Abstract
Background and Purpose. Skin wound healing is a dynamic process driven by molecular events responsible for the morphofunctional repair of the injured tissue. In a systematic review, we analyzed the relevance of plant fractions and isolates on skin wound healing. By revising preclinical investigations with murine models, we investigated if the current evidence could support clinical trials. Methods. Studies were selected in the MEDLINE/PubMed and Scopus databases according to the PRISMA statement. All 32 identified studies were submitted to data extraction and the methodological bias was investigated according to ARRIVE strategy. Results. The studies demonstrated that plant fractions and isolates are able to modulate the inflammatory process during skin wound healing, being also effective in attenuating the oxidative tissue damage in the scar tissue and stimulating cell proliferation, neoangiogenesis, collagen synthesis, granulation tissue expansion, reepithelialization, and the wound closure rate. However, we identified serious methodological flaws in all studies, such as the high level of reporting bias and absence of standardized experimental designs, analytical methods, and outcome measures. Conclusion. Considering these limitations, the current evidence generated from flawed methodological animal studies makes it difficult to determine the relevance of herbal medicines to treat skin wounds and derails conducting clinical studies.
1. Introduction
The skin wound healing is a dynamic and complex process divided into three complementary stages: inflammatory, proliferative, and maturation. The inflammatory phase comprehends the intense leucocytes recruitment to the wound area, removal of cellular and extracellular matrix debris, and syntheses of regulatory molecules such as cytokines and chemokines [1, 2]. The proliferative phase progresses with an intense proliferation and migration of fibroblasts, endothelial cells, and keratinocytes as well as formation of the granulation tissue (rich in type III collagen) and progressive reepithelialization [1–3]. At the maturation phase, type III collagen is gradually replaced by type I collagen, which originates more thicker and resistant collagen fibers [2–4].
It has been demonstrated that flaws on the leukocyte recruitment and function can impair the healing process due to reductions in the synthesis of regulatory molecules that drives the extracellular matrix assembly [5–7] and neoangiogenesis [8]. In this way, the development of drugs and alternative treatments that favor the migration and cellular activity during the inflammatory and proliferation phases may enhance the skin wound repair.
Skin wounds represent a serious health problem worldwide frequently associated with high costs and inefficient treatments [9, 10]. The use of herbal drugs is opening a new perspective for the treatment of skin wounds, mainly in developing countries. Once herbal strategies represent a simple pharmacological option, 80% of the population uses herbal drugs in their health care [1]. Although several plant species are currently used in the popular medicine to treat skin wounds worldwide [11–14], the scientific evidence that supports this practice is scarce. Thus, determining the security and efficiency of herbal drugs is an urgent and challenging task, which is essential to develop new technologies and products potentially applied in wound care.
In general, the healing properties of plant products are related to specific secondary metabolites, especially tannins, saponins, flavonoids, and alkaloids [11, 47, 48]. Plant products present a broad spectrum of biological functions such as astringent, antimicrobial, antioxidant, and anti-inflammatory [49–54] functions, which has been systematically associated with the beneficial effects in stimulating the healing process [49, 52, 54]. Before extrapolation to the human condition, preclinical researches using animal models have been useful for testing the toxicological security and biological effects of plant fractions and isolated molecules with potential applicability in the treatment of skin wounds [11, 52].
Despite the increasing number of experimental trials in the last decade, few advances were observed in the treatment of skin wounds, especially in humans. Considering that studies using animal models are conceived to support clinical investigations, there is a clear limitation in translating the findings obtained from animal models of wound healing to the human context. Considering that herbal drugs are extensively used in the popular medicine, we still do not know where the gap is that hinders the implementation of experimental findings for the development of innovations and technologies potentially useful in the clinical management of skin wounds. Thus, we systematically revised preclinical studies with murine models that investigated the effects of plant fractions and isolated molecules on the treatment of skin wounds. Beyond determining the relevance of plant derivatives in the skin repair, we analyzed the methodological quality of all preclinical studies identified, especially considering that the quality of evidence generated from flawed methodological studies could compromise the generalizability of the findings and derail conducting clinical studies.
2. Materials and Methods
2.1. Search Strategy
Research papers that investigate the action of plant fractions and isolated molecules in murine models of skin wound healing, published until 09/04/2015 (15:05:23), were recovered and independently analyzed by three researchers (FBL, MMS, and RVG). The search strategy was constructed by four components: “animals (filter),” “injury (wounds),” “organ (skin),” and “plants extract (isolates and fractions).” The filters were developed from PubMed database according to the hierarchical distribution of Medical Subject Headings [MeSH Terms]. A standardized search filter for animal studies was applied in PubMed database [55]. The same search strategy was adapted and used to recover studies in the Scopus platform. The standard animal filter provided by Scopus was used. The complete search strategy is described in Table S1 in Supplementary Material available online at http://dx.doi.org/10.1155/2016/4916068. Language restrictions were applied to recover only articles in English, Spanish, and Portuguese.
2.2. Selection Strategy
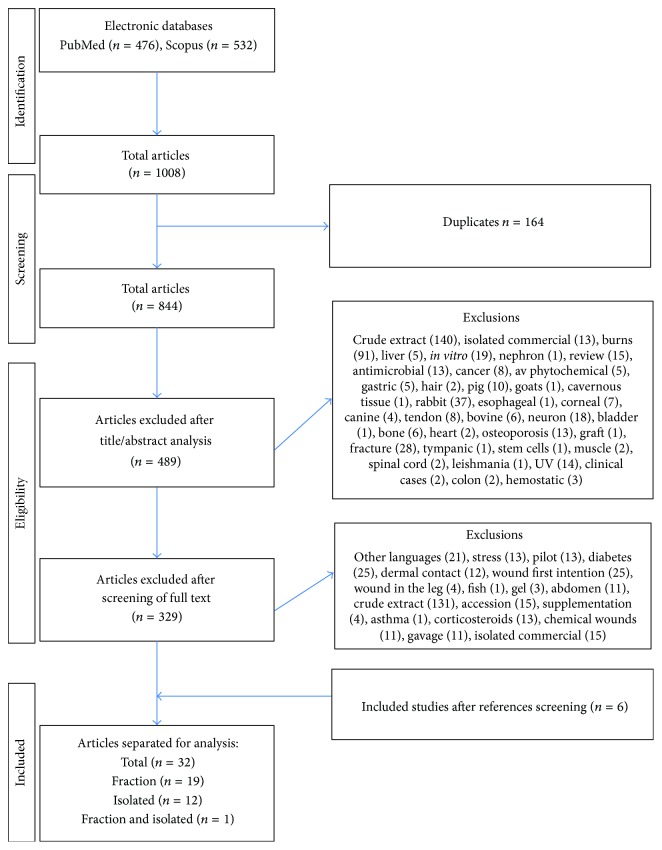
An initial selection based on title and abstract [TIAB] was independently conducted by the researchers (FBL, MMS, and RDN). Duplicate studies were removed and only studies investigating the effect of fractions and isolated molecules from plant extracts in murine models of skin wound healing were considered. After the initial search, all relevant studies were recovered in full text and evaluated by eligibility criteria. Works containing unrefined extracts, commercial isolates, in vitro assays, humans, nontraumatic injuries, other animal models, first intention wounds, metabolic diseases associates, and secondary studies (i.e., letter to the editor, note, review, and editorial) were excluded (Figure 1).
Figure 1.
Flowchart of the strategy applied to recover preclinical studies according to the PRISMA statement.
2.3. Data Extraction
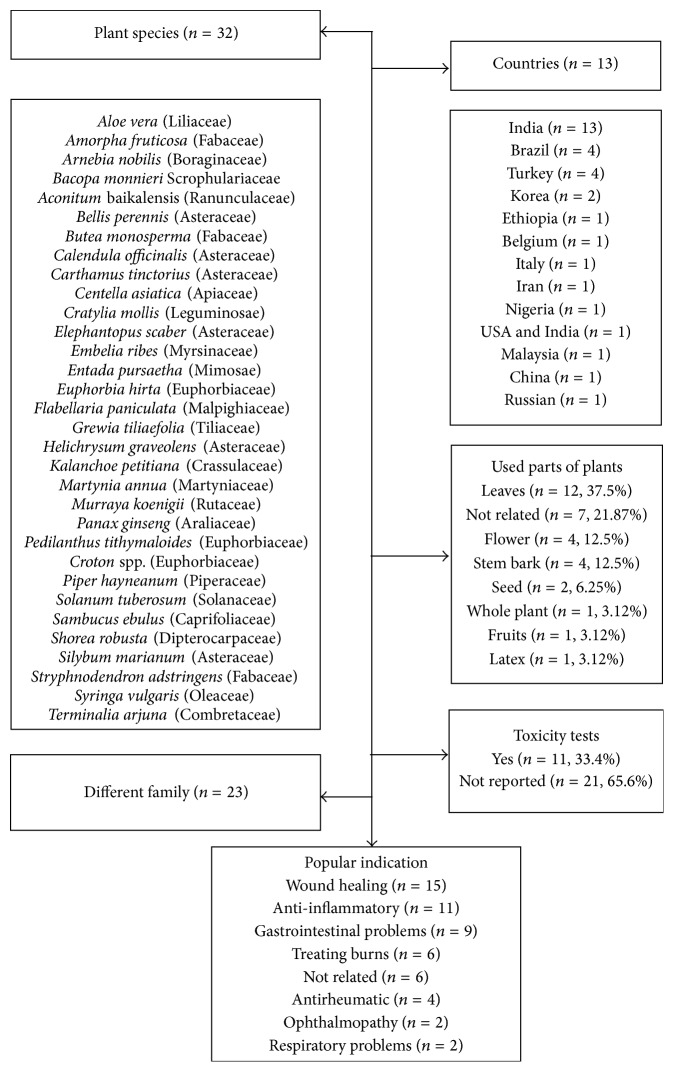
Data were extracted and tabulated in a descriptive way (Tables 1(a), 1(b), 2(a), and 2(b)). The characteristics investigated were publication characteristics (author, title, publication year, and country); research methods (control group, randomization, experimental procedures, and blind evaluation of the results); experimental model (animal, number of animals, sex, age, weight, species, acclimation period, animal's housing, number of animals per cage and experimental groups, food supply, temperature, and light cycle); plants (plant's species, isolates, fractions, dose, toxicity test, exotic/native plant, popular name, utilized part of the plant, and popular indication); wounds description (wound area, measurement interval, and treatment duration) (Tables 1(a), 1(b), 2(a), and 2(b)). In a comprehensive approach, ethnobotanical/ethnopharmacological aspects were also investigated as follows: plant's species investigated (geographic distribution and existence or not of bioprospecting), popular indication, and reports of toxicity tests (Figure 2).
Table 1.
(a) Description of the main characteristics of the studies with fractions obtained from plant extracts. (b) Description of the main characteristics of the studies with isolated molecules from plant extracts.
(a).
| Reference | Country | Animal strain | Animal number | Sex | Age | Weight | Experimental groups | Animal number per group | Numbers of animals per box | Treatment control group | Plant species | Native/exotic | Used parts | Fractions | Dose | Popular indication | Wound area | Measurement interval | Wound area calculation | Treatment time |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bastos et al., 2011 [15] | Brazil | Wistar rats | 12 | ♂ | 4 mo | 300–320 g | 4 | 3 | 1 | Miconazole and nonionic cream | Piper hayneanum (Piperaceae) | ? | L | [CHCl3-EtOAc 1 : 1 (haulm, A) and CHCl3-MeOH 1 : 1 (routs, B) | ? | Anti-inflammatory, infectious skin diseases and healing, wounds, hematoma, and ecchymosis | 6 mm2 | Daily | ? | 15 days |
|
| ||||||||||||||||||||
| Shukla et al., 1999 [16] | India | Sprague Dawley rats |
? | ♂ | ? | 200–220 g | 3 | ? | 1 | Saline solution | Centella asiatica (Apiaceae) | ? | ? | Asiaticoside | 20 μL of 0.2% | Healing activity | 8 mm2 | 7/7 days | ? | 14 days |
|
| ||||||||||||||||||||
| Muralidhar et al., 2011 [17] | India | Wistar rats |
42 | ? | ? | 150–200 g | 7 | 6 | ? | Ointment base | Butea monosperma (Fabaceae) | N | Sb | PETFR: petroleum ether fraction BENFR: benzene fraction, CHLFR: chloroform fraction, and ACEFR: acetone fraction |
200 mg/kg- | Antitumor, antiulcer, antifungal, and antidiarrheal activities | 500 mm2 | 4/4 days | ? | 16 days |
|
| ||||||||||||||||||||
| Süntar et al., 2013 [18] | Turkey | Sprague Dawley rats |
? | ♂ | ? | 160–180 g | ? | 6 | ? | Ointment base | Helichrysum graveolens (Asteraceae) | N | F | Hg-hexane; Hg-CH2Cl2; Hg-EtOAc, Hg-BuOH; Hg-R-H2O; Hg-Fr.B1; Hg-Fr.B2, Hg-Fr.B3, Hg-Fr.A, Hg-Fr.B; and Hg-Fr.C | 0.5 g | Antimicrobial, antioxidant, anti-inflammatory, sedative, antidiabetic, and cytotoxic activities | 5 mm2 | Daily | Reduction in wounded area, using AutoCAD program | 12 days |
|
| ||||||||||||||||||||
| Mekonnen, et al., 2013 [19] | Ethiopia | Swiss mice/Wistar rats |
24 | ? | 8–10 wk/3–5 mo | 30–40 g/180–200 g | 4 | 6 | 1 | Sodium carboxyl methyl cellulose xerogel and nitrofurazone | Kalanchoe petitiana (Crassulaceae) | N | L | Methanolic and chloroform fractions were 16%, 8.76%, 7.5%, and 5.6%, respectively | ? | Wound healing, hemorrhoids, and antibacterial activities | 312 mm2 | Daily | % wound contraction = wound area on day 0 − wound area on day n/wound area on day 0 × 100 | 10 days |
|
| ||||||||||||||||||||
| Pieters et al., 1995 [20] | Belgium | Wistar rats |
? | ♀ | ? | 250–300 g | 20 | 2 | 1 | Not treated | Croton spp. (Euphorbiaceae) | E | Lx | Polyphenolic: PEG ointment, PEG 400 10% | 0,5 mL/2x day | Wound healing | 3 cm | Daily | ? | 18 days |
|
| ||||||||||||||||||||
| Korkina et al., 2007 [21] | Italy | Wistar rats |
40 | ♂ | ? | 350–400 g | 5 | 10 | ? | Saline solution | Syringa vulgaris (Oleaceae) | N | F | Two phenylpropanoid glycosides: verbascoside and teupolioside | 100 μL (0.2 mg/mL) | Wound healing, anti-inflammatory, antirheumatic, antipyretic, and antifungal activities | 2.25 cm2 | 4/4 days | The recorded wounds were measured by planimetry using special computer program | 8 days |
|
| ||||||||||||||||||||
| Bigoniya et al., 2013 [22] | India | Wistar rats |
30 | ♂ | ? | 175 ± 10 g | 5 | 6 | ? | Vehicle (not related) | Euphorbia hirta (Euphorbiaceae) | N | Wp | Flavonoid fraction (EHTF) | ? | Antimicrobial, antifungal, antiviral, anti-inflammatory, antiarthritic, and antioxidant activities | 500 mm2 | 4/4 days | ? | 20 days |
|
| ||||||||||||||||||||
| Lodhi et al., 2011 [23] | India | Wistar rats |
30 | ♂♀ | ? | 150–200 g | 5 | 6 | 1 | Not treated | Martynia annua (Martyniaceae) | N | L | M. annua fraction: MAF-A, MAF-B, and MAF-C | ? | ? | 500 mm2 | 2/2 days | % Wound contraction = healed area/total wound area × 100 | 20 days |
|
| ||||||||||||||||||||
| Tabandeh et al., 2013 [24] | Iran | Wistar rats |
60 | ♂ | ? | 200 ± 50 g | 4 | 15 | ? | Saline solution | Silybum marianum (Asteraceae) | E | ? | Flavonoid silibinin (SB) | 10% and 20% SB powder | Hepatoprotective and liver regenerating activities | 1 cm | Daily | ? | 30 days |
|
| ||||||||||||||||||||
| Sonmez et al., 2015 [25] | Turkey | Wistar rats |
24 | ♂ | ? | 180–260 g | 3 | 8 | ? | Saline solution | Solanum tuberosum (Solanaceae) | ? | ? | Polysaccharide hemostat (APH) | 3 mg of wheat meal in group 2 and 3 mg of APH in powder form | ? | 2 × 2 × 2 cm | 3, 7, and 14 days | Percentage of contraction = [100 − (total wound area on the 14th day/total wound area on the 3rd day) × 100] | 14 days |
|
| ||||||||||||||||||||
| Karakaş et al., 2012 [26] | Turkey | Wistar rats |
12 | ♂ | ? | 200–250 g | 2 | 12 and 8 | ? | Not treated | Bellis perennis (Asteraceae) | E | F | n-Butanol fraction | ? | Activities in sore throat, headache, eczema, skin boils, and gastritis | 4 mm2 | 1, 5, 10, and 30 days | Percentage of wound area = wound area in day/wound area in the first day × 100; percentage of wound healing = 100 − percentage of wound area | 30 days |
|
| ||||||||||||||||||||
| Choi et al., 2001 [27] | Korea | Hairless mice | 10 | ♂ | ? | ? | 2 | 10 | ? | Vehicle (not related) | Aloe vera (Liliaceae) | ? | ? | Glycoprotein fraction named G1G1M1DI2 | 10 mg/g ointment Gentamicin 0.1%, every day |
Wound healing, thermal injury healing, anti-inflammation, and immunomodulation activities | 154 mm2 | Daily | ? | 8 days |
|
| ||||||||||||||||||||
| Parente et al., 2011 [28] | Brazil | Wistar rats |
36 | ♀ | 60 days | 160–190 g | 2 | 18 and 6 | 1 | Distilled water | Calendula officinalis (Asteraceae) | E | F | DCF: dichloromethane fraction at 1%; HCF: hexane fraction at 1% | ? | Anti-inflammatory, first-degree burns, and skin rashes activities | 1 cm | 4, 7, and 14 days | ? | 14 days |
|
| ||||||||||||||||||||
| Olugbuyiro et al., 2010 [29] | Nigeria | Wistar rats | 16 | ♂ | ? | 250–300 g | 2 | 4 | 1 | Gentamicin and saline solution | Flabellaria paniculata (Malpighiaceae) | N | L | Chloroform fraction and aqueous fraction | 100 mg/mL | Activities in skin infections, wounds and sores, and dysentery | 2 × 2 cm | 7, 12, 14, and 18 days | ? | 18 days |
|
| ||||||||||||||||||||
| Süntar et al., 2010 [30] | Turkey | Sprague-Dawley rats/Swiss mice | ? | ♂ | ? | 160–180 g/20–25 g | 9 | 6 | ? | Not treated | Sambucus ebulus (Caprifoliaceae) | N | L | Polyamide column fractions from the methanolic extract (Fr A, B, C, D, and E) | 0,5 g | Hemorrhoids, rheumatic pain, treating burns, infectious wounds, edema, eczema, urticarial, and inflammations | 5 mm2 | 2/2 days | Wound contraction was calculated as percentage of the reduction in wounded area | 12 days |
|
| ||||||||||||||||||||
| Kim et al., 2013 [31] | Korea | Hairless mice | 10 | ♀ | 2 mo | ? | 2 | 5 | ? | Matrigel solution | Panax ginseng (Araliaceae) | ? | L | Ginsenoside Rd | 10 mL | Strengthening immune system and atherosclerosis activities | ? | 3/3 days | ? | 9 days |
|
| ||||||||||||||||||||
| Chaudhari et al., 2006 [32] | India | Wistar rats |
30 | ♂♀ | ? | 180–250 g | 5 | 6 | ? | Soft paraffin (85%), cetostearyl alcohol (5%), hard paraffin (5%), and wool fat (5%) | Terminalia arjuna (Combretaceae) | N | Sb | Fraction I hydroalcohol Fraction II phytoconstituents extraction of tannins Fraction III consisted of saponins | 0.5 g | Diuretic, cooling, aphrodisiac, expectorant, antidysenteric, urinary astringent, antioxidant, and antibacterial activities | 4 cm2 | 2/2 days | ? | 16 days |
|
| ||||||||||||||||||||
| Swamy et al., 2006 [33] | India | Wistar rats |
24 | ♂♀ | ? | 150–200 g | 4 | 6 | ? | Framycetin ointment | Embelia ribes (Myrsinaceae) | ? | L | Embelin | 4 mg/mL of 0.2% sodium alginate gel | Anti-inflammatory to relieve rheumatism and fever activities | 500 mm2 | 4/4 days | ? | 16 days |
|
| ||||||||||||||||||||
| Hernandes et al., 2010 [34] | Brazil | Wistar rats |
15 | ♂ | ? | 180–200 g | 3 | 5 | 1 | Ointment base | Stryphnodendron adstringens (Fabaceae) | ? | Sb | EtOAc fraction | ? | Antioxidant, cicatrizant, and anti-inflammatory activities | 7 mm2 | 4, 7, and 10 days | ? | 10 days |
|
| ||||||||||||||||||||
| Sidhu et al., 1999 [35] | USA and India | Sprague Dawley rats | ? | ♂ | ? | 250–300 g | 4 | ? | 1 | Vehicle PBS | Arnebia nobilis (Boraginaceae) | N | ? | Arnebin-1 (5,8-dihydroxy-2-(19-b,b-dimethylaryoxy-49- methylpent-3-enyl)-1,4-naphthoquinone | ? | ? | 8 mm2 | Daily | ? | 11 days |
|
| ||||||||||||||||||||
| Paramesha et al., 2015 [36] | India | Wistar rats |
18 | ? | ? | 150–200 g | 3 | 6 | ? | Sodium alginate | Carthamus tinctorius (Asteraceae) | N | L | Dehydroabietylamine of C. tinctorius L., var. Annigeri-2 | 50 g to get 0.2% (w/w) ointment gel | Laxative, appetizer, and diuretic also useful in urorrhea and ophthalmopathy activities | ? | 4/4 days | ? | 16 days |
|
| ||||||||||||||||||||
| Nagappan et al., 2012 [37] | Malaysia | Sprague Dawley rats | 84 | ♀ | ? | 200–250 g | 7 | 12 | 1 | Not treated | Murraya koenigii (Rutaceae) | N | L | Carbazole alkaloids mahanine (1) (0.40%) (C23H25NO2), mahanimbicine (2) (0.24%) (C23H25NO), and mahanimbine (3) (0.66%) | Mahanine (1) (0.40%), (2) (0.24%), and (3) (0.66%) (w/w) | Stimulants, tonics, treating influenza, fever, and bronchial asthma activities | 8 mm2 | Daily | % of wound contraction = Ø of wound area − Ø of unhealed w.a./diameter of w.a. (wound area) × 100% | 18 days |
|
| ||||||||||||||||||||
| Qu et al., 2013 [38] | China | Sprague Dawley rats | 54 | ♂ | ? | 200–220 g | 9 | 6 | 1 | Vaseline | Amorpha fruticosa (Fabaceae) | N | Fr | 6a,12a–dehydroamorphin, D–3–O–methyl–chiro–inositol, Kaempferol-3-gluco- 7-rhamnoside,7,2′,4′,5′-tetrom–ethoxyoflavone, dehydrosermundone, tephrosin, 7,4′-dimethoxyisoflavone | ? | ? | 500 mm2 | 2/2 days | Percent wound contraction = (original wound area − unhealed area)/original wound area × 100% | 22 days |
(b).
| References | Country | Animal strain | Animal number | Sex | Age | Weight | Experimental groups | Animal number per group | Numbers of animals per box | Treatment control group | Plant species | Native/exotic | Used parts | Isolated | Dose | Popular indication | Wound area | Measurement interval | Wound area calculation | Treatment time |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ghosh et al., 2012 [39] | India | Wistar rats/Swiss albino mice | 36 | ♂ | ? | 150–180 g/20–25 g | 6 | 6 | ? | Ointment base | Pedilanthus tithymaloides (Euphorbiaceae) | N | L | 2-(3,4-Dihydroxy-phenyl)-5,7-dihydroxy-chromen-4-one, 1, 2-tetradecanediol, and 1-(hydrogen sulfate) | 50 mg | Antiviral, antibacterial, antihemorrhagic, antitumor, abortive, and anti-inflammatory activities | 500 mm2 | 3/3 days | (%) wound contraction 1/4 (initial × final wound area) × 100 | 21 days |
|
| ||||||||||||||||||||
| Mukherjee et al., 2013 [40] | India | Swiss albino mice/Wistar rats | ? | ? | ? | 18–20 g/150–180 g | 10 | ? | ? | Ointment base and povidone iodine | Shorea robusta (Dipterocarpaceae) | N | L | Compound I: bioactive bergenin and compound II: triterpene ursolic acid |
0.025 g of isolated compounds 1 and 2 mixed with 10 g ointment base | Wounds and burn healing | 6 cm | 3/3 days | Two-day interval/(wound area on day 0 × wound area on day n)/wound area on day 0 × 100 | ? |
|
| ||||||||||||||||||||
| Melo et al., 2011 [41] | Brazil | Swiss mice | 30 | ♀ | 12 wk | 45.0 ± 2.0 g | 4 | ? | 1 | NaCl | Cratylia mollis (Leguminoceae) | N | S | Cramoll 1,4 lectin |
100 g/mL | ? | 0.5 cm | 2, 7, and 12 days | A = π × R × r | 12 days |
|
| ||||||||||||||||||||
| Pieters et al., 1995 [20] | Belgium | Wistar rats | 40 | ♀ | ? | 250–300 g | 20 | 2 | 1 | Not treated | Croton spp. (Euphorbiaceae) | E | Lx | 3′,4-0-Dimethylcedrusin, taspine hydrochloride | 0,5 mL/2x day | Wound healing activities | 3 cm | Daily | ? | 18 days |
|
| ||||||||||||||||||||
| Ahamed et al., 2008 [42] | India | Wistar rats |
24 | ♂♀ | ? | 240–250 g | 4 | 6 | 6 | Tween 80 | Grewia tiliaefolia (Tiliaceae) | N | Sb | Gulonic acid γ-lactone (GAGL) | 50 mg/1x day | Burns, skin diseases, inflammation, diarrhea and pruritus, chronic wounds, and gastric ulcers | ? | 4/4 days | ? | 16 days |
|
| ||||||||||||||||||||
| Zyuz'kov et al., 2012 [43] | Russian | ?/mice | 136 | ♂ | 2 mo | 22–24 g | 6 | ? | ? | Water | Aconitum baikalensis (Ranunculaceae) | ? | ? | Songorine, napelline, hypaconitine, 12-epinapelline N-oxide, and mesaconitine | 30 mL | ? | 10 × 10 mm | Daily | ? | 16 days |
|
| ||||||||||||||||||||
| Singh et al., 2005 [44] | India | Wistar rats |
20 | ♂♀ | ? | 150–200 g | 5 | 6 | ? | Tragacanth | Elephantopus scaber (Asteraceae) | N | L | Deoxyelephantopin | 50 mg | Dysuria, diarrhea, dysentery, stomach pain; eczema and ulcers, and wound healing | 500 mm2 | 4/4 days | ? | 14 days |
|
| ||||||||||||||||||||
| Sharath et al., 2010 [45] | India | Wistar rats |
? | ♂♀ | ? | 200–250 g | 2 | 5 | ? | Nitrofurazone | Bacopa monnieri (Scrophulariaceae) | N | ? | Bacoside-A | 200 mg | Laxative, ulcers, anemia, leucoderma, and scabies | 500 mm2 | 4/4 days | ? | 16 days |
|
| ||||||||||||||||||||
| Vidya et al., 2012 [46] | India | Wistar rats |
30 | ? | ? | 160–200 g | 4 | 6 | ? | Nitrofurazone | Entada pursaetha (Mimosaceae) | N | S | Entadamide, phaseoloidin, and entagenic acid | ? | Cancer, dropsy, eye diseases, wounds, snake bite, respiratory problems, and antibacterial | 500 mm2 | 4/4 days | ? | 16 days |
Ad: adults; wk: week; mo: month; d: days; ♂: male; ♀: female; N: native; E: exotic; L: leaves; F: flowers; Sb: stem bark; S: seed; Wp: whole plant; Fr: fruits; Lx: latex; ?: not related.
Table 2.
(a) Main parameters analyzed in the studies demonstrating the action of fractions from plants in the treatment of skin wounds in murine models. (b) Main parameters analyzed in the studies demonstrating the action of isolated molecules from plants in the treatment of skin wounds in murine models.
(a).
| Reference | Wound closure analysis | Reepithelialization analysis | Oxidative stress | Granulation tissue fill | Tensile strengths |
|---|---|---|---|---|---|
| Bastos et al., 2011 | ? | Fractions A and B: moderated 9 days Fractions A and B: 100% 15 days |
? | After 15 days in the treated rats, the wound healing process by stimulating different biological events such as network of fibrin, epithelialization, granulation tissue, neovascularization, and wound contraction | ? |
|
| |||||
| Shukla et al., 1999 | ? | ? |
Increased: Superoxide dismutase (35%), catalase (67%), and glutathione peroxidase (49%) Reduced: Glutathione (17%) |
? | ? |
|
| |||||
| Muralidhar et al., 2011 | Petroleum ether fraction: (86.83 ± 0.87%) 16 days Benzene fraction: (86.67 ± 0.67%) 16 days Chloroform fraction: (88.0 ± 0.57%) 16 days Acetone fraction: (96.0 ± 0.37%) 16 days Control: (85.17 ± 0.79%) 16 days |
Epithelialization in days
Petroleum ether fraction: 21.17 ± 0.48% Benzene fraction: 21.67 ± 0.42% Chloroform fraction: 21.83 ± 0.48% Acetone fraction: 16.67 ± 0.42% Control: 22.0 ± 0.37% |
? |
Hydroxyproline content (μg/mg)
Petroleum ether fraction: 21.57 ± 0.21 Benzene fraction: 20.96 ± 0.08 Chloroform fraction: 21.84 ± 0.08 Acetone fraction: 23.50 ± 0.17 Control: 21.48 ± 0.17 |
Petroleum ether fraction: 155.83 ± 2.26 g Benzene fraction: 151.0 ± 2.59 g Chloroform fraction: 163.33 ± 1.33 g Acetone fraction: 212.83 ± 2.02 g Control: 147.33 ± 1.23 g |
|
| |||||
| Süntar et al., 2013 |
Wound area (mm
2
) ± SEM (contraction%) in 12 days
Hg-MeOH: 0.96 ± 0.30 (65.71%) Hg-Hexane: 2.37 ± 0.11 (15.36%) Hg-CH2Cl2: 2.35 ± 0.29 (16.07%) Hg-EtOAc: 1.47 ± 0.32 (47.50%) Hg-BuOH: 1.74 ± 0.48 (37.86%) Hg-R-H2O: 2.63 ± 0.17 (6.07%) Hg-Fr.A: 2.20 ± 0.39 (20.29%) Hg-Fr.B: 1.65 ± 0.09 (40.22%) Hg-Fr.C: 1.83 ± 0.14 (33.69%) Control: 2.76 ± 0.30 (6.44%) |
Tissues treated with Hg-MeOH, Hg-EtOAc, and Hg-Fr.B demonstrated good wound recovery with faster reepithelialization compared to the other groups tested | ? |
Hydroxyproline content (μg/mg)
Hg-MeOH: 26.3 ± 1.0 Hg-Hexane: 18.5 ± 2.1 Hg-CH2Cl2: 19.7 ± 1.9 Hg-EtOAc: 31.2 ± 0.9 Hg-BuOH: 15.6 ± 1.8 Hg-R-H2O: 13.3 ± 1.8 Hg-Fr.A: 15.4 ± 1.2 Hg-Fr.B: 25.5 ± 1.2 Hg-Fr.C: 16.3 ± 1.9 Control: 8.9 ± 2.1 |
Hg-MeOH: 30.11% Hg-Hexane: 17.5% Hg-CH2Cl2: 15.2% Hg-EtOAc: 28.5% Hg-BuOH: 25.8% Hg-R-H2O: 11.6% Hg-Fr.A: 13.9% Hg-Fr.B: 25.2% Hg-Fr.C: 21.3% Control: 5.8% |
|
| |||||
| Mekonnen et al. 2013 |
Wound contraction in 12 days
Chloroform: xerogel: (77.517 ± 1.88), 5%: (79.91 ± 71.30), and 10%: (82.63 ± 1.74) Methanol: simple ointment: (86.21 ± 1.5), 5%: (90.86 ± 0.21), and 10%: (92.09 ± 2.00) Control: (96.63 ± 0.32) |
Epithelialization in days
Chloroform: xerogel: (17.83 ± 0.30), 5%: (17.16 ± 0.60), and 10%: (16.83 ± 0.65) Methanol: simple ointment: (17.33 ± 0.33), 5%: (15.66 ± 0.21), and 10%: (15.33 ± 0.66) Positive control: (14.00 ± 0.44) |
? |
Hydroxyproline content (μg/mg)
Chloroform: xerogel: (3.01 ± 0.46), 5%: (5.83 ± 0.79), and 10%: (7.08 ± 2.08) Methanol: simple ointment: (3.29 ± 0.66), 5%: (11.01 ± 0.53), and 10%: (15.33 ± 0.66) Control: (12.57 ± 2.59) |
Chloroform: xerogel: 190.83 ± 15.62 g (14.26%), 5%: 238.33 ± 22.86 g (24.89%), and 10%: 265.00 ± 33.04 g (38.86%) Methanol: simple ointment: 201.50 ± 10.05 g (20.65%), 5%: 322.00 ± 23.63 g (59.80%), and 10%: 336.83 ± 28.39 g (67.16%) Control: 402.33 ± 30.26 g |
|
| |||||
| Pieters et al., 1995 | PEG ointment: (70%) 15 days PEG 400 10%: (80%) 15 days Polyphenolic fraction from dragon's blood in H2O: (90%) 15 days Control: (60%) 15 days |
PEG ointment: ++ (15 days) PEG 400 10%: ++ (15 days) Polyphenolic fraction from dragon's blood in H2O: ++ (15 days) Control: + (15 days) |
? |
Crust presence
PEG ointment: after 4 days PEG 400 10%: after 5 days Polyphenolic fraction from dragon's blood in H2O: after 1 day Control: after 3 days |
? |
|
| |||||
| Korkina et al., 2007 | Both verbascoside 56% (46,29 ± 12,21%) 8 days Both verbascoside 97% (124,29 ± 31,23%) 8 days Teupolioside 70% (78,39 ± 21,75%) 8 days Teupolioside 97% (98,45 ± 24,26%) 8 days Control (150,16 ± 65,46%) 8 days |
? |
Lipid peroxidation
Both verbascoside 56% (7,4 ± 0,6%) Both verbascoside 97% (5,8 ± 0,4%) Teupolioside 70% (12,0 ± 0,7%) Teupolioside 97% (9,4 ± 0,6%) Control: (10,3 ± 1,0) Glutathione (GST) Both verbascoside 56% (3,0 ± 1,3%) Both verbascoside 97% (5,1 ± 1,3%) Teupolioside 70% (3,4 ± 1,3%) Teupolioside 97% (5,9 ± 1,2%) Control: (3,6 ± 1.3%) Superoxide dismutases Both verbascoside 56% (2,5 ± 0,1%) Both verbascoside 97% (2,2 ± 0,1%) Teupolioside 70% (3,1 ± 0,3%) Teupolioside 97% (1,0 ± 0,1%) Control: (4,5 ± 0,5%) |
? | ? |
|
| |||||
| Bigoniya et al., 2013 | EHTF 200 (71,01 ± 4,25%) 16 days EHTF 400 (69,98 ± 3,34%) 16 days EHTF 600 (6,02 ± 0,79%) 16 days Control (71,65 ± 3,21%) 16 days |
EHTF 200 (19,66 ± 2,85%) EHTF 400 (19,50 ± 2,63%) EHTF 600 (17,50 ± 1,56%) Control (21,50 ± 1,22%) |
Vehicle control: catalase (0,46 ± 0,02%); SOD (1,15 ± 0,12%), and total protein (2,60 ± 0,06%) EHTF 200: catalase (0,45 ± 0,03%), SOD (1,16 ± 0,06%), and total protein (2,69 ± 0,07%) EHTF 400: catalase (0,52 ± 0,09%), SOD (2,63 ± 0,15%), and total protein (3,34 ± 0,05%) EHTF 600: catalase (0,75 ± 0,19%), SOD (5,06 ± 0,09%), and total protein (4,02 ± 0,03%) |
Hydroxyproline content
EHTF 200 (15,89 ± 1,28%) EHTF 400 (17,89 ± 2,26%) EHTF 600 (24,14 ± 2,23%) Control (16,09 ± 1,35%) |
? |
|
| |||||
| Lodhi et al., 2011 | MAF A (100,00%) 20 days MAF B (100,00%) 20 days MAF C (100,00%) 18 days Control (90,37 ± 2,07%) 20 days |
MAF A and B (20 days) MAF C (18 days) Control (24 days) |
? |
Hydroxyproline content: MAF A (37,11 ± 1,25%) MAF B (32,86 ± 0,85%) MAF C (42,01 ± 0,82%) Control (21,74 ± 1,85%) Protein content MAF A (56,30 ± 0,55%) MAF B (52,50 ± 1,70%) MAF C (83,60 ± 0,72%) Control (47,30 ± 1,72%) |
MAF A (603,00 ± 12,01%) MAF B (635,00 ± 9,68%) MAF C (850,00 ± 11,89%) Control (423,00 ± 10,96%) |
|
| |||||
| Tabandeh et al., 2013 | Silibinin 10%: 100% (18 days) Silibinin 20%: 100% (22 days) Control: 100% (26 days) |
? | ? |
Content N-acetyl glucosamine and n-acetyl galactosamine: silibinin 10 and 20% ↑ compared with the control groups at days 10, 20, and 30 Hydroxyproline and collagen content: silibinin 10 and 20% ↑ compared with the control groups at days 10, 20, and 30 |
? |
|
| |||||
| Sonmez et al., 2015 | Absorbable polysaccharide haemostat (APH): (94.74 ± 0.02%) 14 days Control: 87.33 ± 0.02% 14 days |
? | ? |
Type 1 collagen
APH: 4.25 Control: 3.25 Fibroblast density APH: 2.87 Control: 1.75 |
? |
|
| |||||
| Karakaş et al., 2012 | HOT: (80%) 30 days HOTBp: (100%) 30 days Control: (80%) 30 days |
? | ? | HOT: ↑ fibroblastic and lymphocytes: 5 days HOTBp: ↑ fibroblastic and lymphocytes: 5 days Control: ↑ fibroblastic and lymphocytes: 5 days HOT: ↑ collagen fibrils: 10 days HOTBp: ↑ collagen fibrils: 10 days Control: ↑ collagen fibrils: 10 days |
? |
|
| |||||
| Choi et al., 2001 | G1G1M1DI2: (98,9%) 8 days Control: (69,5%) 8 days |
Epithelialization in 8 days
G1G1M1DI2: 98,9% Control: 69,5% |
? |
EGF receptor
G1G1M1DL2 0,5%: (113%) G1G1M1DL2 50%: (220 ± 8%) Control: 100% Fibronectin G1G1M1DL2 0,5%: (294 ± 34%) G1G1M1DL2 50%: (408 ± 80%) Control: 100% Fibronectin receptor G1G1M1DL2 0,5%: (159 ± 11%) G1G1M1DL2 50%: (220 ± 19%) Control: 100% |
? |
|
| |||||
| Parente et al., 2011 | ? | ? | ? | Number of blood vessels HCF 1 (0/4) DCF 2 (0/13) Control 2 (0/13) Days 4 and 7: presence of fibrin in both groups |
? |
|
| |||||
| Olugbuyiro et al., 2010 |
Flabellaria paniculata
Chloroform fraction: 0.0 (100%) 14 days Aqueous fraction: 25.0 ± 3.0% (71.4%) 14 days Control: 87.5 ± 7.5% |
Flabellaria paniculata on noninfected rat wounds
Chloroform fraction: (14.0 ± 0.0%) Aqueous fraction: (21.5 ± 0.5%) Control: (24.5 ± 0.5%) |
? | ? | ? |
|
| |||||
| Süntar et al., 2010 | mm2 (%) Fr.A: 1.60 ± 1.53 (44.4%) Fr.B: 1.59 ± 0.11 (44.8%) Fr.C: 0.99 ± 0.31 (65.6%) Fr.D: 0.77 ± 0.03 (73.3%) Fr.E: 1.98 ± 0.63 (31.3%) Control: 2.88 ± 0.72 (17.5%) |
? | ? | ? | mm2 (%) Fr.A: 21.52 ± 1.15 (13.9%) Fr.B: 24.97 ± 3.18 (32.3%) Fr.C: 25.63 ± 1.43 (35.8%) Fr.D: 26.61 ± 2.05 (40.9%) Fr.E: 22.95 ± 2.73 (21.6%) Control: 18.88 ± 2.67 (16.3%) |
|
| |||||
| Kim et al., 2013 | The ginsenoside Rd-treated wounds were significantly smaller than the wounds treated with control Matrigel on days 6 and 9 | ? | ? | Ginsenoside Rd ↑ proliferation and migration fibroblasts; ginsenoside Rd at 0.1–10 mM ↑ collagen type I protein and ↓ MMP-1 protein in fibroblasts | ? |
|
| |||||
| Chaudhari et al., 2006 | ? | Fraction I: 9 days Fraction II: 23 days Fraction III: 20 days |
? | Fraction I increase in hexosamine Fractions II and III did not reveal increase in the hexosamine content of granulation tissue |
Fraction I: 719.33 g ± 0.88 Fraction II: 572.33 g ± 2.46 Fraction III: 590.33 g ± 1.87 |
|
| |||||
| Swamy et al., 2006 | Embelin: (98.50% ± 1.64) 16 days Control: (85.33% ± 3.66) 16 days |
Epithelialization in days
Embelin: 18.17 ± 1.47 Control: 20.33 ± 2.66 |
? | Granulation tissue showed complete healing with more fibroblasts, collagen, and increased number of blood vessels | Embelin: 528.00 g ± 15.85 Control: 374.67 g ± 5564 |
|
| |||||
| Hernandes et al., 2010 | The 1% ethyl-acetate fraction from Stryphnodendron adstringens did not influence wound contraction | No difference in the length of newly formed epithelium was found between the treated and control wounds | ? | ? | ? |
(b).
| Reference | Wound closure analysis | Reepithelialization analysis | Oxidative stress | Granulation tissue fill | Tensile strengths |
|---|---|---|---|---|---|
| Sidhu et al., 1999 | Arnebin-1 reduced wound width wounds compared with control | Arnebin-1: 7 days Control: only epithelial migration over the dermis |
? | The organization of the granulation tissue was more advanced in arnebin-1-treated wounds with thick bundles of well-aligned collagen compared with controls | ? |
|
| |||||
| Paramesha et al., 2015 | Dehydroabietylamine: (97.78% ± 2.15) 16 days Control: (82.92% ± 1.83) 16 days |
Epithelialization in days
Dehydroabietylamine: 17.67 ± 2.62 Control: 23.17 ± 1.14 |
? |
Hydroxyproline content (µg/100 g)
Dehydroabietylamine: 2106,50 ± 2,62 Control: 1369,67 ± 10,54 |
Dehydroabietylamine: 425.67 g ± 10.03 Control: 277.00 g ± 9.39 |
|
| |||||
| Nagappan et al., 2012 | Mahanine and mahanimbicine: (88.5% ± 2.03 to 93% ± 2.04) 16 days Control: (82.7% ± 2.13) 16 days |
Mahanine and mahanimbicine: 18 days Control: 18 days |
? |
Collagen deposition
Mahanine and mahanimbicine: (65.63% ± 0.87 to 67.76% ± 0.85) 21 days and (81.56% ± 1.04 to 88.54% ± 1.34) 28 days Control: (61.84% ± 0.94) 21 days and (78.06% ± 1.22) 28 days |
? |
|
| |||||
| Qu et al., 2013 | Compound I to compound VII: (96.8% ± 1.9 to 87.0% ± 2.6) 16 days Control: (87.2% ± 3.1) 16 days |
Compound I and compound V: 18 days Control and other groups: 22 days |
? |
Hydroxyproline content (mg/g tissue)
Compound I to compound VII: 58.4 ± 3.7 to 80.3 ± 4.4 Control: 60.2 ± 4.1 |
Compound I to compound VII: 431.5 g ± 8.3 to 547.3 g ± 7.9 Control: 436.5 g ± 7.6 |
|
| |||||
| Ghosh et al., 2012 | Compound I to compound II: (100%) 18 days Control: (100%) 22 days |
Compound I: 17.16 ± 0.4 days Compound II: 17.25 ± 0.25 days Control: 22.00 ± 0.1 days |
? | Compounds I and II: fibrous connective tissue with strong collagenation Control: fibrosis and more aggregation of macrophages with less collagen fibers |
Compound I: 565.10 g ± 3.1 Compound II: 561.12 g ± 3.9 Control: 372.13 g ± 3.23 |
|
| |||||
| Mukherjee et al., 2013 | Compound I (2,5%): (89.91% ± 0.55) 18 days Compound II (2,5%): (97.89% ± 0.77) 18 days Control: (75.44% ± 0.37) 18 days |
Compound I (2,5%): 17.16 ± 0.4 days Compound II (2,5%): 16.01 ± 0.33 days Control: 21.00 ± 0.11 days |
? |
Hydroxyproline content (mg/g tissue)
Compound I (2,5%): 158.23 ± 0.44 Compound II (2,5%): 198.16 ± 0.33 Control: 151.9 ± 2.69 |
Compound I (2,5%): 538.00 g ± 1.89 Compound II (2,5%): 535.12 g ± 3.59 Control: 322.39 g ± 2.66 |
|
| |||||
| Melo et al., 2011 | Cramoll 1,4: (100%) 10 days Control: (100%) 12 days |
? | ? |
Crust presence: cramoll 1,4: 13.1 ± 7.02 Control: 5.4 ± 3.3 Collagen presence: cramoll 1,4: (higher collagen deposition and annex sprouts) 12 days Control: (matrix poor in collagen fibers) 12 days |
? |
|
| |||||
| Pieters et al., 1995 | 3′,4-0-Dimethylcedrusin: (85%) 15 days Taspine: (75%) 15 days Control: (60%) 15 days |
3′,4-0-Dimethylcedrusin: ++ (15 days) Taspine: + (15 days) Control: + (15 days) |
? |
Crust presence
3′,4-0-Dimethylcedrusin: after 5 days Taspine: after 5 days Control: after 3 days |
? |
|
| |||||
| Ahamed et al., 2009 | Gulonic acid γ-lactone: (94.02% ± 0.20) 16 days Control: (79.53% ± 0.97) 16 days |
Epithelialization in days
Gulonic acid γ-lactone: 18.62 ± 0.21 Control: 22.59 ± 0.15 |
? |
Hydroxyproline content (µg/100 g)
Gulonic acid γ-lactone: 780.48 ± 50.73 Control: 346.15 ± 14.54 Fibroblast count/high power field × 400 Gulonic acid γ-lactone: 53.26 ± 2.37 Control: 97.53 ± 4.26 Blood vessel count/high power field × 400 Gulonic acid γ-lactone: 21.94 ± 1.15 Control: 11.63 ± 1.11 |
Gulonic acid γ-lactone: 561.12 g ± 5.18 Control: 327.63 g ± 6.37 |
|
| |||||
| Zyuz'kov et al., 2012 | Songorine: 100% (9–16 days) Napelline: 100% (9–16 days) Hypaconitine: 100% (9–16 days) 12-Epinapelline N-oxide: 89.93% ± 5.53 (9–16 days) Mesaconitine: 97.8% ± 2.2 (9–16 days) Control: 89.72% ± 4.72 (9–16 days) |
Songorine-napelline-hypaconitine Newly formed epithelium by the wound edges represented a cell layer of varying thickness without vertical anisomorphism: 5 days |
? |
Leukocytic infiltration
Songorine: reduction (3 days) Napelline: reduction (3 days) Hypaconitine: reduction (3 days) 12-Epinapelline N-oxide: ?/mesaconitine: ?/control: ? Counts of fibroblasts Songorine: increased (3 days) Napelline: increased (3 days) Hypaconitine: increased (3 days) 12-Epinapelline N-oxide: ?/mesaconitine: ?/control: ? |
? |
|
| |||||
| Singh et al., 2005 | Deoxyelephantopin: 98.8% ± 0.35 (16 days) Control: 85.8% ± 0.69 (16 days) |
Epithelialization in days
Deoxyelephantopin: 14.0 ± 0.26 Control: 20.0 ± 0.86 |
? | Deoxyelephantopin: ↓ macrophages and ↑ collagen formation Control: ↑ macrophages and ↓ collagen formation |
Deoxyelephantopin: 412.0 g ± 11.37 Control: 298.6 g ± 8.48 |
|
| |||||
| Sharath et al., 2010 | Bacoside-A: 98.18% ± 0.05 (16 days) Control: 85.22% ± 0.02 (16 days) |
Epithelialization in days
Bacoside-A: 18.30 ± 0.01 Control: 20.20 ± 0.04 |
? | Bacoside-A: ↑ blood vessels and ↑ collagen formation Control: ↑ inflammatory cells, ↓ blood vessels, and ↓ collagen formation |
Bacoside-A: 538.47 g ± 0.14 Control: 380.48 g ± 0.11 |
|
| |||||
| Vidya et al., 2012 | Entadamide: 92.22% ± 0.05 (16 days) Phaseoloidin: 88.50 ± 0.10 (16 days) Entagenic acid: 96.08% ± 0.04 (16 days) Control: 83.31% ± 1.06 (16 days) |
Epithelialization in days
Entadamide: 19.92 ± 0.01 Phaseoloidin: 21.16 ± 0.02 Entagenic acid: 18.08 ± 0.01 Control: 24.00 ± 0 |
? |
Hydroxyproline content (µg/100 g)
Entadamide: 1891.17 ± 2.75 Phaseoloidin: 1690.33 ± 2.80 Entagenic acid: 2001.33 ± 3.53 Control: 1369.67 ± 10.54 |
Entadamide: 463.33 g ± 4.48 Phaseoloidin: 450.17 g ± 7.55 Entagenic acid: 549.83 g ± 2.21 Control: 260.83 g ± 14.05 |
Figure 2.
Summary of the studies describing the plants species, families, used parts of each species, toxicity tests, and popular indications.
2.4. Analysis of Bias
The articles quality was analyzed by the criteria described on the ARRIVE platform (Animal Research: Reporting of In Vivo Experiments). These criteria are based on short descriptions that indicate essential characteristics of all studies with animal models, such as theoretical and methodological basis, research objective, refinement of the analytical methods, statistical design, sample calculations, and measure outcomes [15]. Recently there has been an increasing interest in the systematic reviews of research involving animals [16]. Considering the purpose of the systematic review on evaluating important aspects of the referenced publications, we built a table summarizing all the aspects investigated as well as their relevance, describing positive and negative characteristics of the recovered studies (Tables 2(a) and 2(b)).
3. Results
3.1. Included Studies
From the PubMed and Scopus database, 1008 articles were recovered. 164 duplicated studies and 489 with thematic inadequacy were excluded after reading the title and abstract. After recovery of 329 articles in full text, 303 studies were excluded for not meeting the eligibility criteria. Thus, 26 studies were included in the systematic review. The reference list of all included studies was carefully analyzed to ensure the identification of additional relevant studies. Thus, six studies were additionally identified and recovered, completing 32 works added to this review. From these studies, 19 studies utilized fractions, 12 studies utilized plant isolates, and 1 study used both fractions and isolates for the treatment of cutaneous wounds (Figure 1).
3.2. Qualitative Analysis
The analyzed studies were conducted in 13 different countries, especially India (40.62%, n = 13), followed by Brazil and Turkey (12.5%, n = 4 each). The most utilized animal models on the experiments were rats (75%, n = 24), followed by mice (12.5%, n = 4) and both (12.5%, n = 4). Considering the animal strain, 65.7% were Wistar rats, 17.14% were Sprague Dawley rats, 11.42% were Swiss mice, and 5.71% were Hairless mice. Half of the experimental models used male animals (n = 16), 15.62% (n = 5) used females, and 18.75% (n = 6) used both sex. 15.62% (n = 5) of all studies did not report this information. The animals' age ranged from 2 to 5 months for rats and from 8 to 12 weeks for mice; however 71.8% (n = 23) of the studies did not relate this information. The weight of rats ranged from 150 to 400 g and the mice weighted between 18 and 40 g, and only 2 studies (6.25%) did not report this data.
More than half of the studies did not describe the popular name of the plant species investigated (59.37%, n = 19). The first treatments utilized on the control group were as follows: 25% (n = 8) used ointment base (which did not have its formulation described), 15.6% (n = 5) used saline solution, 9.4% (n = 3) used nitrofurazone, and 6.2% (n = 2) utilized distilled water. Only 3.1% (n = 1) did not present the treatment for the control group. The other works utilized miconazole and nonionic cream; gentamicin; Matrigel solution; soft paraffin (85%), cetostearyl alcohol (5%), hard paraffin (5%), and wool fat (5%); framycetin ointment; PBS; sodium alginate; Vaseline; Tween 80; tragacanth; povidone iodine ointment; madecassol and ointment base; chlorocresol BP 0.1% mentioned only once, representing 40.6% of all included studies (n = 13). 62.5% (n = 20) of the plant species were native and 12.5% (n = 4) were exotic and 25% (n = 8) of the studies did not describe this characteristic.
Investigated wound area presented a large variation (5 mm2 to 600 mm2), and 9.37% (n = 3) of the studies did not describe this data. The calculations used to measure the wound area were described in only 59.37% (n = 19) of the studies. All the works described the interval in which the wound area was measured, and the most common interval was daily, 31.25% (n = 10), followed by measurements taken each 4 days, 28.12% (n = 9) (Tables 1(a) and 1(b)). From the 32 species of plants, 23 different families were reported, and the main ones are Asteraceae 18.75% (n = 6), Euphorbiaceae 9.37% (n = 3), Leguminosae 6.25% (n = 2), and Fabaceae 6.25% (n = 2), and the other families, Liliaceae, Boraginaceae, Scrophulariaceae, Ranunculaceae, Apiaceae, Myrsinaceae, Mimosae, Malpighiaceae, Tiliaceae, Crassulaceae, Martyniaceae, Rutaceas, Araliaceae, Piperaceae, Solanaceae, Caprifoliaceae, Dipterocarpaceae, Oleaceae, and Combretaceae, were mentioned once and represent 59.37% (n = 19) of the included studies. The most used plant structures were the leaves representing 37.5% (n = 12), followed by the flowers 12.5% (n = 4), bole bark 12.5% (n = 4), and seeds 6.25% (n = 2). The fruit, the whole plant, and the latex were mentioned once, representing 3.12% (n = 1) each. However, 21.87% (n = 7) of the studies did not mention this information. Considering the popular indication, healing effects were described in 46.87% (n = 15) of the studies, followed by anti-inflammatory effects 34.37% (n = 11), treatment of gastrointestinal diseases 28.12% (n = 9), burns 18.75% (n = 6), and antirheumatic 12.5% (n = 4) and ophthalmological diseases 6.25% (n = 2). 18.75% (n = 6) of the studies did not report the popular indication. Only 33.4% (n = 11) of the studies report toxicity tests (Figure 2).
3.3. Bias Analysis
Among the analyzed works, 78.12% presented a title coherent to the text, 90.6% presented abstracts containing the objectives, methods, main results, and conclusions, and 75% presented an introduction with sufficient scientific base. All studies described ethical approval and no work reported a blind controlled study. Most studies (87.5%) related to the therapeutic dose administered (90.62%) reported the route of administration and (96.87%) the treatment duration. The choice of administration route was not justified in any study. Most studies (96.87%) reported the investigated animal strain. The sex and weight were reported in 84.37% and 93.75% of the works, respectively, but only 31.25% provided information about the age of the animals. 59.37% of the studies provided information about the experimental conditions (temperature, humidity, light cycles, feed, and water). A statistical analysis was conducted by all studies, but only 68.75% of the studies specified the data analyzed. 84.37% of the studies reported the number of animals in each group. No study reported mortality or modifications on the experimental protocol by adverse events. A coherent interpretation of the results and direct relationship between objectives and hypothesis were described in 75% of all included studies (Table S2).
In general, the animals treated with isolates and fractions of plants presented an elevated closure rate of the wound, representing 72.72% of the studies [17, 18, 20, 23–27, 29, 30, 33, 35–46], increase in tissue reepithelialization (30.3%) [15, 18–20, 23, 27, 35, 38, 43], increase of the traction strength on the cicatricle tissue (75.75%) [15, 17–20, 22–25, 27, 31, 33–46], greater content and organization of the extracellular matrix on fast expansion of the granulation tissue (42.42%) [17, 18, 23, 30, 32, 33, 36, 38–40, 42, 44–46], and stimulation of the activity of endogenous antioxidant enzymes (9.09%) [16, 21, 22] (Tables 2(a) and 2(b)).
4. Discussion
The use of plant based strategies is opening a new perspective for the treatment of skin wounds, mainly in developing countries, once it represents a simple, low cost, and affordable therapy [1, 7, 56–58]. There are several studies indicating beneficial effects of herbal medicines in all phases of the healing process. In fact, most of the studies included in this systematic review reported that plant fractions and isolates were able to improve the skin wound healing. Apparently, these medicines were especially favorable in controlling the cutaneous inflammatory and oxidative response and in stimulating the granulation tissue formation, collagen maturation, and reepithelialization.
In this review, we did not include studies testing crude plant extracts, since the chemical characterization of the extracts makes it difficult to determine the herbal components responsible for the effects reported. Even in case of including only studies with murine models, different animal strains were observed. This aspect makes the generalizability of the results difficult, since the biological variability directly influences the response to the treatments. In addition, among the 32 analyzed studies, there were large methodological variation and discrepancies in the measure outcomes. An evident example was the wide variation in wound area and time of wound closure. These considerations are important because they are directly associated with the tensile force experienced by the tissue, which profoundly affect the speed and quality of skin repair [59, 60]. Our findings show that 20% of the studies that utilized fractions neglected the analysis of wound closure, an essential piece of information to assess the ability of any intervention to stimulate the healing process. In addition, the interval between measurements of wound area and the used protocols for the calculations were variable, representing methodological flaws that compromise the study reproduction and generalizability of the findings [61, 62].
Considering that the reepithelization and organization of the granulation tissue are fundamental aspects to understand how chemical substances act to stimulate wound healing, only 60% of all studies analyzed the reepithelialization rate and 75% evaluated the molecular components of the extracellular matrix, especially collagen. These parameters indicate if the wound closure follows a normal process, in which the newly formed tissue gradually develops drastic structural changes to reconstitute the morphofunctional characteristics of the intact skin. Works which demonstrated the importance of these analyses assert that the type and quantity of collagen fibers deposited on the tissue can be used as a marker of tissue mechanical resistance [2, 3, 9, 58]. The connective and epithelial tissues form a support structure to promote the correct closure of the wound [57, 63], reducing the chances of opportunistic infections in the wounded area [38, 64]. During the formation of granulation tissue, there is predominance of sulfated molecules which attract water, facilitating the cellular migration, and also serve as a support structure for the first formed collagen (type III collagen) [65]. There are enough evidences that the synthesis and differentiation of cells and matrix components are crucial for a normal wound closure [60]. It is already known that the oxidative stress induces cell damage, lipid, protein, and nucleic acids oxidation [66, 67]. It is recognized that cutaneous trauma increases the tissue oxidative stress in the wounded area [66–69]. Although reactive species are able to activate cell signaling pathways and stimulate cell proliferation, differentiation, and neoangiogenesis, excessive production of these molecules inhibits the healing process, especially by inducing cell death and molecular damage in the extracellular matrix [23, 70, 71]. Thus, there is a notorious importance in analyzing the redox balance during skin repair. However, from all analyzed studies, only 15% investigated the oxidative status. This is a surprising finding, since the antioxidant effect is a pivotal mechanism indicated in several studies to support the applicability of plant extracts in the treatment of tissue damage, including skin wounds [66–69]. Another fundamental result on the cutaneous repair process is the restoration of the biomechanical properties, especially the tensile force of the newly formed tissue, which provides functional estimates on the quality of the healing process [37]. In this review, only 35% of all studies investigating plant fractions evaluated the traction resistance of the scar tissue, aspects investigated in 61.53% of the studies with plants isolates.
In our findings, we see that the majority of the studies used male animals, an aspect potentially associated with the hormonal stability, which is not observed in female animals due to the estrous cycle [72]. The use of rats as the experimental model was higher (75%), aspect potentially related to the large body area needed to perform experimental wounds (1 to 5). Thus, it is possible to construct a larger number of wounds and to use a smaller number of animals in each group. In addition, in rats it is possible to collect enough fragments in order to fully analyze the healing process. Another interesting piece of data was the age of the animals, which presented a large variation (rats, 2 to 8 weeks; mice, 5 to 12 weeks). However, 71.8% of the studies did not report this information, making it difficult to establish a temporal basis to determine the effectivity of the herbal treatments investigated. More than half of the studies (59.37%) did not describe the popular name of the plant species. The large number of works that did not describe important variables such as age of the animals and plant species represents a concerning number, once these characteristics are of great importance to ensure the study reproducibility and to allow the elaboration of broad reports with a critical review of the findings [15]. The orientation cited in the ARRIVE guideline describes the minimum information that all scientific publications using animals should include. This guide also brings items that help to understand the quality of the writing and potential methodological bias that compromise the quality of the evidence [15]. The work title should refer the readers to a brief summary of the article content, providing keywords and terms that could be researched in electronic databases [73]. Only 78.12% of the studies presented a coherent title, while 90.6% presented abstracts with clear information relative to the objectives, methods, main results, and conclusions. Furthermore, 75% presented introduction with enough scientific base, which can make it harder for the reader to understand the relevance of the study. Another observation made through ARRIVE guide refers to the health conditions of the animals during the experiment. Thus, aspects such as information about environmental conditions (temperature and humidity), mortality, feeding, randomization, and reactions indicative of systemic or local toxicity were neglected in most studies, demonstrating that the report bias is a serious limitation of these preclinical tests that compromise the reliability of the results and the quality of the evidence [74].
5. Conclusions
The current evidence indicates that fractions and isolated molecules from plant extracts stimulate the healing process in cutaneous wounds. Apparently, the main effects of these herbal medicines are associated with the stimulation of collagen synthesis, expansion of the granulation tissue, reepithelialization, modulation of the inflammatory response, and oxidative stress during tissue repair. Together, these effects promote increase of the speed of wound closure and the biomechanical resistance of newly formed tissue. However, the serious methodological flaws and report bias observed in most included studies make the current evidence fragile. Thus, the relevance of fractions and isolated molecules from plant extracts in the treatment of skin wound cannot be accurately determined. Considering these limitations, it seems impossible to use these evidences to construct a rational basis that supports clinical studies. Therefore, there is an urgent need to improve research reports in experimental studies with herbal medicines in murine models of skin wound healing. This task requires a collective effort of authors, journal editors, reviewers, and financial organisms, to ensure the reproducibility, reliability, and generalizability of the evidence, fundamental elements to determine to what extent herbal medicines are promising in the treatment of skin wounds.
Supplementary Material
The full search strategy is presented in Table S1. Keywords were obtained in the MeSH (Medical Subject Headings) database, which is the U.S. National Library of Medicine (NLM) controlled vocabulary thesaurus used for indexing manuscripts in PubMed. In the additional databases used to recover relevant articles, the keywords were adapted acording the search algoritm adopted in the search plataforms.
Table S2 shows the results of bias analysis. All criteria investigated were based on ARRIVE guideline, which states the essential elements that should be reported in In Vivo animal experiments.
Acknowledgments
This work received financial support from the Brazilian agency Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) (FAPEMIG Process: APQ 00685-14).
Competing Interests
The authors declare that there are no competing interests regarding the publication of this paper.
References
- 1.Bahramsoltani R., Farzaei M. H., Rahimi R. Medicinal plants and their natural components as future drugs for the treatment of burn wounds: an integrative review. Archives of Dermatological Research. 2014;306(7):601–617. doi: 10.1007/s00403-014-1474-6. [DOI] [PubMed] [Google Scholar]
- 2.Nazaruk J., Galicka A. The influence of selected flavonoids from the leaves of Cirsium palustre (L.) Scop. on collagen expression in human skin fibroblasts. Phytotherapy Research. 2014;28(9):1399–1405. doi: 10.1002/ptr.5143. [DOI] [PubMed] [Google Scholar]
- 3.Gonçalves R. V., Novaes R. D., Matta S. L. P., Benevides G. P., Faria F. R., Pinto M. V. M. Comparative study of the effects of gallium-aluminum-arsenide laser photobiomodulation and healing oil on skin wounds in wistar rats: a histomorphometric study. Photomedicine and Laser Surgery. 2010;28(5):597–602. doi: 10.1089/pho.2009.2669. [DOI] [PubMed] [Google Scholar]
- 4.Bowden L. G., Maini P. K., Moulton D. E., et al. An ordinary differential equation model for full thickness wounds and the effects of diabetes. Journal of Theoretical Biology. 2014;361:87–100. doi: 10.1016/j.jtbi.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Lee C.-H., Chang S.-H., Chen W.-J., et al. Augmentation of diabetic wound healing and enhancement of collagen content using nanofibrous glucophage-loaded collagen/PLGA scaffold membranes. Journal of Colloid and Interface Science. 2015;439:88–97. doi: 10.1016/j.jcis.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 6.Mikaili P., Moloudizargari M., Aghajanshakeri S. Treatment with topical nitroglycerine may promote the healing process of diabetic foot ulcers. Medical Hypotheses. 2014;83(2):172–174. doi: 10.1016/j.mehy.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Tan Y. Q., Wang K. Y., Wang N., Li G., Liu D. Ectopic expression of human acidic fibroblast growth factor 1 in the medicinal plant, Salvia miltiorrhiza, accelerates the healing of burn wounds. BMC Biotechnology. 2014;14(1, article 74):1–10. doi: 10.1186/1472-6750-14-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosa A. D. S., Bandeira L. G., Monte-Alto-Costa A., Romana-Souza B. Supplementation with olive oil, but not fish oil, improves cutaneous wound healing in stressed mice. Wound Repair and Regeneration. 2014;22(4):537–547. doi: 10.1111/wrr.12191. [DOI] [PubMed] [Google Scholar]
- 9.Xie Z., Paras C. B., Weng H., et al. Dual growth factor releasing multi-functional nanofibers for wound healing. Acta Biomaterialia. 2013;9(12):9351–9359. doi: 10.1016/j.actbio.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bueno F. G., Panizzon G. P., Mello E. V. S. D. L., et al. Hydrolyzable tannins from hydroalcoholic extract from Poincianella pluviosa stem bark and its wound-healing properties: phytochemical investigations and influence on in vitro cell physiology of human keratinocytes and dermal fibroblasts. Fitoterapia. 2014;99:252–260. doi: 10.1016/j.fitote.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Abenavoli L., Capasso R., Milic N., Capasso F. Milk thistle in liver diseases: past, present, future. Phytotherapy Research. 2010;24(10):1423–1432. doi: 10.1002/ptr.3207. [DOI] [PubMed] [Google Scholar]
- 12.Oryan A., Naeini A. T., Nikahval B., Gorjlan E. Effects of aqueous extract of Aloe vera on experimental cutaneous wound healing in rat. Veterinarski Arhiv. 2010;80(4):509–522. [Google Scholar]
- 13.Chaturvedi A. P., Kumar M., Tripathi Y. B. Efficacy of Jasminum grandiflorum L. leaf extract on dermal wound healing in rats. International Wound Journal. 2013;10(6):675–682. doi: 10.1111/j.1742-481x.2012.01043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pazyar N., Yaghoobi R., Rafiee E., Mehrabian A., Feily A. Skin wound healing and phytomedicine: a review. Skin Pharmacology and Physiology. 2014;27(6):303–310. doi: 10.1159/000357477. [DOI] [PubMed] [Google Scholar]
- 15.Kilkenny C., Browne W. J., Cuthill I. C., Emerson M., Altman D. G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biology. 2010;8(6) doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yun Y., Kim K., Choi I., Ko S.-G. Topical herbal application in the management of atopic dermatitis: a review of animal studies. Mediators of Inflammation. 2014;2014:8. doi: 10.1155/2014/752103.752103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shukla A., Rasik A. M., Dhawan B. N. Asiaticoside-induced elevation of antioxidant levels in healing wounds. Phytotherapy Research. 1999;13(1):50–54. doi: 10.1002/(sici)1099-1573(199902)13:1<50::aid-ptr368>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 18.Muralidhar A., Babu K. S., sankar T. R., Reddanna P., Latha J. Evaluation of wound healing properties of bioactive fractions from the extract of Butea monosperma (lam) stem bark. International Journal of Phytomedicine. 2011;3(1):41–49. [Google Scholar]
- 19.Süntar I., Akkol E. K., Keles H., Yesilada E., Sarker S. D. Exploration of the wound healing potential of Helichrysum graveolens (Bieb.) Sweet: isolation of apigenin as an active component. Journal of Ethnopharmacology. 2013;149(1):103–110. doi: 10.1016/j.jep.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Mekonnen A., Sidamo T., Asres K., Engidawork E. In vivo wound healing activity and phytochemical screening of the crude extract and various fractions of Kalanchoe petitiana A. Rich (Crassulaceae) leaves in mice. Journal of Ethnopharmacology. 2013;145(2):638–646. doi: 10.1016/j.jep.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Pieters L., De Bruyne T., Van Poel B., et al. In vivo wound healing activity of Dragon's blood (Croton spp.), a traditional South American drug, and its constituents. Phytomedicine. 1995;2(1):17–22. doi: 10.1016/s0944-7113(11)80043-7. [DOI] [PubMed] [Google Scholar]
- 22.Korkina L. G., Chik E. V. M., Suprun M. V., et al. Molecular mechanisms underlying wound healing and anti-inflammatory properties of naturally occurring biotechnologically produced phenylpropanoid glycosides. Cellular and Molecular Biology. 2007;53(5):84–91. [PubMed] [Google Scholar]
- 23.Bigoniya P., Agrawal S., Verma N. K. Potential wound healing activity of Euphorbia hirta Linn total flavonoid fraction. International Journal of Pharmaceutical Sciences Review and Research. 2013;22(2):149–156. [Google Scholar]
- 24.Lodhi S., Singhai A. K. Preliminary pharmacological evaluation of Martynia annua Linn leaves for wound healing. Asian Pacific Journal of Tropical Biomedicine. 2011;1(6):421–427. doi: 10.1016/s2221-1691(11)60093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tabandeh M. R., Oryan A., Alipour A. M., Naieni A. T. Silibinin regulates matrix metalloproteinase 3 (stromelysine1) gene expression, hexoseamines and collagen production during rat skin wound healing. Phytotherapy Research. 2013;27(8):1149–1153. doi: 10.1002/ptr.4839. [DOI] [PubMed] [Google Scholar]
- 26.Sonmez E., Turkdogan K. A., Civelek C., et al. The efficacy of absorbable polysaccharide haemostats in wound healing. Blood Coagulation & Fibrinolysis. 2015;26(1):50–53. doi: 10.1097/mbc.0000000000000177. [DOI] [PubMed] [Google Scholar]
- 27.Karakaş F. P., Karakaş A., Boran Ç., Türker A. U., Yalçin F. N., Bilensoy E. The evaluation of topical administration of Bellis perennis fraction on circular excision wound healing in Wistar albino rats. Pharmaceutical Biology. 2012;50(8):1031–1037. doi: 10.3109/13880209.2012.656200. [DOI] [PubMed] [Google Scholar]
- 28.Choi S.-W., Son B.-W., Son Y.-S., Park Y.-I., Lee S.-K., Chung M.-H. The wound-healing effect of a glycoprotein fraction isolated from aloe vera. British Journal of Dermatology. 2001;145(4):535–545. doi: 10.1046/j.1365-2133.2001.04410.x. [DOI] [PubMed] [Google Scholar]
- 29.Parente L. M. L., Júnior R. D. S. L., Tresvenzol L. M. F., Vinaud M. C., De Paula J. R., Paulo N. M. Wound healing and anti-inflammatory effect in animal models of Calendula officinalis L. growing in Brazil. Evidence-Based Complementary and Alternative Medicine. 2012;2012:7. doi: 10.1155/2012/375671.375671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olugbuyiro J. A. O., Abo K. A., Leigh O. O. Wound healing effect of Flabellaria paniculata leaf extracts. Journal of Ethnopharmacology. 2010;127(3):786–788. doi: 10.1016/j.jep.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 31.Süntar I. P., Akkol E. K., Yalçin F. N., Koca U., Keleş H., Yesilada E. Wound healing potential of Sambucus ebulus L. leaves and isolation of an active component, quercetin 3-O-glucoside. Journal of Ethnopharmacology. 2010;129(1):106–114. doi: 10.1016/j.jep.2010.01.051. [DOI] [PubMed] [Google Scholar]
- 32.Kim W.-K., Song S.-Y., Oh W. K., et al. Wound-healing effect of ginsenoside Rd from leaves of Panax ginseng via cyclic AMP-dependent protein kinase pathway. European Journal of Pharmacology. 2013;702(1–3):285–293. doi: 10.1016/j.ejphar.2013.01.048. [DOI] [PubMed] [Google Scholar]
- 33.Chaudhari M., Mengi S. Evaluation of phytoconstituents of Terminalia arjuna for wound healing activity in rats. Phytotherapy Research. 2006;20(9):799–805. doi: 10.1002/ptr.1857. [DOI] [PubMed] [Google Scholar]
- 34.Kumara Swamy H. M., Krishna V., Shankarmurthy K., et al. Wound healing activity of embelin isolated from the ethanol extract of leaves of Embelia ribes Burm. Journal of Ethnopharmacology. 2007;109(3):529–534. doi: 10.1016/j.jep.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Hernandes L., da Silva Pereira L. M., Palazzo F., de Mello J. C. P. Wound-healing evaluation of ointment from Stryphnodendron adstringens (barbatimão) in rat skin. Brazilian Journal of Pharmaceutical Sciences. 2010;46(3):431–436. doi: 10.1590/s1984-82502010000300005. [DOI] [Google Scholar]
- 36.Sidhu G. S., Singh A. K., Banaudha K. K. Accelerates normal and hydrocortisone-induced impaired wound healing. The Journal of Investigative Dermatology. 1999;113(5):773–781. doi: 10.1046/j.1523-1747.1999.00761.x. [DOI] [PubMed] [Google Scholar]
- 37.Paramesha M., Ramesh C. K., Krishna V., Kumar Swamy H. M., Aditya Rao S. J., Hoskerri J. Effect of dehydroabietylamine in angiogenesis and GSK3-β inhibition during wound healing activity in rats. Medicinal Chemistry Research. 2015;24(1):295–303. doi: 10.1007/s00044-014-1110-1. [DOI] [Google Scholar]
- 38.Nagappan T., Segaran T. C., Wahid M. E. A., Ramasamy P., Vairappan C. S. Efficacy of carbazole alkaloids, essential oil and extract of Murraya koenigii in enhancing subcutaneous wound healing in rats. Molecules. 2012;17(12):14449–14463. doi: 10.3390/molecules171214449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qu X., Diao Y., Zhang Z., Wang S., Jia Y. Evaluation of anti-bacterial and wound healing activity of the fruits of Amorpha fruticosa L. African Journal of Traditional, Complementary, and Alternative Medicines. 2013;10(3):458–468. doi: 10.4314/ajtcam.v10i3.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghosh S., Samanta A., Mandal N. B., Bannerjee S., Chattopadhyay D. Evaluation of the wound healing activity of methanol extract of Pedilanthus tithymaloides (L.) Poit leaf and its isolated active constituents in topical formulation. Journal of Ethnopharmacology. 2012;142(3):714–722. doi: 10.1016/j.jep.2012.05.048. [DOI] [PubMed] [Google Scholar]
- 41.Mukherjee H., Ojha D., Bharitkar Y. P., et al. Evaluation of the wound healing activity of Shorea robusta, an Indian ethnomedicine, and its isolated constituent(s) in topical formulation. Journal of Ethnopharmacology. 2013;149(1):335–343. doi: 10.1016/j.jep.2013.06.045. [DOI] [PubMed] [Google Scholar]
- 42.Melo C. M. L. D., Porto C. S., Júnior M. R. M., et al. Healing activity induced by Cramoll 1,4 lectin in healthy and immunocompromised mice. International Journal of Pharmaceutics. 2011;408(1-2):113–119. doi: 10.1016/j.ijpharm.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 43.Ahamed B. M. K., Krishna V., Malleshappa K. H. In vivo wound healing activity of the methanolic extract and its isolated constituent, gulonic acid γ-lactone, obtained from Grewia tiliaefolia . Planta Medica. 2009;75(5):478–482. doi: 10.1055/s-0029-1185315. [DOI] [PubMed] [Google Scholar]
- 44.Zyuz'kov G. N., Krapivin A. V., Nesterova Y. V., et al. Mechanisms of regeneratory effects of Baikal aconite diterpene alkaloids. Bulletin of Experimental Biology and Medicine. 2012;153(6):847–851. doi: 10.1007/s10517-012-1841-2. [DOI] [PubMed] [Google Scholar]
- 45.Singh S. D. J., Krishna V., Mankani K. L., Manjunatha B. K., Vidya S. M., Manohara Y. N. Wound healing activity of the leaf extracts and deoxyelephantopin isolated from Elephantopus scaber Linn. Indian Journal of Pharmacology. 2005;37(4):238–242. doi: 10.4103/0253-7613.16570. [DOI] [Google Scholar]
- 46.Sharath R., Harish B. G., Krishna V., Sathyanarayana B. N., Kumara Swamy H. M. Wound healing and protease inhibition activity of bacoside-A, isolated from Bacopa monnieri Wettest. Phytotherapy Research. 2010;24(8):1217–1222. doi: 10.1002/ptr.3115. [DOI] [PubMed] [Google Scholar]
- 47.Martins M. D., Marques M. M., Bussadori S. K., et al. Comparative analysis between Chamomilla recutita and corticosteroids on wound healing. An in vitro and in vivo study. Phytotherapy Research. 2009;23(2):274–278. doi: 10.1002/ptr.2612. [DOI] [PubMed] [Google Scholar]
- 48.Manjunatha B. K., Vidya S. M., Krishna V., Mankani K. L., Jagadeesh Singh S. D., Manohara Y. N. Comparative evaluation of wound healing potency of Vitex trifolia L. and Vitex altissima L. . Phytotherapy Research. 2007;21(5):457–461. doi: 10.1002/ptr.2094. [DOI] [PubMed] [Google Scholar]
- 49.Roy A., Saraf S. Limonoids: overview of significant bioactive triterpenes distributed in plants kingdom. Biological and Pharmaceutical Bulletin. 2006;29(2):191–201. doi: 10.1248/bpb.29.191. [DOI] [PubMed] [Google Scholar]
- 50.Miot H. A., Batistella R. F., Batista K. D. A., et al. Comparative study of the topical effectiveness of the Andiroba oil (Carapa guianensis) and DEET 50% as repellent for Aedes sp. Revista do Instituto de Medicina Tropical de São Paulo. 2004;46(5):253–256. doi: 10.1590/s0036-46652004000500004. [DOI] [PubMed] [Google Scholar]
- 51.Huemer H. P. Possible immunosuppressive effects of drug exposure and environmental and nutritional effects on infection and vaccination. Mediators of Inflammation. 2015;2015:7. doi: 10.1155/2015/349176.349176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shetty S., Udupa S., Udupa L. Evaluation of antioxidant and wound healing effects of alcoholic and aqueous extract of Ocimum sanctum Linn in rats. Evidence-Based Complementary and Alternative Medicine. 2008;5(1):95–101. doi: 10.1093/ecam/nem004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parry J., Su L., Moore J., et al. Chemical compositions, antioxidant capacities, and antiproliferative activities of selected fruit seed flours. Journal of Agricultural and Food Chemistry. 2006;54(11):3773–3778. doi: 10.1021/jf060325k. [DOI] [PubMed] [Google Scholar]
- 54.Shi J., Yu J., Pohorly J. E., Kakuda Y. Polyphenolics in grape seeds—biochemistry and functionality. Journal of Medicinal Food. 2003;6(4):291–299. doi: 10.1089/109662003772519831. [DOI] [PubMed] [Google Scholar]
- 55.Hooijmans C. R., Tillema A., Leenaars M., Ritskes-Hoitinga M. Enhancing search efficiency by means of a search filter for finding all studies on animal experimentation in PubMed. Laboratory Animals. 2010;44(3):170–175. doi: 10.1258/la.2010.009117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu X.-B., Luo X.-Q., Gu S.-Y., Xu J.-H. The effects of Polygonum cuspidatum extract on wound healing in rats. Journal of Ethnopharmacology. 2012;141(3):934–937. doi: 10.1016/j.jep.2012.03.040. [DOI] [PubMed] [Google Scholar]
- 57.Gonçalves R. V., da Matta S. L. P., Novaes R. D., Leite J. P. V., Peluzio M. D. C. G., Vilela E. F. Bark extract of Bathysa cuspidata in the treatment of liver injury induced by carbon tetrachloride in rats. Brazilian Archives of Biology and Technology. 2014;57(4):504–513. doi: 10.1590/s1516-89132014005000019. [DOI] [Google Scholar]
- 58.Sarandy M. M., Novaes R. D., Da Matta S. L. P., et al. Ointment of Brassica oleracea var. capitata matures the extracellular matrix in skin wounds of wistar rats. Evidence-Based Complementary and Alternative Medicine. 2015;2015:9. doi: 10.1155/2015/919342.919342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park S. A., Raghunathan V. K., Shah N. M., et al. PDGF-BB does not accelerate healing in diabetic mice with splinted skin wounds. PLoS ONE. 2014;9(8) doi: 10.1371/journal.pone.0104447.e104447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu J., Yu A., Qi B., Li Z., Hu X. Effects of negative pressure wound therapy on mesenchymal stem cells proliferation and osteogenic differentiation in a fibrin matrix. PloS ONE. 2014;9(9) doi: 10.1371/journal.pone.0107339.e107339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yonehiro L., Burleson G., Sauer V. Use of a new acellular dermal matrix for treatment of nonhealing wounds in the lower extremities of patients with diabetes. Wounds. 2014;26(5):E39–E47. [PubMed] [Google Scholar]
- 62.Simera I., Altman D. G. Writing a research article that is ‘fit for purpose’: EQUATOR Network and reporting guideline. Evidence-Based Medicine. 2009;14(5):132–134. doi: 10.1136/ebm.14.5.132. [DOI] [PubMed] [Google Scholar]
- 63.Xavier M., David D. R., De Souza R. A., et al. Anti-inflammatory effects of low-level light emitting diode therapy on achilles tendinitis in rats. Lasers in Surgery and Medicine. 2010;42(6):553–558. doi: 10.1002/lsm.20896. [DOI] [PubMed] [Google Scholar]
- 64.Lerman G., Sharon M., Leibowitz-Amit R., Sidi Y., Avni D., Martelli F. The crosstalk between IL-22 signaling and miR-197 in human keratinocytes. PLoS ONE. 2014;9(9):1–13. doi: 10.1371/journal.pone.0107467.e107467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Assis De Brito T. L., Monte-Alto-Costa A., Romana-Souza B. Propranolol impairs the closure of pressure ulcers in mice. Life Sciences. 2014;100(2):138–146. doi: 10.1016/j.lfs.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 66.Silveira P. C. L., Silva L. A., Tuon T., Freitas T. P., Streck E. L., Pinho R. A. Effects of low-level laser therapy on epidermal oxidative response induced by wound healing. Revista Brasileira de Fisioterapia. 2009;13(4):281–287. [Google Scholar]
- 67.Servetto N., Cremonezzi D., Simes J. C., et al. Evaluation of inflammatory biomarkers associated with oxidative stress and histological assessment of low-level laser therapy in experimental myopathy. Lasers in Surgery and Medicine. 2010;42(6):577–583. doi: 10.1002/lsm.20910. [DOI] [PubMed] [Google Scholar]
- 68.Guo S., DiPietro L. A. Factors affecting wound healing. Journal of Dental Research. 2010;89(3):219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scioli M. G., Giudice P. L., Bielli A., et al. Propionyl-L-carnitine enhances wound healing and counteracts microvascular endothelial cell dysfunction. PLoS ONE. 2015;10(10):1–20. doi: 10.1371/journal.pone.0140697.e0140697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dröge W. Free radicals in the physiological control of cell function. Physiological Reviews. 2002;82(1):47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 71.Ewertowska M., Mikołajczak P. Ł., Okulicz-kozaryn I., Stachecki B., Murias M., Jodynis-Liebert J. Different response of antioxidant defense system to acamprosate in ethanol preferring and non-preferring rats. Acta Poloniae Pharmaceutica—Drug Research. 2015;72(3):439–445. [PubMed] [Google Scholar]
- 72.Peplow P. V., Chung T.-Y., Baxter G. D. Laser photobiomodulation of proliferation of cells in culture: a review of human and animal studies. Photomedicine and Laser Surgery. 2010;28(supplement 1):S3–S40. doi: 10.1089/pho.2010.2771. [DOI] [PubMed] [Google Scholar]
- 73.Fox C. W., Burns C. S. The relationship between manuscript title structure and success: editorial decisions and citation performance for an ecological journal. Ecology and Evolution. 2015;5(10):1970–1980. doi: 10.1002/ece3.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sampaio R. F., Mancini M. C. Estudos de revisão sistemática: um guia para síntese criteriosa da evidência científica. Revista Brasileira de Fisioterapia. 2007;11(1):83–89. doi: 10.1590/s1413-35552007000100013. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The full search strategy is presented in Table S1. Keywords were obtained in the MeSH (Medical Subject Headings) database, which is the U.S. National Library of Medicine (NLM) controlled vocabulary thesaurus used for indexing manuscripts in PubMed. In the additional databases used to recover relevant articles, the keywords were adapted acording the search algoritm adopted in the search plataforms.
Table S2 shows the results of bias analysis. All criteria investigated were based on ARRIVE guideline, which states the essential elements that should be reported in In Vivo animal experiments.