Abstract
Alport syndrome, a type IV collagen disorder, manifests as glomerular disease associated with hearing loss with thickening of the glomerular and strial capillary basement membranes (SCBMs). We have identified a role for endothelin-1 (ET-1) activation of endothelin A receptors (ETARs) in glomerular pathogenesis. Here we explore whether ET-1 plays a role in strial pathology. Wild type (WT) and Alport mice were treated with the ETAR antagonist, sitaxentan. The stria vascularis was analyzed for SCBM thickness and for extracellular matrix (ECM) proteins. Additional WT and Alport mice were exposed to noise or hypoxia and the stria analyzed for hypoxia-related and ECM genes. A strial marginal cell line cultured under hypoxic conditions, or stimulated with ET-1 was analyzed for expression of hypoxia-related and ECM transcripts. Noise exposure resulted in significantly elevated ABR thresholds in Alport mice relative to wild type littermates. Alport stria showed elevated expression of collagen α1(IV), laminin α2, and laminin α5 proteins relative to WT. SCBM thickening and elevated ECM protein expression was ameliorated by ETAR blockade. Stria from normoxic Alport mice and hypoxic WT mice showed upregulation of hypoxia-related, ECM, and ET-1 transcripts. Both ET-1 stimulation and hypoxia up-regulated ECM transcripts in cultured marginal cells. We conclude that ET-1 mediated activation of ETARs on strial marginal cells results in elevated expression of ECM genes and thickening of the SCBMs in Alport mice. SCBM thickening results in hypoxic stress further elevating ECM and ET-1 gene expression, exacerbating strial pathology.
Keywords: Alport syndrome, stria vascularis, basement membrane
Introduction
Alport syndrome is characterized by delayed onset and progressive glomerular disease associated with progressive, often high frequency hearing loss. It is the result of mutations in basement membrane type IV collagen genes. About 80 percent of the cases are X-linked and associated with mutations in the COL4A5 gene (Barker et al., 1990), and the remainder autosomal recessive, and associated with mutations in either COL4A3 or COL4A4 genes (Lemmink et al., 1994; Mochizuki et al., 1994). Mutations in any of these genes results in the absence of all three proteins in basement membranes where they are present, due to an obligatory association to form type IV collagen heterotrimers (LeBleu et al., 2010). The basement membranes where these collagens are normally found are now comprised of the ubiquitous collagen α1(IV)/α2(IV) network in Alport patients.
In the glomerulus, and likely in the stria vascularis as well, this change in basement membrane composition results in a thinner structure with fewer interchain disulfide crosslinks (Gunwar et al., 1998). The consequences of this difference are two-fold. 1)The resulting capillary basement membrane is likely more elastic, which would be expected to impart higher than normal biophysical strain on glomerular cells, and 2) collagen α1(IV)/α2(IV) networks (as opposed to collagen α3(IV)/α4(IV)/α5(IV) networks in normal mammals) are now in contact with glomerular podocytes, which may alter cell signaling. With regard to biomechanics, it has been shown in Alport mouse models that renal pathology is markedly accelerated in hypertensive compared to normotensive mice (Meehan et al., 2009) and measures of Young's modulus, which is a metric for the biophysical rigidity, is reduced in Alport glomeruli relative to wild type glomeruli (Wyss et al., 2011). With regard to cell signaling, it was recently shown using super resolution microscopy, that the collagen α1(IV)/α2(IV) network in Alport mice is proximate to the podocyte pedicle surfaces, placing it in position to interact with podocyte cell surface receptors (Suleiman et al., 2013). One of these collagen receptors, the discoidin domain receptor 1 (DDR1), when mutated in mice, results in both GBM and SCBM dysmorphologies that are similar (but not identical) to those observed in Alport mice (Meyer zum Gottesberge et al., 2008; Gross et al., 2004. Another podocyte collagen receptor, integrin α2β1, when mutated ameliorates the Alport glomerular phenotype, extending lifespan by 20% (Rubel et al., 2014).
Strial capillary basement membranes are thickened in Alport mice and demonstrate elevated levels of ECM proteins (Cosgrove et al., 1998; Gratton et al., 2005), similar to the Alport GBM (Cosgrove et al., 1996). Both the stria vascularis and the glomerulus show dysregulation of matrix metalloproteinases (Gratton et al., 2005; Rao et al., 2006; Cosgrove et al., 2008), and treatment of these mice with metalloproteinase inhibitors ameliorates glomerular disease progression (Rao et al., 2006; Zeisberg et al., 2006), suggesting that dysregulation of basement membrane homeostasis may play a significant role in the progression of the disease.
We previously showed that laminin 211 deposition in the GBM by mesangial filopodia activates focal adhesion kinase (FAK) on glomerular podocytes (Zallocchi et. Al., 2013; Delimont et al., 2014), and more recently that endothelin-1-mediated activation of the endothelin A receptors (ETARs) on glomerular mesangial cells activates the invasion of glomerular capillaries by mesangial filopodia (Dufek et al., 2016). Blocking either FAK or ETARs ameliorated the dysregulation of pro-pathologic genes, including matrix metalloproteinases (MMPs) in glomerular podocytes and significantly improved GBM architecture and function in these same mice. In the present study, we demonstrate that FAK is also activated in the strial capillaries and endothelin-1 is induced in the Alport stria. ETARs are present on strial marginal cells and basal cells. Laminin α2, α5, and type IV collagen are induced at both the mRNA and protein levels in Alport stria relative to wild type stria, and this matrix accumulation can be blocked by treating animals with the ETAR antagonist, sitaxentan normalizing the SCBM thickness. We developed a strial marginal cell line to further explore this mechanism. Treating these cells with ET-1 induced the same ECM genes known to be elevated in vivo. Pre-treatment of these cells with sitaxentan prior to addition of ET-1 blocked induction of these genes. These same genes, including ET-1, are induced under conditions of hypoxia, in both the marginal cell culture system and in vivo in the stria vascularis. Our findings suggest that strial pathology and glomerular pathology in Alport mice share a common mechanism that hinges on ETAR activation by ET-1.
Methods
Mice
129 Sv autosomal Alport mice were developed here (Cosgrove et al., 1996). The Alport mice used at the Gratton lab were derived from the Cosgove colony. All mice were on a pure 129 Sv genetic background and maintained in house. The H-2Kb-tsA58 transgenic (immorto) mouse (Jackson Laboratories, Bar Harbor, ME) was used for strial explants to develop the strial marginal cell line. All procedures involving animals were conducted in accordance of an approved IACUC protocols at both sites: BoysTown National Research Hospital and Saint Louis University. Every effort was made to minimize usage as well as minimize any pain or distress.
Treatment of mice with sitaxentan
Sitaxentan (Sigma, St. Louis, MO) was solubilized in water and frozen in aliquots at 1 mg/ml. Animals were administered the drug at 10mg/kg daily by oral gavage from 2 weeks (pre-proteinuric) to 7 weeks (near end stage) of age.
Strial microdissection
A detailed description of the procedure for strial microdissection was described previously (Gratton et al., 2005).
Real time qRT-PCR analysis
Micro dissected striae were placed in Trizol™ (Ambion) and RNA isolated utilizing Direct·zol™ RNA Mini Prep Kit (Cat. # R2050 ZYMO Research, Irvine, CA). cDNA (from cells and stria) was generated using Superscript®III First-Strand (Cat. #18-080-05 Invitrogen, Waltham, MA). qPCR was run with the following TaqMan® Gene Expression Assays (Cat.# 4331182 Applied Biosystems, Waltham, MA) : Col4α1 Mm0120125_m1, ET-1 Mm00438656_m1, GLUT 1 Mm0441473_m1, HIF1α Mm00468869_m1, Lamα1 Mm01226102_m1, Lamα2 Mm01193171_m1, Lamα5 Mm01222046_g1, NFKbia Mm0477798_m and VEGF1 Mm01281449_m1.
Noise exposure
A subgroup of randomly selected Alport and wildtype mice were exposed to a moderate noise with a 106 dB SPL, octave band of noise (OBN) centered at 10 kHz for 10 hours. Immediately prior to exposure, the mice were hydrated with 2ml sterile lactated Ringers s.c. and are then placed into individual wire-mesh cages containing food. Following the noise exposure, the mouse wass removed for physiological assessment (ABR) or was euthanized for ultrastructural or biochemical analysis of the cochlear lateral wall.
ABR Testing
Animals were sedated (Avertin [2,2,2-Tribromoethanol, Sigma Aldrich, St. Louis, MO] 0.4 mg/gm i.p.) with supplemental injections (0.5 original dose) as needed. Anesthetized animals were placed in a head holder with the right ear directed superiorward in an acoustically shielded sound chamber. Body temperature was maintained at 37°C using a thermoelectrical feedback system (FHC, Bowdoin, ME). Heart function was monitored auditorilly. Tone pips at half-octave intervals (8-32kHz) were synthesized, shaped (1 ms cosine ramps, 2 ms plateau), and delivered via the Tucker-Davis ABR workstation. The stimuli were delivered via a customized high frequency transducer at a rate of 12/sec. Sound pressure levels (dB SPL) of the tone pips were calibrated using a 6.25 mm condenser microphone (ACO-Pacific, Belmont, CA) positioned at the approximate location of the animal's head. The ABR was recorded using needle electrodes positioned subdermally at the vertex (active, noninverting), at the bulla (reference, inverting) and over the musculature of the rump (ground). Differentially recorded voltages were amplified, filtered (30 Hz – 3 kHz), digitized, and averaged using the Tucker-Davis ABR Workstation software. Each ABR waveform was averaged over a 16 ms time window and resolved after 512 – 1024 samples. Artifact rejection was used to enhance reliability and validity of the ABR. The intensity of the tonal stimuli was decreased in 10 dB steps and varied in 5 dB steps for stimuli near threshold. Threshold was defined as half the interval between lowest stimulus level producing a replicable response waveform and the stimulus level below which no reproducible response is elicited.
A subgroup of randomly selected Alport and wildtype mice underwest ABR testing prior to, immediately after (Day 0) and five days (Day 5) after exposure to the moderate noise
Immunofluorescence analysis
Cochleae were perfused with 4% paraformaldehyde and then decalcified overnight in 150mM EDTA on a rotator at 4°C. The samples were transferred to 15% sucrose for 1 hour and then into 30% sucrose for 2 hours. Samples were mounted in OCT and frozen at −80°C. Cochleae were sectioned at 8-μm and dipped in cold acetone. After air drying 2 hours, slides were rehydrated with 1X PBS. Cochleae sections used for collagen IV immunostaining were first denatured with acid urea (6M urea, 0.1M glycine, pH 3.5) for 1 hour at 4°C and then rinsed with 1X PBS. Slides were stained with one of the following antibodies: rat anti-mouse Laminin α2 antibody (L0663, Sigma-Aldrich, St. Louis, MO, USA) at 1:500, rabbit anti-mouse Laminin α5 antibody (a gift from Jeff Miner, Washington University) at 1:1500, rabbit anti-mouse Phospho-FAK397 antibody (44-625G, Thermo Fisher Scientific, Waltham, MA, USA) at 1:25, rabbit anti-mouse Collagen α1IV antibody (T40261R, Biodesign, Saco, ME, USA) at 1:500 or sheep anti-mouse Endothelin R Type A antibody (NB600-836, Novus Biologicals, Littleton, CO, USA) at 1:200. Sections were incubated overnight at 4°C in a humidified chamber with their primary antibodies diluted in 0.1% PBST (Triton X-100) + 5% FBS. Slides were rinsed with 1X PBS and incubated with the appropriate Alexa Fluor secondary antibodies at 1:500 for 1 hour at room temperature. The slides were rinsed again with 1X PBS and mounted with Vectashield Mounting Medium with Dapi (H-1200, Vector Laboratories, Burlingame, CA, USA).
Confocal Microscopy
Confocal images captured using a Leica TCS SP8 MP confocal imaging system, using a 63x NA: 1.4 oil or 10x NA: 0.3 objective. Final figures were assembled using Adobe Photoshop and Illustrator software (Adobe Systems, CA).
Transmission Electron Microscopy
Preparation of the cochlea for transmission electron microscopy was conducted as described previously (Gratton et al., 2005). Thin sections (70 nm) of the scala media cut in the mid-modiolar plane were mounted on copper grids, counterstained with uranyl acetate and lead citrate and examined at 40k magnification in a Hitachi H-7500 transmission electron microscope. Digitized images were obtained and archived with an ORCA camera with IC-PCI framegrabber and AMT 12-HR software. Measurements of basement membrane width were made at approximately 500nm intervals around the strial capillary profile.
Derivation of Strial marginal Cells
The micro dissected stria vascularis from an H-2Kb-tsA58 transgenic (immorto) mouse cochlea was cut into several pieces and placed onto BD Cell Tak™ (BD Biosciences, Bedford, MA.) coated- tissue culture dishes. Outgrowths were propagated at 33°C in DMEM/F-12 Media (Gibco, Grand Island, NY.) supplemented with 15% heat-inactivated fetal bovine serum (Atlanta Biologicals, Atlanta, GA.), 50 units/ml mouse recombinant γ-interferon (Calbiochem, La Jolla, CA.) and penicillin, streptomycin, glutamine (Gibco). Outgrowths were dissociated with 0.05% trypsin, 0.02% EDTA (Gibco) and cloned by limiting dilution. Fifty clones were subsequently frozen back, twenty of which were expanded for further characterization by RT-PCR and immunofluorescence. To expand, sub confluent (≤80%) clonal cultures were dissociated and replated 1:5 in 10% FBS, 10 U/ml γ-interferon containing medium on dishes coated with rat tail Collagen Type 1 (Corning, Bedford, MA.) at 33°C. To induce differentiation, dissociated cells were plated in 5% FBS- containing medium without γ- interferon, placed at 37°C for at least seven days.
Hypoxia Induction
Sub-confluent Strial Marginal cells plated on Collagen Type 1 from rat tail (Corning, Bedford, MA.) were differentiated (≥ 9 days at 37°, 5 % FBS-containing media, less α-interferon) placed on 0.5% FBS-containing media overnight and the next day placed in a hypoxia chamber (Cat.#27310; Billups-Rothenburg, Dell Mar, CA). Utilizing two independent flow meters (Billups-Rothenburg) attached separately to compressed nitrogen and carbon-dioxide gas, the chamber was flushed for 10 minutes with 95% ↑N2/ 5%↑CO2 at a flow rate of ~20 liters/min. Immediately after purging the chamber was sealed and placed in a conventional CO2 incubator. The system was re-flushed after 1 hour, as recommend by the manufacturer. RNA was collected, at indicated time points, by removing cultures from chamber and immediately applying TRIzol®Reagent (Ambion, TX) to cells. Normoxia control culture(s) were placed in an identical chamber left “open” to CO2 incubator conditions.
Sitaxentan/ET-1 Treatment of strial marginal cells
Differentiated Strial Marginal cells were exposed to 10 μM Sitaxentan (Sigma, St Louis, MO.) for one hour and then treated with 1 μg/mL ET-1 (Cat.# 55553 Syd Labs, Boston, MA.). Non-treated control and ET-1 alone treatment were also performed. RNA was collected, at indicated time points, as before, and analyzed by real time RT-PCR for ECM transcripts.
Statistical analysis
The measures of SCBM thickness were subjected to 1-way ANOVA analysis with the factor of genotype/treatment (, Kruskal-Wallis, SigmaStat, Jandel, San Jose, CA) followed by post-hoc multiple comparisons (Dunn's Method). For ABR analysis, hearing loss incurred at each timepoint for the Alport mice and their age-matched WT controls was subjected to ANOVA with factors of test frequency, and strain/test day with post-hoc comparisons made using the Holm-Sidak test (SigmaStat, Jandel, San Jose, CA). Significance was set at a p value ≤ 0.05. For all other analyses, one or two way (as appropriate) Student's t-test was used. Significance was set at the probability level of 0.05
Results
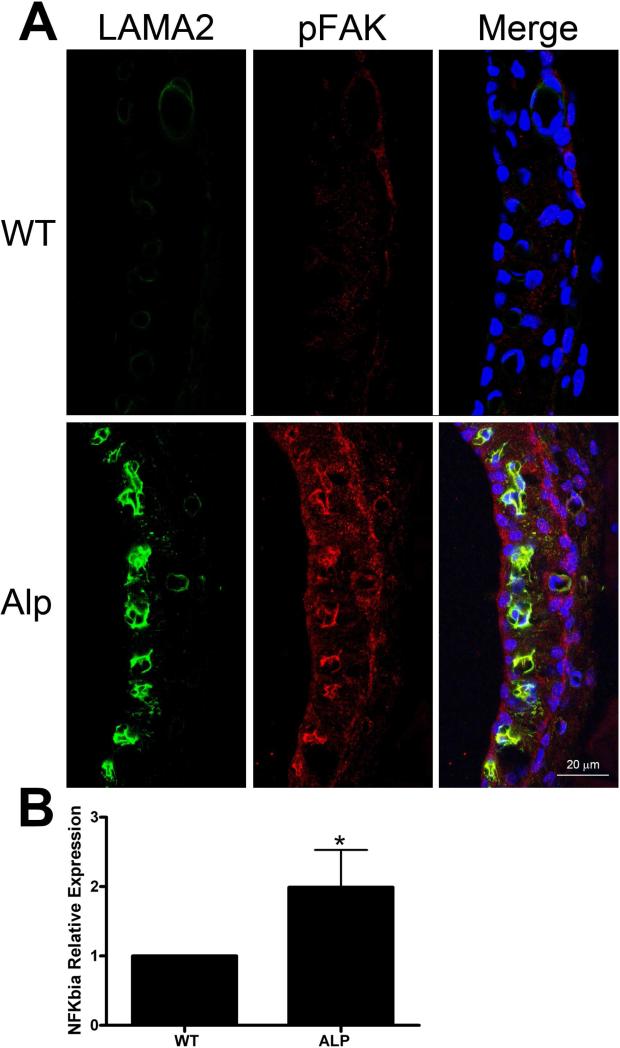
Strial capillary basement membrane thickening in the Alport mouse model was reported previously (Cosgrove et al., 1998; Gratton et al., 2005). In the study by Gratton et al. (2005), colloidal gold TEM was used to show that collagen α1(IV)/α2(IV), laminin α1, and laminin α5 are present at higher levels in the Alport stria compared to wild type controls. Laminin 211, which was shown to activate FAK, resulting in a pro-inflammatory signal cascade in glomerular podocytes was never examined in this study (Delimont et al., 2014). To address this, mid-modiolar cross sections of wild type and Alport cochleae were dual immunostained using antibodies specific for either laminin α2 chain or activated FAK (pFAK397). The lateral walls are shown in Figure 1A. It is clear that laminin α2 is up-regulated in the Alport stria vascularis in the regions surrounding the strial capillaries, where it co-localizes with pFAK397. This is similar to what we have previously documented in the renal glomerulus (Delimont et al., 2014). Figure 1B shows that the I kappa B-alpha (NFkappaBIA) mRNA expression in isolated stria from Alport mice is elevated relative to wild type mice, consistent with pro-inflammatory NFkappaB activation in the Alport stria (Bottero et al., 2003).
Figure 1.
Laminin 211 accumulates in the SCBM of Alport mice activating FAK and pro-inflammatory NF-kappaB. Panel A: Wild type and Alport stria vascularis from 7 week old mice were immunostained using antibodies specific for laminin α2 chain and antibodies that detect the activated form of FAK, pFAK397. Panel B: RNA from wild type and Alport stria vascularis was analyzed for the NF-kappaBIa transcript by real time RT-PCR. Asterisk denotes statistical significance (P<0.05).
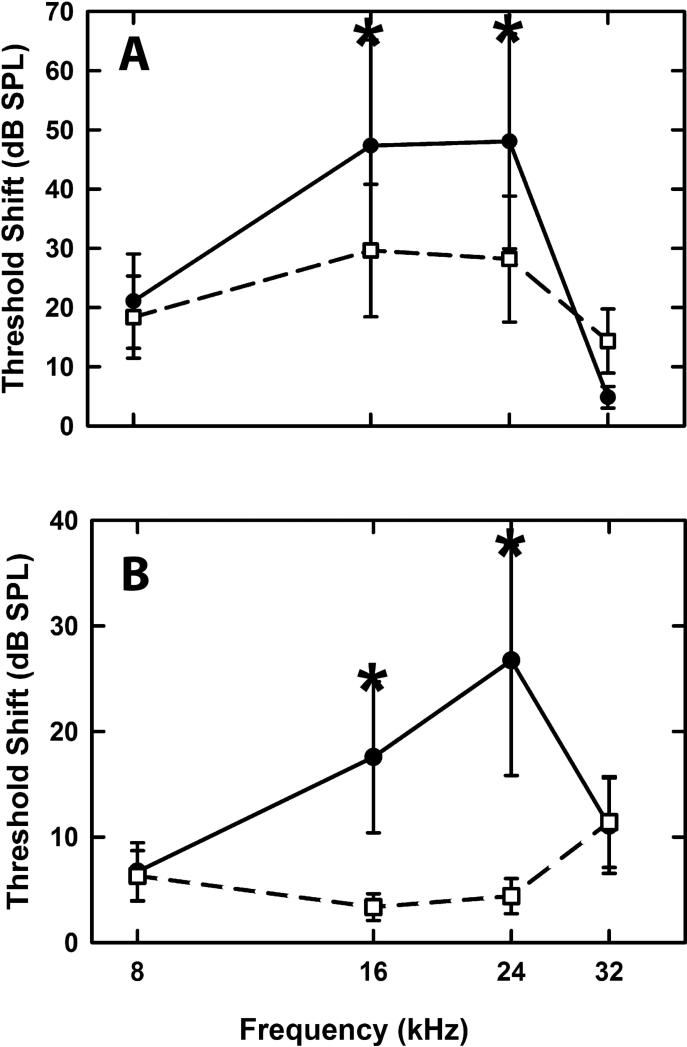
Alport mice show only mild progressive hearing loss (Cosgrove et al., 1998). We surmised that if thickened strial capillary basement membranes altered vascular permeability, the strial cells might be predisposed to metabolic stresses. To test this, we subjected wild type and Alport mice to noise exposure (8-17 kHz, 106 dB SPL) for 10 hrs. Hearing loss was assessed immediately post noise, and again at 5 days post noise by auditory brainstem response (ABR). Figure 2A shows that immediately post noise the Alport mice show significantly elevated thresholds (2-way ANOVA: F(9,92) = 4748.774, p < 0.001) at the middle frequency ranges (16 and 24 kHz), when compared to wild type mice (p<0.05, Holm-Sidak). Figure 2B shows that by 5 days post-noise exposure, wild type mice have returned to normal thresholds, while Alport mice again show significantly elevated thresholds at 16 and 24 kHz (p<0.05, Holm-Sidak), likely reflecting permanent noise-induced damage.
Figure 2.
Noise exposure results in significantly elevated thresholds for Alport mice relative to wild type mice. Mice were exposed to noise (8-17 kHz, 106 dB SPL) for 10 hrs. Hearing was assessed using the ABR immediately post-noise exposure (panel A) and again at 5 days post-noise exposure (panel B). Asterisks denote frequencies that contributed to the statistically significant differences noted overall between the wild type and Alport mice(P<0.05).
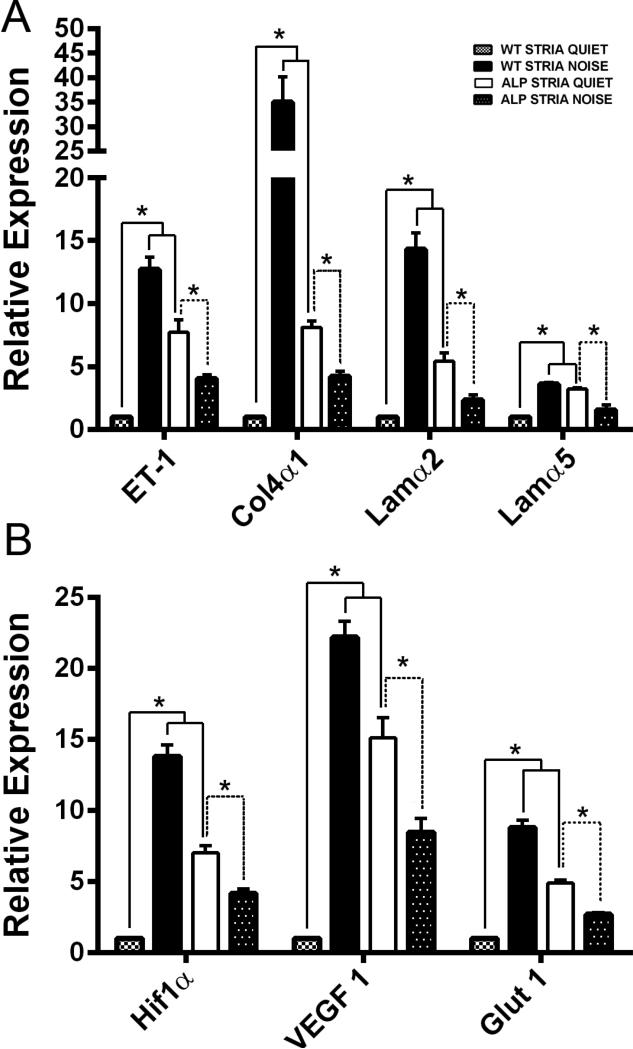
More recently we have shown that endothelin-1 (ET-1) activates the accumulation of abnormal GBM laminins (including laminin 211) and initiates epithelial cell dysfunction in the renal glomerulus via activation of the endothelin A receptor subclass (Dufek et al., 2016). Blocking ETARs using sitaxentan resulted in the inhibition of ECM accumulation in the GBM and amelioration of glomerular disease initiation and progression. We analyzed expression of ET-1 and ECM genes encoding proteins known to accumulate in the SCBM of Alport mice using RNA isolated from microdissected stria vascularis from 7 week old wild type and Alport mice plus or minus noise exposure as described above. The results in Figure 3A show that both ET-1 mRNA and mRNA encoding ECM proteins known by immunogold analysis to accumulate in Alport stria (Gratton et al., 2005), are significantly higher in Alport stria vascularis compared to stria from age/strain matched wild type mice. Furthermore, exposure of wild type mice to noise significantly elevates these same transcripts. Interestingly, while expression of these transcripts in the stria from Alport mice exposed to noise is still elevated compared to wild type mice under quiet conditions, Alport noise is actually lower than Alport quiet, again likely reflecting noise-induced damage to the strial cells.
Figure 3.
The stria vascularis in Alport mice is under metabolic/oxidative stress and overexpresses transcripts encoding ECM molecules. Stria vascularis was isolated from 7 week Alport mice, strain and age-matched wild type mice, and wild type mice exposed to noise (see methods). RNA was analyzed by real time RT-PCR for transcripts encoding endothelin-1, the ECM molecules known to accumulate in the SCBM (laminin α2, laminin α5, and collagen α1(IV), Panel A), or for hypoxia related factors Hif-1α and VEGF or glucose transporter-1 (Glut1) (Panel B), which is up-regulated under conditions of metabolic stress. Asterisks denotes statistically significant differences (P<0.01).
Thickened SCBMs would be expected to impart metabolic stresses leading to a hypoxic environment in the stria vascularis. Metabolic stresses caused by intense noise exposure have been documented in the stria vascularis, and lead to upregulation of hypoxia inducible factor 1α (Hif1-α) and vascular endothelial growth factor (VEGF) (Shi, 2009). We compared stria vascularis from non-noise exposed and noise exposed wild type mice to stria vascularis from non-noise and noise-exposed Alport mice for expression of mRNA encoding Hif1-α and VEGF. Glucose transporter-1, expressed by strial capillary endothelial cells (Ando et al., 2008), was also measured, as it would be expected to be up-regulated under conditions of metabolic stress (Barnes et al., 2002). The results in Figure 3B show that all three transcripts are significantly elevated in stria vascularis from both non-noise exposed Alport mice and noise exposed wild type mice compared to non-noise exposed wild type mice, suggesting that the SCBM thickening in the Alport stria vascularis results in a hypoxic microenvironment which results in metabolic stress. Like the ECM and ET-1 transcripts, expression hypoxia-related genes were lower in stria from noise-exposed Alport mice compared Alport mice from quiet conditions. This may reflect the already stressed condition of the stria in Alport mice, and along with the data for these same mice in Figure 3A, may reflect damage to the strial cells. These observations in Alport quiet versus Alport noise stria are consistent with the permanent shift in ABR thresholds observed in Figure 2B.
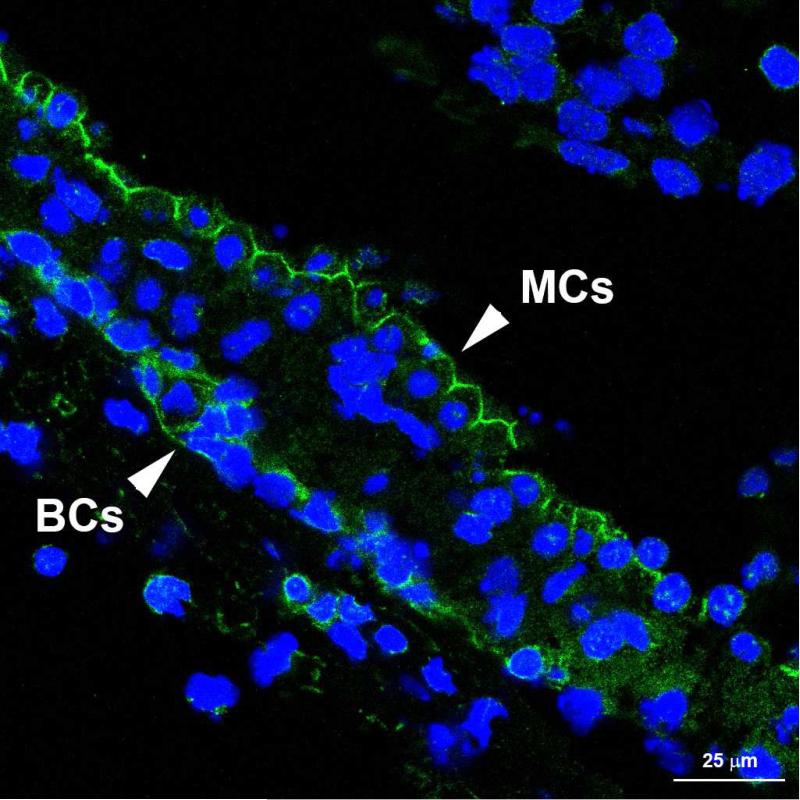
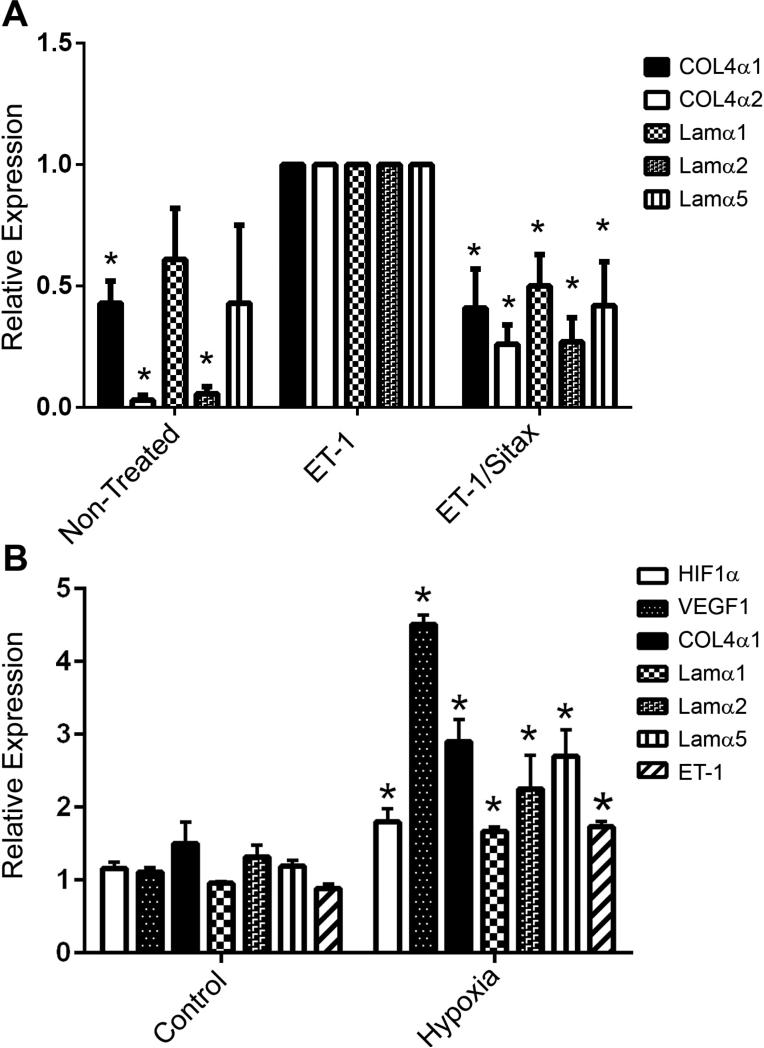
In the renal glomerulus, ET-1 mediated ECM and pro-inflammatory cytokine induction is regulated by ETAR signaling. Blocking ETARs using the small molecule inhibitor, Sitaxentan, ameliorates glomerular and renal disease in Alport mice (Dufek et al., 2016). We examined the cellular distribution of ETARs in the stria vascularis in adult mice by immunohistochemistry. The antibodies would only work in unfixed tissue, so freshly microdissected stria vascularis was used. Figure 4 demonstrates that only the strial marginal cells and basal cells express ETARs. To address the role of ET-1 mediated ETAR activation in the regulation of ECM genes we developed a strial marginal cell line from the immortomouse. Strial explants from adult mice were adhered to tissue culture plates using Cell-Tak (Corning, Inc.) and outgrowths cloned twice. Individual clones were characterized by real time PCR using primers to detect the strial cell-specific markers. One clone was selected that was positive for all the strial marginal cell-specific markers and expressing very low to undetectable levels for intermediate/basal cells markers shown in Table 1. This clone was expanded, differentiated, and stimulated with ET-1 in the presence or absence of the ETAR receptor antagonist, sitaxentan. RNA from these cells was then analyzed by real time RT-PCR for the ECM proteins shown in Figure 5A. The results show that the transcripts encoding the ECM proteins known to accumulate in the Alport stria vascularis are expressed in strial marginal cell cultures and are up-regulated by stimulating the cells with ET-1. Co-incubation of ET-1 with sitaxentan significantly reduced expression of these same ECM transcripts, supporting the notion that ET-1 regulates ECM expression in strial marginal cells via ETAR signaling.
Figure 4.
Endothelin A receptors are expressed by strial basal cells and strial marginal cells. The lateral wall with the intact stria vascularis was dissected from unfixed cochlea of a 4 week old wild type mouse and cryosections immunostained using antibodies specific for ETARs. Both marginal cells (MCs) and basal cells (BCs) were immunopositive.
Table 1.
RT PCR Markers for Marginal Cell Clone Characterization.
| Transcript | Strial Cell Expression | Reference |
|---|---|---|
| − Connexin 26 | Basal/Intermediate | Liu YP, et al. 2008 |
| + Dclkl | Marginal | Girotto G, et al. 2014 |
| − Glut-1 | Basal/Endothelial | Barnes K, et al. 2002 |
| + KCNQ 1 | Marginal | Hur DG, et al. 2007 |
| + KCNE 1 | Marginal | Hur DG, et al. 2007 |
| − KCNJ10 (Kir4.1) | Intermediate | Jin Z, et al. 2006 & Chen J, et al. 2014 |
| + KvLQT1 (IsK) | Marginal | Teixeira M, et al. 2006 & Tu TY, et al. 1999 |
| + Na, K- ATPase | Marginal | Crouch JJ, et al. 1997 |
| − PANx2 | Basal | Wang XH, et al. 2009 |
| + SIRT1 | Intermediate/ Marginal | Takumida M, et al. 2014 |
| + KCNK1 (TWIK-1) | Marginal | Nicolas MT, et al. 2003 |
| + ZO-1 | Marginal | Suzuki T, et al. 2001 |
− = low expression; + = high expression
Figure 5.
Stria marginal cells respond to both ET-1 and hypoxia by up regulating expression of ECM genes, and the response can be blunted by ETAR blockade. Panel A. Differentiated strial marginal cell cultures were stimulated with ET-1 in the presence or absence of sitaxentan. RNA was analyzed for the indicated ECM transcripts. Panel B. Differentiated strial marginal cells were cultured under normoxic or hypoxic conditions (for 24 hours) and RNA analyzed for the indicated transcripts. Asterisks denote statistically significant differences (P<0.05).
We exposed these same cells to hypoxic conditions to determine whether hypoxia (metabolic stress) itself might contribute to the induction of genes encoding ECM proteins, as is suggested from the in vivo noise exposure studies (Figure 3B). The results in Figure 5B show that hypoxia related genes (Hif1-α and VEGF) were induced in cells cultured under hypoxic conditions. Messenger RNAs for both ET-1 and ECM proteins were also significantly induced, suggesting that once established, the metabolic stresses in Alport stria might themselves contribute to the SCBM thickening and stria pathology.
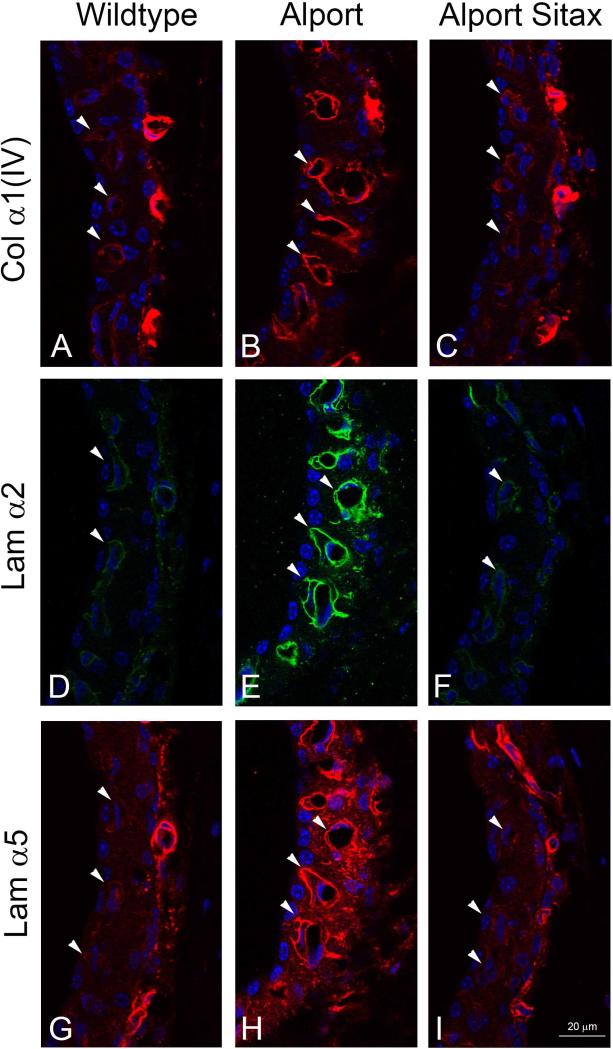
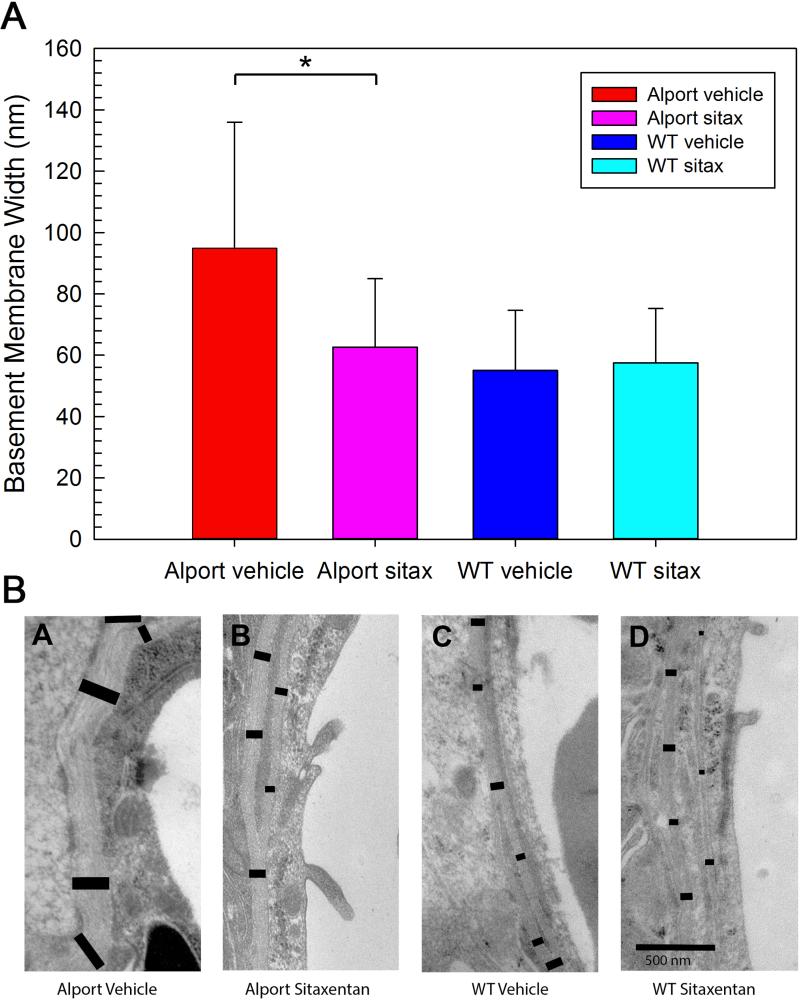
Lastly, we aimed to determine whether ETAR blockade in vivo would prevent the accumulation of ECM proteins in the SCBM as well as prevent SCBM thickening. Wild type and Alport mice were treated with sitaxentan (10 mg/kg) or vehicle from 2 to 7 weeks of age. One ear was processed for transmission electron microscopy and the other processed for immunofluorescence analysis. The results in Figure 6 show that collagen α1(IV), laminin α2, and laminin α5 are all significantly elevated in the Alport stria relative to the wild type stria, and treatment with sitaxentan prevents the accumulation of these matrix proteins in the SCBMs. It is notable in these immunostains that immunostaining intensity in the capillaries of the spiral ligament is not affected by sitaxentan treatment. TEM analysis shows that untreated Alport SCBMs are significantly thicker (H =, 1227.979, 1 df, p= <0.001 than that for either treated or untreated wild type mice. Sitaxentan treatment prevents SCBM thickening (Figure 7A). Figure 7B shows an example of the differences in SCBM thickness for the wildtype and Alport mice; the black bars delineate the BM width and illustrate how the SCBM morphometric measures were performed. Collectively the data in Figures 6 and 7 suggest SCBM thickening is caused by ETAR-mediated ECM gene dysregulation in strial marginal cells, which underlies SCBM thickening and strial dysfunction.
Figure 6.
Endothelin A receptor blockade prevents accumulation of ECM in the Alport strial capillary basement membranes. Alport mice were treated with sitaxentan or vehicle from 2 weeks to 7 weeks of age. Mid-modiolar cryosections were immunostained with antibodies against type IV collagen α1 chain (col α1(IV)), laminin α2 chain (Lam α2), or laminin α5 chain (Lam α5). All sections were stained at the same time and microscope settings standardized to the vehicle-treated Alport cochleae. Arrow heads denote strial capillary basement membranes. Note that vessels in the spiral ligament do not show changes in staining intensity upon sitaxentan treatment, and thus serve as a control.
Figure 7.
Endothelin blockade ameliorates strial capillary basement membrane thickening in Alport mice. Panel A. Morphometric analysis of strial capillary basement membrane thickness was done using transmission electron micrographs for multiple capillaries from at least 4 independent animals per group. Panel B. An example of the methodology used for morphometric analysis of SCBM thickness. Asterisk denotes a statistically significant difference (P<0.05).
Discussion
This study demonstrates that progressive SCBM thickening, which results in metabolic and oxidative stress in the stria vascularis of Alport mice, evolves through a mechanism that is remarkably similar to the mechanism of glomerular basement membrane disease (Dufek et al., 2016); Endothelin-1 mediated activation of ETARs on strial marginal cells resulting in elevated expression of ECM proteins that accumulate in the SCBMs. We show using both in vivo and in vitro systems, that ETAR blockade results in reduced ECM protein expression, normalization of SCBM thickness and reduced expression of mRNAs encoding ECM proteins that accumulate in the SCBMs. The link between ET-1-mediated ETAR activation and ECM protein accumulation in fibrotic mechanisms is well established (Shi-Wen et al., 2001; Xu et al., 2004), however attempts to control or reverse the fibrotic process by way of ETAR blockade have produced disappointing results (Rodriguez-Pascual et al., 2014). In the Alport mouse model ETAR blockade produced impressive results in preventing SCBM thickening and ECM accumulation in the SCBMs.
Strial pathology associated with Alport syndrome has been understudied relative to Alport glomerular pathology and the literature on inner ear pathology associated with Alport syndrome is not without controversy. The only ultrastructural defect observed in the Alport mouse cochlea was a thickening and lamination of the strial capillary basement membranes associated with endothelial cell swelling (Cosgrove et al., 1998). This defect is very similar to one described in humans with Alport syndrome many years ago (Weidauer et al., 1976). More recently in a study conducted on archived celloidin-embedded human temporal bones from Alport patients, the authors concluded that a shared defect of splitting of the basilar membrane and cellular infilling of the tunnel of Nuel might predict a defect in cochlear mechanics (Merchant et al., 2004). One important difference between the two studies is that Weidauer and Arnold fixed the tissue within one hour of death, immediately removed the membranous tissue and prepared it for transmission electron microscopy.
It is important to note that Merchant et al. (2004) claimed not to observe SCBM thickening in the human samples. What the authors failed to recognize is that the SCBM has two layers; an inner layer adjacent to the endothelium and an outer layer that lies on the outer surface of the pericytes (Neng et al., 2013). The outer layer, which forms contacts with strial marginal cell basolateral infoldings, is thickened in the Alport mice (Cosgrove et al., 1998). This layer is also thickened in the samples from Alport patients as is clearly evident in Merchant et al., and in Weidauer and Arnolds study.
Lastly, if defective cochlear mechanics were responsible for hearing loss in human Alport patients, as hypothesized by Merchant et al. (2004), one would expect that ALL Alport patients with hearing loss would have impaired otoaccoustic emissions (OAEs). Moon et al. (2009) found that OAEs had no predictive value in young Alport patients with documented hearing loss. As patients get older OAEs do change, likely due to hair cell loss, as documented in Merchant's study.
Our findings may have broader relevance than Alport syndrome. Strial capillary basement membrane thickening has been associated with diabetic hearing loss in humans (Akinpelu et al., 2014) and implicated in presbycusis, where strial atrophy is often observed (Nelson et al., 2006). In aging gerbil models thickening of the strial capillary basement membranes is observed and associated with the accumulation of laminin (Gratton et al., 1996; Thomopoulos et al., 1997; Sakaguchi et al., 1997). It would be of interest to explore whether the ET-1/ETAR axis for ECM dysregulation plays a role in SCBM thickening in models for these forms of hearing loss.
ET-1 mediated ETAR activation is the earliest event we have documented to be associated with glomerular pathology in Alport mice. The fact that this pathway appears activated in the stria vascularis in Alport mice is remarkable and suggests the possibility for the identification of single-target therapeutic approaches that may attenuate/arrest both the glomerular and strial pathologies in their pre-initiated state. This approach has the potential for preserving both hearing and kidney function in patients afflicted with the disease. Studies are under way aimed at determining whether endothelin blockade in Alport mice results in normalization of hearing, susceptibility to noise-induced hearing loss, endocochlear potentials, and measures of metabolic stress. These studies will define whether ETAR antagonism is a potential therapeutic strategy to prevent hearing loss associated with Alport syndrome.
Highlights.
-Strial capillary basement membrane thickening underlies cochlear pathology in Alport syndrome.
-Endothelin-1 up-regulates genes encoding ECM molecules in strial marginal cells.
-blocking the endothelin A receptor in vitro or in vivo prevents elevates expression of ECM components in the strial capillary basement membranes and ameliorates SCBM thickening in the mouse model.
Acknowledgements
The authors thank John (Skip) Kennedy for assistance with figure preparation. This work was supported by NIH R01 DK055000 (to DC) and R01 DC015385 (to DC and MAG). Confocal microscopy was conducted at the Integrated Biomedical Imaging Facility, Creighton University, Omaha, NE (GM103427, GM110768, GM103427 of the NIGMS of NIH, and the Creighton University School of Medicine). Structured illumination microscopy was conducted at the Advanced Microscopy Core Facility, UNMC, Omaha, NE. Support for the UNMC Advanced Microscopy Core Facility was provided by the Nebraska Research Initiative, the Fred and Pamela Buffett Cancer Center Support Grant (P30CA036727), and an Institutional Development Award (IDeA) from the NIGMS of the NIH (P30GM10639). Transmission electron microscopy was conducted at the Microscopy and Digital Imaging Core of the Research Center for Auditory and Vestibular Studies at Washington University (P30 DC004665).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: Daniel Meehan conducted numerous experiments, helped with the conceptual design, and edited the writing; Duane Delimont performed husbandry, genotyping, and edited the writing; Brianna Dufek performed immunohistology, confocal microscopy analysis, and edited the writing; Marisa Zallocchi performed immunohistochemistry and helped with early conceptual design, and edited the writing; Grady Phillips performed TEM and morphometric analysis of the results; Michael Anne Gratton participated in conceptual design, directed research conducted at Saint Louis University, performed strial microdissections, oversaw noise exposures and the ABR testing, and participated in the writing of the manuscript; Dominic Cosgrove directed the research, assembled figures and led the effort in writing and assembly of the final manuscrip
The authors have no conflicts of interest with regard to this work.
Literature Cited
- Akinpelu OV, Ibrahim F, Waissbluth S, Daniel SJ. Histopathologic changes in the cochlea associated with diabetes mellitus—a review. Otol. Neurotol. 2014;35:764–774. doi: 10.1097/MAO.0000000000000293. [DOI] [PubMed] [Google Scholar]
- Ando M, Edamatsu M, Fukuizumi S, Takeuchi S. Cellular localization of facilitated glucose transporter 1 (GLUT-1) in the cochlear stria vascularis: its possible contribution to the transcellular glucose pathway. Cell Tissue Res. 2008;331:763–769. doi: 10.1007/s00441-007-0495-2. [DOI] [PubMed] [Google Scholar]
- Ando M, Edamatsu M, Fukuizumi S, Takeuchi S. Cellular localization of facilitated glucose transporter 1 (GLUT-1) in the cochlear stria vascularis: its possible contribution to the transcellular glucose pathway. Cell Tissue Res. 2008;331:763–769. doi: 10.1007/s00441-007-0495-2. [DOI] [PubMed] [Google Scholar]
- Barker DF, Hostikka SL, Zhou J, Chow LT, Oliphant AR, Gerken SC, Gregory MC, Skolnick MH, Atkin CL, Tryggvason K. Identification of mutations in the COL4A5 collagen gene in Alport Syndrome. Science. 1990;8:1224–1227. doi: 10.1126/science.2349482. [DOI] [PubMed] [Google Scholar]
- Barnes K, Ingram JC, Porras OH, Barros LF, Hudson ER, Fryer LG, Foufelle F, Carling D, Hardie DG, Baldwin SA. Activation of GLUT1 by metabolic and osmotic stress: potential involvement of AMP-activated protein kinase (AMPK). J. Cell Sci. 2002;115:2433–2442. doi: 10.1242/jcs.115.11.2433. [DOI] [PubMed] [Google Scholar]
- Bottero V, Imbert V, Frelin C, Formento JL, Peyron JF. Monitoring NF-kappa B transactivation potential via real-time PCR quantification of l kappa B-alpha gene expression. Mol. Diagn. 2003;7:187–194. doi: 10.1007/BF03260037. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhao HB. The role of an inwardly rectifying K(+) channel (Kir4.1) in the inner ear and hearing loss. Neuroscience. 2014;265:137–46. doi: 10.1016/j.neuroscience.2014.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D, Meehan DT, Delimont D, Pozzi A, Chen X, Rodgers KD, Tempero RM, Zallocchi M, Rao VH. Integrin alpha1beta1 regulates matrix metalloproteinases via P38 mitogen-activated protein kinase in mesangial cells: implications for Alport syndrome. Am. J. Pathol. 2008;172:761–773. doi: 10.2353/ajpath.2008.070473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D, Meehan DT, Grunkemeyer JA, Kornak JM, Sayers R, Hunter WJ, Samuelson GC. Collagen COL4A3 knockout: a mouse model for autosomal Alport syndrome. Genes Dev. 1996;10:2981–2992. doi: 10.1101/gad.10.23.2981. [DOI] [PubMed] [Google Scholar]
- Cosgrove D, Samuelson G, Meehan DT, Miller C, McGee J, Walsh EJ, Siegel M. Ultrastructural, physiological, and molecular defects in the inner ear of a gene-knockout mouse model for autosomal Alport syndrome. Hear Res. 1998;121:84–98. doi: 10.1016/s0378-5955(98)00069-0. [DOI] [PubMed] [Google Scholar]
- Crouch JJ, Sakaguchi N, Lytle C, Schulte BA. Immunohistochemical localization of the Na-K-Cl co-transporter (NKCC1) in the gerbil inner ear. J. Histochem. Cytochem. 1997;45:773–778. doi: 10.1177/002215549704500601. [DOI] [PubMed] [Google Scholar]
- Delimont D, Dufek BM, Meehan DT, Zallocchi M, Gratton MA, Phillips G, Cosgrove D. Laminin α2-mediated focal adhesion kinase activation triggers Alport glomerular pathogenesis. PLoS One. 2014;9:e99083. doi: 10.1371/journal.pone.0099083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufek B, Meehan D, Delimont D, Cheung L, Gratton MA, Phillips G, Song W, Liu S, Cosgrove D. Endothelin A receptor activation on mesangial cells initiates Alport glomerular disease. Kidney Int. 2016 doi: 10.1016/j.kint.2016.02.018. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girotto G, Vuckovic D, Buniello A, Lorente-Canovas B, Lewis M, Gasparini P, Steel KP. Expression and replication studies to identify new candidate genes involved in normal hearing function. PLos One. 2014;9:e85352. doi: 10.1371/journal.pone.0085352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton MA, Rao VH, Meehan DT, Askew C, Cosgrove D. Matrix metalloproteinase dysregulation in the stria vascularis of mice with Alport syndrome: implications for capillary basement membrane pathology. Am. J. Pathol. 2005;166:1465–1474. doi: 10.1016/S0002-9440(10)62363-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton MA, Schmiedt RA, Schulte BA. Age-related decreases in endocochlear potential are associated with vascular abnormalities in the stria vascularis. Hear. Res. 1996;102:181–190. doi: 10.1016/s0378-5955(96)90017-9. [DOI] [PubMed] [Google Scholar]
- Gross O, Beirowski B, Harvey SJ, McFadden C, Chen D, Tam S, Thorner PS, Smyth N, Addicks K, Bloch W, Ninomiya Y, Sado Y, Weber M, Vogel WF. DDR1-deficient mice show localized subepithelial GBM thickening with focal loss of slit diaphragms and proteinuria. Kidney Int. 2004;66:102–111. doi: 10.1111/j.1523-1755.2004.00712.x. [DOI] [PubMed] [Google Scholar]
- Gunwar S, Ballester F, Noelken ME, Sado Y, Ninomiya Y, Hudson BG. Glomerular basement membranse. Identification of a novel disulfide-cross-linked network of alpha3, Alpha4, and alpha5 chains of type IV collagen and its implications for the pathogenesis of Alport syndrome. J. Biol. Chem. 1998;273:8767–8775. doi: 10.1074/jbc.273.15.8767. [DOI] [PubMed] [Google Scholar]
- Hur DG, Lee JH, Oh SH, Kim YH, Lee JH, Shin DH, Chang SO, Kim CS. KCNQ1/KCNE1 K+ channel and P2Y4 receptor are co-expressed from the time of birth in the apical membrane of rat strial marginal cells. Acta Otolaryngol. Suppl. 2007;558:30–35. doi: 10.1080/03655230701624830. [DOI] [PubMed] [Google Scholar]
- Jin Z, Wei D, Järlebark L. Developmental expression and localization of KCNJ10 K+ channels in the guinea pig inner ear. Neuroreport. 2006;17:475–479. doi: 10.1097/01.wnr.0000208999.25234.91. [DOI] [PubMed] [Google Scholar]
- LeBleu V, Sund M, Sugimoto H, Birrane G, Kanasaki K, Finan E, Miller CA, Gattone VH, 2nd, McLaughlin H, Shield CF, 3rd, Kalluri R. Identification of the NC1 domain of α3 chain as critical for α3α4α5 type IV collagen network assembly. J. Biol. Chem. 2010;285:41874–41885. doi: 10.1074/jbc.M110.149534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmink HH, Mochizuki T, van den Heuvel LP, Schröder CH, Barrientos A, Mochizuki T, van Oost BA, Brunner HG, Reeders ST, Smeets HJ. Mutations in the type IV collagen alpha 3 (COL4A3) gene in autosomal recessive Alport syndrome. Hum. Mol. Genet. 1994;3:1269–1273. doi: 10.1093/hmg/3.8.1269. [DOI] [PubMed] [Google Scholar]
- Liu YP, Zhao HB. Cellular characterization of Connexin26 and Connexin30 expression in the cochlear lateral wall. Cell Tissue Res. 2008;333:395–403. doi: 10.1007/s00441-008-0641-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan DT, Delimont D, Cheung L, Zallocchi M, Sansom SC, Holzclaw JD, Rao V, Cosgrove D. Biomechanical strain causes maladaptive gene regulation, contributing factor in Alport glomerular disease. Kidney Int. 2009;76:968–976. doi: 10.1038/ki.2009.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant SN, Burgess BJ, Adams JC, Kashtan CE, Gregory MC, Santi PA, Colvin R, Collins B, Nadol JB., Jr. Temporal bone histopathology in Alport syndrome. Laryngoscope. 2004;114:1609–1618. doi: 10.1097/00005537-200409000-00020. [DOI] [PubMed] [Google Scholar]
- Meyer zum Gottesberge AM, Gross O, Becker-Lendzian U, Massing T, Vogel WF. Inner ear defects and hearing loss in mice lacking the collagen receptor DDR1. Lab. Invest. 2008;88:27–37. doi: 10.1038/labinvest.3700692. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Lemmink HH, Mariyama M, Antignac C, Gubler MC, Pirson Y, Verellen-Dumoulin C, Chan B, Schröder CH, Smeets HJ, Reeders ST. Identification of mutations in the alpha 3(IV) and alpha 4(IV) collagen genes in autosomal recessive Alport syndrome. Nat. Genet. 1994;8:77–81. doi: 10.1038/ng0994-77. [DOI] [PubMed] [Google Scholar]
- Moon IS, Bang MY, Shim DB, Shin SH, Choi JY. Severe to profound hearing loss in patients with progressed Alport's syndrome. Acta Otolaryngol. 2009;129:982–987. doi: 10.1080/00016480802545588. [DOI] [PubMed] [Google Scholar]
- Nelson EG, Hinojosa R. Presbycusis: a human temporal bone study of individuals with downward sloping audiometric patterns of hearing loss and review of the literature. Laryngoscope. 2006;116:1–12. doi: 10.1097/01.mlg.0000236089.44566.62. [DOI] [PubMed] [Google Scholar]
- Neng L, Zhang F, Kachelmeier A, Shi X. Endothelial cell, pericyte, and perivascular resident macrophage-type melanocyte interactions regulate cochlear intrastrial fluid-blood barrier permeability. J. Assoc. Res. Otolaryngol. 2013;14:175–185. doi: 10.1007/s10162-012-0365-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas MT, Barhanin J, Reyes R, Demêmes D. Cellular localization of TWIK-1, a two-pore-domain potassium channel in the rodent inner ear. Hear. Res. 2003;181:20–26. doi: 10.1016/s0378-5955(03)00162-x. [DOI] [PubMed] [Google Scholar]
- Rao VH, Meehan DT, Delimont D, Nakajima M, Wada T, Gratton MA, Cosgrove D. Role for macrophage metalloelastase in glomerular basement membrane damage associated with Alport syndrome. Am. J. Pathol. 2006;169:32–46. doi: 10.2353/ajpath.2006.050896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Pascual F, Busnadiego O, González-Santamaría J. The profibrotic role of endothelin-1: is the door still open for the treatment of fibrotic diseases? Life Sci. 2014;118:156–164. doi: 10.1016/j.lfs.2013.12.024. [DOI] [PubMed] [Google Scholar]
- Rubel D, Frese J, Martin M, Leibnitz A, Girgert R, Miosge N, Eckes B, Müller GA, Gross O. Collagen receptors integrin α2β1 and discoidin domain receptor 1 regulate maturation of the glomerular basement membrane and loss of integrin α2β1 delays kidney fibrosis in COL4A3 knockout mice. Matrix Biol. 2014;34:13–21. doi: 10.1016/j.matbio.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Sakaguchi N, Spicer SS, Thomopoulos GN, Schulte BA. Increased laminin deposition in capillaries of the stria vascularis of quiet-aged gerbils. Hear Res. 1997;105:44–56. doi: 10.1016/s0378-5955(96)00180-3. 1997. [DOI] [PubMed] [Google Scholar]
- Shi X. Cochlear pericyte responses to acoustic trauma and the involvement of hypoxia-inducible factor-1alpha and vascular endothelial growth factor. Am. J. Pathol. 2009;174:1692–1704. doi: 10.2353/ajpath.2009.080739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi-Wen X, Denton CP, Dashwood MR, Holmes AM, Bou-Gharios G, Pearson JD, Black CM, Abraham DJ. Fibroblast matrix gene expression and connective tissue remodeling: role of endothelin-1. J. Invest. Dermatol. 2001;116:417425. doi: 10.1046/j.1523-1747.2001.01256.x. [DOI] [PubMed] [Google Scholar]
- Suleiman H, Zhang L, Roth R, Heuser JE, Miner JH, Shaw AS, Dani A. Nanoscale protein architecture of the kidney glomerular basement membrane. Elife. 2013;2:e01149. doi: 10.7554/eLife.01149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Oyamada M, Takamatsu T. Different regulation of connexin26 and ZO-1 in cochleas of developing rats and of guinea pigs with endolymphatic hydrops. J. Histochem. Cytochem. 2001;49:573–586. doi: 10.1177/002215540104900504. [DOI] [PubMed] [Google Scholar]
- Takumida M, Takumida H, Anniko M. Localization of sirtuins in the mouse inner ear. Acta Otolaryngol. 2014;134:331–338. doi: 10.3109/00016489.2013.861928. [DOI] [PubMed] [Google Scholar]
- Teixeira M, Viengchareun S, Butlen D, Ferreira C, Cluzeaud F, Blot-Chabaud M, Lombès M, Ferrary E. Functional lsK/KvLQT1 potassium channel in a new corticosteroid-sensitive cell line derived from the inner ear. J. Biol. Chem. 2006;281:10496–10507. doi: 10.1074/jbc.M512254200. [DOI] [PubMed] [Google Scholar]
- Thomopoulos GN, Spicer SS, Gratton MA, Schulte BA. Age-related thickening of basement membrane in stria vascularis capillaries. Hear. Res. 1997;111:31–41. doi: 10.1016/s0378-5955(97)00080-4. [DOI] [PubMed] [Google Scholar]
- Tu TY, Chiu JH, Chang TJ, Yang AH, Lien CF. Expression of lsK protein mRNA in cultured rat strial marginal cells. Acta Otolaryngol. 1999;119:544–549. doi: 10.1080/00016489950180766. [DOI] [PubMed] [Google Scholar]
- Wang XH, Streeter M, Liu YP, Zhao HB. Identification and characterization of pannexin expression in the mammalian cochlea. J. Comp. Neurol. 2009;512:336–346. doi: 10.1002/cne.21898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidauer H, Arnold W. [Morphological changes in the inner ear of Alport's syndrome (author's transl)]. Laryngol. Rhinol. Otol. (Stuttg) 1976;55:6–16. [PubMed] [Google Scholar]
- Wyss HM, Henderson JM, Byfield FJ, Bruggeman LA, Ding Y, Huang C, Suh JH, Franke T, Mele E, Pollak MR, Miner JH, Janmey PA, Weitz DA, Miller RT. Biophysical properties of normal and diseased renal glomeruli. Am. J. Physiol. Cell Physiol. 2011;300:C397–C405. doi: 10.1152/ajpcell.00438.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SW, Howat SL, Renzoni EA, Holmes A, Pearson JD, Dashwood MR, Bou-Gharios G, Denton CP, du Bois RM, Black CM, Leask A, Abraham DJ. Endothelin-1 induces expression of matrix-associated genes in lung fibroblasts through MEK/ERK. J. Biol. Chem. 2004;279:23098–23103. doi: 10.1074/jbc.M311430200. [DOI] [PubMed] [Google Scholar]
- Zallocchi M, Johnson B, Meehan DT, Delimont D, Cosgrove D. α1β1 integrin/Rac1-dependent mesangial invasion of glomerular capillaries in Alport syndrome. Am. J. Pathol. 2013;183:1269–1280. doi: 10.1016/j.ajpath.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg M, Khurana M, Rao VH, Cosgrove D, Roughier JP, Werner MC, Shield CF, 3rd, Werb Z, Kalluri R. Stage-specific action of matrix metalloproteinases influences progressive hereditary kidney disease. PLoS Med. 2006;3:e100. doi: 10.1371/journal.pmed.0030100. [DOI] [PMC free article] [PubMed] [Google Scholar]