Abstract
Recognition of histone marks by reader modules is thought to be at the heart of epigenetic mechanisms. These protein domains are considered to function by targeting regulators to chromosomal loci carrying specific histone modifications. This is important for proper gene regulation as well as propagation of epigenetic information. The NuA4 acetyltransferase complex contains two of these reader modules, an H3K4me3-specific plant homeodomain (PHD) within the Yng2 subunit and an H3K36me2/3-specific chromodomain in the Eaf3 subunit. While each domain showed a close functional interaction with the respective histone mark that it recognizes, at the biochemical level, genetic level (as assessed with epistatic miniarray profile screens), and phenotypic level, cells with the combined loss of both readers showed greatly enhanced phenotypes. Chromatin immunoprecipitation coupled with next-generation sequencing experiments demonstrated that the Yng2 PHD specifically directs H4 acetylation near the transcription start site of highly expressed genes, while Eaf3 is important downstream on the body of the genes. Strikingly, the recruitment of the NuA4 complex to these loci was not significantly affected. Furthermore, RNA polymerase II occupancy was decreased only under conditions where both PHD and chromodomains were lost, generally in the second half of the gene coding regions. Altogether, these results argue that methylated histone reader modules in NuA4 are not responsible for its recruitment to the promoter or coding regions but, rather, are required to orient its acetyltransferase catalytic site to the methylated histone 3-bearing nucleosomes in the surrounding chromatin, cooperating to allow proper transition from transcription initiation to elongation.
INTRODUCTION
Chromatin is a dynamic nucleoprotein structure that compacts the long DNA molecule into the small nuclear space, simultaneously creating a mechanism to regulate DNA access to machineries implicated in essential nuclear processes, namely, transcription, DNA replication, DNA repair, and meiotic recombination. Modulation of the chromatin structure is highly regulated by four broad classes of nuclear factors: chromatin remodelers, histone chaperones, histone variants, and histone modifiers. These players essentially target nucleosomes, basic units of chromatin consisting of 146 bp of DNA wrapped around an octamer of histone proteins. Histone modifiers catalyze posttranslational modifications (PTM), including acetylation, phosphorylation, and methylation, that can directly affect DNA-histone contacts (e.g., acetylation) and/or create a binding platform for PTM readers (1, 2). These readers correspond to proteins/complexes containing distinct domains/modules that can bind specific modified histone residues. Such motifs include bromodomains that recognize acetylated residues; chromodomains (CHD); PWWP, Tudor, and MBT domains for methylated residues; BRCT, BIR, and 14-3-3 domains for phosphorylated histones; and plant homeodomain (PHD) fingers that can bind different modification states, depending on the subfamily. Recent years have seen an increasing number of studies dissecting the functions and regulation of histone modifiers and histone marks and how these marks are interpreted. Interestingly, many histone-modifying complexes harbor reader modules allowing cross talk between epigenetic marks or propagation of the PTM deposited (3).
The NuA4 (nucleosome acetyltransferase of histone 4 [H4]) complex is a highly conserved histone modifier that was shown to contain several protein motifs with specific binding properties, including PHD fingers, CHD, SANT, and YEATS domains (4). Noticeably, NuA4 contains two of the three CHD-containing proteins in budding yeast, namely, Eaf3 and Esa1. Esa1 is the only essential acetyltransferase in Saccharomyces cerevisiae and is the catalytic subunit of the large NuA4 complex (5–7), while Eaf3 is also a subunit of the RPD3S histone deacetylase complex, implicated in the repression of cryptic transcription over the coding region of active genes (8).
NuA4 acetylates lysines on the N-terminal tails of H4, H2A, and H2AZ (Htz1) histones (7, 9–12), and its histone acetyltransferase (HAT) activity is important for the regulation of gene transcription (13–21) and also for the cellular response to DNA damage, recombination, and DNA double-strand break repair (22–27). More recently, NuA4 was also shown to regulate life span and autophagy through its ability to acetylate nonhistone proteins (28–30). While the NuA4 complex was first demonstrated to be recruited to specific gene promoters, where it locally acetylates nucleosomal histones (9, 14, 15, 18), more recent studies have shown that NuA4 is also present on actively transcribed coding sequences (open reading frames [ORFs]) (19, 20). Its enrichment at promoters correlates with an increase of histone acetylation and chromatin relaxation, allowing access to DNA for the transcription machinery, and its targeting to promoters is thought to be mediated by transcription activators (13, 15, 21, 31). However, NuA4 harbors several subunits carrying PTM-binding motifs (Fig. 1A), and it is tempting to speculate that these specific binding domains may have an important role in NuA4 recruitment and localization on chromatin. The Eaf3 chromodomain has been shown to recognize and bind methylated H3K36 residues catalyzed by the Set2 methyltransferase (8, 32, 33), while the Yng2 plant homeodomain can bind Set1-dependent methylated H3K4 (34, 35). Interestingly, active promoter regions are enriched in H3K4me3 and active coding regions are enriched in H3K36me3, two marks recognized by NuA4 subunits that could potentially mediate NuA4 targeting to specific chromatin regions.
FIG 1.
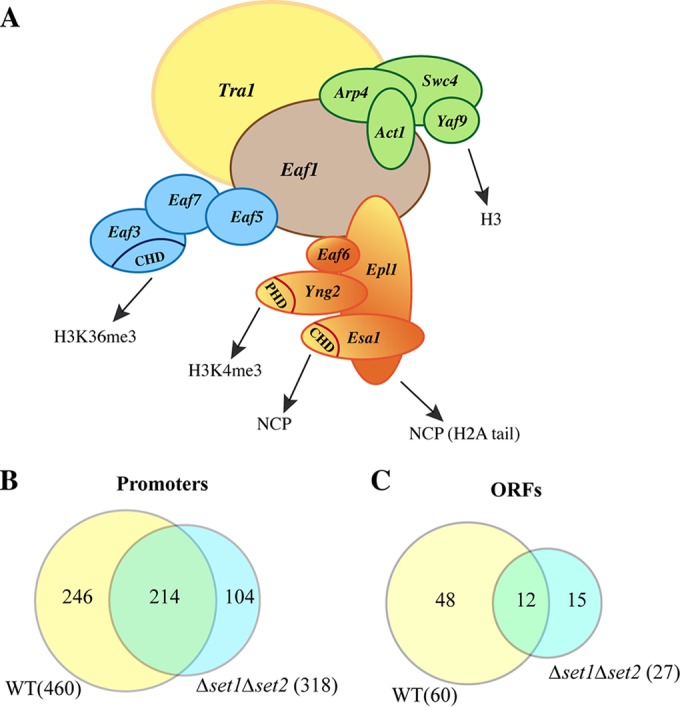
The NuA4 complex contains multiple specific binding modules, including methyl mark-binding motifs, but its localization on the genome is not dependent on H3K4 and H3K36 methylation. (A) Schematic representation of the submodule association forming the NuA4 complex and the specific binding domains (CHD and PHD) that recognize/bind histones, nucleosome core particles (NCP), or specific histone modifications. (B and C) Venn diagrams of Eaf1 ChIP on chip comparing Eaf1 enrichment on promoters (B) or gene coding regions (C) in WT cells (yellow) and Δset1 Δset2 mutant cells missing H3K4 and H3K36 methylation marks (blue). The yellow and blue areas correspond to Eaf1-enriched promoters and ORFs exclusively in WT and Δset1 Δset2 cells, respectively, whereas the overlapping area in green corresponds to the Eaf1-enriched regions in both types of cells.
In light of this, we decided to focus our study on NuA4 Eaf3 and Yng2 subunits containing methyl mark reader modules. We investigated how the properties of Yng2 and Eaf3 binding to methylated H3 tails affect NuA4 function. We demonstrate that the Eaf3 interaction with H3K36me3 and Yng2 binding to H3K4me3 alter NuA4 HAT activity on chromatin in vitro. Using growth assays and synthetic genetic arrays (epistatic miniarray profile [E-MAP] screens), we show that the Yng2 PHD function is directly linked to the Bre1/Rad6-Set1/COMPASS axis and early steps of the transcription process. Importantly, genome-wide chromatin immunoprecipitation (ChIP) coupled with next-generation sequencing (ChIP-seq) data indicate that Yng2 binding to H3K4me3 does not significantly affect NuA4 binding to promoters but is important for histone H4 acetylation near the transcription start site (TSS). In contrast, the presence of Eaf3 in NuA4 appears to be critical for proper acetylation toward the 3′ end of actively transcribed coding sequences, again without significantly affecting the association of the complex with these regions. Interestingly, our results demonstrate that the combination of Eaf3 and Yng2 interactions with their respective methyl marks on histone H3 is not important for NuA4 localization on the genome but is required for proper H4 acetylation throughout the transcription unit.
MATERIALS AND METHODS
Yeast strains, mutagenesis, and growth assays.
All the yeast strains used in this study are presented in Table S1 in the supplemental material. Gene deletion, tagging, or punctual mutations were performed using standard yeast genetic protocols (36, 37). The Yng2 W247A and 6K/R-A mutations were integrated in the genome at the yng2 endogenous locus and created by homologous recombination of two PCR products: one sequence containing the mutation(s) and one sequence carrying the NatR selection cassette (from plasmid p4339), inserted at the 5′ end of the gene. The low-copy-number (ARS/CEN) yeast vectors (pFL36) for expression of the mutant Eaf3 protein lacking its chromodomain (the vectors contained an N-terminal 2× Flag tag and the EAF3 promoter) were constructed following standard procedures and PCR-mediated deletion of the sequence coding for amino acids 77 to 120. Growth assays were performed using exponentially growing cells harvested when the optical density at 600 nm (OD600) was 0.5 and resuspended in 1 ml of sterile water. Then, 10-fold serial dilutions of these cells were spotted on yeast extract-peptone-dextrose (YPD) plates containing or not containing different drugs (6 mM caffeine, 3% formamide, 5, 15, 20, or 25 nM rapamycin, 130 mM hydroxyurea, 0.03% methyl methanesulfonate) or on plates with synthetic complete medium without Leu for cells containing the pFL36-Flag-Eaf3 plasmid, and the cells were grown at 30°C or the temperature indicated below.
Recombinant GST purification and peptide pulldown and phosphatidylinositol phosphate (PIP) binding assays.
For recombinant glutathione S-transferase (GST)-tagged protein purification, 1 liter of Escherichia coli BL21 bacteria at an OD600 of 0.5, induced for 3 h at 37°C with IPTG (isopropyl-β-d-thiogalactopyranoside; 200 μg/ml), were harvested and washed with 20 mM HEPES, pH 7.5, 200 mM NaCl. Cell pellets were resuspended in lysis buffer (20 mM HEPES, pH 7.5, 200 mM NaCl, 1 mM phenylmethylsulfonyl fluoride [PMSF], 2 mg/liter leupeptin, 2 mg/liter pepstatin, 2 mg/liter benzamidine, 2 mg/liter aprotinin, 10 mM β-mercaptoethanol), flash-frozen in liquid nitrogen, thawed on ice, and incubated for 10 min at room temperature with 500 μg/ml lysozyme. After addition of 0.1% NP-40, cells were sonicated 3 times for 10 s each time, and NP-40 was added to a 0.5% final concentration with Triton X-100 at a 1% final concentration. The lysates were gently shaken for 1.5 h at 4°C and then for 15 to 30 min at room temperature and centrifuged. The proteins in the supernatant were incubated for 2 h at 4°C with glutathione-Sepharose 4B beads and washed with lysis buffer, and GST-tagged proteins were eluted with elution buffer (50 mM Tris-HCl, pH 8, 10 mM glutathione, 5% glycerol) and analyzed on SDS-polyacrylamide gels with coloration with Coomassie stain.
Peptide pulldown assays were performed as previously described (38) with a few modifications. Briefly, 500 ng of biotinylated histone H3 peptides (amino acids 1 to 21 or 21 to 44) was incubated with 1 μg of purified recombinant GST-protein in binding buffer (300 mM NaCl, 50 mM Tris, pH 7.5, 0.05% NP-40, 100 μg/ml bovine serum albumin [BSA], 1 mM PMSF) at 4°C for 2 h and for 1 h with streptavidin-coupled magnetic beads (Invitrogen). The beads were then washed four times with binding buffer, and GST-bound proteins were subjected to Western blotting using an anti-GST antibody (1/5,000; J. Lavoie).
For GST-binding assays on a PIP membrane, 2 μg/ml of GST-tagged purified protein was incubated overnight at 4°C with PIP membranes (P-6001; Echelon Biosciences) that had been preblocked for 1 h in 3% BSA (fatty acid free; Sigma) and Tris-buffered saline–Tween 20. The membranes were washed to remove unbound GST-tagged protein, and bound proteins were then detected by Western blotting using anti-GST antibody (1/5,000; J. Lavoie) and incubation for 1 h at room temperature.
Affinity purification of protein complexes.
Tandem affinity purification of complexes was performed as described previously (39) with a few modifications reported elsewhere (12, 20). Cultures of 250 ml of exponentially growing cells were harvested at an OD600 of 1 to 2 and washed in 10 mM Tris, pH 8, 350 mM NaCl, and cell lysis was achieved with glass beads at 4°C in lysis buffer (10 mM Tris-HCl, pH 8, 350 mM NaCl, 10% glycerol, 0.1% NP-40, 1 mM PMSF, 0.5 mM dithiothreitol [DTT], 2 μg/ml pepstatin, 2 μg/ml leupeptin, 5 μg/ml aprotinin, 5 mM β-glycerophosphate, 5 mM Na butyrate). After 20 min of centrifugation at 20,000 × g at 4°C, cell lysates were incubated overnight with magnetic beads manually prebound with IgG (as described by Mitchell et al. [16]) or IgG-Sepharose beads. The beads were then washed, and complexes tagged by tandem affinity purification (TAP) were eluted with tobacco etch virus (TEV) recombinant enzyme in TEV elution buffer (10 mM Tris-HCl, pH 8, 150 mM NaCl, 0.5 mM EDTA, pH 8, 0.5 mM DTT). The eluted complexes were incubated for 20 min at 4°C with protein A-Sepharose beads to eliminate the remaining free IgG. For IgG-Sepharose-purified complexes, a calmodulin step was added. Eluted complexes were mixed with 3 volumes of calmodulin binding buffer (10 mM Tris-HCl, pH 8, 150 mM NaCl, 1 mM Mg acetate, 2 mM CaCl2, 1 mM imidazole, 10 mM β-mercaptoethanol, 0.1% NP-40), 3 μl of 1 M CaCl2/ml of the flowthrough recovered, and 50 μl of calmodulin affinity resin (Stratagene). After an overnight incubation on a wheel at 4°C, the resin was washed in the same buffer. For elution, 75 μl of calmodulin elution buffer (10 mM Tris-HCl, pH 8, 150 mM NaCl, 1 mM Mg acetate, 20 mM EGTA, pH 8, 1 mM imidazole, 10 mM β-mercaptoethanol, 0.1% NP-40, 5% glycerol, 1 mM PMSF, 2 μg/ml pepstatin, 2 μg/ml leupeptin, 5 μg/ml aprotinin, 5 mM β-glycerophosphate, 5 mM Na butyrate) was added to the resin, and elution was performed for 1 h at 4°C. Second and third elutions were performed as described above. The complexes that eluted with calmodulin and TEV were separated by SDS-PAGE and analyzed by silver staining to verify their composition and purity.
Purification of native chromatin and histone acetyltransferase assays.
Purification of yeast oligonucleosomes from nuclei was performed by the protocol previously reported (40). After fractionation by gel filtration (Superose 6; Amersham Biosciences), fractions containing short oligonucleotides (with DNA sizes of between 300 and 2,000 bp) were pooled and concentrated, and histone purity was confirmed by SDS-PAGE and Coomassie staining. Purified oligonucleosomes were also subjected to Western blotting to verify the posttranslational modifications. Histones were separated on an 18% acrylamide-SDS gel, transferred onto a nitrocellulose membrane, and blotted with anti-acetylated H4K5 (anti-H4K5ac), anti-H4K8ac, anti-H4K12ac, and anti-H4K16ac (from Serotech) and anti-H3 (Abcam catalog number ab1791), anti-H3K4me2 (Millipore catalog number 07-030), anti-H3K36me2 (Millipore catalog number 07-274), and anti-H3K79me (Abcam catalog number ab3594).
HAT assays on native chromatin were performed essentially as previously reported (7). TAP-purified complexes were normalized either by Western blotting using anti-Esa1 antibody or by their HAT activity on free core histone purified from HeLa cells. For kinetic assay, purified complexes were preincubated with the native chromatin substrate for 15 min at room temperature in HAT buffer (50 mM Tris-HCl, pH 8, 50 mM KCl plus NaCl, 0.1 mM EDTA, 5% glycerol, 1 mM DTT, 1 mM PMSF, 20 mM Na butyrate) before adding [3H]acetyl coenzyme A ([3H]acetyl-CoA; 0.125 μCi). For time course assays, reactions were stopped at the times indicated below by spotting the assay mixture on p81 filters, and the counts were determined in scintillation liquid as described previously (41).
E-MAP.
E-MAP screens were performed and normalized as described previously (42). Using a Singer robot, the mutants indicated below were crossed to a library of 1,536 mutants (42) covering a number of GO categories, including RNA processing, kinases/phosphatases, and chromatin biology. The mutants contained either complete deletions or decreased abundance by mRNA perturbation (DAmP) alleles of the indicated genes. All strains were screened in triplicate, with each triplicate containing three technical replicates. Colony size was used to determine the S score, a modified t-statistic measure which captures both the confidence and the strength of the genetic interaction. Scores greater than 2.0 or less than −2.5 are considered significant (43). The full data set is included in Table S2 in the supplemental material.
ChIP on chip.
Chromatin immunoprecipitation of NuA4 with anti-Eaf1 antibody or preimmune serum from the same rabbit (12) was carried out with wild-type (WT) and mutant yeast cells grown in YPD medium. The subsequent genome-wide location analyses were performed with a tiling microarray covering the whole genome, with one PCR probe per ORF and one PCR probe per intergenic region being used. Regions of significant enrichment were determined by measuring the ratio of the anti-Eaf1 signal versus the preimmune signal in WT or set1 set2 double mutant cells, and enrichment was considered significant when the ratio (Cy3/Cy5 in duplicate with reciprocal labeling) was greater than 2 and the adjusted P value was <0.05. The full data set is presented in Tables S3 and S4 in the supplemental material.
ChIP-seq and data analysis.
Chromatin immunoprecipitations were performed essentially as previously described (20). Chromatin samples were prepared from 200 ml of exponentially growing cells using the standard protocol. One hundred micrograms of each chromatin was immunoprecipitated using anti-Myc (2 μl; catalog number 9E10; Babco), anti-H4K8ac (0.5 μl; catalog number 07328, lot 2433445; Upstate), anti-H4 (1.5 μl; catalog number 7311, lot GR-125550-1; Abcam), and anti-RNA polymerase II (Pol II; 8WG16; 2 μl; Covance) antibodies, followed by incubation with protein A magnetic beads (Invitrogen) for H4K8ac, H4, and RNA Pol II or with protein G magnetic beads for anti-Myc. ChIP efficiency was verified by real-time quantitative PCR on single loci using a LightCycler apparatus (Roche), and 25 to 30 ng of purified DNA was used to create ChIP libraries. Illumina next-generation sequencing was performed as 50-bp single-end reads (HiSeq 2000 sequencing system; McGill University Genome Innovation Centre).
For every ChIP-seq experiment, we mapped reads to the April 2011 build of the S. cerevisiae genome (sacCer3) using the bowtie (version 0.12.8) program (44). All the alignments were converted into the number of reads per million reads using the BEDTools (version 2.16.2) program (45). We then used the deepTools program (46) to correct the acetylation ChIP-seq signal to the H4 level by determining the ratio of the acetylation signal to the corresponding H4 signal using the bamCompare function. This ratio was finally divided by the mean ratio for a 4-kb region in the middle of FMP27, a gene known to be expressed at very low levels. The RNA Pol II ChIP-seq signals are based on biological replicates after subtraction of the signal in the middle of FMP27. To calculate the statistically significant variations of Pol II signals over the first and last halves of the ORFs, the distribution of the median signal over the region between the various mutants and the wild-type condition was compared using a Wilcoxon signed-rank test. The Myc ChIP-seq signal was obtained after subtraction of the signal from a corresponding experiment with untagged proteins. All gene body summary plots were generated using a custom R script directly from the output files produced by the function heatmapper of the deepTools program. We used the yeast gene expression data of Holstege et al. (47) for stratification of the gene body signal into quartiles of transcription levels (with 25% of genes being in each quartile).
Accession number(s).
The full data set has been deposited in the GEO public database under accession number GSE77945.
RESULTS
Methylation of H3K4 and H3K36 is not required for NuA4 binding to the genome.
In order to determine if the transcription-linked methylation of H3K4 and H3K36 is important for NuA4 localization on the genome, we initially performed a ChIP on chip experiment in cells in which both histone methyltransferases (HMTs) responsible for methylation of these residues, Set1 and Set2, respectively, were deleted. We used an antibody against the Eaf1 scaffold subunit, as it is exclusive to NuA4 (12), and compared signal enrichment over that for preimmune serum on promoters and open reading frames (ORFs) between WT and Δset1 Δset2 cells (Fig. 1B and C). As expected, we observed that NuA4 was detected on promoter regions of highly transcribed genes as well as coding regions (460 and 60 enriched regions, respectively, using a 2-fold enrichment cutoff; see Table S3 in the supplemental material) (20, 48, 49). Interestingly, a large portion of these enriched regions was still detected in cells lacking both the Set1 and Set2 HMTs (see Table S4). In fact, when the enrichment cutoff was increased to 3-fold, no significant difference between WT and mutant cells could be seen (∼120 enriched promoters; data not shown). These results suggest that methylation of H3K4 and H3K36 is not essential for NuA4 overall localization on gene promoters. While it is difficult to draw conclusions from the small number of enriched coding regions detected, the loss of Set1/Set2 generates an apparent decrease in the number of Eaf1-enriched ORFs, indicating that methylation of H3K4 and HeK36 may influence NuA4 recruitment or retention on a subset of gene coding sequences, as was previously described for GAL1 and ADH1 (50).
Eaf3 binding to H3K36me influences NuA4 HAT activity in vitro.
To better understand the link between NuA4 and H3K4/H3K36 methylation, we analyzed the function of the CHD-containing Eaf3 and PHD-containing Yng2 subunits. Eaf3 harbors several specific domains: a chromodomain of the chromo barrel type at its N terminus, a putative DNA binding region, and a large highly conserved MRG domain (Fig. 2A) (51). We have previously shown that Eaf3 is part of the TINTIN trimeric subcomplex (Eaf5-Eaf7-Eaf3), which associates with NuA4 through Eaf5 binding to the scaffold subunit Eaf1 (20). Furthermore, Eaf3 is also a subunit of the Rpd3S deacetylase complex and was shown to be involved in the binding/retention of Rpd3S on coding regions of active genes to repress spurious transcription (8, 11, 32, 52, 53).
FIG 2.
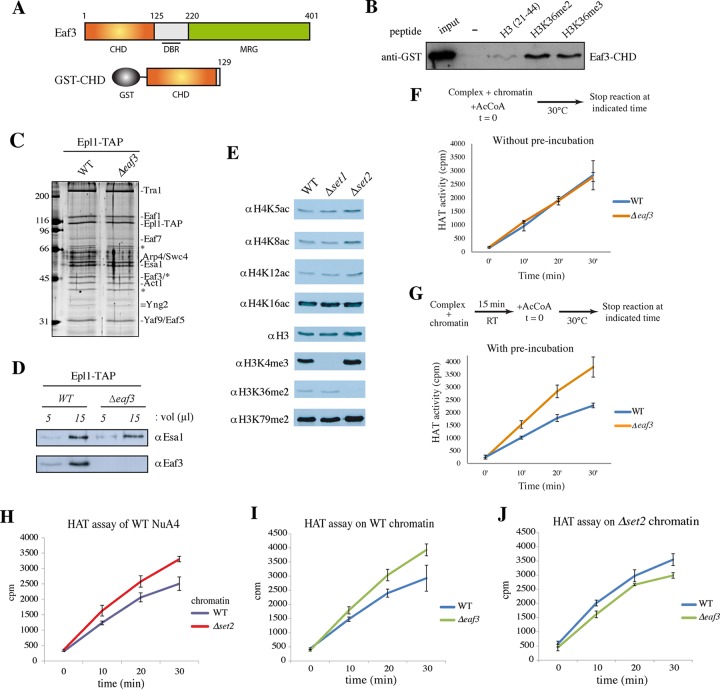
The Eaf3 chromodomain (CHD) binds H3K36 methylated residues and affects NuA4 HAT activity in vitro. (A) Schematic representation of CHD-containing protein Eaf3. The Eaf3 protein also contains a central DNA binding region (DBR) (51) and an MRG domain. For GST-CHD construction, the first 1 to 129 amino acids was used to test the CHD binding specificity. (B) Peptide pulldown assay using purified GST-CHD of Eaf3 and H3 (amino acids 21 to 44) peptides that were unmodified or that contained K36me2 and K36me3. Binding to the peptides was detected by Western blotting using an anti-GST antibody and compared to the signal achieved with 50% of the input. (C) Silver-stained gel of NuA4 complexes purified by tandem affinity purification using the Epl1 (Epl1-TAP) subunit from WT and Δeaf3 cells. Molecular size markers (in kilodaltons) are indicated on the left, and bands corresponding to subunits are identified on the right. Asterisks indicate nonspecific bands, one of which masked Eaf3. (D) Western blot analysis of the complexes shown in panel C performed using anti-Esa1 and anti-Eaf3 antibodies. (E) Western blot analysis of native chromatin (oligonucleosomes) purified from the indicated yeast strains and used as the substrate for HAT assays. Antibodies against the indicated histone marks were used to verify the global impact of the Δset1 and Δset2 mutants. (F and G) Kinetics of HAT assays using WT and Δeaf3 NuA4 complexes on native wild-type chromatin. Schematic representations of the experimental steps of HAT assay kinetics without or with 15 min of preincubation at room temperature (RT) of enzyme with chromatin are shown on the top of panels F and G, respectively (t = 0, time zero). In both experiments (F and G), the reactions were stopped at the indicated times (0, 10, 20, and 30 min) by spotting on p81 membranes, and the incorporation of acetyl-CoA (AcCoA) was quantified in scintillation liquid. Purified complexes were normalized on the basis of both the Esa1 signals in the Western blots and their activity on free histones. Error bars correspond to standard deviations between independent assays. (H to J) Kinetics of HAT assays with NuA4 complexes purified from WT and Δeaf3 cells on native chromatin purified from WT and Δset2 cells. NuA4 complexes were preincubated with the chromatin substrate for 15 min at room temperature, and at time zero, acetyl-CoA was added to the reaction mixture. The reactions were stopped at the indicated times, and the reaction mixtures were processed as described in the legend to panel G. The activities of WT NuA4 on WT versus Δset2 chromatin (H), of WT NuA4 versus Δeaf3 NuA4 on WT chromatin (I), and of WT versus Δeaf3 NuA4 on Δset2 chromatin (J) were tested.
First, we used recombinant GST-Eaf3-CHD in histone peptide pulldown assays to confirm its specificity for di- and trimethylated H3K36 (Fig. 2B). Then, in order to evaluate the impact of Eaf3 CHD binding to H3K36me on NuA4 activity, we performed time course histone acetyltransferase assays on native yeast chromatin using complexes purified from WT and Δeaf3 strains. As we have previously demonstrated, Eaf3 is not essential for the NuA4 structure or NuA4 assembly, as its absence does not lead to the loss of any other subunit of the complex (20) (Fig. 2C and D). We also purified yeast native chromatin from WT and histone methylation-defective cells (Δset1 and Δset2 cells) for use as the most physiological substrate in HAT kinetic assays (compared to homogeneous/single-sequence recombinant nucleosome core particles with methyl-lysine analogs) (Fig. 2E) (note that histone H4 acetylation sites are not globally affected in these mutant chromatins). Interestingly, using WT chromatin, we detected a clear effect of Eaf3 loss only when the NuA4 complex was preincubated with the oligonucleosomes for 15 min before addition of acetyl-CoA, allowing an interaction to occur and stabilize before the onset of acetylation (compare Fig. 2F and G). Then, using chromatin from WT and Δset2 cells, we determined that the absence of H3K36 methylation allowed NuA4 to acetylate more rapidly (Fig. 2H). This result suggests that H3K36 methylation directly affects NuA4 HAT activity on nucleosomal histones through local retention, which would have a negative impact on free diffusion along chromatin and, therefore, processive acetylation. To verify whether this effect is linked to H3K36me binding by Eaf3, we compared the results of HAT assays with NuA4 complexes lacking the Eaf3 subunit on WT and Δset2 chromatin (Fig. 2I and J). As was shown in Fig. 2G, we observed that the absence of Eaf3 led to the more rapid acetylation of wild-type chromatin, similar to the effect of H3K36me0 in Fig. 2H. Then, to assess the combined effect on processive acetylation of chromatin by NuA4 in the absence of H3K36me or Eaf3, we tested the HAT activity of WT and Δeaf3 NuA4 on Δset2 chromatin. As shown in Fig. 2I and J, NuA4 lacking Eaf3 did not acetylate chromatin more rapidly than the WT complex when H3K36me was absent from its substrate. These results indicate a functional redundancy between Eaf3 in NuA4 and the H3K36me mark during acetylation of chromatin. While the methylation of H3K4 and H3K36 was previously shown to affect NuA4 binding to TAP-purified nucleosomes in vitro (50), these data demonstrate that Eaf3 can play an important role in NuA4 activity, allowing significant local retention on H3K36me-rich regions of chromatin (after preincubation) and therefore decreasing processive global acetylation in our in vitro assay.
Functional dissection of the Yng2 C terminus for binding to H3K4me3 and phospholipids.
To further understand the impact of histone methylation on NuA4 function, we also analyzed the PHD-containing protein Yng2. This subunit has been shown to be important for gene activation and histone H4 acetylation in vivo and essential for acetylation of nucleosomes in vitro (9, 54–57). Yng2 is part of the highly conserved ING tumor suppressor family, and its PHD motif has been shown to directly bind H3K4me3 (34, 35). Dissection of the C-terminal domains of Yng2 showed that it contains a PHD motif followed by a frequently associated polybasic region (PBR). Other PHD/PBR-containing proteins, such as ING2, Pf1, and TAF3, have been shown to bind phosphoinositides (phosphatidylinositol biphosphate or phosphatidylinositol triphosphate), and this binding is important for phospholipid-dependent nuclear signaling and gene expression, arguably through cross talk with recognition of H3K4 by the PHD (58–62). In order to evaluate the function and the biochemical properties of these domains, we generated mutants with mutations in these two regions (Fig. 3A). We mutated one critical amino acid in the PHD (W247A) and 6 basic residues, lysines and arginines, into alanines in the PBR (the mutant is named PIP and is comparable to the loss-of-function K/R-A mutants generated in Pf1 or ING2 PBRs) (59, 60). We confirmed by peptide pulldown that the C terminus of Yng2 (containing both PHD and PBR motifs) strongly bound H3K4me3 in vitro, while the W247A mutation abolished this specific binding (Fig. 3B, lane 2 versus lane 4) (35). In contrast, mutation of the PBR motif did not affect the binding to H3K4me3 peptides (Fig. 3B, lane 2 versus lane 3). In parallel, using the same constructs, we measured potential binding to phospholipids using a membrane spotted with various lipids, as represented in Fig. 3C. This binding assay clearly shows that the PHD/PBR C-terminal region of Yng2 recognizes specific phospholipids with a clear preference for phosphatidylinositol 3-phosphate and weaker binding to phosphatidylinositol 4-phosphate, phosphatidylinositol 5-phosphate, and maybe phosphatidylinositol 3,4,5-triphosphate. Importantly, this binding is not affected by the W247A PHD mutation but is abolished by the mutation of the 6 basic residues in the PBR (Fig. 3C). Together, these results reveal distinct features of these two closely packed binding domains in Yng2, clearly distinguishing the roles of histone and phospholipid binding.
FIG 3.
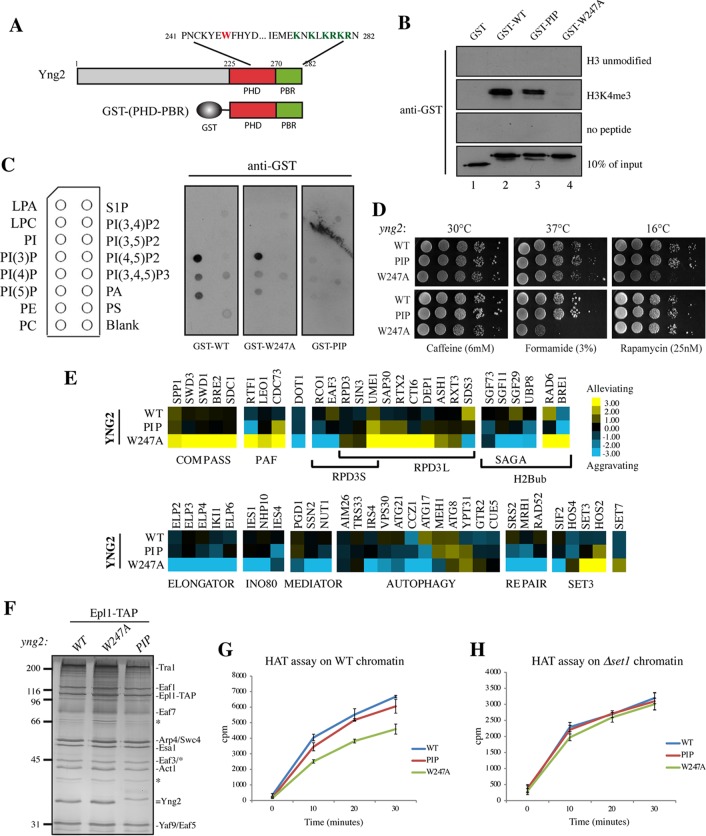
The Yng2 C terminus contains two functionally distinct domains, PHD and PBR. (A) Schematic representation of the Yng2 PHD and PIP-binding domains depicting the amino acid sequence with mutated W247 in the PHD (red) and the 6K/R mutated in the PBR (green), corresponding to K274, K276, K278, R279, K280, and R282. The GST-(PHD-PBR) construction used in the in vitro assays whose results are presented in panels B and C is also schematically represented. (B) Peptide pulldown assay of purified GST-Yng2 WT or the 6K/R-A (designated PIP) or W247A mutant with H3K4me3 and unmodified H3 peptides. Specific binding was detected by Western blotting using an anti-GST antibody. (Note that the slightly less apparent binding of the PIP mutant is explained by the smaller amount of input protein.) (C) Lipid dot blots were probed with purified GST-Yng2-(PHD-PBR) WT or the W247A or PIP mutant, as indicated, and specific binding to lipids was detected with an anti-GST antibody. A schematic representation of the overlay is given on the left, and the definitions of the abbreviations are as follows: LPA, lysophosphatidic acid; LPC, lysophosphatidylcholine; PI, phosphatidylinositol; PI(3)P, phosphatidylinositol 3-phosphate; PI(4)P, phosphatidylinositol 4-phosphate; PI(5)P, phosphatidylinositol 5-phosphate; PE, phosphatidylethanolamine; PC, phosphatidylcholine. (D) Spot assays showing the effect of PIP and W247A mutations on cell growth. Tenfold serial dilutions of the indicated cells were grown for 2 to 10 days on YPD at either 30°C, 37°C, or 16°C (top) or at 30°C on the indicated drug-containing medium (bottom). (E) Subsets of genetic interaction profiles for WT and the yng2-PIP and yng2-W247A mutants. Blue and yellow represent aggravating and alleviating genetic interactions, respectively (i.e., slower or faster growth/fitness compared to the baseline expected from the accumulation of the individual effects of the single mutations). Strong positive genetic interactions between the yng2-W247A mutant and subunits of the COMPASS or PAF complex and strong negative interactions with subunits of Elongator or INO80 factors were observed, while genetic interactions of these factors with the yng2-PIP mutant were rare and only moderate. (F) Silver-stained gel of NuA4 complexes purified from WT cells and yng2-W247A and yng2-PIP mutant cells and used in the HAT assays. Note the similar level of Yng2 protein in wild-type and mutant complexes and the shift in migration of the PIP mutant because of the loss of a positive charge. Molecular size markers (in kilodaltons) are indicated on the left, and bands corresponding to subunits are identified on the right. (G and H) Kinetics of acetylation by the NuA4 complexes shown in panel F on native chromatin purified from WT (G) and Δset1 mutant (H) cells. After 15 min of preincubation at room temperature of complexes with the substrate, the reactions were stopped at the indicated times and processed as described in the legend to Fig. 2. NuA4 complexes were normalized according to their HAT activity on free histones purified from HeLa cells.
The Yng2 PHD is functionally linked to transcription-related processes and can affect NuA4 activity in vitro.
To study the role of Yng2 PHD/PBR motifs in vivo, we generated yeast strains carrying the W247A and 6K/R-A (PIP) mutations. Cells expressing either mutant protein did not present any visible growth defect in rich medium (YPD at 30°C), at 37°C, or in caffeine- and rapamycin-containing media (Fig. 3D). However, cells expressing the W247A mutant exhibited clear growth defects at 16°C and on formamide-containing rich medium, and these defects were often linked to mutants defective in transcription-related processes and the response to environmental stress. In contrast, cells expressing the Yng2 PIP mutant did not show such phenotypes, indicating independent function.
To gain more specific insight into the functions of the Yng2 PHD and PBR, we performed an epistatic miniarray profile (E-MAP) analysis (42) of Yng2 mutant strains and compared the results to those for the wild type (Fig. 3E; see also Fig. S1 and Table S2 in the supplemental material). The results indicated that the W247A and PIP mutants have very different genetic interaction profiles. While Yng2-PIP mutant cells resembled wild-type cells, the Yng2-W247A mutant showed very strong positive genetic interactions with gene-encoding subunits of the COMPASS histone methyltransferase complex responsible for H3K4 methylation. In addition, they also demonstrated robust positive interactions with subunits of the PAF complex or Bre1/Rad6, responsible for H2B ubiquitination, both of which are essential for H3K4 trimethylation by COMPASS at the beginning of transcriptionally active genes. In contrast, the W247A mutant presented strong negative interactions with the SAGA H2B-deubiquitinase module, Elongator, RPD3S, INO80, and Mediator subunits, all of which are involved in transcription initiation or elongation. Interestingly, negative interactions with genes involved in autophagy and DNA repair, both of which are processes in which NuA4 has been implicated but which are thought to be independent of H3K4me binding (24, 30), were also detected. These results suggest that the Yng2 PHD functions mainly near the transcription start sites of active genes, where H3K4me3 is found to be more enriched, in agreement with its binding specificity. This genetic analysis also indicates that the PBR domain is not closely tied to the function of the PHD, nor is it clearly associated with any pathway.
While the Yng2 protein is critical for NuA4 HAT activity on nucleosomes in vitro and histone H4 acetylation in vivo (54, 56, 63), deletion of its PHD-containing C terminus does not affect global acetylation, NuA4 activity, or nucleosome binding in vitro (55, 57). We revisited this question using NuA4 complexes purified from cells expressing WT Yng2 and the W247A and PIP Yng2 mutants (Fig. 3F). Those complexes showed similar levels of associated Yng2 proteins (see also the Western blots in Fig. 4E) and were normalized according to their HAT activity on free histones. Time course HAT assays were performed after preincubation with purified native chromatin, as described above (Fig. 2G to H). As shown in Fig. 3G, we observed that NuA4 containing the Yng2-W247A mutant was less processive at acetylation of chromatin, while the PIP mutant was similar to the WT. However, this different kinetic of the W247A mutant was lost when Δset1 chromatin (lacking H3K4me) (Fig. 2E) was used as the substrate, showing that all three complexes acetylated similarly (Fig. 3H). In comparison, the activity of the NuA4 complex carrying the PIP mutant was not affected by the presence of H3K4me in chromatin. As for Eaf3 CHD and H3K36me, these results demonstrate a redundancy between the Yng2 PHD and H3K4me in regulating NuA4 activity in vitro, supporting their direct binding and functional interaction in the context of the native HAT complex and chromatin substrate. Interestingly, Yng2 PHD prebinding to H3K4me stimulated NuA4 processivity in vitro, while the Eaf3 CHD association with H3K36me tended to restrict it in our assay (Fig. 2). This differential effect of Yng2 and Eaf3 observed with native substrates could reflect the distinct distribution patterns of H3K4me3 and H3K36me2/3 on the yeast genome, with the former being localized near promoters/transcription start sites, while the latter covers larger regions over the body of the genes. It could also likely reflect the higher affinity of the Yng2 PHD for H3K4me3 compared to the affinity of Eaf3 CHD for H3K36me2/3 during the preincubation (equilibrium dissociation constants, 5 μM versus ∼300 μM [62, 64]).
FIG 4.
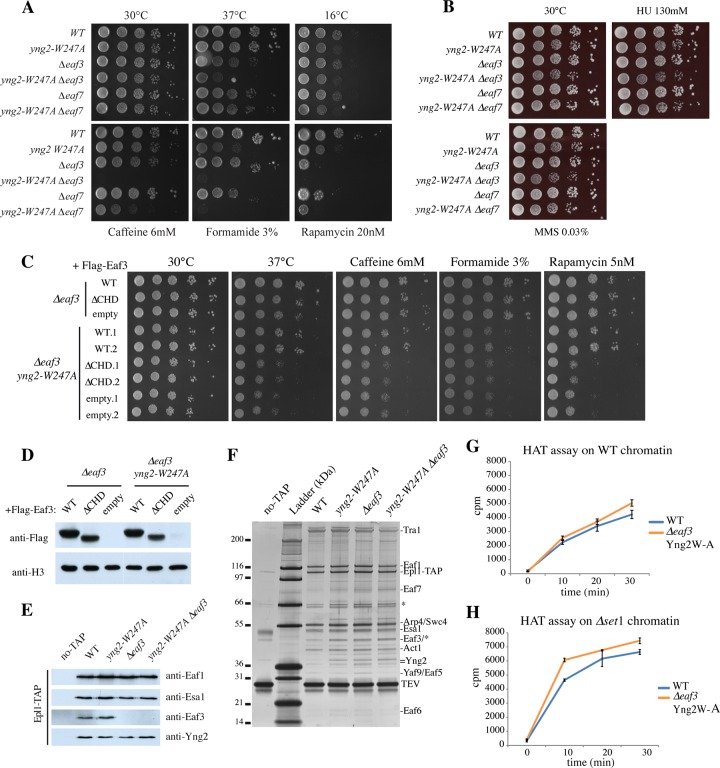
The Yng2 PHD and CHD-containing Eaf3 genetically and functionally interact in NuA4 function/activity. (A) Spot assays showing the effect of the W247A mutation of yng2 alone or in combination with deletion of eaf3 or eaf7 on cell growth. Tenfold serial dilutions of WT (strain QY3207), yng2-W247A (strain QY3208), Δeaf3 (strain QY3209), yng2-W247A Δeaf3 (strain QY3210), Δeaf7 (strain QY3219), and yng2-W247A Δeaf7 (strain QY3220) cells were grown for 2 to 10 days on YPD at the indicated temperatures (top) or at 30°C on the indicated drug-containing YPD (bottom). (B) Growth assays using the cells mentioned in panel A, which were spotted on the DNA-damaging agents methyl methanesulfonate (MMS; 0.03%) and hydroxyurea (HU; 130 mM). The cells were grown for 2 to 3 days at 30°C. (C) Spot assays with cells producing Eaf3 but lacking its chromodomain. Tenfold serial dilutions of Δeaf3 and Δeaf3 yng2-W247A cells containing plasmid pFL36 expressing Flag-Eaf3 WT or ΔCHD (amino acids 77 to 120) or the empty plasmid were grown on minimum medium (synthetic complete medium without Leu) at 30°C and 37°C and at 30°C on minimum medium containing the indicated drugs. (Note that the different levels of sensitivity to drugs compared to those in panel A are due to the use of minimal versus rich medium.) (D) Western blot analysis of wild-type and mutant Eaf3 expression in whole-cell extracts from cells used in the assay whose results are presented in panel C. Anti-Flag signals are compared to the anti-histone H3 signal as a loading control. (E) Western blot analysis of NuA4 complexes purified from WT, yng2-W247A, Δeaf3, and yng2-W247A Δeaf3 cells, using antibodies against the indicated subunits. no-TAP, a fraction purified from untagged cells by the method used for tagged cells. (F) Silver-stained gel of the NuA4 complexes analyzed in panel E and used in the HAT assays. Molecular size markers (in kilodaltons) are indicated on the left, and subunits are identified on the right. (G and H) Kinetics of HAT assays using NuA4 complexes purified from WT and yng2-W247A (Yng2W-A) Δeaf3 cells on native chromatin purified from WT (G) and Δset1 mutant (H) cells. After preincubation at room temperature for 15 min, acetyl-CoA was added to the reaction mixture, and the reactions were stopped at the indicated time points. Purified complexes were normalized on the basis of their activity on free histones. Values are based on those from two independent assays, and standard deviations are shown.
CHD-containing Eaf3 and the Yng2 PHD genetically interact and independently influence NuA4 activity in vitro.
After separately examining the interactions between NuA4 subunits and histone methylation marks, we investigated the combined functions of the Yng2 PHD and Eaf3 CHD. First, we analyzed the phenotypes of cells carrying both the yng2-W247A and Δeaf3 mutations and compared them to those of cells with each of the single mutations. As mentioned above, Eaf3 is also part of the RPD3S histone deacetylase complex, and a significant portion of the phenotypes observed in Δeaf3 cells may be due to the function of Eaf3 in this complex. To distinguish these roles, we also used a Δeaf7 mutant that specifically and exclusively removes Eaf3 from the NuA4 complex without affecting its presence in the RPD3S complex (Fig. 4A and B) (20). This is an important control, as Δeaf3 and Δeaf7 cells show some distinct phenotypes; e.g., both are sensitive to rapamycin, while they show distinct growth defects on formamide and caffeine (see also reference 20). Interestingly, when the eaf3 and eaf7 deletions were combined with the yng2-W247A mutation, growth was normal in rich medium but almost impossible on medium with caffeine, formamide, and rapamycin, supporting a functional link between the two chromatin reader modules within NuA4 during transcription (Fig. 4A). It is important to note that the increased temperature sensitivity at 37°C was restricted to eaf3/yng2-W247A mutants and not eaf7/yng2-W247A mutants and therefore implicates the RPD3S function instead of NuA4. In parallel, sensitivity to cold was restricted to the Yng2 PHD function, and no significant sensitivity to DNA-damaging agents was detected (Fig. 4B). In order to clearly implicate the Eaf3 CHD in the phenotypes that we observed, we also produced cells carrying a deletion of the chromodomain (which is episomal in a Δeaf3 background) alone or in combination with yng2-W247A and analyzed growth on the different media (Fig. 4C and D). Interestingly, cells expressing Eaf3 ΔCHD showed phenotypes comparable to those of cells completely lacking Eaf3, i.e., slow growth at 37°C and on medium containing caffeine, formamide, and rapamycin when combined with the yng2-W247A mutation. These results argue that the phenotypes of cells lacking Eaf3 are directly linked to the function mediated by its chromodomain.
In order to begin to discern the combined roles of the Yng2 PHD and Eaf3 CHD in NuA4 acetyltransferase activity, time course HAT assays were again carried out, but in this case they were carried out with NuA4 complexes purified from WT and Δeaf3 yng2-W247A cells (Fig. 4E to H). After preincubation with native nucleosomes, the NuA4 complex lacking both H3K4me3 and H3K36me2/3 binding motifs acetylated with kinetics similar to those of the wild-type complex, with slightly higher activity being detected only at the last time point (Fig. 4G). When tested on chromatin lacking H3K4me (purified from Δset1 cells), NuA4 from yng2-W247A Δeaf3 cells did seem to acetylate chromatin more efficiently than the wild-type complex (Fig. 4H). These in vitro results seem to represent the sum of the opposite effects seen in the assays with the single mutants, canceling each other on WT chromatin (Fig. 4G versus 2I and 3G), while showing the impact of the Eaf3 absence only when the H3K4me-Yng2 effect is taken out (Yng2 mutant on set1 chromatin) (Fig. 4H versus 2I and 3H). This suggests that Eaf3-CHD/H3K36me2/3 and Yng2-PHD/H3K4me3 interactions are independent yet additive in their impact on chromatin acetylation by NuA4 in this experimental setting. This likely reflects again that the H3K36me2/3 mark covers large coding regions compared to H3K4me3, which is localized primarily near the transcription start sites.
Recognition of H3K4me3 and H3K36me2/3 is important for NuA4 HAT activity across the entire transcription unit.
After studying the biochemical properties and phenotypes attributed to the two methylated H3-binding motifs in NuA4, we took a genome-wide approach to study their roles in NuA4 histone acetyltransferase activity, RNA Pol II occupancy, and NuA4 localization using chromatin immunoprecipitation coupled with next-generation sequencing (ChIP-seq). We recently reported that, under normal growth conditions, NuA4 is enriched on the promoter and coding regions of the most highly transcribed genes in the genome, a large portion of which code for ribosomal proteins (20). First, we analyzed the histone H4 acetylation profiles after correction for nucleosome occupancy (H4K8ac/total H4) in order to understand whether the recognition of methyl marks by Eaf3 and/or the Yng2 PHD is important for local NuA4-dependent acetylation (Fig. 5A). Genes were separated into 4 equal groups according to transcription levels. Figure 5A presents the metagene profiles of the group of 25% of genes most highly transcribed in the presence of NuA4 (the profiles of the remaining 3 groups are shown in Fig. S2A in the supplemental material). As expected, under wild-type conditions, H4K8ac-bearing nucleosomes were most enriched over the promoter regions and transcription start sites (TSS) of these highly transcribed genes (Fig. 5A, black line). Interestingly, cells expressing the Yng2 PHD mutant showed a clearly localized decrease in the level of H4 acetylation at the TSS and the beginning of the coding regions (Fig. 5A, red line). This result indicates that the Yng2 PHD is important for nucleosome acetylation by NuA4 over these regions enriched in H3K4me3. In comparison, Δeaf7 cells, which lack Eaf3 in NuA4, did not show such an H4K8ac decrease at the TSS but instead exhibited a significant loss of acetylation over the coding regions, with a larger difference being noted toward the 3′ end (Fig. 5A, green line). Once again, this result correlates the decrease of acetylation with the regions enriched in the histone mark (H3K36me2/3) targeted by the reader module that is mutated in NuA4.
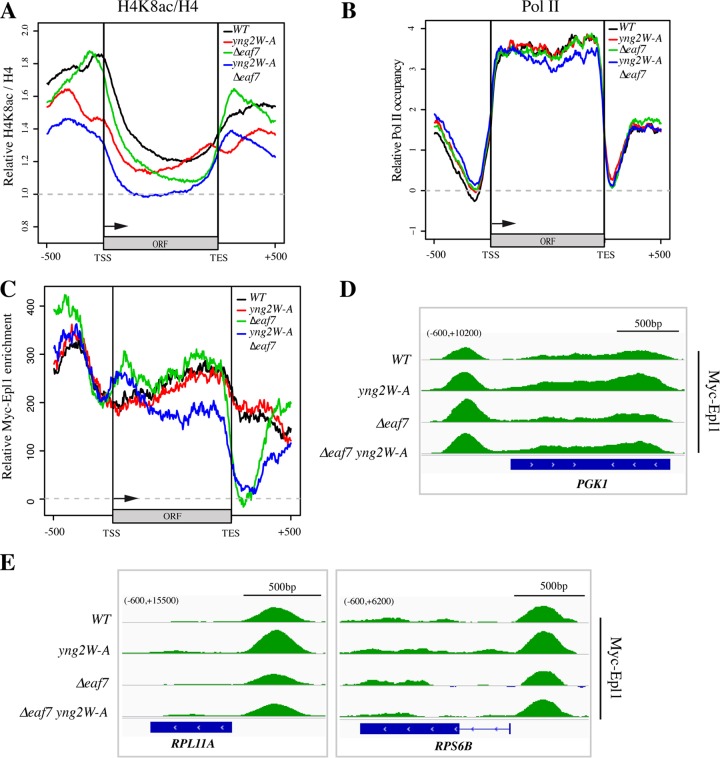
FIG 5.
Yng2 and Eaf3 are important for NuA4 activity on specific regions of the transcription unit but not for its association. (A and B) Metagene analysis of H4 acetylation and Pol II signals from ChIP-seq performed with WT, yng2-W247A, Δeaf7, and yng2-W247A Δeaf7 strains. Genes were divided into four equal groups according to their transcriptional frequency (47). The average profiles obtained for the 25% most transcribed genes are shown here (the average profiles for the three other groups are shown in Fig. S2A and B in the supplemental material). The analyzed region covers the sequence 500 bp upstream of the TSS and ORF and 500 bp downstream of the transcription end site (TES). (A) The average profiles of the H4K8ac ChIP-seq signals corrected on the basis of the total H4 signal divided by the mean ratio for a 4-kb region in the middle of the FMP27 gene, which has a low level of transcription. (B) Pol II occupancy profiles correspond to the average of the signals obtained with the anti-total Pol II antibody (8WG16) from which the mean Pol II signals of a 4-kb region in the middle of FMP27 were subtracted. The drop of the signal of RNA Pol II over the 3′ half of the ORFs in the yng2-W247A Δeaf7 double mutant cells (blue) was statistically significant (P = 0.006). (C) Metagene profiles of Myc-Epl1 signals in WT, yng2-W247A, Δeaf7, and yng2-W247A Δeaf7 cells in the 25% most active genes. Relative Myc-Epl1 enrichment corresponds to the Myc signals in the indicated strains from which the Myc signals obtained in untagged cells were subtracted. The drop of signal detected downstream of the ORFs in the Δeaf7 cells, while likely specific, could be exaggerated by the very low Myc-Epl1 signal over these regions (see the text). (D and E) Examples of ChIP-seq profiles of Myc-Epl1 enrichment on three highly transcribed genes, PGK1, RPL11A, and RPS6B. Myc-Epl1 enrichment corresponds to the Myc signals obtained when the signals for an untagged strain are subtracted. The numbers in parentheses at the top left of each panel correspond to the range of values on the y axis.
Only the simultaneous loss of NuA4 binding to H3K4me3 and H3K36me2/3 marks significantly affects RNA Pol II enrichment on the coding region of genes.
Surprisingly, the localized defects in H4 acetylation around the TSS in the Yng2 PHD mutant or the body of the genes in the Δeaf7 cells did not translate into any apparent change in RNA Pol II occupancy on these highly transcribed genes (as determined by monitoring Rbp1, the largest subunit of Pol II) (Fig. 5B). This is supported by the normal growth of these mutant cells in rich medium (see above). Mutants with a combination of mutations, on the other hand, did show a decreased occupancy of RNA Pol II toward the 3′ end of the genes (Fig. 5B, blue line) (P = 0.006 for the difference over the 3′ half of the ORFs). This condition correlates with the combined loss of H4 acetylation throughout the promoters, TSS, and coding regions, basically accumulating the individual effects of the yng2 and eaf7 single mutations and exacerbating them (Fig. 5A, blue line). Together these results indicate that the Yng2 PHD and Eaf3 CHD are important for NuA4-dependent H4 acetylation on distinct regions of transcribed genes, consistent with their histone mark specificities. However, only the combined loss of these reader modules has a detectable impact on polymerase occupancy on the body of the genes. Essentially, these results indicate that recognition of H3K4me3 by Yng2 and H3K36me2/3 by Eaf3 allows NuA4 to acetylate H4 tails across promoter and coding sequences and suggest that overlapping regions containing both histone marks may be key to regulating transcription elongation.
Methylated histone reader modules within NuA4 are not responsible for its recruitment to promoters or coding regions.
Considering the effects of the yng2-W247A and Δeaf7 mutations on H4 acetylation at TSS and ORFs and their combined impact on Pol II occupancy over highly transcribed genes, we also performed ChIP-seq analysis of NuA4 itself in these mutant cells. This is basically reevaluating the importance of methylated histones on NuA4 localization described in Fig. 1, albeit with incomparable resolution and through mutation of NuA4 reader modules instead of the histone marks. Using a Myc-tagged Epl1 subunit as the bait for ChIP, we detected not only NuA4 enrichment on the promoter regions of actively transcribed genes but also significant signals over the coding regions (Fig. 5C to E; see also Fig. S2C in the supplemental material), as we previously reported (20). When analyzing a subset of highly transcribed genes, such as the ribosomal protein genes RPL11A, RPS6B, RPL15A, and RPP1B or the PGK1 gene, we did not detect major changes in Epl1/NuA4 occupancy in either cells with a single yng2-W247A or Δeaf7 mutation or cells with double yng2-W247A and Δeaf7 mutations (Fig. 5D and E; see also Fig. S2C). Metagene analysis showed that Epl1/NuA4 remains highly enriched over the promoter regions of highly transcribed genes, supporting the ChIP on chip data presented in Fig. 1 and arguing that the combined loss of H3K4me and H3K36me has no significant consequence on overall NuA4 recruitment/binding to its target genes. Importantly, there was also little to no impact of the Yng2 PHD and Eaf3 CHD on the NuA4 association with the coding regions of highly transcribed genes. These results indicate that recognition of H3K4me and H3K36me by NuA4 reader modules is not important for its recruitment/binding to coding regions but is critical for accurate acetylation of the underlying nucleosomes (Fig. 5A). The drawing of conclusions on the impact of the yng2-W247A eaf7 double mutant on NuA4 localization over the coding region may be hampered by an indirect effect. Metagene analysis did show a significant decrease of NuA4 over the middle and 3′ end of transcribed regions in double mutant cells (Fig. 5C). This would suggest that the combined recognition of H3K4me and H3K36me is important for the NuA4 association with the body of transcribed genes. On the other hand, we have shown that Eaf7 associates with RNA Pol II during transcription elongation and that it is important for its localization on the coding regions (20). Therefore, the reduction in Pol II occupancy seen in double mutant cells (Fig. 5B) could be responsible for the decreased detection of NuA4, or vice versa. In parallel, the apparent strong loss of an Epl1-Myc signal just downstream of the ORFs in Δeaf7 mutant cells requires further investigation, as it could be related to a previously reported link between Eaf7 and nuclear exosome/3′ end formation (20). Nevertheless, taken together, these results argue that neither Yng2 binding to H3K4me3 nor Eaf3 binding to H3K36me3 is essential for NuA4 localization on its target genes, promoter, TSS, or coding regions. Yng2 PHD binding to H3K4me3 is instead important to direct NuA4 acetyltransferase activity toward the TSS region and 5′ end of the genes, while Eaf3 CHD binding to H3K36me2/3 directs acetylation of nucleosomes downstream on the coding region.
DISCUSSION
The work presented here investigated the function of Yng2 and Eaf3, two subunits of the essential NuA4 acetyltransferase complex. While NuA4-dependent histone acetylation has recently been linked to the histone H3 methylation state (50), we dissected the function of these two subunits that contain methyl mark-binding motifs. Using in vitro assays with native reagents and phenotypic/genetic analyses, we demonstrate the importance of the Yng2 PHD and the Eaf3 CHD in the recognition of H3K4me3 and H3K36me2/3, respectively. Both reader modules affect the kinetics of nucleosome acetylation by NuA4 in vitro, and their combined loss renders cells highly sensitive to drugs that disrupt various pathways linked to gene expression. E-MAP analysis of the Yng2 PHD mutant confirmed its close functional relationship with the molecular players involved in H3K4 trimethylation near the TSS of active genes, i.e., the Rad6/Bre1-H2BK123ub, PAF, and COMPASS complexes. Finally, genome-wide ChIP analyses demonstrated the importance of the two reader modules for localized NuA4-dependent acetylation along the transcription unit but not for the association of the HAT complex per se. Thus, NuA4 reader modules are not required for recruitment/localization of the complex on the genome in vivo but, rather, help orient the acetyltransferase activity toward the surrounding methylated nucleosomes. Therefore, the localization of NuA4 on the promoter region would be largely dependent on a recruiting DNA-bound factor, while the association with the coding region would depend on the interaction with the CTD of the advancing polymerase (19, 20). The primary dependence on the association with the CTD, rather than binding to H3K36me, has also been demonstrated for the Eaf3-containing RPD3S complex (65, 66). These studies showed that the interaction of Eaf3 with methylated nucleosomes was required for histone deacetylation but not the recruitment of RPD3S, analogous to what we observed here for NuA4.
During the last steps of preparation of the manuscript, a report implicating the combined action of Eaf3 CHD/H3K36me and Yng2 PHD/H3K4me in assisting NuA4 function during the repair of DNA double-strand breaks was published (67). While the concept is interesting, in our hands we could not detect any increased sensitivity to DNA-damaging agents for either our single or double mutant cells (20, 54) (Fig. 4B), and we decided not to pursue this line of investigation. In addition, it is important to point out that the work presented in the report does not distinguish Eaf3 function in NuA4 HAT versus RPD3S histone deacetylase. Nevertheless, Set1/H3K4me and Set2/H3K36me have previously been implicated in the DNA damage response (e.g., see reference 68) and could be used by NuA4 reader modules to modify chromatin near damage sites or to activate expression of DNA repair genes.
Alternative aspects of the NuA4 structure could play roles in its binding to specific regions of the genome. A short region of the Epl1 N terminus was shown to be required for binding to nucleosomes and the histone H2A tail, an interaction essential for acetylation of nucleosomal H4 (57, 69, 70). The N terminus of Yng2 is also required for efficient acetylation of nucleosomes, but the function of these domains cannot distinguish global acetylation by the Piccolo NuA4 complex and targeted acetylation by NuA4 (9, 55, 71). The Yaf9 YEATS domain was also shown to be structured similarly to the ASF1 histone chaperone and to bind histones (72). While these binding surfaces are important, they cannot account for the association with specific regions of the genome. Interestingly, Esa1, NuA4's catalytic subunit, also contains a chromodomain of the chromo barrel type, like Eaf3 does. Mutations in this domain are lethal, and it is specifically required to acetylate nucleosome substrates in vitro but not free histones (55, 70). Furthermore, this domain requirement is not dependent on a specific histone mark, as the experiments were performed with recombinant nucleosomes. It has been proposed that this type of chromo barrel/Tudor-like domain can recognize DNA and RNA (73, 74), including that of Esa1 (75; C. Gallo, N. Lacoste, J. Côté, and J. Smith, unpublished data). Strikingly, the mammalian NuA4 complex, Tip60-p400, was recently shown to bind R loops formed by nascent transcripts at the beginning of genes, which explains in part its transcription-dependent binding to these regions (76). It will be very interesting to verify whether this occurs in yeast and if it is dependent on the Esa1 chromodomain. This could lead to a three-step model for the NuA4 association with genes: first, recruitment by a DNA-bound transcription factor to the promoter; second, binding to nascent transcripts near the transcription start site; and third, association with the Ser2-P CTD of advancing RNA Pol II on the downstream coding region. In this model, recognition of methylated histone marks along the way by the Yng2 PHD and Eaf3 CHD directs NuA4 acetyltransferase activity to the underlying nucleosomes to favor transcription.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Stéphane Allard, Andréanne Auger, Xue Cheng, and Nancy Lévesque for initial experiments/technical support in this project. We thank Amine Nourani for comments on the text.
M.J.A. was supported by a Frederick Banting and Charles Best Canada graduate scholarship. M.S.K. holds the Canada Research Chair in Social Epigenetics, and J.C. holds the Canada Research Chair in Chromatin Biology and Molecular Epigenetics.
Funding Statement
This work was funded by grants from the Canadian Institutes of Health Research (CIHR) to François Robert (MOP-82891), to Michael S. Kobor (MOP-119383), and to Jacques Côté (MOP-14308 and FDN-334002).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.00112-16.
REFERENCES
- 1.Yun M, Wu J, Workman JL, Li B. 2011. Readers of histone modifications. Cell Res 21:564–578. doi: 10.1038/cr.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Musselman CA, Lalonde ME, Cote J, Kutateladze TG. 2012. Perceiving the epigenetic landscape through histone readers. Nat Struct Mol Biol 19:1218–1227. doi: 10.1038/nsmb.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang T, Cooper S, Brockdorff N. 2015. The interplay of histone modifications—writers that read. EMBO Rep 16:1467–1481. doi: 10.15252/embr.201540945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doyon Y, Cote J. 2004. The highly conserved and multifunctional NuA4 HAT complex. Curr Opin Genet Dev 14:147–154. doi: 10.1016/j.gde.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Smith ER, Eisen A, Gu W, Sattah M, Pannuti A, Zhou J, Cook RG, Lucchesi JC, Allis CD. 1998. ESA1 is a histone acetyltransferase that is essential for growth in yeast. Proc Natl Acad Sci U S A 95:3561–3565. doi: 10.1073/pnas.95.7.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke AS, Lowell JE, Jacobson SJ, Pillus L. 1999. Esa1p is an essential histone acetyltransferase required for cell cycle progression. Mol Cell Biol 19:2515–2526. doi: 10.1128/MCB.19.4.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allard S, Utley RT, Savard J, Clarke A, Grant P, Brandl CJ, Pillus L, Workman JL, Cote J. 1999. NuA4, an essential transcription adaptor/histone H4 acetyltransferase complex containing Esa1p and the ATM-related cofactor Tra1p. EMBO J 18:5108–5119. doi: 10.1093/emboj/18.18.5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carrozza MJ, Li B, Florens L, Suganuma T, Swanson SK, Lee KK, Shia WJ, Anderson S, Yates J, Washburn MP, Workman JL. 2005. Histone H3 methylation by Set2 directs deacetylation of coding regions by Rpd3S to suppress spurious intragenic transcription. Cell 123:581–592. doi: 10.1016/j.cell.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 9.Boudreault AA, Cronier D, Selleck W, Lacoste N, Utley RT, Allard S, Savard J, Lane WS, Tan S, Cote J. 2003. Yeast enhancer of polycomb defines global Esa1-dependent acetylation of chromatin. Genes Dev 17:1415–1428. doi: 10.1101/gad.1056603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Babiarz JE, Halley JE, Rine J. 2006. Telomeric heterochromatin boundaries require NuA4-dependent acetylation of histone variant H2A.Z in Saccharomyces cerevisiae. Genes Dev 20:700–710. doi: 10.1101/gad.1386306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keogh MC, Mennella TA, Sawa C, Berthelet S, Krogan NJ, Wolek A, Podolny V, Carpenter LR, Greenblatt JF, Baetz K, Buratowski S. 2006. The Saccharomyces cerevisiae histone H2A variant Htz1 is acetylated by NuA4. Genes Dev 20:660–665. doi: 10.1101/gad.1388106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Auger A, Galarneau L, Altaf M, Nourani A, Doyon Y, Utley RT, Cronier D, Allard S, Cote J. 2008. Eaf1 is the platform for NuA4 molecular assembly that evolutionarily links chromatin acetylation to ATP-dependent exchange of histone H2A variants. Mol Cell Biol 28:2257–2270. doi: 10.1128/MCB.01755-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Utley RT, Ikeda K, Grant PA, Cote J, Steger DJ, Eberharter A, John S, Workman JL. 1998. Transcriptional activators direct histone acetyltransferase complexes to nucleosomes. Nature 394:498–502. doi: 10.1038/28886. [DOI] [PubMed] [Google Scholar]
- 14.Reid JL, Iyer VR, Brown PO, Struhl K. 2000. Coordinate regulation of yeast ribosomal protein genes is associated with targeted recruitment of Esa1 histone acetylase. Mol Cell 6:1297–1307. doi: 10.1016/S1097-2765(00)00128-3. [DOI] [PubMed] [Google Scholar]
- 15.Nourani A, Utley RT, Allard S, Cote J. 2004. Recruitment of the NuA4 complex poises the PHO5 promoter for chromatin remodeling and activation. EMBO J 23:2597–2607. doi: 10.1038/sj.emboj.7600230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell L, Lambert JP, Gerdes M, Al-Madhoun AS, Skerjanc IS, Figeys D, Baetz K. 2008. Functional dissection of the NuA4 histone acetyltransferase reveals its role as a genetic hub and that Eaf1 is essential for complex integrity. Mol Cell Biol 28:2244–2256. doi: 10.1128/MCB.01653-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joo YJ, Kim JH, Kang UB, Yu MH, Kim J. 2011. Gcn4p-mediated transcriptional repression of ribosomal protein genes under amino-acid starvation. EMBO J 30:859–872. doi: 10.1038/emboj.2010.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uprety B, Sen R, Bhaumik SR. 2015. Eaf1p is required for recruitment of NuA4 in targeting TFIID to the promoters of the ribosomal protein genes for transcriptional initiation in vivo. Mol Cell Biol 35:2947–2964. doi: 10.1128/MCB.01524-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ginsburg DS, Govind CK, Hinnebusch AG. 2009. NuA4 lysine acetyltransferase Esa1 is targeted to coding regions and stimulates transcription elongation with Gcn5. Mol Cell Biol 29:6473–6487. doi: 10.1128/MCB.01033-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossetto D, Cramet M, Wang AY, Steunou AL, Lacoste N, Schulze JM, Cote V, Monnet-Saksouk J, Piquet S, Nourani A, Kobor MS, Cote J. 2014. Eaf5/7/3 form a functionally independent NuA4 submodule linked to RNA polymerase II-coupled nucleosome recycling. EMBO J 33:1397–1415. doi: 10.15252/embj.201386433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng X, Auger A, Altaf M, Drouin S, Paquet E, Utley RT, Robert F, Cote J. 2015. Eaf1 links the NuA4 histone acetyltransferase complex to Htz1 incorporation and regulation of purine biosynthesis. Eukaryot Cell 14:535–544. doi: 10.1128/EC.00004-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bird AW, Yu DY, Pray-Grant MG, Qiu Q, Harmon KE, Megee PC, Grant PA, Smith MM, Christman MF. 2002. Acetylation of histone H4 by Esa1 is required for DNA double-strand break repair. Nature 419:411–415. doi: 10.1038/nature01035. [DOI] [PubMed] [Google Scholar]
- 23.Choy JS, Kron SJ. 2002. NuA4 subunit Yng2 function in intra-S-phase DNA damage response. Mol Cell Biol 22:8215–8225. doi: 10.1128/MCB.22.23.8215-8225.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Downs JA, Allard S, Jobin-Robitaille O, Javaheri A, Auger A, Bouchard N, Kron SJ, Jackson SP, Cote J. 2004. Binding of chromatin-modifying activities to phosphorylated histone H2A at DNA damage sites. Mol Cell 16:979–990. doi: 10.1016/j.molcel.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Lin YY, Qi Y, Lu JY, Pan X, Yuan DS, Zhao Y, Bader JS, Boeke JD. 2008. A comprehensive synthetic genetic interaction network governing yeast histone acetylation and deacetylation. Genes Dev 22:2062–2074. doi: 10.1101/gad.1679508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.House NC, Yang JH, Walsh SC, Moy JM, Freudenreich CH. 2014. NuA4 initiates dynamic histone H4 acetylation to promote high-fidelity sister chromatid recombination at postreplication gaps. Mol Cell 55:818–828. doi: 10.1016/j.molcel.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bennett G, Papamichos-Chronakis M, Peterson CL. 2013. DNA repair choice defines a common pathway for recruitment of chromatin regulators. Nat Commun 4:2084. doi: 10.1038/ncomms3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin YY, Lu JY, Zhang J, Walter W, Dang W, Wan J, Tao SC, Qian J, Zhao Y, Boeke JD, Berger SL, Zhu H. 2009. Protein acetylation microarray reveals that NuA4 controls key metabolic target regulating gluconeogenesis. Cell 136:1073–1084. doi: 10.1016/j.cell.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu JY, Lin YY, Sheu JC, Wu JT, Lee FJ, Chen Y, Lin MI, Chiang FT, Tai TY, Berger SL, Zhao Y, Tsai KS, Zhu H, Chuang LM, Boeke JD. 2011. Acetylation of yeast AMPK controls intrinsic aging independently of caloric restriction. Cell 146:969–979. doi: 10.1016/j.cell.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yi C, Ma M, Ran L, Zheng J, Tong J, Zhu J, Ma C, Sun Y, Zhang S, Feng W, Zhu L, Le Y, Gong X, Yan X, Hong B, Jiang FJ, Xie Z, Miao D, Deng H, Yu L. 2012. Function and molecular mechanism of acetylation in autophagy regulation. Science 336:474–477. doi: 10.1126/science.1216990. [DOI] [PubMed] [Google Scholar]
- 31.Brown CE, Howe L, Sousa K, Alley SC, Carrozza MJ, Tan S, Workman JL. 2001. Recruitment of HAT complexes by direct activator interactions with the ATM-related Tra1 subunit. Science 292:2333–2337. doi: 10.1126/science.1060214. [DOI] [PubMed] [Google Scholar]
- 32.Joshi AA, Struhl K. 2005. Eaf3 chromodomain interaction with methylated H3-K36 links histone deacetylation to Pol II elongation. Mol Cell 20:971–978. doi: 10.1016/j.molcel.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 33.Keogh MC, Kurdistani SK, Morris SA, Ahn SH, Podolny V, Collins SR, Schuldiner M, Chin K, Punna T, Thompson NJ, Boone C, Emili A, Weissman JS, Hughes TR, Strahl BD, Grunstein M, Greenblatt JF, Buratowski S, Krogan NJ. 2005. Cotranscriptional set2 methylation of histone H3 lysine 36 recruits a repressive Rpd3 complex. Cell 123:593–605. doi: 10.1016/j.cell.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 34.Shi X, Hong T, Walter KL, Ewalt M, Michishita E, Hung T, Carney D, Pena P, Lan F, Kaadige MR, Lacoste N, Cayrou C, Davrazou F, Saha A, Cairns BR, Ayer DE, Kutateladze TG, Shi Y, Cote J, Chua KF, Gozani O. 2006. ING2 PHD domain links histone H3 lysine 4 methylation to active gene repression. Nature 442:96–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi X, Kachirskaia I, Walter KL, Kuo JH, Lake A, Davrazou F, Chan SM, Martin DG, Fingerman IM, Briggs SD, Howe L, Utz PJ, Kutateladze TG, Lugovskoy AA, Bedford MT, Gozani O. 2007. Proteome-wide analysis in Saccharomyces cerevisiae identifies several PHD fingers as novel direct and selective binding modules of histone H3 methylated at either lysine 4 or lysine 36. J Biol Chem 282:2450–2455. doi: 10.1074/jbc.C600286200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Longtine MS, McKenzie A III, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, Pringle JR. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14:953–961. [DOI] [PubMed] [Google Scholar]
- 37.Tong AH, Evangelista M, Parsons AB, Xu H, Bader GD, Page N, Robinson M, Raghibizadeh S, Hogue CW, Bussey H, Andrews B, Tyers M, Boone C. 2001. Systematic genetic analysis with ordered arrays of yeast deletion mutants. Science 294:2364–2368. doi: 10.1126/science.1065810. [DOI] [PubMed] [Google Scholar]
- 38.Avvakumov N, Lalonde ME, Saksouk N, Paquet E, Glass KC, Landry AJ, Doyon Y, Cayrou C, Robitaille GA, Richard DE, Yang XJ, Kutateladze TG, Cote J. 2012. Conserved molecular interactions within the HBO1 acetyltransferase complexes regulate cell proliferation. Mol Cell Biol 32:689–703. doi: 10.1128/MCB.06455-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puig O, Caspary F, Rigaut G, Rutz B, Bouveret E, Bragado-Nilsson E, Wilm M, Seraphin B. 2001. The tandem affinity purification (TAP) method: a general procedure of protein complex purification. Methods 24:218–229. doi: 10.1006/meth.2001.1183. [DOI] [PubMed] [Google Scholar]
- 40.Lacoste N, Utley RT, Hunter JM, Poirier GG, Cote J. 2002. Disruptor of telomeric silencing-1 is a chromatin-specific histone H3 methyltransferase. J Biol Chem 277:30421–30424. doi: 10.1074/jbc.C200366200. [DOI] [PubMed] [Google Scholar]
- 41.Brownell JE, Allis CD. 1995. An activity gel assay detects a single, catalytically active histone acetyltransferase subunit in Tetrahymena macronuclei. Proc Natl Acad Sci U S A 92:6364–6368. doi: 10.1073/pnas.92.14.6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collins SR, Roguev A, Krogan NJ. 2010. Quantitative genetic interaction mapping using the E-MAP approach. Methods Enzymol 470:205–231. doi: 10.1016/S0076-6879(10)70009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fiedler D, Braberg H, Mehta M, Chechik G, Cagney G, Mukherjee P, Silva AC, Shales M, Collins SR, van Wageningen S, Kemmeren P, Holstege FC, Weissman JS, Keogh MC, Koller D, Shokat KM, Krogan NJ. 2009. Functional organization of the S. cerevisiae phosphorylation network. Cell 136:952–963. doi: 10.1016/j.cell.2008.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Langmead B, Trapnell C, Pop M, Salzberg SL. 2009. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quinlan AR, Hall IM. 2010. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramirez F, Dundar F, Diehl S, Gruning BA, Manke T. 2014. deepTools: a flexible platform for exploring deep-sequencing data. Nucleic Acids Res 42:W187–W191. doi: 10.1093/nar/gku365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holstege FC, Jennings EG, Wyrick JJ, Lee TI, Hengartner CJ, Green MR, Golub TR, Lander ES, Young RA. 1998. Dissecting the regulatory circuitry of a eukaryotic genome. Cell 95:717–728. doi: 10.1016/S0092-8674(00)81641-4. [DOI] [PubMed] [Google Scholar]
- 48.Robert F, Pokholok DK, Hannett NM, Rinaldi NJ, Chandy M, Rolfe A, Workman JL, Gifford DK, Young RA. 2004. Global position and recruitment of HATs and HDACs in the yeast genome. Mol Cell 16:199–209. doi: 10.1016/j.molcel.2004.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Venters BJ, Wachi S, Mavrich TN, Andersen BE, Jena P, Sinnamon AJ, Jain P, Rolleri NS, Jiang C, Hemeryck-Walsh C, Pugh BF. 2011. A comprehensive genomic binding map of gene and chromatin regulatory proteins in Saccharomyces. Mol Cell 41:480–492. doi: 10.1016/j.molcel.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ginsburg DS, Anlembom TE, Wang J, Patel SR, Li B, Hinnebusch AG. 2014. NuA4 links methylation of histone H3 lysines 4 and 36 to acetylation of histones H4 and H3. J Biol Chem 289:32656–32670. doi: 10.1074/jbc.M114.585588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruan C, Lee CH, Cui H, Li S, Li B. 2015. Nucleosome contact triggers conformational changes of Rpd3S driving high-affinity H3K36me nucleosome engagement. Cell Rep 10:204–215. doi: 10.1016/j.celrep.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li B, Gogol M, Carey M, Lee D, Seidel C, Workman JL. 2007. Combined action of PHD and chromo domains directs the Rpd3S HDAC to transcribed chromatin. Science 316:1050–1054. doi: 10.1126/science.1139004. [DOI] [PubMed] [Google Scholar]
- 53.Li B, Gogol M, Carey M, Pattenden SG, Seidel C, Workman JL. 2007. Infrequently transcribed long genes depend on the Set2/Rpd3S pathway for accurate transcription. Genes Dev 21:1422–1430. doi: 10.1101/gad.1539307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nourani A, Doyon Y, Utley RT, Allard S, Lane WS, Cote J. 2001. Role of an ING1 growth regulator in transcriptional activation and targeted histone acetylation by the NuA4 complex. Mol Cell Biol 21:7629–7640. doi: 10.1128/MCB.21.22.7629-7640.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Selleck W, Fortin I, Sermwittayawong D, Cote J, Tan S. 2005. The Saccharomyces cerevisiae Piccolo NuA4 histone acetyltransferase complex requires the Enhancer of Polycomb A domain and chromodomain to acetylate nucleosomes. Mol Cell Biol 25:5535–5542. doi: 10.1128/MCB.25.13.5535-5542.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Loewith R, Meijer M, Lees-Miller SP, Riabowol K, Young D. 2000. Three yeast proteins related to the human candidate tumor suppressor p33(ING1) are associated with histone acetyltransferase activities. Mol Cell Biol 20:3807–3816. doi: 10.1128/MCB.20.11.3807-3816.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chittuluru JR, Chaban Y, Monnet-Saksouk J, Carrozza MJ, Sapountzi V, Selleck W, Huang J, Utley RT, Cramet M, Allard S, Cai G, Workman JL, Fried MG, Tan S, Cote J, Asturias FJ. 2011. Structure and nucleosome interaction of the yeast NuA4 and Piccolo-NuA4 histone acetyltransferase complexes. Nat Struct Mol Biol 18:1196–1203. doi: 10.1038/nsmb.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stijf-Bultsma Y, Sommer L, Tauber M, Baalbaki M, Giardoglou P, Jones DR, Gelato KA, van Pelt J, Shah Z, Rahnamoun H, Toma C, Anderson KE, Hawkins P, Lauberth SM, Haramis AP, Hart D, Fischle W, Divecha N. 2015. The basal transcription complex component TAF3 transduces changes in nuclear phosphoinositides into transcriptional output. Mol Cell 58:453–467. doi: 10.1016/j.molcel.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bua DJ, Martin GM, Binda O, Gozani O. 2013. Nuclear phosphatidylinositol-5-phosphate regulates ING2 stability at discrete chromatin targets in response to DNA damage. Sci Rep 3:2137. doi: 10.1038/srep02137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaadige MR, Ayer DE. 2006. The polybasic region that follows the plant homeodomain zinc finger 1 of Pf1 is necessary and sufficient for specific phosphoinositide binding. J Biol Chem 281:28831–28836. doi: 10.1074/jbc.M605624200. [DOI] [PubMed] [Google Scholar]
- 61.Reynoird N, Gozani O. 2014. Nuclear PI5P, Uhrf1, and the road not taken. Mol Cell 54:901–903. doi: 10.1016/j.molcel.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pena PV, Davrazou F, Shi X, Walter KL, Verkhusha VV, Gozani O, Zhao R, Kutateladze TG. 2006. Molecular mechanism of histone H3K4me3 recognition by plant homeodomain of ING2. Nature 442:100–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Choy JS, Tobe BT, Huh JH, Kron SJ. 2001. Yng2p-dependent NuA4 histone H4 acetylation activity is required for mitotic and meiotic progression. J Biol Chem 276:43653–43662. doi: 10.1074/jbc.M102531200. [DOI] [PubMed] [Google Scholar]
- 64.Sun B, Hong J, Zhang P, Dong X, Shen X, Lin D, Ding J. 2008. Molecular basis of the interaction of Saccharomyces cerevisiae Eaf3 chromo domain with methylated H3K36. J Biol Chem 283:36504–36512. doi: 10.1074/jbc.M806564200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Govind CK, Qiu H, Ginsburg DS, Ruan C, Hofmeyer K, Hu C, Swaminathan V, Workman JL, Li B, Hinnebusch AG. 2010. Phosphorylated Pol II CTD recruits multiple HDACs, including Rpd3C(S), for methylation-dependent deacetylation of ORF nucleosomes. Mol Cell 39:234–246. doi: 10.1016/j.molcel.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Drouin S, Laramee L, Jacques PE, Forest A, Bergeron M, Robert F. 2010. DSIF and RNA polymerase II CTD phosphorylation coordinate the recruitment of Rpd3S to actively transcribed genes. PLoS Genet 6:e1001173. doi: 10.1371/journal.pgen.1001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Su WP, Hsu SH, Chia LC, Lin JY, Chang SB, Jiang ZD, Lin YJ, Shih MY, Chen YC, Chang MS, Yang WB, Hung JJ, Hung PC, Wu WS, Myung K, Liaw H. 2016. Combined interactions of plant homeodomain and chromodomain regulate NuA4 activity at DNA double-strand breaks. Genetics 202:77–92. doi: 10.1534/genetics.115.184432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wysocki R, Javaheri A, Allard S, Sha F, Cote J, Kron SJ. 2005. Role of Dot1-dependent histone H3 methylation in G1 and S phase DNA damage checkpoint functions of Rad9. Mol Cell Biol 25:8430–8443. doi: 10.1128/MCB.25.19.8430-8443.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lalonde ME, Avvakumov N, Glass KC, Joncas FH, Saksouk N, Holliday M, Paquet E, Yan K, Tong Q, Klein BJ, Tan S, Yang XJ, Kutateladze TG, Cote J. 2013. Exchange of associated factors directs a switch in HBO1 acetyltransferase histone tail specificity. Genes Dev 27:2009–2024. doi: 10.1101/gad.223396.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang J, Tan S. 2013. Piccolo NuA4-catalyzed acetylation of nucleosomal histones: critical roles of an Esa1 Tudor/chromo barrel loop and an Epl1 Enhancer of Polycomb A (EPcA) basic region. Mol Cell Biol 33:159–169. doi: 10.1128/MCB.01131-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Friis RM, Wu BP, Reinke SN, Hockman DJ, Sykes BD, Schultz MC. 2009. A glycolytic burst drives glucose induction of global histone acetylation by picNuA4 and SAGA. Nucleic Acids Res 37:3969–3980. doi: 10.1093/nar/gkp270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang AY, Schulze JM, Skordalakes E, Gin JW, Berger JM, Rine J, Kobor MS. 2009. Asf1-like structure of the conserved Yaf9 YEATS domain and role in H2A.Z deposition and acetylation. Proc Natl Acad Sci U S A 106:21573–21578. doi: 10.1073/pnas.0906539106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Akhtar A, Zink D, Becker PB. 2000. Chromodomains are protein-RNA interaction modules. Nature 407:405–409. doi: 10.1038/35030169. [DOI] [PubMed] [Google Scholar]
- 74.Kim D, Blus BJ, Chandra V, Huang P, Rastinejad F, Khorasanizadeh S. 2010. Corecognition of DNA and a methylated histone tail by the MSL3 chromodomain. Nat Struct Mol Biol 17:1027–1029. doi: 10.1038/nsmb.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shimojo H, Sano N, Moriwaki Y, Okuda M, Horikoshi M, Nishimura Y. 2008. Novel structural and functional mode of a knot essential for RNA binding activity of the Esa1 presumed chromodomain. J Mol Biol 378:987–1001. doi: 10.1016/j.jmb.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 76.Chen PB, Chen HV, Acharya D, Rando OJ, Fazzio TG. 2015. R loops regulate promoter-proximal chromatin architecture and cellular differentiation. Nat Struct Mol Biol 22:999–1007. doi: 10.1038/nsmb.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.