ABSTRACT
This study evaluated the inhibitory effect of cinnamon oil against Escherichia coli O157:H7 Shiga toxin (Stx) production and further explored the underlying mechanisms. The MIC and minimum bactericidal concentration (MBC) of cinnamon oil against E. coli O157:H7 were 0.025% and 0.05% (vol/vol), respectively. Cinnamon oil significantly reduced Stx2 production and the stx2 mRNA expression that is associated with diminished Vero cell cytotoxicity. Consistently, induction of the Stx-converting phage where the stx2 gene is located, along with the total number of phages, decreased proportionally to cinnamon oil concentration. In line with decreased Stx2 phage induction, cinnamon oil at 0.75× and 1.0× MIC eliminated RecA, a key mediator of SOS response, polynucleotide phosphorylase (PNPase), and poly(A) polymerase (PAP I), which positively regulate Stx-converting phages, contributing to reduced Stx-converting phage induction and Stx production. Furthermore, cinnamon oil at 0.75× and 1.0× MIC strongly inhibited the qseBC and luxS expression associated with decreased AI-2 production, a universal quorum sensing signaling molecule. However, the expression of oxidative stress response genes oxyR, soxR, and rpoS was increased in response to cinnamon oil at 0.25× or 0.5× MIC, which may contribute to stunted bacterial growth and reduced Stx2 phage induction and Stx2 production due to the inhibitory effect of OxyR on prophage activation. Collectively, cinnamon oil inhibits Stx2 production and Stx2 phage induction in E. coli O157:H7 in multiple ways.
IMPORTANCE This study reports the inhibitory effect of cinnamon oil on Shiga toxin 2 phage induction and Shiga toxin 2 production. Subinhibitory concentrations (concentrations below the MIC) of cinnamon oil reduced Stx2 production, stx2 mRNA expression, and cytotoxicity on Vero cells. Subinhibitory concentrations of cinnamon oil also dramatically reduced both the Stx2 phage and total phage induction in E. coli O157:H7, which may be due to the suppression of RNA polyadenylation enzyme PNPase at 0.25× to 1.0× MIC and the downregulation of bacterial SOS response key regulator RecA and RNA polyadenylation enzyme PAP I at 0.75× or 1.0× MIC. Cinnamon oil at higher levels (0.75× and 1.0× MIC) eliminated quorum sensing and oxidative stress. Therefore, cinnamon oil has potential applications as a therapeutic to control E. coli O157:H7 infection through inhibition of bacterial growth and virulence factors.
INTRODUCTION
Escherichia coli O157:H7 is an important foodborne pathogen that is associated with hemorrhagic colitis, hemolytic-uremic syndrome (HUS), and death (1). More than 63,000 cases of foodborne illness are estimated to be caused by E. coli O157:H7 annually in the United States (2). Outbreaks have been associated with ground beef, ready-to-eat salad, cheese, apple juice, and other foods (3–6).
Shiga toxin (Stx) is a major virulence factor of E. coli O157:H7. Its production in the gastrointestinal tract in conjunction with other virulence factors induces hemorrhagic colitis, and its entry into the circulatory system can lead to life-threatening HUS (7, 8). Genes that encode Stx are located in prophages integrated into the E. coli O157:H7 chromosome (9). The activation of Stx prophage, phage DNA replication, and subsequent bacterial cell lysis results in Stx release (10). Importantly, released Stx phages are able to convert commensal E. coli into new Stx-producing E. coli (STEC) (11), facilitating the spread of STEC strains (12). Prophage induction can be caused by factors provoking SOS response, which activates the RecA protein, resulting in self-cleavage of the phage-encoded cI repressor, initiation of phage transcription, and further the transcription of stx genes (13). Quorum sensing (QS) is a global virulence regulator of E. coli O157:H7 as well as a positive regulator of bacterial oxidative stress response (14), which activates the SOS stress response through both LuxS/autoinducer 2 (AI-2) signaling and QseBC signaling (15, 16). OxyR-mediated oxidative stress inhibits prophage induction in λ-phage-infected E. coli MG1655 (17). Our previous study showed that lytic development of the Stx2-converting phage is positively regulated by polynucleotide phosphorylase (PNPase), an important enzyme in bacterial RNA polyadenylation (18). Dysfunction of another RNA polyadenylation enzyme, poly(A) polymerase (PAP I), has also been reported to downregulate the formation of the Stx-converting phage and subsequent Stx production (19).
Treatment of E. coli O157:H7 infection is difficult since many antibiotics are shown to induce SOS response and enhance Stx production both in vitro and in vivo (20, 21). In light of the restrictions of antibiotic treatments in E. coli O157:H7 infection, an alternative antimicrobial intervention is urgently needed that ideally can inhibit not only E. coli O157:H7 growth but also Stx production. Cinnamon is an antimicrobial spice that has been widely used for thousands of years. It is known that cinnamon oil inhibits the growth of many foodborne pathogens, including E. coli O157:H7 (22, 23), non-O157 STEC (24), and other pathogenic E. coli (25). In addition, cinnamaldehyde, the major component of cinnamon oil, suppresses E. coli biofilm formation and attachment to epithelial cells (26, 27). However, there is no information available regarding the effect of cinnamon oil on the expression of key virulence factors in E. coli O157:H7. Therefore, the objectives of this study were to evaluate the effect of cinnamon oil against E. coli O157:H7 Stx production and further explore the potential mechanisms.
MATERIALS AND METHODS
Bacteria strain.
E. coli O157:H7 EDL933 was obtained from the STEC center at Michigan State University. E. coli MG-1655 (ATCC 700926) was obtained from the American Type Culture Collection. The strains were grown in LB broth at 37°C and 250 rpm overnight.
Antimicrobial effects.
Cinnamon oil containing 60% trans-cinnamaldehyde (24) was used in this study. Disc diffusion, the MIC, minimum bactericidal concentration (MBC), growth curve, and death curve were measured as previously described (24). Briefly, for the disc diffusion assay, 2.5, 5, 10, or 20 μl of 4% (vol/vol) cinnamon oil (in 10% dimethyl sulfoxide [DMSO] and 0.5% Tween 80) was loaded onto paper discs placed on a Mueller-Hinton agar plate seeded with ∼1 × 104 CFU of E. coli O157:H7. Twenty microliters of 10% DMSO-0.5% Tween 80 was used as the vehicle control for this study. Three replicates were used for each treatment in the disc diffusion assay. For MIC and MBC, 5 × 105 CFU/ml E. coli O157:H7 was treated with cinnamon oil ranging from 0.003125% to 1.6% in Mueller-Hinton broth containing 0.15% (wt/vol) agar as a stabilizer (28) for 24 h at 37°C in 96-well plates. The lowest concentration of cinnamon oil without visible bacterial growth is considered to be the MIC, while the concentration that eliminated bacterial growth is regarded as the MBC detected by plating onto LB agar plates. A cinnamon oil concentration lower than the MIC is defined as a subinhibitory concentration (29–31). Six replicates were used for each treatment in the MIC and MBC assay. The growth curve was measured hourly by analyzing turbidity at an optical density at 600 nm (OD600) for 24 h, which was conducted under the initial E. coli O157:H7 level of 5 × 105 or 1 × 107 CFU/ml in LB broth with 0.15% (wt/vol) agar (LBA) supplemented with cinnamon oil at 0×, 0.25×, 0.5×, 0.75×, or 1.0× MIC. Six replicates were used for each treatment in the growth curve. The death curve was determined by adding 1 × 107 CFU/ml E. coli O157:H7 into LBA broth containing cinnamon oil at 0×, 1×, 2×, or 3× MBC. The survival bacteria were enumerated at 0, 15, 30, 60, and 120 min postincubation in LBA broth with respective cinnamon oil concentrations. Four replicates were used for each treatment in the death curve. The experiments were repeated three times independently.
Cinnamon oil treatment of E. coli O157:H7 for all mechanistic study.
E. coli O157:H7 from cultures grown overnight in LB was adjusted to 5 × 105 CFU/ml and subcultured in 5 ml of LB broth containing cinnamon oil at 0×, 0.25×, 0.5×, 0.75×, and 1.0× MIC in 15-ml test tubes for 6 and 12 h at 37°C with shaking at 250 rpm when samples were analyzed for CFU enumeration, protein production, gene expression, cytotoxicity, phage titer, bacteriophage stx2 DNA content, and the AI-2 assay.
Immunoblotting analysis.
Immunoblotting was conducted as previously described (32). Briefly, 6-h and 12-h bacterial protein samples (4 replicates for each treatment) were separated by 10% (wt/vol) SDS polyacrylamide gels, transferred to a nitrocellulose membrane, and subjected to primary and secondary antibody incubation and enhanced chemiluminescence (ECL) incubation (GE Healthcare, Buckinghamshire, United Kingdom) for band visualization. Primary poly(A) polymerase (PAP I) polyclonal antibody (Santa Cruz Biotechnology, Inc., Dallas, TX), RecA monoclonal antibody (Enzo Life Sciences, Inc., Farmingdale, NY), RpoS monoclonal antibody (Santa Cruz Biotechnology), monoclonal antibody against the Stx2 A subunit (Toxin Technology, Inc., Sarasota, FL), and PNPase polyclonal antibody (Thermo Fisher Scientific, Rockford, IL) were used at 1:1,000 or 1:2,000 dilutions per manufacturer recommendations. Band density was first corrected with the total protein obtained by quantifying the density of all protein bands existing in a dedicated protein sample after Coomassie staining of the gel following SDS-PAGE (33, 34) and was then normalized to that of the control. The experiment was repeated three times independently.
RNA extraction and reverse transcription real-time PCR.
Total RNA was extracted from bacteria 12 h postincubation with cinnamon oil using the RNeasy Protect bacteria minikit (Qiagen, Valencia, CA). There are 4 replicates for each treatment. RNA concentration was measured by a NanoDrop 2000c spectrophotometer (Thermo Scientific, Waltham, MA), and 500 ng of RNA was reverse transcribed using a QuantiTect reverse transcription kit (Qiagen). We then performed quantitative reverse transcription-PCR (RT-PCR) of selected genes using a CFX96 real-time PCR detection system (Bio-Rad, Hercules, CA) following our published procedure (35). The primers used are listed in Table 1, and 16S rRNA was used as a housekeeping gene. The mRNA expression of selected genes was normalized to the control.
TABLE 1.
Primer sets used for reverse transcription-quantitative PCR
| Gene name | Product size (bp) | Direction | Sequence |
|---|---|---|---|
| 16S rRNA | 132 | Forward | AGACCAAAGAGGGGGACCT |
| Reverse | TTCCAGTGTGGCTGGTCAT | ||
| hfq | 154 | Forward | ATGGCTAAGGGGCAATCTTT |
| Reverse | GGCTGACCGTGTTTTTCAAC | ||
| luxS | 152 | Forward | TGGAAGACGTGCTGAAAGTG |
| Reverse | TTCTTCGTTGCTGTTGATGC | ||
| oxyR | 79 | Forward | GGCAAATTCGTAAGCTGGAA |
| Reverse | GCCTGGGTGAACAACACTTT | ||
| qseB | 137 | Forward | GGCGAACCCTTAACACTGAA |
| Reverse | ACGGCATTACTGGTGACCTC | ||
| qseC | 147 | Forward | GGCACAGACGCCAAATAAAT |
| Reverse | GCAAAACCTTCCCGTTGATA | ||
| rpoS | 123 | Forward | TATTCGTTTGCCGATTCACA |
| Reverse | GGCTTATCCAGTTGCTCTGC | ||
| soxR | 157 | Forward | AGCGGCGTTATAAACGTGAT |
| Reverse | CATTGGGACGAAAGCTGTTT | ||
| stx2 | 133 | Forward | CGTCACTCACTGGTTTCATCAT |
| Reverse | TCTGTATCTGCCTGAAGCGTAA |
Cytotoxicity.
Bacterial cultures at 6 h or 12 h after cinnamon oil treatment were centrifuged at 10,000 × g at 4°C for 10 min. The supernatants were 1:1 mixed with high-glucose Dulbecco modified Eagle medium (DMEM) (Sigma, St. Louis, MO), containing 1% fetal bovine serum (FBS) (Sigma). Vero cells (3 × 104 cells/ml) were seeded into 96-well plates, incubated at 37°C with 5% CO2 for 24 h, and then treated with the above-described toxin preparation for 12 h at 37°C with 5% CO2. Vero cells treated with different concentrations of cinnamon oil were used as negative controls. Lactate dehydrogenase (LDH) was measured using the Cytotoxicity Detection Kit from Roche (Indianapolis, IN) per manufacturer recommendations. LDH is a cytoplasmic enzyme present in Vero cells and is released into extracellular space when the cell membrane is damaged (36). Therefore, released LDH activity was used to predict cytotoxicity. The OD values at 492 nm of the respective treatments were first normalized by log10 CFU to rule out the influence of the population size and then normalized to the control cells. There are 8 replicates for each treatment in each independent study. Experiments were repeated independently three times.
Phage enumeration.
PFU were determined as previously described (11, 18). Since no detectable phage was found in the E. coli O157:H7 culture 6 h postsubculture in LB broth containing various concentrations of cinnamon oil, PFU were only analyzed at 12 h. Briefly, E. coli O157:H7 culture at 12 h after cinnamon oil treatment (4 replicates per treatment) was centrifuged at 10,000 × g and 4°C for 5 min. The resulting supernatant was serial diluted in a phage buffer containing 10 mM CaCl2 and 5 mM MgSO4. The diluent was mixed with 0.9 ml of E. coli MG1655 culture and 5 ml of tempered top agar (0.7% [wt/vol] LB agar supplemented with 10 mM CaCl2 and 10 mM MgSO4); this mixture was immediately poured onto a bottom layer of agar (1.5% LB agar containing 3 μg/ml chloramphenicol) (35). Plates were incubated at 37°C for ∼36 to ∼48 h to enumerate PFU. Results were normalized by log10 CFU to rule out the influence of the population size. Experiment was repeated three times independently.
Quantitative PCR analysis of Stx2 bacteriophage.
E. coli O157:H7 cultures at 12 h after cinnamon oil treatment (4 replicates per treatment) were centrifuged at 10,000 × g and 4°C for 5 min. Supernatants were filtered through a 0.22-μm-pore-size filter. The resulting filtrate was centrifuged at 60,000 × g at 4°C for 1 h using an L7-80R centrifuge (Beckman, Palo Alto, CA). The pellets were resuspended in deionized water and then treated with DNase I for 2 h at 37°C to digest genomic DNA. The above-described phage preparations were boiled at 100°C for 5 min to denature DNase I and release phage DNA and were then used as the templates for the quantitative PCR for the phage stx2 gene. Results were first adjusted with bacterial number to rule out the influence of the population size and then normalized to control. Experiments were repeated three times independently.
AI-2 assay.
The AI-2 assay was performed as described previously (37). Briefly, ∼5 × 105 CFU/ml E. coli O157:H7 was cultured in LB with 0.5% (wt/vol) glucose (LBG), containing various cinnamon oil concentrations, for 12 h at 37°C under shaking at 250 rpm and then centrifuged at 10,000 × g for 5 min. The resulting supernatant was incubated with Vibrio harveyi to assess the AI-2 level since V. harveyi expresses luminescence in response to AI-2 (38). Luminescence was measured using a BioTek microplate reader (Synergy H1; BioTek, Winooski, VT). V. harveyi isolates incubated with different concentrations of cinnamon oil were used as negative controls. The luminescence of different treatments minus the luminescence of corresponding negative controls was used to rule out the influence of cinnamon oil on V. harveyi luminescence production. The resulting luminescence values were adjusted to bacterial number to eliminate the effect of population size on AI-2 level. The AI-2 levels of the different treatments were expressed relative to that of the control group. Eight replicates were used for each treatment. The experiment was repeated three times independently.
Statistical analysis.
Data were analyzed using GLM from Statistical Analysis Systems (SAS, Cary, NC). Mean values were compared using the least significant difference (LSD) multiple-comparison test (P < 0.05). The mean ± standard error of the mean (SEM) is reported.
RESULTS
Cinnamon oil inhibits the growth of E. coli O157:H7.
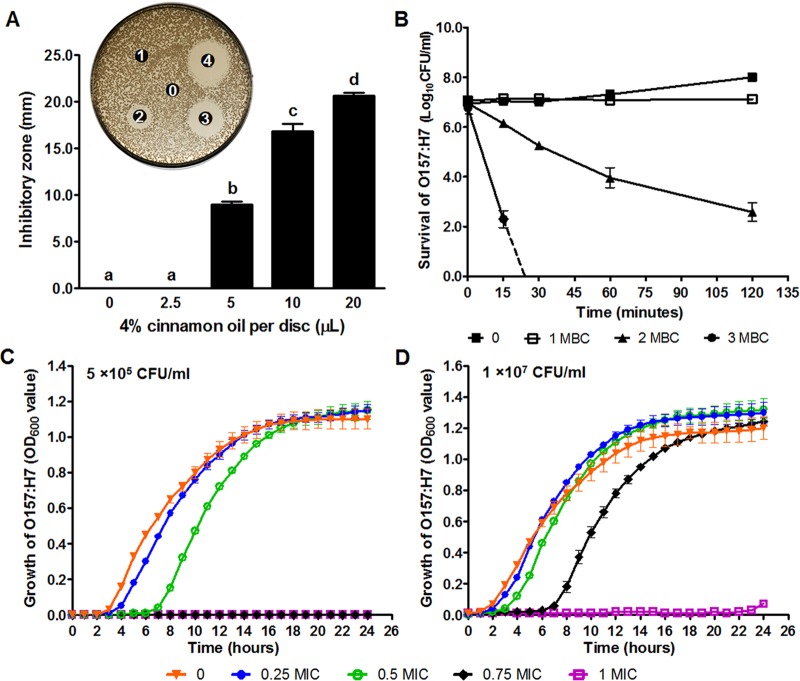
The disc diffusion assay indicated that as little as 5 μl of 4% (vol/vol) cinnamon oil per disc has an inhibitory effect against E. coli O157:H7, resulting in a 9.0 ± 0.3 mm zone of inhibition (Fig. 1A). Volumes of 10 μl and 20 μl of 4% (vol/vol) cinnamon oil per disc resulted in 16.8 ± 0.4 mm and 20.7 ± 0.2 mm inhibition zones, respectively (Fig. 1A). The MIC and MBC of cinnamon oil against E. coli O157:H7 were 0.025% (vol/vol) and 0.05% (vol/vol), respectively. Antibiotics are clinically used to treat infection; however, commonly used antibiotics, such as ciprofloxacin, can induce Stx production (21, 39). It is of great necessity to find alternative compounds that can kill the bacteria in a short time without inducing Shiga toxin production. Therefore, the death curve was further analyzed, indicating that cinnamon oil at 1× MBC (0.05%, vol/vol) failed to reduce the bacterial number at the initial population of 1 × 107 CFU/ml during 2 h of incubation, 2× MBC (0.1%, vol/vol) showed a bactericidal effect within 15 min, and 3× MBC (0.15%, vol/vol) eliminated all of the bacteria in 30 min (Fig. 1B). In the growth curve, bacterial growth in LB without cinnamon oil had a 2-h lag phase at the low inoculation level and a 1-h lag phase at the high inoculation level (Fig. 1C and D). At 0.25× MIC, the bacterial lag phase was extended for ∼1 h at the low inoculation level, but there was no influence on the bacterial lag phase at the high inoculation level (Fig. 1C and D). At 0.5× MIC, the bacterial lag phase was elongated by ∼4 h and 1 h for the low and high inoculation levels, respectively (Fig. 1C and D). At 0.75× MIC, stunted bacterial growth was observed at the low inoculation level for at least 24 h; a prolonged bacterial lag phase of ∼6 h was observed at the high inoculation level (Fig. 1C and D). With cinnamon oil at 1× MIC, complete inhibition of bacterial growth at both inoculation levels for at least 22 h was observed (Fig. 1C and D).
FIG 1.
Antimicrobial activity of cinnamon oil against E. coli O157:H7. (A) Disc diffusion assay. Disc 0, vehicle control; disc 1, 2.5 μl of 4% (vol/vol) cinnamon oil; disc 2, 5 μl of 4% (vol/vol) cinnamon oil; disc 3, 10 μl of 4% (vol/vol) cinnamon oil; disc 4, 20 μl of 4% (vol/vol) cinnamon oil. (B) Death curve of E. coli O157:H7 in LB broth supplemented with different concentrations of cinnamon oil as indicated. (C) Growth curve of bacteria at initial population of 5 × 105 CFU/ml. (D) Growth curve of bacteria at the inoculation level of 1 × 107 CFU/ml. Histogram bars with the same letter do not differ significantly at a P value of 0.05.
Cinnamon oil reduced Stx2 production by E. coli O157:H7.
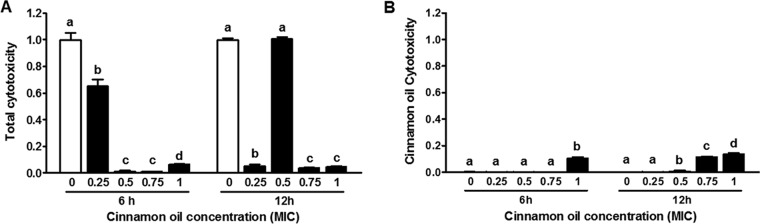
Subinhibitory concentrations of cinnamon oil stunted E. coli O157:H7 growth under shaking in a dose-dependent manner. The initial population was 5.87 ± 0.02 log10 CFU/ml (Fig. 2A). After 6 h of incubation, bacterial populations treated with cinnamon oil at 0×, 0.25×, 0.5×, 0.75×, and 1× MIC were 8.80 ± 0.02, 8.70 ± 0.01, 7.59 ± 0.03, 5.96 ± 0.01, and 5.87 ± 0.01 log10 CFU/ml, respectively (Fig. 2A). After 12 h of incubation, bacterial populations treated with cinnamon oil at 0×, 0.25×, 0.5×, 0.75×, and 1× MIC were 8.79 ± 0.03, 8.79 ± 0.04, 8.69 ± 0.4, 5.99 ± 0.04, and 5.88 ± 0.02 log10 CFU/ml, respectively (Fig. 2A). The bacterial population treated with cinnamon oil at 0.75× MIC increased only slightly at both time points compared with the initial population, while 1× MIC treatment completely inhibited bacterial growth (Fig. 2A). The Stx2 protein at 0 h was below the detection level for all treatments. Inhibition of Stx2 production occurred in a concentration-dependent fashion with cinnamon oil, except for the 0.5× MIC treatment for 12 h (Fig. 2B). At 0.75× and 1× MIC, bacterial Stx2 production was nondetectable at both 6 h and 12 h of incubation (Fig. 2B). Consistently, stx2 mRNA expression decreased to nondetectable levels at 6 h and 12 h after treatment with cinnamon oil at 0.75× and 1× MIC (Fig. 2C). Reduced Stx2 production with cinnamon oil treatment was further confirmed by a Vero cell cytotoxicity assay as indicated by LDH level, which was used to measure the Stx secreted outside the bacteria (Fig. 3A). LDH levels decreased with increasing cinnamon oil concentration except for the 6-h 1× MIC cinnamon oil treatment and 12-h 0.5× MIC cinnamon oil treatment (Fig. 3A). Cinnamon oil at 1× MIC exhibited a cytotoxic effect on Vero cells after 6 h of incubation (Fig. 3B); at 0.5× MIC, it showed cytotoxicity on Vero cells after 12 h of interaction, and the cytotoxicity effect increased in a dose-dependent manner (Fig. 3B).
FIG 2.
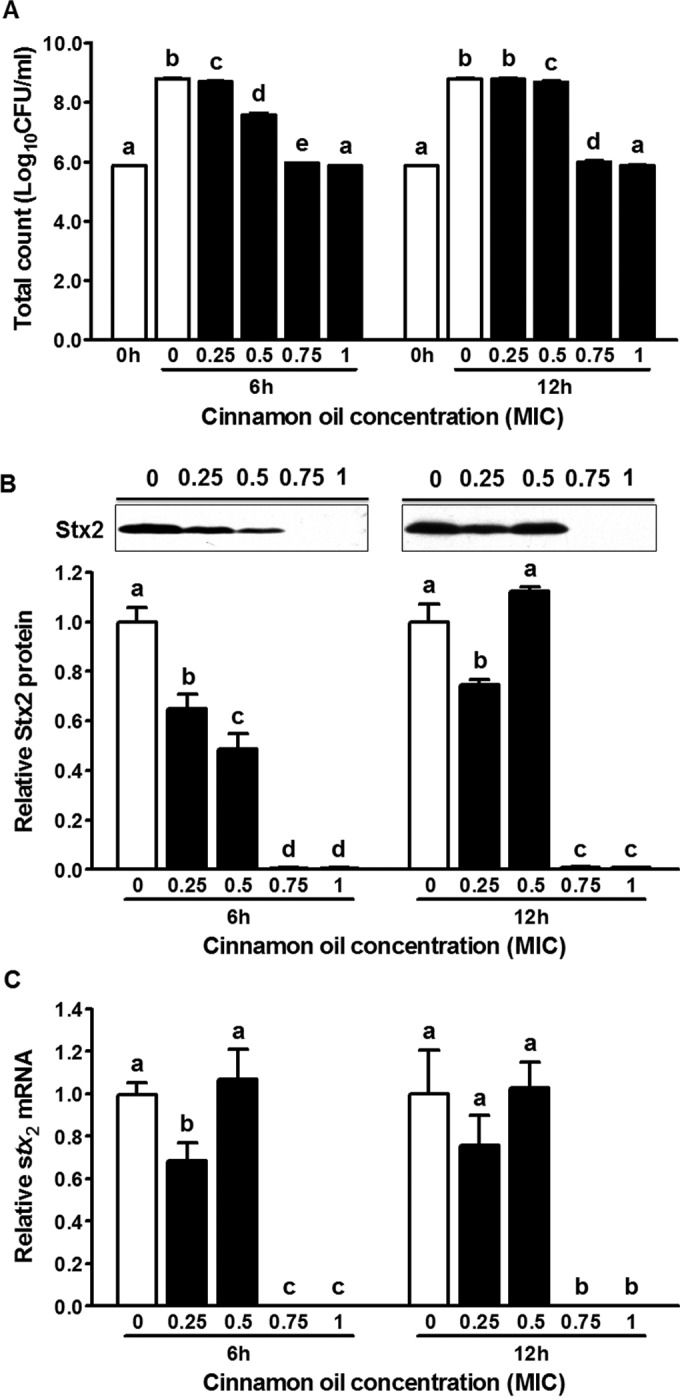
Effect of the subinhibitory concentration of cinnamon oil on Stx2 production and stx2 mRNA expression by E. coli O157:H7 after 6 h and 12 h of incubation in LB broth. (A) Total count after 0 h, 6 h, and 12 h of incubation. (B) Stx2 production statistical data and representative Western blot image. (C) stx2 mRNA expression. Histogram bars with the same letter do not differ significantly at a P value of 0.05.
FIG 3.
Effect of cinnamon oil on E. coli O157:H7 cytotoxicity on Vero cells. (A) Total cytotoxicity. (B) Cinnamon oil cytotoxicity. Histogram bars with the same letter do not differ significantly at a P value of 0.05.
Cinnamon oil decreases lambdoid bacteriophage and Stx2 prophage induction.
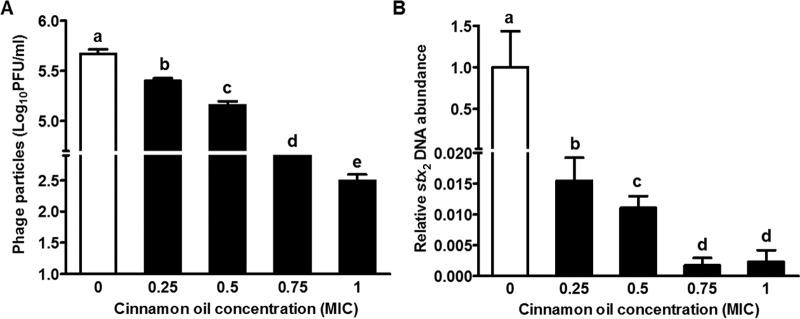
Phage titers in the 12-h culture suspension decreased with concentrations of cinnamon oil in a dose-dependent manner (Fig. 4A). Cinnamon oil at 0.75× and 1× MIC resulted in about 2- and 3-log reductions in PFU, respectively (Fig. 4A). Consistently, Stx2 prophage induction was reduced in a dose-dependent manner as indicated by decreased Stx2 phage DNA abundance (Fig. 4B).
FIG 4.
Effect of cinnamon oil on total phage titer and the Stx2-producing phage after a 12-h interaction in LB broth. (A) Phage titer as indicated by log10 PFU per ml. (B) Stx2 phage abundance as indicated by stx2 phage DNA abundance. Histogram bars with the same letter do not differ significantly at a P value of 0.05.
Cinnamon oil impairs SOS response and RNA polyadenylation of E. coli O157:H7.
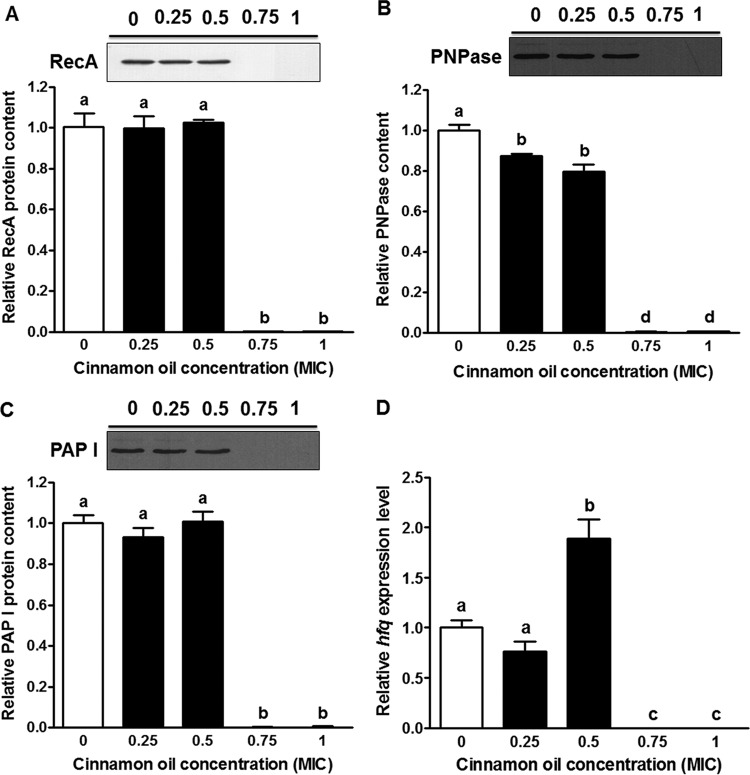
The prophage is derepressed by the activated RecA protein, an important factor in bacterial SOS response, by inducing the cleavage of the cI repressor (13). With cinnamon oil at 0.75× and 1× MIC, RecA production was eliminated, while at 0.25× and 0.5× MIC, no effect was observed (Fig. 5A), indicating that additional factors are involved with the inhibitory effect of Stx2 prophage induction. Previous studies showed that dysfunction of PNPase or PAP I, important enzymes in RNA polyadenylation, impaired the lytic development of the Stx2 phage (18, 19). Therefore, we evaluated the production of PNPase and PAP I proteins as well as the expression of hfq mRNA, which is an important factor involved in the function of both enzymes. The PNPase protein level was decreased by cinnamon oil in a dose-dependent manner (Fig. 5B). Low concentrations of cinnamon oil had no effect on PAP I production, but at 0.75× and 1× MIC it reduced PAP I production to a trace level (Fig. 5C). Consistently, the hfq mRNA level was below detection in the groups treated with cinnamon oil at 0.75× or 1× MIC (Fig. 5D) but increased in response to 0.5× MIC (Fig. 5D).
FIG 5.
Effect of cinnamon oil on SOS response and RNA polyadenylation in E. coli O157:H7 after a 12-h interaction in LB broth. (A) RecA production statistical data and representative Western blot image. (B) PNPase production statistical data and representative Western blot image. (C) PAP I production statistical data and representative Western blot image. (D) hfq mRNA expression. Histogram bars with the same letter do not differ significantly at a P value of 0.05.
Impact of cinnamon oil on QS and oxidative stress response.
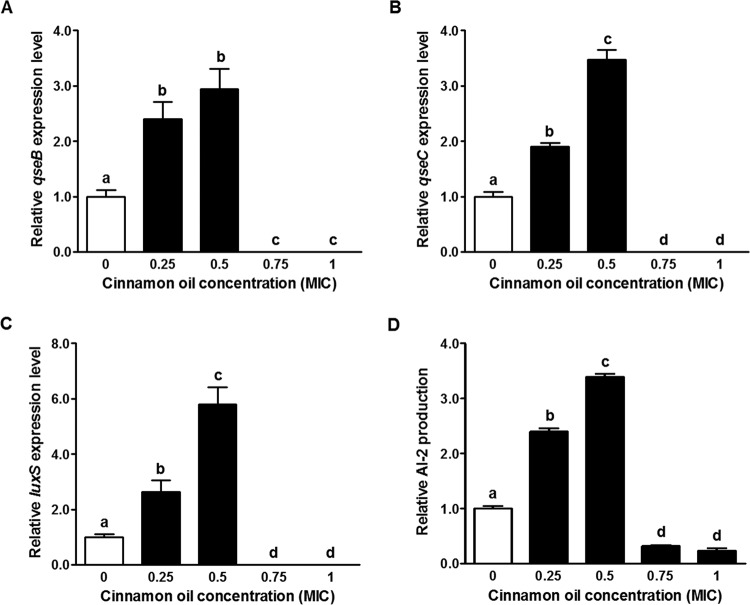
SOS response as well as subsequent Stx2 production is regulated by QS through QseBC and LuxS/AI-2 (15, 16). Twelve-hour treatments with cinnamon oil at 0.75× or 1× MIC decreased qseB, qseC, and luxS mRNA levels below detection, while lower concentrations enhanced these levels (Fig. 6A to C). Similarly, AI-2 production was reduced dramatically in the groups treated with cinnamon oil at 0.75× or 1× MIC but was enhanced in the groups treated with 0.25× or 0.5× MIC (Fig. 6D).
FIG 6.
Effect of cinnamon oil on E. coli O157:H7 quorum sensing. (A) qseB gene expression. (B) qseC gene expression. (C) luxS gene expression. (D) Relative AI-2 production. Histogram bars with the same letter do not differ significantly at a P value of 0.05.
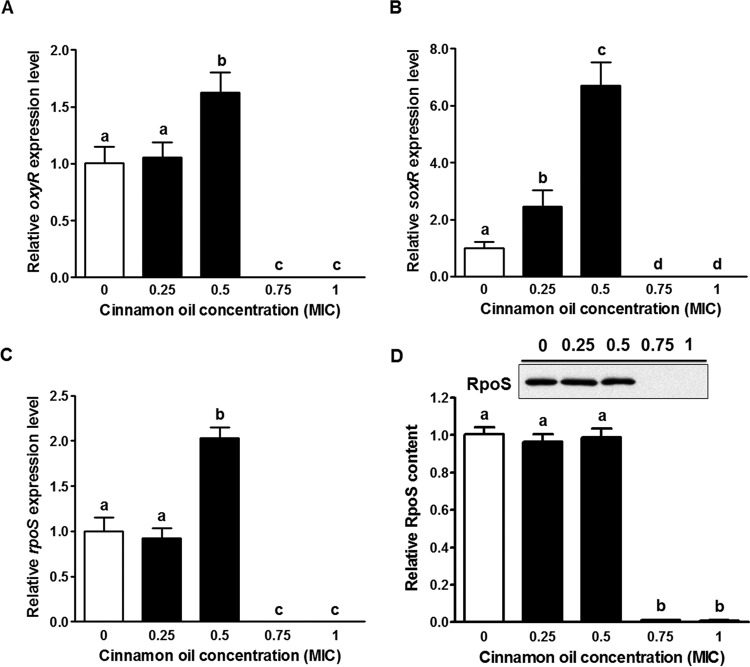
Since QS is a positive regulator of the oxidative stress response (14), we further evaluated the effects of cinnamon oil on three major oxidative stress regulons of E. coli: OxyR, SoxR, and RpoS (41). Using cinnamon oil at 0.75× or 1× MIC, treatments for 12 h reduced oxyR, soxR, and rpoS mRNA to below detectable levels (Fig. 7A to C). However, cinnamon oil at 0.5× MIC increased the expression of oxyR, soxR, and rpoS by 1.5-, 6.7-, and 2.0-fold, respectively (Fig. 7A to C). Furthermore, cinnamon oil treatments at 0.75× and 1× MIC completely eliminated RpoS production (Fig. 7D), while neither 0.25× nor 0.5× MIC treatment affected the RpoS protein level in E. coli O157:H7 (Fig. 7D).
FIG 7.
Oxidative stress response of E. coli O157:H7 after a 12-h interaction with cinnamon oil in LB broth. (A) oxyR mRNA expression. (B) soxR mRNA expression. (C) rpoS mRNA expression. (D) RpoS production statistical data and representative Western blot image. Histogram bars with the same letter do not differ significantly at a P value of 0.05.
DISCUSSION
Stx production is central to the pathogenesis of E. coli O157:H7, which is associated with bloody diarrhea, hemorrhagic colitis, and hemolytic-uremic syndrome (HUS) (42). In recent years, new STEC serotypes have emerged, culminating in the 2011 E. coli O104:H4 outbreak that involved more than 4,000 persons in 16 countries (43). E. coli O104:H4 appears to be an enteroaggregative E. coli (EAEC) acquiring stx via horizontal gene transfer (44), which can also occur between STEC and nonpathogenic E. coli (11), facilitating the spread of stx genes and increasing the pathogenic potential of STEC. Therefore, exploring an effective method to control Stx prophage activation and related Stx production in STEC is of great necessity.
Cinnamon oil likely inhibits Stx2 production through repressing Stx2 prophage lytic development.
Cinnamon oil has shown an inhibitory effect against nonpathogenic E. coli (45, 46) and E. coli O157:H7 (22, 23, 47). The MIC of cinnamon oil against E. coli O157:H7 was 0.025% (vol/vol), which is the same as the MIC of cinnamon cassia oil against E. coli O157:H7 S0575 (48) as well as the CDC's “top-six” non-O157 STEC (24) but lower than the reported 0.05% (vol/vol) against E. coli O157:H7 ATCC 35150 (48) or E. coli O157:H7 EDL933 (47). This may be due to the difference in the cinnamon oil used as well as the interstrain heterogeneity in response to cinnamon. In this study, the cinnamon oil tested has ∼60% cinnamaldehyde (24), which has broad-spectrum antimicrobial activity against various bacteria and fungi (45, 49). Cinnamaldehyde was reported to suppress stx2 expression in E. coli O157:H7 (50), but this study examined the effect of cinnamon oil or its major component cinnamaldehyde on E. coli O157:H7 Stx2 protein production and Stx2 phage induction. Our previous study indicated that different E. coli O157:H7 strains (EDL933, Sakai, 86-24) behaved similarly with regard to Stx2 phage DNA abundance and Stx2 protein production (18). Therefore, in this study, we only chose EDL933 for the impacts of cinnamon oil on E. coli O157:H7 Stx2 production and activation.
The MIC and subinhibitory concentrations (0.25× and 0.75× MIC) of cinnamon oil eliminated or inhibited stx2 mRNA expression and Stx2 production in E. coli O157:H7, which were associated with decreased Vero cell cytotoxicity. Similarly, natural compounds, such as coumarin derivative esculetin and allspice extract, decreased stx2 expression or Stx2 production in E. coli O157:H7 (51, 52). In addition, we found that higher concentrations (0.75× and 1.0× MIC) of cinnamon oil itself showed slight cytotoxicity on Vero cells. In line with this, 0.015% (vol/vol) Cinnamomum zeylanicum oil exhibited a cytotoxic effect on both F2048 and 5RP7 cells after 24 h of incubation (53). Interestingly, a 6-h treatment with cinnamon oil at 0.5× MIC had no effect on stx2 expression but reduced Stx2 production. This may be due to delays between transcription and translation, posttranslational modifications, and/or protein degradation (54). A 12-h treatment with cinnamon oil at 0.5× MIC had no effect on stx2 gene expression, Stx2 production, or Vero cell cytotoxicity. This may be because 0.5× MIC treatment induced quorum sensing that positively regulated Stx2 production (15, 16), balancing the inhibitory effect of cinnamon oil.
Stx2 is encoded by stx2 genes in prophages, which are silent during lysogeny due to the presence of the repressor cI protein (55). However, once the lytic cycle of the prophage is induced, it leads to self-cleavage of the cI repressor, prophage replication, transcription of the stx gene, production of Stx, lysis of E. coli O157:H7, and subsequent release of Stx (56, 57). In this study, not only did the abundance of the Stx2-converting phage decrease, the total number of phage decreased proportionally with the increase of cinnamon oil concentration, which explained the reduction of Stx2 production. A similar phenomenon was observed in that subinhibitory concentrations of phenethyl isothiocyanate, which is a derivative of glucosinolate found in cruciferous vegetables, significantly reduced the amount of Stx-encoding phage and stx expression in E. coli O157:H7 (58).
Inhibitory effect of cinnamon oil on Stx2 phage lytic cycle may be due to the impaired SOS response and RNA polyadenylation of E. coli O157:H7.
Previous study shows that the Stx2 production in recA mutant strains of E. coli O157:H7 EDL 933 and 86-24 strains was much lower than that of the wild-type strains; complementing the mutant strains with the recA vector restored the Stx2 production level (59). This is likely because RecA plays a positive role in Stx2 prophage induction (60). Therefore, suppression of RecA production by cinnamon oil at 0.75× or 1× MIC supported the dramatic reduction of the Stx2 phage in those treatments but failed to explain the inhibition of the Stx2 phage at lower concentrations. Polynucleotide phosphorylase (PNPase) is an important enzyme in RNA polyadenylation. Previously we demonstrated that PNPase plays a vital role in Stx2 prophage induction (18). PAP I can bind to the 3′ terminus of mRNA and further polyadenylates the mRNA, generating substrates readily degraded by PNPase (40). Dysfunction of PAP I has also been reported to inhibit the induction of the Stx-converting phage (19). Consistent with the inhibition of Stx2 prophage induction and reduction of Stx2 phage abundance, cinnamon oil at 0.75× or 1× MIC eliminated both PAP I and PNPase content. In addition, low concentrations of cinnamon oil caused a dose-dependent reduction of PNPase production, explaining the reduction of the Stx2 phage in E. coli O157:H7 even at 0.25× and 0.5× MIC. This is further supported by the increased expression of the hfq gene with the above treatments, which negatively affects the enzymatic activity of PNPase (40).
Impacts of cinnamon oil on bacterial communication and subsequent oxidative stress response.
As a global regulator of E. coli O157:H7, QS plays a positive role in Stx2 production (15, 16). In E. coli, there are mainly two types of QS systems, bacterium-bacterium communication through AI-2 synthesized by the LuxS protein and bacterium-host communication through AI-3 sensed by a two-component system QseBC (8). QseBC is activated by the LuxS/AI-2 system (61). Mutation of the luxS or qseBC gene in E. coli O157:H7 strain 86-24 significantly downregulated recA and stx gene expression as well as Stx2 protein content (15, 16). In this study, high concentrations of cinnamon oil (0.75× and 1× MIC) abolished luxS, qseB, and qseC gene expression, likely contributing to reductions in RecA level, stx2 gene expression, and subsequent Stx2 production. Inhibition of QS by cinnamon oil at 0.75× and 1× MIC was further confirmed by the reduction of the AI-2 level. Of note, QS positively regulates the virulence of E. coli O157:H7 (15), and inactivation of QS by cinnamon oil may impair other bacterial pathogenesis as well. Interestingly, lower concentrations of cinnamon oil (0.25× and 0.5× MIC) stimulated QS-related gene expression as well as AI-2 production in a dose-dependent manner. This may be due to the increased expression of the hfq gene, which is a positive regulator of QS (62). Nevertheless, the increased QS at low cinnamon oil levels failed to affect RecA content.
Quorum sensing enhances the bacterial oxidative stress response (14). High concentrations of cinnamon oil inhibited the oxidative stress response through elimination of the mRNA levels of genes encoding the oxidative stress response regulons as well as RpoS protein production. Similarly, cinnamaldehyde at ∼100 mg/liter downregulates the proteins involved in oxidative stress response in Cronobacter sakazakii (63). Low cinnamon oil concentrations, especially 0.5× MIC, however, increased the oxidative stress response gene expression, which is consistent with a previous report showing that 2 h of cinnamaldehyde (200 mg/liter) exposure caused oxidative stress responses in E. coli O157:H7 (64). Oxidative stress plays a positive role in stx2 gene expression (65). An induced oxidative stress response together with QS after a 0.5× MIC treatment may contribute to the increased stx2 expression and Stx2 production. Oxidative stress also induces RecA production and the SOS response to repair the oxidative DNA damage (66). However, incomplete repair leads to a double-stranded DNA break and accelerates cell death (67). In our study, although the induced oxidative stress after treatment with cinnamon oil at 0.25× or 0.5× MIC failed to increase RecA production, it may contribute to the inhibition of bacterial growth. On the other hand, OxyR has been reported to bind to the pM promoter region, influence cI mRNA expression, and reduce prophage activation (17). Thus, the enhanced expression of oxyR, soxR, and rpoS may also contribute to the reduced Stx-converting phage induction and subsequent Stx production in the low-concentration cinnamon oil treatment groups. These data indicate the complex nature of the effects of cinnamon oil on QS, oxidative stress, Stx2 prophage induction, and Stx2 production. Of importance, RpoS is the master regulator of the general stress response in E. coli, and induction of RpoS allows bacteria to be more resistant to the stress they first encounter as well as other environmental stresses, including starvation, oxidation, high or low temperature, and others (68). We speculate that cinnamon oil may weaken the other stress responses of E. coli O157:H7 through the inactivation of rpoS.
In conclusion, cinnamon oil effectively inhibited the growth of E. coli O157:H7. In LB medium, cinnamon oil at a concentration as low as 0.25× MIC reduced Stx2 production, stx2 mRNA expression, and cytotoxicity on Vero cells. Cinnamon oil also dramatically reduced both Stx2 phage induction and total phage induction in E. coli O157:H7. The inhibitory effect may be due to the suppression of bacterial SOS response key regulator RecA and vital RNA polyadenylation enzymes (PNPase and PAP I). Cinnamon oil at 0.75× and 1× MIC eliminated QS, contributing to the inhibition of Stx2 production. The subsequent oxidative stress response was also strongly suppressed by those treatments. With cinnamon oil at 0.25× or 0.5× MIC, the bacterial oxidative stress response was slightly induced; however, this may contribute to inhibition of prophage induction as well. Taken together, these results indicate that cinnamon oil inhibits Stx2 production and Stx2 phage induction in E. coli O157:H7 via multiple mechanisms. It has potential applications as a therapeutic agent to control E. coli O157:H7 infection through inhibition of both bacterial growth and virulence factors.
ACKNOWLEDGMENTS
This activity was funded by the Emerging Research Issues Competitive Grant and the BioAg Grant Program from the College of Agricultural, Human, and Natural Resource Sciences at Washington State University.
We declare no competing financial interests.
REFERENCES
- 1.Jacob ME, Foster DM, Rogers AT, Balcomb CC, Shi X, Nagaraja TG. 2013. Evidence of non-O157 Shiga toxin-producing Escherichia coli in the feces of meat goats at a U.S. slaughter plant. J Food Prot 76:1626–1629. doi: 10.4315/0362-028X.JFP-13-064. [DOI] [PubMed] [Google Scholar]
- 2.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis 17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King LA, Loukiadis E, Mariani-Kurkdjian P, Haeghebaert S, Weill FX, Baliere C, Ganet S, Gouali M, Vaillant V, Pihier N, Callon H, Novo R, Gaillot O, Thevenot-Sergentet D, Bingen E, Chaud P, De Valk H. 2014. Foodborne transmission of sorbitol-fermenting Escherichia coli O157:[H7] via ground beef: an outbreak in northern France, 2011. Clin Microbiol Infect 20:O1136–O1144. doi: 10.1111/1469-0691.12736. [DOI] [PubMed] [Google Scholar]
- 4.McCollum JT, Williams NJ, Beam SW, Cosgrove S, Ettestad PJ, Ghosh TS, Kimura AC, Nguyen L, Stroika SG, Vogt RL, Watkins AK, Weiss JR, Williams IT, Cronquist AB. 2012. Multistate outbreak of Escherichia coli O157:H7 infections associated with in-store sampling of an aged raw-milk Gouda cheese, 2010. J Food Prot 75:1759–1765. doi: 10.4315/0362-028X.JFP-12-136. [DOI] [PubMed] [Google Scholar]
- 5.Vojdani JD, Beuchat LR, Tauxe RV. 2008. Juice-associated outbreaks of human illness in the United States, 1995 through 2005. J Food Prot 71:356–364. [DOI] [PubMed] [Google Scholar]
- 6.Marder EP, Garman KN, Ingram LA, Dunn JR. 2014. Multistate outbreak of Escherichia coli O157:H7 associated with bagged salad. Foodborne Pathog Dis 11:593–595. doi: 10.1089/fpd.2013.1726. [DOI] [PubMed] [Google Scholar]
- 7.Griffin PM, Tauxe RV. 1991. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol Rev 13:60–98. [DOI] [PubMed] [Google Scholar]
- 8.Smith JL, Fratamico PM, Gunther NW IV. 2014. Shiga toxin-producing Escherichia coli. Adv Appl Microbiol 86:145–197. doi: 10.1016/B978-0-12-800262-9.00003-2. [DOI] [PubMed] [Google Scholar]
- 9.O'Brien AD, Newland JW, Miller SF, Holmes RK, Smith HW, Formal SB. 1984. Shiga-like toxin-converting phages from Escherichia coli strains that cause hemorrhagic colitis or infantile diarrhea. Science 226:694–696. doi: 10.1126/science.6387911. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt H. 2001. Shiga toxin-converting bacteriophages. Res Microbiol 152:687–695. doi: 10.1016/S0923-2508(01)01249-9. [DOI] [PubMed] [Google Scholar]
- 11.Yue WF, Du M, Zhu MJ. 2012. High temperature in combination with UV irradiation enhances horizontal transfer of stx2 gene from E. coli O157:H7 to non-pathogenic E. coli. PLoS One 7:. doi: 10.1371/journal.pone.0031308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gamage SD, Patton AK, Hanson JF, Weiss AA. 2004. Diversity and host range of Shiga toxin-encoding phage. Infect Immun 72:7131–7139. doi: 10.1128/IAI.72.12.7131-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Los JM, Los M, Wegrzyn G. 2011. Bacteriophages carrying Shiga toxin genes: genomic variations, detection and potential treatment of pathogenic bacteria. Future Microbiol 6:909–924. doi: 10.2217/fmb.11.70. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Contreras R, Nunez-Lopez L, Jasso-Chavez R, Kwan BW, Belmont JA, Rangel-Vega A, Maeda T, Wood TK. 2015. Quorum sensing enhancement of the stress response promotes resistance to quorum quenching and prevents social cheating. ISME J 9:115–125. doi: 10.1038/ismej.2014.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sperandio V, Torres AG, Giron JA, Kaper JB. 2001. Quorum sensing is a global regulatory mechanism in enterohemorrhagic Escherichia coli O157:H7. J Bacteriol 183:5187–5197. doi: 10.1128/JB.183.17.5187-5197.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes DT, Clarke MB, Yamamoto K, Rasko DA, Sperandio V. 2009. The QseC adrenergic signaling cascade in enterohemorrhagic E. coli (EHEC). PLoS Pathog 5:. doi: 10.1371/journal.ppat.1000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glinkowska M, Los JM, Szambowska A, Czyz A, Calkiewicz J, Herman-Antosiewicz A, Wrobel B, Wegrzyn G, Wegrzyn A, Los M. 2010. Influence of the Escherichia coli oxyR gene function on lambda prophage maintenance. Arch Microbiol 192:673–683. doi: 10.1007/s00203-010-0596-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu J, Zhu MJ. 2015. Defects in polynucleotide phosphorylase impairs virulence in Escherichia coli O157:H7. Front Microbiol 6:806. doi: 10.3389/fmicb.2015.00806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nowicki D, Bloch S, Nejman-Falenczyk B, Szalewska-Palasz A, Wegrzyn A, Wegrzyn G. 2015. Defects in RNA polyadenylation impair both lysogenization by and lytic development of Shiga toxin-converting bacteriophages. J Gen Virol 96:1957–1968. doi: 10.1099/vir.0.000102. [DOI] [PubMed] [Google Scholar]
- 20.Kimmitt PT, Harwood CR, Barer MR. 2000. Toxin gene expression by Shiga toxin-producing Escherichia coli: the role of antibiotics and the bacterial SOS response. Emerg Infect Dis 6:458–465. doi: 10.3201/eid0605.000503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Mcdaniel AD, Wolf LE, Keusch GT, Waldor MK, Acheson DW. 2000. Quinolone antibiotics induce Shiga toxin-encoding bacteriophages, toxin production, and death in mice. J Infect Dis 181:664–670. doi: 10.1086/315239. [DOI] [PubMed] [Google Scholar]
- 22.Friedman M, Henika PR, Mandrell RE. 2002. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J Food Prot 65:1545–1560. [DOI] [PubMed] [Google Scholar]
- 23.Smith-Palmer A, Stewart J, Fyfe L. 1998. Antimicrobial properties of plant essential oils and essences against five important food-borne pathogens. Lett Appl Microbiol 26:118–122. doi: 10.1046/j.1472-765X.1998.00303.x. [DOI] [PubMed] [Google Scholar]
- 24.Sheng L, Zhu MJ. 2014. Inhibitory effect of Cinnamomum cassia oil on non-O157 Shiga toxin-producing Escherichia coli. Food Control 46:374–381. doi: 10.1016/j.foodcont.2014.05.050. [DOI] [Google Scholar]
- 25.Zhu H, Du M, Fox LK, Zhu MJ. 2016. Bactericidal effects of Cinnamon cassia oil against bovine mastitis bacterial pathogens. Food Control 66:291–299. doi: 10.1016/j.foodcont.2016.02.013. [DOI] [Google Scholar]
- 26.Amalaradjou MA, Narayanan A, Venkitanarayanan K. 2011. Trans-cinnamaldehyde decreases attachment and invasion of uropathogenic Escherichia coli in urinary tract epithelial cells by modulating virulence gene expression. J Urol 185:1526–1531. doi: 10.1016/j.juro.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 27.Amalaradjou MA, Narayanan A, Baskaran SA, Venkitanarayanan K. 2010. Antibiofilm effect of trans-cinnamaldehyde on uropathogenic Escherichia coli. J Urol 184:358–363. doi: 10.1016/j.juro.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 28.Mann CM, Markham JL. 1998. A new method for determining the minimum inhibitory concentration of essential oils. J Appl Microbiol 84:538–544. doi: 10.1046/j.1365-2672.1998.00379.x. [DOI] [PubMed] [Google Scholar]
- 29.Fonseca AP, Extremina C, Fonseca AF, Sousa JC. 2004. Effect of subinhibitory concentration of piperacillin/tazobactam on Pseudomonas aeruginosa. J Med Microbiol 53:903–910. doi: 10.1099/jmm.0.45637-0. [DOI] [PubMed] [Google Scholar]
- 30.Braga PC, Sala MT, Dal Sasso M. 1999. Pharmacodynamic effects of subinhibitory concentrations of rufloxacin on bacterial virulence factors. Antimicrob Agents Chemother 43:1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maggiolo F, Taras A, Frontespezi S, Legnani MC, Silanos MA, Pravettoni G, Suter F. 1994. Pharmacodynamic effects of subinhibitory concentrations of imipenem on Pseudomonas aeruginosa in an in vitro dynamic-model. Antimicrob Agents Chemother 38:1416–1418. doi: 10.1128/AAC.38.6.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu MJ, Olsen SA, Sheng L, Xue Y, Yue W. 2015. Antimicrobial efficacy of grape seed extract against Escherichia coli O157:H7 growth, motility and Shiga toxin production. Food Control 51:177–182. doi: 10.1016/j.foodcont.2014.11.024. [DOI] [Google Scholar]
- 33.Yasukawa T, Kanei-Ishii C, Maekawa T, Fujimoto J, Yamamoto T, Ishii S. 1995. Increase of solubility of foreign proteins in Escherichia coli by coproduction of the bacterial thioredoxin. J Biol Chem 270:25328–25331. doi: 10.1074/jbc.270.43.25328. [DOI] [PubMed] [Google Scholar]
- 34.Bos MP, Tefsen B, Geurtsen J, Tommassen J. 2004. Identification of an outer membrane protein required for the transport of lipopolysaccharide to the bacterial cell surface. Proc Natl Acad Sci U S A 101:9417–9422. doi: 10.1073/pnas.0402340101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harris SM, Yue WF, Olsen SA, Hu J, Means WJ, McCormick RJ, Du M, Zhu MJ. 2012. Salt at concentrations relevant to meat processing enhances Shiga toxin 2 production in Escherichia coli O157:H7. Int J Food Microbiol 159:186–192. doi: 10.1016/j.ijfoodmicro.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 36.Burd JF, Usategui-Gomez M. 1973. A colorimetric assay for serum lactate dehydrogenase. Clin Chim Acta 46:223–227. doi: 10.1016/0009-8981(73)90174-5. [DOI] [PubMed] [Google Scholar]
- 37.Surette MG, Bassler BL. 1998. Quorum sensing in Escherichia coli and Salmonella Typhimurium. Proc Natl Acad Sci U S A 95:7046–7050. doi: 10.1073/pnas.95.12.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bassler BL, Greenberg EP, Stevens AM. 1997. Cross-species induction of luminescence in the quorum-sensing bacterium Vibrio harveyi. J Bacteriol 179:4043–4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bielaszewska M, Idelevich EA, Zhang W, Bauwens A, Schaumburg F, Mellmann A, Peters G, Karch H. 2012. Effects of antibiotics on Shiga toxin 2 production and bacteriophage induction by epidemic Escherichia coli O104:H4 strain. Antimicrob Agents Chemother 56:3277–3282. doi: 10.1128/AAC.06315-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohanty BK, Maples VF, Kushner SR. 2004. The Sm-like protein Hfq regulates polyadenylation dependent mRNA decay in Escherichia coli. Mol Microbiol 54:905–920. doi: 10.1111/j.1365-2958.2004.04337.x. [DOI] [PubMed] [Google Scholar]
- 41.Chiang SM, Schellhorn HE. 2012. Regulators of oxidative stress response genes in Escherichia coli and their functional conservation in bacteria. Arch Biochem Biophys 525:161–169. doi: 10.1016/j.abb.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Karmali MA, Steele BT, Petric M, Lim C. 1983. Sporadic cases of haemolytic-uraemic syndrome associated with faecal cytotoxin and cytotoxin-producing Escherichia coli in stools. Lancet i:619–620. [DOI] [PubMed] [Google Scholar]
- 43.Centers for Disease Control and Prevention. 2013. Outbreak of Escherichia coli O104:H4 infections associated with sprout consumption-Europe and North America, May–July 2011. Centers for Disease Control and Prevention, Atlanta, GA. [PMC free article] [PubMed] [Google Scholar]
- 44.Bloch SK, Felczykowska A, Nejman-Falenczyk B. 2012. Escherichia coli O104:H4 outbreak—have we learnt a lesson from it? Acta Biochim Pol 59:483–488. [PubMed] [Google Scholar]
- 45.Ooi LS, Li Y, Kam SL, Wang H, Wong EY, Ooi VE. 2006. Antimicrobial activities of cinnamon oil and cinnamaldehyde from the Chinese medicinal herb Cinnamomum cassia Blume. Am J Chin Med 34:511–522. doi: 10.1142/S0192415X06004041. [DOI] [PubMed] [Google Scholar]
- 46.Hili P, Evans CS, Veness RG. 1997. Antimicrobial action of essential oils: the effect of dimethylsulphoxide on the activity of cinnamon oil. Lett Appl Microbiol 24:269–275. doi: 10.1046/j.1472-765X.1997.00073.x. [DOI] [PubMed] [Google Scholar]
- 47.Oussalah M, Caillet S, Lacroix M. 2006. Mechanism of action of Spanish oregano, Chinese cinnamon, and savory essential oils against cell membranes and walls of Escherichia coli O157:H7 and Listeria monocytogenes. J Food Prot 69:1046–1055. [DOI] [PubMed] [Google Scholar]
- 48.Mith H, Dure R, Delcenserie V, Zhiri A, Daube G, Clinquart A. 2014. Antimicrobial activities of commercial essential oils and their components against food-borne pathogens and food spoilage bacteria. Food Sci Nutr 2:403–416. doi: 10.1002/fsn3.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Helander IM, Alakomi H-L, Latva-Kala K, Mattila-Sandholm T, Pol I, Smid EJ, Gorris LGM, Von Wright A. 1998. Characterization of the action of selected essential oil components on Gram-negative bacteria. J Agric Food Chem 46:3590–3595. doi: 10.1021/jf980154m. [DOI] [Google Scholar]
- 50.Kim YG, Lee JH, Kim SI, Baek KH, Lee J. 2015. Cinnamon bark oil and its components inhibit biofilm formation and toxin production. Int J Food Microbiol 195:30–39. doi: 10.1016/j.ijfoodmicro.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 51.Lee JH, Kim YG, Cho HS, Ryu SY, Cho MH, Lee J. 2014. Coumarins reduce biofilm formation and the virulence of Escherichia coli O157:H7. Phytomedicine 21:1037–1042. doi: 10.1016/j.phymed.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 52.Takemasa N, Ohnishi S, Tsuji M, Shikata T, Yokoigawa K. 2009. Screening and analysis of spices with ability to suppress verocytotoxin production by Escherichia coli O157. J Food Sci 74:M461–M466. doi: 10.1111/j.1750-3841.2009.01326.x. [DOI] [PubMed] [Google Scholar]
- 53.Unlu M, Ergene E, Unlu GV, Zeytinoglu HS, Vural N. 2010. Composition, antimicrobial activity and in vitro cytotoxicity of essential oil from Cinnamomum zeylanicum Blume (Lauraceae). Food Chem Toxicol 48:3274–3280. doi: 10.1016/j.fct.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 54.de Sousa Abreu R, Penalva LO, Marcotte EM, Vogel C. 2009. Global signatures of protein and mRNA expression levels. Molecular Biosystems 5:1512–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ptashne M, Hopkins N. 1968. The operators controlled by the lambda phage repressor. Proc Natl Acad Sci U S A 60:1282–1287. doi: 10.1073/pnas.60.4.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johannes L, Romer W. 2010. Shiga toxins—from cell biology to biomedical applications. Nat Rev Microbiol 8:105–116. doi: 10.1038/nrmicro2279. [DOI] [PubMed] [Google Scholar]
- 57.Tyler JS, Mills MJ, Friedman DI. 2004. The operator and early promoter region of the Shiga toxin type 2-encoding bacteriophage 933W and control of toxin expression. J Bacteriol 186:7670–7679. doi: 10.1128/JB.186.22.7670-7679.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nowicki D, Maciag-Dorszynska M, Kobiela W, Herman-Antosiewicz A, Wegrzyn A, Szalewska-Palasz A, Wegrzyn G. 2014. Phenethyl isothiocyanate inhibits Shiga toxin production in enterohemorrhagic Escherichia coli by stringent response induction. Antimicrob Agents Chemother 58:2304–2315. doi: 10.1128/AAC.02515-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fuchs S, Muhldorfer I, Donohue-Rolfe A, Kerenyi M, Emody L, Alexiev R, Nenkov P, Hacker J. 1999. Influence of RecA on in vivo virulence and Shiga toxin 2 production in Escherichia coli pathogens. Microb Pathog 27:13–23. doi: 10.1006/mpat.1999.0279. [DOI] [PubMed] [Google Scholar]
- 60.Matsushiro A, Sato K, Miyamoto H, Yamamura T, Honda T. 1999. Induction of prophages of enterohemorrhagic Escherichia coli O157:H7 with norfloxacin. J Bacteriol 181:2257–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sperandio V, Torres AG, Kaper JB. 2002. Quorum sensing Escherichia coli regulators B and C (QseBC): a novel two-component regulatory system involved in the regulation of flagella and motility by quorum sensing in E. coli. Mol Microbiol 43:809–821. doi: 10.1046/j.1365-2958.2002.02803.x. [DOI] [PubMed] [Google Scholar]
- 62.Kendall MM, Gruber CC, Rasko DA, Hughes DT, Sperandio V. 2011. Hfq virulence regulation in enterohemorrhagic Escherichia coli O157:H7 strain 86-24. J Bacteriol 193:6843–6851. doi: 10.1128/JB.06141-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Amalaradjou MA, Venkitanarayanan K. 2011. Effect of trans-cinnamaldehyde on reducing resistance to environmental stresses in Cronobacter sakazakii. Foodborne Pathog Dis 8:403–409. doi: 10.1089/fpd.2010.0691. [DOI] [PubMed] [Google Scholar]
- 64.Visvalingam J, Hernandez-Doria JD, Holley RA. 2013. Examination of the genome-wide transcriptional response of Escherichia coli O157:H7 to cinnamaldehyde exposure. Appl Environ Microbiol 79:942–950. doi: 10.1128/AEM.02767-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mei GY, Tang J, Carey C, Bach S, Kostrzynska M. 2015. The effect of oxidative stress on gene expression of Shiga toxin-producing Escherichia coli (STEC) O157:H7 and non-O157 serotypes. Int J Food Microbiol 215:7–15. doi: 10.1016/j.ijfoodmicro.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 66.Lee W, Lee DG. 2014. Lycopene-induced hydroxyl radical causes oxidative DNA damage in Escherichia coli. J Microbiol Biotechnol 24:1232–1237. doi: 10.4014/jmb.1406.06009. [DOI] [PubMed] [Google Scholar]
- 67.Foti JJ, Devadoss B, Winkler JA, Collins JJ, Walker GC. 2012. Oxidation of the guanine nucleotide pool underlies cell death by bactericidal antibiotics. Science 336:315–319. doi: 10.1126/science.1219192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Battesti A, Majdalani N, Gottesman S. 2011. The RpoS-mediated general stress response in Escherichia coli. Annu Rev Microbiol 65:189–213. doi: 10.1146/annurev-micro-090110-102946. [DOI] [PMC free article] [PubMed] [Google Scholar]