and conclusions
In this review, we examined the role of basolateral epithelial K+ channels in the DCT and CCD. As described above, there are numerous interactions between dietary K+ intake, and the control of electrolyte homeostasis and blood pressure regulation, respectively. It is clear that basolateral epithelial K+ channels play vital roles in the kidney electrolyte homeostasis. While there is not much information available, so far, this is an emerging and growing field of research. More molecular and animal studies are clearly needed to increase our knowledge about the function of these channels in the kidney. This is especially important for identification of regulatory mechanism of the basolateral K+ channels, which is poorly understood. For instance, there is still missing link in the chain of events relating increases in interstitial K+ concentration to inhibition of NCC activity in DCT although plausible steps include membrane potential to intracellular Cl− concentration to WNK 1/4 to SPARK and OxSR1 to NCC phosphorylation. Furthermore, additional mechanistic studies are required to precisely uncover molecular mechanisms controlling these channels in CDs. It is expected that novel pharmacological tools modulating basolateral K+ channels, and especially Kir4.1 and Kir5.1, will be developed. Targeting these channels may be attractive for treatment of hypertension as well as some renal diseases. Therefore, these channels warrant further studies to uncover their role in cardiorenal physiology.
Acknowledgments
We apologize to the investigators of K+ epithelial transport whose relevant publications were inadvertently not directly discussed. Nicholas Burgraff and Denisha Spires (MCW) are greatly appreciated for proof reading of this manuscript. Research in the author’s laboratories was supported by the National Institutes of Health grants HL108880, HL122662 (to A. Staruschenko), DK095029 (to Oleh Pochynyuk), American Diabetes Association grant 1-15-BS-172, American Heart Association (16EIA26720006) (to A. Staruschenko), and Medical College of Wisconsin Neuroscience Research Center/Advancing a Healthier Wisconsin pilot grant #9520217 (to Oleg Palygin).
Footnotes
Conflict of Interest
There are no conflicts of interests.
References
- Alewine C, Kim BY, Hegde V, Welling PA. Lin-7 targets the Kir 2.3 channel on the basolateral membrane via a L27 domain interaction with CASK. Am J Physiol Cell Physiol. 2007;293:C1733–1741. doi: 10.1152/ajpcell.00323.2007. [DOI] [PubMed] [Google Scholar]
- Alewine C, Olsen O, Wade JB, Welling PA. TIP-1 has PDZ scaffold antagonist activity. Mol Biol Cell. 2006;17:4200–4211. doi: 10.1091/mbc.E06-02-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazúa-Valenti S, Chávez-Canales M, Rojas-Vega L, González-Rodríguez X, Vázquez N, Rodríguez-Gama A, Argaiz ER, Melo Z, Plata C, Ellison DH, García-Valdés J, Hadchouel J, Gamba G. The effect of WNK4 on the Na+–Cl− cotransporter is modulated by intracellular chloride. J Am Soc Nephrol. 2015;26:1781–1786. doi: 10.1681/ASN.2014050470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhave G, Chauder BA, Liu W, Dawson ES, Kadakia R, Nguyen TT, Lewis LM, Meiler J, Weaver CD, Satlin LM, Lindsley CW, Denton JS. Development of a selective small-molecule inhibitor of Kir1.1, the renal outer medullary potassium channel. Mol Pharmacol. 2011;79:42–50. doi: 10.1124/mol.110.066928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockenhauer D, Feather S, Stanescu HC, Bandulik S, Zdebik AA, Reichold M, Tobin J, Lieberer E, Sterner C, Landoure G, Arora R, Sirimanna T, Thompson D, Cross JH, van’t Hoff W, Al Masri O, et al. Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med. 2009;360:1960–1970. doi: 10.1056/NEJMoa0810276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boim MA, Ho K, Shuck ME, Bienkowski MJ, Block JH, Slightom JL, Yang Y, Brenner BM, Hebert SC. ROMK inwardly rectifying ATP-sensitive K+ channel. II. Cloning and distribution of alternative forms. Am J Physiol. 1995;268:F1132–1140. doi: 10.1152/ajprenal.1995.268.6.F1132. [DOI] [PubMed] [Google Scholar]
- Canessa CM, Horisberger JD, Rossier BC. Epithelial sodium channel related to proteins involved in neurodegeneration. Nature. 1993;361:467–470. doi: 10.1038/361467a0. [DOI] [PubMed] [Google Scholar]
- Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- Cha SK, Huang C, Ding Y, Qi X, Huang CL, Miller RT. Calcium-sensing receptor decreases cell surface expression of the inwardly rectifying K+ channel Kir4.1. J Biol Chem. 2011;286:1828–1835. doi: 10.1074/jbc.M110.160390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciampolillo F, McCoy DE, Green RB, Karlson KH, Dagenais A, Molday RS, Stanton BA. Cell-specific expression of amiloride-sensitive, Na+-conducting ion channels in the kidney. Am J Physiol. 1996;271:C1303–1315. doi: 10.1152/ajpcell.1996.271.4.C1303. [DOI] [PubMed] [Google Scholar]
- D’Adamo MC, Shang L, Imbrici P, Brown SD, Pessia M, Tucker SJ. Genetic inactivation of Kcnj16 identifies Kir5.1 as an important determinant of neuronal PCO2/pH sensitivity. J Biol Chem. 2011;286:192–198. doi: 10.1074/jbc.M110.189290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirkan A, Henneman P, Verhoeven A, Dharuri H, Amin N, van Klinken JB, Karssen LC, de Vries B, Meissner A, Göraler S, van den Maagdenberg AMJM, Deelder AM, C’t Hoen PA, van Duijn CM, van Dijk KW. Insight in genome-wide association of metabolite quantitative traits by exome sequence analyses. PLoS Genet. 2015;11:e1004835. doi: 10.1371/journal.pgen.1004835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derst C, Hirsch JR, Preisig-Muller R, Wischmeyer E, Karschin A, Doring F, Thomzig A, Veh RW, Schlatter E, Kummer W, Daut J. Cellular localization of the potassium channel Kir7.1 in guinea pig and human kidney. Kidney Int. 2001;59:2197–2205. doi: 10.1046/j.1523-1755.2001.00735.x. [DOI] [PubMed] [Google Scholar]
- Dong K, Yan Q, Lu M, Wan L, Hu H, Guo J, Boulpaep E, Wang W, Giebisch G, Hebert SC, Wang T. Romk1 knockout mice do not produce Bartter phenotype but exhibit impaired K excretion. J Biol Chem. 2016;291:5259–5269. doi: 10.1074/jbc.M115.707877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison DH, Terker AS, Gamba G. Potassium and its discontents: new insight, new treatments. J Am Soc Nephrol. 2016;27:981–989. doi: 10.1681/ASN.2015070751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenthal B, Kulaveerasingam D, Lingappa L, Shah MA, Brueton L, Wassmer E, Ognjanovic M, Dorison N, Reichold M, Bockenhauer D, Kleta R, Zdebik AA. KCNJ10 mutations disrupt function in patients with EAST syndrome. Nephron Physiol. 2011;119:p40–p48. doi: 10.1159/000330250. [DOI] [PubMed] [Google Scholar]
- Frindt G, Palmer LG. Effects of insulin on Na and K transporters in the rat CCD. Am J Physiol Renal Physiol. 2012;302:F1227–F1233. doi: 10.1152/ajprenal.00675.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furutani K, Ohno Y, Inanobe A, Hibino H, Kurachi Y. Mutational and in silico analyses for antidepressant block of astroglial inward-rectifier Kir4.1 channel. Mol Pharmacol. 2009;75:1287–1295. doi: 10.1124/mol.108.052936. [DOI] [PubMed] [Google Scholar]
- Garcia-Filho E, Malnic G, Giebisch G. Effects of changes in electrical potential difference on tubular potassium transport. Am J Physiol. 1980;238:F235–246. doi: 10.1152/ajprenal.1980.238.3.F235. [DOI] [PubMed] [Google Scholar]
- González-Núñez D, Morales-Ruiz M, Leivas A, Hebert SC, Poch E. In vitro characterization of aldosterone and cAMP effects in mouse distal convoluted tubule cells. Am J Physiol Renal Physiol. 2004;286:F936–F944. doi: 10.1152/ajprenal.00070.2003. [DOI] [PubMed] [Google Scholar]
- Gray DA, Frindt G, Zhang YY, Palmer LG. Basolateral K+ conductance in principal cells of rat CCD. Am J Physiol Renal Physiol. 2005;288:F493–F504. doi: 10.1152/ajprenal.00301.2004. [DOI] [PubMed] [Google Scholar]
- Hamilton KL, Devor DC. Basolateral membrane K+ channels in renal epithelial cells. Am J Physiol Renal Physiol. 2012;302:F1069–1081. doi: 10.1152/ajprenal.00646.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch J, Schlatter E. K+ channels in the basolateral membrane of rat cortical collecting duct. Pflugers Arch. 1993;424:470–477. doi: 10.1007/BF00374910. [DOI] [PubMed] [Google Scholar]
- Huang C, Sindic A, Hill CE, Hujer KM, Chan KW, Sassen M, Wu Z, Kurachi Y, Nielsen S, Romero MF, Miller RT. Interaction of the Ca2+-sensing receptor with the inwardly rectifying potassium channels Kir4.1 and Kir4.2 results in inhibition of channel function. Am J Physiol Renal Physiol. 2007;292:F1073–F1081. doi: 10.1152/ajprenal.00269.2006. [DOI] [PubMed] [Google Scholar]
- Ito M, Inanobe A, Horio Y, Hibino H, Isomoto S, Ito H, Mori K, Tonosaki A, Tomoike H, Kurachi Y. Immunolocalization of an inwardly rectifying K+ channel, K(AB)-2 (Kir4.1), in the basolateral membrane of renal distal tubular epithelia. FEBS Lett. 1996;388:11–15. doi: 10.1016/0014-5793(96)00502-9. [DOI] [PubMed] [Google Scholar]
- Juang JM, Lu TP, Lai LC, Ho CC, Liu YB, Tsai CT, Lin LY, Yu CC, Chen WJ, Chiang FT, Yeh SF, Lai LP, Chuang EY, Lin JL. Disease-targeted sequencing of ion channel genes identifies de novo mutations in patients with non-familial Brugada syndrome. Sci Rep. 2014;4:6733. doi: 10.1038/srep06733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B. Les constantes electriques de la membrane du muscle. Arch Sci Physiol. 1949;2:285–299. [Google Scholar]
- Kieferle S, Fong P, Bens M, Vandewalle A, Jentsch TJ. Two highly homologous members of the ClC chloride channel family in both rat and human kidney. Proc Natl Acad Sci U S A. 1994;91:6943–6947. doi: 10.1073/pnas.91.15.6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofuji P, Ceelen P, Zahs KR, Surbeck LW, Lester HA, Newman EA. Genetic inactivation of an inwardly rectifying potassium channel (Kir4.1 subunit) in mice: phenotypic impact in retina. J Neurosci. 2000;20:5733–5740. doi: 10.1523/JNEUROSCI.20-15-05733.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster JC, Bentle KA, Nichols CG, Ho K. Assembly of ROMK1 (Kir 1.1a) inward rectifier K+ channel subunits involves multiple interaction sites. Biophys J. 1998;74:1821–1829. doi: 10.1016/S0006-3495(98)77892-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachheb S, Cluzeaud Fó, Bens M, Genete M, Hibino H, Lourdel Sp, Kurachi Y, Vandewalle A, Teulon J, Paulais M. Kir4.1/Kir5.1 channel forms the major K+ channel in the basolateral membrane of mouse renal collecting duct principal cells. Am J Physiol Renal Physiol. 2008;294:F1398–F1407. doi: 10.1152/ajprenal.00288.2007. [DOI] [PubMed] [Google Scholar]
- Larsen CK, Jensen IS, Sorensen MV, de Bruijn PI, Bleich M, Praetorius HA, Leipziger J. Hyperaldosteronism following decreased renal K+ excretion in KCNMB2 knock-out mice. Am J Physiol Renal Physiol. 2016 doi: 10.1152/ajprenal.00010.2016. [DOI] [PubMed] [Google Scholar]
- Le Maout S, Brejon M, Olsen O, Merot J, Welling PA. Basolateral membrane targeting of a renal-epithelial inwardly rectifying potassium channel from the cortical collecting duct, CCD-IRK3, in MDCK cells. Proc Natl Acad Sci U S A. 1997;94:13329–13334. doi: 10.1073/pnas.94.24.13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Maout S, Welling PA, Brejon M, Olsen O, Merot J. Basolateral membrane expression of a K+ channel, Kir 2.3, is directed by a cytoplasmic COOH-terminal domain. Proc Natl Acad Sci U S A. 2001;98:10475–10480. doi: 10.1073/pnas.181481098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WS, Hebert SC. ROMK inwardly rectifying ATP-sensitive K+ channel. I. Expression in rat distal nephron segments. Am J Physiol. 1995;268:F1124–1131. doi: 10.1152/ajprenal.1995.268.6.F1124. [DOI] [PubMed] [Google Scholar]
- Lin DH, Sterling H, Yang B, Hebert SC, Giebisch G, Wang WH. Protein tyrosine kinase is expressed and regulates ROMK1 location in the cortical collecting duct. Am J Physiol Renal Physiol. 2004;286:F881–F892. doi: 10.1152/ajprenal.00301.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DH, Yue P, Rinehart J, Sun P, Wang Z, Lifton R, Wang WH. Protein phosphatase 1 modulates the inhibitory effect of With-no-Lysine kinase 4 on ROMK channels. Am J Physiol Renal Physiol. 2012;303:F110–F119. doi: 10.1152/ajprenal.00676.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DH, Yue P, Yarborough O, Scholl UI, Giebisch G, Lifton RP, Rinehart J, Wang WH. Src-family protein tyrosine kinase phosphorylates WNK4 and modulates its inhibitory effect on KCNJ1 (ROMK) Proc Natl Acad Sci U S A. 2015;112:4495–4500. doi: 10.1073/pnas.1503437112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BC, Yang LL, Lu XY, Song X, Li XC, Chen G, Li Y, Yao X, Humphrey DR, Eaton DC, Shen BZ, Ma HP. Lovastatin-induced phosphatidylinositol-4-phosphate 5-kinase diffusion from microvilli stimulates ROMK channels. J Am Soc Nephrol. 2015;26:1576–1587. doi: 10.1681/ASN.2013121326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourdel S, Paulais M, Cluzeaud F, Bens M, Tanemoto M, Kurachi Y, Vandewalle A, Teulon J. An inward rectifier K+ channel at the basolateral membrane of the mouse distal convoluted tubule: similarities with Kir4-Kir5.1 heteromeric channels. J Physiol. 2002;538:391–404. doi: 10.1113/jphysiol.2001.012961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Giebisch G, Wang W. Nitric oxide-induced hyperpolarization stimulates low-conductance Na+ channel of rat CCD. Am J Physiol Renal Physiol. 1997a;272:F498–F504. doi: 10.1152/ajprenal.1997.272.4.F498. [DOI] [PubMed] [Google Scholar]
- Lu M, Giebisch G, Wang W. Nitric oxide links the apical Na+ transport to the basolateral K+ conductance in the rat cortical collecting duct. J Gen Physiol. 1997b;110:717–726. doi: 10.1085/jgp.110.6.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Wang W. Protein kinase C stimulates the small-conductance K+ channel in the basolateral membrane of the CCD. Am J Physiol. 1996a;271:F1045–1051. doi: 10.1152/ajprenal.1996.271.5.F1045. [DOI] [PubMed] [Google Scholar]
- Lu M, Wang WH. Nitric oxide regulates the low-conductance K+ channel in basolateral membrane of cortical collecting duct. Am J Physiol. 1996b;270:C1336–1342. doi: 10.1152/ajpcell.1996.270.5.C1336. [DOI] [PubMed] [Google Scholar]
- McCormick JA, Ellison DH. Distal convoluted tubule. Compr Physiol. 2015;5:45–98. doi: 10.1002/cphy.c140002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough AA, Geering K, Farley RA. The sodium pump needs its beta subunit. FASEB J. 1990;4:1598–1605. doi: 10.1096/fasebj.4.6.2156741. [DOI] [PubMed] [Google Scholar]
- Méndez-González MP, Kucheryavykh YV, Zayas-Santiago A, Vélez-Carrasco W, Maldonado-Martínez G, Cubano LA, Nichols CG, Skatchkov SN, Eaton MJ. Novel KCNJ10 variations compromise function of inwardly rectifying potassium channel 4.1. J Biol Chem. 2016;291:7716–7726. doi: 10.1074/jbc.M115.679910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar ID, Taylor HC, Cooper GJ, Kibble JD, Robson L. A Kir2.3-like K+ conductance in mouse cortical collecting duct principal cells. J Membr Biol. 2006;211:173–184. doi: 10.1007/s00232-006-0036-z. [DOI] [PubMed] [Google Scholar]
- Nichols CG, Lopatin AN. Inward rectifier potassium channels. Annu Rev Physiol. 1997;59:171–191. doi: 10.1146/annurev.physiol.59.1.171. [DOI] [PubMed] [Google Scholar]
- Nwaobi SE, Lin E, Peramsetty SR, Olsen ML. DNA methylation functions as a critical regulator of Kir4.1 expression during CNS development. Glia. 2014;62:411–427. doi: 10.1002/glia.22613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwaobi SE, Olsen ML. Correlating gene-specific DNA methylation changes with expression and transcriptional activity of astrocytic KCNJ10 (Kir4.1) J Vis Exp. 2015;103:e52406. doi: 10.3791/52406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno Y, Hibino H, Lossin C, Inanobe A, Kurachi Y. Inhibition of astroglial Kir4.1 channels by selective serotonin reuptake inhibitors. Brain Res. 2007;1178:44–51. doi: 10.1016/j.brainres.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Olsen O, Liu H, Wade JB, Merot J, Welling PA. Basolateral membrane expression of the Kir2.3 channel is coordinated by PDZ interaction with Lin-7/CASK complex. Am J Physiol Cell Physiol. 2002;282:C183–195. doi: 10.1152/ajpcell.00249.2001. [DOI] [PubMed] [Google Scholar]
- Ookata K, Tojo A, Suzuki Y, Nakamura N, Kimura K, Wilcox CS, Hirose S. Localization of inward rectifier potassium channel Kir7.1 in the basolateral membrane of distal nephron and collecting duct. J Am Soc Nephrol. 2000;11:1987–1994. doi: 10.1681/ASN.V11111987. [DOI] [PubMed] [Google Scholar]
- Palygin O, Levchenko V, Ilatovskaya DV, Barnett JL, Geurts AM, Jacob HJ, Staruschenko A. Renal phenotype of inwardly rectifying potassium channel Kcnj16 (Kir5.1) knockout in the Dahl salt-sensitive rats. Hypertension. 2015;66:A113. [Google Scholar]
- Parrock S, Hussain S, Issler N, Differ AM, Lench N, Guarino S, Oosterveld MJS, Keijzer-Veen M, Brilstra E, van Wieringen H, Konijnenberg AY, Amin-Rasip S, Dumitriu S, Klootwijk E, Knoers N, Bockenhauer D, et al. KCNJ10 mutations display differential sensitivity to heteromerisation with KCNJ16. Nephron Physiol. 2013;123:7–14. doi: 10.1159/000356353. [DOI] [PubMed] [Google Scholar]
- Paulais M, Bloch-Faure M, Picard N, Jacques T, Ramakrishnan SK, Keck M, Sohet F, Eladari D, Houillier P, Lourdel Sp, Teulon J, Tucker SJ. Renal phenotype in mice lacking the Kir5.1 (Kcnj16) K+ channel subunit contrasts with that observed in SeSAME/EAST syndrome. Proc Natl Acad Sci U S A. 2011;108:10361–10366. doi: 10.1073/pnas.1101400108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov TS, Ilatovskaya DV, Levchenko V, Li L, Ecelbarger CM, Staruschenko A. Regulation of ENaC in mice lacking renal insulin receptors in the collecting duct. FASEB J. 2013a;27:2723–2732. doi: 10.1096/fj.12-223792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov TS, Levchenko V, O’Connor PM, Ilatovskaya DV, Palygin O, Mori T, Mattson DL, Sorokin A, Lombard JH, Cowley AW, Jr, Staruschenko A. Deficiency of renal cortical EGF increases ENaC activity and contributes to salt-sensitive hypertension. J Am Soc Nephrol. 2013b;24:1053–1062. doi: 10.1681/ASN.2012080839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson PB. Good publication practice in physiology 2015. Acta Physiol. 2015;215:163–164. doi: 10.1111/apha.12606. [DOI] [PubMed] [Google Scholar]
- Pessia M, Imbrici P, D’Adamo MC, Salvatore L, Tucker SJ. Differential pH sensitivity of Kir4.1 and Kir4.2 potassium channels and their modulation by heteropolymerisation with Kir5.1. J Physiol. 2001;532:359–367. doi: 10.1111/j.1469-7793.2001.0359f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessia M, Tucker SJ, Lee K, Bond CT, Adelman JP. Subunit positional effects revealed by novel heteromeric inwardly rectifying K+ channels. EMBO J. 1996;15:2980–2987. [PMC free article] [PubMed] [Google Scholar]
- Pluznick JL, Wei P, Grimm PR, Sansom SC. BK-β1 subunit: immunolocalization in the mammalian connecting tubule and its role in the kaliuretic response to volume expansion. Am J Physiol Renal Physiol. 2005;288:F846–F854. doi: 10.1152/ajprenal.00340.2004. [DOI] [PubMed] [Google Scholar]
- Raphemot R, Lonergan DF, Nguyen TT, Utley T, Lewis LM, Kadakia R, Weaver CD, Gogliotti R, Hopkins C, Lindsley CW, Denton JS. Discovery, characterization, and structure-activity relationships of an inhibitor of inward rectifier potassium (Kir) channels with preference for Kir2.3, Kir3.x, and Kir7.1. Front Pharmacol. 2011;2:75. doi: 10.3389/fphar.2011.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichold M, Zdebik AA, Lieberer E, Rapedius M, Schmidt K, Bandulik S, Sterner C, Tegtmeier I, Penton D, Baukrowitz T, Hulton SA, Witzgall R, Ben-Zeev B, Howie AJ, Kleta R, Bockenhauer D, et al. KCNJ10 gene mutations causing EAST syndrome (epilepsy, ataxia, sensorineural deafness, and tubulopathy) disrupt channel function. Proc Natl Acad Sci U S A. 2010;107:14490–14495. doi: 10.1073/pnas.1003072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieg T, Vallon V, Sausbier M, Sausbier U, Kaissling B, Ruth P, Osswald H. The role of the BK channel in potassium homeostasis and flow-induced renal potassium excretion. Kidney Int. 2007;72:566–573. doi: 10.1038/sj.ki.5002369. [DOI] [PubMed] [Google Scholar]
- Rubera I, Loffing J, Palmer LG, Frindt G, Fowler-Jaeger N, Sauter D, Carroll T, McMahon A, Hummler E, Rossier BC. Collecting duct-specific gene inactivation of αENaC in the mouse kidney does not impair sodium and potassium balance. J Clin Invest. 2003;112:554–565. doi: 10.1172/JCI16956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala-Rabanal M, Kucheryavykh LY, Skatchkov SN, Eaton MJ, Nichols CG. Molecular mechanisms of EAST/SeSAME syndrome mutations in Kir4.1 (KCNJ10) J Biol Chem. 2010;285:36040–36048. doi: 10.1074/jbc.M110.163170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlatter E, Lohrmann E, Greger R. Properties of the potassium conductances of principal cells of rat cortical collecting ducts. Pflugers Arch. 1992;420:39–45. doi: 10.1007/BF00378639. [DOI] [PubMed] [Google Scholar]
- Scholl UI, Choi M, Liu T, Ramaekers VT, Hausler MG, Grimmer J, Tobe SW, Farhi A, Nelson-Williams C, Lifton RP. Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proc Natl Acad Sci U S A. 2009;106:5842–5847. doi: 10.1073/pnas.0901749106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepúlveda FV, Pablo Cid L, Teulon J, Niemeyer MI. Molecular aspects of structure, gating, and physiology of pH-sensitive background K2P and Kir K+-transport channels. 2015;95:179–217. doi: 10.1152/physrev.00016.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaats GG, Wheway G, Foletto V, Szymanska K, van Balkom BWM, Logister I, Den Ouden K, Keijzer-Veen MG, Lilien MR, Knoers NV, Johnson CA, Giles RH. Screen-based identification and validation of four new ion channels as regulators of renal ciliogenesis. J Cell Sci. 2015;128:4550–4559. doi: 10.1242/jcs.176065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, Ziegler U, Odermatt A, Loffing-Cueni D, Loffing J. Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int. 2013;83:811–824. doi: 10.1038/ki.2013.14. [DOI] [PubMed] [Google Scholar]
- Staruschenko A. Regulation of transport in the connecting tubule and cortical collecting duct. Compreh Physiol. 2012;2:1541–1584. doi: 10.1002/cphy.c110052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S, Ohno Y, Lossin C, Hibino H, Inanobe A, Kurachi Y. Inhibition of astroglial inwardly rectifying Kir4.1 channels by a tricyclic antidepressant, nortriptyline. J Pharmacol Exp Ther. 2007;320:573–580. doi: 10.1124/jpet.106.112094. [DOI] [PubMed] [Google Scholar]
- Su X-T, Zhang C, Wang L, Gu R, Lin D-H, Wang W-H. The disruption of KCNJ10 (Kir4.1) stimulates the expression of ENaC in the collecting duct. Am J Physiol Renal Physiol. 2016 doi: 10.1152/ajprenal.00584.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanya AR, Ellison DH. Distal convoluted tubule. Clin J Am Soc Nephrol. 2014;9:2147–2163. doi: 10.2215/CJN.05920613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takumi T, Ishii T, Horio Y, Morishige KI, Takahashi N, Yamada M, Yamashita T, Kiyama H, Sohmiya K, Nakanishi S, Kurachi Y. A novel ATP-dependent inward rectifier potassium channel expressed predominantly in glial cells. J Biol Chem. 1995;270:16339–16346. doi: 10.1074/jbc.270.27.16339. [DOI] [PubMed] [Google Scholar]
- Tanemoto M. Regulatory mechanism of “K+-recycling” for Na+-reabsorption in renal tubules. Clin Exp Nephrol. 2007;11:1–6. doi: 10.1007/s10157-006-0447-2. [DOI] [PubMed] [Google Scholar]
- Tanemoto M, Abe T, Uchida S, Kawahara K. Mislocalization of K+ channels causes the renal salt wasting in EAST/SeSAME syndrome. FEBS Lett. 2014;588:899–905. doi: 10.1016/j.febslet.2014.02.024. [DOI] [PubMed] [Google Scholar]
- Tanemoto M, Fujita A, Higashi K, Kurachi Y. PSD-95 mediates formation of a functional homomeric Kir5.1 channel in the brain. Neuron. 2002;34:387–397. doi: 10.1016/s0896-6273(02)00675-x. [DOI] [PubMed] [Google Scholar]
- Tang X, Hang D, Sand A, Kofuji P. Variable loss of Kir4.1 channel function in SeSAME syndrome mutations. Biochem Biophys Res Comm. 2010;399:537–541. doi: 10.1016/j.bbrc.2010.07.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi J, Yoshitomi K, Imai M. K+ channel currents in basolateral membrane of distal convoluted tubule of rabbit kidney. Am J Physiol. 1989;256:F246–254. doi: 10.1152/ajprenal.1989.256.2.F246. [DOI] [PubMed] [Google Scholar]
- Terker AS, Zhang C, McCormick JA, Lazelle RA, Zhang C, Meermeier NP, Siler DA, Park HJ, Fu Y, Cohen DM, Weinstein AM, Wang WH, Yang CL, Ellison DH. Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab. 2015;21:39–50. doi: 10.1016/j.cmet.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp S, Tucker SJ, Gourine AV. Respiratory responses to hypercapnia and hypoxia in mice with genetic ablation of Kir5.1 (Kcnj16) Exp Physiol. 2011;96:451–459. doi: 10.1113/expphysiol.2010.055848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker SJ, Imbrici P, Salvatore L, D’Adamo MC, Pessia M. pH dependence of the inwardly rectifying potassium channel, Kir5.1, and localization in renal tubular epithelia. J Biol Chem. 2000;275:16404–16407. doi: 10.1074/jbc.C000127200. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang C, Su X, Lin DH, Wang W. Caveolin-1 deficiency inhibits the basolateral K+ channels in the distal convoluted tubule and impairs renal K+ and Mg2+ transport. J Am Soc Nephrol. 2015;26:2678–2690. doi: 10.1681/ASN.2014070658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Hebert SC, Giebisch G. Renal K+ channels: structure and function. Annu Rev Physiol. 1997;59:413–436. doi: 10.1146/annurev.physiol.59.1.413. [DOI] [PubMed] [Google Scholar]
- Wang WH. Regulation of the hyperpolarization-activated K+ channel in the lateral membrane of the cortical collecting duct. J Gen Physiol. 1995;106:25–43. doi: 10.1085/jgp.106.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WH, Giebisch G. Regulation of potassium (K) handling in the renal collecting duct. Pflugers Arch. 2009;458:157–168. doi: 10.1007/s00424-008-0593-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WH, McNicholas CM, Segal AS, Giebisch G. A novel approach allows identification of K channels in the lateral membrane of rat CCD. Am J Physiol. 1994;266:F813–822. doi: 10.1152/ajprenal.1994.266.5.F813. [DOI] [PubMed] [Google Scholar]
- Wei Y, Liao Y, Zavilowitz B, Ren J, Liu W, Chan P, Rohatgi R, Estilo G, Jackson EK, Wang WH, Satlin LM. Angiotensin II type 2 receptor regulates ROMK-like K+ channel activity in the renal cortical collecting duct during high dietary K+ adaptation. Am J Physiol Renal Physiol. 2014;307:F833–843. doi: 10.1152/ajprenal.00141.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welling PA. Primary structure and functional expression of a cortical collecting duct Kir channel. Am J Physiol. 1997;273:F825–836. doi: 10.1152/ajprenal.1997.273.5.F825. [DOI] [PubMed] [Google Scholar]
- Welling PA. Roles and regulation of renal K channels. Annu Rev Physiol. 2016;78:415–435. doi: 10.1146/annurev-physiol-021115-105423. [DOI] [PubMed] [Google Scholar]
- Wen D, Cornelius RJ, Rivero-Hernandez D, Yuan Y, Li H, Weinstein AM, Sansom SC. Relation between BK-α/β4-mediated potassium secretion and ENaC-mediated sodium reabsorption. Kidney Int. 2014;86:139–145. doi: 10.1038/ki.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DM, Lopes CMB, Rosenhouse-Dantsker A, Connelly HL, Matavel A, O-Uchi J, McBeath E, Gray DA. Molecular basis of decreased Kir4.1 function in SeSAME/EAST syndrome. J Am Soc Nephrol. 2010;21:2117–2129. doi: 10.1681/ASN.2009121227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woda CB, Bragin A, Kleyman TR, Satlin LM. Flow-dependent K+ secretion in the cortical collecting duct is mediated by a maxi-K channel. Am J Physiol Renal Physiol. 2001;280:F786–F793. doi: 10.1152/ajprenal.2001.280.5.F786. [DOI] [PubMed] [Google Scholar]
- Woda CB, Leite M, Jr, Rohatgi R, Satlin LM. Effects of luminal flow and nucleotides on [Ca2+]i in rabbit cortical collecting duct. Am J Physiol Renal Physiol. 2002;283:F437–F446. doi: 10.1152/ajprenal.00316.2001. [DOI] [PubMed] [Google Scholar]
- Zaika O, Mamenko M, Boukelmoune N, Pochynyuk O. IGF-1 and insulin exert opposite actions on ClC-K2 activity in the cortical collecting ducts. Am J Physiol Renal Physiol. 2015;308:F39–F48. doi: 10.1152/ajprenal.00545.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaika O, Palygin O, Tomilin V, Mamenko M, Staruschenko A, Pochynyuk O. Insulin and IGF-1 activate Kir4.1/5.1 channels in cortical collecting duct principal cells to control basolateral membrane voltage. Am J Physiol Renal Physiol. 2016a;310:F311–321. doi: 10.1152/ajprenal.00436.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaika O, Tomilin V, Mamenko M, Bhalla V, Pochynyuk O. New perspective of ClC-Kb/2 chloride channel physiology in the distal renal tubule. Am J Physiol Renal Physiol. 2016b doi: 10.1152/ajprenal.00577.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaika OL, Mamenko M, Palygin O, Boukelmoune N, Staruschenko A, Pochynyuk O. Direct inhibition of basolateral Kir4.1/5.1 and Kir4.1 channels in the cortical collecting duct by dopamine. Am J Physiol Renal Physiol. 2013;305:F1277–1287. doi: 10.1152/ajprenal.00363.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Wang L, Su XT, Lin DH, Wang WH. KCNJ10 (Kir4.1) is expressed in the basolateral membrane of the cortical thick ascending limb. Am J Physiol Renal Physiol. 2015;308:F1288–F1296. doi: 10.1152/ajprenal.00687.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Wang L, Thomas S, Wang K, Lin DH, Rinehart J, Wang WH. Src family protein tyrosine kinase regulates the basolateral K channel in the distal convoluted tubule (DCT) by phosphorylation of KCNJ10 protein. J Biol Chem. 2013;288:26135–26146. doi: 10.1074/jbc.M113.478453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Wang L, Zhang J, Su XT, Lin DH, Scholl UI, Giebisch G, Lifton RP, Wang WH. KCNJ10 determines the expression of the apical Na-Cl cotransporter (NCC) in the early distal convoluted tubule (DCT1) Proc Natl Acad Sci U S A. 2014;111:11864–11869. doi: 10.1073/pnas.1411705111. [DOI] [PMC free article] [PubMed] [Google Scholar]