ABSTRACT
Fusarium langsethiae is a fungal pathogen of cereal crops that is an increasing problem in northern Europe, but much of its epidemiology is poorly understood. The species produces the mycotoxins T-2 and HT-2, which are highly toxic. It was hypothesized that grain aphids, Sitobion avenae, may transmit F. langsethiae inoculum between wheat plants, and a series of transmission experiments and volatile chemical analyses was performed to test this. Manual translocation of aphids from inoculated to uninfected hosts resulted in pathogen DNA accumulation in hosts. However, the free movement of wingless aphids from infected to healthy plants did not. The addition of winged aphids reared on F. langsethiae-inoculated wheat seedlings to wheat plants also did not achieve successful pathogen transfer. While our data suggested that aphid transmission of the pathogen was not very efficient, we observed an increase in disease when aphids were present. After seedling inoculation, an increase in pathogen DNA accumulation in seedling leaves was observed upon treatment with aphids. Furthermore, the presence of aphids on wheat plants with F. langsethiae-inoculated ears not only led to a rise in the amount of F. langsethiae DNA in infected grain but also to an increase in the concentrations of T-2 and HT-2 toxins, with more than 3-fold higher toxin levels than diseased plants without aphids. This work highlights that aphids increase the susceptibility of wheat host plants to F. langsethiae and that aphid infestation is a risk factor for accumulating increased levels of T-2 and HT-2 in wheat products.
IMPORTANCE Fusarium langsethiae is shown here to cause increased contamination levels of grain with toxins produced by fungus when aphids share the host plant. This effect has also recently been demonstrated with Fusarium graminearum, yet the two fungal species show stark differences in their effect on aphid populations. In both cases, aphids improve the ability of the pathogens to cause and initiate Fusarium head blight (FHB) disease in wheat, but F. langsethiae may be able to act as a dispersal agent. F. langsethiae contributes harmful toxins to wheat grain that need to be controlled, but as yet, its epidemiology is unresolved. This work reveals insights into the role aphids play in promoting the successful colonization of this species in wheat and the benefit of controlling aphid populations on crops that are at high risk of FHB.
INTRODUCTION
Fusarium head blight (FHB) is a fungal disease of cereal crops that is caused by a pathogen complex comprising several species from the genus Fusarium (1) and two from the genus Microdochium (2). Fusarium species produce mycotoxins upon infecting host plants, which can be harmful if consumed by mammals, with different health impacts depending on the specific toxins produced (3). As well as causing mycotoxin contamination, infection can also lead to reduced yield and grain quality. The disease affects crops in all major cereal-growing regions worldwide, and different species prevail in different geographic areas (4). Globally, the most dominant and aggressive pathogens are Fusarium graminearum and Fusarium culmorum, which produce the mycotoxins deoxynivalenol (DON) and nivalenol (NIV) (5); however, weaker pathogens, such as Fusarium langsethiae (6) or Fusarium poae, are able to coexist on infected hosts alongside the more aggressive species and, in doing so, contribute to the mycotoxin contamination of the grain (7). F. langsethiae is a newly identified species, first described in 2004 (8), that has been observed in Norway (9), Sweden (10), Denmark (11), the United Kingdom (12), Poland (13), Belgium (14), Italy (15), Germany (16), Russia (17), and Finland (18). There is also evidence that F. langsethiae is increasing in importance in northern Europe (19) with confirmed hosts, including oats, wheat, and barley (20).
Previously, F. langsethiae was considered to be one and the same as F. poae due to its similar microconidial morphology, although phylogenetically, F. langsethiae is more closely related to Fusarium sporotrichioides than F. poae (21). The mycotoxin profile reflects this, as the highly toxic T-2 and HT-2 type A trichothecenes (22) are produced by both F. langsethiae and F. sporotrichioides (12, 23). Levels of T-2 and HT-2 are not yet under European Union regulation, but it is likely that they will be soon. The indicative limits of these toxins reflect the lower level of toxins detected in wheat than in oats (6, 12, 24, 25) and that the processing of oats leads to a great reduction in toxin contamination (26), i.e., 1,000 μg kg−1 in unprocessed oats compared to 100 μg kg−1 in wheat.
The epidemiology of F. langsethiae has been challenging to unravel since the species causes few outwards symptoms of FHB in cereals and has preferential pathogenicity toward oats and then barley and wheat (25). However, the mycotoxins that it produces in cereals mean that it is a major threat to grain production (27, 28). The ability of F. langsethiae to successfully cause diseases of the Fusarium complex was investigated under a controlled environment by Imathiu et al. (29), who inoculated oat (cv. Gerald) and wheat (cv. Claire) seeds with fungal conidia and observed that F. langsethiae failed to cause visible seedling blight or subsequent head blight symptoms. However, only diseased tissues were used to reisolate the pathogen, and the success of F. langsethiae colonization of nonsymptomatic seedling tissues was not assessed. While this study provides evidence against F. langsethiae being pathogenic to seedlings, it does not exclude the possibility that seeds may be congenitally colonized with the species, that cryptic infection may be possible, or that other biological processes, such as herbivory or wounding, may be needed to facilitate infection. Indeed, detached leaf assays demonstrated that F. langsethiae required a wound site to cause lesions on wheat leaves (25), and wounding of wheat glumes increased symptom development and pathogen DNA accumulation in ears with F. langsethiae inoculation (30).
Under field conditions, weather patterns play a role in which Fusarium species contribute the most to FHB disease and mycotoxin accumulation at different times. Edwards et al. (31) reported that following a wet harvest in 2004, United Kingdom wheat was highly contaminated with NIV and DON but T-2 and HT-2 were not present in large amounts. Conversely, T-2 and HT-2 were at their highest levels in 2005, which had been a dry summer. The observed pattern of mycotoxin accumulation may represent the ability of different Fusarium spp. to dominate the FHB pathogen complex when conditions reflect the differing optimal conditions of the species; for example, the DON-producing F. graminearum requires a high water availability to grow and produce mycotoxins (32). Increased T-2 and HT-2 during drier weather may indicate that species capable of surviving on marginal water availability, such as F. langsethiae, outcompete the more frequently dominant species during drought stress. The optimum temperature for F. langsethiae growth and T-2 and HT-2 production is also lower than that for F. graminearum growth and DON production (23, 32), and accordingly, this species is of greatest concern in cooler, drier geographical regions (19).
Drier weather also favors increased levels of insect pests, such as aphids. English grain aphids, Sitobion avenae, have been described as ear-feeders (33) that are more strongly influenced by the volatile organic chemical (VOC) emissions of wheat ears over wheat seedlings (34) and have been recorded as being present during ear emergence in Belgian wheat fields (35). We hypothesized that, because they occur on wheat ears alongside FHB pathogens, grain aphids may influence disease epidemiology, and we have recently shown that FHB caused by F. graminearum was influenced by aphid activity when the insect and pathogen shared the same host plant (36). Our previous study showed that grain aphids were unable to transmit F. graminearum between wheat ears but that coincidence of aphids and the pathogen lead to increased disease severity, fungal biomass, and DON accumulation in hosts. While oat is a more suitable host plant for F. langsethiae, in order to compare the role of aphids in the epidemiology of these two diverse FHB pathogens, wheat was also used in the present study. F. graminearum is a species that produces large macroconidia, which are severalfold larger than the microconidia produced by F. langsethiae (37). The smaller spore size and the prevalence of F. langsethiae in dry seasons are factors that may make F. langsethiae more compatible for insect transmission by aphids than F. graminearum.
The interactions of insect herbivores with host plants can be influenced by a number of sensory factors, with olfactory detection of host volatile odors being just one way that herbivores can recognize suitable host plants (38). Aphids are repelled by volatile chemical emissions from wheat hosts that are infected with F. graminearum (36), and as such, aphids would be expected to avoid hosts infected with this species. While this may provide a stimulus to induce emigration from infected hosts, this is unlikely to directly affect the spread of the disease, as aphids were shown to be incapable of transmitting the pathogen. Studying the response of aphids to volatiles from F. langsethiae-infected wheat plants would provide more information about the compatibility of the insect to fulfill the role of vector to the pathogen. The present study aimed to (i) assess if aphids can transmit F. langsethiae from diseased to healthy wheat plants and seedlings, (ii) compare the speed of disease progression and mycotoxin levels in infected plants and seedlings with and without cohabiting aphids, and (iii) elucidate the nature of diseased host-insect volatile chemical interactions.
MATERIALS AND METHODS
Wheat plants at ear emergence.
Wheat seed (cv. Gallant), treated with 10 g of prothioconazole and 50 g clothianidin 100 kg−1 seed (Redigo Deter; Bayer CropScience), was sown in compost (Levington's F2+S) and vernalized for 6 weeks at 4°C and then potted into individual pots (5 liters) and grown in a glasshouse at a maximum 18°C, minimum 15°C daytime temperature and a maximum 12°C, minimum 10°C nighttime temperature with a 16-h photoperiod until growth stage (GS) 59 (ear emergence) (39).
Wheat seedlings.
Wheat seeds (cv. Gallant) were surface sterilized in sodium hypochlorite for 2 min followed by three 2-min washes in distilled water and dried by blotting. Individual seeds were sown into compost (Levington's F2+S) in seedling trays comprising 3 by 4 modules of size 3 by 3 by 10 cm (length by width by depth). Seedlings were grown in a controlled environment chamber at 18°C daytime temperature and 12°C nighttime temperature under a 12-h photoperiod for 14 days until the three-leaf growth stage (GS13) (39).
Preparation of fungal inoculum.
Single-spore isolates of Fusarium langsethiae from the University of Nottingham collection (numbers 154, 221, and 229), which had originally been isolated from wheat or oat samples, were grown on potato-dextrose agar (PDA) plates for 10 days, and plugs of colonized agar (10 per flask) were used to inoculate carboxymethyl cellulose liquid media flasks (40). Sterile L-shaped spreaders were added to flasks to increase turbulence, and flasks were sealed with cotton wool and foil and shaken at 90 rpm at 23°C for 4 days. After this time, the flask contents were filtered through two layers of sterile muslin, and spore concentrations were calculated under a light microscope with a Neubauer hemocytometer (Marienfeld-Superior, Germany). Spore suspensions were adjusted to 1 × 106 spores ml−1 for ear inoculations and 1 × 107 spores ml−1 for seedling inoculations, with a composite inoculum suspension made by combining the spores of the three isolates together in equal measure.
Aphid rearing.
Grain aphids, Sitobion avenae, were obtained from colonies maintained at Rothamsted Research (Harpenden, United Kingdom) and reared on wheat seedlings (cv. Gallant) in cages within a glasshouse that was under the same temperature and light settings as those for plant growth (18°C daytime temperature and 12°C nighttime temperature; 12 h/12 h light/dark photoperiod) at the University of Nottingham (Sutton Bonington, United Kingdom).
Transmission of Fusarium langsethiae via manual movement of apterous (wingless) aphids from infected to healthy host ears (experiment 1).
Wheat ears were infected with a suspension of Fusarium langsethiae spores by spray inoculation until runoff. Ears were bagged in polyethylene bags for 7 days and then grown for a further 7 days while disease became established in the host. Approximately 20 apterous (wingless) adult aphids were placed on infected ears (n = 6) and trapped on the ear in netting sealed at the base of the ear. After a 72-h feeding period, aphids were removed from the diseased plants by carefully handling them by the rear limbs using sterile needle-nosed forceps. Ten individuals were transferred to each new healthy host ear (n = 6) and again trapped there inside netting sealed below the ear. After 72 h on the host ears, the aphids were removed. A disease-free aphid control treatment was prepared in the same way, but the initial aphid host ears were mock inoculated with water. Two further treatments were prepared. A pathogen-only treatment was prepared by point inoculation of ears at anthesis (GS65) with spore suspension, with 10 μl added to each of the 10 spikelets per ear, and a negative control was prepared as in the pathogen-only treatment but using distilled water instead of the spore suspension.
Following the application of treatments, ears were assessed visually for disease symptoms (dark lesions at the base of spikelets or bleached spikelets). The number of symptomatic spikelets was taken as a proportion of the total number of spikelets per ear to calculate disease severity (%). Assessments were conducted at nine time points, roughly twice weekly, beginning at 4 days after inoculation (DAI) until 29 DAI. Ears were then left to develop until maturity when they were harvested, freeze-dried whole, and milled without threshing to obtain flour samples. From the flour, DNA extractions were performed and samples were analyzed by two technical repeats of quantitative PCR (qPCR) as described below.
Volatile collection and olfactometer bioassays.
Under the same controlled environment chamber conditions as those described above, four ears per wheat plant were spray inoculated with F. langsethiae spore suspension or mock inoculated with sterile distilled water. Following inoculation, ears were bagged in polyethylene for 72 h, and then bags were removed and plants were incubated for a further 14 days to allow the infection to develop until lesions were visible on the infected ears. The volatile organic chemical (VOC) emissions were then collected using the headspace sampling methods described previously (36, 41), and VOC samples were used in 4-arm olfactometer bioassays using individual alate (winged) Sitobion avenae (n = 10) with one treated arm and three solvent arms. The assays were conducted over a period of 16 min, rotating the chamber every 2 min to eliminate lighting bias, at 23°C under uniform lighting; the chamber was lined with filter paper (Whatman no. 1), and air was drawn out through the center at a rate of 350 ml min−1. After bioassays, which used either diseased or healthy host volatiles, comparative bioassays were performed that directly compared the preference of aphids for healthy versus diseased volatiles. The olfactometer bioassays were performed in the same manner as above (n = 10), except two oppositely positioned arms were loaded with each of the healthy and diseased host volatiles, and the remaining two were treated only with dichloromethane solvent.
Microscopy.
Aphids were added to colonies of F. langsethiae grown on PDA for 7 days and were removed with forceps after 24 h and stored at −20°C for up to 3 days until staining. To detect fungal hyphae on or within the aphids, aphid preps were stained with Malachite green (2 ml of 1% Malachite green solution in 95% ethanol, 50 ml deionized water, 40 ml glycerol, 10 ml of 1% acid fuchsin, 2.5 ml lactic acid, and 5 g phenol) for 2 min. The fluorescence was visualized by a Leica SP2 confocal microscope using a 543-nm excitation wavelength.
Inoculation of wheat seedlings with Fusarium langsethiae and transmission between seedlings by apterous Sitobion avenae (experiment 2).
Wheat seedlings were placed into individual plastic boxes that were 25 by 25 by 15 cm (length by width by height) with netting panels in the lid and absorbent paper on the bottom. Seedlings (n = 10) were infested with aphids by placing a colonized wheat leaf from the rearing cage in proximity to seedling leaves to allow aphids to move onto the new hosts. A further set of seedlings (n = 5) was kept aphid-free during this period as a control. After a period of 48 h, the detached leaf that was used to import aphids to the seedlings was removed. Of the seedlings infested with aphids, half (n = 5) were then cleared of aphids by hand. All of the seedlings were then removed from their containers and spray inoculated with Fusarium langsethiae spore suspension until runoff, using a plastic sheet to prevent runoff reaching the compost, and replaced in the containers for 7 days. A second 14-day-old seedling was added to the opposite side of each container to avoid direct contact between seedling leaves. The experiment was terminated 21 days after the addition of the second seedling, the target of the transmission experiment (target seedlings), by manual removal of any aphids and excision at the base of all of the leaves of the seedlings. Green leaf material was stored at −18°C until DNA extraction.
Transmission of Fusarium langsethiae via natural movement of apterous aphids from infected to healthy host plants (experiment 3).
Wheat plants at ear emergence (GS59) were inoculated on all ears by spray inoculation until runoff with Fusarium langsethiae spore suspension and bagged in polyethylene bags for 7 days. A single infected plant was placed into each of the 20 compartments of the large timber-framed and net-covered cages that were 105 by 30 by 120 cm (length by width by height). Each cage was accessible on the narrow side by a Velcro-sealed door and fed with automatic watering drippers. Infected plants were placed furthest from the cage door, and into half of the cages (n = 10), aphids were added to the plant ears according to a randomized design by placing a colonized wheat leaf from the rearing colony onto the infected ears, which were held in place between the top spikelets. Aphids were allowed to move to the new host for a period of 48 h before the removal of the detached leaves. Seven days after the addition of the aphids, a second wheat plant, uninoculated and at ear emergence (GS59), was then added to all of the cages and positioned near the cage door at a distance of approximately 1 m from the infected plant in each cage. Visual disease assessments were conducted from 4 days after the addition of the target plant to each cage (4 DAI) at twice weekly intervals to 35 DAI. Disease symptoms (lesions and bleaching) were recorded for each ear of each plant that had emerged when plants were added to the cages. Disease severity was calculated for individual ears and averaged over the whole plant. Plants were allowed to mature before harvesting, with each ear harvested individually, freeze-dried, and milled without threshing to gain flour samples that were used in DNA and mycotoxin extractions.
Primary transmission of Fusarium langsethiae via alate Sitobion avenae (experiment 4).
To prepare the alate aphids, wheat seedlings were grown to create the feedstock for two aphid rearing cages. Seedlings were grown under standard conditions as described. One pot was spray inoculated with sterile distilled water for disease-free control aphids, and the other pot was spray inoculated with Fusarium langsethiae spore suspension until runoff. Seedlings were added to rearing cages along with aphids 24 h after inoculation. Following the addition of the pots of seedlings to their separate rearing cages and the colonization of the seedlings with aphids for a period of 7 days, colonized plants were resprayed with spore suspension or distilled water. Colonized plants were maintained for another 5 weeks to induce crowding and encourage the production of alate morphs. Leaf material from the rearing cages was sampled 4 weeks after the addition of aphids to the cages for use in DNA extraction, as described for the seedling transmission experiment, to confirm the infection status of each cage.
Single wheat plants at ear emergence (GS59; n = 20) were added to each of the 20 individual compartments of a large timber-framed cage (as for experiment 3). Ten alate aphids were harvested from the rearing cages with a vacuum pooter (Watkins & Doncaster, United Kingdom) and released into the cage compartments. Aphids from the infected rearing colony were used in half of the cages (n = 10), and the other half was treated with aphids from the control colony according to a randomized design. No visual assessment of plants was conducted to prevent the need to open the cages and the escape of winged aphids. When all tillers were mature, the oldest 4 ears per plant were harvested, freeze-dried, and milled into flour for use in DNA and mycotoxin extractions.
DNA extraction and quantification of Fusarium langsethiae DNA by qPCR.
DNA extractions were performed on 6, 5, 10, and 10 biological replicates from experiments 1, 2, 3, and 4, respectively. Freeze-dried samples of whole wheat were milled without threshing in a centrifugal mill (ZM 200; Retsch GmbH, Germany). Flour (0.5 g) was shaken with cetyltrimethylammonium bromide (CTAB) buffer (3.75 ml) in 15-ml tubes and incubated at 65°C for 2 h. Tubes were then chilled on ice, and potassium acetate was added (1.25 ml). The extraction then proceeded as previously described (42). For green leaf material, the oldest three seedling leaves were chopped finely with scissors, and 0.5 g of leaf material was added to CTAB (3.75 ml) with a sterile ball bearing and shaken mechanically for two 20-s intervals using a FastPrep-24 homogenizer (MP Biomedicals, USA). Extraction proceeded as described for flour samples from this point. The concentration of DNA was determined by spectrophotometry measuring the absorbance at 260-, 280-, 328-, and 360-nm wavelengths using a CARY 50 probe UV-visible spectrophotometer (Varian, CA, USA). Simple Reads software was used to calculate the DNA concentration from absorbance values, and then sample concentrations were adjusted to 20 ng μl−1 and stored at 4°C until use in PCR assays.
Sample DNA was analyzed with diagnostic PCR using internal transcribed spacer (ITS) 4 and 5 primers to confirm the presence of fungal DNA as described previously (43). Samples were then analyzed further to quantify the amount of Fusarium langsethiae DNA present by qPCR, using species-specific primers FlangF3 (5′-CAAAGTTCAGGGCGAAAACT-3′) and LanspoR1 (5′-TACAAGAAGACGTGGCGATAT-3′) and a previously described cycling program (44), which amplified a 310-bp product. Tenfold dilutions of pure F. langsethiae were included in the assay to generate a standard curve from 1 to 10−4 ng μl−1. Two technical PCR repeats of each biological replicate were performed for each experiment. The limit of quantification for the assay was 0.005 pg ng−1.
Mycotoxin extraction from flour samples and analysis by LC-MS/MS.
Flour samples (12.5 g) were spiked with 13C-labeled DON toxin internal standard (Biopure; Romer Labs, Runcorn, United Kingdom) to produce a final concentration of 20 μg kg−1 and blended with 50 ml methanol (100%) for 3 min. Samples were filtered through filter paper (Whatman no. 1), and 2 ml of the filtrate made up to 50 ml with phosphate-buffered saline (PBS) buffer, which was then filtered through fiberglass. The filtrate (20 ml) was passed over a cleanup column (DZT MS-Prep; R-Biopharm, Germany) by gravity over a 30-min period, and then the column was washed with sterile water prior to the elution of samples by passing through 1 ml methanol (100%). Samples were dried under a stream of nitrogen gas and then redissolved in 500 μl methanol (10%) in preparation for liquid chromatography-tandem mass spectrometry (LC-MS/MS).
The LC-MS/MS analysis was performed on an Agilent 1100 series LC system (Agilent Technologies, Germany) coupled with a triple quadrupole Micromass Quattro Ultima v4.0 SP4 (Waters) and equipped with Luna C18 100 Å LC (5 μm; 250 by 3 mm) column (Phenomenex, Torrance, CA, USA). The flow rate was set at 0.5 ml min−1, and the injection volume was 30 μl. Mobile phase A was 90% water with 10% methanol, and mobile phase B was 100% methanol. A linear binary gradient was applied from 0% to 100% phase B within 15 min. The content of phase B was held for 7 min and then lowered to 0% within 20 s followed by equilibration of the column for 5 min.
Quantitative determination of all compounds was performed by operating the mass spectrometer in electrospray ionization (ESI) positive and negative ionization mode. Optimized instrument settings include a capillary voltage of 3.5 kV, a source temperature of 100°C, a desolvation temperature of 450°C, a desolvation gas flow rate of 672 liters h−1 cone voltage, a cone gas flow rate of 76 liters h−1, and a multiplier of 650 V. Argon was used as the collision gas at a pressure sufficient to increase the collision cell Pirani gauge to a reading of 0.0874 Pa. Parent/daughter ions for T-2, HT-2, and 13C-labeled DON were detected at 489/245, 447/354, and 279/230 m/z, respectively, with a dwell time of 0.1 s. MassLynx 4.0 software was used for data acquisition and processing. Quantification of the samples was carried out using a matrix of standards prepared in-house. The limit of detection for both T-2 and HT-2 was 10 μg kg−1.
Toxicity of T-2 to Sitobion avenae through artificial feeding.
To determine the toxicity of T-2 on Sitobion avenae, an artificial feeding assay was performed. Sucrose solution (0.5 g ml−1) was spiked with T-2 toxin in various concentrations, ranging from 1 μg ml−1 down in 10-fold dilutions to 1 ng ml−1. Toxin-free sucrose solution was used as a control. Sachets of feeding solution were prepared in Parafilm suspended over Perspex rings (approximately 2 cm tall with a 2-cm inner radius). Ten adult aphids were added to the underside of each sachet, sealed at the base of the tube, and allowed to feed for a total of 7 days. The feeding apparatus was covered with a green acetate sheet and placed under direct lighting for a 12-h photoperiod in a controlled environment chamber with a daytime temperature of 18°C and a nighttime temperature of 12°C. The number of living and dead aphids was recorded after 24 h, 48 h, and 7 days, and any newly born nymphs were removed. To calculate the mortality, the number of dead aphids as a proportion of the starting population was subjected to Abbot's correction for mortality, where the control mortality (Mc) is deducted from the treatment mortality (Mt) and from 100 to correct the numerator and the denominator of the percentage calculation. This can be summarized in the following formula: corrected mortality = [(Mt − Mc)/(100 − Mc)] × 100.
The concentration at which 50% corrected mortality was observed, the EC50, was calculated from the gradient of the slope of the trendline generated by plotting the means for corrected mortality against the log10-transformed concentrations of T-2.
Statistics.
Disease progress was analyzed with repeated measurements analysis of variance (ANOVA), and calculated values for the area under the disease progress curve (AUDPC) were analyzed using general ANOVA for experiments with multiple treatments, or t tests for experiments with two treatments (and in experiment 1 where disease symptoms were only present in two treatments), along with disease severity at individual time points, pathogen DNA, and [T-2 + HT-2] concentration data. To normalize the residuals, DNA and toxin concentrations were log10 transformed and disease severity data were angularly transformed. Aphid mortality data were analyzed with general ANOVA separately for each time point and, due to the Abbot's correction analysis, were only performed on data from T-2-containing diets. All statistical analyses were conducted using Genstat v15.2 (Harpenden, United Kingdom).
RESULTS
Experiment 1: transmission of Fusarium langsethiae via manual movement of apterous (wingless) aphids from infected to healthy host ears.
Infection levels were low for all treatments as expected for this pathogen species. The final disease severity (29 DAI) was zero for the mock inoculated and aphid-only treatments so these were excluded, and aphid transmission and point inoculated treatments were compared using two sample t tests. The final disease severity observed was similar for the aphid transmission treatment (12.0%) and the point inoculated treatment (10.1%), which were not significantly different from one another (P = 0.781). Values calculated for the area under the disease progress curve (AUDPC) were also not significantly different between treatments (P = 0.288), and the overall mean AUDPC across both symptomatic treatments was 96.8.
Only samples from ears from point inoculated and aphid transmission treatments accumulated detectable levels of F. langsethiae DNA. The mean pathogen DNA concentrations of 0.019 pg F. langsethiae DNA ng−1 of total extracted DNA for point inoculated samples and 0.014 pg ng−1 from the aphid transmission treatment were not significantly different from one another.
Aphid behavioral responses to VOCs of hosts with or without Fusarium langsethiae infection.
There was no significant difference in time spent in areas of the olfactometer treated with healthy wheat VOC samples compared to that spent in areas treated only with solvent. There was also no significant difference in time spent in areas treated with F. langsethiae-infected host VOC samples compared to that of solvent-treated areas. In comparative bioassays, the time spent by aphids in areas treated with F. langsethiae-infected host volatiles, healthy host volatiles, or solvent was also not significantly different.
Confocal microscopy.
Hyphae were observed on the external surfaces of aphids that had walked on F. langsethiae colonies. Hyphal threads were observed in greatest density on the legs (Fig. 1A) and siphunculi of S. avenae. Microconidia were observed in the stain washings (Fig. 1B) but were not observed on the external surfaces of the aphids.
FIG 1.
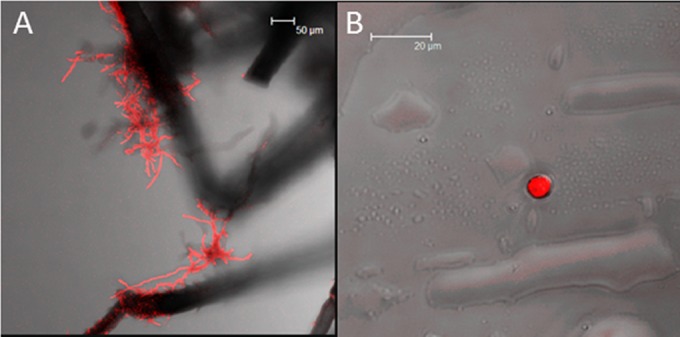
Confocal microscopy of (A) S. avenae, which had walked on F. langsethiae colonies, showing fungal hyphae on the aphid legs and (B) F. langsethiae microconidia obtained from residual stain solution (stained fungal materials fluoresced red).
Experiment 2: inoculation of wheat seedlings with Fusarium langsethiae and transmission between seedlings by apterous (wingless) Sitobion avenae.
Aphids moved quickly from the inoculated plant to the target seedlings and multiplied to over 100 aphids per seedling on the new seedling within 10 days for all replicates. Despite this, no pathogen DNA was detected in target seedlings; thus, transmission of Fusarium langsethiae between inoculated and susceptible wheat seedlings was not observed in any treatment, with or without aphids present.
Seedlings that were treated with aphids prior to spray inoculation with Fusarium langsethiae spores were observed to have elevated levels of pathogen DNA in leaf tissue (P = 0.043). Seedlings with aphid exposure throughout the experiment had a mean pathogen DNA concentration of 0.148 pg ng−1, and those with aphid exposure for only 48 h prior to inoculation had 0.137 pg ng−1, values which were not significantly different from one another. Seedlings without aphids had a 3-fold lower mean level of pathogen DNA of 0.044 pg ng−1 (Fig. 2, log10-transformed data shown).
FIG 2.
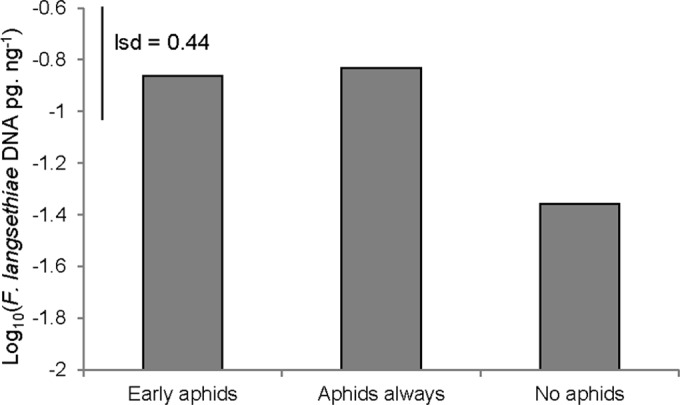
F. langsethiae DNA concentration (pg ng−1) (log10-transformed data) in inoculated wheat seedlings (experiment 2) with aphid infestation prior to inoculation (early aphids), continued aphid infestation both before and after inoculation (aphids always), and without aphid infestation (no aphids). lsd, least significant difference.
Experiment 3: transmission of Fusarium langsethiae via natural movement of apterous (wingless) aphids from infected to healthy host plants.
Wheat plants placed at a distance of 1 m from Fusarium langsethiae-infected wheat plants with or without aphids present showed no significant difference in the concentration of pathogen DNA. There was also no difference in the number of tillers per plant that became infected between the two treatments, and neither was there a significant difference in AUDPC or the final disease severity at 28 days after inoculation. Low levels of pathogen DNA and visual signs of disease were observed in the target plants, with a mean concentration of 0.01 pg ng−1 for target plants across both treatments when uninfected tillers were excluded. The final disease severity was 0.6% and 0.4% for plants with and without aphids present, respectively.
Wheat plants that were spray inoculated with Fusarium langsethiae spores did show differences in fungal biomass and mycotoxin contamination when aphids were present compared to plants without aphids. The presence of aphids significantly increased the concentrations of F. langsethiae DNA (P = 0.015) and T-2 plus HT-2 (P < 0.001) (Fig. 3) in infected flour samples. For pathogen DNA concentrations, aphid-free inoculated wheat had a mean of 3.12 pg ng−1 compared to a mean of 5.57 pg ng−1 in inoculated plants to which aphids were added. Mean T-2 and HT-2 toxin concentrations for aphid-free inoculated plants were 15.2 μg kg−1 compared to 52.2 μg kg−1 when aphids were present. Levels of T-2 and HT-2 were increased 2.7 and 7.6 times, respectively, when aphids were present. Furthermore, a significant, strong, and positive relationship was observed between log10-transformed concentrations of pathogen DNA and T-2 plus HT-2 in both aphid-free and aphid-infested plants (P < 0.001; R2 = 0.78) (Fig. 4). Data from each group were best fitted to parallel lines, with a higher intercept being fitted to aphid infested plants.
FIG 3.
Concentrations of (left axis) F. langsethiae DNA (pg ng−1) (log10 transformed) (P = 0.015) and (right axis) T-2 + HT-2 toxins (μg kg−1) (log10 transformed) (P < 0.001) in inoculated feedstock plants in the wingless aphid transmission experiment (experiment 3) for plants with and without aphid infestation. Asterisks represent the level of significant difference (*, 0.05 > P > 0.01; ***, P < 0.001).
FIG 4.
Linear regression fit to parallel lines between log10-transformed concentrations of F. langsethiae DNA (pg ng−1) and T-2 + HT-2 toxins (μg kg−1) in the ears of inoculated wheat plants in the wingless aphid transmission experiment. Closed circles, aphid-free plants; open circles, aphid-infested plants. R2 = 0.78; P < 0.001.
Experiment 4: primary transmission of Fusarium langsethiae via alate (winged) Sitobion avenae.
Leaf material from the F. langsethiae inoculated aphid rearing cage had 0.10 pg ng−1 of F. langsethiae DNA. In leaf material from the healthy rearing cage, no F. langsethiae DNA was detected. No F. langsethiae DNA was detected in real-time PCR assays using DNA samples extracted from wheat ears treated with alate aphids reared on either F. langsethiae-infected wheat seedlings or healthy wheat seedlings.
Toxicity of T-2 on Sitobion avenae.
After 7 days of aphid feeding on T-2-spiked sucrose solution, the concentration of T-2 in the aphid diet was a significant factor accounting for aphid mortality (P = 0.007). The concentration of T-2 that induced 50% mortality, the EC50, after 7 days of feeding was 407 pg ml−1 (Table 1). At 24 h and 48 h of artificial feeding, no significant change in mortality was observed between T-2 treatments.
TABLE 1.
Mortality rate associated with T-2 for Sitobion avenae during the artificial feeding assay using T-2 toxin-spiked sucrose solution
| Concn of T-2 (pg ml−1) or other parameter | Abbott's corrected mortality (%) after 7 daysa |
|---|---|
| 1 | 5 A |
| 10 | 11.4 AB |
| 100 | 40 BC |
| 1,000 | 60 C |
| 5% LSDb | 30.2 |
| P value | 0.007 |
| EC50 (pg ml−1) | 407 |
Data marked with different uppercase letters are significantly different.
LSD, least significant difference.
DISCUSSION
The current results show that aphid infestation results in a significantly increased fungal biomass of F. langsethiae and type A trichothecenes, T-2 and HT-2, in wheat. Aphid-infested diseased plants had more than 3-fold-higher T-2 + HT-2 mycotoxin levels than diseased plants without aphids as well as elevated levels of fungal biomass. The amount of T-2 plus HT-2 in 1 of the 10 aphid-infested inoculated wheat plants breached the recommended tolerable daily limit of 100 μg per kg. A similar finding has recently been reported for F. graminearum in association with grain aphids on wheat (36). The authors showed a rise in DON and pathogen DNA in ears treated with aphids and F. graminearum and that increased time of aphid colonization prior to inoculation led to increased pathogen DNA accumulation. The present study goes one step further by examining the impact of aphids on fungal biomass accumulation in seedlings as well as ear infection. It was not previously known if F. langsethiae was capable of colonizing wheat seedlings systemically as a result of seed infection; infection attempts have only been measured visually, and no symptoms were seen (29). This study showed that seedling colonization measured by pathogen DNA can be achieved using spray inoculation with a high concentration of inoculum (107 spores ml−1) and that pretreatment of seedlings with aphids increases fungal biomass accumulation 4-fold in seedlings following inoculation. Thus, the effect of elevated biomass of F. langsethiae with aphids feeding on hosts that become colonized is consistent across the two different wheat developmental stages that were tested.
Volatile organic chemical (VOC) emissions from wheat ears infected with F. langsethiae produced no change in aphid behavior during olfactometer bioassay experiments. This is in contrast to F. graminearum-infected wheat plants, which produced repellent VOCs (36). F. graminearum rapidly produces visible symptoms, including ear bleaching, which reduces the green area of the ear available for aphid feeding. As F. langsethiae produces fewer or no symptoms, the ear remains green for longer. This too has implications for the compatibility of aphids to interact with hosts infected with F. langsethiae and potentially act as a vector. Our current data show that aphids do not distinguish between hosts infected with F. langsethiae and healthy ones. It is likely, based on our results, that aphids are not repelled by F. langsethiae-infected wheat volatiles because the plants remain good quality hosts unlike F. graminearum-infected plants, which have lower aphid survival (36).
Another difference between the infection process of F. graminearum and F. langsethiae is the production of type A trichothecenes, T-2 and HT-2, during F. langsethiae infection compared to that of type B trichothecenes, such as DON, which are produced by F. graminearum. Accordingly, the role of DON in the production of host volatile metabolites versus that of T-2 and HT-2 may differ, as may the comparative toxicity to aphids. Data presented in this work show that aphids were sensitive to T-2 after prolonged exposure to high concentrations of this mycotoxin. After 48 h, the concentration of T-2 in artificial diets did not influence aphid mortality. This is in contrast to the previously observed effect of elevated mortality of aphid populations after feeding on wheat plants infected with F. graminearum for the same short time frame of 48 h (36). Thus, while aphids were sensitive to T-2, the effect on aphid populations may be less when feeding on hosts infected with F. langsethiae than when feeding on hosts infected with F. graminearum.
Early indications from the manual transmission experiment showed that grain aphids are capable of picking up sufficient inoculum from F. langsethiae-infected wheat ears to induce disease when directly placed on a new susceptible host ear. Low levels of visible symptoms were observed, as is typical for F. langsethiae infection in wheat (28). However, both the disease severity and the level of pathogen DNA within the ears were raised in the aphid transmission treatment group compared to those of controls treated with aphids from healthy previous hosts and were comparable to those of point inoculated positive controls. DNA levels were low in the experiment and suggest that point inoculation is a less robust method for inducing F. langsethiae disease in wheat ears than spray inoculation. The level of disease induced by aphid transmission with manual transfer between hosts was not significantly different from that induced by point inoculation and indicates that there is potential for aphid bodies to act as suitable vehicles for F. langsethiae inoculum given an unhindered route to the susceptible host or secondary spread on the ear. Microscopy of aphids from F. langsethiae colonies showed that hyphae may become entangled on the aphid body and legs and that microconidia may be washed from the surfaces of aphids. This provides support for the possibility of aphid bodies to acquire F. langsethiae inoculum from an abundant source. Microconidia were not able to be visualized on/in aphid bodies after staining yet were found in the washing residues, implying that the retention of spores on aphid surfaces may potentially be easily disrupted and short-lived in a field environment. More robust retention of hyphae was observed on aphid appendages; however, whether infection could be initiated is not clear. Transmission experiments that simulate the natural movement of aphids from colonized plant to target plant did not provide further evidence that transmission of F. langsethiae is possible by either apterous or alate aphids. Apterous aphids move slowly from plant to plant to relieve crowding on any one host and do not possess wings to move directly to wheat ears without moving up the length of the host plant to reach them. Emigrating aphids that had previously colonized infected host tissues may have originated from the site of infection with sufficient levels of inoculum on their bodies to infect a new host; by the end of the journey to the new host, this capacity to transmit appears to have diminished. It remains possible that these results would be affected by decreasing the distance of the infected and healthy hosts from 1 m to a distance more typical of field conditions or with greater wind currents that can assist the translocation of aphids from infected ears to susceptible ones.
Alate aphids present an opportunity for aphids to reach new host tissues without the need for crawling long distances and potentially losing carried inoculum in the process. However, from the experiment performed, in which alate aphids were reared from F. langsethiae-infected seedlings, the aphids were unable to transmit the inoculum when brought into proximity with a new host. Contact between immigrating alate aphids and wheat ears was not guaranteed, so it remains possible that aphids chose not to alight on wheat ears but may have colonized other parts of the plant first, with growing aphid colonies then spreading to the ears of the target plants. Also, while seedlings were successfully inoculated by F. langsethiae spores, the nature of the fungal growth through seedling tissues and the level of spore production of the pathogen on the surfaces of the seedlings are not known. It has been discussed whether F. langsethiae can exist as a nonpathogenic endophyte (45). As such, the failure of alate aphids to transmit inoculum may have been due to the use of foliar tissues as a source of the fungus rather than infected ears. Further work to examine the infection process of F. langsethiae in wheat seedlings and the nature of the relationship between the host and the fungus is required to clarify the appropriateness of the method used in this instance.
From these results, the major implications for F. langsethiae epidemiology are that wheat seedlings that are attacked by aphids, either late in summer providing there is a mild autumn or early in the spring if a mild winter, may be more vulnerable to opportunistic attack by F. langsethiae and that ears colonized with aphids may be at greater risk of accumulating dangerous levels of mycotoxins upon fungal infection. It has been mentioned previously that the mycotoxin output of F. langsethiae cannot be predicted by visual disease assessment (27), but this study and others (11, 12) provide support for a strong correlation to be expected between pathogen DNA levels at harvest and T-2 and HT-2 contamination. If aphid presence on wheat can increase the susceptibility of the host to accumulate increased levels of T-2 and HT-2 during F. langsethiae infection, it is possible that this same effect may persist across other cereal hosts. Of particular interest for investigation would be oats, which are a more compatible host for F. langsethiae (26, 45). Control of aphids in high-risk geographical locations may be of benefit for the limitation of toxin levels in grain and grain products.
This study, along with the previously described study on F. graminearum (36), shows that the effect of aphid feeding on increased host susceptibility to FHB is not limited to a single Fusarium species; other fungal pathogen infections may also be enhanced in aphid-infested wheat hosts. While the mechanism by which aphids can increase the susceptibility of the host toward F. langsethiae is not addressed in this study, this pathogen species has been observed to be unable to produce lesions in detached wheat leaves without a wound site (26), indicating that physical degradation of tissues upon aphid feeding may contribute to improved infection success by the pathogen. While aphids do not produce large mechanical wounds, components of aphid saliva are able to alter host biochemistry so as to cause discoloration in the tissues surrounding feeding sites and can influence plant defense responses (46, 47). Suppression of host defenses or upregulation of plant defense pathways in response to aphid attack that operate antagonistically with the pathways responsible for coping with attack by fungal pathogens may lead to a muted defense response to the fungal attack and increased disease (48). It has been shown that the salicylic acid pathway is important in wheat defense against Fusarium spp. (49), and it is possible that aphid attack may suppress these defenses because aphid saliva contains proteins that suppress plant defense (50).
In conclusion, despite indications that grain aphids may be compatible vectors of F. langsethiae between wheat plants, experiments that mimicked the natural movement of aphids between host plants in the field did not show aphid transmission. However, aphid herbivory on hosts that became infected from other sources was shown to increase the amount of fungal biomass accumulated in wheat seedling foliar tissues and in ears as well as the concentration of T-2 and HT-2 toxin in infected grain. In light of this, given that aphids are not repelled by infected hosts, as has been shown to occur with the more aggressive FHB pathogen F. graminearum, our study indicates that persistent interaction between infected hosts and aphids may be possible and that this interaction may lead to an increase in the toxin contamination of wheat crops. These findings help to fill knowledge gaps surrounding the life cycle and epidemiology of this newly identified pathogen species, particularly with respect to seedling infection and the role that aphids may play in enhancing the infection success of this weak pathogen of wheat.
ACKNOWLEDGMENTS
We thank Mark Meacham for technical assistance.
This work was supported by a Lawes' Trust PhD studentship. Rothamsted Research receives grant-aided support from the Biotechnology and Biological Sciences Research Council (BBSRC).
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.Wegulo SN, Baenziger PS, Nopsa JH, Bockus WW, Hallen-Adams H. 2015. Management of Fusarium head blight of wheat and barley. Crop Prot 73:100–107. doi: 10.1016/j.cropro.2015.02.025. [DOI] [Google Scholar]
- 2.Glynn NC, Edwards SG. 2010. Evaluation of PCR assays for quantifying seed-borne infection by Fusarium and Microdochium seedling blight pathogens. J Appl Microbiol 108:81–87. doi: 10.1111/j.1365-2672.2009.04410.x. [DOI] [PubMed] [Google Scholar]
- 3.Marin S, Ramos AJ, Cano-Sancho G, Sanchis V. 2013. Mycotoxins: occurrence, toxicology, and exposure assessment. Food Chem Toxicol 60:218–237. doi: 10.1016/j.fct.2013.07.047. [DOI] [PubMed] [Google Scholar]
- 4.Parry DW, Jenkinson P, McLeod L. 1995. Fusarium ear blight (scab) in small-grain cereals—a review. Plant Pathol 44:207–238. doi: 10.1111/j.1365-3059.1995.tb02773.x. [DOI] [Google Scholar]
- 5.Goswami RS, Kistler HC. 2004. Heading for disaster: Fusarium graminearum on cereal crops. Mol Plant Pathol 5:515–525. doi: 10.1111/j.1364-3703.2004.00252.x. [DOI] [PubMed] [Google Scholar]
- 6.Imathiu SM, Edwards SG, Ray RV, Back MA. 2013. Fusarium langsethiae—a HT-2 and T-2 toxins producer that needs more attention. J Phytopathol 161:1–10. doi: 10.1111/jph.12036. [DOI] [Google Scholar]
- 7.Logrieco A, Bottalico A, Mule G, Moretti A, Perrone G. 2003. Epidemiology of toxigenic fungi and their associated mycotoxins for some Mediterranean crops. Eur J Plant Pathol 109:645–667. doi: 10.1023/A:1026033021542. [DOI] [Google Scholar]
- 8.Torp M, Nirenberg HI. 2004. Fusarium langsethiae sp. nov. on cereals in Europe. Int J Food Microbiol 95:247–256. doi: 10.1016/j.ijfoodmicro.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 9.Halstensen AS, Nordby K-C, Klemsdal SS, Elen O, Clasen P-E, Eduard W. 2006. Toxigenic Fusarium spp. as determinants of trichothecene mycotoxins in settled grain dust. J Occup Environ Hyg 3:651–659. doi: 10.1080/15459620600987431. [DOI] [PubMed] [Google Scholar]
- 10.Fredlund E, Gidlund A, Sulyok M, Borjesson T, Krska R, Olsen M, Lindblad M. 2013. Deoxynivalenol and other selected Fusarium toxins in Swedish oats—occurrence and correlation to specific Fusarium species. Int J Food Microbiol 167:276–283. doi: 10.1016/j.ijfoodmicro.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen LK, Jensen JD, Nielsen GC, Jensen JE, Spliid NH, Thomsen IK, Justesen AF, Collinge DB, Jorgensen LN. 2011. Fusarium head blight of cereals in Denmark: species complex and related mycotoxins. Phytopathology 101:960–969. doi: 10.1094/PHYTO-07-10-0188. [DOI] [PubMed] [Google Scholar]
- 12.Edwards SG, Imathiu SM, Ray RV, Back M, Hare MC. 2012. Molecular studies to identify the Fusarium species responsible for HT-2 and T-2 mycotoxins in UK oats. Int J Food Microbiol 156:168–175. doi: 10.1016/j.ijfoodmicro.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 13.Lenc L. 2015. Fusarium head blight (FHB) and Fusarium populations in grain of winter wheat grown in different cultivation systems. J Plant Prot Res 55:94–109. [Google Scholar]
- 14.Dedeurwaerder G, Ghysselinckx J, Hellin P, Janssen F, Duvivier M, Legreve A. 2014. Detection of Fusarium langsethiae on wheat in Belgium. Eur J Plant Pathol 139:453–455. doi: 10.1007/s10658-014-0419-4. [DOI] [Google Scholar]
- 15.Infantino A, Santori A, Aureli G, Belocchi A, De Felice S, Tizzani L, Lattanzio VMT, Haidukowski M, Pascale M. 2015. Occurrence of Fusarium langsethiae strains isolated from durum wheat in Italy. J Phytopathol 163:612–619. doi: 10.1111/jph.12361. [DOI] [Google Scholar]
- 16.Denschlag C, Rieder J, Vogel RF, Niessen L. 2014. Real-time loop-mediated isothermal amplification (LAMP) assay for group specific detection of important trichothecene producing Fusarium species in wheat. Int J Food Microbiol 177:117–127. doi: 10.1016/j.ijfoodmicro.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Minaeva LP, Korotkevich YV, Sheveleva SA. 2013. Rapid determination of food grain infection by fungi Fusarium and their species detection (part 1). Vopr Pitan 82:61–66. (In Russian.) [PubMed] [Google Scholar]
- 18.Yli-Mattila T, Paavanen-Huhtala S, Jestoi M, Parikka P, Hietaniemi V, Gagkaeva T, Sarlin T, Haikara A, Laaksonen S, Rizzo A. 2008. Real-time PCR detection and quantification of Fusarium poae, F. graminearum, F. sporotrichioides and F. langsethiae in cereal grains in Finland and Russia. Arch Phytopathol Plant Prot 41:243–260. [Google Scholar]
- 19.Kokkonen M, Ojala L, Parikka P, Jestoi M. 2010. Mycotoxin production of selected Fusarium species at different culture conditions. Int J Food Microbiol 143:17–25. doi: 10.1016/j.ijfoodmicro.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 20.Torp M, Adler A. 2004. The European Sporotrichiella project: a polyphasic approach to the biology of a new Fusarium species. Int J Food Microbiol 95:241–245. doi: 10.1016/j.ijfoodmicro.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 21.Yli-Mattila T, Mach RL, Alekhina IA, Bulat SA, Koskinen S, Kullnig-Gradinger CM, Kubicek CP, Klemsdal SS. 2004. Phylogenetic relationship of Fusarium langsethiae to Fusarium poae and Fusarium sporotrichioides as inferred by IGS, ITS, beta-tubulin sequences and UP-PCR hybridization analysis. Int J Food Microbiol 95:267–285. doi: 10.1016/j.ijfoodmicro.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Wang Z, Beier RC, Shen J, De Smet D, De Saeger S, Zhang S. 2011. T-2 toxin, a trichothecene mycotoxin: review of toxicity, metabolism and analytical methods. J Agric Food Chem 59:3441–3453. doi: 10.1021/jf200767q. [DOI] [PubMed] [Google Scholar]
- 23.Nazari L, Pattori E, Terzi V, Morcia C, Rossi V. 2014. Influence of temperature on infection, growth, and mycotoxin production by Fusarium langsethiae and F. sporotrichioides in durum wheat. Food Microbiol 39:19–26. doi: 10.1016/j.fm.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 24.European Commission. 2013. Commission recommendation of 27th March 2013 on the presence of T-2 and HT-2 toxin in cereals and cereal products. 2013/165/EU. European Commission, Brussels, Belgium. [Google Scholar]
- 25.Scudamore K, Baillie H, Patel S, Edwards SG. 2007. The occurrence and fate of Fusarium mycotoxins during commercial processing of oats in the UK. Food Addit Contam 24:1374–1385. doi: 10.1080/02652030701509972. [DOI] [PubMed] [Google Scholar]
- 26.Imathiu SM, Ray RV, Back M, Hare MC, Edwards SG. 2009. Fusarium langsethiae pathogenicity and aggressiveness towards oats and wheat in wounded and unwounded in vitro detached leaf assays. Eur J Plant Pathol 124:117–126. doi: 10.1007/s10658-008-9398-7. [DOI] [Google Scholar]
- 27.Opoku N, Back M, Edwards SG. 2013. Development of Fusarium langsethiae in commercial cereal production. Eur J Plant Pathol 136:159–170. doi: 10.1007/s10658-012-0151-x. [DOI] [Google Scholar]
- 28.Imathiu SM, Ray RV, Back MI, Hare MC, Edwards SG. 2013. A survey investigating the infection of Fusarium langsethiae and production of HT-2 and T-2 mycotoxins in UK oat fields. J Phytopathol 161:553–561. doi: 10.1111/jph.12105. [DOI] [Google Scholar]
- 29.Imathiu SM, Hare MC, Ray RV, Back M, Edwards SG. 2010. Evaluation of pathogenicity and aggressiveness of F. langsethiae on oat and wheat seedlings relative to known seedling blight pathogens. Eur J Plant Pathol 126:203–216. doi: 10.1007/s10658-009-9533-0. [DOI] [Google Scholar]
- 30.Ajigboye OO, Bousquet L, Murchie EH, Ray RV. 2016. Chlorophyll fluorescence parameters allow the rapid detection and differentiation of plant responses in three different wheat pathosystems. Funct Plant Biol 43:356–369. doi: 10.1071/FP15280. [DOI] [PubMed] [Google Scholar]
- 31.Edwards SG, Barrier-Guillot B, Clasen PE, Hietaniemi V, Pettersson H. 2009. Emerging issues of HT-2 and T-2 toxins in European cereal production. World Mycotoxin J 2:173–179. doi: 10.3920/WMJ2008.1126. [DOI] [Google Scholar]
- 32.Hope R, Aldred D, Magan N. 2005. Comparison of environmental profiles for growth and deoxynivalenol production by Fusarium culmorum and F. graminearum on wheat grain. Lett Appl Microbiol 40:295–300. doi: 10.1111/j.1472-765X.2005.01674.x. [DOI] [PubMed] [Google Scholar]
- 33.Wratten SD. 1975. Nature of effects of aphids Sitobion avenae and Metopolophium dirhodum on growth of wheat. Ann Appl Biol 79:27–34. doi: 10.1111/j.1744-7348.1975.tb01518.x. [DOI] [PubMed] [Google Scholar]
- 34.De Zutter N, Audenaert K, Haesaert G, Smagghe G. 2012. Preference of cereal aphids for different varieties of winter wheat. Arthropod-Plant Interact 6:345–350. doi: 10.1007/s11829-012-9184-5. [DOI] [Google Scholar]
- 35.De Roissart A, Audenaert K, Smagghe G, Haesaert G. 2010. Monitoring wheat fields in Belgium to assess the role of aphids in the spread of Fusarium, abstr 4274808. Abstr 11th European Seminar, Radzików, Poland. [Google Scholar]
- 36.Drakulic J, Caulfield J, Woodcock C, Jones SPT, Linforth R, Bruce TJA, Ray RV. 2015. Sharing a host plant (wheat Triticum aestivum) increases the fitness of Fusarium graminearum and the severity of Fusarium head blight but reduces the fitness of grain aphids (Sitobion avenae). Appl Environ Microbiol 81:3492–3501. doi: 10.1128/AEM.00226-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leslie JF, Summerell BA. 2006. The Fusarium laboratory manual. Blackwell, Oxford, United Kingdom. [Google Scholar]
- 38.de Vos M, Jander G. 2010. Volatile communication in plant-aphid interactions. Curr Opin Plant Biol 13:366–371. doi: 10.1016/j.pbi.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Zadoks JC, Chang TT, Konzak CF. 1974. A decimal code for growth stages of cereals. Weed Res 14:415–421. doi: 10.1111/j.1365-3180.1974.tb01084.x. [DOI] [Google Scholar]
- 40.Tuite J. 1969. Plant pathological methods: fungi and bacteria. Burgess Publishing Company, Minneapolis, MN. [Google Scholar]
- 41.Birkett MA, Bruce TJA, Martin JL, Smart LE, Oakley J, Wadhams LJ. 2004. Responses of female orange wheat blossom midge, Sitodiplosis mosellana, to wheat panical volatiles. J Chem Ecol 30:1319–1328. doi: 10.1023/B:JOEC.0000037742.05022.9f. [DOI] [PubMed] [Google Scholar]
- 42.Edwards SG, Pirgozliev SR, Hare MC, Jenkinson P. 2001. Quantification of trichothecene-producing Fusarium species in harvested grain by competitive PCR to determine efficacies of fungicides against Fusarium head blight of winter wheat. Appl Environ Microbiol 67:1575–1580. doi: 10.1128/AEM.67.4.1575-1580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p 315–322. In Innis MA, Gelfand DH, Sninsky JJ, White TJ (ed), PCR protocols: a guide to methods and applications. Academic Press, San Diego, CA. [Google Scholar]
- 44.Wilson A, Simpson D, Chandler E, Jennings P, Nicholson P. 2004. Development of PCR assays for the detection and differentiation of Fusarium sporotrichioides and Fusarium langsethiae. FEMS Microbiol Lett 233:69–76. doi: 10.1016/j.femsle.2004.01.040. [DOI] [PubMed] [Google Scholar]
- 45.Divon HH, Razzaghian J, Udnes-Aamot H, Klemsdal SS. 2012. Fusarium langsethiae (Torp and Nirenberg), investigation of alternative infection routes in oats. Eur J Plant Pathol 132:147–161. doi: 10.1007/s10658-011-9858-3. [DOI] [Google Scholar]
- 46.Petersson J, Tjallingii WF, Hardie J. 2007. Aphids as crop pests, p 87–113. In VanEmden HF, Harrington R (ed), Host-plant selection and feeding. CABI Publishing, Wallingford, United Kingdom. [Google Scholar]
- 47.Walling LL. 2008. Avoiding effective defenses: strategies employed by phloem-feeding insects. Plant Physiol 146:859–866. doi: 10.1104/pp.107.113142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Biere A, Bennett AE. 2013. Three-way interactions between plants, microbes and insects. Funct Ecol 27:567–573. doi: 10.1111/1365-2435.12100. [DOI] [Google Scholar]
- 49.Makandar R, Nalam VJ, Lee H, Trick HN, Dong Y, Shah J. 2012. Salicylic acid regulates basal resistance to Fusarium head blight in wheat. Mol Plant Microbe Interact 25:431–439. doi: 10.1094/MPMI-09-11-0232. [DOI] [PubMed] [Google Scholar]
- 50.Elzinga DA, de Vos M, Jander G. 2014. Suppression of plant defenses by a Myzus persicae (green peach aphid) salivary effector protein. Mol Plant Microbe Interact 27:747–756. doi: 10.1094/MPMI-01-14-0018-R. [DOI] [PMC free article] [PubMed] [Google Scholar]


