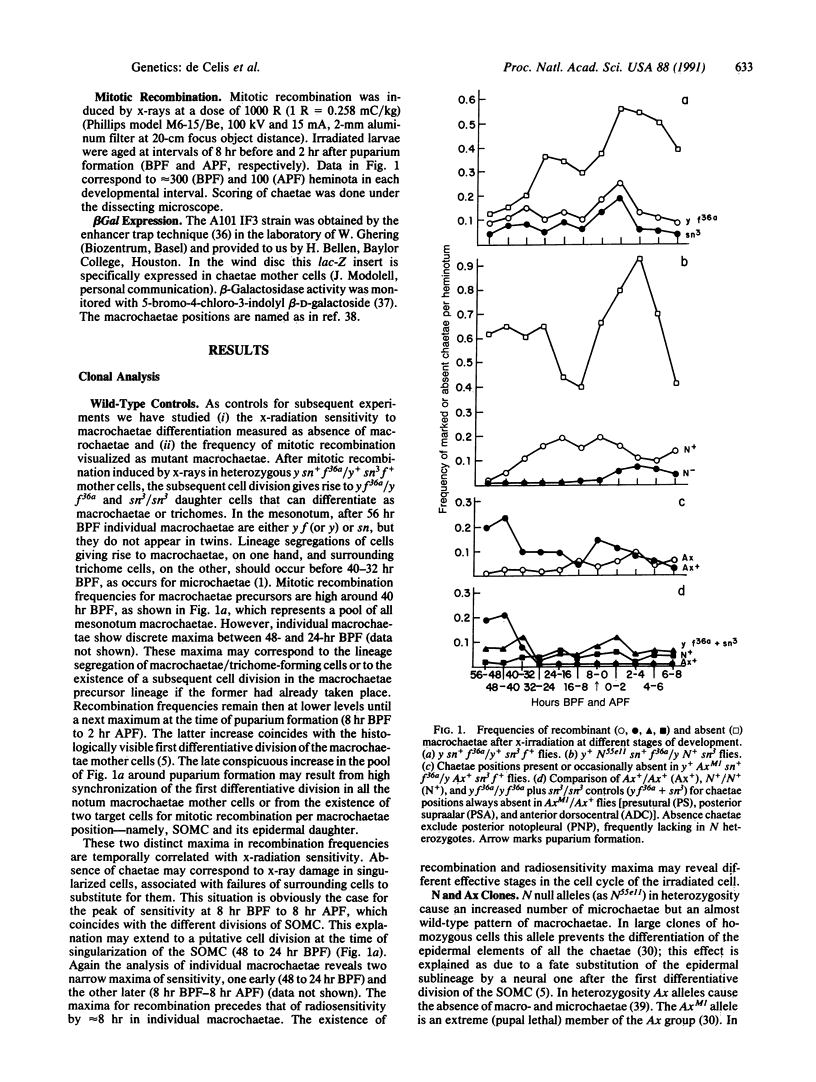
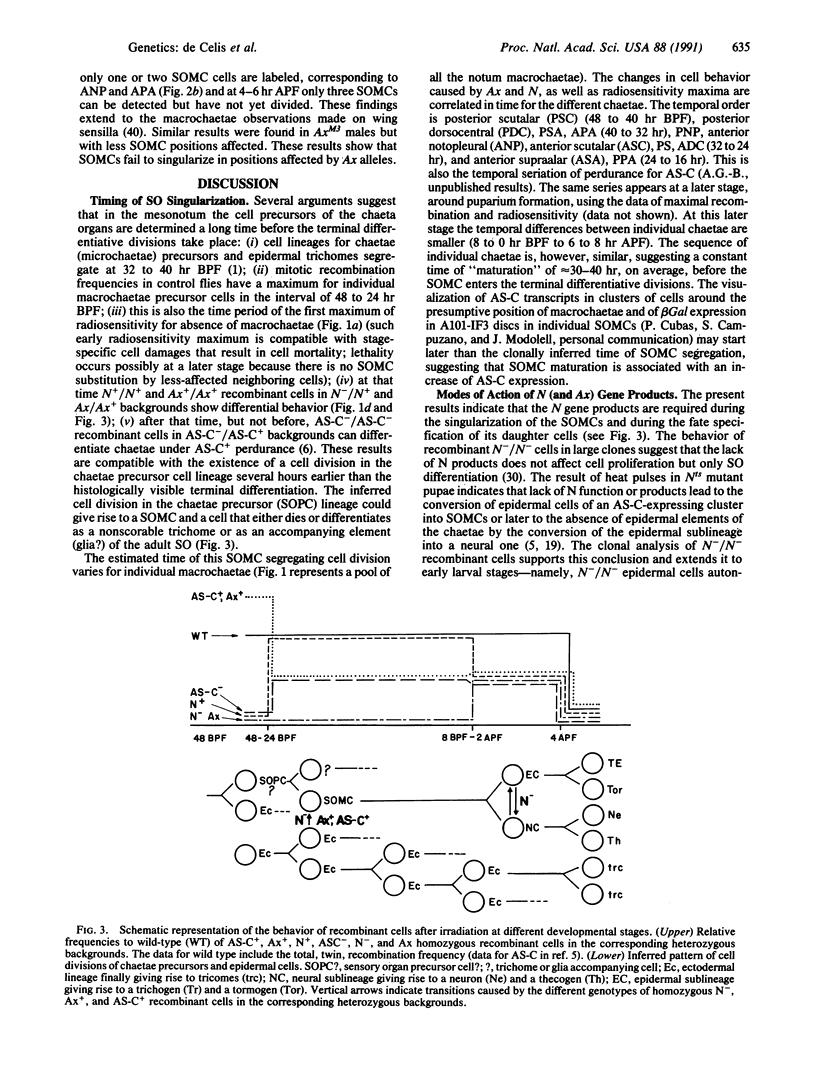
Abstract
The gene Notch (N) codes for a transmembrane protein with an extracellular domain that has homologies to epidermal growth factors and an intracellular domain that could be involved in signal transduction. N null alleles cause the transformation of most epidermal cells into neuroblasts in central and peripheral nervous systems. Alleles of the same gene, called Abruptex (Ax), that map to the extracellular domain of N protein cause the absence of adult sensory organs. Both types of alleles show cell autonomy in mosaic analysis carried out in the last stages of the formation of adult sensory organs. The phenotypes are different: cells lacking N gene products differentiate as sensory organ mother cells early and as its neural sublineage later, whereas in the homozygous Ax condition epidermal cells do not enter the sensory organ mother cell pathway. The results indicate that N gene products act internally in the cell, probably as receptors of intercellular signals both in sensory organ mother cell singularization and in fate specification of its daughter cells. Ax mutations behave as an excess of N+ function in this signal transduction process. N proteins modified by these mutations act as constitutively activated.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso M. C., Cabrera C. V. The achaete-scute gene complex of Drosophila melanogaster comprises four homologous genes. EMBO J. 1988 Aug;7(8):2585–2591. doi: 10.1002/j.1460-2075.1988.tb03108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S. The molecular biology of the Notch locus and the fine tuning of differentiation in Drosophila. Trends Genet. 1988 Apr;4(4):95–100. doi: 10.1016/0168-9525(88)90096-0. [DOI] [PubMed] [Google Scholar]
- Cabrera C. V., Martinez-Arias A., Bate M. The expression of three members of the achaete-scute gene complex correlates with neuroblast segregation in Drosophila. Cell. 1987 Jul 31;50(3):425–433. doi: 10.1016/0092-8674(87)90496-x. [DOI] [PubMed] [Google Scholar]
- Campos-Ortega J. A. Cellular interactions during early neurogenesis of Drosophila melanogaster. Trends Neurosci. 1988 Sep;11(9):400–405. doi: 10.1016/0166-2236(88)90077-x. [DOI] [PubMed] [Google Scholar]
- Campuzano S., Carramolino L., Cabrera C. V., Ruíz-Gómez M., Villares R., Boronat A., Modolell J. Molecular genetics of the achaete-scute gene complex of D. melanogaster. Cell. 1985 Feb;40(2):327–338. doi: 10.1016/0092-8674(85)90147-3. [DOI] [PubMed] [Google Scholar]
- Dietrich U., Campos-Ortega J. A. The expression of neurogenic loci in imaginal epidermal cells of Drosophila melanogaster. J Neurogenet. 1984 Dec;1(4):315–332. doi: 10.3109/01677068409107094. [DOI] [PubMed] [Google Scholar]
- Fehon R. G., Kooh P. J., Rebay I., Regan C. L., Xu T., Muskavitch M. A., Artavanis-Tsakonas S. Molecular interactions between the protein products of the neurogenic loci Notch and Delta, two EGF-homologous genes in Drosophila. Cell. 1990 May 4;61(3):523–534. doi: 10.1016/0092-8674(90)90534-l. [DOI] [PubMed] [Google Scholar]
- Garcia-Bellido A., Dapena J. Induction, detection and characterization of cell differentiation mutants in Drosophila. Mol Gen Genet. 1974;128(2):117–130. doi: 10.1007/BF02654485. [DOI] [PubMed] [Google Scholar]
- Garcia-Bellido A., Merriam J. R. Parameters of the wing imaginal disc development of Drosophila melanogaster. Dev Biol. 1971 Jan;24(1):61–87. doi: 10.1016/0012-1606(71)90047-9. [DOI] [PubMed] [Google Scholar]
- García-Bellido A., Santamaria P. Developmental Analysis of the Achaete-Scute System of DROSOPHILA MELANOGASTER. Genetics. 1978 Mar;88(3):469–486. doi: 10.1093/genetics/88.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghysen A., Dambly-Chaudiere C. Genesis of the Drosophila peripheral nervous system. Trends Genet. 1989 Aug;5(8):251–255. doi: 10.1016/0168-9525(89)90097-8. [DOI] [PubMed] [Google Scholar]
- Ghysen A., O'Kane C. Neural enhancer-like elements as specific cell markers in Drosophila. Development. 1989 Jan;105(1):35–52. doi: 10.1242/dev.105.1.35. [DOI] [PubMed] [Google Scholar]
- González F., Romani S., Cubas P., Modolell J., Campuzano S. Molecular analysis of the asense gene, a member of the achaete-scute complex of Drosophila melanogaster, and its novel role in optic lobe development. EMBO J. 1989 Dec 1;8(12):3553–3562. doi: 10.1002/j.1460-2075.1989.tb08527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartenstein V., Posakony J. W. Development of adult sensilla on the wing and notum of Drosophila melanogaster. Development. 1989 Oct;107(2):389–405. doi: 10.1242/dev.107.2.389. [DOI] [PubMed] [Google Scholar]
- Hoppe P. E., Greenspan R. J. Local function of the Notch gene for embryonic ectodermal pathway choice in Drosophila. Cell. 1986 Aug 29;46(5):773–783. doi: 10.1016/0092-8674(86)90353-3. [DOI] [PubMed] [Google Scholar]
- Jiménez F., Campos-Ortega J. A. A region of the Drosophila genome necessary for CNS development. Nature. 1979 Nov 15;282(5736):310–312. doi: 10.1038/282310a0. [DOI] [PubMed] [Google Scholar]
- Kelley M. R., Kidd S., Deutsch W. A., Young M. W. Mutations altering the structure of epidermal growth factor-like coding sequences at the Drosophila Notch locus. Cell. 1987 Nov 20;51(4):539–548. doi: 10.1016/0092-8674(87)90123-1. [DOI] [PubMed] [Google Scholar]
- Kidd S., Baylies M. K., Gasic G. P., Young M. W. Structure and distribution of the Notch protein in developing Drosophila. Genes Dev. 1989 Aug;3(8):1113–1129. doi: 10.1101/gad.3.8.1113. [DOI] [PubMed] [Google Scholar]
- Kidd S., Kelley M. R., Young M. W. Sequence of the notch locus of Drosophila melanogaster: relationship of the encoded protein to mammalian clotting and growth factors. Mol Cell Biol. 1986 Sep;6(9):3094–3108. doi: 10.1128/mcb.6.9.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd S., Lockett T. J., Young M. W. The Notch locus of Drosophila melanogaster. Cell. 1983 Sep;34(2):421–433. doi: 10.1016/0092-8674(83)90376-8. [DOI] [PubMed] [Google Scholar]
- Kopczynski C. C., Alton A. K., Fechtel K., Kooh P. J., Muskavitch M. A. Delta, a Drosophila neurogenic gene, is transcriptionally complex and encodes a protein related to blood coagulation factors and epidermal growth factor of vertebrates. Genes Dev. 1988 Dec;2(12B):1723–1735. doi: 10.1101/gad.2.12b.1723. [DOI] [PubMed] [Google Scholar]
- Markopoulou K., Artavanis-Tsakonas S. The expression of the neurogenic locus Notch during the postembryonic development of Drosophila melanogaster and its relationship to mitotic activity. J Neurogenet. 1989 Sep;6(1):11–26. doi: 10.3109/01677068909107097. [DOI] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989 Mar 10;56(5):777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- O'Kane C. J., Gehring W. J. Detection in situ of genomic regulatory elements in Drosophila. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9123–9127. doi: 10.1073/pnas.84.24.9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palka J., Schubiger M., Schwaninger H. Neurogenic and antineurogenic effects from modifications at the Notch locus. Development. 1990 May;109(1):167–175. doi: 10.1242/dev.109.1.167. [DOI] [PubMed] [Google Scholar]
- Romani S., Campuzano S., Macagno E. R., Modolell J. Expression of achaete and scute genes in Drosophila imaginal discs and their function in sensory organ development. Genes Dev. 1989 Jul;3(7):997–1007. doi: 10.1101/gad.3.7.997. [DOI] [PubMed] [Google Scholar]
- Romani S., Campuzano S., Modolell J. The achaete-scute complex is expressed in neurogenic regions of Drosophila embryos. EMBO J. 1987 Jul;6(7):2085–2092. doi: 10.1002/j.1460-2075.1987.tb02474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel M. F., Downing J. R., Rettenmier C. W., Sherr C. J. A point mutation in the extracellular domain of the human CSF-1 receptor (c-fms proto-oncogene product) activates its transforming potential. Cell. 1988 Dec 23;55(6):979–988. doi: 10.1016/0092-8674(88)90243-7. [DOI] [PubMed] [Google Scholar]
- Shellenbarger D. L., Mohler J. D. Temperature-sensitive periods and autonomy of pleiotropic effects of l(1)Nts1, a conditional notch lethal in Drosophila. Dev Biol. 1978 Feb;62(2):432–446. doi: 10.1016/0012-1606(78)90226-9. [DOI] [PubMed] [Google Scholar]
- Technau G. M., Campos-Ortega J. A. Cell autonomy of expression of neurogenic genes of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4500–4504. doi: 10.1073/pnas.84.13.4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villares R., Cabrera C. V. The achaete-scute gene complex of D. melanogaster: conserved domains in a subset of genes required for neurogenesis and their homology to myc. Cell. 1987 Jul 31;50(3):415–424. doi: 10.1016/0092-8674(87)90495-8. [DOI] [PubMed] [Google Scholar]
- Vässin H., Bremer K. A., Knust E., Campos-Ortega J. A. The neurogenic gene Delta of Drosophila melanogaster is expressed in neurogenic territories and encodes a putative transmembrane protein with EGF-like repeats. EMBO J. 1987 Nov;6(11):3431–3440. doi: 10.1002/j.1460-2075.1987.tb02666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton K. A., Johansen K. M., Xu T., Artavanis-Tsakonas S. Nucleotide sequence from the neurogenic locus notch implies a gene product that shares homology with proteins containing EGF-like repeats. Cell. 1985 Dec;43(3 Pt 2):567–581. doi: 10.1016/0092-8674(85)90229-6. [DOI] [PubMed] [Google Scholar]
- Xu T., Rebay I., Fleming R. J., Scottgale T. N., Artavanis-Tsakonas S. The Notch locus and the genetic circuitry involved in early Drosophila neurogenesis. Genes Dev. 1990 Mar;4(3):464–475. doi: 10.1101/gad.4.3.464. [DOI] [PubMed] [Google Scholar]






