Abstract
Recent studies have identified the osteoblast as an insulin responsive cell that participates in global energy homeostasis. Here, we show that glucose transporter-4 (Glut4) is required for insulin-dependent uptake and oxidation of glucose in mature osteoblasts. In primary cultures of mouse osteoblasts, insulin increased uptake and oxidation of 14C-glucose in a dose-dependent fashion but did not significantly affect uptake or oxidation of 14C-oleate. In vitro, undifferentiated osteoblasts expressed 3 high-affinity Gluts: Glut1, Glut4, and Glut3. However, although levels of Glut1 and Glut3 remained constant during the course of osteoblast differentiation, Glut4 expression increased by 5-fold in association with enhanced insulin-stimulated glucose uptake. Glut4 ablation in osteoblasts in vitro eliminated insulin-stimulated glucose uptake, reduced proliferation and diminished measures of osteoblast maturation. In vivo, Glut4 expression was observed in osteoblasts, osteocytes, and chondrocytes at a level approaching that observed in adjacent skeletal muscle. To determine the importance of Glut4 in bone in vivo, we generated mice lacking Glut4 in osteoblasts and osteocytes (ΔGlut4). ΔGlut4 mice exhibited normal bone architecture but exhibited an increase in peripheral fat in association with hyperinsulinemia, β-cell islet hypertrophy, and reduced insulin sensitivity. Surprisingly, the expression of insulin target genes in liver, muscle, and adipose from ΔGlut4 mice were unchanged or increased, indicating that alterations in glucose homeostasis were the result of reduced clearance by bone. These findings suggest that Glut4 mediates insulin-stimulated glucose uptake by mature osteoblasts/osteocytes and that the magnitude of glucose use by bone cells is sufficient to impact global glucose disposal in the mouse.
Osteoblasts are specialized mesenchymal cells that originate from marrow stromal precursors and function to produce new bone (1). During periods of active bone remodeling, osteoblast precursors migrate to newly eroded bone where they differentiate into polarized cuboidal cells and elaborate a highly compact organic matrix composed primarily of collagen type I and then coordinate its mineralization. Fully differentiated osteoblasts exhibit abundant mitochondria consistent with the increased bioenergetic demand during this synthetic phase of their life span (2). A fraction of mature osteoblasts become embedded in mineralized bone and terminally differentiate into osteocytes. The longevity of osteocytes and their maintenance of an extensive lacunar-canalicular network also suggest the need for substantial energy generating potential. Moreover, the sheer size of the skeleton and the density of resident osteocytes and osteoblasts, particularly during periods of active bone formation and remodeling, suggest that these cell populations may require a large supply of energy-rich molecules to maintain skeletal architecture.
A growing body of evidence supports the idea that the skeleton is an endocrine organ that releases hormones instrumental to the coordination of fuel distribution and consumption (3, 4). For example, adipose-derived leptin alters bone mass through a hypothalamic-osteoblast endocrine loop and leptin's effects on insulin secretion are influenced by osteoblastic activity (5, 6). In addition, 2 independent but highly complementary studies (7, 8) recently demonstrated that insulin signaling in osteoblasts regulates the production and bioavailability of the hormone osteocalcin, which in turn acts in an endocrine fashion to regulate pancreatic insulin secretion and peripheral insulin responsiveness (3). Subsequent work added important confirmation of osteocalcin's endocrine actions by showing that infusion of undercarboxylated osteocalcin into wild-type mice is able to increase pancreatic insulin secretion (9). These studies strongly suggest that osteoblasts and osteocytes participate in the homeostatic control of global glucose homeostasis.
Despite this increased appreciation of the importance of the skeleton to whole-body metabolism, relatively little attention has been paid to understanding bone cell metabolism and energy expenditure (EE). The cessation of bone formation in grossly undernourished children and adults with anorexia nervosa (10, 11) suggests the need for osteoblasts to attain sufficient nutrient supplies for normal function. Early studies using relatively crude bone cell populations or endochondral bone chips suggested that the osteoblast generates energy primarily through glycolytic pathways (12, 13). Osteoblasts contain the enzymatic requirements for glycolysis; metabolize glucose to form lactate, and both insulin and parathyroid hormone stimulate the uptake of 2-deoxyglucose (14–16). These data imply that osteoblasts must also express the components required for the uptake of sufficient quantities of glucose, including the glucose transporters (Gluts), to facilitate bone formation.
In the current studies, we sought to characterize the mechanisms responsible for glucose uptake and oxidation by primary mouse osteoblasts. We report that both basal and insulin-stimulated glucose oxidation increase as osteoblasts mature in association with increased expression of Glut4. Elimination of Glut4 expression abolishes insulin-stimulated uptake and attenuates osteoblast mineralization. Remarkably, mice deficient in Glut4 in osteoblasts develop peripheral adiposity in association with mild hyperinsulinemia and marked insulin resistance. These data suggest that the osteoblast lineage contributes to the clearance of glucose in response to insulin and likely functions in the regulation of whole-body metabolism beyond the secretion of osteocalcin.
Materials and Methods
Animal models
The Institutional Animal Care and Use Committee of the Johns Hopkins University approved all procedures involving mice. Glut4 was disrupted in the osteoblast by crossing Glut4flox/flox mice maintained on a 129SV background (17–19) with osteocalcin-Cre transgenic mice maintained on a C57Bl/6 background (20). The genotyping strategy is available upon request. Specificity of recombination was assessed using a PCR-based strategy and primer pairs that flanked the floxed allele. Glut4-Cre;Rosa26-RFP reporter mice were kindly provided by Dr Domenico Accilli (21). Mice containing floxed alleles of the insulin receptor (IR) (IRflox/flox) have been described previously (22). Mice were fed a standard chow diet (Extruded Global Rodent Diet; Harlan Laboratories).
Osteoblast culture
Primary osteoblasts were isolated from calvarial bones of 1- to 4-day-old neonatal pups from mixed sex litters via serial digestion in 1.8-mg/mL collagenase type I according to standard techniques. To abolish Glut4 expression in vitro, osteoblasts containing floxed alleles were infected with adenovirus encoding Cre-recombinase or green fluorescent protein as a control (Vector Biolabs). Infection with 100 multiplicity of infection was used in all studies and efficiency of gene deletion was confirmed by PCR and immunoblotting. Osteoblast proliferation was assessed by flow cytometry (FACSCAlibur; BD Biosciences) after staining bromodeoxyuridine (BrdU)-labeled osteoblasts (10μM) with anti-BrdU-Allophycocyanin and 7-amino-actinomycin D. A total of 20 000 events were collected for each sample. For differentiation experiments, osteoblasts were grown to confluence and then cultured for 7 or 14 days in the presence of 10mM β-glycerol phosphate and 50-μg/mL ascorbic acid. Alkaline phosphatase and Alizarin Red S staining were carried out according to standard techniques.
Gene expression studies
Total RNA was extracted from tissues or osteoblasts grown in vitro using the TRIzol method (Life Technologies). One microgram of pure RNA was reverse transcribed using the iScript cDNA synthesis system (Bio-Rad). Two microliters of cDNA were then subjected to PCR amplification using iQ SYBR Green Supermix (Bio-Rad). Primer sequences were obtained from PrimerBank (http://pga.mgh.harvard.edu/primerbank/index.html). Reactions were normalized to endogenous β-actin reference transcript. Cellular protein was collected in Triton X-100 lysis buffer and prepared for immunoblotting according to standard technique. Antibodies specific for Glut4 (2213, AB_823508), IR (3025, AB_2280448), IGF-1 receptor (3018, AB_10693453), and β-actin (3700, AB_2242334) were obtained from Cell Signaling Technologies. To assess Glut4 translocation, osteoblast cultures were treated with 10nM insulin for 30 minutes and then labeled with Sulfo-NHS-SS-Biotin (Cell Surface Protein Isolation kit; Thermo Scientific) for an additional 30 minutes to tag cell surface proteins. Labeled protein was then purified with NeutrAvidin resin according to the manufacturer's instructions (Thermo Scientific) and subjected to immunoblotting.
Glucose uptake and oxidation studies
Glucose uptake was assessed as previously described (23, 24). Briefly, osteoblast cultures were incubated in MEM (1-g/L glucose)/0.1% BSA at 37°C for 2 hours. The cells were then washed with Krebs-Ringers-HEPES buffer (25mM HEPES-NaOH [pH 7.4], 120mM NaCl, 5mM KCl, 1.2mM MgSO4, 1.3mM CaCl2, and 1.3mM KH2PO4) containing no glucose and incubated for an additional 2 hours. Insulin (10nM–100nM; Sigma-Aldrich) was added for 15 minutes before incubating cells in KRH containing 2-deoxy-D-[3H]-glucose for 5 minutes. An additional group was treated with cytochalasin B (10μM) to assess nonspecific uptake. Uptake of 3H-glucose was detected using a Beckman scintillation counter and normalized to protein content.
Fatty acid and glucose oxidation were measured in flasks with stoppers equipped with center wells (25). Cultures were treated with insulin before incubating at 37°C in media containing 0.5mM L-carnitine, 0.2% BSA, and either 14C-oleate (PerkinElmer), 14C-glucose, or 3H-acetate (14). CO2 was captured and counted by the addition of 1N perchloric acid to the reaction mixture and 1M NaOH to the center well containing Whatman filter paper. The acidified reaction mixture was incubated overnight at 4°C and centrifuged at 4000 rpm for 30 minutes before aliquots of the supernatant were counted for 14C-labeled acid soluble metabolites
Computed tomography (CT) imaging and histomorphometry
Male control and transgenic mice were sacrificed at the indicated time to assess bone structure using a SkyScan1172 high-resolution Micro-CT scanner (ìCT, Bruker) in accordance with the recommendations of the American Society for Bone and Mineral Research (26). Bones were scanned at 50 keV and 200 mA using a 0.5-mm aluminum filter with an isotropic voxel size of 10 μm. The resulting 2-dimensional cross-sectional images are shown in gray scale. Trabecular bone parameters were assessed in the distal femur 500 μm proximal to the growth plate and extending for 2 mm (200 CT slices). Cortical bone parameters were assessed at the femoral midshaft and represent an average of 50 CT slices (500 μm).
Metabolic studies
Weekly measurements were performed to assess changes in body mass. At necropsy, tissues were collected and weights were normalized to body mass. Body composition was measured by quantitative nuclear magnetic resonance (Echo MRI). Blood glucose was measured using a OneTouch Ultra hand-held glucose monitor (LifeScan) in random-fed animals or after an overnight fast. Random-fed and fasting serum insulin levels were determined by ELISA (Alpco). Pancreata were fixed and stained, and islet morphometry was assessed as previously described (27). The antiinsulin antibody was obtain from DAKO (A056401, AB_2617169). Frozen sections of gonadal white adipose tissue were stained with hematoxylin and eosin. Indirect calorimetry was conducted in an open-flow indirect calorimeter (Comprehensive Lab Animal Monitoring System; Columbus Instruments). Calorimetry, daily body weight, and daily food intake data were acquired during a 4-day experimental period. Data from the first 3 days were used to confirm acclimation to the calorimetry chamber, and the fourth day was used for analyses. Rates of O2 consumption (VO2) (mL/kg·h) and CO2 production were measured for each chamber every 20 minutes throughout the study. Respiratory exchange ratio (RER) (RER = CO2 production/VO2) was calculated by Oxymax software (v. 4.90) to estimate relative oxidation of carbohydrate (RER = 1.0) vs fat (RER approaching 0.7), not accounting for protein oxidation. EE was calculated as EE = VO2 × (3.815 + [1.232 × RER]) (28) and normalized for subject lean body mass.
For glucose tolerance testing, mice were fasted overnight (16 h) and injected ip with glucose at a dose of 2-g/kg body weight, and blood glucose concentrations were measured at the indicated time points. Insulin tolerance tests were performed by ip administration of insulin (1-U/kg body weight) to mice after a 6-hour fast followed by measurement of blood glucose concentrations at the indicated time points. Area under the curve was calculated using Prism GraphPad. Whole-body insulin sensitivity was assessed by hyperinsulinemic-euglycemic clamp and in vivo glucose uptake. The clamp was conducted in overnight-fasted, conscious mice by the National Mouse Metabolic Phenotyping Center at the University of Massachusetts Medical School (29, 30). Briefly, mice were anesthetized with an ip injection of ketamine (100-mg/kg body weight) and xylazine (10-mg/kg body weight) 4–5 days before the procedure, and an indwelling catheter was inserted into the right internal jugular vein. A 2-hour hyperinsulinemic-euglycemic clamp was performed in conscious, overnight-fasted mice with primed and continuous infusion of insulin (Humulin; Lilly) at a rate of 2.5 mU kg−1 per min−1. Glucose metabolism was estimated with a continuous infusion of [3-3H]glucose (PerkinElmer Life Sciences) for 2 hours before (1850 Bq/min) and throughout the clamps (3700 Bq/min).
Statistical analysis
All results are expressed as mean ± SEM. For comparisons between 2 groups of data, the Student's t test was used; P < .05 was considered statistically significant.
Results
Insulin stimulates glucose uptake and oxidation by osteoblasts
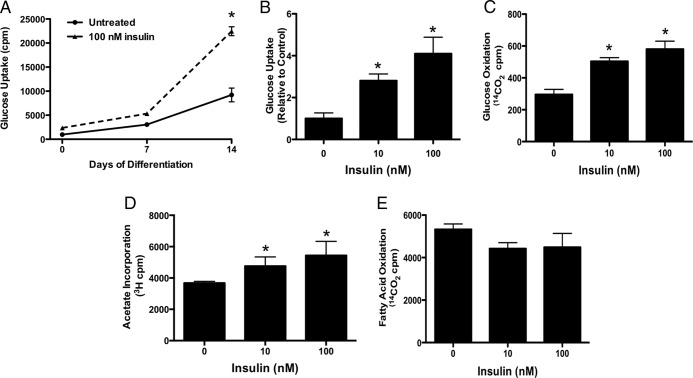
To begin to interrogate the mechanism by which insulin stimulates glucose acquisition, we used radiolabeled tracers to assess the effect of insulin on osteoblast metabolism during in vitro differentiation. Basal and insulin-stimulated 2-deoxy-D-(3H)-glucose uptake were low in freshly plated cells, but both basal and insulin-stimulated uptake increased dramatically as osteoblasts differentiated (Figure 1, A and B). At least a portion of the glucose taken up by osteoblasts after insulin stimulation enters the citric acid cycle as insulin dose dependently enhanced glucose oxidation as indexed by the conversion of 14C-glucose to 14C-labeled CO2 (Figure 1C). 3H-acetate incorporation (Figure 1D), an indicator of de novo fatty acid synthesis, was also increased by insulin stimulation, but oxidation of fatty acids was not affected (Figure 1E). Taken together, these data indicate that glucose but not fatty acids is likely to fuel the anabolic actions of insulin in the skeleton.
Figure 1.
Insulin stimulates glucose uptake and oxidation in osteoblasts. A, Uptake of 2-deoxy-D-(3H)-glucose in response to insulin on day 0, 7, and 14 of differentiation. B, Uptake of 2-deoxy-D-(3H)-glucose by osteoblasts in response to the indicated concentration of insulin. Results are normalized to untreated cells (0nM insulin). C, Conversion of 14C-glucose to 14CO2 was used to assess glucose oxidation after insulin stimulation. D, Fatty acid incorporation was assessed by labeling osteoblasts with 14C-acetate after insulin stimulation. E, Fatty acid oxidation was assessed by measuring the conversion of 14C-oleate to 14CO2. Results for B–D are presented as cpm normalized to protein levels; *, P < .05.
Glut4 is expressed by osteoblasts and is required for insulin-stimulated glucose uptake
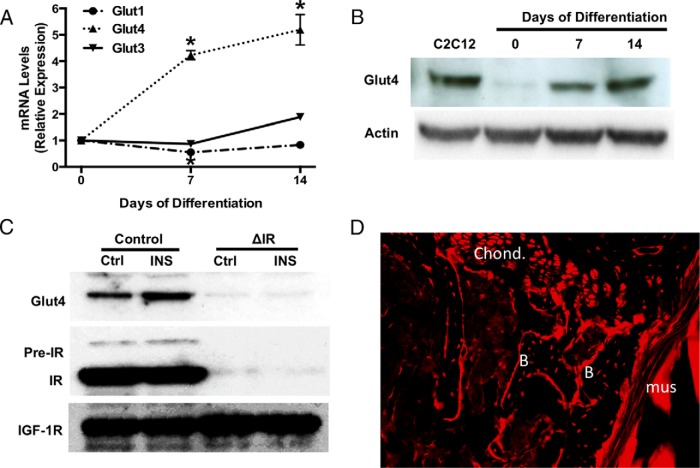
We next investigated the mechanism of insulin-stimulated glucose transport in osteoblasts. In undifferentiated osteoblast cultures, transcripts for 3 high-affinity Gluts, Glut1, Glut4, and Glut3, were detectable by quantitative polymerase chain reaction (qPCR) (Figure 2A). Expression of Glut2 transcripts was not detectable in undifferentiated cells or after osteogenic induction (data not shown). Intriguingly, although Glut1 and Glut3 mRNA levels remained relatively constant over 14 days of osteoblast differentiation (Figure 2A), Glut4 mRNA levels increased more than 5-fold (Figure 2A). These data, together with equivalent changes in Glut4 protein evident during the course of differentiation (Figure 2B), parallel the increases in insulin-stimulated 2-deoxy-glucose uptake during osteoblast maturation (Figure 1A). Importantly, insulin stimulation increased the presence of Glut4 in the membrane of control osteoblasts but, as expected, had no effect on IR-deficient osteoblasts (Figure 2C). To confirm that Glut4 was also expressed by osteoblasts in vivo, we examined tissue sections prepared from the femur of Glut4-Cre;Rosa26-RFP mice (21). In these mice, Cre-recombinase expression is driven by the Glut4 promoter and results in the recombination of the Rosa26 locus and the expression of a red fluorescent protein (RFP) reporter gene. As shown in Figure 2D, osteoblasts, osteocytes, and chondrocytes express RFP, indicative of Glut4 promoter activity in these cells.
Figure 2.
Expression of a functional Glut4 in osteoblasts. A, Relative levels of Glut1, Glut3, and Glut4 mRNA determined by qPCR during osteoblast differentiation in vitro. Glut2 was not detectable. B, Immunoblot analysis of Glut4 expression in extracts isolated at the indicated times during osteoblast differentiation. Lysates from C2C12 myoblasts were used as a positive control. C, Immunoblotting of plasma membrane proteins in control and IR-deficient osteoblasts treated with insulin (10nM). IGF-1R was used as a loading control. D, Representative tissue section from the femur of Glut4-Cre;Rosa26-RFP mice, in which RFP expression denotes Glut4 promoter activity. B, bone; Chond, chondrocyte; Mus, muscle. No RFP expression was detected in the skeletal tissue of Cre-negative mice (data not shown).
Glut4 is required for late stage differentiation of osteoblasts in vivo but does not alter bone acquisition
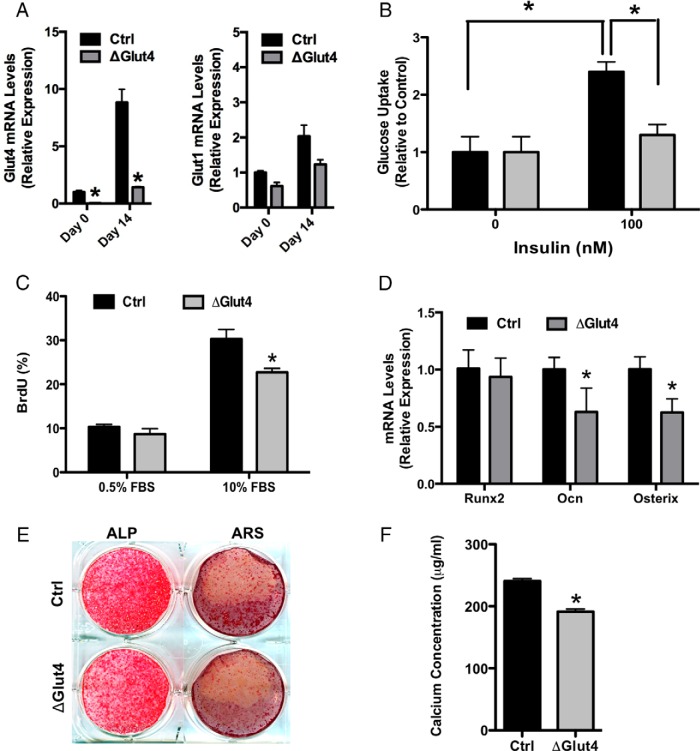
To determine the significance of Glut4 expression by osteoblasts, we examined the requirement for this Glut in insulin-stimulated glucose uptake and osteoblast differentiation in vitro. Calvarial osteoblasts were isolated from Glut4flox/flox mice (17–19) and infected with adenovirus expressing Cre-recombinase to disrupt Glut4 expression (ΔGlut4) or green fluorescent protein as a control (Figure 3A). Cre-mediated knockdown of Glut4 did not induce a compensatory change in the mRNA levels of Glut1 (Figure 3A) or affect basal glucose uptake levels (Figure 3B), which are likely to be governed by the activity of Glut1 (31). However, loss of Glut4 function largely abolished insulin-stimulated uptake of 2-deoxy-D-(3H)-glucose (Figure 3B). Cultures of ΔGlut4 osteoblasts also exhibited modest but significant reductions in serum-induced proliferation (Figure 3C), alkaline phosphatase staining (Figure 3E), and the expression of osterix and osteocalcin (Figure 3D) relative to controls. Moreover, Alizarin staining for matrix mineralization was significantly reduced relative to controls (Figure 3, D and E). These results suggest that the expression of Glut4 is necessary for optimal osteoblast differentiation, at least in vitro.
Figure 3.
Glut4 expression facilitates late stage osteoblast differentiation in vitro. A, Analysis of Glut4 and Glut1 mRNA by qPCR in control and ΔGlut4 before and after 14 days of differentiation of osteoblast cultures. B, Uptake of 2-deoxy-D-[3H]-glucose by control and ΔGlut4 osteoblasts in response to the indicated concentration of insulin; *, P < .05. C, Flow cytometric analysis of BrdU incorporation in control and ΔGlut4 osteoblasts cultured in the presence of 0.5% or 10% fetal bovine serum (FBS). D, qPCR analysis of Runx2, osteocalcin (Ocn), and osterix mRNA levels in control and ΔGlut4 osteoblasts after 14 days of differentiation. E, Alkaline phosphatase (ALP) and Alizarin Red staining (ARS) of control and ΔGlut4 osteoblasts after 14 days of differentiation. F, Quantification of deposited calcium in control and ΔGlut4 osteoblasts; *, P < .05.
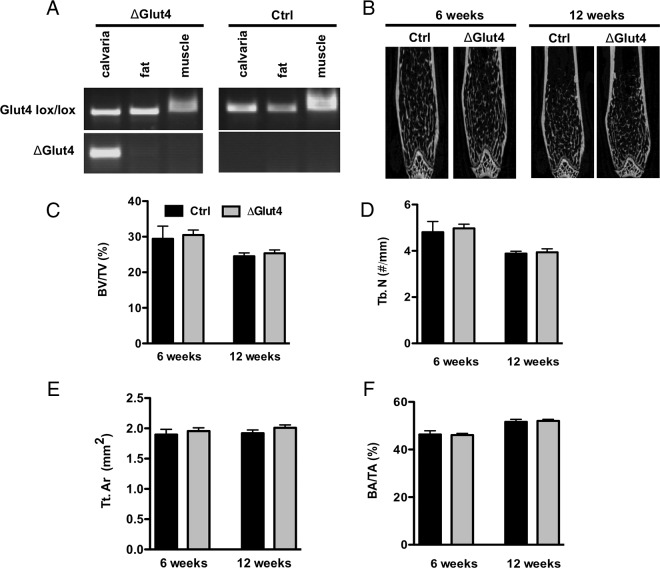
We next generated mice in which the Glut4 gene was disrupted specifically in osteoblasts and osteocytes (ΔGlut4) to define the requirement for Glut4 expression by osteoblasts during postnatal bone acquisition. Glut4flox/flox mice (17–19) were crossed with osteocalcin-Cre mice (20) and allele-specific PCR confirmed that recombination occurred only in skeletal tissue (Figure 4A). Male control and ΔGlut4 mice were selected for detailed analysis. Despite significant reductions in osteoblast performance observed in cultured Glut4-deficient osteoblasts, micro-CT analysis of femoral bone morphometry at 6 and 12 weeks indicated no major differences in cortical or trabecular bone structure in ΔGlut4 mice when compared with controls (Figures 4, B–F). Histomorphometric parameters were also similar between mutant and control mice, although there was a trend towards a reduction in osteoblast number per bone surface, mineralizing surface, and bone formation rate (Supplemental Table 1).
Figure 4.
Bone morphometry in ΔGlut4 mice. A, Allele-specific PCR analysis of recombination of the Glut4flox allele in control and ΔGlut 4 mice. Note the recombined PCR product in the lower panel is only evident in the calvarial tissue of the ΔGlut4 mice. B, Representative micro-CT images of 6- and 12-week-old male control and ΔGlut4 femurs. Micro-CT scanning was used to quantify bone volume per tissue volume (BV/TV) (C) and trabecular number (Tb.N) (D) in the distal femur, and cortical tissue area (Tt.Ar) (E) and bone area per tissue area (BA/TA) (F) at the femoral middiaphysis (n = 4–9 mice).
Loss of Glut4 in osteoblasts results in peripheral insulin resistance
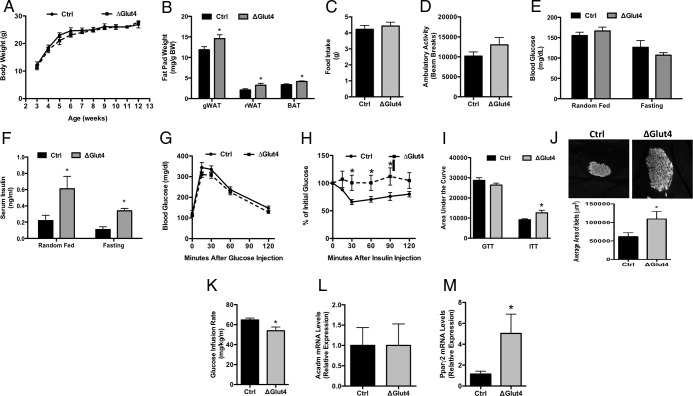
Despite the lack of a clear defect in skeletal development, we continued our analysis of ΔGlut4 mice to determine whether Glut4-deficiency in osteoblasts and osteocytes impacted whole-body metabolism. Over the first 3 months of life, ΔGlut4 mice and their control littermates exhibited equivalent body weight (Figure 5A). However, at necropsy 12-week-old Glut4-deficient mice exhibited significant increases in the weights of both white and brown adipose depots when compared with controls (Figure 5B). Analyses of body composition by quantitative nuclear magnetic resonance, which showed strong trends towards increased fat mass (6.730 ± 0.80% vs 8.87 ± 0.80%, P = .07) and reduced lean mass (77.48 ± 0.90% vs 74.82 ± 0.89%, P = .06) in ΔGlut4 mice relative to controls (data not shown), confirmed these data. These changes in body composition occurred without significant changes in food intake (Figure 5C), ambulatory activity (Figure 5D), or detectable changes in whole-body EE when assessed by indirect calorimetry (data not shown).
Figure 5.
Disturbances in global glucose metabolism in ΔGlut4 mice. A, Body weight of male control and ΔGlut4 mice (n = 8–11 mice). B, Fat pad mass at 12 weeks of age (n = 12–17 mice). Fat pad mass is normalized to body mass. C, Food intake measured in the CLAMS system (n = 8–9 mice). D, Ambulatory activity measured by beam breaks in the CLAMS system (n = 8–9 mice). E, Random-fed and fasting blood glucose levels in control and ΔGlut4 mice (n = 6–10 mice). F, Serum insulin levels in control and ΔGlut4 mice (n = 5 mice). G, Glucose tolerance testing was performed at 12 weeks of age after fasting overnight (n = 8–10 mice). H, Insulin tolerance testing (ITT) was performed after fasting for 4 hours (n = 6–8 mice). I, Area under the curve analysis for GTT and ITT studies. J, Representative insulin-stained β-cell islets from 12-week-old control (top) and ΔGlut4 (bottom) mice and quantitation of islet area (n = 6 mice). Glucose infusion rate (K) was assessed during euglycemic hyperinsulinemic clamps. L, qPCR analysis of Acadm mRNA levels in the skeletal muscle of 12-week-old control and ΔGlut4 mice (n = 5 mice). M, qPCR analysis of Pparγ2 mRNA levels in the gonadal fat pad of 12-week-old control and ΔGlut4 mice (n = 5 mice).
Blood glucose levels in ΔGlut4 mice were comparable with those in control littermates in both the fasting and random-fed state (Figure 5E), but serum insulin levels were significantly elevated (Figure 5F), suggestive of the development of insulin resistance. In accordance with this idea, ΔGlut4 mice exhibited a normal response in standard glucose tolerance testing (Figure 5, G and I), but significant reductions in insulin sensitivity during insulin tolerance testing (Figure 5, H and I) and hypertrophy of pancreatic β-cell islets (Figure 5J). Moreover, the rate of glucose infusion necessary to maintain euglycemia in clamp studies (Figure 5K) was significant reduced in ΔGlut4 mice when compared with that for littermate controls (64.7 ± 1.9 vs 53.9 ± 3.7 mg/kg·min, P < .05). Interestingly, the development of insulin resistance in the mutant mice was not associated with a reduction in the sensitivity of skeletal muscle or white adipose tissue as the expression of insulin-sensitive genes were unaffected or increased (Figure 5, L and M). Therefore, the loss of Glut4 activity in osteoblasts and consequent reductions in glucose acquisition by osteoblasts appears to be sufficient to influence global glucose disposal and contribute to the development of insulin resistance without affecting other glucose using tissues.
Discussion
The emerging paradigm involving the skeleton in the homeostatic control of global energy homeostasis carries with it the obvious implication that bone cells consume substantial amounts of fuel to generate the energy required for bone formation. The results from this study provide strong evidence that insulin-stimulated glucose uptake in mature osteoblasts depends on Glut4 and that loss of this pathway in vivo impacts global glucose metabolism.
Our finding that insulin stimulates glucose oxidation supports results from early studies conducted in crude bone cell populations and bone chips (12, 13), which indicated the presence of enzymatic components for both aerobic and anaerobic glycolysis, including insulin-dependent glucose oxidation (14–16). Importantly, insulin-stimulated glucose uptake rose progressively and was highest in fully differentiated osteoblasts, which accords with the greater fuel demands likely required by mature osteoblasts for matrix production and mineral deposition; these data also complement the increase in mitochondrial mass associated with osteoblast differentiation. Immature osteoblasts have also been reported to contain granules that stain positively for glycogen, which decrease in size as the cells mature (14, 32), further supporting the importance of glucose oxidation in differentiated osteoblasts. Although insulin also stimulated osteoblast uptake of fatty acids it had little effect on fatty acid oxidation. Previous studies in intact mice show that bone takes up a significant fraction of postprandial lipoprotein (33) and that osteoblasts grown in the absence of lipoproteins exhibit severe proliferation defects (34). Thus, it appears that osteoblasts use fatty acids as an energy source for ATP production through insulin-independent mechanisms.
Several lines of evidence from our studies suggest that Glut4 mediates insulin-dependent glucose uptake in mature osteoblasts. First, although 3 Gluts were expressed in primary osteoblasts, Glut4 expression uniquely rose in parallel with the increased insulin-stimulated glucose oxidation and osteoblast differentiation. The fact that maximal expression of Glut4 coincides with maximal insulin-induced glucose acquisition in fully mature osteoblasts strongly suggests an important role for this transporter in the energy requirements associated with matrix production and mineralization. Furthermore, insulin treatment induced Glut4 translocation to the plasma membrane in close association with increased 2-deoxy-glucose uptake. Finally, elimination of Glut4 in osteoblasts in vitro eliminated insulin-stimulated glucose uptake.
It is important to emphasize that our experimental approach did not test the possible contribution of Glut1 to insulin-dependent glucose transport in osteoblasts. The fact that Glut1 is well expressed in immature osteoblasts suggests that this transporter may function to promote glucose oxidation for energy needed for more general cellular processes such as proliferation. Indeed, a recent study by Wei et al reported that undifferentiated osteoblasts preferentially take up glucose via Glut1 but in an insulin-independent manner (31). They further demonstrated that Glut1 mediated glucose metabolism in osteoprogenitors is essential for Runx2 to exert transcriptional control of collagen type I synthesis and osteoblast differentiation.
The lack of a discernable bone phenotype in the ΔGlut4 mice was somewhat surprising considering the reductions in matrix mineralization observed in the mutant osteoblasts in vitro. Although not formally tested in this study, it is possible that that up-regulation of other glucose transport mechanisms or conversion to fatty acid oxidation might have compensated for the loss of Glut4 to enable normal osteoblast differentiation in vivo. Nonetheless, the changes in body composition and metabolic perturbations in the ΔGlut4 mice suggest that loss of insulin-dependent glucose uptake by Glut4 in osteoblasts impacts global glucose homeostasis. In this regard, comparison of the metabolic phenotype of the Glut4 mutants with that previously reported in mice lacking IR in osteoblasts (8) reveals important differences that inform on the mechanisms accounting for the different phenotypes in these mice. For example, although both Glut4 and IR mutants both exhibit blunted responses to insulin during insulin tolerance testing, the IR mice are also hyperglycemic, glucose intolerant, and have low circulating insulin, features not observed in the Glut4 mutants. Thus, the reduced insulin sensitivity in IR mutants appears to reflect insulin resistance in peripheral tissues, which arises secondary to defective osteocalcin production and impaired insulin secretory reserve. By contrast, the osteoblast-pancreas loop appears to be intact in the Glut4 mutant mice and indeed circulating levels of insulin are appropriately elevated in the face of insulin resistance. Also, although standard markers of insulin responsiveness in tissues from the IR mutants such as adipose peroxisome proliferator-activated receptor (Ppar)γ2 are reduced in accordance with tissue resistance to insulin, adipose Pparγ2 expression is up-regulated in the Glut 4 mutants, indicating appropriate responsiveness of fat to prevailing insulin levels. It would therefore appear that the failure of Glut4 mutants to lower circulating glucose as indicated by insulin tolerance testing and hyperinsulinemic-euglycemic clamp results at least in part from decreased glucose disposal by osteoblasts rather than from global insulin resistance in the periphery. The increased fat mass in the Glut4 mutants may therefore be the result of increased glucose uptake by adipose. Current studies to test this possibility are underway using PET-CT with radiolabeled tracers (35).
In summary, the results presented here identify for the first time the presence of a functional insulin-responsive Glut4 transporter in osteoblasts and establish its importance in the regulation of whole-body glucose homeostasis. These findings further emphasize the importance of insulin responsive skeletal cells as a component of a newly appreciated endocrine network critical for regulating global energy homeostasis.
Acknowledgments
We thank Dr B. Kahn for supplying the Glut4 floxed mouse and D. Accili for the Glut4 reporter mouse.
Author contributions: T.L.C. and R.C.R. designed the study. Z.L., J.L.F., and R.C.R. collected the data. Z.L., R.C.R., G.W.W., M.J.W., and T.L.C. analyzed and interpreted the data. Z.L., R.C.R,. and T.L.C. drafted and reviewed the manuscript. All authors approved the final version of the manuscript for submission. T.L.C. takes responsibility for the integrity of the data analysis.
This work was supported by the Merit Review Grant BX001234 from the Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development (to T.L.C.) and by National Institutes of Health Grants DK099134 (to R.C.R.), NS072241 (to M.J.W.), and DK093000 (to J.K.K.). T.L.C. is a recipient of a Research Career Scientist Award from the Department of Veterans Affairs. Support was also provided by the Johns Hopkins University-University of Maryland Diabetes Research Center Grant DK079637.
Disclosure Summary: The authors have nothing to disclose.
Appendix
See Table 1.
Table 1.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used | Antibody Registry ID |
|---|---|---|---|---|---|---|
| Glut4 | Glut4 (1F8) | Cell Signaling Technologies, 2213 | Mouse; monoclonal | 1:1000 | AB_823508 | |
| IR | IR-β (4B8) | Cell Signaling Technologies, 3025 | Rabbit; monoclonal | 1:2000 | AB_2280448 | |
| IGF-1 receptor | IGF-1Rβ (111A9) | Cell Signaling Technologies, 3018 | Rabbit; monoclonal | 1:2000 | AB_10693453 | |
| Actin | β-Actin (8H10D10) | Cell Signaling Technologies, 3700 | Mouse; monoclonal | 1:10 000 | AB_2242334 | |
| Insulin | Insulin | DAKO, A056401-2 | Guinea pig; polyclonal | 1:1000 | AB_2617169 |
Footnotes
- BrdU
- bromodeoxyuridine
- CT
- computed tomography
- EE
- energy expenditure
- Glut
- glucose transporter
- IR
- insulin receptor
- PPAR
- peroxisome proliferator-activated receptor
- qPCR
- quantitative polymerase chain reaction
- RER
- respiratory exchange ratio
- RFP
- red fluorescent protein
- VO2
- O2 consumption.
References
- 1. Karsenty G. Bone formation and factors affecting this process. Matrix Biol. 2000;19(2):85–89. [DOI] [PubMed] [Google Scholar]
- 2. Pritchard JJ. A cytological and histochemical study of bone and cartilage formation in the rat. J Anat. 1952;86(3):259–277. [PMC free article] [PubMed] [Google Scholar]
- 3. Lee NK, Sowa H, Hinoi E, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130(3):456–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yoshikawa Y, Kode A, Xu L, et al. Genetic evidence points to an osteocalcin-independent influence of osteoblasts on energy metabolism. J Bone Miner Res. 2011;26(9):2012–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hinoi E, Gao N, Jung DY, et al. The sympathetic tone mediates leptin's inhibition of insulin secretion by modulating osteocalcin bioactivity. J Cell Biol. 2008;183(7):1235–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ducy P, Amling M, Takeda S, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100(2):197–207. [DOI] [PubMed] [Google Scholar]
- 7. Ferron M, Wei J, Yoshizawa T, et al. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142(2):296–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fulzele K, Riddle RC, DiGirolamo DJ, et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2010;142(2):309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferron M, McKee MD, Levine RL, Ducy P, Karsenty G. Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone. 2011;50(2):568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bolton JG, Patel S, Lacey JH, White S. A prospective study of changes in bone turnover and bone density associated with regaining weight in women with anorexia nervosa. Osteoporos Int. 2005;16(12):1955–1962. [DOI] [PubMed] [Google Scholar]
- 11. Nussbaum M, Baird D, Sonnenblick M, Cowan K, Shenker IR. Short stature in anorexia nervosa patients. J Adolesc Health Care. 1985;6(6):453–455. [DOI] [PubMed] [Google Scholar]
- 12. Borle AB, Nichols N, Nichols G., Jr Metabolic studies of bone in vitro. II. The metabolic patterns of accretion and resorption. J Biol Chem. 1960;235:1211–1214. [PubMed] [Google Scholar]
- 13. Borle AB, Nichols N, Nichols G., Jr Metabolic studies of bone in vitro. I. Normal bone. J Biol Chem. 1960;235:1206–1210. [PubMed] [Google Scholar]
- 14. Schmid C, Steiner T, Froesch ER. Parathormone promotes glycogen formation from [14C]glucose in cultured osteoblast-like cells. FEBS Lett. 1982;148(1):31–34. [DOI] [PubMed] [Google Scholar]
- 15. Schmid C, Steiner T, Froesch ER. Insulin-like growth factors stimulate synthesis of nucleic acids and glycogen in cultured calvaria cells. Calcif Tissue Int. 1983;35(4–5):578–585. [DOI] [PubMed] [Google Scholar]
- 16. Felix R, Neuman WF, Fleisch H. Aerobic glycolysis in bone: lactic acid production by rat calvaria cells in culture. Am J Physiol. 1978;234(1):C51–C55. [DOI] [PubMed] [Google Scholar]
- 17. Abel ED, Kaulbach HC, Tian R, et al. Cardiac hypertrophy with preserved contractile function after selective deletion of GLUT4 from the heart. J Clin Invest. 1999;104(12):1703–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Graham TE, Kahn BB. Tissue-specific alterations of glucose transport and molecular mechanisms of intertissue communication in obesity and type 2 diabetes. Horm Metab Res. 2007;39(10):717–721. [DOI] [PubMed] [Google Scholar]
- 19. Kim JK, Zisman A, Fillmore JJ, et al. Glucose toxicity and the development of diabetes in mice with muscle-specific inactivation of GLUT4. J Clin Invest. 2001;108(1):153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang M, Xuan S, Bouxsein ML, et al. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem. 2002;277(46):44005–44012. [DOI] [PubMed] [Google Scholar]
- 21. Lin HV, Ren H, Samuel VT, et al. Diabetes in mice with selective impairment of insulin action in Glut4-expressing tissues. Diabetes. 2011;60(3):700–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brüning JC, Michael MD, Winnay JN, et al. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell. 1998;2(5):559–569. [DOI] [PubMed] [Google Scholar]
- 23. Kanda H, Tamori Y, Shinoda H, et al. Adipocytes from Munc18c-null mice show increased sensitivity to insulin-stimulated GLUT4 externalization. J Clin Invest. 2005;115(2):291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wei Z, Peterson JM, Lei X, et al. C1q/TNF-related protein-12 (CTRP12), a novel adipokine that improves insulin sensitivity and glycemic control in mouse models of obesity and diabetes. J Biol Chem. 2012;287(13):10301–10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ellis JM, Wong GW, Wolfgang MJ. Acyl coenzyme A thioesterase 7 regulates neuronal fatty acid metabolism to prevent neurotoxicity. Mol Cell Biol. 2013;33(9):1869–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25(7):1468–1486. [DOI] [PubMed] [Google Scholar]
- 27. Hussain MA, Porras DL, Rowe MH, et al. Increased pancreatic β-cell proliferation mediated by CREB binding protein gene activation. Mol Cell Biol. 2006;26(20):7747–7759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lusk G. The Elements of the Science of Nutrition. Philadelphia, PA: WB Saunders; 1928. [Google Scholar]
- 29. Kim JK. Hyperinsulinemic-euglycemic clamp to assess insulin sensitivity in vivo. Methods Mol Biol. 2009;560:221–238. [DOI] [PubMed] [Google Scholar]
- 30. Cho YR, Kim HJ, Park SY, et al. Hyperglycemia, maturity-onset obesity, and insulin resistance in NONcNZO10/LtJ males, a new mouse model of type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;293(1):E327–E336. [DOI] [PubMed] [Google Scholar]
- 31. Wei J, Shimazu J, Makinistoglu MP, et al. Glucose uptake and Runx2 synergize to orchestrate osteoblast differentiation and bone formation. Cell. 2015;161(7):1576–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Scott BL, Glimcher MJ. Distribution of glycogen in osteoblasts of the fetal rat. J Ultrastruct Res. 1971;36(5):565–586. [DOI] [PubMed] [Google Scholar]
- 33. Niemeier A, Niedzielska D, Secer R, et al. Uptake of postprandial lipoproteins into bone in vivo: impact on osteoblast function. Bone. 2008;43(2):230–237. [DOI] [PubMed] [Google Scholar]
- 34. Catherwood BD, Addison J, Chapman G, Contreras S, Lorang M. Growth of rat osteoblast-like cells in a lipid-enriched culture medium and regulation of function by parathyroid hormone and 1,25-dihydroxyvitamin D. J Bone Miner Res. 1988;3(4):431–438. [DOI] [PubMed] [Google Scholar]
- 35. Zoch ML, Abou DS, Clemens TL, Thorek DL, Riddle RC. In vivo radiometric analysis of glucose uptake and distribution in mouse bone. Bone Res. 2016;4:16004. [DOI] [PMC free article] [PubMed] [Google Scholar]