ABSTRACT
Legionella pneumophila proliferates in freshwater environments at temperatures ranging from 25 to 45°C. To investigate the preference of different sequence types (ST) for a specific temperature range, growth of L. pneumophila serogroup 1 (SG1) ST1 (environmental strains), ST47, and ST62 (disease-associated strains) was measured in buffered yeast extract broth (BYEB) and biofilms grown on plasticized polyvinyl chloride in flowing heated drinking water originating from a groundwater supply. The optimum growth temperatures in BYEB were approximately 37°C (ST1), 39°C (ST47), and 41°C (ST62), with maximum growth temperatures of 42°C (ST1) and 43°C (ST47 and ST62). In the biofilm at 38°C, the ST47 and ST62 strains multiplied equally well compared to growth of the environmental ST1 strain and an indigenous L. pneumophila non-SG1 strain, all attaining a concentration of approximately 107 CFU/cm−2. Raising the temperature to 41°C did not impact these levels within 4 weeks, but the colony counts of all strains tested declined (at a specific decline rate of 0.14 to 0.41 day−1) when the temperature was raised to 42°C. At this temperature, the concentration of Vermamoeba vermiformis in the biofilm, determined with quantitative PCR (qPCR), was about 2 log units lower than the concentration at 38°C. In columns operated at a constant temperature, ranging from 38 to 41°C, none of the tested strains multiplied in the biofilm at 41°C, in which also V. vermiformis was not detected. These observations suggest that strains of ST47 and ST62 did not multiply in the biofilm at a temperature of ≥41°C because of the absence of a thermotolerant host.
IMPORTANCE Growth of Legionella pneumophila in tap water installations is a serious public health concern. The organism includes more than 2,100 varieties (sequence types). More than 50% of the reported cases of Legionnaires' disease are caused by a few sequence types which are very rarely detected in the environment. Strains of selected virulent sequence types proliferated in biofilms on surfaces exposed to warm (38°C) tap water to the same level as environmental varieties and multiplied well as pure culture in a nutrient-rich medium at temperatures of 42 and 43°C. However, these organisms did not grow in the biofilms at temperatures of ≥41°C. Typical host amoebae also did not multiply at these temperatures. Apparently, proliferation of thermotolerant host amoebae is needed to enable multiplication of the virulent L. pneumophila strains in the environment at elevated temperatures. The detection of these amoebae in water installations therefore is a scientific challenge with practical implications.
INTRODUCTION
The genus Legionella currently includes ca. 63 (sub)species (https://www.dsmz.de), at least 20 of which have been implicated in legionellosis (1). Legionella pneumophila is responsible for more than 90% of the reported cases of Legionnaires' disease (LD) in Europe (2), the United States (3), Canada (4), and Japan (5). This species includes 15 polyclonal serogroups (SGs). Most of the clinical isolates represent SG1, which is present worldwide and is also the most commonly cultured SG from environmental samples (6, 7, 8, 9, 10, 11). The use of monoclonal antibodies (MAbs) revealed more heterogeneity in L. pneumophila, and nine phenons reacting with different MAbs have been defined within SG1 (12). A vast majority of clinical SG1 isolates are MAb3/1 positive, but this type is significantly less frequently isolated from environmental samples (7, 8, 10, 12, 13). The introduction of molecular methods enabled a further refinement of the classification of representatives of L. pneumophila. Currently, multilocus sequence-based typing is used for this purpose (14), and in August 2016 more than 2,100 sequence types (STs) of L. pneumophila had been registered (http://bioinformatics.phe.org.uk/legionella/legionella_sbt/php/sbt_homepage.php). L. pneumophila SG1 strains identified as ST37, ST47, and ST62 accounted for nearly half of 167 unrelated clinical isolates in the United Kingdom and Wales, collected in the period 2000 to 2008 (8). ST47 also is a predominant ST in clinical samples in the Netherlands (15), France (16), and Belgium (17). The large outbreak of LD at a flower show in the Netherlands in 1999 (18) was caused by ST62. This ST is also responsible for the cooling tower-associated outbreaks in Ulm, Germany (19), and Quebec, Canada (20). The relatively high case fatality rates in these outbreaks underline the high virulence of ST62. However, of the three STs most frequently observed in clinical samples in the United Kingdom and Wales, only one strain (ST47) was isolated once from an outbreak-unrelated environmental sample (8). Also in France (21), the Netherlands (15, 22), the United States (13), and Canada (4), ST47 and ST62 were not or were only incidentally observed in environmental samples. Contrastingly, L. pneumophila SG1 ST1, the most frequently isolated environmental ST, accounts for a relatively small proportion of the clinical isolates (8, 13, 15), suggesting that ST1 strains are less virulent than strains of ST47, ST62, and a number of other STs. Possible explanations for the apparent absence of the clinically most significant STs in environmental samples include (i) their presence in low numbers compared to numbers of other STs, in particular ST1, (ii) a less efficient recovery in standard culture-based detection procedures than environmental strains, and (iii) a higher susceptibility to control measures, e.g., disinfectants and heat (8).
A temperature range of 25 to 45°C is given for growth of L. pneumophila, with an optimum of 36 ± 1°C (23). Growth of L. pneumophila in pure culture has been observed at 15°C (24), but it is generally accepted that temperatures of ≥25°C are required for its growth in freshwater environments (1, 25, 26). This notion is supported by observations that at temperatures of ≤20°C, L. pneumophila SG1 is digested by amoebae serving as its host at an elevated temperature (24, 27, 28). L. pneumophila SG1 multiplied at 42°C in a batch test with Vermamoeba vermiformis, previously named Hartmannella vermiformis (29), but not at 43°C (24). Konoshi et al. (30) observed growth of pure cultures of L. pneumophila SG1 strains at 43°C and not at 44°C, but growth of L. pneumophila SG1 (Philadelphia strain 1) has been observed in a chemostat at 44°C (31). Clinical isolates of L. pneumophila SG1 and SG6, respectively, showed more growth in pure culture at 44°C and 45°C than two environmental isolates of these SGs although they showed clearly less growth than at 37°C (32). Furthermore, growth of an environmental strain of L. pneumophila SG1 at 45°C in coculture with a cyanobacterium (Fischerella sp.) and associated microbiota has been reported (33).
These observations indicate that the maximum growth temperature of L. pneumophila is not clearly defined and may differ for different SGs and STs. Furthermore, the influence of temperature on a pure culture may differ from the influence on growth in association with a protozoan host (24). The ability to multiply at a temperature above 37°C is a prerequisite for a microorganism to thrive in the human body when temperature rises in response to infection. Therefore, it is hypothesized that virulent L. pneumophila STs may have higher optimum and maximum growth temperatures than environmental STs. The objectives of our study were the following: (i) to establish the influence of temperature on the growth of L. pneumophila SG1 isolates belonging to ST1, ST47, and ST62 in a culture medium; (ii) to determine the ability of isolates of ST47 and ST62 to multiply at 38°C in a biofilm exposed to flowing tap water in comparison to growth of ST1 isolates; and (iii) to assess the ability of these organisms to multiply in a biofilm at temperatures of >40°C.
MATERIALS AND METHODS
Strains.
The L. pneumophila strains used in the experiments are listed in Table 1, with information about their identities and origins.
TABLE 1.
Selected Legionella pneumophila SG1 strains used in this study
| L. pneumophila ST | Strain codea | Origin |
|---|---|---|
| 1 | LP17 | Tap water installation |
| 1 | LP25 | Tap water installation |
| 1 | LP26 | Tap water installation |
| 1 | LP27 | Tap water installation |
| 1 | NA | Tap water installation |
| 1 | LP5 | Clinical sampleb |
| 47 | LP7 | EWGLI, 004 Lyon (France)d |
| 47 | NA | Clinical sampleb |
| 47 | NA | Clinical sampleb |
| 62 | LP3 | Whirlpoolc |
| 62 | LP8 | EWGLI, 010 London (UK) |
| 62 | NA | Clinical sampleb |
| 62 | NA | Clinical sampleb |
NA, not applicable.
Provided by the National Institute for Public Health and the Environment (RIVM), Bilthoven, the Netherlands.
Outbreak related.
EWGLI, European Working Group for Legionella Infections.
Culture media.
Buffered charcoal yeast extract (BCYE) agar with antibiotics was used for determining the colony counts of Legionella spp. (34). Buffered yeast extract broth (BYEB) was used for testing the influence of temperature on the growth of the L. pneumophila strains. BYEB was prepared by adding yeast extract (10 g), ACES [N-(2-acetamido)-2-aminoethanesulfonic acid] buffer (10 g), and 50 ml 1 M KOH to 920 ml of demineralized water. After this solution was sterilized (15 min at 121 ± 3°C) and cooled down to room temperature, 10 ml of membrane-sterilized (0.2-μm pore size) demineralized water with 0.6 g of l-cysteine-HCl, 10 ml of membrane-sterilized demineralized water with 0.37 g of iron(III) pyrophosphate, and 10 ml of membrane-sterilized demineralized water with 1.5 g of 2-oxoglutaric acid were added aseptically, and the pH was adjusted to 6.8 ± 0.2 at 25°C.
Growth in BYEB.
Colonies of the L. pneumophila strains were collected from BCYE agar and cultured in BYEB for 3 days at 36 ± 2°C. Growth rate measurements in BYEB (20 ml) were conducted in duplicate or triplicate in sterile Erlenmeyer flasks (250 ml) with side arms, using a shaking water bath (Thermolab GFL 1083) (temperature constancy, ±0.1°C) at 100 rpm. Growth was recorded by periodic measurement of the optical density at 600 nm (OD600) of the cultures and the blanks with a spectrophotometer (Novasopec II). Growth level measurements were conducted in BYEB (10 ml) in aluminum-capped glass culture tubes (16 by 147 mm) incubated in a water bath (Grant W28 and 150) (temperature constancy, ±0.1°C) containing demineralized water. One day prior to incubation, the water temperature was determined with a calibrated electric thermometer (Testo 110), adjusted to the desired value (±0.1°C), and monitored with a calibrated thermocouple (type Pt100 class B, 1/3 DIN) placed in a reference tube. Growth in the tubes was recorded at the same time each day for up to 4 days. At the end of the test, samples from the tubes were streaked on BCYE agar, BCYE agar without cysteine, and Lab Lemco broth agar (Oxoid) to check for contamination.
Legionella growth in biofilm.
Glass columns (length, 83 cm; inner diameter [i.d.], 2.5 cm), each containing ca. 40 cylindrical pieces (length, ca. 2 cm) of plasticized polyvinyl chloride (pPVC) (diameter, 1.8 cm; wall thickness, 0.2 cm) on top of each other in tap water recirculating via a stainless steel pipe (100 cm; i.d., 0.95 cm) and Teflon tubing (70 cm; i.d., 0.95 cm), were used to obtain biofilms growing under controlled conditions. The tap water, originating from a groundwater supply treated without disinfectant (see Table S1 in the supplemental material), was heated separately in each column by an external metal heating element combined with a thermocouple-based temperature control (accuracy, ±0.5%). The columns were placed in a dark room to prevent light access. In the first experiment, comprising nine columns, growth of L. pneumophila strains LP3 (ST62), LP7 (ST47), LP8 (ST62), and LP25 (ST1) in the biofilm was tested at 38 ± 0.2°C, and subsequently the effect of a temperature increase from 38 to 41 and 42°C was determined. To promote biofilm formation on the pPVC pieces, tap water was recirculated at a flow rate of 150 liter h−1 at 25°C and at 120 ml min−1 of water replacement, corresponding with an average retention time of about 4.5 min. One pPVC piece from each column was investigated at day 7 for the presence of L. pneumophila by quantitative PCR (qPCR), and the biofilm concentration was determined using ATP analysis. L. pneumophila was detected in one column, subsequently labeled column 1. At day 9, the water temperature of all columns was set at 38 ± 0.2°C, and each of the selected L. pneumophila strains cultured in BYEB at 36 ± 2°C for 3 days was inoculated into two separate columns (labeled columns 2 to 9) by adding 1 ml of BYEB (approximately 9 × 108 CFU). The presence of L. pneumophila in the inoculated columns was confirmed by qPCR at day 15. Subsequently, water and pPVC pieces were sampled weekly from each column and analyzed for the colony count of Legionella and the concentration of ATP. The identity of the L. pneumophila strain in each of the columns was established with amplified fragment length polymorphism (AFLP) analysis of colonies grown on BCYE agar. Water temperature in the odd-numbered columns, except column 1, was adjusted to 41°C at day 63 and to 42°C at day 84. Details of the experimental setup are presented in Table S2 in the supplemental material.
A second biofilm experiment was designed to test the influence of temperature on the replication of strains LP3, LP7, LP8, LP25, and also LP27 (ST1) in the biofilm on pPVC at a range of constant temperatures. The strains were grown separately in biofilms on pPVC pieces in five columns operated at 38°C for 42 days, as described for the first experiment. Thereafter, all pPVC pieces were removed from the columns, which then were flushed with hot (70°C) tap water for 2 h. Subsequently, one pPVC piece from each of the columns, with an L. pneumophila concentration of 2 × 106 to 3 × 107 CFU cm−2 in the biofilm, was placed at the bottom of the cleaned columns to achieve a mixed inoculum, and also 30 fresh pPVC pieces were added. On the same day, water temperature in the columns was fixed at 38, 39, 40, 40.5, and 41°C at a recirculation rate of 150 liter h−1 and a water replacement rate of 120 ml min−1. Temperature variation in these columns monitored with a calibrated thermocouple for more than 60 days was less than ±0.2°C.
Biofilm analysis.
Attached biomass was removed from the pPVC pieces submerged in 60 ml of autoclaved tap water by using an electric toothbrush (Braun Oral B), with an autoclaved brush head, for 1 min. The obtained suspension, contained in a closed vial with the brush head and the pPVC piece, was treated with high-energy sonication (HES) using a Branson Sonifier ultrasonic cell disruptor (model W-250D; Branson Ultrasonics Corporation, USA) for 1 min at a constant 20-kHz frequency and an adjusted 90-W power output (45% amplitude). The suspension was kept on ice during and after HES treatment and subsequently was analyzed for ATP (35) either by colony counts of Legionella on BCYE agar by spreading 0.1 ml directly on the plates in triplicate or, less frequently, by qPCR for L. pneumophila as described previously (36). Incidentally, the total cell count (TCC) in the biofilm suspension was determined by acridine orange staining and fluorescence microscopy (37), combined with the heterotrophic plate count (HPC) on R2A agar incubated at 25°C for 10 days. AFLP analysis of selected L. pneumophila colonies and pure cultures was conducted as described elsewhere (38). The concentrations of V. vermiformis and Acanthamoeba spp. in the biofilm suspension were determined by qPCR as described earlier (39). The carbohydrate (CH) concentration was determined with a colorimetric method (40), and the concentrations of iron and manganese were determined by standardized methods (41). All concentrations were expressed in units per square centimeter of pPVC.
Statistics.
For statistical analysis of biofilm parameters, Student's t test was used on normally distributed data, eventually obtained after log transformation, verified with a Shapiro-Wilk test. The F test was used to test on equality of variances. Testing was two sided, with 95% confidence.
RESULTS
Growth in BYEB.
Characteristically shaped relations between temperature and growth rate were obtained, which differed for the tested strains (Fig. 1). The ST1 strains had a maximum specific growth rate (μmax) of ca. 0.40 h−1 (LP26) and 0.45 h−1 (LP25) at ca. 37°C. The μmax of strain LP7 was ca. 0.45 h−1 at ca. 39°C, and for strain LP8, the μmax was ca. 0.53 h−1 at ca. 41°C. At 42°C the growth rates of strains LP3, LP7, and LP8 were about three to five times higher than the growth rates of strains LP25 and LP26. At 44°C no growth (OD of <0.1) was observed within 3 days.
FIG 1.
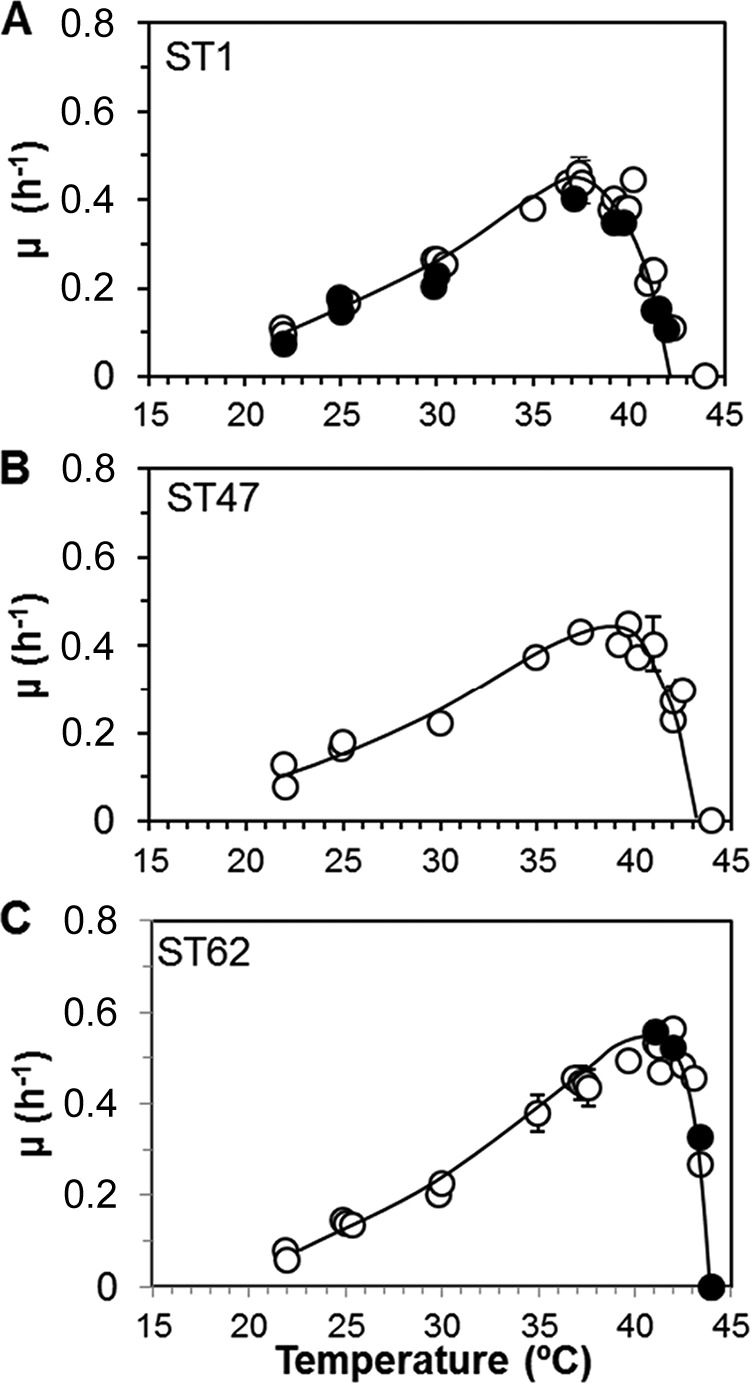
Specific growth rates (μ h−1) of L. pneumophila SG1 strains in buffered yeast extract broth at a range of temperatures. (A) Sequence type (ST) 1 strains LP25 (○) and LP26 (●). (B) ST47 strain LP7. (C) ST62 strains LP3 (●) and LP8 (○).
Growth tests with these and additional strains were conducted in BYEB in culture tubes. Figure 2 shows the average level of growth of the strains belonging to a specific ST recorded after 72 h (ST47 and ST62) or 96 h(ST1) of incubation. Three ST1 strains, including LP25 and LP26, did not grow at 42°C (OD of <0.1), but three other environmental strains, including LP27, showed some growth (OD of 0.16) at this temperature after 96 h. Strains of ST47 and ST62 multiplied at 43°C, with strongest growth of the ST62 strains, but at 44°C almost no growth (OD of <0.1) was observed. These observations demonstrate that the temperatures for optimum and maximum growth differ between the tested L. pneumophila STs and possibly within ST1.
FIG 2.
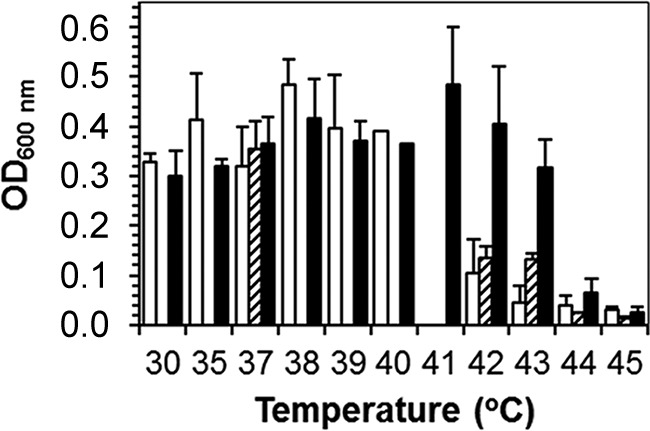
Growth (optical density [OD]) of strains of L. pneumophila ST1 (white bars; n = 6), ST47 (dashed bars; n = 3), and ST62 (black bars; n = 4) in BYEB after 72 h or 96 h (ST1) of incubation at different temperatures. All strains were tested only at 37, 42, 43, and 44°C. Bars show the average values ± standard deviations for strains of the indicated STs. The origins of the strains are shown in Table 1.
Biofilm formation on pPVC in flowing tap water.
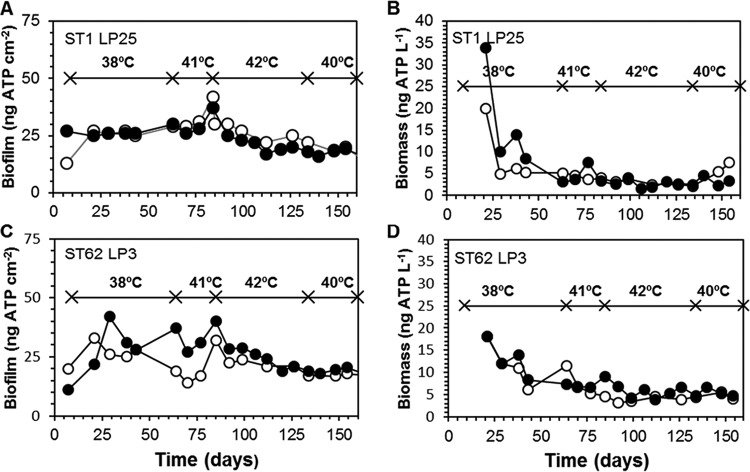
Biofilm developed within 1 week on pPVC, and maximum ATP concentrations of 20 to 40 ng cm−2 were observed within 1 month (Fig. 3). After about 3 months, the biofilm concentration declined slowly to a level of 15 to 20 ng of ATP cm−2. ATP concentrations in the water from the columns showed a clearer decline after having reached a maximum level within 3 weeks (Fig. 3). After 2 months the average concentration in the water stabilized at 5.5 ± 1.1 ng ATP liter−1. After 3 months of operation, the amount of biomass (nanograms of ATP) attached per square centimeter in the columns at a water temperature of 38°C was 4 to 7.5 times higher than the suspended-biomass concentration (nanograms of ATP per liter) in the water (Table 2). TCCs in the biofilm at day 112 were between 5 × 108 and 1.3 × 109 cells cm−2, and the HPCs constituted about 20% of the TCC values. The different temperature regimes had no significant effect on the biofilm concentration (ATP) and the HPC value, but the TCCs at day 112 in the columns at 42°C were significantly higher than in the columns operated at 38°C (Table 2). The carbohydrate (CH) concentration in the biofilm at day 165 ranged from 22 to 148 μg cm−2. The average concentration in the columns operated at 38°C did not differ significantly from the concentration in the columns operated at 38 to 42°C, but the concentrations of iron, manganese, and copper were about 50% less in the columns operated at an elevated temperature (Table 2).
FIG 3.
Concentration of attached (A and C) and planktonic (B and D) biomass in the columns with pieces of plasticized PVC (pPVC) operated at 38°C (○) and at various temperatures (●), as indicated. Columns were operated at 25°C from day 0 to day 9.
TABLE 2.
Characteristics of the biofilm on plasticized polyvinyl chloride
| Column(s) | Temp (°C) | ATP concn (avg ± SD) |
Day 112 profile |
Day 165 profile |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Biofilm (ng/cm2) | Water (ng/liter) | Ratio B/Wa | TCC (no./cm2)b | HPC (CFU/cm2)c | TCC (no./cm2) | CH (μg/cm2)d | Fe (μg/cm2) | Mn (μg/cm2) | Cu (μg/cm2) | ||
| 1 | 38 | 27 ± 7 | 4.9 ± 2.0 | 6.8 ± 2.2 | 7.0 × 108 | 3.0 × 108 | 7.2 × 108 | 118 | 40 | 1.5 | 32 |
| 2 | 38 | 24 ± 11 | 4.6 ± 2.5 | 4.7 ± 2.3 | 6.7 × 108 | 1.4 × 108 | 5.0 × 108 | 108 | 38 | 1.4 | 28 |
| 3 | 38–42 | 22 ± 8 | 6.5 ± 3.7 | 4.6 ± 2.0 | 9.4 × 108 | 2.2 × 108 | 6.4 × 108 | 60 | 21 | 0.7 | 15 |
| 4 | 38 | 25 ± 7 | 4.3 ± 1.6 | 7.5 ± 3.2 | 5.3 × 108 | 1.3 × 108 | 5.1 × 108 | 96 | 42 | 1.8 | 35 |
| 5 | 38–42 | 23 ± 5 | 4.3 ± 3.2 | 7.7 ± 3.1 | 1.3 × 109 | 2.2 × 108 | 1.3 × 109 | 84 | 19 | 0.6 | 17 |
| 6 | 38 | 22 ± 6 | 5.7 ± 2.7 | 5.0 ± 1.5 | 9.5 × 108 | 2.0 × 108 | 6.9 × 108 | 132 | 30 | 1.1 | 24 |
| 7 | 38–42 | 26 ± 8 | 6.7 ± 2.4 | 4.3 ± 1.3 | 1.2 × 109 | 4.1 × 108 | 1.7 × 109 | 148 | 17 | 0.3 | 15 |
| 8 | 38 | 29 ± 8 | 7.3 ± 1.9 | 4.0 ± 1.1 | 5.3 × 108 | 2.2 × 108 | 4.3 × 108 | 113 | 25 | 0.9 | 15 |
| 9 | 38–42 | 22 ± 9 | 5.8 ± 1.4 | 3.1 ± 4.4 | 9.4 × 108 | 1.3 × 108 | 3.6 × 108 | 22 | 8.9 | 0.4 | 7 |
| All (avg ± SD) | 38 | 25.4 ± 2.8 | 5.4 ± 1.2 | 5.6 ± 1.5 | (6.8 ± 1.7) × 108 | (2.0 ± 0.7) × 108 | (5.7 ± 1.3) × 108 | 113 ± 13 | 35 ± 7.2 | 1.3 ± 0.35 | 27 ± 7.8 |
| All (avg ± SD) | 38–42 | 23.2 ± 1.8 | 5.8 ± 1.1 | 4.9 ± 2.0 | (11 ± 1.8) × 108e | (2.5 ± 1.2) × 108 | (10 ± 6.1) × 108 | 79 ± 53 | 16.5 ± 5.3e | 0.5 ± 0.2e | 13.5 ± 4.4e |
Ratio of concentrations in biofilm (B) and water (W) from day 92 to day 154.
TCC, total cell count.
HPC, heterotrophic plate count.
CH, carbohydrate.
Significantly different (P < 0.05) from concentrations in columns operated at 38°C.
L. pneumophila in biofilm and water.
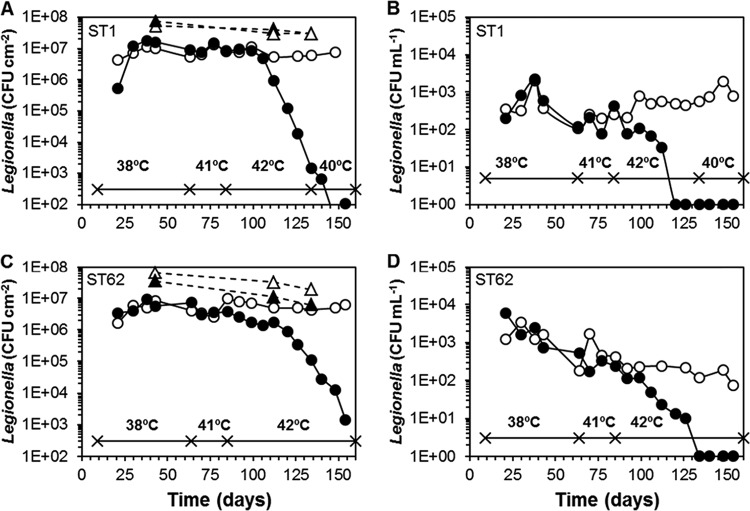
An indigenous L. pneumophila was observed at a low concentration in the biofilm (25 gene copies cm−2) on day 7 in one of the columns. This column (column 1) therefore was not inoculated with an L. pneumophila culture. L. pneumophila was detected by qPCR in the water collected from each of the columns (1.8 × 104 to 1.4 × 107 gene copies liter−1) at day 15 and was cultured from the biofilm on pPVC at day 21 in all columns. The L. pneumophila colonies on the BCYE plates showed uniform morphologies in each of the inoculated columns, and AFLP analysis revealed that the fingerprints of the bacteria in the inoculated columns were similar to those of the L. pneumophila strains added to the columns at day 9 (see Fig. S1 in the supplemental material). The indigenous strain in column 1 was identified as L. pneumophila non-SG1, with an allelic profile of as-yet-undefined ST (see Table S2). A second colony type on the BCYE plates of column 1 was identified as Legionella anisa. This organism was present at low numbers in the biofilm and the water (see Fig. S2). Four weeks after inoculation, L. pneumophila attained a maximum level of approximately 1 × 107 to 2 × 107 CFU cm−2 in the biofilm in all columns (Fig. 4; see also Fig. S2 and S3 in the supplemental material). In the columns operated at 38°C, this level declined by about 50% in the experimental period. The concentration of L. pneumophila in the water from the columns was >103 CFU ml−1 in the first 2 months of operation and declined thereafter to less than 5 × 102 CFU ml−1 in most cases (Fig. 4; see also Fig. S2 and S3). Increasing the temperature to 41 ± 0.2°C in the odd-numbered columns (except column 1) did not affect the colony counts of L. pneumophila in the biofilm within 4 weeks (Fig. 4; see also Fig. S3). Therefore, water temperature in these columns was further raised to 42 ± 0.2°C. At this temperature, a slow decline of the L. pneumophila colony counts in the biofilm was observed after about 2 weeks, followed by a more rapid decline resulting in a 3- to 4-log reduction at day 133. The specific decline rate ranged from 0.14 day−1 (LP3) and 0.28 day−1 (LP8 and LP25) to 0.41 day−1 (LP7). Changing the water temperature to 40 ± 0.2°C at day 135 resulted only in the columns with LP7 and LP8 in a small increase of the L. pneumophila colony counts within 3 weeks (see Fig. S3 in the supplemental material). The AFLP patterns of L. pneumophila colonies incubated at day 126 confirmed the presence of the inoculated strains, but in column 8, inoculated with LP7 and operated at 38°C, an L. pneumophila SG1 ST1 strain was present (see Fig. S1). Analysis of five colonies from column 8 on day 154 confirmed that L. pneumophila SG1 ST1 had become the predominating L. pneumophila.
FIG 4.
Growth of L. pneumophila ST1 (strain LP25) and ST62 (strain LP3) in the biofilm on pPVC (A and C) and water (B and D) in the columns operated at 38°C (○) and in the columns operated at the indicated temperatures (●). The columns were operated at 25°C from day 0 to day 9. The L. pneumophila concentration determined by qPCR (number of gene copies per square centimeter) is shown for the columns operated at 38°C (△) and the columns operated at the other indicated temperatures (▲). Colony counts below the detection level in water (3.3 CFU ml−1) are shown as 1 CFU ml−1.
The number of gene copies of L. pneumophila in the biofilm determined at day 43 for each of the columns and on days 112 and 134 for the columns operated at 38°C was (overall) 5.8 ± 1.0 times higher than the colony counts of L. pneumophila (Fig. 4; see also Fig. S3C in the supplemental material). At day 134, the gene copy concentrations in the columns operated at 42°C did not show a clear decline, and the gene copy number/CFU count ratio had increased to 56 (column 7), 1,500 (column 9), 2 × 104 (column 5), and 2.6 × 105 (column 3). The concentration of V. vermiformis in the biofilms at 42°C on day 112, at the onset of the colony count decline, was significantly lower (P < 0.05) than the concentration in the columns operated at 38°C (Table 3). Acanthamoeba spp. were not detected (<5 cell eq cm−2).
TABLE 3.
Legionella and free-living amoebae in the biofilm on plasticized polyvinyl chloride
| Column no. | L. pneumophila serogroup or ST (strain) | Temp (°C) |
Legionella concn |
V. vermiformis concn at day 112 (cell eq/cm2)c | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Biofilm (CFU/cm2) |
Water (CFU/ml) |
Biofilm/water ratio |
No. of CFU/pg of ATP (avg ± SD)b | |||||||
| 50th percentile | 90th percentile | 50th percentile | 90th percentile | 50th percentile | 90th percentile | |||||
| 1 | L. pneumophila non-SG1 | 38 | 1.2 × 107 | 1.7 × 107 | 290 | 8.9 × 103 | 1.3 × 105 | 2.1 × 105 | 431 ± 177 (433) | 380 |
| 2 | ST62 (LP8) | 38 | 9.8 × 106 | 1.7 × 107 | 720 | 6.4 × 103 | 6.9 × 104 | 1.1 × 105 | 454 ± 150 (405) | 1,390 |
| 3 | ST62 (LP8) | 38–42 | NAa | NA | NA | NA | NA | NA | NA | 6 |
| 4 | ST1 (LP25) | 38 | 7.7 × 106 | 1.2 × 107 | 370 | 1.5 × 103 | 1.2 × 104 | 3.9 × 104 | 305 ± 95 (261) | 190 |
| 5 | ST1 (LP25) | 38–42 | NA | NA | NA | NA | NA | NA | NA | 13 |
| 6 | ST62 (LP3) | 38 | 5.0 × 106 | 8.1 × 106 | 240 | 1.7 × 103 | 3.0 × 104 | 5.8 × 104 | 263 ± 60 (253) | 240 |
| 7 | ST62 (LP3) | 38–42 | NA | NA | NA | NA | NA | NA | NA | 6 |
| 8 | ST47 (LP7) | 38 | 8.6 × 106 | 1.4 × 107 | 740 | 2.1 × 103 | 1.3 × 104 | 2.7 × 104 | 302 ± 199 (322) | 450 |
| 9 | ST47 (LP7) | 38–42 | NA | NA | NA | NA | NA | NA | NA | <3 |
NA, not applicable/not analyzed.
Values in parentheses represent the 50th percentile.
V. vermiformis concentrations, expressed as cell equivalents (eq), at 38°C differ significantly (P < 0.05) from those in the columns operated at 42°C. Concentrations are means of duplicate qPCR tests in single samples with a relative standard deviation of 13 to 32%. Acanthamoeba spp. were not detected (<5 cell eq cm−2).
The influence of temperature on the replication of L. pneumophila in the biofilm on pPVC was tested in five columns, each inoculated with a mixture of five L. pneumophila strains representing three STs grown separately in the biofilm at 38°C. The water temperature in the columns was maintained at 38, 39, 40, 40.5, and 41°C, respectively. The biofilm concentrations in these columns were all at the same levels (23 ± 6 ng ATP cm−2) (results not shown). Rapid growth of L. pneumophila was observed in the biofilm at 38 and 39°C, but the colony counts declined at 41°C (Fig. 5). AFLP analysis of five colonies from each column grown on BCYE agar on day 37 revealed that L. pneumophila ST62 (strain LP3 and/or LP8) predominated in the columns operated at 38, 39, 40, and 40.5°C (see Fig. S4 in the supplemental material). The AFLP patterns of four out of five tested colonies from the column operated at 41°C were similar to the AFLP pattern of L. pneumophila ST1 (strains LP25 and LP27), and the pattern of one colony was similar to that of L. pneumophila ST62 (strain LP3 and/or LP8). The concentration of V. vermiformis in the biofilm at day 24 ranged from 1,110 ± 300 cell eq cm−2 (38°C) to 490 ± 10 cell eq cm−2 (39°C), 310 ± 60 cell eq cm−2 (40°C), 120 ± 20 cell eq cm−2 (40.5°C), and <1 cell eq cm−2 at 41°C and correlated significantly (P < 0.01) with temperature (see Fig. S5). V. vermiformis was also detected in the biofilm at day 50, except in the column operated at 41°C. Acanthamoeba spp. were not detected in the biofilm. These observations demonstrate that none of the tested L. pneumophila strains or V. vermiformis and Acanthamoeba spp. replicated at 41°C in the biofilm on tap water-exposed pPVC, thus confirming the colony count decline observed at 42°C in the first biofilm experiment.
FIG 5.
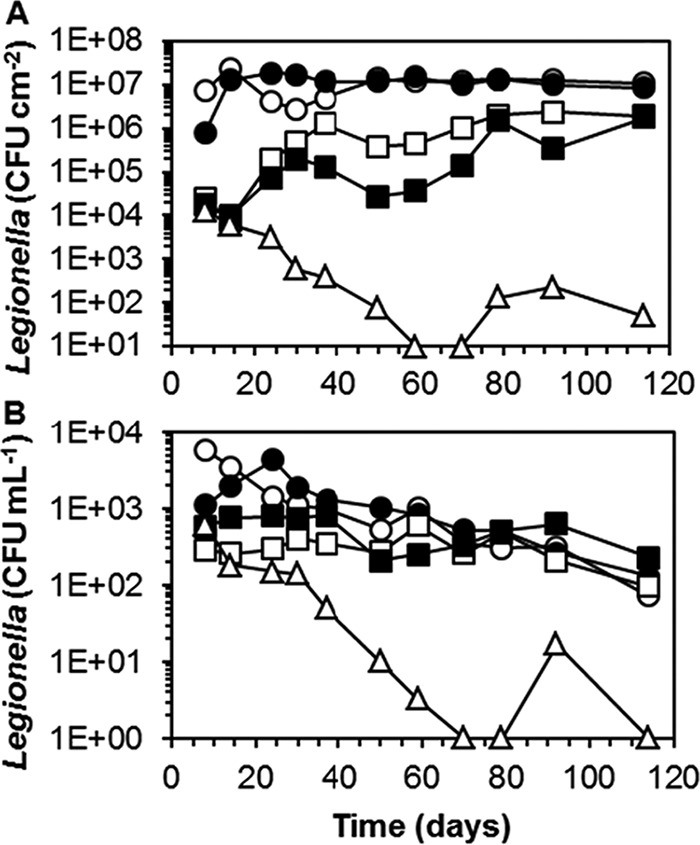
Colony counts of L. pneumophila in the biofilm on pPVC (A) and in the water (B) in the columns operated at a constant temperature ranging from 38°C to 41°C. Colony counts below the detection level in water (3.3 CFU ml−1) are shown as 1 CFU ml−1. Symbols are as follows: ○, 38°C; ●, 39°C; □, 40°C; ■, 40.5°C; △, 41°C.
DISCUSSION
Influence of temperature on growth of Legionella spp. in water systems.
A large diversity of Legionella spp., many of which are as yet uncultured, is ubiquitous in freshwater environments at temperatures of <20°C (36, 42, 43, 44, 45, 46). L. pneumophila is not cultured or only sporadically cultured from these low-temperature environments. In drinking water distributed at 21 to 31°C, a large variety of Legionella spp. was cultured, but L. pneumophila was not observed (47). In three tropical water supplies (30 to 35°C), L. pneumophila was the predominant cultured Legionella species in most samples (48). Uncultured Legionella non-pneumophila species predominated in an acidic biofilm community at 30 to 38°C (49). Cultured Legionella non-pneumophila species predominated in groundwater from a hydrothermal source at ca. 38°C, whereas L. pneumophila predominated in water from a source at ca. 45°C (50). Elevated L. pneumophila colony counts were also observed in residential hot water systems at a water temperature ranging from 35 to 45°C (51). These and other observations indicate a transition from a large diversity of mostly yet undefined Legionella spp. at temperatures of <20°C to a diversity of L. pneumophila SGs and STs at temperatures of >30°C. The environmental temperature therefore is an essential parameter for controlling L. pneumophila in engineered water systems, and temperature management, aiming at a cold water temperature of <20 to 25°C and a hot water temperature of >60°C, is included in guidelines and national regulations (52, 53, 54, 55). However, the information about the relationship between the environmental temperature and the Legionella community composition in natural and engineered water systems is still fragmented. The lack of information can be attributed to (i) absence of temperature data in reports on environmental Legionella surveys, (ii) fluctuations in the environmental water temperature, including discrepancies between the temperature of the water sampled and the temperature at the site of growth, and (iii) difficulties encountered by collecting quantitative data about the Legionella non-pneumophila species and the large diversities of L. pneumophila SGs and STs in these environments. These difficulties are also associated with the use of culture-independent and culture-based methods. The requirement of specific protozoan hosts for growth (56) may also complicate the elucidation of temperature effects on growth of L. pneumophila in water systems.
Growth of L. pneumophila STs in BYEB and biofilms at different temperatures.
The μmax values of the L. pneumophila strains in BYEB correspond with minimum generation times of approximately 1.3 to 1.7 h, which are below the generation time (2 h) observed inside human monocytes at 37°C (57). BYEB obviously is a well-suited growth medium, even supporting growth at 22°C (Fig. 1). As shown in Fig. 1 and 2, the influence of temperature on growth in BYEB differs between L. pneumophila STs and possibly within STs. However, accurate establishment of the optimum growth temperature (±0.5°C) and the maximum growth rate requires more measurements at temperatures of >35°C. The optimum growth temperature of the ST1 strains in BYEB was approximately 37°C. Growth was slow at 42°C (Fig. 1), and a few environmental isolates did not grow in the tubes at this temperature, suggesting that ST1 is heterogeneous in terms of temperature tolerance. Heterogeneity within ST1 has been demonstrated by monoclonal antibody typing (8, 58, 59) and more recently also by whole-genome sequencing (60). The ST62 strains, with an optimum temperature of approximately 41°C, multiplied rapidly at 42°C and relatively well at 43°C, but almost no growth (OD of <0.1) was observed at 44°C (Fig. 1 and 2). Hence, L. pneumophila ST62, which is associated with LD outbreaks at a high case fatality rate after exposure to aerosols from whirlpools and cooling towers (18, 19, 20), is able to multiply at higher temperatures than ST1.
The high biofilm concentration on pPVC and the high colony counts of L. pneumophila on pPVC are consistent with earlier findings (61). The strong biofilm formation on pPVC, which is caused by the release of biodegradable plasticizers, mostly phthalate esters (62), makes the material unsuited for application in plumbing systems. The use of pPVC-based garden hoses may lead to exposure to elevated numbers of legionellae (63). The CFU/ATP ratios of the indigenous L. pneumophila non-SG1 and L. pneumophila ST1, ST47, and ST62 strains in the biofilm on pPVC at 38°C (Table 3) show that these organisms attained similar levels of growth in the biofilm at this temperature. These ratios, which remained fairly constant during the test period, are about two orders of magnitude higher than those observed at a 10-times-lower biofilm concentration on cross-linked polyethylene and stainless steel in a model warm-water installation (35). A high biofilm concentration therefore may be associated with a high efficiency of L. pneumophila proliferation. The similarity of the CFU/ATP ratios as well as the similar ratios of the number of gene copies and the colony count of L. pneumophila in the biofilm at 38°C indicates that the recovery of the ST47 and ST62 isolates on the BCYE medium did not differ from the recovery of the ST1 strain and the indigenous L. pneumophila. Therefore, the low isolation frequency of ST47 and ST62 from environmental samples in comparison to that of ST1 (8, 15) most likely is not caused by a limited ability to multiply in a biofilm or by a low culturability. The predominance of ST62 grown in the biofilm in the columns inoculated with different strains indicates that this organism outcompeted ST1 and ST7 under the experimental conditions at temperatures ranging from 38 to 40.5°C.
Protozoan host.
The more rapid growth of the ST62 and ST47 strains than that of ST1 in BYEB at a temperature of ≥40°C may be an indication of an environmental niche at elevated temperatures. However, no growth was observed in the biofilm at 41°C (Fig. 5), and the colony counts of all the tested strains declined at 42°C (Fig. 4; see also Fig. S3 in the supplemental material). The number of gene copies of L. pneumophila in the columns operated at 42°C remained at about the same level as that observed in the columns at 38°C (Fig. 4; see also Fig. S3). Furthermore, in these columns the washout rate, estimated from the water replacement rate (120 ml min−1), and the colony counts in the biofilm and the water were low (<0.02 day−1), and the biofilm concentration (ATP) did not decrease after the temperature rise. Hence, the decline of the colony counts of the L. pneumophila STs in the biofilm at 42°C represents a loss of culturability. The concentration of metals, including copper, in the biofilm at this temperature was lower than that at 38°C (Table 2) and therefore did not cause this loss of culturability. Culturability loss of L. pneumophila has also been observed in sterilized water stored at temperatures ranging from 20 to 42°C and was attributed to starvation (64, 65, 66). Addition of amoebae resulted in an increase of culturable bacteria by intracellular replication (64, 67). These observations are consistent with the need of a protozoan host for replication of the fastidious L. pneumophila in the aquatic environment (1, 68), including biofilms (61, 69). The culturability loss of L. pneumophila ST47 and ST62 in the biofilm at 42°C and the inability to grow in the biofilm at 41°C therefore may be explained by the absence of a protozoan host proliferating at these temperatures.
V. vermiformis was detected in the biofilm at 38°C, but Acanthamoeba spp. were not observed (Table 3), which is consistent with the inability of most of these species to grow at temperatures of >37°C (70, 71). A number of observations indicate that V. vermiformis most likely served as a host for the L. pneumophila strains in the biofilm on pPVC. First, L. pneumophila did not multiply in the biofilm grown at 41°C (Fig. 5), in which V. vermiformis was not detected. Second, the concentration of V. vermiformis in the columns at 42°C at day 112 at the onset of the colony count decline of L. pneumophila was about 2 log units lower than the concentration in the columns operated at 38°C (Table 3). Moreover, V. vermiformis is the most commonly observed host amoeba for L. pneumophila in tap water (39, 48, 61, 68) and also a predominating amoeba in hot water installations (72, 73).
The description of V. vermiformis strain CDC-19 (ATCC 50237) isolated from water associated with nosocomial legionellosis (74) does not provide information about its temperature range. Buse and Ashbolt (56) reported that this strain multiplied at 37°C with Escherichia coli as prey but not at 41°C. Furthermore, no growth or very poor growth of L. pneumophila was observed at 41°C in coculture with two V. vermiformis strains isolated from tap water installations (75). However, strains of V. vermiformis that can grow at 44°C have been isolated from hot water installations together with strains that were unable to multiply at this temperature (72, 73). The latter type predominated on moist surfaces at lower temperatures (72). Apparently, V. vermiformis is heterogeneous in its ability to grow at an elevated temperature. V. vermiformis in the biofilms on pPVC probably originated from the groundwater supply because the organism is ubiquitous in treated groundwater in temperate climates (76, 77). This V. vermiformis may be adapted to a relatively low temperature and apparently did not proliferate at ≥41°C. The inability of L. pneumophila LP7 (ST47), LP3, and LP8 (both ST62) to replicate in the biofilm at 41°C and their culturability decrease at 42°C, in contrast with optimal growth in BYEB at these temperatures, therefore most likely are due to the absence of a thermotolerant host. The significantly higher TCC values in the biofilm at day 112 in the columns operated at 42°C than in the columns operated at 38°C (Table 2) are consistent with reduced protozoan grazing at 42°C.
The experimental observations and literature data suggest a transition at ca. 40 to 41°C from growth of commonly occurring free-living amoebae to amoebae adapted to an elevated temperature. It is hypothesized that growth of the highly virulent L. pneumophila ST62 or ST47 in water systems depends on a combination of factors, including (i) the presence of these STs, (ii) a temperature close to their optimum growth temperatures, and (iii) the presence of a protozoan host with the ability to proliferate at this temperature. The niche favoring competition of these and other STs with an optimum growth temperature of ≥40°C may cover a temperature range of only a few degrees Celsius because no growth of the strains was observed at 44°C and because strains of more commonly observed STs may grow rapidly at a temperature of <40°C. The public health impact of ST47, ST62, and other virulent STs warrants further research on their occurrence as well as on the presence and identity of thermotolerant free-living amoebae in (water entering) engineered water systems in relation to water temperature.
Supplementary Material
ACKNOWLEDGMENTS
We are much indebted to Anita van der Veen-Lugtenberg and Sarah Lahondès for punctual analyses and performance of the experiments, to Ronald Italiaander for preparing the AFLP dendrograms, to Harry van Wegen and Sidney Meijering for constructing the temperature-controlled columns, and to Hein van Lieverloo for statistical analysis of the biofilm parameters.
Funding Statement
This study was conducted within the framework of the Joint Research Program of the Water Supply Companies in the Netherlands, with financial support of the Inspectorate of Housing, Spatial Planning and Environment.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01107-16.
REFERENCES
- 1.Fields BS, Benson RF, Besser R. 2002. Legionella and Legionnaires' disease: 25 years of investigation. Clin Microbiol Rev 15:506–526. doi: 10.1128/CMR.15.3.506-526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Centre for Disease Prevention and Control. 2014. Legionnaires' disease in Europe 2012. Surveillance report. European Centre for Disease Prevention and Control, Stockholm, Sweden: http://ecdc.europa.eu/en/publications/publications/legionnaires-disease-surveillance-2012.pdf. [Google Scholar]
- 3.Yu VL, Plouffe JF, Pistoris MC, Stout JE, Schousboe M, Widmer A, Summersgill J, File T, Heath CM, Paterson DL, Cheresky A. 2002. Distribution of Legionella species and serogroups isolated by culture in patients with sporadic community-acquired legionellosis: an international collaborative survey. J Infect Dis 186:127–128. doi: 10.1086/341087. [DOI] [PubMed] [Google Scholar]
- 4.Tijet N, Tang P, Romilowych M, Duncan C, Ng V, Fisman DN, Jamieson F, Low DE, Guyard C. 2010. New endemic Legionella pneumophila serogroup 1 clones, Ontario, Canada. Emerg Infect Dis 16:447–454. doi: 10.3201/eid1603.081689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amemura-Maekawa J, Kura F, Helbig JH, Chang B, Kaneko A, Watanabe Y, Isobe J, Nukina M, Nakajima H, Kawano K, Tada Y, Watanabe H, Working Group for Legionella in Japan . 2010. Characterization of Legionella pneumophila isolates from patients in Japan according to serogroups, monoclonal antibody subgroups and sequence types. J Med Microbiol 59:653–659. doi: 10.1099/jmm.0.017509-0. [DOI] [PubMed] [Google Scholar]
- 6.Lück PC, Helbig JH. 2003. Typing of Legionella strains isolated from patients and environmental sources in Germany, 1990–2000, p 267–270. In Marre R, Kwaik YA, Bartlett C, Cianciotto NP, Fields BS, Frosch M, Hacker J, Lück R (ed), Legionella. ASM Press, Washington, DC. [Google Scholar]
- 7.Doleans A, Aurell H, Reyrolle M, Lina G, Freney J, Vandenesch F, Etienne J, Jarraud S. 2004. Clinical and environmental distributions of Legionella strains in France are different. J Clin Microbiol 42:458–460. doi: 10.1128/JCM.42.1.458-460.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrison TG, Afshar B, Doshi N, Fry NK. 2009. Distribution of Legionella pneumophila serogroups, monoclonal antibody subgroups and DNA sequence types in recent clinical and environmental isolates from England and Wales (2000–2008). Eur J Clin Microbiol Infect Dis 28:781–791. doi: 10.1007/s10096-009-0705-9. [DOI] [PubMed] [Google Scholar]
- 9.Lee HK, Shim JI, Kim HE, Yu JY, Kang YH. 2010. Distribution of Legionella species from environmental water sources of public facilities and genetic diversity of L. pneumophila serogroup 1 in South Korea. Appl Environ Microbiol 76:6547–6554. doi: 10.1128/AEM.00422-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amemura-Maekawa J, Kikukawa K, Helbig JH, Kaneko S, Susuki-Hashimoto A, Furuhata K, Chang B, Murai M, Ichinose M, Ohnishi M, Kura F, Working Group for Legionella in Japan . 2012. Distribution of monoclonal antibody subgroups and sequence based types among Legionella pneumophila serogroup 1 isolates derived from cooling tower water, bath water and soil in Japan. Appl Environ Microbiol 78:4263–4270. doi: 10.1128/AEM.06869-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin T, Zhou H, Ren H, Guan H, Li M, Zhu B, Shao Z. 2014. Distribution of sequence based types of Legionella pneumophila serogroup 1 strains isolated from cooling towers, hot springs, and potable water systems in China. Appl Environ Microbiol 80:2150–2157. doi: 10.1128/AEM.03844-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helbig JH, Bernander S, Castellani Pastoris M, Etienne J, Gaia V, Lindsay D, Lück PC, Marques T, Mentula S, Peeters MF, Pelaz C, Struelens M, Uldum SA, Wewalka G, Harrison TG. 2002. Pan-European study on culture-proven Legionnaires' disease: distribution of Legionella pneumophila serogroups and monoclonal subgroups. Eur J Microbiol Infect Dis 21:710–716. doi: 10.1007/s10096-002-0820-3. [DOI] [PubMed] [Google Scholar]
- 13.Kozak-Muiznieks NA, Lucas CE, Brown E, Pondo T, Taylor TH, Frace M, Miskowski D, Winchell JM. 2014. Prevalence of sequence types among clinical and environmental isolates of Legionella pneumophila serogroup 1 in the United States from 1982 to 2012. J Clin Microbiol 52:201–211. doi: 10.1128/JCM.01973-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaia V, Fry NK, Afshar B, Lück PC, Meugnier H, Etienne J, Peduzzi R, Harrison TG. 2005. Consensus sequence-based scheme for epidemiological typing of clinical and environmental isolates of Legionella pneumophila. J Clin Microbiol 43:2047–2052. doi: 10.1128/JCM.43.5.2047-2052.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Euser SM, Bruin JP, Brandsema P, Reijnen L, Boers SA, den Boer JW. 2013. Legionella prevention in the Netherlands: an evaluation using genotype distribution. Eur J Clin Microbiol Infect Dis 32:1017–1022. doi: 10.1007/s10096-013-1841-9. [DOI] [PubMed] [Google Scholar]
- 16.Campese C, Bitar D, Jarraud S, Maine C, Forey F, Etienne J, Desenclos JC, Saura C, Che D. 2011. Progress in the surveillance and control of Legionella infection in France, 1998–2008. Int J Infect Dis 15:e30–e37. doi: 10.1016/j.ijid.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Vekens E, Soetens O, de Mendonça R, Echahidi F, Roisin R, Deplano A, Eeckhout I. 2012. Sequence-based typing of Legionella pneumophila serogroup 1 clinical isolates from Belgium between 2000 and 2010. Euro Surveill 17:1–6. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20302. [PubMed] [Google Scholar]
- 18.Den Boer JW, Yzerman EP, Schellekens J, Lettinga KD, Boshuizen HC, van Steenbergen JE, Bosman A, van den Hof S, van Vliet HA, Peeters MF, van Ketel RJ, Speelman P, Kool JL, Conyn-van Spaendonk MA. 2002. A large outbreak of Legionnaires' disease at a flower show, the Netherlands, 1999. Emerg Infect Dis 8:37–43. doi: 10.3201/eid0801.010176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Von Baum H, Härter G, Essig A, Lück C, Gonser T, Embacher A, Brockmann S. 2010. Preliminary report: outbreak of Legionnaires' disease in the cities of Ulm and Neu-Ulm in Germany, December 2009–January 2010. Euro Surveill 15:1–2. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19472. [PubMed] [Google Scholar]
- 20.Lévesque S, Plante PL, Mendis N, Cantin P, Marchand G, Charest H, Raymond F, Huot C, Goupil-Sormany I, Desbiens F, Faucher CP, Corbeil J, Tremblay C. 2014. Genomic characterization of a large outbreak of Legionella pneumophila serogroup 1 strains in Quebec City, 2012. PLoS One 9:e103852. doi: 10.1371/journal.pone.0103852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ginevra C, Forey F, Campèse C, Reyrolle M, Che D, Etienne J, Jarraudet S. 2008. Lorraine Strain of Legionella pneumophila serogroup 1, France. Emerg Infect Dis 14:673–675. doi: 10.3201/eid1404.070961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schalk JAC, Euser SM, van Heinsbergen E, Bruin JP, den Boer JW, de Roda Husman AM. 2014. Soil as a source of Legionella pneumophila sequence type 47. Int J Infect Dis 27:18–19. doi: 10.1016/j.ijid.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Garrity GM, Bell JA, Tilburn T. 2005. Order VI Legionellales family 1. Legionellaceae, p 210–237. In Brenner DJ, Krieg NR, Staley JT (ed), Bergey's manual of systematic bacteriology, 2nd ed, vol 2, part B Springer-Verlag, New York, NY. [Google Scholar]
- 24.States SJ, Podorski A, Conley LF, Young WD, Wadowsky RM, Dowling JN, Kuchta JM, Navratil JS, Yee RB. 1993. Temperature and the survival and multiplication of Legionella pneumophila associated with Hartmannella vermiformis, p 147–149. In Barberee JM, Breiman RF, Dufour AP (ed), Legionella: current status and emerging perspectives. ASM Press, Washington, DC. [Google Scholar]
- 25.Yee RB, Wadowsky RM. 1982. Multiplication of Legionella pneumophila in unsterilized tap water. Appl Environ Microbiol 43:1330–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wadowsky RM, Wolford R, McNamara AM, Yee R. 1985. Effect of temperature, pH and oxygen level on the multiplication of naturally occurring Legionella pneumophila in potable water. Appl Environ Microbiol 49:1197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anand CM, Skinner RA, Malic A, Kurtz JB. 1983. Interaction of L. pneumophila and a free living amoeba (Acanthamoeba palestinensis). J Hyg (Lond) 91:167–178. doi: 10.1017/S0022172400060174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohno A, Kato N, Sakamoto R, Kimura S, Yamaguchi K. 2008. Temperature dependent parasitic relationship between Legionella pneumophila and a free-living amoeba (Acanthamoeba castellani). Appl Environ Microbiol 74:4585–4588. doi: 10.1128/AEM.00083-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smirnov A. 2011. A revised classification of naked lobose amoebae (Amoebozoa: Lobosa). Protist 162:545–570. doi: 10.1016/j.protis.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Konishi T, Yamashiro T, Koide M, Nishizono A. 2006. Influence of temperature on growth of Legionella pneumophila biofilm determined by precise temperature gradient incubator. J Biosci Bioeng 101:478–484. doi: 10.1263/jbb.101.478. [DOI] [PubMed] [Google Scholar]
- 31.Berg JD, Hoff JC, Roberts PV, Matin A. 1985. Growth of Legionella pneumophila in continuous culture. Appl Environ Microbiol 49:1534–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kusnetsov JM, Ottoila E, Martikainen PJ. 1996. Growth, respiration and survival of Legionella pneumophila at high temperatures. J Appl Bacteriol 81:341–347. doi: 10.1111/j.1365-2672.1996.tb03517.x. [DOI] [PubMed] [Google Scholar]
- 33.Tison DL, Hope DH, Cherry WB, Fliermans CB. 1980. Growth of Legionella pneumophila in association with blue-green algae (cyanobacteria). Appl Environ Microbiol 39:456–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.International Organization for Standardization. 2004. Water quality: detection and enumeration of Legionella. Part 2: direct membrane filtration method for waters with low bacterial counts. ISO 11731-2:2004. International Organization for Standardization, Geneva, Switzerland. [Google Scholar]
- 35.van der Kooij D, Veenendaal HR, Scheffer WJH. 2005. Biofilm formation and multiplication of Legionella in a model warm-water system with pipes of copper, stainless steel and cross-linked polyethylene. Water Res 39:2789–2798. doi: 10.1016/j.watres.2005.04.075. [DOI] [PubMed] [Google Scholar]
- 36.Wullings BA, Bakker G, van der Kooij D. 2011. Concentration and diversity of uncultured Legionella spp. in two unchlorinated drinking water supplies with different concentrations of natural organic matter. Appl Environ Microbiol 77:634–641. doi: 10.1128/AEM.01215-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hobbie JE, Daley RJ, Jasper S. 1977. Use of nucleopore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol 33:1225–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magic-Knezev A, Wullings B, van der Kooij D. 2009. Polaromonas and Hydrogenophaga species are the predominant bacteria cultured from granular activated carbon filters in water treatment. J Appl Microbiol 107:1457–1467. doi: 10.1111/j.1365-2672.2009.04337.x. [DOI] [PubMed] [Google Scholar]
- 39.Valster RM, Wullings BA, van der Kooij D. 2010. Detection of protozoan hosts for Legionella pneumophila in engineered water systems by using a biofilm batch test. Appl Environ Microbiol 76:7144–7153. doi: 10.1128/AEM.00926-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. 1956. Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- 41.International Organization for Standardization. 2003. Water quality: application of inductively coupled plasma mass spectrometry (ICP-MS). Part 2: determination of 62 elements. ISO 17294-2:2003. International Organization for Standardization, Geneva, Switzerland. [Google Scholar]
- 42.Calvo-Bado LA, Morgan JAW, Sergeant M, Pettitt TR, Whipps JM. 2003. Molecular characterization of Legionella populations present within slow sand filters used for fungal plant pathogen suppression in horticulture crops. Appl Environ Microbiol 69:533–541. doi: 10.1128/AEM.69.1.533-541.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brooks T, Osicki RA, Springthorpe VS, Sattar SA, Filion L, Abrial D, Riffard S. 2004. Detection and identification of Legionella species from groundwaters. J Toxicol Environ Health A 67:1845–1859. doi: 10.1080/15287390490492449. [DOI] [PubMed] [Google Scholar]
- 44.Wullings BA, van der Kooij D. 2006. Occurrence and genetic diversity of uncultured Legionella spp. in drinking water treated at temperatures below 15°C. Appl Environ Microbiol 72:157–160. doi: 10.1128/AEM.72.1.157-166.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carvalho FRS, Nastasi FR, Gamba RC, Foronda AS, Pellizari VH. 2008. Occurrence and diversity of Legionellaceae in polar lakes of the Antarctic peninsula. Curr Microbiol 57:294–300. doi: 10.1007/s00284-008-9192-y. [DOI] [PubMed] [Google Scholar]
- 46.Parthuisot N, West NJ, Lebaron P, Baudart J. 2010. High diversity and abundance of Legionella spp. in a pristine river and impact of seasonal and antropogenic effects. Appl Environ Microbiol 76:8201–8210. doi: 10.1128/AEM.00188-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pryor M, Springthorpe S, Riffard S, Brooks T, Huo Y, Davis G, Sattar SA. 2004. Investigation of opportunistic pathogens in municipal drinking water under different supply and treatment regimes. Water Sci Technol 50:83–90. [PubMed] [Google Scholar]
- 48.Valster RM, Wullings BA, van den Berg R, van der Kooij D. 2011. Relationships between free-living protozoa, cultivable Legionella spp. and water quality characteristics in three drinking-water supplies in the Caribbean. Appl Environ Microbiol 77:7321–7328. doi: 10.1128/AEM.05575-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheehan KB, Henson JM, Ferris MJ. 2005. Legionella species diversity in an acidic biofilm community in Yellowstone National Park. Appl Environ Microbiol 71:507–511. doi: 10.1128/AEM.71.1.507-511.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Costa J, Tiago I, da Costa MS, Veríssiomo A. 2005. Presence and persistence of Legionella spp. in groundwater. Appl Environ Microbiol 71:663–671. doi: 10.1128/AEM.71.2.663-671.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mathys W, Stanke J, Harmuth M, Junge-Mathys E. 2008. Occurrence of Legionella in two hot water systems of single-family residences in suburbs of two German cities with special reference to solar and district heating. Int J Hyg Environ Health 211:179–185. doi: 10.1016/j.ijheh.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 52.Bartram J, Chartier Y, Lee JV, Pond K, Surman-Lee S (ed). 2007. Legionella and the prevention of legionellosis. World Health Organization, Geneva, Switzerland: http://www.who.int/water_sanitation_health/emerging/legionella.pdf. [Google Scholar]
- 53.Health and Safety Executive. 2014. Legionnaires' disease. Part 2: the control of Legionella bacteria in hot and cold water systems. Health and Safety Executive, London, United Kingdom: http://www.hse.gov.uk/legionnaires/hot-and-cold.htm. [Google Scholar]
- 54.American Society of Heating, Refrigerating and Air Conditioning Engineers, Inc. 2011. BSR/ASHRAE standard 188P. Proposed new standard 188, prevention of legionellosis associated with building water systems (second public review). American Society of Heating, Refrigerating and Air Conditioning Engineers, Inc., Atlanta GA. [Google Scholar]
- 55.Atsma JJ. 2011. Regeling legionellapreventie in drinkwater en warm tapwater. Staatscourant 2011 nr 10828. Ministry of Infrastructure and the Environment, The Hague, the Netherlands. [Google Scholar]
- 56.Buse HY, Ashbolt NJ. 2011. Differential growth of Legionella pneumophila strains within a range of amoebae at various temperatures associated with in-premise plumbing. Lett Appl Microbiol 53:217–224. doi: 10.1111/j.1472-765X.2011.03094.x. [DOI] [PubMed] [Google Scholar]
- 57.Horwitz MA, Silverstein SC. 1980. Legionnaires' disease bacterium (Legionella pneumophila) multiplies intracellularly in human monocytes. J Clin Invest 66:441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kozak NA, Benson RF, Brown E, Alexander NT, Taylor TH, Shelton BG, Fields BS. 2009. Distribution of Lag-1 alleles and sequence-based types among Legionella pneumophila serogroup 1 clinical and environmental isolates in the United States. J Clin Microbiol 47:2525–2535. doi: 10.1128/JCM.02410-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ginevra C, Jacotin N, Diancourt L, Guigon G, Arquilliere R, Meugnier H, Descours G, Vandenesch F, Etienne J, Lina G, Caro V, Jarraud S. 2012. Legionella pneumophila sequence type 1/Paris pulsotype subtyping by spoligotyping. J Clin Microbiol 50:696–701. doi: 10.1128/JCM.06180-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Underwood AP, Jones G, Mentasi M, Fry NK, Harrison TG. 2013. Comparison of the Legionella pneumophila population structure as determined by sequence-based typing and whole genome sequencing. BMC Microbiol 13:302–321. doi: 10.1186/1471-2180-13-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuiper MW, Wullings BA, Akkermans ADL, Beumer RR, van der Kooij D. 2004. Intracellular proliferation of Legionella pneumophila in Hartmannella vermiformis in aquatic biofilms grown on plasticized polyvinyl chloride. Appl Environ Microbiol 70:6826–6833. doi: 10.1128/AEM.70.11.6826-6833.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marcilla A, Garcia S, Garcia-Quesada JC. 2004. Study of the migration of PVC plasticizers. J Anal Appl Pyrolysis 71:457–463. doi: 10.1016/S0165-2370(03)00131-1. [DOI] [Google Scholar]
- 63.Thomas JM, Thomas J, Stuetz RM, Ashbolt NJ. 2014. Your garden hose: a potential health risk due to Legionella spp. growth facilitated by free-living amoebae. Environ Sci Technol 48:10456–10464. doi: 10.1021/es502652n. [DOI] [PubMed] [Google Scholar]
- 64.Steinert M, Emödy L, Amann R, Hacker J. 1997. Resuscitation of viable but nonculturable Legionella pneumophila Philadelphia JR32 by Acanthamoeba castellanii. Appl Environ Microbiol 63:2047–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.James BW, Mauchline WS, Dennis PJ, Keevil CW, Wait R. 1999. Poly-3-hydroxybutyrate in Legionella pneumophila, an energy source for survival in low nutrient environments. Appl Environ Microbiol 65:822–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ohno A, Kato N, Yamada K, Yamaguchi K. 2003. Factors influencing survival of Legionella pneumophila serotype 1 in hot spring water and tap water. Appl Environ Microbiol 69:2540–2547. doi: 10.1128/AEM.69.5.2540-2547.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sanden GN, Morrill WE, Fields BS, Breiman RF, Barbaree JM. 1992. Incubation of water samples containing amoebae improves detection of legionellae by the culture method. Appl Environ Microbiol 58:2001–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wadowsky RM, Butler LJ, Cook MK, Verma SM, Paul MA, Fields BS, Keleth G, Sukora JL, Yee RB. 1988. Growth-supporting activity for Legionella pneumophila in tap water cultures and implication of hartmannellid amoebae as growth factors. Appl Environ Microbiol 54:2677–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murga R, Forster TS, Brown E, Pruckler JM, Fields BS, Donlan RM. 2001. Role of biofilms in the survival of Legionella pneumophila in a model potable-water system. Microbiology 147:3121–3126. doi: 10.1099/00221287-147-11-3121. [DOI] [PubMed] [Google Scholar]
- 70.Griffin JL. 1972. Temperature tolerance of pathogenic and nonpathogenic free-living amoebas. Science 178:869–870. doi: 10.1126/science.178.4063.869. [DOI] [PubMed] [Google Scholar]
- 71.De Jonckheere JF. 1980. Growth characteristics, cytopathic effect in cell culture, and virulence in mice of 36 type strains belonging to 19 different Acanthamoeba spp. Appl Environ Microbiol 39:681–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rohr U, Weber S, Michel R, Selenka F, Wilhelm M. 1998. Comparison of free-living amoebae in hot water systems of hospitals with isolates from moist areas by identifying genera and determining temperature tolerance. Appl Environ Microbiol 64:1822–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thomas V, Herrera-Rimann K, Blanc DS, Greub G. 2006. Biodiversity of amoebae and amoeba-resisting bacteria in a hospital water network. Appl Environ Microbiol 72:2428–2438. doi: 10.1128/AEM.72.4.2428-2438.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fields BS, Nerad TA, Sawyer TK, King CH, Barbaree JM, Martin WT, Morrill WE, Sanden GN. 1990. Characterization of an axenic strain of Hartmannella vermiformis obtained from an investigation of nosocomial legionellosis. J Protozool 3:581–583. [DOI] [PubMed] [Google Scholar]
- 75.Kuchta JM, States SJ, Wadowsky RM, Byers TJ. 1998. Interactions of Legionella pneumophila with Hartmannella vermiformis including the efficacy of chlorine and silver ions to disrupt the intra-amoebic multiplication of L. pneumophila. Recent Res Dev Microbiol 2:405–425. [Google Scholar]
- 76.Michel R, Hoffmann R, Gless A, Müller KD. 1995. Prevalence of Acanthamoebae, Naegleriaea and other free-living amoebae in the course of water treatment in three well waterworks. Acta Hydrochim Hydrobiol 23:202–211. doi: 10.1002/aheh.19950230503. [DOI] [Google Scholar]
- 77.Valster RM, Wullings BA, Bakker G, Smidt H, van der Kooij D. 2009. Free-living protozoa in two unchlorinated drinking water supplies identified by phylogenetic analysis of 18S rRNA gene sequences. Appl Environ Microbiol 75:4736–4746. doi: 10.1128/AEM.02629-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.