Abstract
The glucose-6-phosphatase catalytic subunit 2 (G6PC2) gene encodes an islet-specific glucose-6-phosphatase catalytic subunit. G6PC2 forms a substrate cycle with glucokinase that determines the glucose sensitivity of insulin secretion. Consequently, deletion of G6pc2 lowers fasting blood glucose (FBG) without affecting fasting plasma insulin. Although chronic elevation of FBG is detrimental to health, glucocorticoids induce G6PC2 expression, suggesting that G6PC2 evolved to transiently modulate FBG under conditions of glucocorticoid-related stress. We show, using competition and mutagenesis experiments, that the synthetic glucocorticoid dexamethasone (Dex) induces G6PC2 promoter activity through a mechanism involving displacement of the islet-enriched transcription factor MafA by the glucocorticoid receptor. The induction of G6PC2 promoter activity by Dex is modulated by a single nucleotide polymorphism, previously linked to altered FBG in humans, that affects FOXA2 binding. A 5-day repeated injection paradigm was used to examine the chronic effect of Dex on FBG and glucose tolerance in wild-type (WT) and G6pc2 knockout mice. Acute Dex treatment only induces G6pc2 expression in 129SvEv but not C57BL/6J mice, but this chronic treatment induced G6pc2 expression in both. In 6-hour fasted C57BL/6J WT mice, Dex treatment lowered FBG and improved glucose tolerance, with G6pc2 deletion exacerbating the decrease in FBG and enhancing the improvement in glucose tolerance. In contrast, in 24-hour fasted C57BL/6J WT mice, Dex treatment raised FBG but still improved glucose tolerance, with G6pc2 deletion limiting the increase in FBG and enhancing the improvement in glucose tolerance. These observations demonstrate that G6pc2 modulates the complex effects of Dex on both FBG and glucose tolerance.
Glucose-6-phosphatase (G6Pase) catalyzes the hydrolysis of glucose-6-phosphate (G6P) to glucose and inorganic phosphate (1). G6Pase is a multicomponent enzyme system located in the endoplasmic reticulum composed of a glucose transporter, a G6P/inorganic phosphate transporter, encoded by the SLC37A4 gene, and a catalytic subunit, of which there are 3 isoforms, G6PC1, glucose-6-phosphatase catalytic subunit 2 (G6PC2), and G6PC3 (1). G6PC2 is expressed exclusively in pancreatic islet β-cells (2, 3), where it is thought to oppose the action of the β-cell glucose sensor, glucokinase, which catalyzes the formation of G6P from glucose (4). This concept is supported by experiments comparing wild-type (WT) and G6pc2 knockout (KO) mouse islets which demonstrate that G6Pase activity (5) and glucose cycling (6) are abolished in G6pc2 KO islets.
Experiments using perfused pancreata and isolated islets demonstrate that deletion of G6pc2 enhances the sensitivity of glucose-stimulated insulin secretion (GSIS) to glucose, by shifting the dose response curve for GSIS to the left, rather than affecting maximal GSIS (5). This has two consequences. First, GSIS is enhanced in G6pc2 KO islets at submaximal but not maximal glucose levels (5). Second, this results in a counterintuitive reduction in fasting blood glucose (FBG) levels in G6pc2 KO mice that is associated with no change in fasting plasma insulin (FPI) levels (5, 7, 8). These observations are consistent with data from genome-wide association studies showing that common single nucleotide polymorphisms (SNPs) in the G6PC2 locus are associated with variations in FBG but not FPI in humans (9, 10).
Because chronically elevated FBG is associated with increased risk for the development of both type 2 diabetes (11) and cardiovascular-associated mortality (12), it implies that high G6PC2 expression is actually detrimental to health over the long term. However, presumably during evolution, the ability of G6PC2 to regulate FBG must have conferred a specific advantage. We recently showed that glucocorticoids induce G6PC2 expression and speculated that G6PC2 evolved to transiently modulate FBG in response to stress (8). We extend that observation here by exploring the mechanism by which the glucocorticoid receptor (GR) regulates G6PC2 promoter activity and how G6pc2 deletion modulates the effects of the synthetic glucocorticoid dexamethasone (Dex) on FBG and glucose tolerance in vivo.
Materials and Methods
Gel retardation assays
Gel retardation assays were used to assess MafA binding exactly as previously described (13).
Cell culture
The βTC-3 and HIT cell lines were cultured in DMEM supplemented with 10% fetal bovine serum. The NIT-1 and βTC-tet cell lines were cultured in Ham's F12 and RPMI 1640 media, respectively, both supplemented with 10% fetal bovine serum. The 832/13 cell line, a generous gift from Dr Chris Newgard, was cultured as described (14).
Fusion gene analyses
The construction of a WT human G6PC2-luciferase fusion gene (13, 15), G6PC2 SNP promoter variants (3, 16), and expression vectors for MafA and Neuro D (13) have all been previously described. Site directed mutations of the MafA-binding site in the G6PC2 promoter were generated using PCR as described (13).
Cells were transfected using Lipofectamine (Promega) according to the manufacturer's instructions and incubated for 18–20 hours in the presence or absence of 250nM Dex before harvesting. Luciferase assays were performed using the Promega Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer's instructions. For comparisons of basal gene expression and Dex-stimulated gene expression, firefly luciferase activity directed by the various fusion gene constructs was expressed relative to Simian virus 40-Renilla luciferase activity in the same sample. Each construct was analyzed in triplicate in multiple transfections, as specified in the figure legends.
Analysis of G6pc2 glucocorticoid response element (GRE) sequences in islet-derived cell lines
Genomic DNA was isolated from the βTC-3, NIT-1, βTC-tet, and HIT cell lines using standard methods. The G6pc2 promoter was amplified from mouse βTC-3, NIT-1, and βTC-tet cell line genomic DNA using PCR in conjunction with the following primers: 5′-CCCAAGCTTCTAGCCAAGCGTAGAGATTGC-3′ (HindIII site underlined) and 5′-CCGCTCGAGAGTCCGGTAGTCCTCCTGCAG-3′ (XhoI site underlined). The G6pc2 promoter was amplified from hamster HIT cell line gDNA using PCR in conjunction with the following primers: 5′-CCCAAGCTTGGCTTTGAGGCTTATTTGACACAG-3′ (HindIII site underlined) and 5′-CCGCTCGAGGTTCTAACCCTGAAGCAACTGGAG-3′ (XhoI site underlined). PCR fragments were digested with HindIII and XhoI and ligated into the pGEM7 vector (Promega) for DNA sequencing.
Mouse islet isolation
Mouse islets were isolated by the Vanderbilt Islet Procurement and Analysis Core as previously described (17–19).
Analysis of gene expression in mouse islets, islet-derived cell lines, mouse pancreas, and liver
Islet and tissue culture cell RNA were isolated using the RNAqueous kit, whereas pancreatic and liver RNA were isolated using the ToTALLY RNA kit (Ambion). Gene expression was quantitated using real-time PCR. Tissue culture cell, pancreatic, and liver gene expression were quantitated after RNA isolation by using the Turbo DNA-free DNAse Treatment kit (Ambion) to remove trace gDNA followed by cDNA generation using the iScript DNA Synthesis kit (Bio-Rad) and then PCR using the deoxyuridine triphosphate-containing FastStart SYBR Green Master Mix in conjunction with Uracil-DNA-Glycosylase (Roche).
Islet gene expression was quantitated using the primer-probe TaqMan approach from Life Technologies as described (18). Fold induction of gene expression was calculated using the 2(−ΔΔC(T)) method (20).
The following primer pairs were used for the analysis of gene expression: mouse G6pc2 forward, 5′-GTCTGTGGGTGGAGCAGGAC-3′ and mouse G6pc2 reverse, 5′-CCCTGATGGTGGTGGCTCTA-3′; mouse Slc37a4 forward, 5′-GCCAGTAAGGCTGCAGTTGG-3′ and mouse Slc37a4 reverse, 5′-TCTGGCTGGCTTACCCTTCA-3′; mouse Ppia forward, 5′-GGCCGATGACGAGCCC-3′ and mouse Ppia reverse, 5′-TGTCTTTGGAACTTTGTCTGCAA-3′; mouse Fkbp5 forward, 5′-AGGCCGTGATTCAGTACAGG-3′ and mouse Fkbp5 reverse, 5′-GAACGACTCTGAGGCTTTGG-3′; mouse Sgk forward, 5′-CGTCCGAACGGGACAACAT-3′ and mouse Sgk reverse, 5′-GTCCACCGTCCGGTCATAC-3′; and mouse G6pc1 forward, 5′-ACCAAGGGAGGAAGGATGGA-3′ and mouse G6pc1 reverse, 5′-CTGCCACCCAGAGGAGATTG-3′.
For the analysis of mouse islet gene expression, TaqMan primers were purchased from Life Technologies. The catalogue numbers are as follows: mouse G6pc2, Mm00491176_m1; and mouse Slc37a4, Mm00484574_m1.
The genes used as internal controls in islet gene expression analyses were as previously reported (18).
Animal care
The animal housing and surgical facilities used for this study meet the standards set by the American Association for the Accreditation of Laboratory Animal Care standards. All protocols were approved by the Vanderbilt University Medical Center Animal Care and Use Committee. Mice were maintained on a standard rodent chow diet (calorie contributions: 28% protein, 12% fat, and 60% carbohydrate [14% disaccharides]; LabDiet 5001). Food and water were provided ad libitum. Adult mice (∼4–7 mo) were used in these studies.
Generation of G6pc2 KO mice
The generation of G6pc2 KO mice on the pure C57BL/6J (5) and 129SvEvBRD (8) genetic backgrounds using a speed congenic breeding strategy has been previously described.
Dex injection paradigm
Mice were injected at 8 am with 0.13-, 1.3-, or 13-μg/g Dex phosphate (2 mg/mL dissolved in PBS) for 4–5 days before ip glucose tolerance tests (IPGTTs). These doses of Dex were based on the results of a previous publication (21).
Physical restraint paradigm
The repeated physical restraint experimental paradigm has been previously described (8, 22). Briefly, mice were immobilized once per day in a 50-mL conical tube for 1 hour from 9 to 10 am for 10 successive days.
Intraperitoneal GTTs
IPGTTs were performed as previously described (8, 23). Glucose concentrations were measured at the times indicated using a Freestyle glucose meter (Abbott Diabetes Care, Inc). Insulin was assayed using RIA by the Vanderbilt Diabetes Center Hormone Assay Core.
Statistical analyses
Gene expression and fusion gene data were analyzed using a Student's t test: 2 sample assuming equal variance. Mouse data were analyzed using a two-way ANOVA assuming normal distribution and equal variance. A post hoc analysis was performed using the Bonferroni correction for multiple comparisons. The level of significance was as indicated.
Results
The GR stimulates G6PC2 promoter activity by displacing MafA
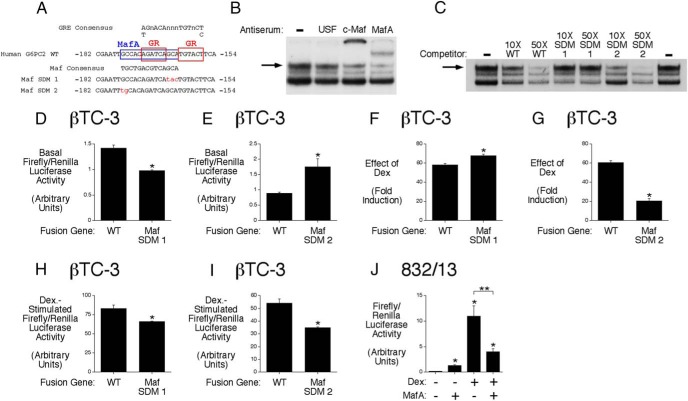
We have previously shown that corticosterone and Dex markedly activate the human G6PC2 promoter through a GRE located between −171 and −157 relative to the transcription start site (8). Sequence analyses suggest that the G6PC2 GRE overlaps a binding site for the islet-enriched transcription factor MafA (Figure 1A) (24). This putative human G6PC2 MafA-binding site shows conservation at 13/14 nucleotides with a previously identified MafA-binding site in the mouse G6pc2 promoter that contributes to basal G6pc2 promoter activity (13). Gel supershift assays confirmed that this human G6PC2 promoter region also binds MafA (Figure 1B). This raised the mechanistic question as to whether MafA and GR compete for binding to this promoter region or whether MafA is acting as an accessory factor to stabilize GR binding and promote glucocorticoid signaling. Thus, although the G6PC2 GRE closely matches the endogenous consensus sequence (Figure 1A), this is not the optimal sequence for high affinity GR binding (25), such that accessory factor involvement is likely required for glucocorticoid signaling (26). To address this question, two G6PC2 promoter site-directed mutants (SDMs) were generated, designated Maf SDMs 1 and 2, which were predicted to reduce or increase MafA binding, respectively, without affecting GR binding (Figure 1A). The Maf SDM 1 mutation disrupts nucleotides that are required for MafA binding, whereas the Maf SDM 2 mutation enhances the G6PC2 MafA-binding site, such that it matches the consensus sequence (Figure 1A). Neither mutation disrupts nucleotides required for GR binding (Figure 1A).
Figure 1.
The GR stimulates G6PC2 promoter activity by displacing MafA. A, The G6PC2 GRE overlaps a binding site for MafA (24). B, Gel retardation supershift assays demonstrate that MafA binds the −182/−154 G6PC2 promoter region. The c-Maf antiserum cross-reacts with MafA (13). A representative gel is shown. C, Gel retardation competition experiments demonstrate that the order of MafA-binding affinity to the sequences shown in A is Maf SDM 2 > WT > Maf SDM 1. A representative gel is shown. D–I, Effect of promoter mutations on basal and Dex-stimulated G6PC2-luciferase fusion gene expression in βTC-3 cells. A reduction in MafA binding (Maf SDM 1) decreases basal expression but has variable effects on the Dex response. An increase in MafA binding (Maf SDM 2) increases basal expression and consistently reduces the Dex response. Results show the mean ± SEM of 3 experiments; *, P < .05 vs control. J, Effect of MafA overexpression on Dex-stimulated G6PC2-luciferase fusion gene expression in 832/13 cells. Overexpression of MafA inhibits G6PC2-luciferase fusion gene expression. Results show the mean ± SEM of 3 experiments; *, P < .05 vs control.
Gel retardation competition assays demonstrated that these mutations have the anticipated effect on MafA binding (Figure 1C and Supplemental Figure 1). When analyzed by transient transfection in βTC-3 cells, the Maf SDM 1 mutation, which reduced MafA binding (Figure 1C), led to reduced basal fusion gene expression (Figure 1D), whereas the Maf SDM 2 mutation, which increased MafA binding (Figure 1C), led to increased basal fusion gene expression (Figure 1E). These data confirm the importance of MafA for basal G6PC2 promoter activity as observed for the mouse G6pc2 promoter (13). These mutations altered G6PC2 promoter activation by Dex (Figure 1, F–I). When data are expressed as fold induction (Figure 1, F and G), the Maf SDM 1 mutation enhanced the Dex response (Figure 1F), whereas the Maf SDM 2 mutation impaired the Dex response (Figure 1G), leading to the conclusion that MafA and GR compete for binding rather than MafA acting as an accessory factor supporting GR binding. In contrast, when data are plotted as maximal induction (Figure 1, H and I), both the Maf SDM 1 (Figure 1H) and Maf SDM 2 (Figure 1I) mutations impaired the Dex response such that only the SDM 2 data clearly support a competition model. However, cotransfection experiments demonstrated that overexpression of MafA (Figure 1J) impairs the Dex response again leading to the conclusion that MafA and GR compete for binding rather than MafA acting as an accessory factor supporting GR binding.
With a view to further exploring this model through the use of chromatin immunoprecipitation assays (27), we also examined Dex-regulated endogenous gene expression in several islet-derived cell lines. Dex induced human G6PC2-luciferase fusion gene expression in the mouse βTC-3 (8), NIT-1 (Supplemental Figure 2A), βTC-tet (Supplemental Figure 2B), and hamster HIT (Supplemental Figure 2C) islet-derived cell lines, with the effect of Dex being enhanced in each case by cotransfection with a plasmid encoding the GR, consistent with previous studies that observed a limiting intracellular concentration of this receptor (28). However, Dex failed to induce endogenous G6pc2 gene expression (Supplemental Figure 3A). In the βTC-3 and NIT-1, but not the βTC-tet and HIT cell lines, the lack of Dex-induced endogenous G6pc2 expression is likely explained by the absence of a consensus GRE in the G6pc2 promoter (Supplemental Figure 3B). However, Dex stimulates endogenous Fkbp5 expression (Supplemental Figure 3C), and Sgk expression (Supplemental Figure 3D), but not Slc37a4 gene expression (Supplemental Figure 3E), in the 3 mouse cell lines, suggesting that G6pc2 and Slc37a4 gene expression are regulated by Dex through mechanisms that are not recapitulated in these islet-derived cell lines. We also considered performing chromatin immunoprecipitation assays in primary islets, but the effects of Dex on G6pc2 (Supplemental Figure 4A), and Slc37a4 (Supplemental Figure 4B), gene expression were transient and reduced in magnitude relative to effects on the endogenous genes in mouse pancreas in vivo (8).
The rs2232316 G6PC2 SNP has opposite effects on basal and Dex-stimulated promoter activity
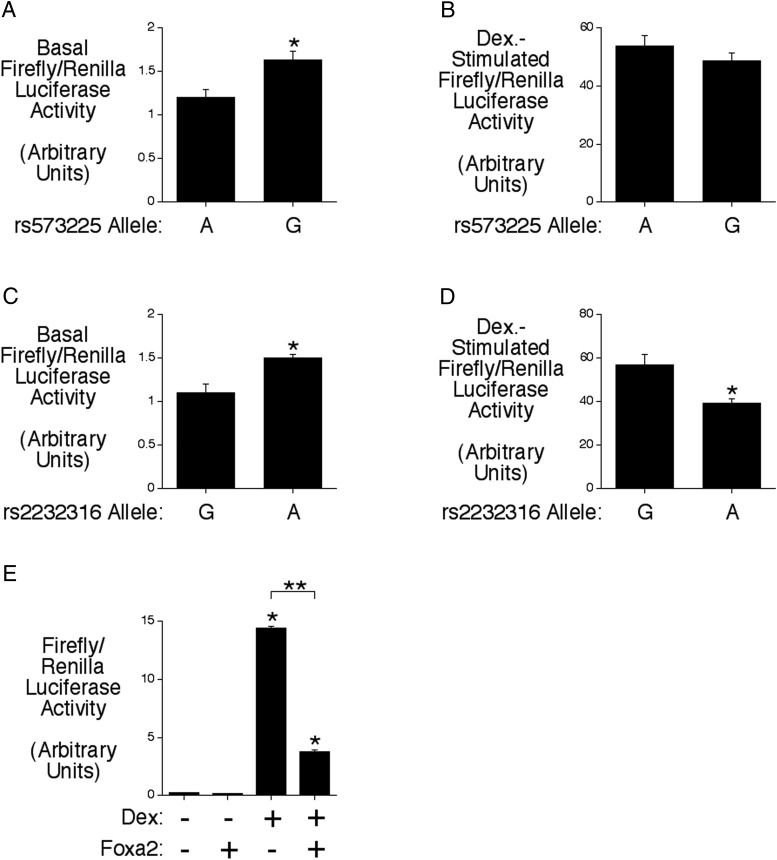
The transcription factor Foxa2 is required for endogenous G6pc2 expression in mouse islets (29) and high G6pc2 promoter activity (13). Because in the related G6pc1 gene Foxa2 can act as an accessory factor to enhance glucocorticoid-stimulated gene expression (30) and because the G6PC2 promoter contains 2 Foxa2-binding sites, located between −261 and −250 and between −246 and −235, we investigated whether Foxa2 also acts as an accessory factor to enhance glucocorticoid-stimulated G6PC2 gene expression. Specifically, we investigated the effect of 2 common SNPs in each of these Foxa2-binding sites, rs573225, located at −259, and rs2232316, located at −238, which we have previously shown affect Foxa2 binding and basal G6PC2 fusion gene expression (3, 16).
The G allele of rs573225 is associated with altered kinetics of Foxa2 binding and increased basal promoter activity (Figure 2A) (3). However, Figure 2B shows that this SNP has no effect on Dex-induced G6PC2-luciferase fusion gene expression. In contrast, although the A allele of rs2232316 is associated with enhanced Foxa2-binding affinity and increased basal promoter activity (Figure 2C) (16), Figure 2D shows that it is actually associated with reduced Dex-induced G6PC2-luciferase fusion gene expression. These data suggest that it is unlikely Foxa2 acts as an accessory factor to enhance Dex-stimulated G6PC2 gene expression through binding to these sites. This conclusion is supported by the observation, from cotransfection experiments, that overexpression of Foxa2 (Figure 2E) impairs the Dex response. Moreover, although rs573225 and rs2232316 are both genetically associated with variations in FBG (3, 16), the data in Figure 2D suggest that whether rs2232316 has a positive or negative influence on FBG may be dependent on glucocorticoid levels.
Figure 2.
The rs2232316 G6PC2 SNP has opposite effects on basal and Dex-stimulated promoter activity. A–D, The influence of the alternate alleles of the rs573225 and rs2232316 SNPs on basal (A and C) and Dex-stimulated (B and D) G6PC2-luciferase fusion gene expression was analyzed by transient transfection in βTC-3 cells. The rs573225-G and rs2232316-A alleles both increase basal expression but only the rs2232316-A allele affects the Dex response. Results show the mean ± SEM of 3 experiments; *, P < .05 vs control. E, Effect of Foxa2 overexpression on Dex-stimulated G6PC2-luciferase fusion gene expression in 832/13 cells. Overexpression of Foxa2 inhibits G6PC2-luciferase fusion gene expression. Results show the mean data ± SD; *, P < .05 vs control.
G6pc2 modulates the effect of Dex on FBG and glucose tolerance in 129SvEv mice
We have previously studied the physiological consequences of glucocorticoid-stimulated 129SvEv G6pc2 expression on FBG in 129SvEv G6pc2 WT and KO mice using a repeated physical restraint experimental paradigm (8). This treatment stimulated endogenous pancreatic G6pc2 and Slc37a4 gene expression in 129SvEv mice but is associated not only with activation of the hypothalamic-pituitary-adrenal axis and elevated endogenous glucocorticoid levels (8) but also with activation of many other autonomic and neuroendocrine axes resulting in the release of epinephrine and norepinephrine (31). The observed induction of G6pc2 expression could, therefore, have been mediated, in part, by factors in addition to glucocorticoids.
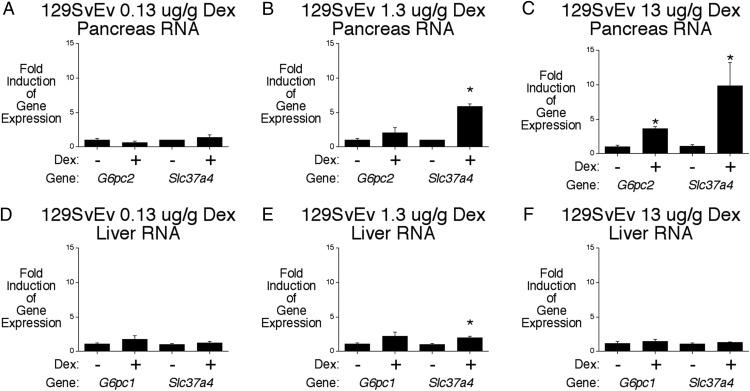
Given this caveat, we examined the impact of G6pc2 deletion on the action of glucocorticoids using an alternate experimental paradigm involving 5 days of once daily Dex injections (21). We first determined the optimal dose of Dex to use for these experiments. Figure 3 shows that a dose of 13-μg/g Dex induced both pancreatic G6pc2 and Slc37a4 expression in WT 129SvEv mice (Figure 3, A–C). This is consistent with our previous studies showing that Dex markedly activates the 129SvEv G6pc2 promoter as well as acutely stimulating, within 3 hours of Dex treatment, endogenous G6pc2 gene expression in primary 129SvEv mouse islets and 129SvEv mouse pancreas in vivo (8). Surprisingly, Dex had little effect on hepatic G6pc1 and Slc37a4 expression in 129SvEv mice in vivo (Figure 3, D–F), despite the fact that both gene promoters are activated by Dex in hepatoma cells (30, 32). This probably is because plasma insulin is elevated in Dex-treated 129SvEv mice (see below) and insulin suppresses both G6pc1 and Slc37a4 gene expression (33, 34).
Figure 3.
Chronic Dex treatment stimulates pancreatic G6pc2 gene expression in 129SvEv mice. Effect of chronic Dex treatment on pancreatic G6pc2 and Slc37a4 (A–C) and hepatic G6pc1 and Slc37a4 (D–F) gene expression in 129SvEv mice. Pancreatic and hepatic RNA were isolated after a 6-hour fast from control mice and mice that had received daily injections of the indicated amount of Dex phosphate for 5 days. Results show the mean ± SEM of 3 experiments; *, P < .05 vs control.
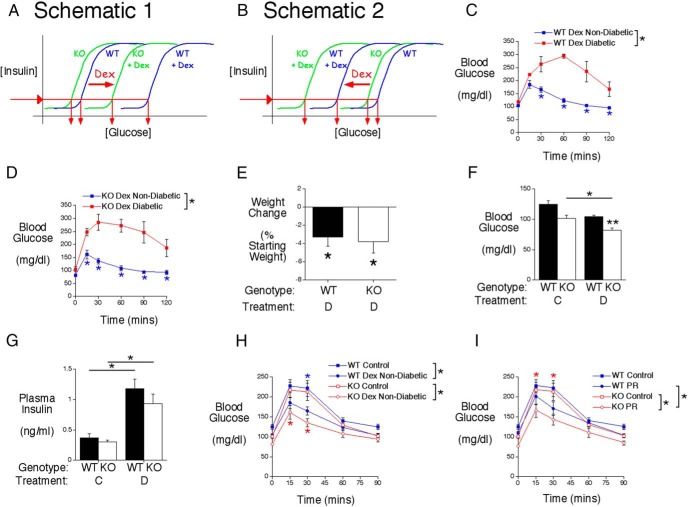
We have previously shown, in mixed genetic background (7), C57BL/6J (5) and 129SvEv (8) mice, that FPI levels are identical in WT and G6pc2 KO mice, but FBG is lower in G6pc2 KO mice. This counterintuitive change in glucose without a change in insulin occurs because deletion of G6pc2 results in a leftward shift in the dose response curve for GSIS rather than a change in maximal GSIS (Figure 4, A and B) (5). We hypothesized that the induction of G6pc2 expression by Dex would enhance this existing difference in FBG between WT and KO mice (Figure 4, A and B). However, because FBG is determined by multiple factors other than G6PC2, we reasoned that the physiological benefit conferred by G6pc2 induction would depend on the overall combined effects of Dex on whole-body glucose metabolism in vivo. We predicted that if Dex results in an elevation in FBG in WT mice, then the induction of G6pc2 expression would contribute to that elevation and it would be prevented or reduced in G6pc2 KO mice (Figure 4A). On the other hand, we predicted that if Dex results in hypoglycemia in G6pc2 KO mice, then the induction of G6pc2 expression in WT mice would limit or prevent hypoglycemia (Figure 4B).
Figure 4.
G6pc2 modulates the effect of Dex on FBG and glucose tolerance in 129SvEv mice. A and B, Schematics proposing that the induction of G6pc2 expression by Dex will increase the difference in FBG between fasted WT and KO mice. The diagrams indicate that the actual values of the x-axis (glucose) may be shifted to the right (A) or left (B), relative to those in control mice, depending on the effects of Dex on other aspects of metabolism. In either case, FPI levels are predicted to not differ between WT and G6pc2 KO mice, as observed in control mice. C, Analysis of glucose tolerance using IPGTTs in 6-hour fasted conscious Dex-treated WT 129SvEv mice. Glucose tolerance was significantly different between nondiabetic Dex-treated (n = 12) and diabetic Dex-treated (n = 5) WT mice based on ANOVA and at the indicated time points based on post hoc analyses (*, P < .05). D, Analysis of glucose tolerance using IPGTTs in 6-hour fasted conscious Dex-treated KO 129SvEv mice. Results show the mean ± SEM. Glucose tolerance was significantly different between nondiabetic Dex-treated (n = 10) and diabetic Dex-treated (n = 5) KO mice based on ANOVA and at the indicated time points based on post hoc analyses (*, P < .05). E, Weight change after 4 days of Dex (D) injections in nondiabetic 129SvEv mice (WT, n = 12; KO, n = 10). Results show the mean ± SEM; *, P < .05 vs initial body weight. F, Glucose levels in 6-hour fasted conscious control (C) (WT, n = 14; KO, n = 12) or Dex-treated nondiabetic (D) (WT, n = 12; KO, n = 10) 129SvEv male mice. Results show the mean ± SEM; *, P < .05 vs control KO; **, P < .05 vs matching WT. G, Insulin levels in 6-hour fasted conscious control (C) (WT, n = 11; KO, n = 10) or Dex-treated nondiabetic (D) (WT, n = 7; KO, n = 6) 129SvEv male mice. Results show the mean ± SEM; *, P < .05 vs control WT or KO. H, Analysis of glucose tolerance using IPGTTs in 6-hour fasted conscious control (WT, n = 14; KO, n = 12) or Dex-treated (WT, n = 12; KO, n = 10) 129SvEv mice. Results show the mean ± SEM. Glucose tolerance was significantly different between control and nondiabetic Dex-treated WT mice and control and nondiabetic Dex-treated KO mice based on ANOVA and at the indicated time points based on post hoc analyses (*, P < .05). I, Analysis of glucose tolerance using IPGTTs in 6-hour fasted conscious control (WT, n = 14; KO, n = 12) or physically restrained (PR) (WT, n = 8; KO, n = 10) 129SvEv mice. Results show the mean ± SEM. Glucose tolerance was significantly different between control and physically restrained KO mice and between physically restrained WT and KO mice based on ANOVA and at the indicated time points based on post hoc analyses (*, P < .05).
Unexpectedly 5/17 129SvEv WT (Figure 4C) and 5/15 129SvEv G6pc2 KO (Figure 4D) mice developed diabetes after Dex treatment, defined here as a blood glucose level of more than 200 mg/dL at the 60-minute time point in an IPGTT. The molecular basis for the development of diabetes in a subset of mice is unknown, although this variable effect of Dex treatment on diabetes incidence resembles that in a previous study by Ogawa et al (35), who observed a 16% incidence of diabetes in Dex-treated Wistar rats.
Considering only the nondiabetic 129SvEv WT and G6pc2 KO mice, prolonged Dex treatment was associated with modest approximately 3% weight loss (Figure 4E). After a 6-hour fast, the difference in FBG between control 129SvEv WT and KO mice was similar to the difference in FBG between Dex-treated 129SvEv WT and KO mice, although only the latter difference was statistically significant (Figure 4F). This likely reflects the fact that large n values are usually required to detect the difference in FBG between control WT and G6pc2 KO mice (5, 7, 8). Figure 4F also shows that FBG was decreased in Dex-treated 129SvEv G6pc2 KO relative to control KO mice. Although FBG was statistically unchanged in Dex-treated 129SvEv WT relative to control WT mice, there was a clear trend towards reduced FBG in WT mice. Overall, these data suggest that the induction of G6pc2 expression by Dex did not markedly enhance the difference in FBG between 129SvEv WT and KO mice. Nevertheless, the presence of G6pc2 in 129SvEv WT mice is clearly protecting against low blood glucose, resembling the schematic shown in Figure 4B. As in control mice, FPI levels did not differ between Dex-treated 129SvEv WT and G6pc2 KO mice (Figure 4G). However, FPI levels were higher in both 129SvEv WT and KO mice after Dex treatment presumably due to the well-characterized induction of insulin resistance by Dex (36). Figure 4B, therefore, represents an oversimplification that does not account for the effect of Dex on insulin signaling.
Interestingly, Dex treatment improved glucose tolerance in both 129SvEv WT and KO mice relative to untreated mice and a clear trend (P < .06) towards a difference in glucose tolerance was apparent between Dex-treated 129SvEv WT and G6pc2 KO mice in contrast to no difference in control mice (Figure 4H). Similarly, after 10 days of repeated physical restraint, glucose tolerance was improved in both 129SvEv WT and KO mice relative to untreated mice and in this case a difference in glucose tolerance was apparent between physically restrained 129SvEv G6pc2 KO mice and WT mice in contrast to no difference in control mice (Figure 4I).
G6pc2 also modulates the effect of Dex on FBG and glucose tolerance in C57BL/6J mice
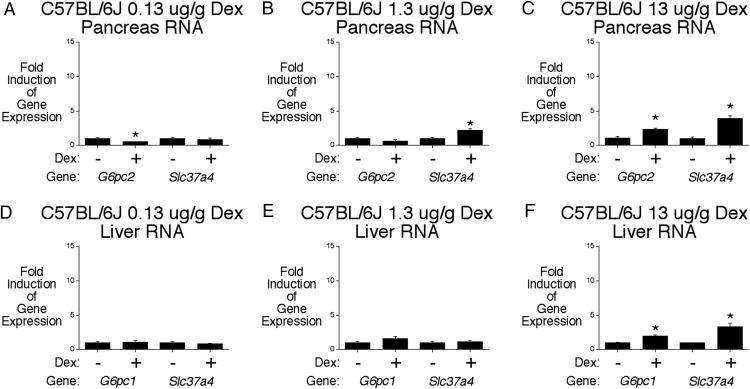
We have previously shown that Dex fails to activate the C57BL/6J G6pc2 promoter or acutely stimulate, within 3 hours of Dex treatment, endogenous G6pc2 gene expression in primary C57BL/6J mouse islets and C57BL/6J mouse pancreas in vivo (8). This difference with 129SvEv mice is due to a SNP that inactivates the GRE in the C57BL/6J G6pc2 promoter (8). Surprisingly, Figure 5 shows that daily injections of a dose of 13-μg/g Dex induced both pancreatic G6pc2 and Slc37a4 expression in C57BL/6J WT mice, although not as markedly as in 129SvEv mice (Figure 3). This observation suggests that the regulation of G6pc2 expression by Dex in vivo is mediated through distinct acute and chronic mechanisms in 129SvEv and C57BL/6J mice (Supplemental Figure 5). In contrast to 129SvEv mice (Figure 3), Dex treatment induced hepatic expression of both G6pc1 and Slc37a4 in C57BL/6J WT mice (Figure 5, D–F); however, because plasma insulin is elevated in Dex-treated C57BL/6J mice (see below) and insulin suppresses both G6pc1 and Slc37a4 gene expression (33, 34), this probably limited the magnitude of the induction.
Figure 5.
Chronic Dex treatment stimulates pancreatic G6pc2 gene expression in C57BL/6J mice. Effect of chronic Dex treatment on pancreatic G6pc2 and Slc37a4 (A–C) and hepatic G6pc1 and Slc37a4 (D–F) gene expression in C57BL/6J mice. Pancreatic and hepatic RNA were isolated after a 6-hour fast from control mice and mice that had received daily injections of the indicated amount of Dex phosphate for 5 days. Results show the mean ± SEM of 3 experiments; *, P < .05 vs control.
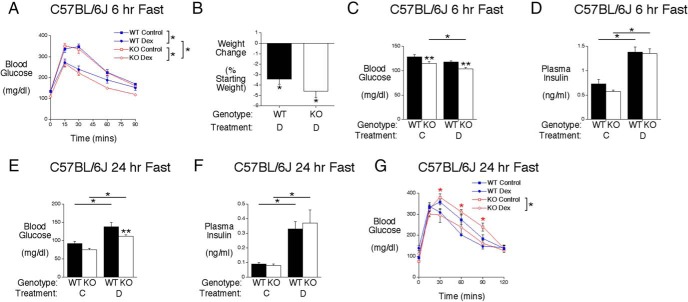
Also, in contrast to 129SvEv mice (Figure 4), no Dex-treated C57BL/6J mice developed diabetes (Figure 6A). Interestingly, as with 129SvEv mice (Figure 4), Dex treatment improved glucose tolerance in both C57BL/6J WT and KO mice relative to untreated mice (Figure 6A). In addition, a difference in glucose tolerance was apparent between Dex-treated C57BL/6J WT and G6pc2 KO mice in contrast to no difference in control mice (Figure 6A).
Figure 6.
The induction of G6pc2 by Dex modulates FBG and glucose tolerance in C57BL/6J mice. A, Analysis of glucose tolerance using IPGTTs in 6-hour fasted conscious control (WT, n = 14; KO, n = 12) or Dex-treated (WT, n = 13; KO, n = 11) C57BL/6J male mice. Results show the mean ± SEM. Glucose tolerance was significantly different between control and Dex-treated mice and between Dex-treated WT and KO mice based on ANOVA; *, P < .05. B, Weight change after 5 days of Dex (D) injections in WT (n = 20) and KO (n = 20) C57BL/6J mice. Results show the mean ± SEM; *, P < .05 vs initial body weight. C, Glucose levels in 6-hour fasted conscious control (C) (WT, n = 12; KO, n = 22) or Dex-treated (D) (WT, n = 36; KO, n = 46) C57BL/6J male mice. Results show the mean ± SEM; *, P < .05 vs control KO; **, P < .05 vs matching WT. D, Insulin levels in 6-hour fasted conscious control (C) (WT, n = 12; KO, n = 22) or Dex-treated (D) (WT, n = 33; KO, n = 42) C57BL/6J male mice. Results show the mean ± SEM; *, P < .05 vs control WT or KO. E, Glucose levels in 24-hour fasted conscious control (C) (WT, n = 9; KO, n = 8) or 24-hour fasted conscious Dex-treated (D) (WT, n = 6; KO, n = 8) C57BL/6J male mice. Results show the mean ± SEM; *, P < .05 vs control WT or KO; **, P < .05 vs matching WT. F, Insulin levels in 24-hour fasted conscious control (C) (WT, n = 9; KO, n = 9) or 24-hour fasted conscious Dex-treated (D) (WT, n = 15; KO, n = 12) C57BL/6J male mice. Results show the mean ± SEM; *, P < .05 vs control WT or KO. G, Analysis of glucose tolerance using IPGTTs in 24-hour fasted conscious control (WT, n = 9; KO, n = 8) or Dex-treated (WT, n = 6; KO, n = 8) C57BL/6J male mice. Results show the mean ± SEM. Glucose tolerance was significantly different between control and Dex-treated KO mice based on ANOVA and at the indicated time points based on post hoc analyses (*, P < .05).
As in 129SvEv mice, prolonged Dex treatment was associated with modest approximately 4% weight loss in C57BL/6J mice (Figure 6B). After a 6-hour fast, the difference in FBG between control C57BL/6J WT and KO mice was similar to the difference in FBG between Dex-treated C57BL/6J WT and KO mice (Figure 6C). Figure 6C also shows that FBG was decreased in Dex-treated C57BL/6J G6pc2 KO relative to control KO mice. Although FBG was statistically unchanged in Dex-treated C57BL/6J WT relative to control WT mice, there was a clear trend towards reduced FBG in C57BL/6J WT mice. Overall, these data suggest that the induction of G6pc2 expression by Dex did not markedly enhance the difference in FBG between C57BL/6J WT and KO mice. Nevertheless, as in 129SvEv mice (Figure 4F), the presence of G6pc2 in C57BL/6J WT mice is clearly protecting against low blood glucose, resembling the schematic shown in Figure 4B. As in control mice, FPI levels did not differ between Dex-treated C57BL/6J WT and G6pc2 KO mice (Figure 6D), although FPI levels were higher in both C57BL/6J WT and KO mice after Dex treatment, again presumably due to the well-characterized induction of insulin resistance by Dex (36).
Strikingly different results were observed in 24-hour fasted mice. Dex treatment increased FBG in both C57BL/6J WT and KO mice, and a difference in FBG was observed between Dex treated, but not control, C57BL/6J WT and KO mice (Figure 6E). These results indicate that, in this context, G6pc2 deletion is limiting the rise in blood glucose induced by Dex treatment, consistent with the schematic shown in Figure 4A. FPI levels did not differ between 24-hour fasted control C57BL/6J WT and KO mice or between Dex-treated C57BL/6J WT and KO mice (Figure 6F), although FPI levels were again higher in both C57BL/6J WT and KO mice after Dex treatment, once again presumably due to the well-characterized induction of insulin resistance by Dex (36). Figure 4A, like Figure 4B, therefore, also represents an oversimplification that does not account for the effect of Dex on insulin signaling.
The reversal in the observed effect of Dex on FBG may be due to the marked decrease in FPI after 24 hours of fasting (Figure 6F) compared with 6 hours of fasting (Figure 6D). Presumably the low FPI in 24-hour fasted Dex-treated C57BL/6J WT and KO mice is insufficient to counteract Dex-induced insulin resistance explaining why FBG is now greater in Dex-treated than control C57BL/6J mice. Despite this reversal in the effect of Dex on FBG in 24- vs 6-hour fasted mice, glucose tolerance was still improved in 24-hour fasted Dex-treated C57BL/6J G6pc2 KO mice with a similar trend in C57BL/6J WT mice (Figure 6G), as was observed in 6-hour fasted mice (Figure 6A). However, in contrast to 6-hour fasted mice (Figure 6A), glucose tolerance was not improved in 24-hour fasted Dex-treated C57BL/6J G6pc2 KO relative to WT mice (Figure 6G).
Discussion
We show here that the synthetic glucocorticoid Dex induces human G6PC2 expression through a mechanism that, competition and mutagenesis studies suggest, involves displacement of the islet-enriched transcription factor MafA by the GR (Figure 1). The effect of Dex on G6PC2 promoter activity is influenced by the rs2232316 SNP, which affects Foxa2 binding and has been linked to variations in FBG (Figure 2) (16). Chronic 5 day treatment with Dex induces G6pc2 expression in both 129SvEv (Figure 3) and C57BL/6J (Figure 5) mouse pancreata in vivo. This contrasts with a selective acute effect of Dex on G6pc2 expression in 129SvEv mice (8). In both 6-hour fasted 129SvEv (Figure 4) and C57BL/6J (Figure 6) mice, the presence of G6pc2 in WT mice served to limit the repression of FBG by Dex. These data closely resemble our studies on 6-hour fasted 129SvEv mice subjected to repeated physical restraint (8), in that the presence of G6pc2 in WT mice again served to limit the repression of FBG observed after physical restraint. These data from 6-hour fasted mice contrast with data from 24-hour fasted C57BL/6J mice (Figure 6), where G6pc2 deletion served to limit the elevation of FBG induced by Dex. Despite the contrasting effects of G6pc2 deletion in 6- vs 24-hour fasted C57BL/6J mice, a unifying theme of these studies is that deletion of G6pc2 lowers FBG regardless of whether the overall whole-body effect of Dex/physical restraint results in reduced or elevated FBG.
In both 6-hour fasted 129SvEv (Figure 4F) and C57BL/6J (Figure 6C) mice, the induction of G6pc2 expression by Dex was insufficient to markedly enhance the difference in FBG between WT and KO mice. In contrast, in 24-hour fasted C57BL/6J mice, the induction of G6pc2 expression by Dex was sufficient to enhance the difference in FBG between C57BL/6J WT and KO mice (Figure 6E). Similarly, in 6-hour fasted 129SvEv mice, the induction of G6pc2 expression by repeated physical restraint was again sufficient to enhance the difference in FBG between 129SvEv WT and KO mice (8). These two latter observations are consistent with the hypothesis that the induction of G6pc2 gene expression by Dex reduces the sensitivity of GSIS to glucose (5, 8). These data suggest that G6PC2 initially evolved to transiently modulate the sensitivity of GSIS to glucose under conditions of glucocorticoid-related stress, in contrast to modern society, where the chronic influence of G6PC2 on FBG affects the risks of cardiovascular-associated mortality and type 2 diabetes. Interestingly, this conclusion is consistent with pathway analyses that demonstrate connectivity between G6PC2 and genes influencing steroid action (37).
In both rodents and humans in vivo, the widely accepted dogma with respect to the effect of Dex/glucocorticoids on glucose metabolism is that they inhibit glucose uptake by inducing insulin resistance (36), stimulate hepatic glucose production (38), and inhibit insulin secretion (35, 39), thereby inducing glucose intolerance (40). These effects result in a transient increase in blood glucose that is considered beneficial during periods of stress (41), although prolonged elevation of glucocorticoids, as occurs in Cushing's disease (42), can lead to diabetes. Our data in 24-hour fasted C57BL/6J mice best resemble normal physiology, because Dex treatment leads to elevated FBG (Figure 6E) although not impaired glucose tolerance (Figure 6G). In contrast, our data in a subset of Dex-injected 129SvEv mice resemble the pathophysiological actions of glucocorticoids, because Dex treatment leads to diabetes (Figure 4, C and D). However, our data in 6-hour fasted mice contrasts with this dogma (Figures 4 and 6), as do studies from other groups (43, 44), and demonstrate the complexity of glucocorticoid physiology by showing that Dex/glucocorticoids can enhance insulin secretion in vivo, beyond the level required to counteract insulin resistance, thereby leading to reduced FBG and improved glucose tolerance. This improved glucose tolerance was observed in 6-hour fasted mice after chronic Dex treatment (Figures 4H and 6A) and after the elevation of endogenous glucocorticoids by physical restraint (Figure 4I). These studies in 6-hour fasted Dex-treated or physically restrained mice, therefore, appear to represent experimental paradigms that provide insight into the function of G6pc2 rather than normal physiology.
The literature on the effects of Dex/glucocorticoids on isolated rodent islets is equally complex with multiple studies reporting that they inhibit (45) or stimulate (46) GSIS in vitro. The explanation for these conflicting observations is unclear; they cannot simply be explained by variations in the duration of Dex/glucocorticoid exposure. Instead, it almost certainly relates to variations in experimental conditions coupled with the kinetic complexity of the transcriptional actions of glucocorticoids (47) and the ability of the GR to also signal through nongenomic mechanisms (48), with the initial health of individuals studied (49) and strain background also being factors in human and mouse studies, respectively.
Data from genome wide association studies data have linked common SNPs in G6PC2 to variations in FBG (9, 10) but not altered glucose tolerance (50–53). In contrast, we observed that glucose tolerance was improved in 129SvEv G6pc2 KO mice relative to WT mice after Dex treatment (Figure 5H) or physical restraint (Figure 5I). Similarly, we observed that glucose tolerance was improved in C57BL/6J G6pc2 KO mice relative to WT mice after Dex treatment (Figure 6A). We hypothesize that the explanation for this apparent difference between the human and mouse data relates to the fact that G6pc2 deletion affects the sensitivity of GSIS to glucose rather than maximal GSIS (5). This means that G6pc2 can influence glucose tolerance if a low amount of glucose is injected in the experiment or if insulin-stimulated glucose disposal has been enhanced, in this case by Dex/glucocorticoids. In both cases, the peak blood glucose will be in a submaximal region of the dose response curve for GSIS, which would be optimal for detecting an effect of G6pc2 deletion on insulin secretion (See Supplemental Figure 6). Consistent with this concept, in those experiments where G6pc2 deletion improved glucose tolerance, the peak glucose concentration in the IPGTT was approximately 150 mg/dL in 129SvEv mice (Figure 5, H and I) and approximately 250 mg/dL in C57BL/6J mice (Figure 6A), which are known to have lower insulin sensitivity (54). This contrasts with peak glucose concentrations of approximately 230 mg/dL (Figure 5, H and I) and approximately 350 mg/dL (Figure 6A) in control 129SvEv and C57BL/6J mice, respectively. Furthermore, in the 24-hour fasted mice where G6pc2 deletion did not improve glucose tolerance, the peak glucose concentration in the IPGTT was almost 400 mg/dL (Figure 6G). This concept is also consistent with previous studies in control, non-Dex-treated mice in which G6pc2 deletion was associated with improved glucose tolerance when a dose of 0.4-g/kg glucose was injected but not higher doses of 0.75 and 2 g/kg (5).
In summary, our data suggest that G6PC2 initially evolved to modulate the sensitivity of GSIS to glucose under conditions of glucocorticoid-induced stress. Future studies will examine whether other hormones/metabolites also regulate G6PC2 gene expression.
Acknowledgments
We thank Susan Hajizadeh and Anastasia Coldren (Vanderbilt University) for performing insulin assays and islet isolations, respectively. We also thank Dr Chris Newgard for generously providing the 832/13 cell line and Alvin C. Powers for helpful discussions on this project.
Author contributions: K.A.B. performed most of the mouse and MafA binding studies and manuscript writing, K.E.S. performed some of the mouse studies, R.A.L. performed studies on MafA and GR binding, C.D. performed the islet gene expression studies, J.K.O. performed the fusion gene studies, O.P.M. contributed to the design of multiple experiments, J.-C.W. contributed to the design of multiple experiments, and R.M.O. performed some of the mouse studies and manuscript writing. R.M.O. is the guarantor of this work, had full access to all the data, and takes full responsibility for the integrity of data and the accuracy of data analysis.
This work was supported by Grants DK92589 (to R.M.O.), DK083591 (to J.-C.W.), DK043748 and DK078188 (to O.P.M.), and DK89572 and DK104211 (to C.D.) and by the Department of Veterans Affairs (C.D.). The Vanderbilt Hormone Assay and Analytical Services Core and the Vanderbilt Islet Procurement and Analysis Core are both supported by the National Institutes of Health (NIH) Grant P60 DK20593 (to the Vanderbilt Diabetes Research Training Center) and by the NIH Grant DK59637 (to the Vanderbilt Mouse Metabolic Phenotyping Center). K.E.S. was supported by the Vanderbilt Molecular Endocrinology Training Program Grant 5T32 DK07563.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Dex
- dexamethasone
- FBG
- fasting blood glucose
- FPI
- fasting plasma insulin
- G6P
- glucose-6-phosphate
- G6Pase
- glucose-6-phosphatase
- G6PC2
- glucose-6-phosphatase catalytic subunit 2
- GR
- glucocorticoid receptor
- GRE
- glucocorticoid response element
- GSIS
- glucose-stimulated insulin secretion
- IPGTT
- ip glucose tolerance test
- KO
- knockout
- SDM
- site-directed mutant
- SNP
- single nucleotide polymorphism
- WT
- wild type.
References
- 1. Hutton JC, O'Brien RM. The glucose-6-phosphatase catalytic subunit gene family. J Biol Chem. 2009;284(43):29241–29245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hutton JC, Eisenbarth GS. A pancreatic β-cell-specific homolog of glucose-6-phosphatase emerges as a major target of cell-mediated autoimmunity in diabetes. Proc Natl Acad Sci USA. 2003;100(15):8626–8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bouatia-Naji N, Bonnefond A, Baerenwald DA, et al. Genetic and functional assessment of the role of the rs13431652-A and rs573225-A alleles in the G6PC2 promoter that strongly associate with elevated fasting glucose levels. Diabetes. 2010;59(10):2662–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Matschinsky FM. Glucokinase, glucose homeostasis, and diabetes mellitus. Curr Diab Rep. 2005(3);5:171–176. [DOI] [PubMed] [Google Scholar]
- 5. Pound LD, Oeser JK, O'Brien TP, et al. G6PC2: a negative regulator of basal glucose-stimulated insulin secretion. Diabetes. 2013;62(5):1547–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wall ML, Pound LD, Trenary I, O'Brien RM, Young JD. Novel stable isotope analyses demonstrate significant rates of glucose cycling in mouse pancreatic islets. Diabetes 2015;64 2129–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang Y, Martin CC, Oeser JK, et al. Deletion of the gene encoding the islet-specific glucose-6-phosphatase catalytic subunit-related protein autoantigen results in a mild metabolic phenotype. Diabetologia. 2007;50(4):774–778. [DOI] [PubMed] [Google Scholar]
- 8. Boortz KA, Syring KE, Dai C, et al. G6PC2 modulates fasting blood glucose in male mice in response to stress. Endocrinology. 2016;157(8):3002–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bouatia-Naji N, Rocheleau G, Van Lommel L, et al. A polymorphism within the G6PC2 gene is associated with fasting plasma glucose levels. Science. 2008;320(5879):1085–1088. [DOI] [PubMed] [Google Scholar]
- 10. Chen WM, Erdos MR, Jackson AU, et al. Variations in the G6PC2/ABCB11 genomic region are associated with fasting glucose levels. J Clin Invest. 2008;118(7):2620–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abdul-Ghani MA, DeFronzo RA. Plasma glucose concentration and prediction of future risk of type 2 diabetes. Diabetes Care. 2009;32(suppl 2):S194–S198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lawes CM, Parag V, Bennett DA, et al. Blood glucose and risk of cardiovascular disease in the Asia Pacific region. Diabetes Care. 2004;27(12):2836–2842. [DOI] [PubMed] [Google Scholar]
- 13. Martin CC, Flemming BP, Wang Y, Oeser J, O'Brien R. Foxa2 and MafA regulate islet-specific glucose-6-phosphatase catalytic subunit-related protein gene expression. J Mol Endocrinol. 2008;41(5):315–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes. 2000;49(3):424–430. [DOI] [PubMed] [Google Scholar]
- 15. Martin CC, Bischof LJ, Bergman B, et al. Cloning and characterization of the human and rat islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP) genes. J Biol Chem. 2001;276(27):25197–25207. [DOI] [PubMed] [Google Scholar]
- 16. Baerenwald DA, Bonnefond A, Bouatia-Naji N, et al. Multiple functional polymorphisms in the G6PC2 gene contribute to the association with higher fasting plasma glucose levels. Diabetologia. 2013;56(6):1306–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brissova M, Shostak A, Shiota M, et al. Pancreatic islet production of vascular endothelial growth factor–a is essential for islet vascularization, revascularization, and function. Diabetes. 2006;55(11):2974–2985. [DOI] [PubMed] [Google Scholar]
- 18. Dai C, Brissova M, Hang Y, et al. Islet-enriched gene expression and glucose-induced insulin secretion in human and mouse islets. Diabetologia. 2012;55(3):707–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brissova M, Aamodt K, Brahmachary P, et al. Islet microenvironment, modulated by vascular endothelial growth factor-A signaling, promotes β cell regeneration. Cell Metab. 2014;19(3):498–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 21. Sadikot RT, Jansen ED, Blackwell TR, et al. High-dose dexamethasone accentuates nuclear factor-κ b activation in endotoxin-treated mice. Am J Respir Crit Care Med. 2001;164(5):873–878. [DOI] [PubMed] [Google Scholar]
- 22. Patel S, Kingsley PJ, Mackie K, Marnett LJ, Winder DG. Repeated homotypic stress elevates 2-arachidonoylglycerol levels and enhances short-term endocannabinoid signaling at inhibitory synapses in basolateral amygdala. Neuropsychopharmacology. 2009;34(13):2699–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pound LD, Sarkar SA, Ustione A, et al. The physiological effects of deleting the mouse slc30a8 gene encoding zinc transporter-8 are influenced by gender and genetic background. PLoS One. 2012;7(7):e40972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kataoka K, Noda M, Nishizawa M. Maf nuclear oncoprotein recognizes sequences related to an AP-1 site and forms heterodimers with both Fos and Jun. Mol Cell Biol. 1994;14(1):700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nordeen SK, Suh BJ, Kühnel B, Hutchison CA., 3rd Structural determinants of a glucocorticoid receptor recognition element. Mol Endocrinol. 1990;4(12):1866–1873. [DOI] [PubMed] [Google Scholar]
- 26. Lucas PC, Granner DK. Hormone response domains in gene transcription. Annu Rev Biochem. 1992;61(Can't get issue from record):1131–1173. [DOI] [PubMed] [Google Scholar]
- 27. Martin CC, Svitek CA, Oeser JK, Henderson E, Stein R, O'Brien RM. Upstream stimulatory factor (USF) and neurogenic differentiation/β-cell E box transactivator 2 (NeuroD/BETA2) contribute to islet-specific glucose-6-phosphatase catalytic-subunit-related protein (IGRP) gene expression. Biochem J. 2003;371(pt 3):675–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vanderbilt JN, Miesfeld R, Maler BA, Yamamoto KR. Intracellular receptor concentration limits glucocorticoid-dependent enhancer activity. Mol Endocrinol. 1(1)987;1:68–74. [DOI] [PubMed] [Google Scholar]
- 29. Gao N, Le Lay J, Qin W, et al. Foxa1 and Foxa2 maintain the metabolic and secretory features of the mature β-cell. Mol Endocrinol. 2010;24(8):1594–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vander Kooi BT, Onuma H, Oeser JK, et al. The glucose-6-phosphatase catalytic subunit gene promoter contains both positive and negative glucocorticoid response elements. Mol Endocrinol. 2005;19(12):3001–3022. [DOI] [PubMed] [Google Scholar]
- 31. Jeong KH, Jacobson L, Pacák K, Widmaier EP, Goldstein DS, Majzoub JA. Impaired basal and restraint-induced epinephrine secretion in corticotropin-releasing hormone-deficient mice. Endocrinology. 2000;141(3):1142–1150. [DOI] [PubMed] [Google Scholar]
- 32. Hiraiwa H, Chou JY. Glucocorticoids activate transcription of the gene for the glucose-6-phosphate transporter, deficient in glycogen storage disease type 1b. DNA Cell Biol. 20(8)01;20:447–453. [DOI] [PubMed] [Google Scholar]
- 33. Hornbuckle LA, Edgerton DS, Ayala JE, et al. Selective, tonic inhibition of G6Pase catalytic subunit but not G6P transporter gene expression by insulin in vivo. Am J Physiol. 2001;281:E713–E725. [DOI] [PubMed] [Google Scholar]
- 34. Hornbuckle LA, Everett CA, Martin CC, et al. Selective stimulation of G-6-Pase catalytic subunit but not G-6-P transporter gene expression by glucagon in vivo and cAMP in situ. Am J Physiol Endocrinol Metab. 2004;286(5):E795–E808. [DOI] [PubMed] [Google Scholar]
- 35. Ogawa A, Johnson JH, Ohneda M, et al. Roles of insulin resistance and β-cell dysfunction in dexamethasone-induced diabetes. J Clin Invest. 1992;90(2):497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kuo T, McQueen A, Chen TC, Wang JC. Regulation of glucose homeostasis by glucocorticoids. Adv Exp Med Biol. 2015;872(Can't get issue from record):99–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Scott RA, Lagou V, Welch RP, et al. Large-scale association analyses identify new loci influencing glycemic traits and provide insight into the underlying biological pathways. Nat Genet. 2012;44(9):991–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McMahon M, Gerich J, Rizza R. Effects of glucocorticoids on carbohydrate metabolism. Diabetes Metab Rev. 1988;4(1):17–30. [DOI] [PubMed] [Google Scholar]
- 39. Delaunay F, Khan A, Cintra A, et al. Pancreatic β cells are important targets for the diabetogenic effects of glucocorticoids. J Clin Invest. 1997;100(8):2094–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rafacho A, Ortsäter H, Nadal A, Quesada I. Glucocorticoid treatment and endocrine pancreas function: implications for glucose homeostasis, insulin resistance and diabetes. J Endocrinol. 2014;223(3):R49–R62. [DOI] [PubMed] [Google Scholar]
- 41. Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol. 2005;67(Can't get issue from record):259–284. [DOI] [PubMed] [Google Scholar]
- 42. Howlett TA, Rees LH, Besser GM. Cushing's syndrome. Clin Endocrinol Metab. 1985;14(4):911–945. [DOI] [PubMed] [Google Scholar]
- 43. Cummings BP, Bremer AA, Kieffer TJ, D'Alessio D, Havel PJ. Investigation of the mechanisms contributing to the compensatory increase in insulin secretion during dexamethasone-induced insulin resistance in rhesus macaques. J Endocrinol. 2013;216(2):207–215. [DOI] [PubMed] [Google Scholar]
- 44. Bates HE, Kiraly MA, Yue JT, et al. Recurrent intermittent restraint delays fed and fasting hyperglycemia and improves glucose return to baseline levels during glucose tolerance tests in the Zucker diabetic fatty rat–role of food intake and corticosterone. Metabolism. 2007;56(8):1065–1075. [DOI] [PubMed] [Google Scholar]
- 45. Lambillotte C, Gilon P, Henquin JC. Direct glucocorticoid inhibition of insulin secretion. An in vitro study of dexamethasone effects in mouse islets. J Clin Invest. 199(3)7;99:414–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hult M, Ortsater H, Schuster G, et al. Short-term glucocorticoid treatment increases insulin secretion in islets derived from lean mice through multiple pathways and mechanisms. Mol Cell Eendocrinol. 2009;301:109–116. [DOI] [PubMed] [Google Scholar]
- 47. John S, Johnson TA, Sung MH, et al. Kinetic complexity of the global response to glucocorticoid receptor action. Endocrinology. 2009;150(4):1766–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Strehl C, Buttgereit F. Unraveling the functions of the membrane-bound glucocorticoid receptors: first clues on origin and functional activity. Ann NY Acad Sci. 2014;1318(Can't get issue from record):1–6. [DOI] [PubMed] [Google Scholar]
- 49. Wajngot A, Giacca A, Grill V, Vranic M, Efendic S. The diabetogenic effects of glucocorticoids are more pronounced in low- than in high-insulin responders. Proc Natl Acad Sci USA. 1992;89(13):6035–6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li X, Shu YH, Xiang AH, et al. Additive effects of genetic variation in Gck and G6pc2 on insulin secretion and fasting glucose. Diabetes. 2009;58(12):2946–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rose CS, Grarup N, Krarup NT, et al. A variant in the G6PC2/ABCB11 locus is associated with increased fasting plasma glucose, increased basal hepatic glucose production and increased insulin release after oral and intravenous glucose loads. Diabetologia. 2009;52(10):2122–2129. [DOI] [PubMed] [Google Scholar]
- 52. Ingelsson E, Langenberg C, Hivert MF, et al. Detailed physiologic characterization reveals diverse mechanisms for novel genetic loci regulating glucose and insulin metabolism in humans. Diabetes. 2010;59(5):1266–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Heni M, Ketterer C, t Hart LM, et al. The impact of genetic variation in the G6PC2 gene on insulin secretion depends on glycemia. J Clin Endocrinol Metab. 2010;95(12):E479–E484. [DOI] [PubMed] [Google Scholar]
- 54. Berglund ED, Li CY, Poffenberger G, et al. Glucose metabolism in vivo in four commonly used inbred mouse strains. Diabetes. 2008;57(7):1790–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]