Abstract
2-Methoxyestradiol (2ME2), a metabolite of 17β-estradiol (E2), exerts bone sparing effects in animal models. We hypothesized that the underlying mechanism is back conversion of 2ME2 to E2, which subsequently acts via estrogen receptor (ER)α. We measured serum E2 levels in orchidectomized wild-type (WT) mice treated with 2ME2 66.6 μg/d or placebo. In placebo-treated animals, E2 was below the detection limit. In 2ME2-treated mice, the serum E2 level was 4.97 ± 0.68 pg/mL. This corresponds to the level found in diesterus in cycling female mice. Next, we investigated bone parameters in orchidectomized WT and ERα knockout mice treated with 2ME2 or placebo for 35 days. 2ME2 (6.66 μg/d) preserved trabecular and cortical bone in WT mice. Trabecular volumetric-bone mineral density was 64 ± 20%, and trabecular bone volume/total volume was 60 ± 20% higher in the metaphyseal region of the femur in the 2ME2 group, compared with placebo (P < .01). Both trabecular number and trabecular thickness were increased (P < .01). Cortical bone mineral content in the diaphyseal region of the femur was 31 ± 3% higher in the 2ME2 group, compared with placebo (P < .001). This was due to larger cortical area (P < .001). Three-point bending showed an increased bone strength in WT 2ME2-treated animals compared with placebo (maximum load [Fmax] +19±5% in the 2ME2 group, P < .05). Importantly, no bone parameter was affected by 2ME2 treatment in ERα knockout mice. In conclusion, 2ME2 treatment of orchidectomized mice results in increased serum E2. ERα mediates the bone sparing effects of 2ME2. The likely mediator of this effect is E2 resulting from back conversion of 2ME2.
2-methoxyestradiol (2ME2) is an endogenous metabolite of 17β-estradiol (E2). It is formed from cytochrome P450-dependent hydroxylation of E2 to the cathecolestrogen 2-hydroxyestradiol (2HE2), which is subsequently methylated by cathecol-O-methyltransferase to form 2ME2 (1, 2). Demethylation back to 2HE2 can occur (3–5), but possible further back conversion to E2 has not been investigated. 2ME2 has a very low affinity for the estrogen receptors (ERs) in vitro (6, 7).
2ME2 exerts antiproliferative and apoptotic effects in a wide variety of tumor cell lines (8–12). These effects are ER independent (6). 2ME2 has been evaluated as an anticancer agent in several clinical phase I and phase II studies, where it has been shown to be well tolerated with no major toxicities (13, 14). Furthermore, administration of 2ME2 has been shown to reduce atherosclerosis in ovariectomized female mice (15).
2ME2 inhibits angiogenesis and endothelial cell proliferation (2, 16, 17) as well as vascular invasion of growth plate cartilage (18). 2ME2 has also been shown to be cytotoxic to osteoclasts in an in vitro setting. This effect was not antagonized by the ER antagonist ICI 182780 (ICI), suggesting that the effect was mediated via an ER-independent mechanism (19). On the other hand, treatment with 2ME2 in estrogen-depleted mice has been shown to activate estrogen-responsive elements in the bone marrow, spleen, and liver, indicating that these effects are ER dependent (20).
2ME2 exhibits uteroproliferative and bone sparing effects in animal models (21, 22). Sibonga et al (21) administered 2ME2 or 17α-ethynyl estradiol by gavage to ovariectomized rats. 2ME2 maintained bone mass after ovariectomy. In young weanling ovary-intact rats, cotreatment with ICI did not clearly antagonize the bone-sparing effects of 2ME2, which could indicate that the 2ME2 effects on bone are not mediated by ERs (21). However, ICI has never been coadministered with 2ME2 in adult gonadectomized animals, and thus these results may not apply to adult bone metabolism. The ERα knockout (KO) mouse offers an alternative way to investigate the involvement of ERα in the bone sparing effects of 2ME2.
The aims of the present study were to 1) investigate whether 2ME2 can be back transformed to E2; and 2) in the case of back transformation investigate whether the bone sparing effects of 2ME2 are mediated via ERα. We therefore administered 2ME2 or placebo to male orchidectomized ERαKO mice and their wild-type (WT) orchidectomized littermates, measured serum E2, and studied the bone phenotype.
Materials and Methods
Animals, treatment, and experimental procedures
10-week-old male WT and ERαKO mice were bilaterally gonadectomized and sc implanted with a slow-release pellet containing 2ME2 (2ME2 6.66 μg/d or 2ME2 66.6 μg/d), or placebo (60-d release; Innovative Research of America). The animals were euthanized after a treatment period of 35 days.
All mice were housed in a temperature- and humidity-controlled room with a 6 am to 6 pm light cycle and consumed a soy-free diet (R70; Lantmännen) and tap water ad libitum. During surgical procedures, the mice were anesthetized (2% isoflurane in air; Baxter Medical), anesthesia depth was monitored by limb withdrawal using toe pinching, and a postoperative analgesic was given sc (buprenorphine 50 g/kg; RB Pharmaceuticals). At the end of the study, mice were euthanized during anesthesia (2% isoflurane in air; Baxter Medical); blood was drawn from the left ventricle. All procedures were approved by the Ethics Committee on Animal Care and Use in Gothenburg and conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (7th edition, 1996) and the directive 2010/63/EU of the European Parliament.
Measurement of estradiol and testosterone
Estradiol and testosterone were measured by gas chromatography-tandem mass spectrometry (GC-MS/MS). In brief, after addition of isotope-labeled standards, steroids were extracted to chlorobutane, purified on a silica column and derivatized using pentafluorobenzyl hydroxylamine hydrochloride followed by pentafluorobenzoyl chloride. Estradiol and testosterone were measured using an Agilent 7000 triple quadrupole MS equipped with a chemical ionization source with ammonia as reagent gas (23).
Assessment of bone parameters
Peripheral quantitative computed tomography (pQCT)
Femur was analyzed by pQCT using a Stratec pQCT XCT Research M densitometer with software version 5.4B (Norland) at a resolution of 70 μm, as described previously (24). Trabecular bone mineral density (BMD) was determined with a metaphyseal scan at a distance from the growth plate corresponding to 3% of the length of the femur. The inner 45% of the area was defined as the trabecular bone compartment. Cortical BMD was determined with a middiaphyseal scan.
Micro-CT (μCT)
μCT analyses were performed by using a Skyscan 1072 scanner (Skyscan N.V.), imaged with an x-ray tube voltage of 100 kV and current 98 μA, with a 1-mm aluminum filter (25). The voxel size was 6.51 μm isotropically. Datasets were reconstructed using a modified Feldkamp algorithm (26) and segmented into binary images using adaptive local thresholding (27). Trabecular bone proximal of the distal growth plate in femur was selected for analyses within a conforming volume of interest (cortical bone excluded) commencing at a distance of 338.5 μm distal of the growth plate, and extending a further longitudinal distance of 488 μm in the proximal direction. Trabecular thickness and separation were calculated by the sphere-fitting local thickness method (28). Cortical bone was analyzed in the mid diaphyseal part of the femur.
Measurements of bone strength
Three-point bending
The 3-point bending test (span length, 5.5 mm; loading speed, 0.155 mm/s) at the mid femur was made by the Instron universal testing machine (Instron 3366; Instron Corp). Based on the recorded load deformation curves, the biomechanical parameters were acquired from raw files produced by Bluehill 2 software version 2.6 (Instron) with custom-made Excel macros.
Statistical analyses
All data are presented as mean ± SEM. Differences between treatment groups are tested using ANOVA with Tukey's post hoc test or t test.
Results
Estradiol and testosterone measurements
Serum for analyses of E2 levels was available from 8 orchidectomized WT mice, out of which 4 were treated with 2ME2 66.6 μg/d and 4 were treated with placebo. Although E2, as expected, was below the limit of detection (0.3 pg/mL) in all placebo-treated animals, the E2 level in 2ME2-treated animals was 4.97 ± 0.68 pg/mL. This corresponds to the level we have found in diestrus in cycling female mice using our equipment as described in Materials and Methods (23). Serum testosterone measurements revealed that both the orchidectomized placebo-treated and orchidectomized 2ME2-treated mice had serum testosterone levels below the limit of detection (<8 pg/mL) of the used high-sensitive GC-MS/MS assay, demonstrating successful gonadectomy.
Body and organ weights
Body and organ weights of WT and ERαKO mice are presented in Table 1. The WT mice in the low-dose, but not in the high-dose, 2ME2 group were slightly heavier than the mice in the placebo group. The mice in the high-dose 2ME2 group had a slightly increased liver weight.
Table 1.
Effect of 2ME2 Treatment on Body and Organ Weights
| WT |
P Value ANOVA | ERαKO |
P Value ANOVA | |||||
|---|---|---|---|---|---|---|---|---|
| Placebo n = 14 | 2ME2 |
Placebo n = 15 | 2ME2 |
|||||
| 6.66 μg/d n = 11 | 66.6 μg/d n = 12 | 6.66 μg/d n = 12 | 66.6 μg/d n = 13 | |||||
| Body weight (g) | 25.1 ± 0.5 | 27.1 ± 0.5b | 24.6 ± 0.3 | .001 | 25.1 ± 1.0 | 25.0 ± 0.6 | 24.1 ± 0.6 | .63 |
| Thymus weight/body weight (g/kg) | 3.8 ± 0.3 | 3.4 ± 0.1 | 3.4 ± 0.2 | .39 | 4.0 ± 0.1 | 4.1 ± 0.1 | 4.2 ± 0.1 | .46 |
| Seminal vesicles/body weight (g/kg) | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.9 ± 0.1 | .95 | 1.3 ± 0.1 | 1.2 ± 0.1 | 1.5 ± 0.3 | .51 |
| Liver weight/body weight (g/kg) | 47.6 ± 1.1 | 50.3 ± 1.3 | 51.3 ± 0.4a | .03 | 49.7 ± 0.6 | 47.8 ± 1.2 | 46.9 ± 1.1 | .11 |
| Femur length (mm) | 15.4 ± 0.1 | 15.7 ± 0.1 | 15.4 ± 0.1 | .07 | 14.9 ± 0.2 | 15.2 ± 0.1 | 14.9 ± 0.1 | .18 |
All values are expressed as mean ± SEM.
P < .05 vs WT placebo.
P < .01 vs WT placebo.
ERα mediates the effects of 2ME2 on bone
Trabecular bone parameters
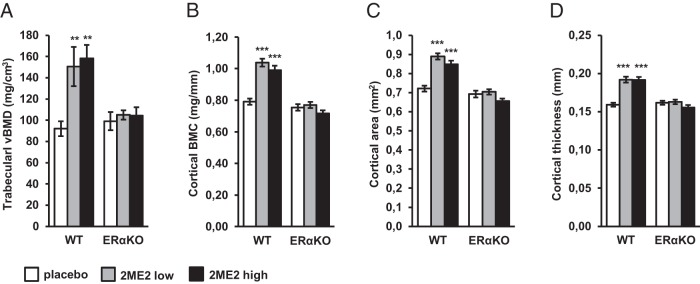
First we measured trabecular vBMD in the distal femur by pQCT. In WT mice, treatment with 2ME2 resulted in a 64 ± 21% and 72 ± 14% increase in trabecular vBMD in the low- and high-dose treatment groups, respectively, compared with placebo (P < .01) (Figure 1A). To dissect the effects of 2ME2 on trabecular bone in more detail, μCT analyses were performed in the low-dose and the placebo treatment groups. Compared with placebo, the 2ME2-treated WT mice had 60 ± 21% higher trabecular bone volume/total volume (P < .01). This was due to an increase in trabecular number (+41 ± 14%) as well as trabecular thickness (+13 ± 4%) (P < .01) (Table 2).
Figure 1.
Effects of 2ME2 on trabecular and cortical bone analyzed by pQCT in the femur in 15-week-old WT and ERαKO mice treated with placebo or 2ME2 (6.66 or 66.6 μg/d) for 5 weeks. Trabecular bone was analyzed in the distal metaphyseal region and cortical bone was analyzed in the diaphyseal region. Values are given as mean ± SEM; **, P < .01 vs WT placebo; ***, P < .001 vs WT placebo. A, Trabecular vBMD. B, Cortical bone mineral content (BMC). C, Cortical area. D, Cortical thickness.
Table 2.
μCT Analyses of Femur
| WT |
P Value t Test | ERαKO |
P Value t Test | |||
|---|---|---|---|---|---|---|
| Placebo | 2ME2, 6.66 μg/d | Placebo | 2ME2, 6.66 μg/d | |||
| Trabecular BV/TV (%) | 7.97 ± 0.66 | 12.73 ± 1.63 | .007 | 10.24 ± 0.84 | 10.85 ± 0.58 | .57 |
| Trabecular number (mm−1) | 1.50 ± 0.10 | 2.11 ± 0.21 | .009 | 1.90 ± 0.13 | 2.01 ± 0.11 | .54 |
| Trabecular thickness (μm) | 52.1 ± 1.3 | 59.0 ± 1.9 | .005 | 53.0 ± 1.1 | 54.0 ± 0.8 | .50 |
| Cortical area (mm2) | 0.61 ± 0.01 | 0.75 ± 0.01 | <.001 | 0.62 ± 0.01 | 0.62 ± 0.01 | .65 |
| Cortical thickness (μm) | 122.5 ± 1.4 | 144.6 ± 2.6 | <.001 | 127.1 ± 2.4 | 127.6 ± 3.1 | .90 |
All values are expressed as mean ± SEM. BV/TV, bone volume/total volume.
In the ERαKO mice, treatment with 2ME2 had no effect on trabecular bone parameters (Figure 1A and Table 2).
Cortical bone parameters
Next, we analyzed the cortical bone compartment in the diaphyseal region of the femur. Results from the pQCT analyses are presented in Figure 1, B–D. Cortical bone mineral content was increased by 31 ± 3% and 25 ± 4% in the low- and high-dose treatment groups, respectively (P < .001) (Figure 1B). This was due to a larger cortical cross sectional area and an increased cortical thickness. The cortical area was increased by 23 ± 2% and 18 ± 3% in the low- and high-dose treatment groups, respectively (P < .001) (Figure 1C). The corresponding figures for cortical thickness were 21 ± 3% and 20 ± 2% (P < .001) (Figure 1D). μCT analyses confirmed the above mentioned results (Table 2). In contrast, 2ME2 had no effect on cortical bone parameters in the ERαKO mice (Figure 1, B–D, and Table 2).
Mechanical strength
Three-point bending test
To analyze whether treatment with 2ME2 makes the bone stronger, we subjected the femurs to the 3-point bending test. Maximal load (Fmax) applied when the bone breaks was 19 ± 5% and 19 ± 3% higher in the animals treated with low- and high-dose 2ME2, respectively, compared with animals treated with placebo (P < .05). In ERαKO mice, there was no significant difference between the placebo and the 2ME2 treatment groups (Figure 2A). This shows that bones of the 2ME2-treated WT mice were indeed stronger than the bones of placebo-treated WT mice. Treatment with 2ME2 did not affect bone stiffness in WT or ERαKO mice (Figure 2B). These results indicate that the increased bone strength in the WT mice treated with 2ME2 was due to an increased amount of bone and not to an affected bone quality.
Figure 2.
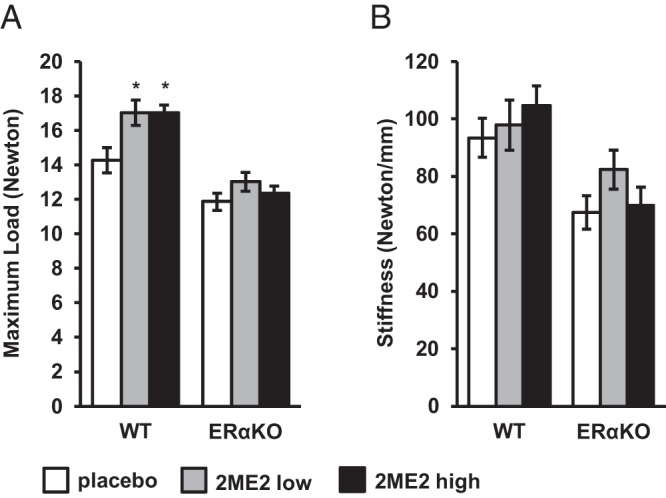
Effects of 2ME2 on bone strength analyzed by 3-point bending of the femur in 15-week-old WT and ERαKO mice treated with placebo or 2ME2 (6.66 or 66.6 μg/d) for 5 weeks. Values are given as mean ± SEM; *, P < .05 vs WT placebo. A, Maximum load (Fmax). B, Stiffness.
Discussion
In this study, we show that 2ME2 can be back converted to E2 and that the bone sparing effects of 2ME2 are mediated via ERα. The most likely mediator of the 2ME2 effects on bone is E2, resulting from back conversion of 2ME2.
It is well known that 2ME2 interacts with ERα to a very low extent. LaVallee et al found the affinity of 2ME2 for ERα to be 500-fold lower than that of E2 (6). It is also known that 2ME2 can be converted to 2-methoxyestrone and that demethylation resulting in 2HE2 and 2-hydroxyestrone can occur. Lippert et al (3) treated rats with 2ME2 by means of sc implanted osmotic pumps and measured metabolites of 2ME2 in urine using GC/MS. Compared with placebo, 2ME2 treatment resulted in significant increases in several metabolites including 2HE2 (3). Evidence of demethylation has also been found in humans (4) and in in vitro settings (5). 2HE2 binds to the ERα. In a study on human breast cancer cells, 2HE2 was found to associate to ERα with the same affinity as E2 (29). Whether further back transformation to E2 can occur has not been established previously.
Our very sensitive GC-MS/MS method provides a technique for measurement of the low levels of E2 present in the serum of mice (23). Using this method, we show for the first time that, in addition to the already known back transformation to 2HE2, substantial further transformation to E2 can occur in vivo. The animals in our study were gonadectomized. Therefore, they have no endogenous production of sex steroids. In line with this, E2 was below the limit of detection in all placebo-treated animals. By contrast, E2 levels in all 2ME2-treated animals were clearly detectable and equivalent to what we have found in intact cycling females during diestrus (23).
Bone phenotype in 2ME2-treated animals has previously been studied in a mouse model of postmenopausal rheumatoid arthritis (22) and in rats (21). Stubelius et al (22) induced arthritis in ovariectomized WT mice, which were subsequently implanted sc with pellets releasing 2ME2 or placebo. Compared with placebo, 2ME2 conserved trabecular and cortical BMD as measured by pQCT, a finding replicated in the WT mice in the present study (22).
Many of the findings in our study are in line with the findings from the study of 2ME2 treatment in weanling, adolescent, and adult ovariectomized rats, performed by Sibonga et al (21). They showed bone sparing effects of 2ME2 in trabecular and cortical bone. When the trabecular bone compartment was investigated more in detail, both trabecular number and trabecular thickness were increased in the adolescent rats, which is consistent with the findings in our mice.
2ME2 has actions that are not mimicked by E2 and that are independent of ERs. For example, antiproliferative effects and induction of apoptosis have been shown both in ER positive and ER-negative cell lines (6). 2ME2 also inhibits osteoclast differentiation and is toxic to mature osteoclasts in vitro, an effect which is not antagonized by the ER antagonist ICI (19). However, findings of activation of estrogen-responsive elements in the bone marrow, spleen and liver indicate that other 2ME2 effects are indeed mediated via ERs (20).
The bone sparing effects of 2ME2 in vivo could thus be either ER dependent or ER independent. In order to explore this, Sibonga et al (21) compared the effects of 2ME2, ICI, and 2ME2 + ICI with placebo in one of their experiments. In that experiment, 2ME2 had no effect on uterine wet weight but significantly reduced bone formation rate compared with placebo. By contrast, ICI deceased uterine wet weight and increased bone formation rate, compared with placebo. When 2ME2 and ICI were coadministered, ICI did not fully antagonize the effects of 2ME2 on bone, although uterine wet weight was clearly decreased in the 2ME2 + ICI group. Thus, these data could indicate that the bone sparing effects of 2ME2 are not mediated via ERs, which is in sharp contrast to our findings in the present study. However, the 2ME2 + ICI experiment was performed only in weanling rats, which were female and ovary intact. They were euthanized already at 4 weeks of age after 7 days of treatment with 2ME2 and/or ICI or placebo (21). Our experiment was performed in adult orchidectomized male mice, which are completely deprived of endogenous sex steroid synthesis. The duration of treatment with 2ME2 was longer in our experiment (35 vs 7 d). Moreover, the ERαKO model has a complete lack of ERα signaling and is not subject to uncertainties regarding dose and treatment regimen of ERα antagonists. Therefore, we believe that it is a robust model for investigating the ERα dependency of 2ME2 effects on bone. ERα is the most important ER for bone, although its effects can be slightly modulated by ERβ (24, 30–33).
It has been shown previously that in orchidectomized mice, estradiol increases bone strength compared with placebo but does not affect qualitative bone parameters in the 3-point bending experiment (25). This fits well with the findings from the 3-point bending test in our study and is another indicator of 2ME2 effects on bone being exerted in the same way as E2 effects, ie, via ERα.
A limitation of the present study is the lack of a sham surgery control group. However, the observed undetectable serum testosterone levels, as analyzed by high-sensitive GC-MS/MS, demonstrate that the gonadectomy was effective in our experiment. This was also supported by the fact that the weights of the seminal vesicles were in the range expected after orchidectomy (34). The male mice in the present study were orchidectomized at 10 weeks of age. This is an age when the skeleton is still growing albeit at a slow rate. Thus, our results in the present study may apply not only to adult bone metabolism but also to skeletal growth.
In conclusion, we demonstrate that 2ME2 treatment of orchidectomized male mice results in increased serum E2 levels. The bone sparing effects of 2ME2 are mediated via ERα. The most likely mediator of this effect is E2 resulting from the back conversion of 2ME2.
Acknowledgments
This work was supported by the Swedish Research Council and by grants from the Swedish Government (under the Avtal om Läkarutbildning och Medicinsk Forskning [Agreement for Medical Education and Research]), the Lundberg Foundation, the Torsten Söderberg Foundation, the Novo Nordisk Foundation, and the Knut and Alice Wallenberg Foundation.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMD
- bone mineral density
- μCT
- micro-CT
- E2
- 17β-estradiol
- ER
- estrogen receptor
- GC-MS/MS
- gas chromatography-tandem mass spectrometry
- 2HE2
- 2-hydroxyestradiol
- ICI
- ICI 182780
- KO
- knockout
- 2ME2
- 2-methoxyestradiol
- pQCT
- peripheral quantitative computed tomography
- vBMD
- volumetric bone mineral density
- WT
- wild type.
References
- 1. Martucci CP, Fishman J. P450 enzymes of estrogen metabolism. Pharmacol Ther. 1993;57(2–3):237–257. [DOI] [PubMed] [Google Scholar]
- 2. Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis. 1998;19(1):1–27. [DOI] [PubMed] [Google Scholar]
- 3. Lippert TH, Adlercreutz H, Berger MR, Seeger H, Elger W, Mueck AO. Effect of 2-methoxyestradiol on the growth of methyl-nitroso-urea (MNU)-induced rat mammary carcinoma. J Steroid Biochem Mol Biol. 2003;84(1):51–56. [DOI] [PubMed] [Google Scholar]
- 4. Lakhani NJ, Sparreboom A, Xu X, et al. Characterization of in vitro and in vivo metabolic pathways of the investigational anticancer agent, 2-methoxyestradiol. J Pharm Sci. 2007;96(7):1821–1831. [DOI] [PubMed] [Google Scholar]
- 5. Zhu BT, Evaristus EN, Antoniak SK, Sarabia SF, Ricci MJ, Liehr JG. Metabolic deglucuronidation and demethylation of estrogen conjugates as a source of parent estrogens and catecholestrogen metabolites in Syrian hamster kidney, a target organ of estrogen-induced tumorigenesis. Toxicol Appl Pharmacol. 1996;136(1):186–193. [DOI] [PubMed] [Google Scholar]
- 6. LaVallee TM, Zhan XH, Herbstritt CJ, Kough EC, Green SJ, Pribluda VS. 2-Methoxyestradiol inhibits proliferation and induces apoptosis independently of estrogen receptors α and β. Cancer Res. 2002;62(13):3691–3697. [PubMed] [Google Scholar]
- 7. Martucci CP, Fishman J. Impact of continuously administered catechol estrogens on uterine growth and luteinizing hormone secretion. Endocrinology. 1979;105(6):1288–1292. [DOI] [PubMed] [Google Scholar]
- 8. Zhu BT, Conney AH. Is 2-methoxyestradiol an endogenous estrogen metabolite that inhibits mammary carcinogenesis? Cancer Res. 1998;58(11):2269–2277. [PubMed] [Google Scholar]
- 9. Pribluda VS, Gubish ER, Jr, Lavallee TM, Treston A, Swartz GM, Green SJ. 2-Methoxyestradiol: an endogenous antiangiogenic and antiproliferative drug candidate. Cancer Metastasis Rev. 2000;19(1–2):173–179. [DOI] [PubMed] [Google Scholar]
- 10. Thaver V, Lottering ML, van Papendorp D, Joubert A. In vitro effects of 2-methoxyestradiol on cell numbers, morphology, cell cycle progression, and apoptosis induction in oesophageal carcinoma cells. Cell Biochem Funct. 2009;27(4):205–210. [DOI] [PubMed] [Google Scholar]
- 11. Zhou NN, Zhu XF, Zhou JM, et al. 2-Methoxyestradiol induces cell cycle arrest and apoptosis of nasopharyngeal carcinoma cells. Acta Pharmacol Sin. 2004;25(11):1515–20. [PubMed] [Google Scholar]
- 12. Mueck AO, Seeger H. 2-Methoxyestradiol–biology and mechanism of action. Steroids. 2010;75(10):625–631. [DOI] [PubMed] [Google Scholar]
- 13. Li L, Bu S, Bäckström T, Landström M, Ulmsten U, Fu X. Induction of apoptosis and G2/M arrest by 2-methoxyestradiol in human cervical cancer HeLaS3 cells. Anticancer Res. 2004;24(2B):873–880. [PubMed] [Google Scholar]
- 14. Tevaarwerk AJ, Holen KD, Alberti DB, et al. Phase I trial of 2-methoxyestradiol NanoCrystal dispersion in advanced solid malignancies. Clin Cancer Res. 2009;15(4):1460–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bourghardt J, Bergström G, Krettek A, Sjöberg S, Borén J, Tivesten A. The endogenous estradiol metabolite 2-methoxyestradiol reduces atherosclerotic lesion formation in female apolipoprotein E-deficient mice. Endocrinology. 2007;148(9):4128–4132. [DOI] [PubMed] [Google Scholar]
- 16. Fotsis T, Zhang Y, Pepper MS, et al. The endogenous oestrogen metabolite 2-methoxyoestradiol inhibits angiogenesis and suppresses tumour growth. Nature. 1994;368(6468):237–239. [DOI] [PubMed] [Google Scholar]
- 17. Yue TL, Wang X, Louden CS, et al. 2-Methoxyestradiol, an endogenous estrogen metabolite, induces apoptosis in endothelial cells and inhibits angiogenesis: possible role for stress-activated protein kinase signaling pathway and Fas expression. Mol Pharmacol. 1997;51(6):951–962. [DOI] [PubMed] [Google Scholar]
- 18. Sibonga JD, Sommer U, Turner RT. Evidence that 2-methoxyestradiol suppresses proliferation and accelerates apoptosis in normal rat growth plate chondrocytes. J Cancer Res Clin Oncol. 2002;128(9):477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maran A, Gorny G, Oursler MJ, et al. 2-methoxyestradiol inhibits differentiation and is cytotoxic to osteoclasts. J Cell Biochem. 2006;99(2):425–434. [DOI] [PubMed] [Google Scholar]
- 20. Stubelius A, Erlandsson MC, Islander U, Carlsten H. Immunomodulation by the estrogen metabolite 2-methoxyestradiol. Clin Immunol. 2014;153(1):40–48. [DOI] [PubMed] [Google Scholar]
- 21. Sibonga JD, Lotinun S, Evans GL, Pribluda VS, Green SJ, Turner RT. Dose-response effects of 2-methoxyestradiol on estrogen target tissues in the ovariectomized rat. Endocrinology. 2003;144(3):785–792. [DOI] [PubMed] [Google Scholar]
- 22. Stubelius A, Andréasson E, Karlsson A, et al. Role of 2-methoxyestradiol as inhibitor of arthritis and osteoporosis in a model of postmenopausal rheumatoid arthritis. Clin Immunol. 2011;140(1):37–46. [DOI] [PubMed] [Google Scholar]
- 23. Nilsson ME, Vandenput L, Tivesten Å, et al. Measurement of a comprehensive sex steroid profile in rodent serum by high-sensitive gas chromatography-tandem mass spectrometry. Endocrinology. 2015;156(7):2492–2502. [DOI] [PubMed] [Google Scholar]
- 24. Windahl SH, Vidal O, Andersson G, Gustafsson JA, Ohlsson C. Increased cortical bone mineral content but unchanged trabecular bone mineral density in female ERβ(−/−) mice. J Clin Invest. 1999;104(7):895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Movérare S, Venken K, Eriksson AL, et al. Differential effects on bone of estrogen receptor α and androgen receptor activation in orchidectomized adult male mice. Proc Natl Acad Sci USA. 2003;100(23):13573–13578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Feldkamp LA, Davis LC, Kress JW. Practical cone-beam algorithm. J Opt Soc Am A. 1984;1(6):612–619. [Google Scholar]
- 27. Waarsing JH, Day JS, Weinans H. An improved segmentation method for in vivo microCT imaging. J Bone Miner Res. 2004;19(10):1640–1650. [DOI] [PubMed] [Google Scholar]
- 28. Hildebrand T, Rüegsegger P. Quantification of bone microarchitecture with the structure model index. Comput Methods Biomech Biomed Engin. 1997;1(1):15–23. [DOI] [PubMed] [Google Scholar]
- 29. Van Aswegen CH, Purdy RH, Wittliff JL. Binding of 2-hydroxyestradiol and 4-hydroxyestradiol to estrogen receptors from human breast cancers. J Steroid Biochem. 1989;32(4):485–492. [DOI] [PubMed] [Google Scholar]
- 30. Windahl SH, Hollberg K, Vidal O, Gustafsson JA, Ohlsson C, Andersson G. Female estrogen receptor β−/− mice are partially protected against age-related trabecular bone loss. J Bone Miner Res. 2001;16(8):1388–1398. [DOI] [PubMed] [Google Scholar]
- 31. Lindberg MK, Movérare S, Skrtic S, et al. Estrogen receptor (ER)- β reduces ERα-regulated gene transcription, supporting a “ying yang” relationship between ERα and ERβ in mice. Mol Endocrinol. 2003;17(2):203–208. [DOI] [PubMed] [Google Scholar]
- 32. Vanderschueren D, Laurent MR, Claessens F, et al. Sex steroid actions in male bone. Endocr Rev. 2014;35(6):906–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nicks KM, Fujita K, Fraser D, et al. Deletion of estrogen receptor β in osteoprogenitor cells increases trabecular but not cortical bone mass in female mice. J Bone Miner Res. 2016;31(3):606–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lindzey J, Wetsel WC, Couse JF, Stoker T, Cooper R, Korach KS. Effects of castration and chronic steroid treatments on hypothalamic gonadotropin-releasing hormone content and pituitary gonadotropins in male wild-type and estrogen receptor-α knockout mice. Endocrinology. 1998;139(10):4092–4101. [DOI] [PubMed] [Google Scholar]