Abstract
Objective
This study explored predictors of improvement after completing a psychodiagnostic screening assessment but before randomization among youth who participated in two pilot randomized controlled trials of omega-3 supplementation and Individual-Family Psychoeducational Psychotherapy (PEP).
Method
Ninety-five youth (56.8% male, 61.1% white) aged 7–14 with mood disorders completed screening and baseline assessments (including Clinical Global Impressions-Improvement [CGI-I], Children’s Depression Rating Scale-Revised [CDRS-R], Young Mania Rating Scale [YMRS]), then were randomized into a 12-week trial of omega-3, PEP, their combination, or placebo.
Results
Between screening and randomization, 35.8% minimally improved (CGI-I=3), 12.6% much improved (CGI-I<3), totaling 48.4% improved. Caregiver post-secondary education (p = .018), absence of attention-deficit/hyperactivity disorder (p = .027), and lower screen depression severity (p = .034) were associated with CGI-Improvement. Caregiver post-secondary education (p = .020) and absence of a disruptive behavior diagnosis (p = .038) were associated with depression severity improvement. Pre-randomization improvement moderated treatment outcomes: among youth who improved pre-randomization, those who received PEP (alone or with omega-3) had more favorable placebo-controlled depression trajectories due to a lack of placebo response.
Conclusions
This open-label trial of psychodiagnostic assessment provides suggestive evidence that psychodiagnostic assessment is beneficial, especially for those with depression and without externalizing disorders. Pre-randomization improvement is associated with better placebo-controlled treatment response. Future research should test alternative hypotheses for change and determine if less intensive (shorter and/or automated) assessments would provide comparable results.
Keywords: assessment, treatment, children, adolescents, mood disorders, treatment moderators
As wait times for a first appointment in mental health clinics often delay treatment at least 8 weeks (Gallucci, Swartz, & Hackerman, 2005; Garry, 2006; Mireau & Inch, 2009; Trusler, Doherty, Mullin, Grant, & McBride, 2006) and may delay treatment up to 6 months in some low-income communities (Cunningham, McKenzie, & Taylor, 2006), initiating a therapeutic diagnostic assessment early in the waiting process might improve functioning for some youth (Arnold, 2013). Delays in treatment are associated with significant public health concerns, including prolonged emotional distress and dysfunction, decreased treatment engagement once services are initiated, and increased risk for harm to the patient or others, physical health risk, and risk of incarceration (Brown, Parker, & Godding, 2002). Given the long wait times for appointments and the associated risks, harnessing of all aspects of therapeutic change, particularly those that could be initiated in a timely manner, could be helpful. Better understanding the effects of a therapeutic psychodiagnostic assessment independent of other interventions (e.g., psychotherapy, psychotropic medication) could have significant benefits to youth and their families.
Previous research has demonstrated the benefits of psychodiagnostic assessment, either on psychosocial outcomes directly or on the therapeutic process of another active treatment (e.g., patient engagement, therapeutic alliance). People often experience improvements in mood after repeated administration of measures, with repeated measurement showing decreases in anxiety and depression scores, potentially due to the benefits of emotional expression and measurement as well as human contact (French & Sutton, 2010; Pennebaker, 1997). Darwin and colleagues (2013) analyzed qualitative responses to psychosocial assessment among pregnant women. They concluded that improvement occurred by increasing participants’ self-awareness and self- management strategies and inducing participants to seek further support from others. Participants also noted the potential therapeutic benefits of speaking to an engaged listener. In a study examining comorbid depression and substance use, participants had, on average, both improved depression scores and lower substance consumption levels post-assessment (Baker et al., 2013). Thus, study participation coupled with the systematic, supportive evaluation of target problems likely contribute to behavioral change and improvements in mood.
A recent meta-analysis of 17 studies on psychological assessment as an intervention found a significant effect of assessment overall (d = 0.42), a large effect on the therapeutic process (d = 1.12), and a small effect on therapy outcomes (d = 0.37) (Poston & Hanson, 2010). While this meta-analysis provides evidence for psychological assessment as a promising intervention, some suggest that Poston and Hanson’s (2010) analyses should be interpreted with caution due to the inclusion of studies with potentially confounding treatment components and the exclusion of negative studies (Lilienfeld, Garb, & Wood, 2011). Further, studies included in the meta-analysis as well as many other studies of therapeutic assessment analyze the benefits of assessment plus feedback; thus, how much benefit is derived from the feedback component (i.e., Barnum effects) rather than the assessment itself is unclear (Lilienfeld et al., 2011; Wood, Garb, Lilienfeld, & Nezworski, 2002). Additionally, Poston and Hanson’s meta-analysis included only adult participants, and 12 of the 17 studies included only college students, potentially limiting the generalizability of the results. Because effective treatment in childhood can reduce the likelihood of having impairing mental health problems later in life (Harrington, Rutter, & Fombonne, 1996), understanding the effects of assessment on youth is essential. Randomized controlled trials (RCT) of the Family Check-Up (FCU), an assessment-based intervention, demonstrated decreased child behavior problems and improved positive parenting strategies in young children and improved parental monitoring and decreased substance use in adolescents compared to controls (Dishion, Nelson, & Kavanagh, 2003; Dishion et al., 2008), providing support for the efficacy of family-centered assessment on behavior problems in youth. However, because the FCU model incorporates multiple sessions (across 2 – 3 years), feedback regarding targeted behaviors and motivational interviewing techniques, additional research is needed to clarify whether assessment, per se impacts outcome or whether feedback or other therapeutic strategies are necessary for assessment benefit.
In clinical trials research, large improvements between the initial screening assessment (when eligibility is determined) and the baseline assessment (at which time randomization occurs) may be problematic for researchers, as these changes limit the ability to demonstrate further improvement during treatment (“floor/ceiling effect”); however, that improvement is a benefit to the participants. Some researchers have posited that the effects of assessment and placebo can be harnessed as an opportunity to promote change as a less demanding intervention (Arnold, 2013). Response in placebo conditions (spaced assessments and a placebo capsule) among youth with mood disorders is associated with several factors including younger age (Bridge, Birmaher, Iyengar, Barbe, & Brent, 2009; Kowatch et al., 1999), lower baseline symptom severity, shorter disorder duration (Bridge et al., 2009; Cohen et al., 2010; Kowatch et al., 1999), lower socioeconomic status (Kowatch et al., 1999), and minority race (Cohen et al., 2010). However, it is unclear how the response to assessment alone may differ from response to placebo plus assessment among youth with mood disorders; no studies known to the authors have explicitly examined factors that predict positive change in youth following the screening assessment of a clinical trial, or therapeutic effects of assessment without feedback for youth with mood disorders. Understanding the effects of diagnostic assessments on youth and identifying predictors of those effects could have important implications for both community-based psychiatric treatment and for clinical trials, which rely on these assessments to determine study eligibility.
Thus, the aims of this study, which can be conceptualized as an open-label trial of the psychodiagnostic screening assessment as a therapeutic tool, were to determine how much children’s mood symptoms improve after assessment, to identify predictors of improvement, and to determine whether and in what way improvement between screening assessment and randomization affects treatment response. We sought to identify participant and family characteristics associated with screen-to-baseline pre-randomization improvement in mood symptoms and global functioning in two randomized controlled pilot trials of omega-3 supplementation and family-focused, cognitive-behavioral psychoeducational psychotherapy (Individual-Family Psychoeducational Psychotherapy [PEP]) for youth with mood disorders (Clinical Trial Identifiers: XXX and XXX). As youth participated in an initial psychodiagnostic screening assessment prior to baseline assessment and randomization, the study design allows for examination of the effect of the screening assessment. We hypothesized that, consistent with findings from studies investigating placebo-response in youth with mood disorders, less severe presentation (lower symptom severity, fewer comorbidities), lower SES (parent education, child insurance status), and younger age would be associated with pre-randomization improvement (change occurring between the psychodiagnostic screening assessment and the baseline visit), while controlling for time between the screening assessment and baseline. Additionally, to help distinguish pre-randomization improvement from regression to the mean as well as to determine how response to an assessment affects treatment outcomes, analyses examined whether pre-randomization improvement moderated the effects of subsequent treatment. Previous research on improvement in psychosocial treatment suggests that atypically low improvement early in treatment might signal potential treatment failures (Lambert, Hansen, & Finch, 2001). Thus, we expected that trial treatment trajectories of youth who responded to an initial psychodiagnostic screening assessment would differ from those who did not; but we had no a priori hypotheses about the nature of such moderation due to the absence of previous work on this relationship.
Method
Participants
Participants, recruited via clinician referrals and community advertisements between July 2011 and May 2014, were 95 children who participated in one of two randomized double-masked placebo-controlled pilot studies conducted at an academic medical center in a Midwestern U.S. city. Eligibility criteria to be randomized in the trial were established at the psychodiagnostic screening assessment and included the diagnosis of a depressive disorder (n = 72; major depressive disorder, dysthymic disorder, depressive disorder not otherwise specified [NOS]) or subsyndromal bipolar disorder (n = 23; bipolar disorder NOS or cyclothymic disorder); youth and at least one parent willing to attend assessments; ages 7–14 years; absence of impairing psychosis or active suicidal ideation; absence of mental health treatment within one month prior to randomization or during the trial (other than study-related interventions); and, among youth with a depressive disorder, Children’s Depression Rating Scale-Revised score ≥ 40.
Procedure
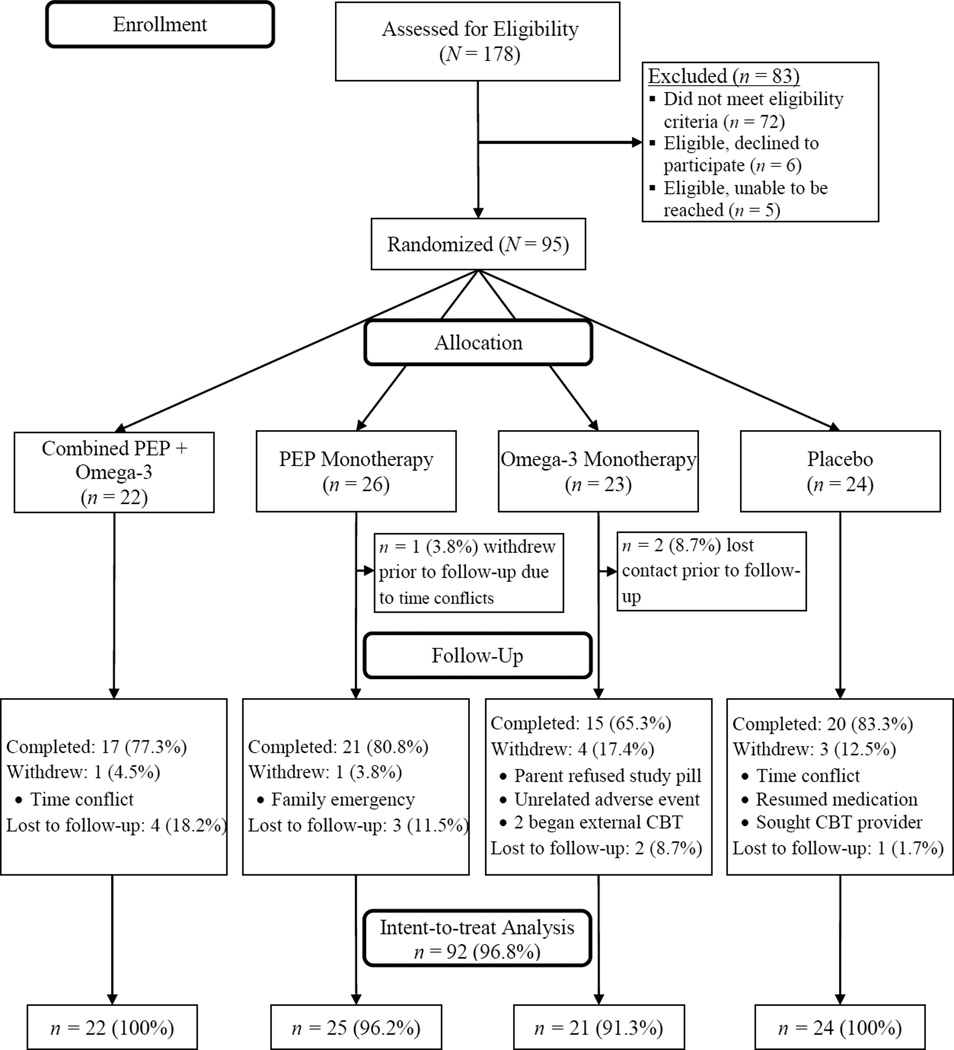
All procedures were approved by the University Human Subjects Review Board; consent and assent was obtained from parents and children, respectively. Recruitment and trial design are explained in detail elsewhere (Fristad et al., 2015). After the psychodiagnostic screening assessment, eligible families attended a baseline visit, then were immediately block-randomized into one of four groups using a 2×2 design (PEP + omega-3 supplementation, PEP + placebo, omega-3 + active monitoring, or placebo + active monitoring). After baseline and randomization (week 0), families participated in five additional follow-up interviews during weeks 2, 4, 6, 9, and 12. Participant flow and attrition throughout the study is depicted in Figure 1.
Figure 1.
CONSORT diagram illustrating participant enrollment, randomization allocation, and study completion status. PEP = Individual Family Psychoeducational Psychotherapy.
Assessments
Interviewers were clinical psychology graduate students and post-doctoral clinicians; interviewer training included didactics, mock interviews, observing and rating videotaped and live interviews. Following the psychodiagnostic screening assessment, interviewers prepared reports to review in a consensus conference with one of two co-principal investigators (co-PIs), during which the Co-PI reviewed and verified symptoms, diagnoses, ratings of global functioning, and appropriateness of study admission. There were similar consensus conferences after participants’ baseline and follow-up visits. Interviewers and the Co-PI involved in assessments were masked to participant randomization.
The psychodiagnostic screening assessment, of primary interest in these analyses, was a thorough assessment of children’s symptoms, global functioning, academic and social (family and peers) functioning, family psychiatric history, and service use throughout their lifetime. Interviews and questionnaires administered during the screening assessment that are pertinent to the current analyses include:
Parents reported information including youths’ sex, age, socioeconomic status, and family structure on a demographics questionnaire.
Parents and youth provided information regarding mood symptoms and severity via semi-structured interviews. The depression (KDRS) and mania (KMRS) modules from the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS) (Ambrosini, Metz, Prabucki, & Lee, 1989; Axelson et al., 2003; Chambers et al., 1985) collected information about youths’ mood symptoms and aided in making mood disorder diagnoses. The KDRS consists of 12 items scored on a 6-point scale from 1 (no symptoms) to 6 (severe symptoms); the KMRS includes 21 items, similarly scored. Both provide ratings of symptoms occurring during a mood episode (i.e., “filtered” ratings). The KDRS has demonstrated excellent test-retest reliability in diagnosing depressive disorders (kappa = 0.90) (Kaufman et al., 1997); the KMRS has excellent inter-rater reliability (intraclass correlation coefficient = 0.97) and convergent validity with the Clinical Global Impressions-Severity scale (r = 0.91) (Axelson et al., 2003). The Children’s Interview for Psychiatric Syndromes-Child and Parent (ChIPS/P-ChIPS), structured diagnostic interviews, were administered to aid in diagnosing comorbid disorders (Weller, Weller, Rooney, & Fristad, 1999a, 1999b). The ChIPS and P-ChIPS have adequate sensitivity, specificity, positive predictive value, and negative predictive value compared to other structured diagnostic interviews (Weller, Weller, Fristad, Rooney, & Schecter, 2000).
Two other scales included “unfiltered” ratings (i.e., symptoms are rated regardless of whether or not they occur in the context of a mood episode) of manic and depressive symptoms. The Children’s Depression Rating Scale-Revised (CDRS-R) is a 17-item clinician-rated depression severity scale, with good convergent and divergent validity as well as adequate inter-rater reliability (r = 0.86) and test-retest reliability (r = 0.81) (Poznanski et al., 1984). It does contain several items not specific to depression (impaired school performance, irritability, insomnia). The Young Mania Rating Scale (YMRS), an 11-item clinician-rated mania severity scale, has demonstrated good reliability (α = 0.91) and discriminant validity (Fristad, Weller, & Weller, 1992; Youngstrom, Danielson, Findling, Gracious, & Calabrese, 2002). However, several items are not specific to mania, including irritability, disruptive-aggressive behavior, lack of insight, distractibility, and psychosis. CDRS-R and YMRS total scores were the a priori selected primary outcome measures for the trial due to their sound psychometric properties and wide use in child psychiatry research (Fristad, Verducci, Walters, & Young, 2009; Kowatch et al., 1999; Miklowitz et al., 2007; TADS Team, 2007; Van Meter, Youngstrom, Demeter, & Findling, 2013).
The Family History Screen (FHS) (Weissman et al., 2000) is a semi-structured interview administered to parents to obtain information on symptoms of 15 psychiatric disorders in the child’s first- and second-degree relatives. The FHS has shown good agreement with best-estimate diagnoses made by independent clinicians, with a 15 month test-retest median kappa of 0.56 (Weissman et al., 2000).
Parents also completed the Understanding Mood Disorders Questionnaire (UMDQ) (Gavazzi, Fristad, & Law, 1997), a parent self-report questionnaire with good internal consistency (α =.73). It contains 20 questions to assess knowledge of mood disorder symptoms, course, and treatment, and a 19-item checklist to ascertain awareness of manic and depressive symptoms. Higher scores reflect a greater understanding; possible scores range from 0 to 59.
At baseline and each follow-up visit, parents and youth completed additional semi-structured interviews (including the KDRS, KMRS, CDRS-R, and YMRS) to assess mood diagnoses and symptom severity throughout the 12-week trial. These interviews also included the Clinical Global Impressions Improvement scale (CGI-I), a clinician-rating of global improvement from 1 (very much improved) to 7 (very much worse). The CGI-I is a commonly used outcome measure in clinical trials for psychiatric conditions with good sensitivity to change (Guy, 1976; Leon et al., 1993; National Institute of Mental Health, 1985; Zaider, Heimberg, Fresco, Schneier, & Liebowitz, 2003). In the current study, CGI-I scores were assigned in consensus conferences with an expert clinician (a co-PI) following the baseline interview to rate participants’ global improvement since the screening assessment (i.e., pre-randomization improvement); participants with scores ≤ 3 were considered improved.
Interventions
IF-PEP is a family-focused, cognitive-behavioral skills-based, manualized psychotherapy (Fristad, Goldberg-Arnold, & Leffler, 2011). Results from a pilot trial indicate medium to large effects of IF-PEP on mood symptoms in youth with bipolar spectrum disorder (Fristad et al., 2015). Families who randomized into IF-PEP were asked to attend two 45–50 minute sessions (one child session, one parent session) weekly. Study therapists were four Ph.D.-level clinicians who were supervised by a Co-PI (XXX). Topics covered in IF-PEP include psychoeducation about symptoms of and treatments for mood disorders; collaborating with the school system; cognitive-behavioral, problem-solving, and communication skills; and safety planning. Details regarding session order and content can be found elsewhere (Fristad et al., 2011). All participants received active monitoring (spaced assessments across the 12-week trial).
Previous research has demonstrated the efficacy of omega-3 fatty acids (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA], in particular) in treating mood symptoms in youth (Clayton et al., 2009; Nemets, Nemets, Apter, Bracha, & Belmaker, 2006; Wozniak et al., 2007). All families received a pill organizer containing study capsules (omega-3 or placebo provided by OmegaBrite; www.omegabrite.com; Las Vegas, NV) and daily multivitamin/mineral tablets to standardize nutrition levels across participants. Youth who randomized into an omega-3 group received two 500 mg capsules of omega-3 twice daily for a total daily dose of 2000 mg (1400 mg EPA, 200 mg DHA, 400 mg other omega-3). Youth randomized to placebo received two capsules twice daily matched to the omega-3 capsules for odor and appearance.
Data Analytic Plan
Frequency of improvement based on CGI-I scores as well as paired t-tests of screen-to-baseline (pre-randomization) change in mood symptom severity (CDRS-R, YMRS) were examined. Additionally, reliable change (RC) indices (Jacobson & Truax, 1991) were calculated to determine rates of reliable pre-randomization improvement, with RC ≥ 1.96 indicating reliable change at the p < .05 level. To identify predictors to include in multivariable models, preliminary chi-square analyses and point-biserial correlations examined bivariate relationships between sample characteristics and pre-randomization global improvement (CGI-I measured at baseline; CGI-I ≤ 3 vs. CGI-I > 3). Predictors of pre-randomization change in mood symptom severity were identified in a series of regression analyses, controlling for screen severity. Sample characteristics that were significantly associated with symptom severity improvement or global improvement in preliminary analyses were included in multivariable logistic (in the case of global improvement) or linear (in the case of CDRS-R and YMRS scores) regression analyses. There were no data missing on screening or baseline CDRS-R or YMRS scores, or baseline CGI-I scores. Multivariable analyses controlled for time (weeks) between screen and baseline assessments. Sample characteristics examined included child sex, age, race, insurance status, primary caregiver education, maternal mood disorder history, child mood disorder and comorbid diagnoses (attention-deficit/hyperactivity disorder [ADHD], disruptive behavior disorder [DBD], anxiety disorder), and parent knowledge of mood disorders (UMDQ).
To determine whether pre-randomization improvement moderated treatment effects, linear mixed effects (LME) models were fit to each of the primary outcomes (CDRS-R, YMRS) using the full intent-to-treat sample. One advantage of LME is that it can accommodate missing data points among participants who have two or more non-missing data points. Intercepts and slopes were modeled as random effects; fixed effects were treatment group (contrast-coded relative to placebo + active monitoring), time since randomization (in weeks), pre-randomization improvement status (CGI-I ≤ 3 vs. CGI-I > 3), group X time, group X pre-randomization improvement, pre-randomization improvement X time, and group X time X pre-randomization improvement. Bivariate, logistic regression, linear regression, and LME analyses were conducted using IBM SPSS Statistics version 22/23. To probe significant moderation effects, simple slopes analysis was conducted using methods described by and Preacher, Curran, and Bauer (2006). Chi-square analyzed whether study attrition differed between youth who improved pre-randomization and those who did not. These exploratory analyses used α < .05 as the cutoff for statistical significance without correction for multiple analyses.
Results
Participants
Demographic and clinical characteristics for the overall sample and by pre-randomization CGI-Improvement status are presented in Table 1. More than half of the participants were male (n = 54; 56.84%); mean age was 11.3 ± 2.2 years. The majority of participants were White/Caucasian (n = 58; 61.05%); 26.32% were Black/African American (n = 25); 11.58% (n = 11) were multi-racial. One-third reported Medicaid as their primary insurance (n = 32; 33.68%); most parents reported post-secondary education (n = 79; 84.04%). On average, there were 3.5 ± 1.8 weeks between screening and baseline assessments (range = 0.9 – 8.0).
Table 1.
Sample Characteristics by Interviewer-Rated Improvement between Screening Assessment and Randomization
| Sample Characteristics | Overall (N = 95) |
Improved Pre- Randomization: CGI≤3 (n = 46) |
Not Improved Pre- Randomization: CGI>3 (n = 49) |
p-value |
|---|---|---|---|---|
| Age M ± SD | 11.3 ± 2.2 | 11.2 ± 2.0 | 11.3 ± 2.4 | .916 |
| Sex: Male | 54 (56.84%) | 26 (56.52%) | 28 (57.14%) | .951 |
| Race | .315 | |||
| White/Caucasian | 58 (61.05%) | 29 (63.04%) | 29 (59.18%) | |
| Black/African American | 25 (26.32%) | 14 (30.43%) | 11 (22.44%) | |
| Bi/multi-racial | 11 (11.58%) | 3 (6.52%) | 8 (16.32%) | |
| Insurance status: Medicaid | 32 (33.68%) | 15 (32.61%) | 17 (34.69%) | .830 |
| Primary caregiver education (n = 94): >High school diploma/GED |
79 (84.04%) | 43 (95.56%) | 36 (73.47%) | .003 |
| Maternal psychiatric history (n = 94) | ||||
| Depression | 36 (38.30%) | 17 (36.96%) | 16 (39.58%) | .793 |
| Bipolar Disorder | 7 (7.45%) | 3 (6.52%) | 4 (8.33%) | .766 |
| Child mood disorder diagnosis | .586 | |||
| Depressive Spectrum Disorder | 72 (75.79%) | 36 (78.26%) | 36 (73.47%) | |
| Subsyndromal Bipolar Disorder | 23 (24.21%) | 10 (21.74%) | 13 (26.53%) | |
| Child comorbid diagnoses | ||||
| Anxiety Disorder | 75 (78.94%) | 37 (80.43%) | 38 (77.55%) | .730 |
| Attention-Deficit/Hyperactivity Disorder | 58 (61.05%) | 23 (50.00%) | 35 (71.42%) | .032 |
| Disruptive Behavior Disorder | 37 (38.95%) | 14 (30.43%) | 23 (46.94%) | .099 |
| CDRS-R | ||||
| At screening M ± SD | 45.1 ± 7.9 | 43.3 ± 7.4 | 46.7 ± 8.0 | .031 |
| At baseline M ± SD |
40.5 ± 10.3 | 34.9 ± 7.6 | 45.8 ± 9.7 | <.001 |
| YMRS | ||||
| At screening M ± SD | 16.6 ± 7.7 | 16.5 ± 7.1 | 16.7 ± 8.3 | .914 |
| At baseline M ± SD | 14.6 ± 7.0 | 13.2 ± 5.4 | 15.9 ± 8.0 | .061 |
| Time between screen and baseline (weeks) M ± SD |
3.5 ± 1.8 | 3.5 ± 1.8 | 3.5 ± 1.8 | .861 |
| Understanding Mood Disorders Questionnaire Score M ± SD |
45.1 ± 9.4 | 46.2 ± 8.8 | 44.2 ± 10.0 | .316 |
Note. All characteristics listed were assessed at the screening assessment unless otherwise indicated. Higher scores on the Understanding Mood Disorders Questionnaire indicate better understanding of mood disorders. Higher scores on the CDRS-R and the YMRS indicate greater severity in depressive and manic symptoms, respectively. CDRS-R = Children’s Depression Rating Scale – Revised; CGI = Clinical Global Impressions - Improvement Scale; YMRS = Young Mania Rating Scale.
Predicting post-screening/pre-randomization improvement
Preliminary analyses
Nearly half the sample (48.42%) experienced at least some improvement between the screening assessment and randomization (CGI-I ≤ 3): 12.63% (n = 12) received ratings of much improved (CGI-I = 2); 35.78% (n = 34) were minimally improved (CGI-I = 3); 45.26% (n = 43) reported no change (CGI-I = 4); and 6.32% (n = 6) were minimally worse (CGI-I = 5). None were rated as very much improved (CGI-I = 1), much worse(CGI-I = 6) or very much worse (CGI-I = 7). Symptom severity, on average, significantly improved pre-randomization on both the CDRS-R [M difference (screen - baseline) = 4.5 ± 6.9, t(94) = 6.42, p < .001, d = 0.49] and YMRS [M difference = 2.0 ± 4.9, t(94) = 4.03, p < .001, d = 0.27]. The three outcomes were closely associated: CGI-Improvement (dichotomized as CGI ≤ 3 vs. CGI > 3) was significantly and positively associated with screen to baseline difference scores for both the CDRS-R (rs = 0.57, p < .001) and the YMRS (rs = 0.29, p = .004).
Interviewer-rated improvement (CGI-I) between the screening assessment and randomization was associated with post-secondary caregiver education (χ2 = 8.53, p = .003), absence of an ADHD diagnosis (χ2 = 4.58, p = .032), and lower screen CDRS-R scores (r = −0.22, p = .031). In preliminary regression analyses, improvement in CDRS-R scores was associated with post-secondary caregiver education (b = 6.29, t = 3.39, p = .001), depressive disorder rather than subsyndromal bipolar disorder (b = −3.75, t = −2.03, p = .046), the absence of a DBD diagnosis (b = 3.38, t = 2.33, p = .022), and greater parent knowledge of mood disorders (b = −0.18, t = −2.30, p = .024). Improvement in YMRS scores was associated with depressive disorder rather than subsyndromal bipolar disorder (b = −4.18, t = −3.16, p = .002) and the absence of an anxiety diagnosis (b = 2.26, t = 2.06, p = .042).
Reliable change scores indicated that 24.21% (n = 23) of youth experienced reliable improvement on at least one measure of mood symptom severity; rates were 10.52% (n = 10) and 16.84% (n = 16) on the CDRS-R and YMRS, respectively.
Multivariable logistic regression analyses
Results of the logistic regression model (χ2(2) = 18.72, p < .001) indicated that post-secondary caregiver education (Odds Ratio[OR] = 7.03, 95% Confidence Interval [CI] = 1.40 – 35.33, p = .018), absence of an ADHD diagnosis (OR = 0.34, 95% CI = 0.13 – 0.89, p = .027), and lower screen CDRS-R scores (OR = 0.93, 95% CI = 0.87 – 1.00, p = .034) continued to be associated with CGI-Improvement.
Results of the linear regression models predicting improvement in CDRS-R and YMRS scores are summarized in Table 2. Having a parent with a post-secondary education (p = .020) and lacking a DBD diagnosis (p = .038) continued to be associated with pre-randomization improvement in CDRS-R scores; but greater parent understanding of mood disorders was only marginally associated (p = .059). Only depressive disorders (not subsyndromal bipolar disorders) were associated with improvement in YMRS scores in the multivariable analyses (p = .006). Notably, time between the screening and baseline assessments was not significantly associated with any measure of improvement.
Table 2.
Predictors of Changes in Mood Severity between Screening Assessment and Randomization (N = 95)*
| Sample characteristics | CDRS-R | YMRS | ||||||
|---|---|---|---|---|---|---|---|---|
| B | 95% CI | t | p | B | 95% CI | t | p | |
| Constant | −0.58 | −12.64 – 11.48 | −0.10 | .924 | 7.19 | 1.97 – 12.42 | 2.73 | .008 |
| Primary caregiver education (reference: >high school diploma) |
4.93 | 0.79 – 9.07 | 2.37 | .020 | -- | -- | -- | -- |
| Depressive disorder (reference: Bipolar disorder) |
−3.33 | −7.20 – 0.53 | −1.72 | .090 | −3.79 | −6.44 – −1.13 | −2.84 | .006 |
| Disruptive behavior disorder (reference: no DBD) |
3.33 | 0.18 – 6.47 | 2.11 | .038 | -- | -- | -- | -- |
| Anxiety disorder (reference: no anxiety disorder) |
-- | -- | -- | -- | 1.65 | −0.49 – 3.79 | 1.54 | .128 |
| Parent understanding of mood disorders | −0.16 | −0.33 – 0.01 | −1.92 | .059 | -- | -- | -- | -- |
| Time between screen and baseline (weeks) | −0.14 | −1.00 – 0.71 | −0.34 | .738 | 0.18 | −0.67 −0.30 | −0.75 | .455 |
Note. CDRS-R = Child Depression Rating Scale, Revised; CI = Confidence Interval; YMRS = Young Mania Rating Scale.
Analyses predicted baseline scores adjusting for participants’ screening assessment scores on the CDRS-R or YMRS.
Pre-randomization improvement as a moderator of trial treatment effects
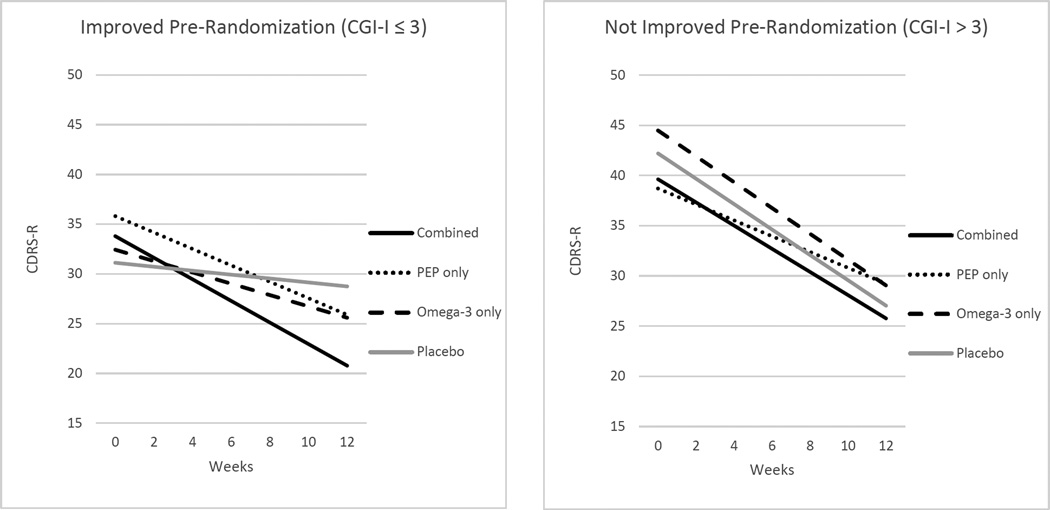
Results of the LME analyses indicated that pre-randomization CGI-Improvement did not significantly moderate treatment effects on the YMRS (p > .05); it did, however, significantly moderate the effects of combined PEP and omega-3 (p = .026) as well as PEP monotherapy (p = .010) relative to placebo on CDRS-R trajectories (Table 3). Figure 2 depicts the simple slopes for each treatment group by pre-randomization improvement status. There was greater placebo-controlled benefit in depression severity in combined treatment (bCombined-Placebo = −0.89 ± 1.11, z = −2.72, p = .006, d = 0.80) and PEP monotherapy (bPEP monotherapy-Placebo = −0.63 ± 1.19, z = −2.01, p = .044, d = 0.53) among youth with clinician-rated improvement pre-randomization. The placebo-controlled effects of omega-3 monotherapy were nonsignificant in youth with pre-randomization improvement (bOmega-3 monotherapy-Placebo = −0.37 ± 1.22, z = −1.01, p = .312, d = 0.31). Of note, baseline scores (intercepts) for the three groups receiving active treatment did not significantly differ from baseline of the placebo group. Among those who did not experience pre-randomization improvement, youth in all four randomized groups improved significantly (ps < .001), with treatment effects relative to placebo nonsignificant.
Table 3.
Results of a Linear Mixed Effects Model Examining Improvement between Screening Assessment and Randomization as a Moderator of the Effects of Treatment on CDRS-R Trajectories (N = 92)
| Parameter | Estimate | df | t | p |
|---|---|---|---|---|
| Treatment Group (reference: placebo + active monitoring) | ||||
| Combined | −2.57 | 111.67 | −0.72 | .473 |
| PEP monotherapy | −3.50 | 113.87 | −1.11 | .270 |
| Omega-3 monotherapy | 2.27 | 115.21 | 0.70 | .489 |
| Time (weeks) | −1.26 | 83.70 | 6.47 | <.001 |
| Pre-Randomization Improvement | −11.09 | 116.21 | −3.32 | .001 |
| Treatment Group X Time | ||||
| Combined X Time | 0.11 | 82.58 | 0.37 | .713 |
| PEP monotherapy X Time | 0.47 | 77.67 | 1.72 | .090 |
| Omega-3 monotherapy X Time | −0.02 | 88.21 | −0.08 | .938 |
| Treatment Group X Pre-Randomization Improvement | ||||
| Combined X Pre-Randomization Improvement | 5.25 | 115.21 | 1.08 | .282 |
| PEP X Pre-Randomization Improvement | 8.19 | 115.14 | 1.76 | .081 |
| Omega-3 X Pre-Randomization Improvement | −0.95 | 119.63 | −0.20 | .845 |
| Pre-Randomization Improvement X Time | 1.07 | 89.73 | 3.50 | .001 |
| Treatment Group X Time X Pre-Randomization Improvement | ||||
| Combined X Time X Pre-Randomization Improvement |
−1.00 | 88.63 | −2.26 | .026 |
| PEP monotherapy X Time X Pre-Randomization Improvement |
−1.10 | 85.92 | −2.64 | .010 |
| Omega-3 monotherapy X Time X Pre-Randomization Improvement |
−0.35 | 97.08 | −0.75 | .456 |
Note. CDRS-R = Children’s Depression Rating Scale-Revised; PEP = Individual-Family Psychoeducational Psychotherapy.
Figure 2.
CDRS-R scores by time and pre-randomization improvement status. For pre-randomization CGI-Improvement scores > 3, the slopes for each treatment group were as follows: combined b = −1.16, SE = 0.22, z = −5.17, p < .0001; PEP monotherapy b = −0.80, SE = 0.20, z = −4.06, p = .0001; omega-3 monotherapy b = −1.29 SE = 0.21, z = −6.07, p < .0001; placebo b = −1.26, SE = 0.20, z = −6.47, p < .0001. For pre-randomization CGI-Improvement scores ≤ 3, the slopes for each treatment group were: combined b = −1.08, SE = 0.23, z = −4.76, p < .0001; PEP monotherapy b = −0.83, SE = 0.21, z = −3.97, p = .0001; omega-3 monotherapy b = −0.57, SE = 0.29, z = −2.00, p = .046; placebo b = −0.20, SE = 0.23, z = −0.84, p = .402. CDRS-R = Children’s Depression Rating Scale – Revised; CGI = Clinical Global Impressions Scale; PEP = Individual Family Psychoeducational Psychotherapy.
Finally, chi-square analysis indicated that trial completion (completed vs. premature termination) did not significantly differ between youth who experienced pre-randomization CGI-improvement (n = 32/46, 69.57% completed) and those who did not (n = 41/49, 83.67% completed), χ2(1) = 2.65, p = .103.
Discussion
On average, youth experienced significant improvements in mood severity after a psychodiagnostic screening assessment, before any study treatment or even feedback about assessments. Nearly half were rated as at least minimally improved (12% much improved) on a categorical measure, and nearly a quarter demonstrated clinically meaningful improvement on the basis of reliable change scores. Thus, if conceptualized as an open-label single-arm trial of psychodiagnostic assessments, these results provide mild additional evidence for assessment as a therapeutic tool. These findings are consistent with prior studies reporting that systematic assessment of a target behavior improves that behavior (Baker et al., 2013; Darwin, McGowan, & Edozien, 2013).
Several characteristics of the screening assessments in these pilot trials may have been inherently therapeutic for participating families. First, scheduling of the screening assessments typically occurred within one week of families contacting the study coordinator. This capitalized on the family’s motivation to seek treatment; previous research has demonstrated that motivation and engagement declines when wait times exceed 4 to 6 weeks (Brown et al., 2002). Second, screening interviewers provided families with any appropriate resources requested by the family or otherwise deemed potentially helpful by the interviewer (e.g., mental health referrals for parents or siblings of the child participant, coaching on how to request school services, referrals to organizations that provide financial resources, etc.). Third, as suggested by previous studies and anecdotal comments when families returned for baseline assessments, the act of reporting on the child’s problems, by promoting reflection, may have increased some parents’ awareness of their children’s emotional state and, as a result, changed behaviors toward their child and other family members prior to the baseline assessment. Finally, youth may have experienced relief after completing a thorough assessment of their problems in an atmosphere that sought to understand their struggles rather than blame or reprimand them for symptomatic behavior.
Assessment appears to be most helpful for youth with less severe clinical presentations. Among youth with mood disorders, those who did not have comorbid ADHD and who had less severe depression were more likely to be rated as improved overall on the pre-randomization CGI-I. Similarly, youth without DBD experienced greater declines in depression severity than youth with DBD. Youth with depressive disorders, rather than subsyndromal bipolar disorders, saw greater improvements on the YMRS, which includes ratings of symptoms such as distractibility, irritability, and disruptive-aggressive behavior that overlap with other common childhood disorders. It is plausible that comorbid behavioral disorders increase clinical complexity and resistance to improvement from a low-intensity intervention.
In addition to child clinical presentation, family-level factors were also associated with improvement. Youth whose primary caregiver had post-secondary education were more likely to improve overall and experienced greater improvement in depression severity. This stands in contrast to study hypotheses and previous research, in which lower socioeconomic status (SES) was associated with response to placebo in youth participating in a fluoxetine trial (Kowatch et al., 1999), as SES and education are highly related. Perhaps response to a single assessment differs from response to multiple, spaced assessments plus placebo. Further study is needed to clarify the role of SES in response to assessment and the interaction of placebo with assessment.
Given long wait-lists for psychotherapy in community clinics and the associated risks to patients, these results suggest that a short-term but thorough and systematic assessment of behaviors germane to a youth’s presenting problems may provide some clinical benefit. In particular, youth with unipolar depression but without a history of behavioral disorders may benefit from such assessments. Such thorough assessments can be expensive and time-consuming for community clinicians (Wood et al., 2002). It would be of interest to study whether or not on-line assessments that would not require a clinician’s time could provide this same benefit, or whether interaction with a clinician is a critical element in the benefit achieved.
In these trials, feedback sessions, which provided focused information regarding the child’s diagnoses and functioning as assessed during the screening assessment, were offered only after baseline to avoid introducing any reporter bias at the baseline interview; thus, it appears that the assessment, per se, rather than just the feedback, may account for some clinical improvement. Additionally, because baseline visits were scheduled at families’ earliest convenience rather than a set time interval, there was substantial variability in time between screening assessments and baseline allowing for examination of the effect of time, which was nonsignificant. In multivariable analyses controlling for the effect of time, clinical and family-level characteristics were associated with improvement. This argues against improvement being solely the natural waning of a mood episode or regression to the mean. The fact that low screening severity predicted improvement also argues against regression to the mean as explanation of pre-randomization improvement. Regression to the mean should show the greatest improvement in the most deviant initial scores (Davis, 1976).
Furthermore, pre-randomization improvement moderated subsequent treatment trajectories. Among youth who benefitted from the psychodiagnostic screening assessment, all but the placebo group experienced a significant decline in depressive severity over the 12-week trial (although only combined or PEP monotherapy demonstrated significantly greater improvement than placebo). This differential response to treatment cannot be explained by differences at baseline (i.e., pre-treatment) depression severity. Participants who had not improved pre-randomization also experienced improvement in their depression severity over the 12-week trial, but with a strong placebo response, the effects of treatment vs. placebo were nonsignificant. Pre-randomization improvement did not moderate treatment effects on manic symptom severity, possibly due to the relatively mild manic symptom severity in these trials.
Overall, these results may have significant implications for clinical trials, as they suggest that response to the screening assessment dampened the placebo effect in the treatment portion of the trial, showing a more robust treatment effect. Results support findings from a meta-analysis of psychopharmacology trials for mood disorders, which indicated that placebo run-in designs (in which all participants initially receive placebo and those who respond are not randomized) generally do not improve trial outcomes compared to trials that do not use a placebo run-in (Trivedi & Rush, 1994). In fact, it was among youth who did experience pre-randomization improvement that treatment efficacy findings were strongest in the current analyses. Thus, it may be worthwhile for future RCTs to consider pre-randomization response as a treatment moderator.
Notably, unlike most studies included in Trivedi and Rush’s (1994) meta-analysis, the current trial focused on youth and non-pharmacological interventions. As such, replication of these findings in RCTs using similar populations and interventions is needed. Future analyses should also seek to better understand why youth who did not improve pre-randomization improved significantly with trial treatment but no more than youth who received only placebo and spaced assessments (e.g., identify mediators/additional moderators of treatment response).
While prior studies have examined benefits of assessment plus placebo, none have identified predictors of benefit from assessment without a placebo among youth with mood disorders. Thus, this study adds to the extant literature by providing information about which youth might benefit most from a therapeutic assessment without placebo treatment. As this is the first study known to the authors to identify predictors of improvement after a single assessment (without placebo or feedback), more work is needed to verify predictors. Such studies might help guide case prioritization and disposition in a clinical setting and potentially minimize negative outcomes associated with delaying care for vulnerable youth; those more likely to respond to assessment might receive less intensive intervention while youth less likely to benefit from assessment could be prioritized for more intensive services.
There are several limitations to this study. Most importantly, as the analyses of pre-randomization effects of assessment constitute a single-arm trial, replication with a control group would strengthen this study’s findings. This study included a fairly diverse sample: nearly 40% identified as belonging to a racial minority group and approximately 34% received Medicaid, supporting the potential generalizability of these findings across demographic characteristics. However, as the focus of these pilot trials was youth with mood disorders, these results may not be generalizable to youth with other primary diagnoses. Additionally, with screening assessments being scheduled flexibly and at families’ earliest convenience and a mean of 3.5 weeks between screening assessments and randomization, trial participants had a fairly short wait time compared to the long wait times common in community clinics. Thus, the results may not be generalizable to settings in which wait times for initial assessment and between an initial assessment and treatment would be longer; longer times might result in a weaker relationship between pre-treatment improvement and treatment trajectories. The study did not include any direct measure of how supportive the assessments were or how often families followed up on recommendations provided by interviewers during the screening assessment; therefore, support and access to resources could not be systematically measured as mechanisms of change in these analyses. A trial designed to measure mechanisms of change will be important to clarify how assessments improve symptoms and/or functioning. Furthermore, because participants were screening for eligibility in a clinical trial, community-based assessment studies are needed to elucidate how these findings translate to youth seeking treatment in the community. Lastly, with these data being derived from pilot studies, the sample size is fairly small to examine treatment moderators; thus, it will be important to replicate the moderator findings in a larger trial.
Conclusion
Assessment may be a useful intervention for youth with mood disorders, particularly those with less severe presentations. Understanding effects of short-term, low-cost interventions could have significant public health implications due to the risk associated with delaying treatment. Assessment may help alleviate some distress while families wait to be seen for more intensive treatment or may potentially serve as an alternative to traditional longer-term therapy for youth with milder symptoms. Future research is needed to determine whether an automated, computerized assessment would provide similar benefit to a clinician-administered assessment.
Clinical Significance.
This study provides preliminary evidence for the efficacy of assessment in treating depressive symptom severity among youth with mood disorders. Findings suggest that less intensive interventions may benefit youth with mild to moderate mood symptom severity and that further study of the therapeutic effects of assessment in youth is warranted.
Acknowledgments
This research was supported by awards from the National Institute of Mental Health (R34 MH090148 and R34 MH85875); the content is solely the responsibility of the authors and does not necessarily represent the official views of NIH. The authors would like to thank the staff who collected data and provided therapy, the families who participated and OmegaBrite, who provided study capsules.
Footnotes
Disclosures: Dr. Arnold has received research funding from Curemark, Forest, Lilly, Neuropharm, Novartis, Noven, Shire, and YoungLiving (as well as NIH and Autism Speaks) and has consulted with or been on advisory boards for Arbor, Gowlings, Neuropharm, Novartis, Noven, Organon, Otsuka, Pfizer, Roche, Seaside Therapeutics, Sigma Tau, Shire, Tris Pharma, and Waypoint and received travel support from Noven. Dr. Fristad receives royalties from Guilford Press, American Psychiatric Press and Child & Family Psychological Services. She has received honoraria from Physicians Postgraduate Press and the American Occupational Therapy Association. Drs. Young, Meers, and Seidenfeld and Mr. Vesco have no financial disclosures to report.
Contributor Information
Andrea S. Young, Department of Psychiatry and Behavioral Health, The Ohio State University, Columbus, Ohio
Molly R. Meers, Department of Psychiatry and Behavioral Health, The Ohio State University, Columbus, Ohio
Anthony T. Vesco, Department of Psychiatry and Behavioral Health, The Ohio State University, Columbus, Ohio
Adina M. Seidenfeld, Department of Psychiatry and Behavioral Health, The Ohio State University, Columbus, Ohio
L. Eugene Arnold, Department of Psychiatry and Behavioral Health, The Ohio State University, Columbus, Ohio.
Mary A. Fristad, Department of Psychiatry and Behavioral Health, The Ohio State University, Columbus, Ohio
References
- Ambrosini PJ, Metz C, Prabucki K, Lee J-C. Videotape reliability of the third revised edition of the K-SADS. Journal of the American Academy of Child and Adolescent Psychiatry. 1989;28(5):723–728. doi: 10.1097/00004583-198909000-00013. [DOI] [PubMed] [Google Scholar]
- Arnold LE. Placebo response and the company it keeps. JAMA Pediatrics. 2013;167(11):1000–1001. doi: 10.1001/jamapediatrics.2013.2704. [DOI] [PubMed] [Google Scholar]
- Axelson D, Birmaher BJ, Brent D, Wassick S, Hoover C, Bridge J, Ryan N. A preliminary study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children mania rating scale for children and adolescents. Journal of Child and Adolescent Psychopharmacology. 2003;13(4):463–470. doi: 10.1089/104454603322724850. [DOI] [PubMed] [Google Scholar]
- Baker AL, Kay-Lambkin FJ, Gilligan C, Kavanagh DJ, Baker F, Lewin TJ. When does change begin following screening and brief intervention among depressed problem drinkers? Journal of Substance Abuse Treatment. 2013;44(3):264–270. doi: 10.1016/j.jsat.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Bridge JA, Birmaher B, Iyengar S, Barbe RP, Brent DA. Placebo response in randomized controlled trials of antidepressants for pediatric major depressive disorder. American Journal of Psychiatry. 2009;166(1):42–49. doi: 10.1176/appi.ajp.2008.08020247. [DOI] [PubMed] [Google Scholar]
- Brown SA, Parker JD, Godding PR. Administrative, clinical, and ethical issues surrounding the use of waiting lists in the delivery of mental health services. Journal of Behavioral Health Services & Research. 2002;29(2):217–228. doi: 10.1007/BF02287708. [DOI] [PubMed] [Google Scholar]
- Chambers WJ, Puig-Antich J, Hirsch M, Paez P, Ambrosini PJ, Tabrizi MA, Davies M. The assessment of affective disorders in children and adolescents by semistructured interview: test-retest reliability of the Schedule for Affective Disorders and Schizophrenia for School-Age Children, Present Episode Version. Arch Gen Psychiatry. 1985;42(7):696–702. doi: 10.1001/archpsyc.1985.01790300064008. [DOI] [PubMed] [Google Scholar]
- Clayton EH, Hanstock TL, Hirneth SJ, Kable CJ, Garg ML, Hazell PL. Reduced mania and depression in juvenile bipolar disorder associated with long-chain omega-3 polyunsaturated fatty acid supplementation. European Journal of Clinical Nutrition. 2009;63(8):1037–1040. doi: 10.1038/ejcn.2008.81. [DOI] [PubMed] [Google Scholar]
- Cohen D, Consoli A, Bodeau N, Purper-Ouakil D, Deniau E, Guile JM, Donnelly C. Predictors of placebo response in randomized controlled trials of psychotropic drugs for children and adolescents with internalizing disorders. Journal of Child and Adolescent Psychopharmacology. 2010;20(1):39–47. doi: 10.1089/cap.2009.0047. [DOI] [PubMed] [Google Scholar]
- Cunningham P, McKenzie K, Taylor EF. The struggle to provide community-based care to low-income people with serious mental illnesses. Health Affairs. 2006;25(3):694–705. doi: 10.1377/hlthaff.25.3.694. [DOI] [PubMed] [Google Scholar]
- Darwin Z, McGowan L, Edozien LC. Assessment acting as intervention: findings from a study of perinatal psychosocial assessment. Journal of Reproductive and Infant Psychology. 2013;31(5):500–511. [Google Scholar]
- Davis CE. The effect of regression to the mean in epidemiologic and clinical studies. American Journal of epidemiology. 1976;104(5):493–498. doi: 10.1093/oxfordjournals.aje.a112321. [DOI] [PubMed] [Google Scholar]
- Dishion TJ, Nelson SE, Kavanagh K. The family check-up with high-risk young adolescents: Preventing early-onset substance use by parent monitoring. Behavior Therapy. 2003;34(4):553–571. [Google Scholar]
- Dishion TJ, Shaw D, Connell A, Gardner F, Weaver C, Wilson M. The family check-up with high-risk indigent families: preventing problem behavior by increasing parents’ positive behavior support in early childhood. Child Development. 2008;79(5):1395–1414. doi: 10.1111/j.1467-8624.2008.01195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fristad MA, Goldberg-Arnold JS, Leffler JM. Psychotherapy for children with bipolar and depressive disorders. New York, NY: Guilford Press; 2011. [Google Scholar]
- Fristad MA, Verducci JS, Walters K, Young ME. Impact of multifamily psychoeducational psychotherapy in treating children aged 8 to 12 years with mood disorders. Arch Gen Psychiatry. 2009;66(9):1013–1021. doi: 10.1001/archgenpsychiatry.2009.112. [DOI] [PubMed] [Google Scholar]
- Fristad MA, Weller EB, Weller RA. The Mania Rating Scale: Can it be used in children? A preliminary report. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31(2):252–257. doi: 10.1097/00004583-199203000-00011. [DOI] [PubMed] [Google Scholar]
- Fristad MA, Young AS, Vesco AT, Nader ES, Healy KZ, Gardner W, Arnold LE. A randomized controlled trial of Individual Family Psychoeducational Psychotherapy & omega-3 fatty acids in youth with subsyndromal bipolar disorder. Journal of Child and Adolescent Psychopharmacology. 2015;25(10):764–774. doi: 10.1089/cap.2015.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallucci G, Swartz W, Hackerman F. Impact of the wait for an initial appointment on the rate of kept appointments at a mental health center. Psychiatric Services. 2005;56(3):344–346. doi: 10.1176/appi.ps.56.3.344. [DOI] [PubMed] [Google Scholar]
- Garry G. An audit of waiting times at a specialist psychotherapy service. Psychiatric Bulletin. 2006;30(5):182–184. [Google Scholar]
- Gavazzi SM, Fristad MA, Law JC. The Understanding Mood Disorders Questionnaire. Psychological Reports. 1997;81(1):172–174. doi: 10.2466/pr0.1997.81.1.172. [DOI] [PubMed] [Google Scholar]
- Guy W. ECDEU assessment manual for psychopharmacology (Department of Health, Education and Welfare Publication No. ADM 76-338) Rockville, MD: US Public Health Service; 1976. [Google Scholar]
- Harrington R, Rutter M, Fombonne E. Developmental pathways in depression: multiple meanings, antecedents, and endpoints. Development and Psychopathology. 1996;8(4):601–616. [Google Scholar]
- Jacobson NS, Truax P. Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. Journal of Consulting and Clinical Psychology. 1991;59(1):12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kowatch RA, Carmody TJ, Emslie GJ, Rintelmann JW, Hughes CW, Rush AJ. Prediction of response to fluoxetine and placebo in children and adolescents with major depression: a hypothesis generating study. J Affect Disord. 1999;54(3):269–276. doi: 10.1016/s0165-0327(98)00205-5. [DOI] [PubMed] [Google Scholar]
- Lambert MJ, Hansen NB, Finch AE. Patient-focused research: Using patient outcome data to enhance treatment effects. Journal of Consulting and Clinical Psychology. 2001;69(2):159–172. [PubMed] [Google Scholar]
- Leon AC, Shear MK, Klerman GL, Portera L, Rosenbaum JF, Goldenberg I. A comparison of symptom determinants of patient and clinican global ratings in patients with panic disorder and depression. Journal of Clinical Psychopharmacology. 1993;12(5):327–331. [PubMed] [Google Scholar]
- Lilienfeld SO, Garb HN, Wood JM. Unresolved questions concerning the effectiveness of psychological assessment as a therapeutic intervention: comment on Poston and Hanson (2010) Psychological Assessment. 2011;23(4):1047–1062. doi: 10.1037/a0025177. [DOI] [PubMed] [Google Scholar]
- Miklowitz DJ, Otto MW, Frank E, Reilly-Harrington NA, Wisniewski SR, Kogan JN, Sachs GS. Psychosocial treatments for bipolar depression: a 1-year randomized trial from the systematic treatment enhancement program. Arch Gen Psychiatry. 2007;64(4):419–426. doi: 10.1001/archpsyc.64.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mireau R, Inch R. Brief Solution-Focused Counseling: A practical effective strategy for dealing with wait lists in community-based mental health services. Social Work. 2009;54(1):63–70. doi: 10.1093/sw/54.1.63. [DOI] [PubMed] [Google Scholar]
- National Institute of Mental Health. Clinical Global Impressions Scale. Psychopharmacology Bulletin. 1985;21(4):839–843. [Google Scholar]
- Nemets H, Nemets B, Apter A, Bracha Z, Belmaker RH. Omega-3 treatment of childhood depression: a controlled, double-blind pilot study. American Journal of Psychiatry. 2006;163(6):1098–1100. doi: 10.1176/ajp.2006.163.6.1098. [DOI] [PubMed] [Google Scholar]
- Poston JM, Hanson WE. Meta-analysis of psychological assessment as a therapeutic intervention. Psychological Assessment. 2010;22(2):203–212. doi: 10.1037/a0018679. [DOI] [PubMed] [Google Scholar]
- Poznanski EO, Grossman JA, Buchsbaum Y, Banegas M, Freeman L, Gibbons R. Preliminary studies of the reliability and validity of the children’s depression rating scale. Journal of the American Academy of Child Psychiatry. 1984;23(2):191–197. doi: 10.1097/00004583-198403000-00011. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics. 2006;31(4):437–448. [Google Scholar]
- TADS Team. The Treatment of Adolescents with Depression Study (TADS): Long-term effectiveness and safety concerns. Arch Gen Psyciatry. 2007;64(10):1132–1143. doi: 10.1001/archpsyc.64.10.1132. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush J. Does a placebo run-in or a placebo treatment cell affect the efficacy of antidepressant medications? Neuropsychopharmacology. 1994;11(1):33–43. doi: 10.1038/npp.1994.63. [DOI] [PubMed] [Google Scholar]
- Trusler K, Doherty C, Mullin T, Grant S, McBride J. Waiting times for primary care psychological therapy and counselling services. Counselling & Psychotherapy Research. 2006;6(1):23–32. [Google Scholar]
- Van Meter A, Youngstrom EA, Demeter C, Findling RL. Examining the validity of cyclothymic disorder in a youth sample: replication and extension. Journal of Abnormal Child Psychology. 2013;41(3):367–378. doi: 10.1007/s10802-012-9680-1. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Wickramaratne P, Adams P, Wolk S, Verdeli H, Olfson M. Brief screening for family psychiatric history: The Family History Screen. Arch Gen Psychiatry. 2000;57(7):675–682. doi: 10.1001/archpsyc.57.7.675. [DOI] [PubMed] [Google Scholar]
- Weller EB, Weller RA, Fristad MA, Rooney MT, Schecter J. Children’s Interview for Psychiatric Syndromes (ChIPS) Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39(1):76–84. doi: 10.1097/00004583-200001000-00019. [DOI] [PubMed] [Google Scholar]
- Weller EB, Weller RA, Rooney MT, Fristad MA. Children’s Interview for Psychiatric Syndromes- Parent Version (P-ChIPS) Washington, D.C.: American Psychiatric Press, Inc; 1999a. [Google Scholar]
- Weller EB, Weller RA, Rooney MT, Fristad MA. Children’s Interview for Psychiatric Syndromes (ChIPS) Washington, D.C.: American Psychiatric Press, Inc; 1999b. [DOI] [PubMed] [Google Scholar]
- Wood JM, Garb HN, Lilienfeld SO, Nezworski MT. Clinical assessment. Annual Review of Psychology. 2002;53:519–543. doi: 10.1146/annurev.psych.53.100901.135136. [DOI] [PubMed] [Google Scholar]
- Wozniak J, Biederman J, Mick E, Waxmonsky J, Hantsoo L, Best C, Laposata M. Omega-3 fatty acid monotherapy for pediatric bipolar disorder: A prospective open-label trial. European Neuropsychopharmacology. 2007;17(6–7):440–447. doi: 10.1016/j.euroneuro.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Youngstrom EA, Danielson CK, Findling RL, Gracious BL, Calabrese JR. Factor structure of the Young Mania Rating Scale for use with youths ages 5 to 17 years. Journal of Clinical Child and Adolescent Psychology. 2002;31(4):567–572. doi: 10.1207/S15374424JCCP3104_15. [DOI] [PubMed] [Google Scholar]
- Zaider TI, Heimberg RG, Fresco DM, Schneier FR, Liebowitz MR. Evaluation of the Clinical Global Impression Scale among individuals with social anxiety disorder. Psychological Medicine. 2003;33(4):611–622. doi: 10.1017/s0033291703007414. [DOI] [PubMed] [Google Scholar]