Abstract
CD4+ regulatory T cells (Tregs) are essential for normal immune surveillance, and their dysfunction can lead to the development of autoimmune diseases, such as type-1 diabetes (T1D). T1D is a T cell-mediated autoimmune disease characterized by islet β cell destruction, hypoinsulinemia, and severely altered glucose homeostasis. Tregs play a critical role in the development of T1D and participate in peripheral tolerance. Pluripotent stem cells (PSCs) can be utilized to obtain a renewable source of healthy Tregs to treat T1D as they have the ability to produce almost all cell types in the body, including Tregs. However, the right conditions for the development of antigen (Ag)-specific Tregs from PSCs (i.e., PSC-Tregs) remain undefined, especially molecular mechanisms that direct differentiation of such Tregs. Auto Ag-specific PSC-Tregs can be programmed to be tissue-associated and infiltrate to local inflamed tissue (e.g., islets) to suppress autoimmune responses after adoptive transfer, thereby avoiding potential overall immunosuppression from non-specific Tregs. Developing auto Ag-specific PSC-Tregs can reduce overall immunosuppression after adoptive transfer by accumulating inflamed islets, which drives forward the use of therapeutic PSC-Tregs for cell-based therapies in T1D.
Keywords: Stem cells, Regulatory T cells, Immunotherapy, Tolerance, T cells, Diabetes
Regulatory T cells (Tregs) are an integral component of the normal immune system and contribute to the maintenance of peripheral tolerance. Tregs can down-regulate immune responses and are essential for immune homeostasis. They can act as key effectors in preventing and treating type-1 diabetes (T1D) (1,2).
Hematopoietic stem cell (HSC)-derived hematopoietic progenitors migrate into the thymus and develop into different types of T cells. The transcription factors Aire (largely expressed in medullary thymic epithelial cells - mTECs) and FoxP3 have key functions in clonal deletion and Treg selection (3). There are links between Aire expression, FoxP3 up-regulation and Treg selection; Aire deficiency affects the negative selection of self-reactive T cells, and FoxP3 controls the development and function of the naturally occurring Tregs (nTregs) (4). Our laboratory has shown the development of stable Tregs from CD4+ T cells by over-expressing FoxP3 and bcl-xL (5).
Recent advances in the use of large-scale in vitro expansion of Tregs followed by in vivo re-infusion of these cells raises the possibility that this strategy may be successfully utilized for the treatment of T1D. Although polyclonally expanded populations of Tregs exhibit suppressive activity, Ag-specific Tregs are more efficient at suppressing local autoimmune disorders such as RA, type-1 diabetes (T1D), inflammatory bowel diseases (IBD), allergic reactions and graft-versus-host disease (GVHD) (6,7,8,9,10,11). In addition, tissue/organ-associated Treg targeting stabilizes FoxP3 expression and avoids induction of a potentially detrimental systemic immunosuppression (12,13). For Treg-based immunotherapy, in vitro generation of tissue/organ (e.g., islets)-associated and non-terminally differentiated effector Tregs for in vivo re-infusion is an optimal approach. However, current methodologies are limited in terms of the capacity to generate, isolate, and expand a sufficient quantity of such Tregs from patients for therapeutic interventions.
There are a number of challenges in Treg-based immunotherapy. First: Only low numbers of Tregs can be harvested from the peripheral blood mononuclear cells (PBMCs). CD4 and CD25 have been used to isolate Tregs for ex vivo expansion. CD4+CD25+ T cells are not homogenous and contain both Tregs and conventional effector T cells (Teffs). Current expansion protocols activate both Tregs and Teffs, and because it takes a longer time for Tregs to enter the S phase of cell cycle, Teffs outgrow Tregs (14). In addition, Tregs can lose suppressive activity after repetitive stimulation with α-CD3 plus α-CD28 Abs with or without rIL-2 in vitro. Second: No approach to date has demonstrated the capacity to isolate the entire Treg population with 100% specificity from patients (the current clinical approach). Even FoxP3 or more recently Eos, a transcriptional factor that is considered the gold standard for identification of Tregs, is expressed transiently in some activated non-regulatory human T cells (15), highlighting the difficulty in both identifying and isolating a pure Treg population. Adoptive transfer of non-regulatory Teffs with Tregs has a potential to worsen autoimmune diseases. Third: Gene transduction of CD4+ T cells from PBMCs with Ag-specific TCR (16) or chimeric Ag receptor (CAR) (17) and/or TCR with FoxP3 elicits the generation of suppressive T cell populations (7) and overcomes the hurdle of the limited numbers of Ag-specific T cells. However, the engineered Tregs express endogenous and exogenous polyclonal TCRs, which reduce their therapeutic potential (the current experimental approach). Also, TCR mispairing is a concern with regards to the safety of TCR gene-transferred Tregs for clinical use, because the formation of new heterodimers of TCR can induce immunopathology (18). Therefore, there is a need to improve this strategy and generate monoclonal Tregs. Fourth: The differentiation state of Tregs is inversely related to their capacity to proliferate and persist. The "right" Tregs resist terminal differentiation, maintain high replicative potential (e.g., expression of common γc, CD132), are less prone to apoptosis (e.g., low expression of PD-1), and have the ability to respond to homeostatic cytokines (19), which facilitates their survival. In addition, the "right" Tregs express high levels of molecules that facilitate their homing to lymph nodes (LNs), such as CD62L and CC-chemokine receptors (e.g., CCR4, CCR7), and maintain stability or plasticity under certain inflammatory conditions. Furthermore, after an effective immune response, the "right" Tregs persist and provide protective immunity. Fifth: Because there are too few cells, harvesting sufficient numbers of tissue-associated Tregs from PBMCs for TCR gene transduction can be problematic.
Taken together, strong arguments support the development of Treg-based therapies in autoimmune diabetes using engineered Tregs. While clinical trials show safety, feasibility, and potential therapeutic activity of Treg-based therapies using this approach, concerns about autoimmunity due to cross-reactivity with healthy tissues remains a major safety issue (20,21). In addition, genetically modified Tregs using current approaches are usually intermediate or later effector Tregs (22), which only have short-term persistence in vivo.
Stem cells have the ability to differentiate into Ag-specific Tregs which can be used for cell-based therapies. To date, pluripotent stem cells (PSCs) are the only source available to generate a high number of the "right" Tregs (11,23). Human iPSCs can be easily generated from patients' somatic cells by transduction of various transcription factors and exhibit characteristics identical to those of embryonic stem cells (ESCs) (24). Many genetic methods as well as protein-based approaches have been developed to produce iPSCs with potentially reduced risks, including that of immunogenicity and tumorigenicity (25). Because of the plasticity and the potential for an unlimited capacity for self-renewal, iPSCs have high potential for advancing the field of cell-based therapies.
Our laboratory was the first to show that the development of Ag-specific iPSC-CTLs or iPSC-Tregs can be used for cell-based therapies of cancers and autoimmune disorders (11,23,26,27); other groups reported similar results (28,29,30). We demonstrated that genetically modified iPSCs with Ag-specific T cell receptor (TCR) and the transcriptional factor FoxP3, followed by differentiation driven by Notch signaling can enable iPSCs to pass hematopoietic and T lineage differentiation checkpoints, resulting in the development of Ag-specific CD4+ Tregs. We have developed a novel system to generate stable auto Ag-specific iPSC-Tregs. Our ongoing studies will validate this system and provide new insights into the methodologies and mechanistic requirements for efficient development of inflamed tissue-associated iPSC-Tregs. Once such strategies become available, there is potential to facilitate the generation of tolerance for autoimmune diabetes. Thus, important advances towards Treg-based immunotherapy in autoimmune disorders are anticipated from the proposed studies.
Signaling mechanisms that direct differentiation of Ag-specific PSC-Tregs remain to be determined. PSCs are exposed to a number of signals responsible for their progression. Although the exact signals are not fully understood, part of the mechanism known to be critical for directing T-cell fate occurs via Notch signaling, an evolutionarily conserved signaling pathway that regulates cell fate decisions in a number of cell and tissue types. Ligand binding by members of the Jagged or Delta-like (DL) families results in the proteolytic cleavage and release of the intracellular fragment of the Notch heterodimer. Translocation to the nucleus then allows for its regulation of gene expression. Notch-1, specifically, is critical for the establishment of T-cell fate. The loss of function results in the blockade of T cell development and enhanced B cell production, while over-expression results in the blockade of B cell lymphopoiesis and leads to the generation of T cells (31). However, the intracellular signaling pathways by which Notch signaling regulates the differentiation of Ag-specific PSC-Tregs remain unknown. PSCs co-cultured on a monolayer of the bone marrow (BM) stromal cell line OP9 cells transfected with the Notch ligand DL1 or 4 exhibit the ability to differentiate into most hematopoietic lineages and T cells (28). Our current studies will determine critical regulations of Hes1 (32), Runx1 (33), survivin (34) and elongation factor-2 (eEF-2) kinase (35) by Notch signaling during the development of auto Ag-specific PSC-Tregs.
While auto Ag-specific iPSC-Tregs have likely therapeutic effects in Treg-based immunotherapy against autoimmunity, the effectiveness is restricted by the demand to develop a great number of cells by complicated and exclusive in vitro differentiation. Furthermore, the extensive period for performing the generation of iPSCs can constraint the practice in personalized treatments. As an alternative, we will implement Treg-based immunotherapy by using the TCR/FoxP3 gene-transduced iPSCs, which have the ability to develop auto Ag-specific iPSC-Tregs in vivo and subsequently control autoimmune diabetes. We will conduct diabetes induction before or after adoptive transfer of the gene-transduced iPSCs. We will then administrate Notch agonists or recombinant cytokines (e.g., rIL-7, rFlt3L) to improve in vivo development of auto Ag-specific iPSC-Tregs. To avoid the potential tumorigenicity of the gene-transduced cells, we will incorporate a suicide gene, the inducible caspase 9 (36), into the vector as this allows the removal of the transduced Tregs by the injection of a bioinert small-molecule dimerizing agent (AP1903) to "shut off" the system.
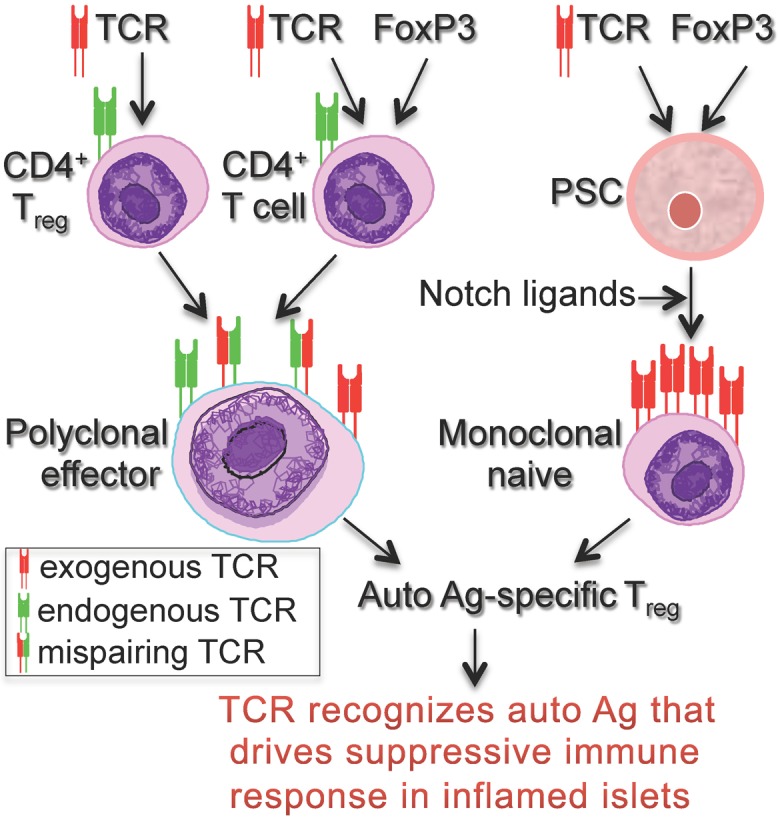
In summary, a current roadblock to progress in the field is the lack of an efficient system to generate the "right" auto Ag-specific Tregs that could be used for cell-based therapies in autoimmune diabetes (Fig. 1). We are using PSC-Tregs to address this limitation, allowing derivation of a large number of stable auto Ag-specific PSC-Tregs for cell-based therapies. Development of such an approach provides an important step toward personalized therapies for T1D.
Figure 1. Populations of auto Ag-specific Tregs that can be generated for cell-based therapies in T1D. Auto Ag specific Tregs can be generated by TCR transduction of CD4+ Tregs, TCR/FoxP3-gene transduction of CD4+ T cells, or TCR/FoxP3-gene transduction of PSCs, followed T-cell differentiation driven by Notch ligands.
ACKNOWLEDGEMENTS
This project is funded, in part, under grants with the National Institute of Health Grant R01AI121180, R21AI109239 and K18CA151798, American Diabetes Association 1-16-IBS-281 and the Pennsylvania Department of Health using Tobacco Settlement Funds.
Abbreviations
- Tregs
regulatory T cells
- T1D
type-1 diabetes
- IBD
inflammatory bowel diseases
- HSC
hematopoietic stem cell
- mTECs
medullary thymic epithelial cell
- nTregs
naturally occurring Tregs
- GVHD
graft-versus-host disease
- PBMCs
peripheral blood mononuclear cells
- Teffs
effector T cells
- TCR
T cell receptor
- CAR
chimeric Ag receptor
- LNs
lymph nodes
- PSCs
pluripotent stem cells
- ESCs
embryonic stem cells
- BM
bone marrow
- DL
Delta-like
- eEF-2
elongation factor-2
References
- 1.Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, Herold KC, Lares A, Lee MR, Li K, Liu W, Long SA, Masiello LM, Nguyen V, Putnam AL, Rieck M, Sayre PH, Tang Q. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med. 2015;7:315ra189. doi: 10.1126/scitranslmed.aad4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahne AE, Klementowicz JE, Chou A, Nguyen V, Tang Q. Therapeutic regulatory T cells subvert effector T cell function in inflamed islets to halt autoimmune diabetes. J Immunol. 2015;194:3147–3155. doi: 10.4049/jimmunol.1402739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hossain DM, Panda AK, Manna A, Mohanty S, Bhattacharjee P, Bhattacharyya S, Saha T, Chakraborty S, Kar RK, Das T, Chatterjee S, Sa G. FoxP3 acts as a cotranscription factor with STAT3 in tumor-induced regulatory T cells. Immunity. 2013;39:1057–1069. doi: 10.1016/j.immuni.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Aschenbrenner K, D'Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, Rolink A, Klein L. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol. 2007;8:351–358. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 5.Haque R, Lei F, Xiong X, Wu Y, Song J. FoxP3 and Bcl-xL cooperatively promote regulatory T cell persistence and prevention of arthritis development. Arthritis Res Ther. 2010;12:R66. doi: 10.1186/ar2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Herwijnen MJ, Wieten L, van der ZR, van Kooten PJ, Wagenaar-Hilbers JP, Hoek A, den BI, Anderton SM, Singh M, Meiring HD, van Els CA, van EW, Broere F. Regulatory T cells that recognize a ubiquitous stress-inducible self-antigen are long-lived suppressors of autoimmune arthritis. Proc Natl Acad Sci U S A. 2012;109:14134–14139. doi: 10.1073/pnas.1206803109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright GP, Notley CA, Xue SA, Bendle GM, Holler A, Schumacher TN, Ehrenstein MR, Stauss HJ. Adoptive therapy with redirected primary regulatory T cells results in antigen-specific suppression of arthritis. Proc Natl Acad Sci U S A. 2009;106:19078–19083. doi: 10.1073/pnas.0907396106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sela U, Olds P, Park A, Schlesinger SJ, Steinman RM. Dendritic cells induce antigen-specific regulatory T cells that prevent graft versus host disease and persist in mice. J Exp Med. 2011;208:2489–2496. doi: 10.1084/jem.20110466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bacher P, Kniemeyer O, Schonbrunn A, Sawitzki B, Assenmacher M, Rietschel E, Steinbach A, Cornely OA, Brakhage AA, Thiel A, Scheffold A. Antigen-specific expansion of human regulatory T cells as a major tolerance mechanism against mucosal fungi. Mucosal Immunol. 2014;7:916–928. doi: 10.1038/mi.2013.107. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen TL, Sullivan NL, Ebel M, Teague RM, DiPaolo RJ. Antigen-specific TGF-beta-induced regulatory T cells secrete chemokines, regulate T cell trafficking, and suppress ongoing autoimmunity. J Immunol. 2011;187:1745–1753. doi: 10.4049/jimmunol.1004112. [DOI] [PubMed] [Google Scholar]
- 11.Haque M, Song J, Fino K, Sandhu P, Song X, Lei F, Zheng S, Ni B, Fang D, Song J. Stem cell-derived tissue-associated regulatory T cells ameliorate the development of autoimmunity. Sci Rep. 2016;6:20588. doi: 10.1038/srep20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Belle TL, Ling E, Haase C, Bresson D, Urso B, von Herrath MG. NKG2D blockade facilitates diabetes prevention by antigen-specific Tregs in a virus-induced model of diabetes. J Autoimmun. 2013;40:66–73. doi: 10.1016/j.jaut.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Takiishi T, Korf H, Van Belle TL, Robert S, Grieco FA, Caluwaerts S, Galleri L, Spagnuolo I, Steidler L, Van HK, Demetter P, Wasserfall C, Atkinson MA, Dotta F, Rottiers P, Gysemans C, Mathieu C. Reversal of autoimmune diabetes by restoration of antigen-specific tolerance using genetically modified Lactococcus lactis in mice. J Clin Invest. 2012;122:1717–1725. doi: 10.1172/JCI60530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogtenhuber C, O'Shaughnessy MJ, Vignali DA, Blazar BR. Outgrowth of CD4low/negCD25+ T cells with suppressor function in CD4+CD25+ T cell cultures upon polyclonal stimulation ex vivo. J Immunol. 2008;181:8767–8775. doi: 10.4049/jimmunol.181.12.8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma MD, Huang L, Choi JH, Lee EJ, Wilson JM, Lemos H, Pan F, Blazar BR, Pardoll DM, Mellor AL, Shi H, Munn DH. An inherently bifunctional subset of Foxp3+ T helper cells is controlled by the transcription factor eos. Immunity. 2013;38:998–1012. doi: 10.1016/j.immuni.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perro M, Tsang J, Xue SA, Escors D, Cesco-Gaspere M, Pospori C, Gao L, Hart D, Collins M, Stauss H, Morris EC. Generation of multi-functional antigen-specific human T-cells by lentiviral TCR gene transfer. Gene Ther. 2010;17:721–732. doi: 10.1038/gt.2010.4. [DOI] [PubMed] [Google Scholar]
- 17.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bendle GM, Linnemann C, Hooijkaas AI, Bies L, de Witte MA, Jorritsma A, Kaiser AD, Pouw N, Debets R, Kieback E, Uckert W, Song JY, Haanen JB, Schumacher TN. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat Med. 2010;16:565–570. doi: 10.1038/nm.2128. [DOI] [PubMed] [Google Scholar]
- 19.Gratz IK, Truong HA, Yang SH, Maurano MM, Lee K, Abbas AK, Rosenblum MD. Cutting Edge: memory regulatory t cells require IL-7 and not IL-2 for their maintenance in peripheral tissues. J Immunol. 2013;190:4483–4487. doi: 10.4049/jimmunol.1300212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuball J, Dossett ML, Wolfl M, Ho WY, Voss RH, Fowler C, Greenberg PD. Facilitating matched pairing and expression of TCR chains introduced into human T cells. Blood. 2007;109:2331–2338. doi: 10.1182/blood-2006-05-023069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Loenen MM, de BR, Amir AL, Hagedoorn RS, Volbeda GL, Willemze R, van Rood J, Falkenburg JH, Heemskerk MH. Mixed T cell receptor dimers harbor potentially harmful neoreactivity. Proc Natl Acad Sci U S A. 2010;107:10972–10977. doi: 10.1073/pnas.1005802107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim YC, Zhang AH, Su Y, Rieder SA, Rossi RJ, Ettinger RA, Pratt KP, Shevach EM, Scott DW. Engineered antigen-specific human regulatory T cells: immunosuppression of FVIII-specific T- and B-cell responses. Blood. 2015;125:1107–1115. doi: 10.1182/blood-2014-04-566786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haque R, Lei F, Xiong X, Bian Y, Zhao B, Wu Y, Song J. Programming of regulatory T cells from pluripotent stem cells and prevention of autoimmunity. J Immunol. 2012;189:1228–1236. doi: 10.4049/jimmunol.1200633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JB, Sebastiano V, Wu G, rauzo-Bravo MJ, Sasse P, Gentile L, Ko K, Ruau D, Ehrich M, van den BD, Meyer J, Hubner K, Bernemann C, Ortmeier C, Zenke M, Fleischmann BK, Zaehres H, Scholer HR. Oct4-induced pluripotency in adult neural stem cells. Cell. 2009;136:411–419. doi: 10.1016/j.cell.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 25.Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–215. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- 26.Lei F, Zhao B, Haque R, Xiong X, Budgeon L, Christensen ND, Wu Y, Song J. In vivo programming of tumor antigen-specific T lymphocytes from pluripotent stem cells to promote cancer immunosurveillance. Cancer Res. 2011;71:4742–4747. doi: 10.1158/0008-5472.CAN-11-0359. [DOI] [PubMed] [Google Scholar]
- 27.Haque M, Song J, Fino K, Sandhu P, Wang Y, Ni B, Fang D, Song J. Melanoma immunotherapy in mice using genetically engineered pluripotent stem cells. Cell Transplant. 2016;25:811–827. doi: 10.3727/096368916X690467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Themeli M, Kloss CC, Ciriello G, Fedorov VD, Perna F, Gonen M, Sadelain M. Generation of tumor-targeted human T lymphocytes from induced pluripotent stem cells for cancer therapy. Nat Biotechnol. 2013;31:928–933. doi: 10.1038/nbt.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vizcardo R, Masuda K, Yamada D, Ikawa T, Shimizu K, Fujii S, Koseki H, Kawamoto H. Regeneration of human tumor antigen-specific T cells from iPSCs derived from mature CD8(+) T cells. Cell Stem Cell. 2013;12:31–36. doi: 10.1016/j.stem.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Saito H, Okita K, Chang K, Ito F. Adoptive transfer of CD8+ T cells generated from induced pluripotent stem cells triggers regressions of large tumors along with immunological memory. Cancer Res. 2016;76:3473–3483. doi: 10.1158/0008-5472.CAN-15-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dervovic DD, Liang HC, Cannons JL, Elford AR, Mohtashami M, Ohashi PS, Schwartzberg PL, Zuniga-Pflucker JC. Cellular and molecular requirements for the selection of in vitro-generated CD8 T cells reveal a role for Notch. J Immunol. 2013;191:1704–1715. doi: 10.4049/jimmunol.1300417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wendorff AA, Koch U, Wunderlich FT, Wirth S, Dubey C, Bruning JC, MacDonald HR, Radtke F. Hes1 is a critical but context-dependent mediator of canonical Notch signaling in lymphocyte development and transformation. Immunity. 2010;33:671–684. doi: 10.1016/j.immuni.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 33.Guo Y, Maillard I, Chakraborti S, Rothenberg EV, Speck NA. Core binding factors are necessary for natural killer cell development and cooperate with Notch signaling during T-cell specification. Blood. 2008;112:480–492. doi: 10.1182/blood-2007-10-120261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lei F, Song J, Haque R, Xiong X, Fang D, Wu Y, Lens SM, Croft M, Song J. Transgenic expression of survivin compensates for OX40-deficiency in driving Th2 development and allergic inflammation. Eur J Immunol. 2013;43:1914–1924. doi: 10.1002/eji.201243081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng Y, Ren X, Yuan Y, Shan Y, Li L, Chen X, Zhang L, Takahashi Y, Yang JW, Han B, Liao J, Li Y, Harvey H, Ryazanov A, Robertson GP, Wan G, Liu D, Chen AF, Tao Y, Yang JM. eEF-2 kinase is a critical regulator of Warburg effect through controlling PP2A-A synthesis. Oncogene. 2016 doi: 10.1038/onc.2016.166. [DOI] [PubMed] [Google Scholar]
- 36.Di SA, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser Y, Martinez C, Straathof K, Liu E, Durett AG, Grilley B, Liu H, Cruz CR, Savoldo B, Gee AP, Schindler J, Krance RA, Heslop HE, Spencer DM, Rooney CM, Brenner MK. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365:1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]


