ABSTRACT
The U.S. lineage, one of the major clades in the Babesia microti group, is known as a causal agent of human babesiosis mostly in the northeastern and upper midwestern United States. This lineage, however, also is distributed throughout the temperate zone of Eurasia with several reported human cases, although convincing evidence of the identity of the specific vector(s) in this area is lacking. Here, the goal was to demonstrate the presence of infectious parasites directly in salivary glands of Ixodes persulcatus, from which U.S. lineage genetic sequences have been detected in Asia, and to molecularly characterize the isolates. Five PCR-positive specimens were individually inoculated into hamsters, resulting in infections in four; consequently, four strains were newly established. Molecular characterization, including 18S rRNA, β-tubulin, and CCT7 gene sequences, as well as Western blot analysis and indirect fluorescent antibody assay, revealed that all four strains were identical to each other and to the U.S. lineage strains isolated from rodents captured in Japan. The 18S rRNA gene sequence from the isolates was identical to those from I. persulcatus in Russia and China, but the genetic and antigenic profiles of the Japanese parasites differ from those in the United States and Europe. Together with previous epidemiological and transmission studies, we conclude that I. persulcatus is likely the principal vector for the B. microti U.S. lineage in Japan and presumably in northeastern Eurasia.
IMPORTANCE The major cause of human babesiosis, the tick-borne blood parasite Babesia microti, U.S. lineage, is widely distributed in the temperate Northern Hemisphere. However, the specific tick vector(s) remains unidentified in Eurasia, where there are people with antibodies to the B. microti U.S. lineage and cases of human babesiosis. In this study, the first isolation of B. microti U.S. lineage from Ixodes persulcatus ticks, a principal vector for many tick-borne diseases, is described in Japan. Limited antigenic cross-reaction was found between the Japan and United States isolates. Thus, current serological tests based on U.S. isolates may underestimate B. microti occurrence outside the United States. This study and previous studies indicate that I. persulcatus is part of the B. microti U.S. lineage life cycle in Japan and, presumably, northeastern Eurasia. This report will be important for public health, especially since infection may occur through transfusion, and also to researchers in the field of parasitology.
INTRODUCTION
Babesia is a protozoan transmitted by ixodid ticks that infects erythrocytes in the host animal. The Babesia microti group is a diverse group of worldwide-distributed parasites that includes various lineages, such as U.S., Kobe, Hobetsu, Munich, monkey/squirrel, and some still-unnamed groups (1–5). The parasites of this group have been detected from various animals, including mouse (U.S., Kobe, Hobetsu, and Munich), rat (U.S. and Kobe), vole (U.S., Hobetsu, and Munich), shrew (U.S., Hobetsu, and Munich), lemming (U.S.), squirrel (monkey/squirrel), and nonhuman primate (monkey/squirrel) (summarized in Zamoto-Niikura et al. [6]). To date, parasites belonging to the U.S. lineage around the world and the Kobe lineage from Japan have been isolated from patients and are apparently pathogenic to humans (5, 7, 8). However, a patient(s) infected with another parasite, such as Hobetsu lineage (9), may emerge as a consequence of improved detection techniques and recent increased attention to emerging tick-borne diseases, such as severe fever with thrombocytopenia syndrome (SFTS), relapsing fever, anaplasmosis, and neoehrlichiosis (10–13).
The largest lineage of the B. microti group, the U.S. lineage, contains B. microti sensu stricto, a causative agent of human babesiosis in the northeastern and upper midwestern United States, where most of the human cases worldwide have been reported. Outside of the United States, a few autochthonous infections have been reported from Canada, Australia, and Germany (7, 8, 14, 15), and antibodies against the U.S. lineage in humans are evident widely in Germany (16), Austria (17), Belgium (18), Switzerland (19), and Mongolia (20). These findings indicate that the B. microti U.S. lineage parasites distributed worldwide are infectious to humans, but pathogenicity may vary among parasite populations. Recently the U.S. lineage parasites were demonstrated to be genetically diverse in β-tubulin and chaperonin containing TCP1 subunit eta (CCT7) gene sequences (21–23). Furthermore, parasites in the U.S. lineage phylogenetically grouped into distinct 3 sublineages, North America, Europe-Central Asia, and East Asia, each of which reflects the geographic origin of the parasites. The relationship between the genetic classification (or the sublineage) and pathogenicity remains unclear.
Identification of tick vectors is important, since they bridge the animal reservoir and humans. The vector's preference for humans (questing behavior), seasonal and regional abundance, geographical distribution, and transmission efficiency as a vector directly or indirectly affect the emergence of human cases (24–27). The B. microti Munich lineage, sequences of which have been detected in various species of rodents from Europe to Russia (summarized in Zamoto-Niikura et al. [6]), is regarded as a nonzoonotic pathogen partly because a nidicolous (nest-dwelling) tick, Ixodes trianguliceps, is considered the principal vector for this lineage (28), and this tick does not infest humans.
Since the B. microti U.S. lineage is distributed widely over the temperate zones of the Northern Hemisphere, species of ticks transmitting parasites of this lineage would be expected to vary. In the U.S., an extensive survey in areas where human babesiosis is endemic demonstrated Ixodes scapularis (formerly I. dammini) as the principal tick vector. The ecology of this tick is well understood. (i) Both nymphal and adult I. scapularis ticks carry the parasites, but nymphs have a higher infection rate and number of developed sporozoites in their salivary glands (29). This is one reason why the infection is usually transmitted through the bite of infected nymphs, although adult ticks occasionally transmit B. microti to humans. (ii) The maximum seasonal activity of I. scapularis nymphs, in May through July, is followed by human infections, which are diagnosed mainly in June, July, or August (27). (iii) The geographical extension of human babesiosis from the northeastern coastal region to inner and southern areas of the U.S. has been partially attributed to geographic expansion of I. scapularis and its deer host (27, 30).
In the Eurasian region where I. scapularis is absent, PCR surveillance and DNA sequence analyses of field-collected ticks have revealed that Ixodes persulcatus (31–33) and Ixodes ricinus (1, 34, 35) in Asia and Europe, respectively, carry the B. microti U.S. lineage. Although experimental transmission of B. microti U.S. lineage to hamsters or gerbils has been shown (33, 36), there is still a lack of biological evidence demonstrating the live pathogen in field-derived specimens. In this study, we provide direct evidence that I. persulcatus ticks carry the infectious B. microti U.S. lineage in their salivary glands. We successfully isolated the B. microti U.S. lineage from field-collected I. persulcatus females in Japan and established 4 strains. We show here the genetic and antigenic features of these strains compared to those previously reported in the U.S. and Europe.
MATERIALS AND METHODS
Field collection.
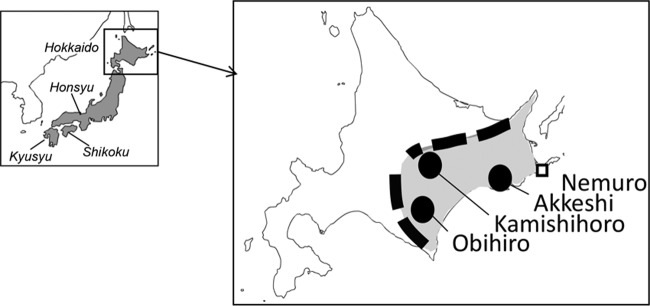
Unfed, host-seeking ticks were collected by flagging vegetation on the sides of forest paths, which were easily accessible by car and on foot. In Japan, B. microti U.S. lineage has been detected in I. persulcatus collected only in the eastern part of Hokkaido (6, 33); thus, 3 areas, including 7 sites, were selected for this study (Fig. 1). The duration times and number of tick collections were recorded at each site. The species identification of ticks was determined morphologically (body size, color, and shape of idiosoma and legs, shape of capitulum, and shape and size of internal spur of coxa I). Microscopically identified female ticks were kept in tightly sealed plastic tubes over a moistened filling of solidified plaster with activated charcoal at 4°C until infested on gerbils. Male ticks were frozen and kept at −30°C until DNAs were extracted.
FIG 1.
Map of Hokkaido, Japan, showing the locations of field collections (circles). The dashed line shows the eastern Hokkaido area and mountain ranges along the border of eastern Hokkaido. Nemuro (square) is the area in which the B. microti U.S. lineage was previously isolated from rodents.
Collection efficiency.
Tick collection efficiency was used to postulate how often wild animals carrying the ticks appeared at the survey site. The collection efficiency was the number of ticks collected divided by duration time (ticks/minute) (Table 1).
TABLE 1.
Ticks collected and screening of male and female adult I. persulcatus ticks for B. microti by nested PCR targeting β-tubulin gene
| Location | Value(s) for: |
|||||
|---|---|---|---|---|---|---|
| Ticks collection (all species) |
I. persulcatus screened for B. microti U.S. lineageg |
|||||
| No. collected | Timea (min) | No. of ticks/min | Total no. screened (no. male/no. female) | No. PCR positive (no. male/no. femaleb) | % of total (% male/% female) | |
| Akkeshi 1 | 387 | 160 | 2.4 | 111 (111/ND) | 1 (1/ND) | 0.9 (0.9/ND) |
| Akkeshi 2 | 67 | 40 | 1.7 | 11 (9/2c) | 1 (1/0) | 9.0 (11.1/0) |
| Kamishihoro 1 | 137 | 80 | 1.7 | 62 (47/16) | 6 (3/3d) | 9.7 (6.4/18.8) |
| Kamishihoro 2 | 35 | 60 | 0.6 | 20 (20/ND) | 0 (0/ND) | 0 (0.0/ND) |
| Obihiro 1 | 35 | 40 | 0.9 | 5 (5/ND) | 0 (0/ND) | 0 (0.0/ND) |
| Obihiro 2 | 88 | 60 | 1.5 | 28 (28/ND) | 0 (0/ND) | 0 (0/ND) |
| Obihiro 3 | 111 | 50 | 2.2 | 56 (33/23) | 9 (7/2e) | 15.3 (21.2/9.0) |
| Total | 860f | 490 | 1.8 | 294 (253/41) | 17 (12/5) | 5.8 (4.7/12.2) |
Time during which ticks were collected at each location.
Whole bodies and salivary glands of males and females, respectively, were used.
Only 2 female ticks were collected.
Two of three were successfully isolated in hamsters.
Two were isolated in hamsters.
I. persulcatus male (n = 253), female (n = 193), and nymph (n = 22); I. ovatus (n = 315) and Hemaphysalis spp. (n = 77).
ND, not determined.
Extraction of DNA from ticks.
DNA extractions were performed as described previously (33). Briefly, individual ticks were homogenized in TNE buffer (10 mM Tris-HCl, 100 mM NaCl, 0.1 mM EDTA, pH 8.0) containing 0.1% sodium dodecyl sulfate (SDS) and digested with proteinase K. DNAs were purified by phenol extraction followed by ethanol precipitation, and the DNA pellet was resuspended in 100 μl of Tris-EDTA (TE) buffer. All DNAs were stored frozen at −30°C until use.
PCR.
To detect B. microti U.S. lineage, lineage-specific PCR based on the β-tubulin gene sequence was performed as described previously (23), using 1 μl of DNA. Briefly, two sets of primers, BmTubu93F/BmTubu897R and Tubu-US5′/Tubu-US3′, were used for primary and nested PCR, respectively. Data collected by the lineage-specific PCR test were used to provide an estimate of infection prevalence. The infection rate (as a percentage) was calculated by comparing the number of PCR samples positive for B. microti group U.S. lineage to the total number of ticks examined.
Experimental animals.
Specific-pathogen-free golden hamsters (slc:Syrian) and gerbils (MON/Jms/GbsSlc) 8 weeks of age were purchased from SLC Inc. (Shizuoka, Japan). Animal experimentation was carried out according to the laboratory animal control guidelines of the National Institute of Infectious Diseases (institutional permission no. 213126 and 114014).
Isolation of B. microti from I. persulcatus.
Isolation of B. microti was done by inoculation of salivary glands as previously described (33). I. persulcatus females collected at the selected site (Obihiro, Akkeshi, and Kamishihoro) (Table 1) were fed on naive gerbils for 3 or 4 days to activate quiescent B. microti sporozoites in the salivary glands into a state of readiness for infection, and then the partially engorged ticks were removed manually. The salivary glands were dissected from the ticks under a stereomicroscope, and the glands from each individual tick were soaked in 500 μl of cold phosphate-buffered saline (PBS) and homogenized by a glass tissue grinder (Radnoti). An aliquot of 100 μl was used for DNA extraction and PCR screening as described above to predict the presence of the B. microti parasites. The remaining 400 μl of the homogenate was inoculated intraperitoneally into individual Syrian hamsters. The hamsters were monitored every 2 or 3 days for 3 months by determining the parasitemia in Giemsa-stained thin blood smears. When the parasitemia in the hamsters reached at least 10%, whole blood was collected by cardiac puncture. The red blood cells (RBCs) were washed 3 times by centrifugation in cold PBS and used to prepare DNA for sequencing, antigen for indirect fluorescent antibody (IFA) test, and antigen for Western blot analysis, and to produce antiserum to B. microti U.S. lineage in hamsters. For the latter, a portion of the washed RBCs was injected intravenously into naive hamsters, and convalescent-phase serum was collected 8 weeks postinfection and stored at −30°C until it was used as described below. It is noted that the isolation was attempted by inoculation of the salivary glands, since we failed to isolate parasites from female ticks by direct infestation (data not shown).
Absence of parasites in the hamsters was determined at 3 months after inoculation by PCR. Nested PCR targeting the 18S rRNA gene was performed on the DNA extracted from erythrocytes as described below.
Western blot analysis.
The antigens described above were separated by SDS-PAGE as described previously (5). The loading volume was compensated based on the percent parasitemia of the blood at harvest from hamsters to prepare erythrocyte antigens (5), except for isolate IpSG13-1-2. Since the parasitemia in the hamster infected with IpSG13-1-2 was 10%, which was the lowest level among the isolates, and since cross-reactivity was relatively low in preliminary experiments, the IpSG13-1-2 sample was loaded at twice the calculated volume. The separated proteins were blotted onto Immobilon-P polyvinylidene difluoride (PVDF) membranes (Millipore), and the membranes were blocked with 5% skim milk in Tris-buffered saline with 0.1% Tween 20. The membranes were incubated first with antisera diluted 1:200 and subsequently with goat polyclonal antibody to Syrian hamster IgG H&L (alkaline phosphatase) (Abcam). Immunoreactive antigens were detected using the 5 bromo-4-chloro-3-indolylphosphate-nitroblue tetrazolium alkaline phosphatase (NBT-BCIP) liquid substrate system (Sigma). IpSG13-10-2 antiserum was used as a representative of antisera raised against B. microti U.S. lineage isolated from I. persulcatus in this study. For negative controls, antigens were prepared from two noninfected hamsters, which had been inoculated with piroplasma-free salivary glands from I. persulcatus females.
IFA tests.
Infected RBCs were mixed with an equal volume of fetal bovine serum, and thin blood smears were made on microscope slides. The slides were transferred into double-distilled water (DDW) to lyse the RBCs and air dried. Hydrophobic circles, used to form wells, were drawn on the slides using a PAP pen (Super PAP pen; Daido, Tokyo, Japan). The slides were placed in a humidified chamber, and 20 μl of serial 2-fold dilutions of serum was added to each well. After 1 h of incubation at 37°C, the slides were washed in PBS, and 20 μl of Alexa Fluor488-conjugated goat anti-hamster IgG (H+L) (Life Technologies) diluted 1:200 in Tris-buffered saline containing 5% Immunoblock (DS Pharma) and Tween 20 was added. The slides were incubated at room temperature for 1 h and washed in PBS. Fluorescent parasites in RBCs were observed with a fluorescence microscope (Olympus IX71) at a magnification of ×200.
Amplification and direct sequencing of 18S rRNA, β-tubulin, and CCT7 gene sequences.
PCR was performed using 1 μl of DNA and primer pairs Piro0F/6R (4), TubuATG5F/Tubu1538R (2), and UScct-1/UScct-4 (22) to amplify 18S rRNA, β-tubulin, and CCT7 gene sequences, respectively. For CCT7, the PCR primer set UScct-2/UScct-3 (22) was used for nested PCR when the primary PCR amplicons were insufficient for direct sequence analysis.
Specific amplicons were subjected to cycle sequencing using the PCR primers and BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems). For CCT7, additional primers UScct-sq1 (5′GCTGCTACGATGCGCGGAAACGACAC3′), UScct-sq2 (5′GAATGCCCTAATACAAAGACCGCAACUSCCT3′), USCCT-sq3R (5′CCTCAGATAATAATCAAATACTATAGGGAAGC3′), and USCCT-sq4R (5′GATGAAATTGATATTAAGCGAGTGGCCAAAG3′) were used for direct sequencing. Sequencing was performed using a 3130 genetic analyzer (Applied Biosystems). All sequences determined in this study were deposited in the DNA Data Bank of Japan (DDBJ). The sequences have been released to the public and are available through GenBank.
Phylogenetic analysis.
The sequences for each gene were aligned with the program CLUSTAL W 2.1 Alignment (http://clustalw.ddbj.nig.ac.jp/) with default settings. Phylogenetic trees were constructed by the neighbor-joining method in the program with 1,000 bootstrap replicates.
Reference parasite strains.
B. microti Gray (37), GI (PRA-398; ATCC), and Nan-H5-2011 (PRA-399; ATCC) strains were used as the northeastern United States B. microti representative. NM69 and AK2273 are strains isolated from Myodes rufocanus and Apodemus speciosus, captured in Nemuro and Akkeshi, Hokkaido, Japan, respectively (Fig. 1) (38).
Accession number(s).
All sequences determined in this study were deposited in the DNA Data Bank of Japan (DDBJ) under accession numbers LC127369 (IpSG13-1-2), LC127370 (IpSG13-10-2), LC127371 (IpSG13-16-2), and LC127372 (IpSG13-18-1) for the 18S rRNA gene; LC127373 (IpSG13-1-2), LC127374 (IpSG13-10-2), LC127375 (IpSG13-16-2), and LC127376 (IpSG13-18-1) for the β-tubulin gene; and LC127377 (IpSG13-1-2), LC127378 (IpSG13-10-2), LC127379 (IpSG13-16-2), and LC127380 (IpSG13-18-1) for CCT7.
RESULTS
Tick collection and PCR screening.
In total, 860 ticks, including Ixodes and Haemaphysalis spp., were collected at 7 sites in 3 areas (Fig. 1 and Table 1). The numbers collected and the amount of time at each survey site are summarized in Table 1. Among all ticks collected, 315 were I. ovatus adults, 253 were I. persulcatus males, and 193 were I. persulcatus females.
Collection efficiency (ticks/minute) varied among the sites (Table 1). Ticks were efficiently collected at Akkeshi 1, Obihiro 3, Akkeshi 2, and Kamishihoro 1 (2.4, 2.2, 1.7 and 1.7 ticks/min, respectively). Collection efficiency at Akkeshi 1 (2.4 ticks/min) was 4 times greater than the lowest efficiency (0.6 ticks/min) at site Kamishihoro 2.
I. persulcatus adult males and females were screened by β-tubulin gene nested PCR for B. microti (Table 1), and infection rates were calculated for I. persulcatus males based on the PCR results. I. persulcatus males positive for B. microti U.S. lineage were found in 4 sites, Akkeshi 1 and 2, Kamishihoro 1, and Obihiro 3, and infection rates ranged from 0.9% at Akkeshi 1 to 21.2% at Obihiro 3 (Table 1). Judging from the infection rate in male ticks and the collection efficiency at each site, females collected in Akkeshi 2, Kamishihoro 1, and Obihiro 3 were selected for parasite isolation. Although Akkeshi 1 had the highest collection efficiency, the infection rate was low (0.9%). Thus, female ticks collected at Akkeshi 1 were not used for parasite isolation. B. microti U.S. lineage was not detected in any of the 315 I. ovatus ticks examined.
Isolation of B. microti U.S. lineage from I. persulcatus.
I. persulcatus females collected at Obihiro 3 were kept at 4°C for 3 to 7 weeks and then fed on gerbils. Out of 23 ticks examined, the salivary glands from 2 ticks, designated IpSG13-1-2 and IpSG13-10-2, were PCR positive (Tables 1 and 2). Two hamsters were inoculated individually with the salivary glands IpSG13-1-2 or IpSG13-10-2 and developed parasitemia 18 and 26 days after inoculation, respectively (Table 2). Hamsters (n = 21) inoculated individually with PCR-negative salivary glands from the remaining 21 ticks remained uninfected for 3 months.
TABLE 2.
Incubation of I. persulcatus and isolation of B. microti
| Salivary gland |
I. persulcatus |
Day of isolation from hamsterb | |
|---|---|---|---|
| Origin of tick | Storagea (wk) | ||
| IpSG13-1-2 | Obihiro 3 | 3 | 18 |
| IpSG13-10-2 | Obihiro 3 | 7 | 26 |
| IpSG13-16-3 | Kamishihoro 1 | 11 | 46 |
| IpSG13-18-1 | Kamishihoro 1 | 19 | 52 |
| IpSG13-19-1 | Kamishihoro 1 | 19 | Failed |
Period during which the ticks were stored at 4°C until used for inoculation.
Number of days until detection of parasites in hamster erythrocytes after salivary gland inoculation.
I. persulcatus females collected at Kamishihoro 1 were kept at 4°C for 11 to 19 weeks (Table 2). Out of 15 examined, the salivary glands from 3 ticks, designated IpSG13-16-3, IpSG13-18-1, and IpSG13-19-1, were PCR positive (Tables 1 and 2). Two hamsters individually inoculated with IpSG13-16-3 and IpSG13-18-1 developed parasitemia 46 and 52 days after inoculation, respectively (Table 2). One hamster inoculated with PCR-positive IpSG13-19-1 salivary glands and 12 hamsters individually inoculated with PCR-negative salivary glands did not develop parasitemia for 3 months after inoculation. Lack of infection in the 13 hamsters was confirmed by nested PCR on peripheral blood samples.
At Akkeshi 2, two I. persulcatus females were collected and examined (Table 2). The salivary glands from 2 ticks were PCR negative, and hamsters individually inoculated with the salivary glands did not develop parasitemia for 3 months after inoculation.
In summary, 4 strains of B. microti U.S. lineage, designated IpSG13-1-2, IpSG13-10-2, IpSG13-16-2, and IpSG13-18-1, were isolated from hamsters by inoculation of infected salivary glands from I. persulcatus ticks (Tables 1 and 2; see also Fig. 6).
FIG 6.
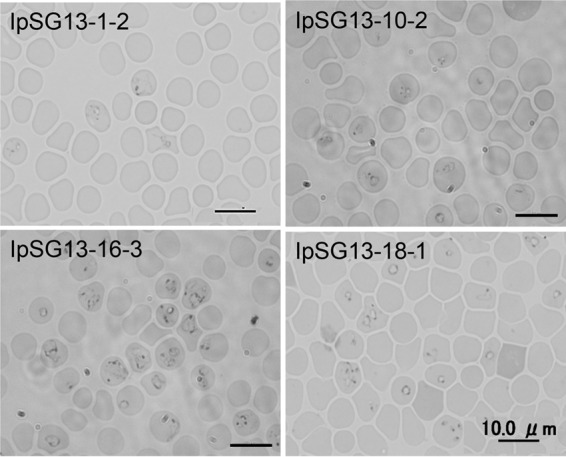
Intraerythrocytic B. microti U.S. lineage parasites in hamster blood isolated from I. persulcatus in Japan. Giemsa stain was used. Bar, 10 μm.
Sequencing analysis of 18S rRNA, β-tubulin, and CCT7 genes.
The 18S rRNA, β-tubulin, and CCT7 genes were successfully amplified from the 4 isolates designated IpSG13-1-2, IpSG13-10-2, IpSG13-16-2, and IpSG13-18-1. For all genes, nucleotide alignments revealed that the sequences from all 4 isolates were identical to each other and to those from B. microti NM69 and AK2273 strains isolated from rodent reservoirs, Myodes rufocanus and Apodemus speciosus, respectively, in Japan (38).
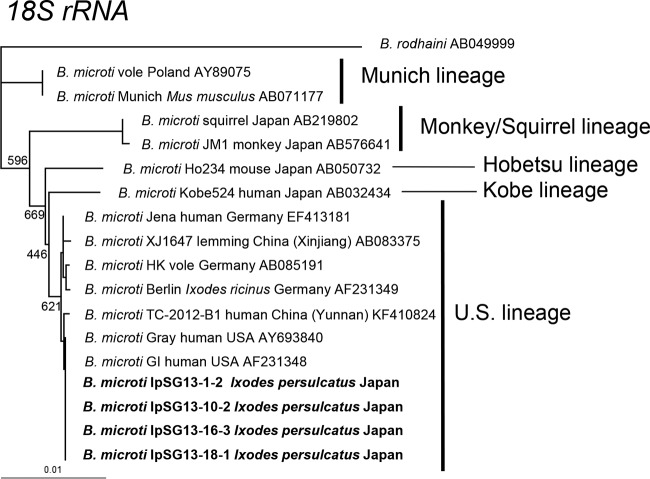
The phylogenetic tree based on the B. microti group 18S rRNA gene was constructed using sequences obtained in this study and sequences available in GenBank (Fig. 2). The B. microti 18S rRNA gene sequences from I. persulcatus were identical to those from humans in the United States (Gray and GI strains) and were placed in the U.S. lineage cluster with those from I. ricinus tick, human, and vole in Germany and from lemming in Xingiang, China (Fig. 2).
FIG 2.
Phylogenetic tree constructed with sequences of the 18S rRNA genes of the B. microti U.S. lineage and other closely related parasites. GenBank accession numbers are given for each strain. Strains for which sequences were determined in this study are shown in boldface. The number on each branch shows the percent occurrence in 1,000 bootstrap replicates. Accession numbers of B. microti IpSG13-1-2, IpSG13-10-2, IpSG13-16-3, and IpSG13-18-1 are LC127369 to LC127372, respectively.
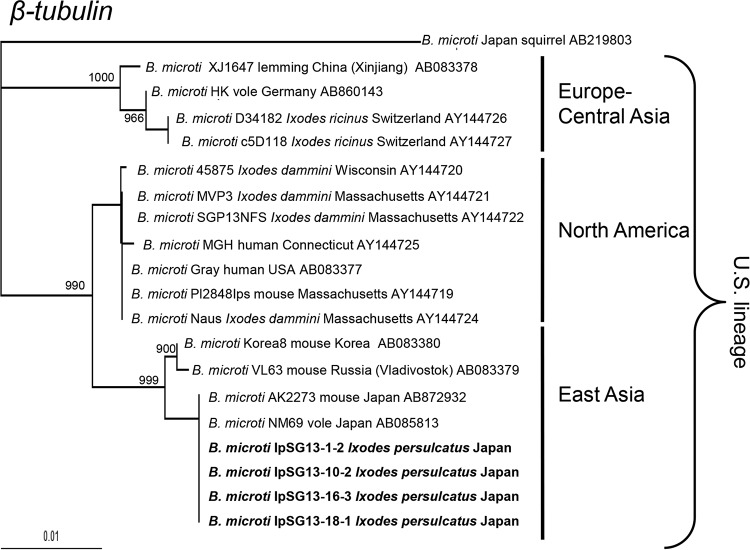
The phylogenetic trees based on the β-tubulin and CCT7 genes were similarly constructed (Fig. 3 and 4) and showed similar topology to each other with high bootstrap values. The B. microti U.S. lineage sequences branched into 3 sublineages, namely, Europe-Central Asia, East Asia, and North America, as shown previously (6, 21, 22). The isolates from I. persulcatus all were placed in the East Asia sublineage, whereas isolates from I. scapularis and I. ricinus ticks belonged to North America and Europe-Central Asia sublineages, respectively (Fig. 3).
FIG 3.
Phylogenetic tree constructed with sequences of the β-tubulin gene of B. microti U.S. lineage. GenBank accession numbers are given for each strain. Strains for which sequences were determined in this study are shown in boldface. The number on each branch shows the percent occurrence in 1,000 bootstrap replicates. Accession numbers of B. microti IpSG13-1-2, IpSG13-10-2, IpSG13-16-3, and IpSG13-18-1 are LC127373 to LC127376, respectively.
FIG 4.
Phylogenetic tree constructed with sequences of the CCT7 genes of B. microti U.S. lineage. GenBank accession numbers are given for each strain. Strains for which sequences were determined in this study are shown in boldface. The number on each branch shows the percent occurrence in 1,000 bootstrap replicates. Accession numbers of B. microti IpSG13-1-2, IpSG13-10-2, IpSG13-16-3, and IpSG13-18-1 are LC127377 to LC127380, respectively.
Antigenic analysis.
To examine the antigenic relationship among the U.S. lineage parasites isolated from I. persulcatus, from rodents (NM69) in Japan, and from humans in the United States (Gray, GI, and Nan-H5-2011), IFA tests were carried out for pairwise comparison. All 4 strains isolated from I. persulcatus showed identical antigenicities; therefore, results for I. persulcatus IpSG13-10-2 are shown as representative (Table 3). The NM69 and IpSG13-10-2 antisera showed high titers of 12,800 against both the NM69 and IpSG13-10-2 parasite antigens (Table 3). Similarly, the United States Gray, GI, and Nan-H5-2011 antisera showed high titers of 12,800 against both homologous and heterologous U.S. parasite antigens for Gray, GI, and Nan-H5-2011 (Table 3). Lower titers resulted with the Japanese NM69 and IpSG 13-10-2 antisera and U.S. Gray, GI, and Nan-H5-2011 antigen and with the U.S. Gray, GI, and Nan-H5-2011 antisera reacted with the Japanese NM69 and IpSG13-10-2 antigen (Table 3). The IpSG 13-10-2 antiserum titers were 1:400 to 1:800 against the Gray, GI, and Nan-H5-2011 strains, which were 16 to 32 times lower than those of the U.S. strain homologous antisera (1:12,800).
TABLE 3.
Results of IFA test with B. microti U.S. lineage parasites
| Seruma | Reciprocal titer against parasite (sublineage): |
||||
|---|---|---|---|---|---|
| IpSG13-10-2 (East Asia) | NM69 (East Asia) | Gray (North America) | Nan-H5-2011 (North America) | GI (North America) | |
| Anti-IpSG13-10-2 | 12,800 | 12,800 | 800 | 800 | 400 |
| Anti-NM69 | 12,800 | 12,800 | 3,200 | 800 | 400 |
| Anti-Gray | 800 | 800 | 12,800 | 12,800 | 12,800 |
| Anti-Nan-H5-2011 | 3,200 | 1,600 | 12,800 | 12,800 | 12,800 |
| Anti-GI | 1,600 | 1,600 | 12,800 | 12,800 | 12,800 |
Convalescent-phase sera from infected hamsters.
Similar results were also obtained in the Western blot analysis (Fig. 5). As in the IFA, the antisera for the 4 strains from I. persulcatus (IpSG13-1-2, IpSG13-10-2, IpSG13-16-2, and IpSG13-18-1) all showed similar reactivity, so anti-B. microti IpSG 13-10-2 is shown as representative in Fig. 5. Among the isolates from I. persulcatus (IpSG13-1-2, IpSG13-10-2, IpSG13-16-2, and IpSG13-18-1) and rodent (NM69 and AK2273) in Japan, many immunodominant antigens were recognized by both B. microti IpSG 13-10-2 and B. microti NM69 antisera irrespective of the isolates. However, the heterologous antiserum was less cross-reactive between the Japanese and United States isolate antigens (Fig. 5).
FIG 5.
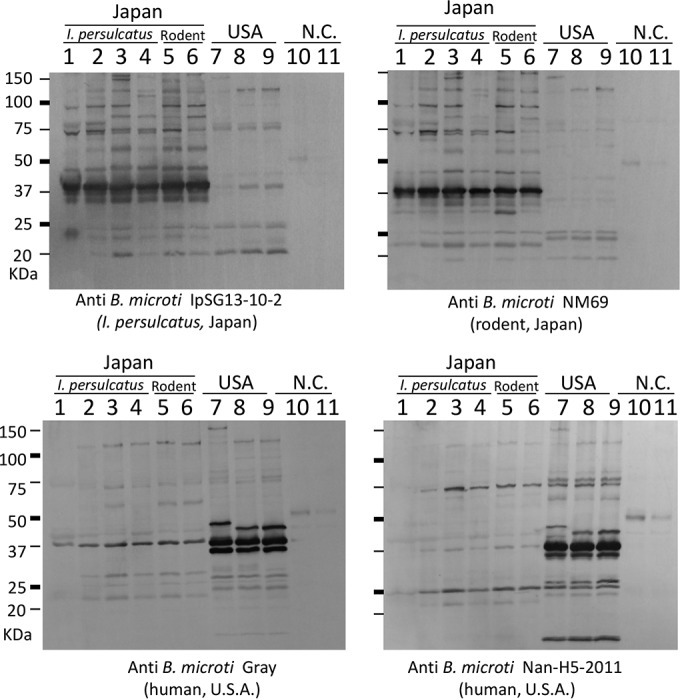
Western blot analysis with the B. microti U.S. lineage isolated from I. persulcatus (IpSG13-1-2, IpSG13-10-2, IpSG13-16-3, and IpSG13-18-1; lanes 1 to 4, respectively), rodents in Japan (NM69 and AK2273; lanes 5 and 6, respectively), and patients in the United States (Gray, GI, and Nan-H5-2011; lanes 7 to 9, respectively). Erythrocytes from noninfected hamsters were used as negative controls (N.C.; lanes 10 and 11). Parasite antigens were detected with anti-IpSG13-10-2 hamster serum (upper left), anti-NM69 hamster serum (upper right), anti-Gray hamster serum (lower left), and anti-Nan-H5-2011 serum (lower right).
In all three strains from the United States, three major antigens (25 to 50 kDa) were recognized by anti-B. microti Gray antisera, only one of which reacted strongly in the Japanese parasites (Fig. 5, lower left). The largest of the three major antigens migrated slower in the Gray strain than those from GI and Nan-H5-2011. This same result was observed with anti-B. microti Nan-H5-2011(Fig. 5, lower right). The reactivity of anti-B. microti GI showed results similar to those of anti-B. microti Nan-H5-2011 (not shown).
DISCUSSION
In this study, we successfully isolated and established B. microti U.S. lineage strains from I. persulcatus ticks and characterized the parasites for the first time (Fig. 6). The isolation was achieved by selection of I. persulcatus females collected in areas where B. microti is highly endemic, allowing the salivary gland sporozoites to mature to the infective state, and then inoculating the infected salivary glands into hamsters. A previous study demonstrated that I. persulcatus was a competent vector for B. microti U.S. lineage under experimental conditions where larval ticks were fed on infected hamsters and nymphs then transmitted B. microti to naive hamsters. The current study proves that B. microti sporozoites in the salivary glands of field-collected I. persulcatus ticks mature to the infective, transmissible stage, resulting in parasitemia in inoculated hamsters. Together with our previous studies (6, 33), the biological isolation and molecular characterization of the B. microti U.S. lineage in this study further supports that I. persulcatus is a competent vector for the B. microti U.S. lineage in Japan.
In the IFA test, the antiserum to strains of U.S. lineage isolated in Japan and the United States showed higher titers against the homologous than heterologous antigen. Furthermore, Western blot analysis showed antibodies raised against Japanese B. microti showed slight reactivity to all three B. microti strains from the United States. These results correspond to our previous findings (38). Serum from rodents captured in Korea and far eastern Russia detected Japanese B. microti strain NM69 in the IFA test but not the B. microti Gray strain from the United States (unpublished data), even though these rodents were genetically proven to be infected with the U.S. lineage (East Asian sublineage) (Fig. 3 and 4). Our results suggest that major antigenic cross-reactivities exist within the same sublineages only. In this regard, the commercially available B. microti IFA kits, which have been used worldwide for serological identification of B. microti infection (16, 18, 20), are based on antigen from parasites isolated in the United States. Thus, diagnostic serological testing outside the United States using these kits may underestimate or even fail to detect antibody titers in patients, and those who have low antibody titers may be disregarded. Therefore, it may be prudent to use parasites locally isolated or in the same sublineages as antigen for serological diagnostics or studies in different geographic locations.
At all survey sites where I. persulcatus ticks were collected, the sympatric tick I. ovatus was also collected. However, unlike I. persulcatus, in which PCR detected the B. microti U.S. lineage, all sympatric I. ovatus ticks tested were negative. This specific I. persulcatus-U.S. lineage relationship was observed in previous studies in Japan (33) and Russia (31). In the Novosibirsk region in Russia, where the B. microti U.S. lineage is distributed in voles, mice, and shrews, I. persulcatus ticks are found to carry U.S. lineages. However, in the Sverdlovsk region where the Munich lineage in the B. microti group (Fig. 2) is distributed in the same reservoir host species, I. persulcatus ticks do not carry the Munich lineage. These results indicate the B. microti U.S. lineage is transmitted specifically by I. persulcatus in Japan, Russia, and presumably other areas in northern Eurasia where I. persulcatus is dominantly distributed.
In Europe, where I. persulcatus and I. scapularis are absent, I. ricinus is supported as a vector for the B. microti U.S. lineage based on PCR detection in field-collected ticks in Germany, Switzerland, and Slovenia (1, 34, 35) and transmission studies in gerbils (36). Thus, the parasites in the U.S. lineage worldwide exist in nature in enzootic cycles involving ticks belonging to the I. ricinus species complex, and Europe-Central Asian, East Asian, and North American sublineages are specifically associated with I. ricinus, I. persulcatus, and I. scapularis (formerly I. dammini), respectively (Fig. 3). This corresponds with the overlapping distribution of the B. microti U.S. lineage parasites (Europe-Central Asia, East Asia, and North American sublineages) and the vector tick species (I. ricinus, I. persulcatus, and I. scapularis, respectively). Investigation in the countries where I. ricinus and I. persulcatus sympatry occur (39), such as Estonia (40), Latvia (41), Finland, Russia (42), and Poland (43), may elucidate the vector competency in nature for transmitting B. microti U.S. lineage parasites on the Eurasian continent.
Careful selection of I. persulcatus ticks, which were collected at rodent-enriched sites where B. microti is highly endemic, was crucial for efficient parasite isolation in this study (Tables 1 and 2). We timed the collection and counted the number of ticks collected at each site. Although sites in the same area were less than a few kilometers apart, they are different in habitat, such as streams and vegetation. Although the number of ticks collected varied among the sites, we could indirectly know how frequently wild animals dropped ticks at each site by calculating collection efficiency as the number of ticks collected per minute (tick density) (Table 1). We had speculated that B. microti could be maintained well in the area where the rodent reservoir and ticks were densely distributed. As we expected, B. microti PCR-positive I. persulcatus males were collected in sites with high collection efficiencies, Akkeshi 1 and 2, Kamishihoro 1, and Obihiro 3 (Table 1). Interestingly, at Akkeshi 1, which had the highest collection efficiency among all sites, only 1 out of 111 I. persulcatus males was PCR positive for B. microti. The B. microti parasite detected in the questing adult tick would have originated from the animal on which the tick fed as a nymph, the previous tick stage. Thus, the ticks collected at the Akkeshi 1 site might have fed on nonreservoir animals, such as deer, raccoon dog, fox, and mink, in their previous stage. Examination of the blood meal remnants in the collected ticks (44) may be useful to identify the source of the previous tick blood meal and to select specimens for parasite isolation in future endeavors.
Among five attempts at parasite isolation in hamsters using tick salivary glands positive for B. microti, four were successful and one failed (Tables 1 and 2). The prepatent period until the parasites were detected at >0.05% parasitemia in the hamsters varied. Isolate IpSG 13-1-2 parasites emerged in the hamsters as early as 18 days after injection of B. microti-positive salivary glands, while IpSG 13-18-1 took 52 days. In our study, there is a direct correlation between the length of storage at 4°C and the length of time before intraerythrocytic parasites are observed in the hamsters. Ticks IpSG13-18-1 and IpSG13-19-1 were both kept at 4°C for 19 weeks before use, and the hamster injected with B. microti-positive salivary glands from IpSG13-19-1 never developed parasitemia throughout the period of this study. Thus, about 19 weeks of storage at 4°C was the threshold for isolating B. microti in hamsters using infected tick salivary glands in this study.
The refrigerated storage period of the ticks might influence parasite activity and survival in the salivary glands. If the infectious parasites in the salivary glands were gradually inactivated and/or reduced in number as I. persulcatus ticks were kept longer at 4°C, it likely would influence how long before the parasite emerged in the hamster RBC. It was reported that hamsters intraperitoneally infected with 25,000 salivarian parasites (sporozoites) from I. scapularis (formerly I. dammini) developed B. microti infection, but an inoculum of 10,000 was ineffective (45), which suggests that a minimum number of surviving parasites was important in the current study. In nature, a similar reduction of parasites in ticks may occur over time. In our studies, B. microti-positive ticks have been abundant in early spring rather than in late spring to early summer (unpublished data). A seasonal prevalence in the infection rate of I. scapularis by B. microti was also observed in ticks collected on Nantucket Island, Massachusetts (26).
I. persulcatus is a major tick species causing human tick bites and is an important vector of many tick-borne diseases, including human Lyme borreliosis (Borrelia afzelii and B. garinii), relapsing fever (B. miyamotoi), tick-borne encephalitis virus (TBEV; far eastern and Siberian subtypes), and neoehrlichiosis (“Candidatus Neoehrlichia mikurensis”) in Russia and in northern regions of China and Japan where the tick is abundant (46–50). Human babesiosis caused by B. microti U.S. lineage has not been clearly documented in these areas, with the exception of China (51).
The study areas are at the feet of mountains and not far from residential areas. In fact, we sometimes found people collecting edible wild plants around our study sites during our field surveys. Inasmuch as B. microti-infected ticks were at these sites, we suspect such persons are at risk of acquiring B. microti infections through tick bites. Human babesiosis has been recognized occasionally by severe infection in asplenic patients and by infection through contaminated blood products from asymptomatic donors (37, 52). Large-scale surveys should be conducted for risk assessment, such as serological screenings for B. microti U.S. lineage in patients with febrile and/or undiagnosed illness as well as in healthy residents who live around I. persulcatus habitats.
ACKNOWLEDGMENTS
We thank Haruyuki Hirata (Rakuno Gakuen University) for his assistance.
This work was partly supported by grants in aid from the Ministry of Health, Labor and Welfare (H25-Shinko-Ippan-008 and H25-Shinko-Shitei-009) and Japan Agency for Medical Research and Development (16768907).
REFERENCES
- 1.Goethert HK, Telford SR III. 2003. What is Babesia microti? Parasitology 127:301–309. doi: 10.1017/S0031182003003822. [DOI] [PubMed] [Google Scholar]
- 2.Hirata H, Kawai S, Maeda M, Jinnai M, Fujisawa K, Katakai Y, Hikosaka K, Tanabe K, Yasutomi Y, Ishihara C. 2011. Identification and phylogenetic analysis of Japanese macaque Babesia-1 (JM-1) detected from a Japanese macaque (Macaca fuscata fuscata). Am J Trop Med Hyg 85:635–638. doi: 10.4269/ajtmh.2011.11-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsuji M, Wei Q, Zamoto A, Morita C, Arai S, Shiota T, Fujimagari M, Itagaki A, Fujita H, Ishihara C. 2001. Human babesiosis in Japan: epizootiologic survey of rodent reservoir and isolation of new type of Babesia microti-like parasite. J Clin Microbiol 39:4316–4322. doi: 10.1128/JCM.39.12.4316-4322.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsuji M, Zamoto A, Kawabuchi T, Kataoka T, Nakajima R, Asakawa M, Ishihara C. 2006. Babesia microti-like parasites detected in Eurasian red squirrels (Sciurus vulgaris orientis) in Hokkaido, Japan. J Vet Med Sci 68:643–646. doi: 10.1292/jvms.68.643. [DOI] [PubMed] [Google Scholar]
- 5.Wei Q, Tsuji M, Zamoto A, Kohsaki M, Matsui T, Shiota T, Telford SR III, Ishihara C. 2001. Human babesiosis in Japan: isolation of Babesia microti-like parasites from an asymptomatic transfusion donor and from a rodent from an area where babesiosis is endemic. J Clin Microbiol 39:2178–2183. doi: 10.1128/JCM.39.6.2178-2183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zamoto-Niikura A, Tsuji M, Qiang W, Hirata H, Nakajima R, Morikawa S, Holman PJ, Ishihara C. 2015. Survey and molecular analysis of Babesia microti group parasites in Japan: strategy and surveying for identification of tick vectors, p 197–213. In Epidemiology II–theory, research and practice, 1st ed iConcept Press Ltd, Brisbane, Australia. [Google Scholar]
- 7.Hildebrandt A, Hunfeld KP, Baier M, Krumbholz A, Sachse S, Lorenzen T, Kiehntopf M, Fricke HJ, Straube E. 2007. First confirmed autochthonous case of human Babesia microti infection in Europe. Eur J Clin Microbiol Infect Dis 26:595–601. doi: 10.1007/s10096-007-0333-1. [DOI] [PubMed] [Google Scholar]
- 8.Senanayake SN, Paparini A, Latimer M, Andriolo K, Dasilva AJ, Wilson H, Xayavong MV, Collignon PJ, Jeans P, Irwin PJ. 2012. First report of human babesiosis in Australia. Med J Aust 196:350–352. doi: 10.5694/mja11.11378. [DOI] [PubMed] [Google Scholar]
- 9.Arai S, Tsuji M, Kaiho I, Murayama H, Zamoto A, Wei Q, Okabe N, Kamiyama T, Ishihara C. 2003. Retrospective seroepidemiological survey for human babesiosis in an area in Japan where a tick-borne disease is endemic. J Vet Med Sci 65:335–340. doi: 10.1292/jvms.65.335. [DOI] [PubMed] [Google Scholar]
- 10.Li H, Zheng YC, Ma L, Jia N, Jiang BG, Jiang RR, Huo QB, Wang YW, Liu HB, Chu YL, Song YD, Yao NN, Sun T, Zeng FY, Dumler JS, Jiang JF, Cao WC. 2015. Human infection with a novel tick-borne Anaplasma species in China: a surveillance study. Lancet Infect Dis 15:663–670. doi: 10.1016/S1473-3099(15)70051-4. [DOI] [PubMed] [Google Scholar]
- 11.Platonov AE, Karan LS, Kolyasnikova NM, Makhneva NA, Toporkova MG, Maleev VV, Fish D, Krause PJ. 2011. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg Infect Dis 17:1816–1823. doi: 10.3201/eid1710.101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welinder-Olsson C, Kjellin E, Vaht K, Jacobsson S, Wenneras C. 2010. First case of human “Candidatus Neoehrlichia mikurensis” infection in a febrile patient with chronic lymphocytic leukemia. J Clin Microbiol 48:1956–1959. doi: 10.1128/JCM.02423-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, Sun YL, Zhang L, Zhang QF, Popov VL, Li C, Qu J, Li Q, Zhang YP, Hai R, Wu W, Wang Q, Zhan FX, Wang XJ, Kan B, Wang SW, Wan KL, Jing HQ, Lu JX, Yin WW, Zhou H, Guan XH, Liu JF, Bi ZQ, Liu GH, Ren J, Wang H, Zhao Z, Song JD, He JR, Wan T, Zhang JS, Fu XP, Sun LN, Dong XP, Feng ZJ, Yang WZ, Hong T, Zhang Y, Walker DH, Wang Y, Li DX. 2011. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med 364:1523–1532. doi: 10.1056/NEJMoa1010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bullard JM, Ahsanuddin AN, Perry AM, Lindsay LR, Iranpour M, Dibernardo A, Van Caeseele PG. 2014. The first case of locally acquired tick-borne Babesia microti infection in Canada. Can J Infect Dis Med Microbiol 25:e87–e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paparini A, Senanayake SN, Ryan UM, Irwin PJ. 2014. Molecular confirmation of the first autochthonous case of human babesiosis in Australia using a novel primer set for the beta-tubulin gene. Exp Parasitol 141:93–97. doi: 10.1016/j.exppara.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Hunfeld KP, Lambert A, Kampen H, Albert S, Epe C, Brade V, Tenter AM. 2002. Seroprevalence of Babesia infections in humans exposed to ticks in midwestern Germany. J Clin Microbiol 40:2431–2436. doi: 10.1128/JCM.40.7.2431-2436.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sonnleitner ST, Fritz J, Bednarska M, Baumgartner R, Simeoni J, Zelger R, Schennach H, Lass-Florl C, Edelhofer R, Pfister K, Milhakov A, Walder G. 2014. Risk assessment of transfusion-associated babesiosis in Tyrol: appraisal by seroepidemiology and polymerase chain reaction. Transfusion 54:1725–1732. doi: 10.1111/trf.12606. [DOI] [PubMed] [Google Scholar]
- 18.Lempereur L, Shiels B, Heyman P, Moreau E, Saegerman C, Losson B, Malandrin L. 2015. A retrospective serological survey on human babesiosis in Belgium. Clin Microbiol Infect 21:e91–e97. [DOI] [PubMed] [Google Scholar]
- 19.Foppa IM, Krause PJ, Spielman A, Goethert H, Gern L, Brand B, Telford SR III. 2002. Entomologic and serologic evidence of zoonotic transmission of Babesia microti, eastern Switzerland. Emerg Infect Dis 8:722–726. doi: 10.3201/eid0807.010459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong SH, Anu D, Jeong YI, Abmed D, Cho SH, Lee WJ, Lee SE. 2014. Molecular detection and seroprevalence of Babesia microti among stock farmers in Khutul City, Selenge Province, Mongolia. Korean J Parasitol 52:443–447. doi: 10.3347/kjp.2014.52.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujisawa K, Nakajima R, Jinnai M, Hirata H, Zamoto-Niikura A, Kawabuchi-Kurata T, Arai S, Ishihara C. 2011. Intron sequences from the CCT7 gene exhibit diverse evolutionary histories among the four lineages within the Babesia microti-group, a genetically related species complex that includes human pathogens. Jpn J Infect Dis 64:403–410. [PubMed] [Google Scholar]
- 22.Nakajima R, Tsuji M, Oda K, Zamoto-Niikura A, Wei Q, Kawabuchi-Kurata T, Nishida A, Ishihara C. 2009. Babesia microti-group parasites compared phylogenetically by complete sequencing of the CCTeta gene in 36 isolates. J Vet Med Sci 71:55–68. doi: 10.1292/jvms.71.55. [DOI] [PubMed] [Google Scholar]
- 23.Zamoto A, Tsuji M, Wei Q, Cho SH, Shin EH, Kim TS, Leonova GN, Hagiwara K, Asakawa M, Kariwa H, Takashima I, Ishihara C. 2004. Epizootiologic survey for Babesia microti among small wild mammals in northeastern Eurasia and a geographic diversity in the beta-tubulin gene sequences. J Vet Med Sci 66:785–792. doi: 10.1292/jvms.66.785. [DOI] [PubMed] [Google Scholar]
- 24.Gray J, Zintl A, Hildebrandt A, Hunfeld KP, Weiss L. 2010. Zoonotic babesiosis: overview of the disease and novel aspects of pathogen identity. Ticks Tick Borne Dis 1:3–10. doi: 10.1016/j.ttbdis.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Herwaldt BL, McGovern PC, Gerwel MP, Easton RM, MacGregor RR. 2003. Endemic babesiosis in another eastern state: New Jersey. Emerg Infect Dis 9:184–188. doi: 10.3201/eid0902.020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piesman J, Mather TN, Dammin GJ, Telford SR III, Lastavica CC, Spielman A. 1987. Seasonal variation of transmission risk of Lyme disease and human babesiosis. Am J Epidemiol 126:1187–1189. [DOI] [PubMed] [Google Scholar]
- 27.Spielman A, Wilson ML, Levine JF, Piesman J. 1985. Ecology of Ixodes dammini-borne human babesiosis and Lyme disease. Annu Rev Entomol 30:439–460. doi: 10.1146/annurev.en.30.010185.002255. [DOI] [PubMed] [Google Scholar]
- 28.Bown KJ, Lambin X, Telford GR, Ogden NH, Telfer S, Woldehiwet Z, Birtles RJ. 2008. Relative importance of Ixodes ricinus and Ixodes trianguliceps as vectors for Anaplasma phagocytophilum and Babesia microti in field vole (Microtus agrestis) populations. Appl Environ Microbiol 74:7118–7125. doi: 10.1128/AEM.00625-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piesman J, Karakashian SJ, Lewengrub S, Rudzinska MA, Spielmank A. 1986. Development of Babesia microti sporozoites in adult Ixodes dammini. Int J Parasitol 16:381–385. doi: 10.1016/0020-7519(86)90118-9. [DOI] [PubMed] [Google Scholar]
- 30.Eskow ES, Krause PJ, Spielman A, Freeman K, Aslanzadeh J. 1999. Southern extension of the range of human babesiosis in the eastern United States. J Clin Microbiol 37:2051–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rar VA, Epikhina TI, Livanova NN, Panov VV. 2011. Genetic diversity of Babesia in Ixodes persulcatus and small mammals from North Ural and West Siberia, Russia. Parasitology 138:175–182. doi: 10.1017/S0031182010001162. [DOI] [PubMed] [Google Scholar]
- 32.Rar VA, Epikhina TI, Livanova NN, Panov VV, Pukhovskaia NM, Vysochina NP, Ivanov LI. 2010. Detection of Babesia spp. DNA in small mammals and ixodic ticks on the territory of north Ural, west Siberia and far east of Russia. Mol Gen Mikrobiol Virusol 2010:26–30. [PubMed] [Google Scholar]
- 33.Zamoto-Niikura A, Tsuji M, Qiang W, Nakao M, Hirata H, Ishihara C. 2012. Detection of two zoonotic Babesia microti lineages, the Hobetsu and U.S. lineages, in two sympatric tick species, Ixodes ovatus and Ixodes persulcatus, respectively, in Japan. Appl Environ Microbiol 78:3424–3430. doi: 10.1128/AEM.00142-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duh D, Petrovec M, Avsic-Zupanc T. 2001. Diversity of Babesia infecting European sheep ticks (Ixodes ricinus). J Clin Microbiol 39:3395–3397. doi: 10.1128/JCM.39.9.3395-3397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zahler M, Rinder H, Gothe R. 2000. Genotypic status of Babesia microti within the piroplasms. Parasitol Res 86:642–646. doi: 10.1007/PL00008545. [DOI] [PubMed] [Google Scholar]
- 36.Gray J, von Stedingk LV, Gurtelschmid M, Granstrom M. 2002. Transmission studies of Babesia microti in Ixodes ricinus ticks and gerbils. J Clin Microbiol 40:1259–1263. doi: 10.1128/JCM.40.4.1259-1263.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saito-Ito A, Tsuji M, Wei Q, He S, Matsui T, Kohsaki M, Arai S, Kamiyama T, Hioki K, Ishihara C. 2000. Transfusion-acquired, autochthonous human babesiosis in Japan: isolation of Babesia microti-like parasites with hu-RBC-SCID mice. J Clin Microbiol 38:4511–4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zamoto A, Tsuji M, Kawabuchi T, Wei Q, Asakawa M, Ishihara C. 2004. U.S.-type Babesia microti isolated from small wild mammals in Eastern Hokkaido, Japan. J Vet Med Sci 66:919–926. doi: 10.1292/jvms.66.919. [DOI] [PubMed] [Google Scholar]
- 39.Vannier E, Krause PJ. 2012. Human babesiosis. N Engl J Med 366:2397–2407. doi: 10.1056/NEJMra1202018. [DOI] [PubMed] [Google Scholar]
- 40.Katargina O, Geller J, Vasilenko V, Kuznetsova T, Jarvekulg L, Vene S, Lundkvist A, Golovljova I. 2011. Detection and characterization of Babesia species in Ixodes ticks in Estonia. Vector Borne Zoonotic Dis 11:923–928. doi: 10.1089/vbz.2010.0199. [DOI] [PubMed] [Google Scholar]
- 41.Katargina O, Russakova S, Geller J, Kondrusik M, Zajkowska J, Zygutiene M, Bormane A, Trofimova J, Golovljova I. 2013. Detection and characterization of tick-borne encephalitis virus in Baltic countries and eastern Poland. PLoS One 8:. doi: 10.1371/journal.pone.0061374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jaaskelainen AE, Sironen T, Murueva GB, Subbotina N, Alekseev AN, Castren J, Alitalo I, Vaheri A, Vapalahti O. 2010. Tick-borne encephalitis virus in ticks in Finland, Russian Karelia and Buryatia. J Gen Virol 91:2706–2712. doi: 10.1099/vir.0.023663-0. [DOI] [PubMed] [Google Scholar]
- 43.Nowak-Chmura M, Siuda K. 2012. Ticks of Poland. Review of contemporary issues and latest research. Ann Parasitol 58:125–155. [PubMed] [Google Scholar]
- 44.Onder O, Shao W, Kemps BD, Lam H, Brisson D. 2013. Identifying sources of tick blood meals using unidentified tandem mass spectral libraries. Nat Commun 4:1746. doi: 10.1038/ncomms2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piesman J, Spielman A. 1982. Babesia microti: infectivity of parasites from ticks for hamsters and white-footed mice. Exp Parasitol 53:242–248. doi: 10.1016/0014-4894(82)90065-0. [DOI] [PubMed] [Google Scholar]
- 46.Hashimoto Y, Kinouchi M, Takahashi H, Matsuo S, Kawagishi N, Kishiyama K, Hirokawa M, Miyamoto K, Iizuka H. 2002. An epidemic study of 700 cases with tick bites in Hokkaido prefecture during the past 6 years: relationship to Lyme disease. Jpn J Dermatol 112:1467–1473. [Google Scholar]
- 47.Mansfield KL, Johnson N, Phipps LP, Stephenson JR, Fooks AR, Solomon T. 2009. Tick-borne encephalitis virus–a review of an emerging zoonosis. J Gen Virol 90:1781–1794. doi: 10.1099/vir.0.011437-0. [DOI] [PubMed] [Google Scholar]
- 48.Masuzawa T. 2004. Terrestrial distribution of the Lyme borreliosis agent Borrelia burgdorferi sensu lato in East Asia. Jpn J Infect Dis 57:229–235. [PubMed] [Google Scholar]
- 49.Takano A, Toyomane K, Konnai S, Ohashi K, Nakao M, Ito T, Andoh M, Maeda K, Watarai M, Sato K, Kawabata H. 2014. Tick surveillance for relapsing fever spirochete Borrelia miyamotoi in Hokkaido, Japan. PLoS One 9:. doi: 10.1371/journal.pone.0104532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wenneras C. 2015. Infections with the tick-borne bacterium Candidatus Neoehrlichia mikurensis. Clin Microbiol Infect 21:621–630. doi: 10.1016/j.cmi.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 51.Zhou X, Xia S, Huang JL, Tambo E, Zhuge HX, Zhou XN. 2014. Human babesiosis, an emerging tick-borne disease in the People's Republic of China. Parasit Vectors 7:509. doi: 10.1186/s13071-014-0509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leiby DA. 2011. Transfusion-transmitted Babesia spp.: bull's-eye on Babesia microti. Clin Microbiol Rev 24:14–28. doi: 10.1128/CMR.00022-10. [DOI] [PMC free article] [PubMed] [Google Scholar]