Abstract
It has been suggested that repeated infection of Opisthorchis viverrini followed by repeated treatment with praziquantel (PZQ) increases risk of development of cholangiocarcinoma (CCA). Evidence for the prediction has accumulated based on findings of indirect approaches involving molecular changes and epidemiological trends. By contrast, here we directly monitored the impact of repeated liver fluke infection and treatment with PZQ on cholangiocarcinogenesis in a rodent model of human opisthorchiasis, using magnetic resonance imaging (MRI) and histopathology. Twenty five Syrian golden hamsters were assigned to five treatment groups: 1) infection with O. viverrini (OV group), 2) treatment with the carcinogen N-nitrosodimethylamine (NDMA) at 12.5 ppm (DMN), 3) O. viverrini infection in tandem with NDMA (OD), 4) O. viverrini infection, NDMA, and treatment with PZQ (ODP), and 5) uninfected, untreated control. The repeated infections were established by intragastric inoculation of 50 metacercariae of O. viverrini to the OV, OD and ODP hamsters at weeks 0, 5 and 10. PZQ at 300 mg/kg body weight was given to each hamster of the ODP group on weeks 4, 9 and 13 (four weeks after each infection). Imaging by MRI was undertaken on weeks 5, 10 and 14 (i.e. one week after each PZQ treatment). MRI revealed that the ODP hamsters did not develop CCA, whereas necropsy at week 40 revealed CCA in hamsters of the OD and DMN groups. Findings for histopathology and for proliferating cell nuclear antigen index conformed to the MRI findings. In overview, and notwithstanding that the immune response of individual hosts may play roles in cholangiocarcinogenesis, three cycles of the infection with O. viverrini followed treatment of the infection with PZQ did not increase the risk of bile duct cancer in this hamster model of liver fluke infection-induced CCA.
Keywords: Repeated infection, Opisthorchis, Praziquantel, MRI, Hamster, Cholangiocarcinoma
1. Introduction
Opisthorchiasis, caused by Opisthorchis viverrini, is a major food-borne trematodiasis in Southeast Asia, especially in the Northeast of Thailand. Infection follows ingestion of uncooked infected fresh-water fish and fish products. Despite its near uniformly fatal outcome in Thailand, CCA as the consequence of chronic opisthorchiasis, and longstanding public health advocacy to warn of the risk of CCA from raw fish products [1–4], many people following treatment continue to consume traditional fish dishes that carry the infective stage of the parasite. Once infected, these people accept treatment with the anthelmintic drug praziquantel (PZQ) to eliminate the parasite; hence they are exposed to repeated cycles of infection and anthelmintic therapy. It has been suggested that such cycles of liver fluke infection and treatment with praziquantel increase the risk of CCA development, based on epidemiological [5] and molecular evidences focusing on inflammation-initiated DNA damage [6].
Pakharukova et al. [7] described a mechanism of anthelmintic action of PZQ against Opisthorchis felineus; PZQ destroyed tegument and musculature of the parasite causing spasm, paralysis and death. It can be anticipated that PZQ acts similarly against O. viverrini [8]. As a result, antigens liberated from the disintegrating flukes stimulate inflammatory cells, such as eosinophils and mast cells that accumulate around the bile ducts. These cells generate free radicals, potentially leading to off-target oxidative DNA damage in bystander cells including cholangiocytes. Inducible nitric oxide synthase (iNOS), a free radical produced by inflammatory cells and biliary epithelial cells, is a potential candidate for this. Thus, a short-term effect of PZQ on the inflammatory induction and increment of oxidative and nitrative stresses was demonstrated by using iNOS, nuclear factor kappa B (NF-kB), and antioxidant enzymes (such as superoxide dismutase, catalase and glutathione peroxidase) [9]. In particular, 8-nitroguanine and 8-oxodG induce G:C ➔ T:A transversion [10]. This DNA adduct could direct the neoplastic transformation and CCA development [11–13].
The process of cholangiocarcinogenesis is not yet well understood, and research is needed into this malignancy, especially given its alarming, negative impact in the residents of opisthorchiasis endemic regions. The hamster model of liver fluke induced CCA is a valuable tool in this endeavor [14], given the limitations on human studies including ethical concern. The Syrian golden hamster (Mesocricetus auratus) infected with O. viverrini develops proliferative changes, including hyperplasia both primary and secondary order bile ducts, adenomatous hyperplasia, metaplasia and periductal fibrosis [14]. Inflammatory cells including lymphocytes, monocytes, macrophages, eosinophils and plasma cells accumulate around the biliary ducts in similar fashion to infected humans.
To visualize the CCA in situ, ultrasonography (US) and magnetic resonance image (MRI) provide potentially non-invasive techniques; indeed, both are used routinely for this indication in the clinic and in the laboratory [15–17]. A benefit of US for this particular task is its combined accessibility and economy of operating costs. However, the resolution of US immensely depends on the specifications of the particular US device and on the skill and knowledge of the operator. Moreover, US cannot quantify well the hepatic fat content or detect minor changes in the liver fat. Therefore, it has limited use for longitudinal clinical studies [18,19]. MRI has been used for early cancer detection, staging and monitoring the therapeutic responses [15,16]. Findings using MRI to sequentially identify the development of CCA in both experimentally infected hamsters and in people in regions endemic for opisthorchiasis have been reported [20,21]. From these studies, it has become apparent that while T1-weighted image could gain a parameter to elucidate CCA in the hepatic parenchyma, T2-weighted image can elucidate changes of the bile duct, such as dilatation, obstruction, cyst, abscess, as well as periductal fibrosis. Accordingly, MRI represents an appropriate tool to investigate hepatobiliary changes in the hamster model of opisthorchiasis-induced bile duct cancer. MRI was deployed, in parallel with histopathological and proliferation analyses, here to investigate and reveal biliary changes in the livers of hamsters repeatedly infected with O. viverrini and repeatedly treated to PZQ to clear the liver fluke infection.
2. Materials and methods
2.1. Hamsters
Twenty-five male Syrian golden hamsters, six-weeks old, weighing 120–150 g, were obtained from the Animal Unit, Faculty of Medicine, Khon Kaen University. The hamsters were housed in conventional conditions, with commercial laboratory rodent food pellets and water provided ad libitum; the experiment continued for a duration of 40 weeks. This study was approved by the Animal Ethics Committee of Khon Kaen University, Khon Kaen, Thailand (AEKKU55/2554, AEKKU52/2553), and procedures followed the Guide for the Care and Use of Laboratory Animals of the National Research Council of Thailand.
2.2. Metacercariae of O. viverrini
Wild cyprinid fish from the endemic area of opisthorchiasis in Khon Kaen Province, Thailand were digested with a 0.25% pepsin–HCl solution and encysted O. viverrini metacercariae isolated, counted and evaluated by microscopic examination for viability, as described [22]. Active (viable) metacercariae were used for the infection of hamsters.
2.3. Experimental design — repeated infections and praziquantel treatments
The 25 hamsters were divided into groups each of five hamsters, including the uninfected group (control group; n = 5 hamsters), O. viverrini infected group (OV; n = 5), NDMA exposed group (DMN; n = 5), O. viverrini infected and NDMA exposed group (OD; n = 5), and OD with PZQ treated group (ODP; n = 5). 12.5 ppm of NDMA in water was given to hamsters in the DMN, OD and ODP groups ad libitum only during days 0 to 56 (i.e. the first eight weeks) of infection. Each animal in the OV and OD groups was infected with 50 O. viverrini metacercariae delivered by intragastric intubation [14] whereas the animal in the ODP group received three consecutive doses of 50 O. viverrini metacercariae at weeks 0, 5 and 10. Concerning PZQ, the five ODP hamsters were treated with 300 mg/kg body weight of PZQ (Sigma®, Switzerland) dissolved in ethanol by intragastric intubation at weeks 4, 9 and 13, i.e. four weeks after each round of infection. Feces were collected for formalin ether concentration test (FECT) to determine the baseline egg count per gram (EPG). Subsequently, the animals were housed without further infection or treatment until week 40 of the study, when the surviving hamsters were euthanized and necropsied.
2.4. Magnetic resonance imaging
MRI was performed to detect the hepatobiliary changes at one week post-treatment of each round (Figs. 1 and 2; 5w = before consecutive cycle, T#1 = week 10, T#2 = week 15 and T#3 = week 20). Prior to performing the MRI, the hamsters were subjected to general anesthesia with 70–90 mg/kg pentobarbital sodium (Nembutal®) delivered intraperitoneally. The hamsters were examined with a 3 T whole-body MR system (AchievaTx; Philips, The Netherlands) with a maximal gradient capability of 80 mT/m and a maximal slew rate of 200 mT/m/s. An 8-channel human wrist coil was used as an animal holder. A hamster was placed in the ventrodorsal or supine position inside the coil with the liver region located in the center of the coil. The abdomen of the hamster was affixed with plastic wrap/cling film to the cradle to reduce artifacts of respiratory movement. Also, the hamster was covered with bedding cloth, aiming to maintain constant body temperature. At the outset, scout images were acquired in three orthogonal planes with a fast low angle shot sequence to locate the liver. Conventional liver MRI protocols were followed: 1) TSE (turbo spin-echo) T2-weighted images with fat suppression SPAIR (spectral selection attenuation inversion recovery) in the axial, sagittal, and coronal orientations (TR 2000 ms, TE 80 ms, inversion delay 120 ms, field of view 100–120 mm, slice thickness 1.5–2 mm, axial matrix 284 × 210, sagittal matrix 240 × 160, coronal matrix 268 × 240) and 2) axial orientation SE (spin echo) T1-weighted images (TR 250 ms, TE 3.53 ms, field of view 100–120 mm, slice thickness 1.5–2 mm, matrix the same as in T2). After the third MRI, further investigation was performed only in groups OD and ODP to monitor long-term changes in the hepatobiliary system.
Fig. 1.
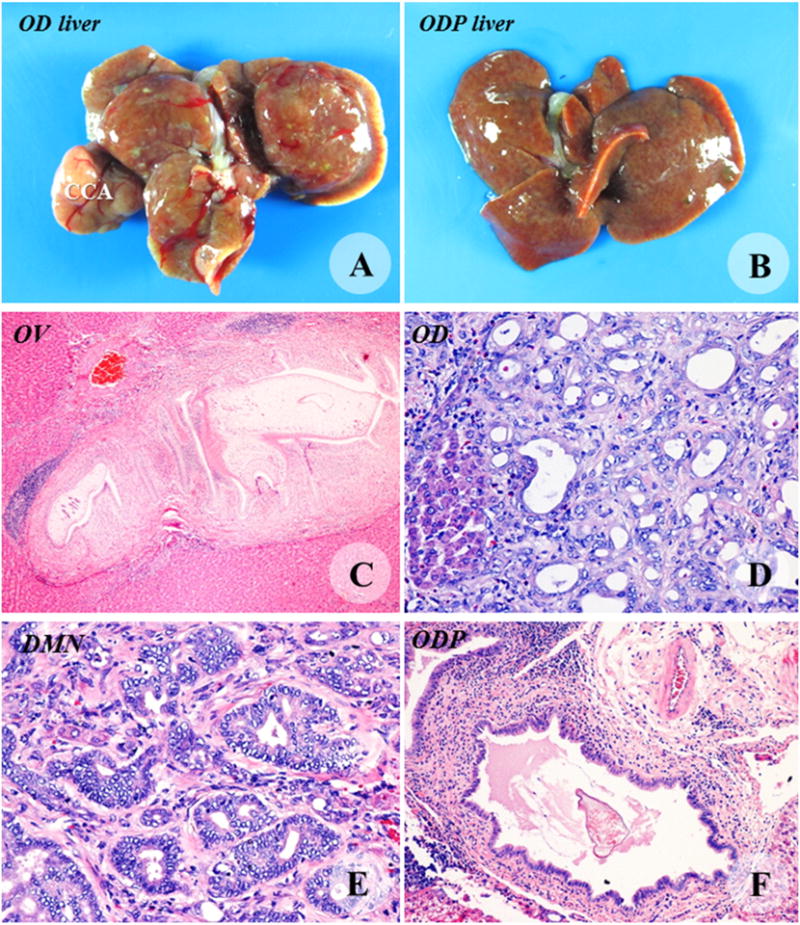
Necropsy and histopathological findings of the livers from the OV (C), OD (A, D), DMN (E) and ODP groups (B, F). CCA (T) was protruding from the middle lobe of the OD liver. The ODP liver contained multiple cysts. Periductal fibrosis and lymphoid aggregation were predominant lesions in the OV hamsters. Histopathology of cholangiocarcinoma (CCA) was shown in the OD and DMN livers. Dead worms were seen often in bile ducts of the liver of hamsters of the ODP group. Original magnification C = 10×, D = 20×, E = 20×, and F = 10×.
Fig. 2.
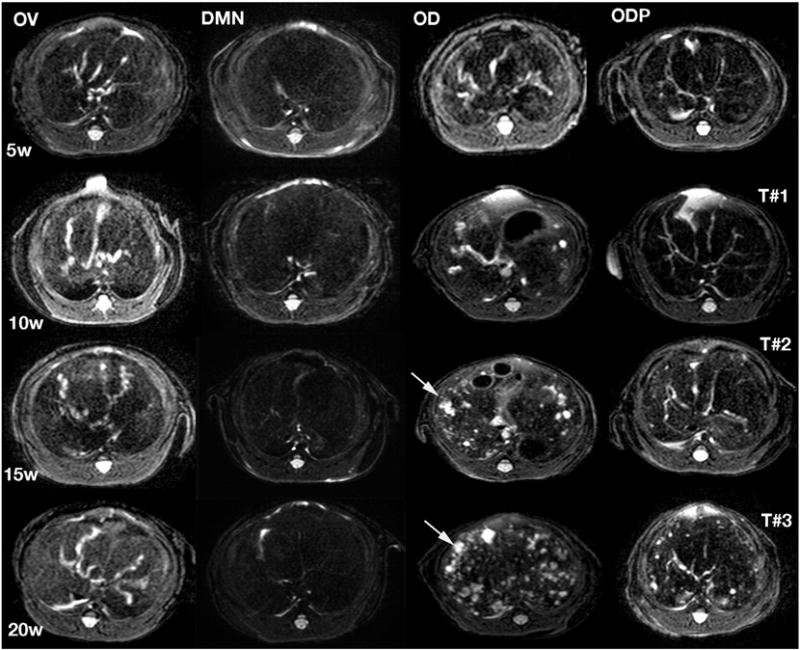
Axial T2 weighed images of hamsters in the OV, DMN, OD and ODP groups. Experimental weeks (W) and order of MRI examination (T#) are labeled on the left and right sides of the figure, respectively. The first MRI (T#1) applied at week five (5W) being shown in the first row could reveal different degrees of hepatobiliary lesions. The animals in the OD group showed the most progressive appearance. Arrows indicated periductal fibrosis and duct dilatation, which increased in the OD hamsters. Hamsters of the ODP group exhibited less severe lesions compared to hamsters in the DMN and OV groups. Progression of the hepatobiliary lesions is in accordance with number of cycles of infections with O. viverrini and treatment with PZQ treatment: the higher number, the more progress.
2.5. Histopathological study
At the conclusion of the study, hamsters were euthanized by an overdose of inhaled ether. Hamsters that died before 20 weeks were also examined. The liver and biliary tract were excised and retained for histopathological and immunohistopathological analyses. Tissue samples were fixed in 10% buffered formalin for 24 h prior to processing for the paraffin block and 4-μm-thick slide with H&E staining.
2.6. Immunohistochemical study for cell proliferation
Proliferative activity of the biliary lesions was investigated by exploration by immunohistochemistry (IHC) of proliferating cell nuclear antigen (PCNA), a nuclear marker of cell proliferation [23,24]. After deparaffinization and rehydration, unstained slides were heated by a high pressure cooker for 3 min to retrieve the antigen. The slides were applied by 3% H2O2 in methanol for peroxidase activity blocking and rinsed with water. Thereafter, slides were incubated in 1% normal horse serum to block non-specific binding sites, and subsequently probed overnight with primary antibody, mouse monoclonal anti-PCNA, clone PC10 (Dako, Denmark) diluted 1:200. Following several washes, the slides were probed with biotinylated goat anti-mouse antibody diluted 1:300 for 30 min before color development in 0.03% diaminobenzidine (DAB) in 0.003% hydrogen peroxide and counterstaining with Meyer’s hematoxylin. Stained slides were evaluated by light microscopy.
2.7. Data evaluation
The MRIs were retrospectively evaluated in parallel by a scientist and by a radiologist, both experienced with diagnosis of hepatobiliary changes and cholangiocarcinoma. The high signal image intensities of the bile ducts from the MRI were used to evaluate inflammation, bile duct dilatation and fibrosis. Changes of the bile duct were graded from 0 to III as follows: grade 0 for the absence of high signal image intensity of the bile ducts, grade I for high signal in one segment of the liver, grade II for high signal in two or three segments of the liver; and grade III for high signal in more than three segments of the liver [20]. Thereafter, the findings from the MRI, histopathology and the IHC studies were correlated and interpreted in combination.
3. Results
One of five hamsters of the OD and ODP groups died from wounds inflicted by infighting among cage mates. At necropsy, CCA was not apparent in these hamsters. Thus, only four of five hamsters of the OD and ODP groups survived until the third MRI scans. However, by the end of the experiment only two animals in each of the OD and ODP had survived; they were euthanized at the end of the experiment. The other groups did not exhibit similar dropout of animals. The mean EPGs of the OD vs. ODP groups were 101 vs. 80.7, 2343 vs. 94.67, 780 vs. 180 and 7838 vs. 0 at weeks 4, 6, 9 and 14, respectively. This reflected the 100% egg reduction rate (ERR) in the ODP group.
3.1. Pathological changes and proliferative activity of biliary cells
Euthanasia and liver necropsy were performed at the conclusion of the experiment. Gross examination of the liver revealed hepatomegaly with rough surface, particularly in hamsters of the OD group. All OD hamsters exhibited evidence of CCA (Fig. 1). Fibrosis and cyst formation were the predominant gross lesion in the liver and the gall bladder in the OV and OD hamsters. More adult worms were found in the gall bladder and large bile duct of hamsters in the OV group than in the OD group. In the DMN group, severe lesion was not apparent in all three MRI investigations; however, CCA development was seemingly expedited after the third MRI. This situation was confirmed at necropsy. CCA was pale and multinodular in appearance, varying in size from a few millimeters up to approximately 1 cm. It contained a range of cystic changes.
Microscopic findings in the livers from the OV hamsters revealed extensive periductal fibrosis with biliary hyperplasia, lymphoid aggregation, duct dilatation and cyst formation. Adult worms were seen in the intrahepatic bile ducts and gall bladders. The lesions in OD hamsters included hyperplasia of the first- and second-order bile ducts, precancerous lesions such as dysplasia, cholangiofibrosis, goblet cell metaplasia, and CCA. Proliferative lesions including dysplasia, hyperplasia, metaplasia and CCA were observed in the livers of the hamsters fed with DMN. Microscopic features of the livers from the ODP group looked similar to those of the OV group but in milder extent. Although some dead worms were seen in the bile ducts of the ODP group, living adult worms were seen in bile ducts of three of the five ODP hamsters. PCNA immunohistochemistry was performed to characterize and quantify proliferation of biliary cells at the end of the study. The PCNA index in the OD, DMN, OV and ODP groups was markedly diminished from 100 to 84, 67 and 21%, respectively.
3.2. Magnetic resonance images
Analysis by MRI commenced at week five post-infection. Infection was established by fecal EPG. Axial T1 and T2 weighted images of the control hamsters showed normal anatomy including the liver, spinal cord, aorta and hepatic veins. Body fat showed higher (brighter) signal intensity than the liver (gray), reflecting its shorter T1 (fat b liver). Aorta and hepatic veins were dark reflecting the high velocity of blood flow. Axial T2 weighted with fat suppression images to show various anatomical structures including the liver was seen darker (lower signal intensity) than the cerebral spinal fluid (CSF) in the spinal column (bright). The dark area around the hamster body reflected the fat suppression effect on the body fat. Coronal T1 and T2 weighted images showed the anatomy including low intensity lung, liver, and kidney. T1 weighted images showed fat around the body of the hamster having higher signal intensity (brighter) than the liver (gray) and kidney. T2 weighted images showed fat around the body having lower signal intensity (dark) reflecting the fat suppression technique. The signal intensity for the liver was lower than the kidney. The higher signal could reflect the density of cells, matrix or fluid accumulation.
Signal intensities of the bile ducts were used to investigate inflammation, bile duct dilatation and fibrosis. All hamsters in the OV, OD and ODP groups were categorized as grade III; more than three segments of the liver displayed a high signal. Bile duct changes were not evident in hamsters of the DMN group. In OV, only inflammation, dilatation and fibrosis changes were seen during the entire study. In contrast, hamsters of the OD group presented inflammation, dilatation and fibrosis changes initially. Examination from the months 2 to 6 could reveal the lesion progression to more severe biliary lesions, such as cysts, abscesses and tumors. In OD, dilatation degree of the intra-and extra-hepatic ducts increased with time, which appeared brighter in the bile duct regions in T2 weighted images. MRI reflected inflammation and dilatation of the intra-hepatic bile ducts, which were seen in different liver lobes of each animal at the same time point. The third MRI examination revealed a small tumor-like mass in one hamster, which was confirmed to be CCA by histopathology following necropsy at the end of the experiment (Fig. 3). Two of the five OD hamsters developed CCA. The dimensions of the tumor in the 14th week sample were comparatively larger than that in the 16th week sample.
Fig. 3.
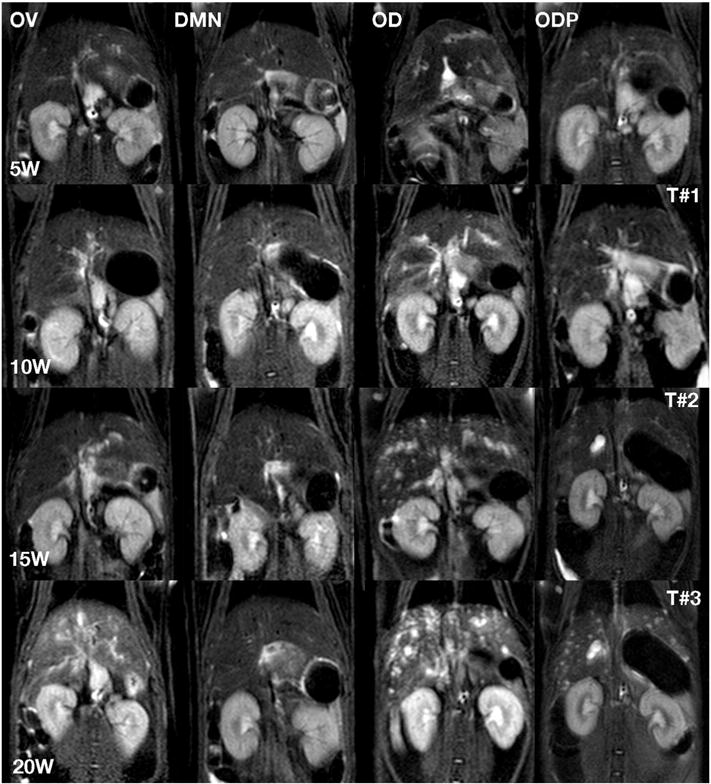
Three coronal planes of MRI scans (T#1, T#2, T#3) at weeks 5, 10, 15 and 20 (5W, 10W, 15W and 20W) after infection for the treatment groups, OV, DMN, OD and ODP. These T2 weighed images documented pathological changes similar to those revealed in Fig. 1. The lesional changes in the ODP livers in the final MRI scan (T#3) were not as markedly progressive as those in the OD liver.
In the ODP group, inflammation, dilatation and fibrosis changes were seen in the livers at the first month as with the OD hamsters. However, the degree of bile duct dilatation and fibrosis was less marked. After the third round of infection/PZQ therapy, one of the hamsters of the ODP group exhibited progression to cystic lesion and bile duct dilatation.
4. Discussion
MRI imaging is a facilitative adjunct tool for the study of progression and stage carcinogenesis, and because of its non-invasive nature, it facilitates investigations that use fewer laboratory animals. The MRI findings revealed that T2 weighted image is appropriate for the monitoring of biliary changes and T1 weighted image is suitable for the observation of tumor mass. These findings are supported by previous reports by colleagues [20]. Moreover, T2 weighted MRI detected early lesions in live O. viverrini-infected hamsters. Such lesions, including inflammation, bile duct dilatation and fibrosis were evident in the first week and their distributions reached grade II–III within five weeks of infection [20]. These studies also deployed MRI to establish grade III distribution, which reflected spread of lesions throughout the livers of the infected hamsters receiving NDMA for five weeks [21]. Here, grade III was evident in the hamsters of the OV, OD and ODP groups whereas DMN did not exhibit abnormalities. The observation of the grade III distribution only in the O. viverrini-infected hamsters of the OV, OD and ODP groups provided strong non-invasive evidence of migration of the parasites throughout the liver via the biliary tract, as shown previously [25]. Lesions caused by the migration of the worms are obviously discrete from those caused by consumption of NDMA in drinking water. Once absorbed, NDMA should affect the entire liver rather the migration trail of the invading parasite.
Similar liver pathology was revealed among the OD, ODP and DMN group hamsters at necropsy after infection for 40 weeks. This outcome was similar to our previous report [14]. OD group hamsters exhibited the most severe hepatobiliary lesions. DMN hamsters presented less severe lesions. However, CCA developed in both groups. At 40 weeks, hamsters of the DMN but not from the ODP group showed CCA. This could result from accumulative carcinogenic effects of NDMA. NDMA mediates DNA damage through DNA methylation and oxidation and its alkylating form induces oxidative stress leading to initiation and progression of neoplasia [26,27]. Despite exposure to a sub-carcinogenic dose of NDMA, cumulative damage from this carcinogen to host DNA of biliary cells may have resulted in malignant transformation and cellular proliferation. Our study and related investigations by others with O. viverrini and Clonorchis sinensis showed that appropriate doses of NDMA induced CCA in the hamster model of liver fluke infection-associated bile duct cancer. Accordingly, 12.5 ppm of NDMA in drinking water ad libitum for eight weeks represents a sub-carcinogenic dose in the hamster [14,28].
Hamsters of the ODP group exhibited the least severe hepatic lesions, not markedly dissimilar to OV hamsters. However, lesions found in the ODP group exposed to multiple infections and treatment with PZQ did not differ from those described by Thamavit et al. [29]. The macroscopic findings conformed to the histopathological findings. Immunohistochemistry for cell proliferation using PCNA demonstrated similar trends to those from the histopathological investigation. Whereas the OD group exhibited the highest PCNA index (100%), the index was seen to decline continuously through the DMN (84%), OV (67%) and ODP (21%) groups. This downward trend in the PCNA index likely reflects the number of worms surviving in the bile duct after the PZQ treatments. Although the EPG value of the ODP hamsters was zero at the conclusion of the study, some adult worms were seen in the bile ducts in the H&E slides. It is not clear whether these worms were indeed already dead at the time of necropsy of the hamsters. Moreover, Sithithaworn et al. [30] reported that number of liver fluke eggs cannot be reliably determined or detected where less than 20 worms remain. However, molecular assays such as PCR could be useful to detect and quantify the infection even in the rat model of clonorchiasis-induced biliary disease, where the numbers of liver flukes are generally small [31], and in analysis of efficacy of treatment of opisthorchiasis in hamsters [32]. The presence of worms in the biliary tree after PZQ indicates that a gastric dose of 300 mg/kg PZQ was ineffective for complete elimination of the worms. The remaining liver flukes continued to inflict pathological changes in the biliary tract.
It has been accepted that inflammation-associated carcinogenesis links with many forms of malignancy, including cholangiocarcinoma. This statement conformed to the findings of the present report where infiltration of the inflammatory cells in hepatic parenchyma, particularly in periperiductal sites was observed (Fig. 1). Chronic or sustained inflammation could generate nitric oxide, superoxide anion, and other reactive oxygen and nitrogen species. These reactive metabolites damage DNA at the genetic and epigenetic levels [33]. Patients exposed to repeated cycles of infection with O. viverrini and treatment with PZQ may be at increased risk of CCA [5,6,9]. PZQ causes the loss of cellular permeability of the liver fluke, influx of Ca2+ that induces tegumental vacuolizing, swelling, blebbing and finally tegumental disruption and musculature of the parasite [34]. Similar outcomes occur during infection with O. felineus [7]. The drug-damaged parasite is ultimately destroyed by the immune cells. Fragments from the disrupted liver fluke can be anticipated to induce inflammation and recruit more inflammatory cells. Humans exposed to cycles of repeated infection and PZQ treatment likewise will be exposed to multiple exposures of disintegrating parasites and antigens. The increase of iNOS expression in the inflamed epithelial cells of the bile duct supports this notion [35]. Free radicals liberated during these processes can damage chromosomal DNA by nitrosation and oxidation, potentially leading to carcinoma in situ and eventual eruption of CCA [6]. Periodic observations of pathological consequences of the adult worms to stimulate hepatobiliary changes were performed after the three infection-treatment cycles until the hamsters were euthanized at week 40. Hamsters of this group did not develop CCA. Inflammation plays a key role in O. viverrini-associated cholangiocarcinogenesis [36–38], and in particular, polymorphisms in immune-related genes such as IL-6 and TGF-beta may play central roles in this outcome [37]. Other risk factors relating to CCA development include DNA polymorphism of detoxifying enzymes, multidrug resistant genes and DNA repair genes [37].
These new findings may be seen as controversial given that the other recent reports indicate increased risk of CCA following cycles of O. viverrini-infection and PZQ [5,6,9]. The MRI images from our hamsters failed to support this notion: carcinogenic lesions of biliary cells in the repeated infection-treatment (ODP) group were less severe than that in the OD group. On the other hand, Thamavit et al. [29] reported that the repeated administration to hamsters of a curative dose of PZQ failed to induce CCA, in like fashion to our present findings. High PZQ dosage regimens could also reduce collagen content within a few weeks after treatment [39]. Moreover, a meta-analysis dealing with studies of the risk of PZQ therapy and CCA development could not conclude whether the number of PZQ treatment could increase the CCA risk; criteria and evidence in each study would be dissimilar and ambiguous [40]. Nonetheless, a recent hospital-based matched case–control study reveals an association between PZQ and CCA [5]. The relationship between the risk of CCA development and the multiple cycles of O. viverrini infection-PZQ treatment in the hamster model cannot entirely model the human situation. Yet studies in this hamster model of longer duration and of individual gene polymorphisms can likely assist to elucidate linkages between repeated infection with O. viverrini and cholangiocarcinogenesis. Investigation involving larger numbers of hamsters will likely provide clarification of the decreased risk of CCA following repeated cycle of O. viverrini infection–PZQ treatment.
Supplementary Material
Acknowledgments
This study was supported by the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), Tropical Medicine Research Center award number P50AI098639, The Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, through the Center of Excellence in Specific Health Problems in Greater Mekong Sub-region Cluster (SHeP-GMS), Khon Kaen University. Facilities were supported by the Department of Radiology, Faculty of Medicine, Khon Kaen University, Thailand.
Appendix A. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.parint.2016.04.012.
References
- 1.Sripa B, Kaewkes S, Sithithaworn P, Mairiang E, Laha T, Smout M, et al. Liver fluke induces cholangiocarcinoma. PLoS Med. 2007;4(7):e201. doi: 10.1371/journal.pmed.0040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sripa B. Concerted action is needed to tackle liver fluke infections in Asia. PLoS Negl Trop Dis. 2008;28(2(5)):e232. doi: 10.1371/journal.pntd.0000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sripa B, Kaewkes S, Intapan PM, Maleewong W, Brindley PJ. Food-borne trematodiases in Southeast Asia epidemiology, pathology, clinical manifestation and control. Adv Parasitol. 2010;72:305–350. doi: 10.1016/S0065-308X(10)72011-X. [DOI] [PubMed] [Google Scholar]
- 4.Sripa B, Brindley PJ, Mulvenna J, Laha T, Smout MJ, Mairiang E, et al. The tumorigenic liver fluke Opisthorchis viverrini—multiple pathways to cancer. Trends Parasitol. 2012;28(10):395–407. doi: 10.1016/j.pt.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamsa-Ard S, Luvira V, Pugkhem A, Luvira V, Thinkhamrop B, Suwanrungruang K, Bhudhisawasdi V. Association between praziquantel treatment and cholangiocarcinoma: a hospital-based matched case–control study. BMC Cancer. 2015;15:776. doi: 10.1186/s12885-015-1788-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinlaor S, Ma N, Hiraku Y, Yongvanit P, Semba R, Oikawa S, et al. Repeated infection with Opisthorchis viverrini induces accumulation of 8-nitroguanine and 8-oxo-7,8-dihydro-2′-deoxyguanine in the bile duct of hamsters via inducible nitric oxide synthase. Carcinogenesis. 2004;25(8):1535–1542. doi: 10.1093/carcin/bgh157. [DOI] [PubMed] [Google Scholar]
- 7.Pakharukova MY, Shilov AG, Pirozhkova DS, Katokhin AV, Mordvinov VA. The first comprehensive study of praziquantel effects in vivo and in vitro on European liver fluke Opisthorchis felineus (Trematoda) Int J Antimicrob Agents. 2015;46(1):94–100. doi: 10.1016/j.ijantimicag.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Day TA, Bennett JL, Pax RA. Praziquantel: the enigmatic antiparasitic. Parasitol Today. 1992;8(10):342–344. doi: 10.1016/0169-4758(92)90070-i. [DOI] [PubMed] [Google Scholar]
- 9.Pinlaor S, Prakobwong S, Hiraku Y, Kaewsamut B, Dechakhamphu S, Boonmars T, et al. Oxidative and nitrative stress in Opisthorchis viverrini-infected hamsters: an indirect effect after praziquantel treatment. Am J Trop Med Hyg. 2008;78:564–573. [PubMed] [Google Scholar]
- 10.Pinlaor S, Pinlaor S, Hiraku Y, Yongvanit P, Tada-Oikawa S, Ma N, Pinlaor P, et al. iNOS-dependent DNA damage via NF-kappaB expression in hamsters infected with Opisthorchis viverrini and its suppression by the antihelminthic drug praziquantel. Int J Cancer. 2006;119:1067–1072. doi: 10.1002/ijc.21893. [DOI] [PubMed] [Google Scholar]
- 11.Correia da Costa JM, Vale N, Gouveia MJ, Botelho MC, Sripa B, Santos LL, et al. Schistosome and liver fluke derived catechol-estrogens and helminth associated cancers. Front Genet. 2014;(5):444. doi: 10.3389/fgene.2014.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jusakul A, Kongpetch S, Teh BT. Genetics of Opisthorchis viverrini-related cholangiocarcinoma. Curr Opin Gastroenterol. 2015;31(3):258–263. doi: 10.1097/MOG.0000000000000162. [DOI] [PubMed] [Google Scholar]
- 13.Brindley PJ, da Costa JM, Sripa B. Why does infection with some helminths cause cancer? Trends Cancer. 2015;1(3):174–182. doi: 10.1016/j.trecan.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tangkawattana S, Kaewkes S, Pairojkul C, Tangkawattana P, Sripa B. Mutations of KRAS and TP53 in a minor proportion of Opisthorchis viverrini-associated cholangiocarcinomas in a hamster model. Asian Pac J Cancer Prev. 2008;9(1):101–106. [PubMed] [Google Scholar]
- 15.Faria SC, Ganesan K, Mwangi I, Shiehmorteza M, Viamonte B, Mazhar S, et al. MR imaging of liver fibrosis: current state of the art. Radiographics. 2009;29(6):1615–1635. doi: 10.1148/rg.296095512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aube C, Moal F, Oberti F, Roux J, Croquet V, Gallois Y, et al. Diagnosis and measurement of liver fibrosis by MRI in bile duct ligated rats. Dig Dis Sci. 2007;52(10):2601–2609. doi: 10.1007/s10620-006-9143-z. [DOI] [PubMed] [Google Scholar]
- 17.Mairiang E, Laha T, Bethony JM, Thinkhamrop B, Kaewkes S, Sithithaworn P, et al. Ultrasonography assessment of hepatobiliary abnormalities in 3359 subjects with Opisthorchis viverrini infection in endemic areas of Thailand. Parasitol Int. 2012;61(1):208–211. doi: 10.1016/j.parint.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roldan-Valadez E, Favila R, Martinez-Lopez M, Uribe M, Mendez-Sanchez N. Imaging techniques for assessing hepatic fat content in nonalcoholic fatty liver disease. Ann Hepatol. 2008;7(3):212–220. [PubMed] [Google Scholar]
- 19.Fass L. Imaging and cancer: a review. Mol Oncol. 2008;2(2):115–152. doi: 10.1016/j.molonc.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanpanich P, Pinlaor S, Charoensuk L, Yongvanit P, Thomas C, Kothan S, et al. MRI and (1)HMRS evaluation for the serial bile duct changes in hamsters after infection with Opisthorchis viverrini. Magn Reson Imaging. 2013;31(8):1418–1425. doi: 10.1016/j.mri.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Hanpanich P, Pinlaor S, Charoensuk L, Yongvanit P, Chamgramol Y, Pairojkul C, et al. MRI and (1)H MRS findings of hepatobilary changes and cholangiocarcinoma development in hamsters infected with Opisthorchis viverrini and treated with N-nitrosodimethylamine. Magn Reson Imaging. 2015;33(9):1146–1155. doi: 10.1016/j.mri.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 22.Sripa B, Kaewkes S. Relationship between parasite-specific antibody responses and intensity of Opisthorchis viverrini infection in hamsters. Parasite Immunol. 2000;22(3):139–145. doi: 10.1046/j.1365-3024.2000.00286.x. [DOI] [PubMed] [Google Scholar]
- 23.Hall PA, Levison DA, Woods AL, Yu CC, Kellock DB, Watkins JA, et al. Proliferating cell nuclear antigen (PCNA) immunolocalization in paraffin sections: an index of cell proliferation with evidence of deregulated expression in some neoplasms. J Pathol. 1990;162(4):285–294. doi: 10.1002/path.1711620403. [DOI] [PubMed] [Google Scholar]
- 24.Moldovan GL, Pfander B, Jentsch S. PCNA, the maestro of the replication fork. Cell. 2007;129(4):665–679. doi: 10.1016/j.cell.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Flavell DJ, Flavell SU, Field GF. Opisthorchis viverrini: the relationship between egg production, worm size and intensity of infection in the hamster. Trans R Soc Trop Med Hyg. 1983;77(4):538–545. doi: 10.1016/0035-9203(83)90132-3. [DOI] [PubMed] [Google Scholar]
- 26.Ahotupa M, Bereziat JC, Bussacchini-Griot V, Camus AM, Bartsch H. Lipid peroxidation induced by N-nitrosodimethylamine (NDMA) in rats in vivo and in isolated hepatocytes free radic. Res Commun. 1987;3:285–291. doi: 10.3109/10715768709069795. [DOI] [PubMed] [Google Scholar]
- 27.Lin H, Hollenberg PF. N-nitrosodimethylamine-mediated formation of oxidized and methylated DNA bases in a cytochrome P450 2E1 expressing cell line. Chem Res Toxicol. 2001;14:562–566. doi: 10.1021/tx0001979. [DOI] [PubMed] [Google Scholar]
- 28.Uddin MH, Choi MH, Kim WH, Jang JJ, Hong ST. Involvement of PSMD10, CDK4, and tumor suppressors in development of intrahepatic cholangiocarcinoma of Syrian golden hamsters induced by Clonorchis sinensis and N-nitrosodimethylamine. PLoS Negl Trop Dis. 2015;27(9(8)):e0004008. doi: 10.1371/journal.pntd.0004008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thamavit W, et al. Repeated exposure to Opisthorchis viverrini and treatment with the antihelminthic praziquantel lacks carcinogenic potential. Carcinogenesis. 1992;13:309–311. doi: 10.1093/carcin/13.2.309. [DOI] [PubMed] [Google Scholar]
- 30.Sithithaworn P, Tesana S, Pipitgool V, Kaewkes S, Pairojkul C, Sripa B, et al. Relationship between faecal egg count and worm burden of Opisthorchis viverrini in human autopsy cases. Parasitology. 1991;102(Pt 2):277–281. doi: 10.1017/s0031182000062594. [DOI] [PubMed] [Google Scholar]
- 31.Rahman SM, Bae YM, Hong ST, Choi MH. Early detection and estimation of infection burden by real-time PCR in rats experimentally infected with Clonorchis sinensis. Parasitol Res. 2011;109(2):297–303. doi: 10.1007/s00436-011-2253-3. [DOI] [PubMed] [Google Scholar]
- 32.Duenngai K, Boonmars T, Sithithaworn J, Sithithaworn P. Diagnosis of early infection and post chemotherapeutic treatment by copro-DNA detection in experimental opisthorchiasis. Parasitol Res. 2013;112(1):271–278. doi: 10.1007/s00436-012-3134-0. [DOI] [PubMed] [Google Scholar]
- 33.Yongvanit P, Pinlaor S, Bartsch H. Oxidative and nitrative DNA damage: key events in opisthorchiasis-induced carcinogenesis. Parasitol Int. 2012;61(1):130–135. doi: 10.1016/j.parint.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 34.Apinhasmit W, Sobhon P. Opisthorchis viverrini: effect of praziquantel on the adult tegument. Southeast Asian J Trop Med Public Health. 1996;27(2):304–311. [PubMed] [Google Scholar]
- 35.Pinlaor S, Hiraku Y, Ma N, Yongvanit P, Semba R, Oikawa S, et al. Mechanism of NO-mediated oxidative and nitrative DNA damage in hamsters infected with Opisthorchis viverrini: a model of inflammation-mediated carcinogenesis. Nitric Oxide. 2004;11(2):175–183. doi: 10.1016/j.niox.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Sripa B, Mairiang E, Thinkhamrop B, Laha T, Kaewkes S, Sithithaworn P, et al. Advanced periductal fibrosis from infection with the carcinogenic human liver fluke Opisthorchis viverrini correlates with elevated levels of interleukin-6. Hepatology. 2009;50:1273–1281. doi: 10.1002/hep.23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sripa B, Thinkhamrop B, Mairiang E, Laha T, Kaewkes S, Sithithaworn P, et al. Elevated plasma IL-6 associates with increased risk of advanced fibrosis and cholangiocarcinoma in individuals infected by Opisthorchis viverrini. PLoS Negl Trop Dis. 2012;6:28, e1654. doi: 10.1371/journal.pntd.0001654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogorodova LM, Fedorova OS, Sripa B, Mordvinov VA, Katokhin AV, Keiser J, et al. Opisthorchiasis: an overlooked danger. PLoS Negl Trop Dis. 2015;9(4):e0003563. doi: 10.1371/journal.pntd.0003563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hutradilok N, Ruenwongsa P, Thamavit W, Upatham ES. Liver collagen in Opisthorchis viverrini infected hamsters following praziquantel treatment. Southeast Asian J Trop Med Public Health. 1983;14(3):290–293. [PubMed] [Google Scholar]
- 40.Kamsa-ard S, Laopaiboon M, Luvira V, Bhudhisawasdi V. Association between praziquantel and cholangiocarcinoma in patients infected with Opisthorchis viverrini: a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2013;14(11):7011–7016. doi: 10.7314/apjcp.2013.14.11.7011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


