Abstract
While decades of research have focused on snake venom proteins, far less attention has been paid to small organic venom constituents. Using mostly pooled samples, we surveyed 31 venoms (six elapid, six viperid, and 19 crotalid) for spermine, spermidine, putrescine, and cadaverine. Most venoms contained all four polyamines, although some in essentially trace quantities. Spermine is a potentially significant component of many viperid and crotalid venoms (≤0.16% by mass, or 7.9 µmol/g); however, it is almost completely absent from elapid venoms assayed. All elapid venoms contained larger molar quantities of putrescine and cadaverine than spermine, but still at levels that are likely to be biologically insignificant. As with venom purines, polyamines impact numerous physiological targets in ways that are consistent with the objectives of prey envenomation, prey immobilization via hypotension and paralysis. Most venoms probably do not contain sufficient quantities of polyamines to induce systemic effects in prey; however, local effects seem probable. A review of the pharmacological literature suggests that spermine could contribute to prey hypotension and paralysis by interacting with N-methyl-d-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, nicotinic and muscarinic acetylcholine receptors, γ-Aminobutyric acid (GABA) receptors, blood platelets, ryanodine receptors, and Ca2+-ATPase. It also blocks many types of cation-permeable channels by interacting with negatively charged amino acid residues in the channel mouths. The site of envenomation probably determines which physiological targets assume the greatest importance; however, venom-induced liberation of endogenous, intracellular stores of polyamines could potentially have systemic implications and may contribute significantly to envenomation sequelae.
Keywords: polyamines, snake venoms, spermine, spermidine, putrescine, cadaverine, titer, pharmacology, envenomation sequelae
1. Introduction
In an ongoing investigation of protein expression regulatory networks in venom glands of the Taiwan habu (Protobothrops mucrosquamatus), we were surprised to discover that the gene for spermine synthase was strongly upregulated, along with genes for well-established venom proteins. This indicated that spermine has some role either in the venom synthetic machinery or is a constituent of the venom itself. A search of the snake venom literature revealed only three papers that mentioned spermine. In 1960, using paper chromatography, Sasaki [1] identified spermine and histamine in the venom of the Taiwan habu (Protobothrops mucrosquamatus). In 1965, Shipolini et al. [2] found that the venom of the Bulgarian viper (Vipera ammodytes ammodytes) contains spermine, but not histamine. No further reports of spermine appeared in the literature until 2007, when Merkel et al. identified spermine as the trypanicidal component in the venom of the viper, Eristocophis macmahoni [3]. While none of the foregoing papers addressed the question of spermine’s function in envenomation, Merkel et al. reported that it constituted 1% of the dry mass of their venom sample. This high titer suggested that spermine has a functional role in envenomation, and that it is not simply a fortuitous trypanicide.
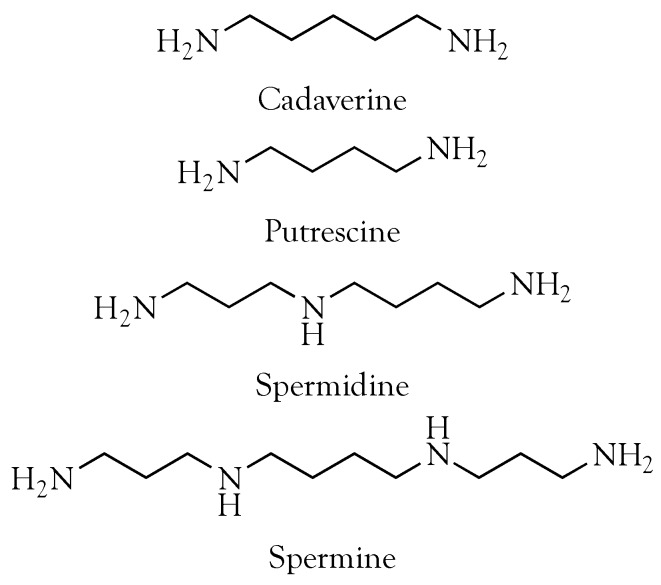
Unlike the arylpolyamines that are abundant components of many spider venoms [4,5,6,7,8], the structures of putrescine, cadaverine, spermidine, and spermine effectively render them invisible at 280 nm (Figure 1), traditionally used in liquid chromatography of snake venom proteins, because that wavelength detects the aromatic amino acids, tryptophan, tyrosine, and phenylalanine. The long-UV invisibility of polyamines, coupled with their small size, probably explains their virtual absence in the snake venom literature.
Figure 1.
Structures of putrescine, cadaverine, spermidine, and spermine. At physiological pH, the amino groups are positively charged.
Because most snakes prey upon mammals, we used a combination of polyamine derivatization and liquid chromatography-mass spectrometry to identify and quantify the four mammalian polyamines spermine, spermidine, putrescine, and cadaverine, not only in the venom of Protobothrops mucrosquamatus, but also in a selection of Old and New World elapid, viperid, and crotalid venoms. Here we confirm their presence in a phylogenetically diverse array of snake venoms. All polyamines display broad taxonomic distributions. The abundance of spermine confirms that it is a significant constituent of many viperid and crotalid snake venoms and implies a functional role in envenomation. Here we present the results of our polyamine taxonomic survey. We review the pharmacology of polyamines that appears pertinent to snake envenomation and we discuss the possible contributions of spermine and other polyamines to prey immobilization.
2. Results
2.1. Detection, Quantification, and Distribution of Polyamines in Snake Venoms
We assayed 31 venoms representing the three major lineages of advanced venomous snakes (Elapidae, Viperinae, and Crotalinae) (Table 1) by LC-MS to determine whether they contained spermine, and if so, to quantify how much was present. In the process we unexpectedly also identified putrescine, spermidine, and cadaverine in all venoms (Figure 1). In fact, most venoms examined contained all four polyamines, but quantities of each varied dramatically among taxa (Table 1).
Table 1.
Polyamine concentrations (µg and nmol polyamine/g venom) in venoms of 31 advanced venomous snake taxa, organized by family. Differences between taxa are large, even interspecifically, as the Crotalus and Protobothrops samples indicate. Elapid venoms are generally depauperate in polyamines. Some values contain more significant figures than are appropriate; however, this has been done to align the values so as to make the table intuitive. Full taxonomic names are provided in Apendixs.
| Snake Taxa | Venom Polyamines (µg/g) | Venom Polyamines (nmol/g) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Spermine | Spermidine | Putrescine | Cadaverine | Total | Spermine | Spermidine | Putrescine | Cadaverine | Total | |
| Elapidae | ||||||||||
| B. multicinctus | 0.006 | 0.000 | 0.083 | 0.038 | 0.128 | 0.032 | 0.000 | 0.944 | 0.376 | 1 |
| D. polylepis | 0.006 | 0.000 | 0.064 | 0.013 | 0.083 | 0.032 | 0.000 | 0.726 | 0.125 | 1 |
| M. surinamensis | 0.006 | 0.000 | 0.064 | 0.013 | 0.083 | 0.032 | 0.000 | 0.726 | 0.125 | 1 |
| N. kaouthia | 0.006 | 0.000 | 0.064 | 0.013 | 0.083 | 0.032 | 0.000 | 0.726 | 0.125 | 1 |
| N. sputatrix | 0.006 | 0.000 | 0.064 | 0.013 | 0.083 | 0.032 | 0.000 | 0.726 | 0.125 | 1 |
| O. hannah | 0.013 | 0.000 | 0.077 | 0.019 | 0.109 | 0.063 | 0.000 | 0.871 | 0.188 | 1 |
| Mean | 0.007 | 0.000 | 0.069 | 0.018 | 0.095 | 0.037 | 0.000 | 0.787 | 0.177 | 1 |
| Std. Deviation | 0.003 | 0.000 | 0.009 | 0.010 | 0.019 | 0.013 | 0.000 | 0.097 | 0.100 | 0 |
| Viperidae | ||||||||||
| B. gabonica | 1.178 | 0.166 | 3.085 | 0.166 | 4.595 | 5.820 | 1.146 | 34.995 | 1.628 | 44 |
| B. nasicornis | 81.158 | 3.104 | 12.864 | 1.568 | 98.694 | 401.099 | 21.370 | 145.933 | 15.345 | 584 |
| C. cerastes | 246.477 | 9.971 | 38.003 | 2.899 | 297.350 | 1218.132 | 68.649 | 431.117 | 28.373 | 1746 |
| D. palestinae | 2.835 | 0.160 | 6.259 | 1.504 | 10.758 | 14.012 | 1.102 | 71.006 | 14.719 | 101 |
| D. r. siamensis | 50.816 | 2.080 | 35.866 | 1.555 | 90.317 | 251.142 | 14.320 | 406.870 | 15.220 | 688 |
| P. fieldi | 0.019 | 0.000 | 0.102 | 0.013 | 0.134 | 0.095 | 0.000 | 1.162 | 0.125 | 1 |
| Mean | 63.747 | 2.580 | 16.030 | 1.284 | 83.642 | 315.050 | 17.764 | 181.847 | 12.569 | 527 |
| Std. Deviation | 95.443 | 3.834 | 16.751 | 1.066 | 113.557 | 471.697 | 26.395 | 190.031 | 10.428 | 664 |
| Crotalidae | ||||||||||
| A. c. contortrix | 220.019 | 0.448 | 6.381 | 1.523 | 228.371 | 1087.347 | 3.084 | 72.386 | 14.907 | 1178 |
| A. p. leucostoma | 80.627 | 0.627 | 1.280 | 0.307 | 82.842 | 398.474 | 4.318 | 14.521 | 3.006 | 420 |
| A. nummifer | 0.051 | 0.006 | 0.077 | 0.013 | 0.147 | 0.253 | 0.044 | 0.871 | 0.125 | 1 |
| B. erythromelas | 0.058 | 0.006 | 0.064 | 0.013 | 0.141 | 0.285 | 0.044 | 0.726 | 0.125 | 1 |
| B. moojeni | 0.608 | 0.083 | 0.493 | 0.102 | 1.286 | 3.005 | 0.573 | 5.590 | 1.002 | 10 |
| B. schlegelii | 104.467 | 1.466 | 6.374 | 1.504 | 113.811 | 516.295 | 10.090 | 72.313 | 14.719 | 613 |
| C. rhodostoma | 67.962 | 0.154 | 6.227 | 1.498 | 75.840 | 335.878 | 1.057 | 70.643 | 14.656 | 422 |
| C. sasai | 0.160 | 0.006 | 0.077 | 0.013 | 0.256 | 0.791 | 0.044 | 0.871 | 0.125 | 2 |
| C. adamanteus | 10.822 | 2.688 | 11.712 | 1.568 | 26.790 | 53.486 | 18.506 | 132.864 | 15.345 | 220 |
| C. cerastes | 3.885 | 2.144 | 7.974 | 1.510 | 15.514 | 19.199 | 14.761 | 90.464 | 14.782 | 139 |
| C. d. terrificus | 30.598 | 12.890 | 23.725 | 1.670 | 68.883 | 151.223 | 88.741 | 269.141 | 16.348 | 525 |
| C. m. pyrrhus | 0.115 | 0.013 | 0.122 | 0.013 | 0.262 | 0.569 | 0.088 | 1.379 | 0.125 | 2 |
| C. v. concolor | 1.984 | 1.062 | 1.216 | 0.019 | 4.282 | 9.805 | 7.314 | 13.795 | 0.188 | 31 |
| C. v. viridis | 2.972 | 1.238 | 1.317 | 0.022 | 5.549 | 14.687 | 8.524 | 14.942 | 0.216 | 38 |
| L. stenophrys | 26.240 | 0.902 | 2.022 | 0.301 | 29.466 | 129.683 | 6.213 | 22.943 | 2.944 | 162 |
| O. okinavensis | 0.090 | 0.006 | 0.070 | 0.019 | 0.186 | 0.443 | 0.044 | 0.799 | 0.188 | 1 |
| P. elegans | 469.459 | 0.179 | 6.214 | 1.504 | 477.357 | 2320.150 | 1.234 | 70.498 | 14.719 | 2407 |
| P. flavoviridis | 17.376 | 0.173 | 6.208 | 1.498 | 25.254 | 85.875 | 1.190 | 70.425 | 14.656 | 172 |
| P. mucrosquamatus | 1602.214 | 11.315 | 61.747 | 14.963 | 1690.240 | 7918.426 | 77.902 | 700.479 | 146.440 | 8843 |
| Mean | 138.932 | 1.864 | 7.542 | 1.477 | 149.815 | 686.626 | 12.830 | 85.561 | 14.454 | 799 |
| Std. Deviation | 372.175 | 3.699 | 14.341 | 3.346 | 390.393 | 1839.355 | 25.468 | 162.691 | 32.745 | 2033 |
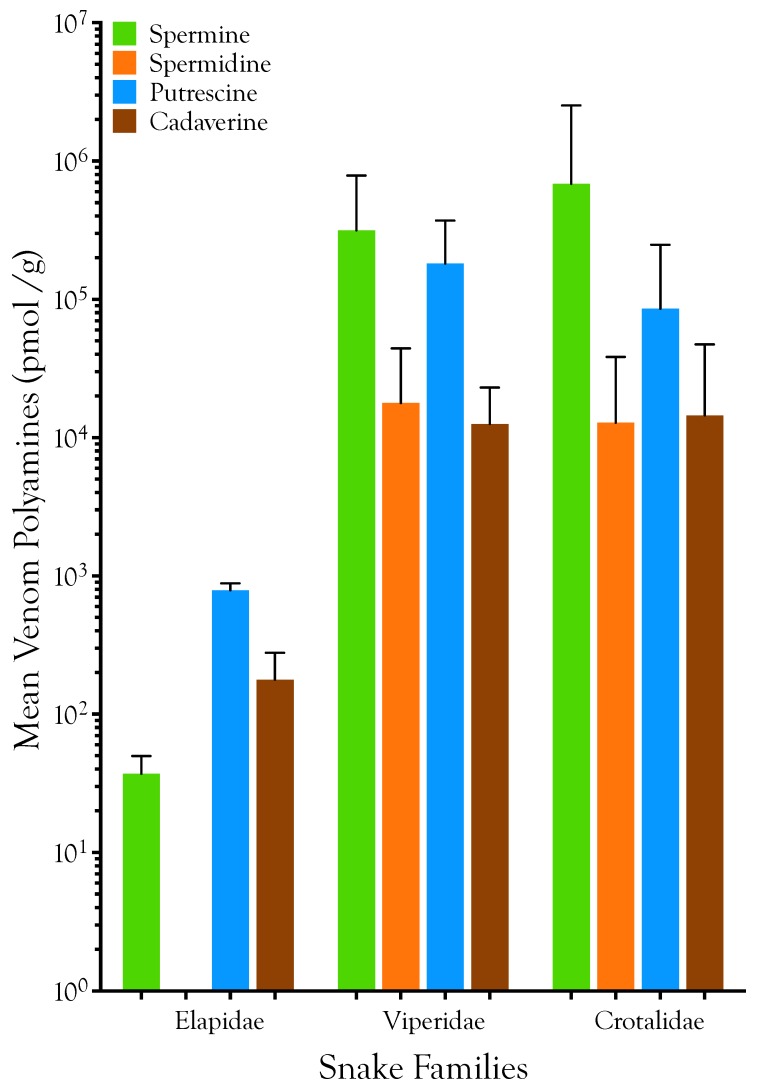
Polyamines are relatively minor components of elapid venoms (Figure 2 and Figure 3; Table 1) and it is doubtful that they serve significant functions in envenomation. Specifically, spermine is almost absent from the six elapid venoms we examined (mean = 0.007 µg/g; range 0.006–0.013 µg/g; n = 6 species; pooled samples). Even though it is an intermediate in the spermine anabolic pathway, spermidine is universally absent in elapid venoms. In contradistinction to most viperid and crotalid venoms, putrescine (mean = 0.069 µg/g; 0.064–0.083 µg/g) and cadaverine (mean = 0.018 µg/g; 0.013–0.038 µg/g) are the dominant polyamines, although in elapid venoms these are still extremely minor venom components at best.
Figure 2.
Polyamines are apparently components of the venoms of all advanced venomous snakes. They are extremely minor constituents of elapid venoms, and venom polyamines probably contribute little to elapid envenomations. Putrescine is the dominant elapid venom polyamine. Viperid and crotalid venom polyamines are much more abundant, but whether they are able to induce significant systemic physiological impairment in prey, independent of other venom constituents, is unclear. However, it is possible that they provoke localized responses, such as skeletal muscle paralysis at the site of injection. Spermine and putrescine are the most significant viperid and crotalid polyamines, but variation between taxa is large. Because polyamine abundance spans several orders of magnitude, bars show the log10 of polyamine concentration in pmol/g venom. Error bars indicate 1 standard deviation.
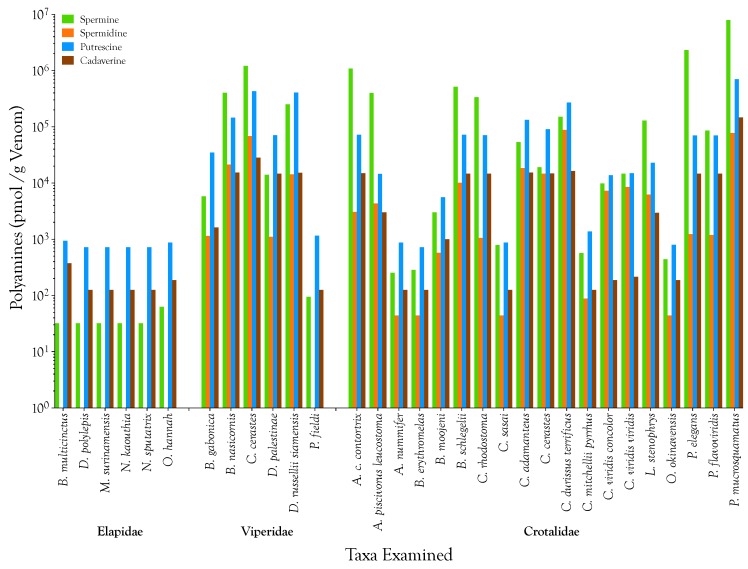
Figure 3.
Polyamine levels in venoms of 31 venomous snake taxa, grouped by family and arranged alphabetically within families. The range of polyamine spanned several orders of magnitude, necessitating a logarithmic Y axis. For that reason, values are expressed as pmol polyamine/g venom. Polyamines are negligible constituents of the elapid venoms examined. They are relatively more abundant in viperid and crotalid venoms, but beyond that it is difficult to discern any distributional patterns at higher taxonomic levels. Interspecific variability, within genera, is considerable, as shown for the genera Bitis, Bothrops, Crotalus, and Protobothrops. Spermine is generally the most abundant polyamine, although in the elapid venoms examined and in our samples of Bitis gabonica, Daboia russellii siamensis, Crotalus durissus terrificus, Crotalus mitchellii pyrrhus, and Crotalus viridis concolor venoms, putrescine was dominant. Spermidine was a significant component only in the venoms of two Crotalus viridis subspecies, from geographically contiguous regions in western Colorado. Significant levels of cadaverine were seen only in Protobothrops mucrosquamatus venom.
Spermine is relatively much more abundant in viperid venoms (µ = 63.8 µg/g; range 0.019–247 µg/g; n = 6 species; pooled and individual samples), and reaches its highest concentrations in some crotalid venoms (µ = 139 µg/g; 0.051–1600 µg/g; n = 19 species; pooled and individual samples). In Protobothrops mucrosquamatus venom, ~7.9 µmol spermine is present per gram of venom (Figure 2 and Figure 3; Table 1).
While spermine is the major polyamine in most venoms examined, putrescine was the most abundant polyamine in all six elapid venoms and in those of Bitis gabonica, Daboia palestinae, Pseudocerastes fieldi, Atropoides nummifer, Bothrops erythromelas, Crotalus adamanteus, Crotalus cerastes, and C. mitchellii pyrrhus (Figure 3; Table 1). Spermidine was a major polyamine only in venoms of Crotalus durissus terrificus and Protobothrops mucrosquamatus. Cadaverine, which is produced from lysine via a different anabolic pathway than the other three polyamines, was a minor to negligible constituent in all venoms examined (Figure 3). It reached its highest levels in the venoms of Protobothrops mucrosquamatus (Figure 3; Table 1).
Merkel et al. reported a value of 9.14 µg/mg (9.14 mg/g) of spermine in the venom of the viperid, Eristocophis macmahoni [3]. This is nearly 6× higher than the highest value recorded in our study (Protobothrops mucrosquamatus) and 37× higher than the highest viperid venom (Bitis gabonica). Merkel et al. have clearly identified spermine in Eristocophis venom; however, we believe that their quantification is spuriously high. Given that their calibration curves look appropriate, several factors may contribute to this discrepancy. First, they did not derivatize their sample, in which case, various polyamines could produce the same fragments [9]. However, the sum of all other polyamines is unlikely to be large enough to account for the difference between their value and ours. In our initial experiments, we did not derivatize the polyamines either. We found that the spermine standard eluted from the C18 column with poor reproducibility. This problem can be exacerbated by other polyamines or other classes of compounds present in venom. Chromatographic methods to circumnavigate this difficulty without derivatization tend to be fairly complex, in part because ion pairing reagents often used to improve chromatographic separations result in signal suppression for basic compounds during mass spectrometry [10]. For this reason, we opted to derivatize our samples. Some derivatization methods in the literature do not go to completion, resulting in multitudes of partial derivatives and rendering quantification impossible. However, the method of Liu et al. [11] yields a single, detectable peak with each of the polyamines assayed. Liu et al. reported a significant increase in sensitivity based upon derivatization with benzoyl chloride; thus our values for spermine ought to have been higher than those of Merkel et al. We cannot explain the difference.
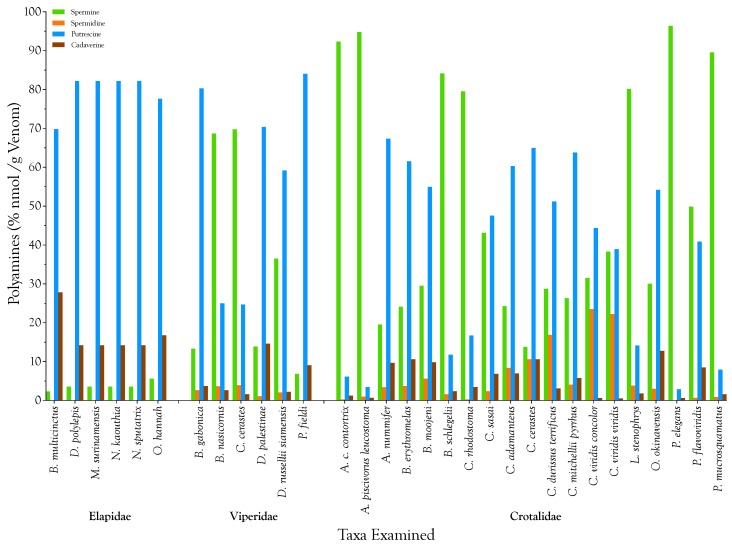
While it is probably meaningless to speak of “polyamine strategies” in relation to elapid venoms, for those species employing significant polyamine titers in their venoms, it is interesting to see how different species have allocated their resources with regard to the polyamine composition of their venoms. For this purpose, total nmol of polyamine per venom sample were calculated. Then each of the four polyamine concentrations was normalized by expressing it as a percentage of the total for that venom sample (Figure 4). All venoms adopted either a putrescine- or spermine-dominant strategy, sometimes to the near exclusion of all other polyamines (Agkistrodon c. contortrix, A. piscivorus leucostoma, Protobothrops elegans, and P. mucrosquamatus) (Figure 4).
Figure 4.
With regard to polyamine composition, venomous snake taxa employ either a putrescine- or a spermine-dominant strategy. Polyamine concentrations in each of 31 venomous snake taxa were normalized by expressing them as percentages of total nmol of polyamine for the taxon (sample) in question; thus all the polyamines in a given sample sum to 100%.
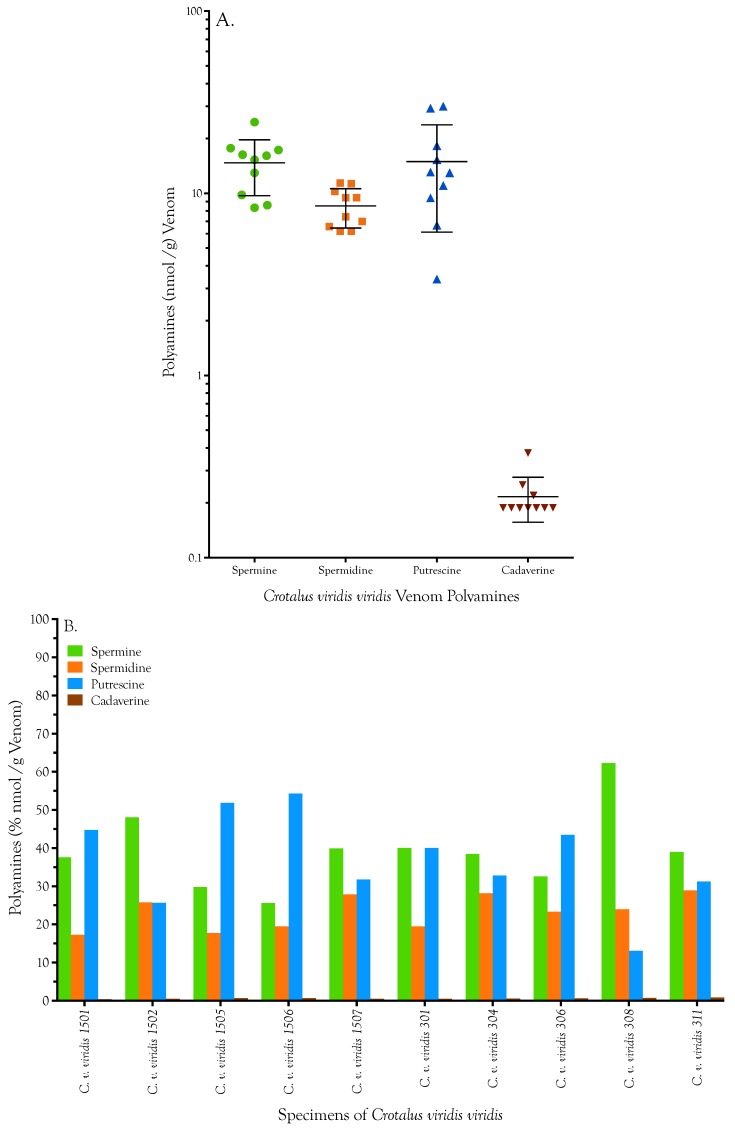
In order to assess the amount of intraspecific variation in polyamine titers, venoms from 10 specimens of Crotalus viridis viridis, representing two western Colorado populations (Buford, Rio Blanco County and Brown’s Park, Moffat County) (Figure 5, Table A1). Two replicates of each specimen were run for each polyamine. We examined the amount of run-to-run variability and intraspecific variation using a multivariate linear model of polyamine concentrations. There was no significant effect of technical replication (p = 0.94), and a small effect of population (p = 0.045). However, the variation between populations was much smaller than that between species (Bartlett’s test p-value < 2.2 × 10−16 in all cases). Replicates of each specimen were highly consistent for all specimens and for all four polyamines (Figure 5, Table A1). In terms of mass, spermine was the most abundant polyamine in all 10 C. v. viridis venoms, while cadaverine was least abundant. However, when considered on a molar basis, the picture changed, except that cadaverine was still least abundant (Figure 5b). On a molar basis, spermine predominated in five specimens, while putrescine was dominant in four. In one specimen they were co-equal.
Figure 5.
Polyamine titers in the venoms of five Crotalus viridis viridis from each of two populations in western Colorado (Buford, Rio Blanco County, CO and Brown’s Park, Moffat County, CO, USA) (nmol polyamine/g venom). (A) Spermine and putrescine showed the highest titers and the greatest dispersion of values. Means for each polyamine are shown ± one standard deviation. (B) Interestingly, in all 10 specimens, on a mass basis, spermine was the most abundant polyamine, while cadaverine was least. However, on a molar basis, spermine was most abundant only in five specimens, while putrescine was most abundant in four.
2.2. Biological Significance of Snake Venom Spermine
Prey classes consumed were documented for 28 of 31 species (Table A2). Personal observations were available for Protobothrops elegans and prey were inferred for the remaining two species, based upon the diets of congeners. Using the R statistical package [12], we fit a multivariate linear model with patterns of polyamine abundance vs. prey consumed by the snake species examined to determine whether the polyamine compositions are related to prey species eaten. However, there was no effect of any prey species on polyamine concentration (p > 0.1 in all cases). There are several possible explanations for this result. We could test only for prey taxa that have been documented. Because such data are not abundant in the literature, prey data compiled here are likely incomplete. That is, many species probably eat other prey types not recorded here. On the other hand, it may not make any difference. Polyamines are clearly minor venom components in many species, and in those species, polyamine abundance is likely to be under minimal selective pressure [13]. Alternatively, there is clearly significant polyamine variation at the family, and perhaps even at the generic level (Figure 4), which could imply the existence of strong phylogenetic constraint acting on these components.
At this point, we cannot categorically exclude the possibility that venom polyamines serve some function in the gland, either to stabilize or temporarily inactivate venom constituents, or to act in a direct regulatory manner on glandular tissue. However, given the phylogenetic patterns mentioned above, we think such explanations for their presence are unlikely.
Quantities of Polyamines Potentially Injected
Hayes [14] reported that prairie rattlesnakes (Crotalus viridis) injected an average of 16 mg of venom when striking mice. In cases involving second bites on the same mouse, that value increased to 22.4 mg, but multiple bites accounted for only about 14% of all feeding strikes. Morrison et al. [15] found that tiger snakes (Notechis scutatus) injected 12.7 mg on average, while taipans (Oxyuranus scutellatus) injected 20.8 mg. Subsequently they reported data for a variety of additional elapids [16]. Death adders (Acanthophis antarcticus), which resemble small viperids, delivered an average of 30.7 mg, while the much larger king brown snakes (Pseudechis australis) averaged 61.6 mg. Other elapids (Pseudonaja textilis, Tropidechis carinatus, Pseudechis collettii, and P. porphyriacus) injected much smaller quantities.
Other factors that influence the amount of venom injected include the temperament and strike behavior of the species, the size of the snake (intraspecific comparisons only), the species and behavior of the prey, and the quantity of venom that the species possesses when the glands are filled to capacity. In regard to the latter variable, over the course of thousands of venom extractions from adult rattlesnakes and other pit vipers, the first author routinely obtained yields of 250–400 µL per extraction (60–140 mg) and one 75-cm Agkistrodon piscivorus once produced an astonishing 1.1 mL (275–385 mg).
If we assume that adult viperids and crotalids are capable of injecting 10–50 mg of venom, using the lowest and highest polyamine titers registered in this study (1.18–8843 nmol/g) (Table 1) we can predict that injections of total polyamines would be in the range of 0.01–442 nmol per injection, or 0.005–221 nmol per fang puncture. For the sake of comparisons, the spermine content of normal erythrocytes is approximately 6–9 nmol/1010 erythrocytes [17,18].
Tabor and Rosenthal [19] reported a transient 26% drop in blood pressure (115 mm Hg to 85 mm) in conscious rats injected i.v. with 0.15 µmol/g of spermine. The response was of the same magnitude as that induced by comparable doses of histamine. The same dose caused 83% mortality in mice due to nephrotoxicity, regardless of injection route (LD50 = 0.128 µmol/g; 2560 nmol/20 g mouse); however, i.v. doses given too rapidly caused immediate death. None of the species examined in this study appear to be capable of injecting quantities of this magnitude into their prey (Table 1).
On the other hand, a snake bite certainly constitutes a rapid injection of a dose, and intravascular punctures undoubtedly occur during predatory strikes. Polyamine oxidation results in the liberation of ammonia, hydrogen peroxide, and acrolein [20,21,22]. Acrolein is highly cytotoxic, but the time course is likely too long to be relevant to envenomation. While small quantities of hydrogen peroxide can probably be metabolized by erythrocyte catalase, oxidation of bolus doses of polyamines probably results in the liberation of lethal quantities of ammonia and peroxide. Moreover, many crotalid venoms contain approximately 100 peptidyl constituents, many of which have pharmacological effects that overlap those of the four polyamines investigated [13,23]. On numerous occasions, the senior author has observed mice bitten by captive rattlesnakes that rolled over or collapsed without taking a single step. Paralysis was effectively instantaneous.
Except in cases involving Cerastes cerastes, Agkistrodon contortrix contortrix, Protobothrops elegans, and P. mucrosquamatus, venom polyamines may exert effects restricted to the bite locale, rather than systemic effects (Figure 2; Table 1). Nonetheless, as will be evident from the discussion of polyamine pharmacology pertinent to envenomation that follows, those local effects should probably not be discounted.
In addition, liberation of endogenous, intracellular polyamine stores from prey tissues, especially leukocytes and erythrocytes [17], by other venom components, could result in a potentially significant contribution to envenomation sequelae, a phenomenon that has been suggested for other types of tissue injury [24]. Polyamine concentrations vary widely between tissue types (from as little as 202 nmol/g of spermine and 145 nmol spermidine in mouse muscle to 5708 nmol/g spermine and 7725 nmol spermidine in the rat prostate) [25]. Comparable values (hundreds of nmoles) have been reported for spermidine in guinea pig tissues [26] and spermine and spermidine in rabbit central and peripheral nervous tissues [27] and the cat brain [28].
Paschen [29] suggested four different mechanisms of polyamine-dependent cell injury. Two of those appear especially pertinent to envenomation: an overactivation of calcium flux resulting in neurotransmitter release and an overactivation of the NMDA receptor complex. Nonetheless, polyamines have various physiological impacts that may contribute to prey immobilization. A brief review of some of these mechanisms is provided below.
2.3. Pharmacology of Spermine that Is Potentially Pertinent to Envenomation
Despite its name, spermine is a constituent of all eukaryotic cells [30,31] and it occurs in vertebrate blood plasma at µM levels [32]. It is also found in synaptic vesicles at concentrations as high as 2.8 mM [33]. Spermine is co-secreted with neurotransmitters [34] and polyamine transport systems exist in glial cells and synaptosomes [33].
Spermine has numerous pharmacological functions that are relevant to snake envenomation of vertebrate and invertebrate prey. Because it is ubiquitous in animal tissues [30,31], like purine nucleosides [35,36], its inclusion in venom represents something of a trump card in the predator-prey arms race. No prey species can possibly develop resistance to it.
2.3.1. Spermine Promotes Hypotension
The acylpolyamines, putrescine, spermidine, and spermine, are constituents of nearly all cells [37]. At physiologic pH, spermine carries a +4 charge and can react ionically with nucleic acids and negatively charged regions of proteins. For this reason, spermine and other polyamines function as Ca2+ antagonists [38,39,40]. De Meis [41] was the first to report that spermine (3 mM) relaxed guinea pig ileum and taenia coli precontracted with acetylcholine, histamine, nicotine, caffeine, or excess potassium. It also relaxed frog skeletal muscle precontracted with acetylcholine, 28 mM potassium, or electrical stimulation. Hashimoto et al. [39] found that 400 µM spermine inhibited spontaneous contractions of rat uterus, an effect that could be overcome by increasing extracellular Ca2+ concentrations. Intravenous injections of spermidine and spermine also induced significant cardiovascular changes in anaesthetized dogs, albeit with lower potencies than catecholamines [42]. Marmo et al. [42] concluded that the hypotensive response was due to histamine release and that i.v. injections of spermine in the vertebral artery elicited hypotensive response via an increase in parasympathetic output. Spermine also decreased baroreceptor reactivity.
Chideckel et al. [43,44] found that polyamines (2 mg/kg) (calculated < 10 µM) relax uterine, gastrointestinal, and respiratory tract smooth muscle, and cause hypotension by reducing peripheral vascular resistance, when infused into rats or dogs. Vasodilation was unaffected by histamine H1 or H2 antagonists, a result that contradicted the conclusion of Marmo et al. Fernández et al. [45] concluded that polyamines inhibit smooth muscle contraction by acting at the plasma membrane to reduce calcium influx. Nilsson et al. [46] reported that 100 µM spermine reduced contractile activity of rat portal vein, and that 1 mM spermine abolished it. Similar results have been obtained using rat bladder strips (10–100 µM spermine) [47] or rat uterus [48]. These effects are evidently mediated by blockade of L-type Ca2+ channels in vascular smooth muscle [49].
Vertebrate arterial endothelial cells express Ca2+-sensing receptors (CaR) [50]. In human embryonic kidney cells (HEK-293) CaRs are activated by increases in spermine concentration [51]. The resulting influx of extracellular Ca2+ liberates IP3- and ryanodine-sensitive intracellular Ca2+ stores [52], leading to the production of nitric oxide [53], and activating IKCa channels [50], resulting in vasodilation.
Spermine also affects cardiac muscle. The negative inotropic effects of 100–500 µM spermine, reported by Ventura et al. [54] are not mediated by nitric oxide or histamine [55]. Guevara-Balcazar et al. found that a P2Y purine receptor antagonist and a nonspecific adenosine receptor antagonist blocked the effects of spermine and concluded that the negative inotropy is mediated at least in part by ATP release.
2.3.2. Spermine Blocks L-Type Voltage-Dependent Calcium Channels, but Exerts Biphasic Effects on N-Type Channels
Six types of voltage-dependent calcium channels (VDCCs), known as L-, N-, P-, Q-, R-, and T-type channels, have been characterized to date [56,57]. When VDCCs open, Ca2+, which is 2 × 104 times more abundant in the extracellular fluid than in the cytoplasm, rushes into the cell [58]. N- (CaV2.2), P-, and Q-type channels (CaV2.1) are primarily responsible for neurotransmitter release at various central sites. R-type channels (CaV2.3) may play a minor role in some cases, but apparently serve primarily to govern synaptic plasticity [56]. It is widely thought that L- (CaV1.1-1.4) and T-type channels (CaV3.1-3.3) generally do not participate in excitatory neurotransmitter release [56,59]. They are present in cardiac and smooth muscle cells and in many neuronal cells. Both open in response to depolarizing stimuli, but T-type channels open at more negative membrane potentials and do so transiently. L-type channels open for longer periods; hence the name. We are unaware of any studies of interactions between spermine and R-type or P-type channels. Herman et al. [60] reported that spermine had no effect on T- or L-type VDCCs in mouse neuroblastoma cells. Eterovic et al. [61] opined that 40% of the calcium channels in rat hippocampal slices were probably of the Q-type, but there do not appear to have been any confirming studies.
One of the earliest reports regarding the action of spermine on VDCCs was that of Pullan et al. [62], who found that spermine competes with ω-conotoxin GVIA at presynaptic N-type VDCCs. Spermine exerts biphasic effects on N-type Ca2+ channels (CaV2.2), which are involved in neurotransmission at many fast synapses, facilitating Ca2+ entry into neuronal cell bodies at nM concentrations, but inhibiting it at µM levels [34].
L-type channels are well known for their role in contraction of smooth (CaV1.2), cardiac (CaV1.1-1.2), and skeletal muscle (CaV1.1), but they also govern liberation of catecholamines from chromaffin cells, dynorphin from rat hippocampal granule cell dendrites, neuropeptides from the posterior pituitary gland, and in at least some cases, excitatory neurotransmitters (e.g., retina) [57,59]. Using the whole-cell patch clamp technique, Bonci et al. reported that L-type channels on presumed dopaminergic neurons of rat midbrain activated NMDA and metabotropic glutamate receptors, via the probable release of aspartate and glutamate.
Schoemaker [63] also concluded that endogenous polyamines function as modulators of L-type VDCCs. Gomez et al. [49] found that spermine inhibited contractions of intestinal smooth muscle in a dose-dependent manner with an EC50 of about 1 mM. Since spermine blocked current induced by 1 µM BAY K 8644, but could not enhance the inhibition caused by 1 µM nifedipine, they concluded that spermine acts by inhibiting Ca2+ currents responsible for generation of action potentials. DiScenna et al. [24] opined that spermine’s reduction of evoked potentials at AMPARs and GABAA receptors is best explained by a blockade of VDCCs, and Eterovic et al. [54] went so far as to suggest that all spermine effects can be explained by inhibition of VDCCs. This conclusion was later supported by Joshi et al. [64] who concluded that in motor neurons, a small portion of the Ca2+ current associated with α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors is attributable to Ca2+-permeable (Type II) AMPA receptors; however, the majority of it is carried by L-type Ca2+ channels activated by AMPA receptors. Cayzac et al. [65] reported that 300 µM spermine inhibited L-type Ca2+ (CaV1.2) channels heterologously expressed in HEK 293 cells, but that 100 nM spermine had no effect, suggesting that at least, CaV1.2 channels do not manifest the biphasic spermine response displayed by N-type channels.
2.3.3. Spermine and Calcium Release (Ryanodine) Receptors
Ryanodine receptors are intracellular Ca2+ receptors that operate by a positive feedback mechanism. When they bind Ca2+ on their cytoplasmic surfaces they depolarize the sarcolemma, releasing far more Ca2+ from sarcoplasmic reticulum and other intracellular Ca2+ stores, resulting in muscle contraction [66]. The mechanisms by which muscle contraction ensues vary between muscle types. Uehara et al. [67] concluded that spermine and other polyamines block the RyR channel, thereby diminishing sarcoplasmic reticulum Ca2+ release. Zarka and Shoshan-Barmatz [68] specifically reported that spermine stimulated binding of ryanodine, an inhibitor of ryanodine receptors, by up to 5-fold, with a concentration of 3.5 mM spermine producing a half-maximal effect.
2.3.4. Spermine Potentiates NMDA Receptors (NMDARs) at µM Concentrations, but Inhibits Them at mM Concentrations
The N-methyl-d-aspartate receptor (NMDAR) contains an integral ion channel that permits entry of Na+, K+, and Ca2+, but is blocked by Mg2+ [69]. Excessive stimulation of NMDARs increases intracellular Ca2+, resulting in excitotoxicity [70]. Responses of Xenopus oocytes expressing whole rat brain or chick cerebellar NMDARs to NMDA and kainate were potentiated by 10–100 µM spermine, suggesting a polyamine binding site on the NMDAR [71]. McGurk et al. likewise found that spermine potentiated responses of NMDAR in the presence of glycine [72]. The latter two studies reported that higher spermine concentrations inhibited responses to glutamate and aspartate, implying different mechanisms of action for excitation and inhibition, a result confirmed by later investigations [73].
Calcium influx is thought to be responsible for the role of NMDARs in long-term depression and long-term potentiation, relevant to learning and memory [74]; however, pre-synaptic NMDARs also modulate synaptic transmission in the spinal cord [75], and NMDARs are responsible for part of the motor drive to respiratory muscles [76,77,78]. More importantly, in all vertebrates from lampreys to primates, NMDARs in the brainstem and spinal cord are involved in coordination of locomotion, regardless of the type of locomotion [79,80].
The polyamine binding site on NMDARs is distinct from the binding sites for glutamate, glycine, Mg2+, Zn2+, and open-channel blockers such as MK-801 [81,82]. Marvizón and Baudry also found that spermine (1 µM–1 mM) increased MK-801 binding to NMDARs in the presence of glutamate and the glycine antagonists, 7-chlorokynurenate (10 µM) or DNQX (10 µM), which glutamate alone does not do. Pullan and Powel [83] showed that 100 µM spermine reduced the binding constant of L-glutamate by about half.
Benveniste and Mayer [84] confirmed that spermine acts at multiple sites on NMDA receptors from rat hippocampal neurons, but also found that spermine exhibits biphasic effects and that it can either potentiate or block ion permeability. They further reported that potentiation results from both an increase in NMDAR affinity for glycine (EC50 = 125 µM) and in an increase in the maximum response to NMDA when NMDARs are saturated with glycine. They concluded that NMDAR blockade is voltage-dependent, and that it is due to polyamine binding to sites inside the ion channel. Robichaud and Boxer [85] found that spermine increased the frequency of spontaneous discharges from rat neocortical slices in Mg2+-free buffer at concentrations below 1 mM, exhibited biphasic effects at 1 mM, and reduced discharge frequency at 3 mM. However, discharge amplitude only diminished in a concentration-dependent manner with spermine concentrations in the range of 300 µM to 3 mM. Araneda et al. [86] also reported biphasic effects of spermine. They found that 300 µM spermine approximately doubled the response to 30 µM NMDA in the presence of 1 µM glycine. In rat hippocampal neurons, extracellular spermine potentiated NMDAR currents by increasing the open channel probability at membrane potentials ranging from −70 to +40 mV (EC50 = 215 µM). They suggested that the channel block at higher spermine concentrations might be due to binding of negatively charged residues in the channel entrance by spermine’s amino groups. Ferchmin et al. [87] reported that spermine is excitotoxic at µM concentrations because it activates NMDARs [88,89], but it neuroprotective at mM concentrations because it blocks the receptor ion channel. Turecek et al. [90] proposed that 10 mM intracellular spermine directly inhibits NMDA receptors in a manner that is different from calcium-induced NMDA receptor inactivation and spermine-induced voltage-dependent inhibition of AMPA/kainate receptors.
Calderon and Lopez-Colome [91] found that spermine inhibited specific [3H]glycine binding to membranes from synaptosomal fractions from the inner (IC50 = 32 µM) and outer (IC50 = 35 µM) plexiform layers of 1–3 day-old chick retinas in a dose-dependent manner. They concluded that their results demonstrate a single spermine binding site on NMDARs in both layers of the chick retina. Mortensen et al. [92] found that spermine binding sites on NMDARs in four human cortical regions showed regional and individual variation in their capacity to enhance binding of the NMDAR open channel blocker, MK-801. Ragnarsson et al. [93] suggested that tissue differences in NMDAR susceptibility to modulation by spermine may result from different NMDAR subunit compositions.
Doyle and Shaw [94] found that 100-µg injections of spermine into the left lateral cerebral ventricle of mice produced two very different phases of CNS excitatory effects. The first, of rapid onset, involved scratching and frequent face washing, while some mice developed clonic convulsions. The second phase involved body tremors that intensified until they climaxed in fatal tonic convulsions. The authors concluded that while the two phases were pharmacologically quite different, both were probably mediated by NMDARs. Kirby and Shaw [95] reported that by virtue of its action at NMDARs, 300 µM spermine causes an increase in the rate of spontaneous epileptiform discharges in epilepsy-prone mice.
Adriani et al. [96] reported that, in mice, NMDA receptor blockade stimulated locomotor activity in a manner similar to MK-801 or dopamine agonists; however, low doses of NMDA or dopamine antagonists impair murine reactivity to spatial changes. Disorientation, rather than stimulation of locomotion would be strategically consonant with the objectives of envenomation, and would be consistent with the locomotor suppression induced by purine nucleosides via central A1 and A2 adenosine receptors [35,97,98,99,100]. Choi [66] opined that NMDA receptor activation might trigger neuronal death more rapidly than AMPA or kainate receptor activation, possibly due to a greater capacity to induce calcium influx and subsequent cytoplasmic calcium concentration.
Hardingham and Bading [101] report that neuronal NMDARs comprise two populations: synaptic receptors, which are neuroprotective, and extrasynaptic receptors that promote excitotoxicity and neuronal death. While the latter diminish during development, they can comprise 75% of all NMDARs. The normal physiological role of extrasynaptic NMDARs is poorly understood. Choi reported that the NMDAR is the primary site for toxic Ca2+ influx [102] and Tymianski et al. found that Ca2+ influx through NMDARs is more effective at inducing neuronal death than that through voltage-gated Ca2+ channels [103].
2.3.5. Spermine Blocks Type II AMPA Receptors (AMPARs)
AMPARs are responsible for fast, excitatory neurotransmission in the vertebrate central nervous system [37]. Rao et al. [104] found that intracerebellar injection of 200 µg spermine attenuated the increased production of cGMP mediated through NMDA and AMPA receptors in response to 5 µg injections of quisqualic acid and D-serine. Brundell et al. [105] proposed that intracellular spermine can bind to both the open and closed configurations of AMPARs in a voltage-independent but use-dependent manner. They found that bath application of 10 µM spermine initially potentiated and then antagonized excitatory post-synaptic currents in locust muscle in a use-dependent fashion. When injected directly into the muscle, the response was similar, except that it was not use-dependent.
Based on the observation that 40 nmol NBQX (2,3-Dihydroxy-6-nitro-7-sulfamoyl- benzo[f]quinoxaline) protected against the effects of 100 nmol of spermine, Otsuki et al. [106] suggested that AMPA receptors as well as NMDA receptors are involved in spermine-induced neurotoxicity in rat striatum. Isa et al. [107] discovered that, in rat hippocampal neurons, current responses mediated by Type II AMPARs were blocked by 170 µM spermine. These receptors have high Ca2+ permeability. In contrast, current responses from Type I AMPARs, which are essentially impermeable to Ca2+, were unaffected. EPSCs in putative non-pyramidal neurons from rat hippocampal slices mediated by Type II AMPARs were largely blocked by 1 mM spermine, while EPSCs resulting from Type I AMPARs were significantly less inhibited. Washburn and Dingledine [108] reported that spermine may bind to GluR2-lacking AMPA receptors (Type II AMPARs) at two or more distinct sites, one close to the cytoplasmic side of the ion channel and the other nearer the outer side of the channel.
Neurons bearing Type II AMPARs are especially susceptible to ischemic cell death. Noh et al. [109] reported that 1-naphthyl acetyl spermine, a selective channel blocker of Type II AMPARs, provided partial protection of rat hippocampal CA1 neurons. Motor neurons are especially vulnerable to AMPA agonists because they express Type II AMPARs [110,111,112].
2.3.6. Spermine and Kainate Receptors
Kainate receptors have nonselective cation channels that are permeable to Ca2+ and they bind intracellular spermine very strongly so that they activate only when hyperpolarized [113]. At present, the effects of extracellular spermine on kainate receptors are unclear, owing to contradictory reports in the literature. Brackley et al. [71] found that in Xenopus oocytes injected with whole rat brain mRNA, responses to L-kainate were potentiated by 10–100 µM spermine. McGurk et al. [72] expressed rat brain glutamate receptors in Xenopus oocytes, but did not find that spermine potentiated the responses to kainate (kainate receptors) or quisqualate (AMPA receptors), as it did to NMDA. Pegg [114] opined that because kainate receptors have been linked to epilepsy-like seizure activity, modulation of kainate receptors could potentially promote seizures.
2.3.7. Spermine Is a Nicotinic Acetylcholine Receptor Antagonist at µM Concentrations, but an Agonist at nM Concentrations
Anis et al. [115] reported that spermine (IC50 = 110 µM) inhibited the binding of both α-bungarotoxin and perhydrohistrionicotoxin to the Torpedo nicotinic acetylcholine receptor (nAChR) in the presence of carbamylcholine, a cholinergic agonist. Szczawinska et al. [116] likewise found that at concentrations above 1 mM, spermine inhibited ion influx and α-bungarotoxin binding to Torpedo electric organ nAChRs, thereby acting as a competitive antagonist of the nAChR. In contrast, at sub-µM concentrations, spermine enhanced cation influx by about 20%, effectively acting as an nAChR agonist. Using an outside-out patch and whole-cell voltage-clamp recordings from cultured Xenopus muscle cells, Hsu [117] obtained similar results, and concluded that the biphasic results resulted from spermine action at different sites.
Haghighi and Cooper [118] found that when spermine was added to the patch electrode in outside-out recordings, it caused a concentration- and voltage-dependent block of ACh-evoked single-channel currents. They conclude that the voltage-dependent block by intracellular spermine underlies inward rectification of neuronal nAChRs. They also found that extracellular spermine blocks both α3β4 (IC50 = 42.3 µM) and α4β2 receptors (IC50 = 40.3 µM) and suggested that extracellular spermine under pathological conditions, could selectively block these receptors. Certainly snake envenomation qualifies as a pathological condition.
On a parallel track, Law et al. [119] found that spermine inhibited the reuptake of choline by rat forebrain synaptosomes (IC50 = 0.22 mM). Dopamine uptake was also inhibited, but about 10-fold less potently.
2.3.8. Spermine and Muscarinic Acetylcholine Receptors
Tsvilovskyy et al. [120] reported that 1 mM extracellular spermine blocks muscarinic receptor controlled Ca2+ currents in guinea pig ileal smooth muscle myocytes in a concentration- and voltage-dependent manner. However, because the inhibition was similar for both carbachol- and GTPgS-evoked currents, they concluded that the blockade was due to a direct action on the ion channel, rather than on the receptor itself.
2.3.9. Spermine Interacts with GABAA Receptors
Intracerebroventricular injection of spermine (1.13 µmol/chick) decreased locomotion, promoted sedation or sleep, and caused a pronounced increase in GABA content of the diencephalon and brainstem [121]. Gilad et al. [122] found that spermine potentiates the binding of diazepam and flunitrazepam to rat forebrain membranes in the presence of 1 mM GABA in a concentration-dependent manner. In the presence of the non-ionic detergent, Triton X-100, larger concentrations of polyamines inhibited binding. Sabato et al. [123] and Martijena et al. [124] had previously reported enhanced binding in the presence of this detergent. Moreover, spermine inhibited binding of the peripheral-type benzodiazepine antagonist, PK 11195, at 50–500 µM concentrations, but not at concentrations above 1 mM. No effects on the binding of GABA, muscimol, or Ro 15–1788 were observed. Di Scenna et al. [24] found that mM concentrations of spermine reduced kainate, AMPA, and GABAA-mediated evoked potentials and concluded that their observations could best be explained by inhibition of presynaptic VDCCs.
2.3.10. Spermine Sensitizes Acid-Sensing Ion Channels
Acid-sensing ion channels of subtype 1a (ASIC1a), which differ from other ASICs in that they conduct Ca2+ as well as Na+, are known to play a major role in ischemic neuronal damage [125]. Duan et al. [126] found that 250 µM extracellular spermine exacerbates ischemic neurotoxicity in mouse cortical neurons by sensitizing ASIC1a channels to extracellular acidosis. Using either a specific ASIC1a antagonist or deletion of the ASIC1 gene, ischemic neuronal damage caused by spermine, both in cultured, dissociated neurons and in a mouse ischemic model, was greatly reduced. Spermine-enhanced ASIC1a activity increases acid-induced neuronal membrane depolarization [126] and cytoplasmic Ca2+ overload [125], the latter being responsible for spermine-exacerbated neuronal damage. Blocked spermine synthesis reduced the damage caused by ASIC1a channels, but not that caused by NMDA receptors [126].
2.3.11. Spermine Sensitizes Capsaicin Receptors
Ahern et al. [127] reported that in sensory neurons from murine nodose ganglia, 100 M spermine alone elicited no detectable current, but markedly enhanced the currents evoked by 10 nM capsaicin 2.8-fold (EC50 = 5 µM). In HEK293 cells, extracellular spermine activated inward currents in voltage-clamped cells. Responses to 500 M and 5 mM spermine elicited currents corresponding to 9% and 29% of that induced by 30 nM capsaicin. Gewehr et al. [128] demonstrated that nociceptive signals are transduced by capsaicin receptors (TRPV1).
2.3.12. Spermine Inhibits Platelet Aggregation
Israels et al. [129] reported that spermine (14.3–47 mM) inhibited 42% to 100% of platelet aggregation induced by thrombin, arachidonic acid, and lysophosphatidic acid. It also inhibited phosphorylation of the myosin light chain induced by thrombin, but not by arachidonic acid or lysophosphatidic acid. Others later reported that spermine (1–10 mM) inhibited thrombin-induced (1.5 nM) platelet activation in a concentration-dependent manner, including 5-hydroxytryptamine release [130,131,132]. Specifically, it blocks thrombin binding to GPIb of resting platelets and fibrinogen binding to GPIIb/IIIa of activated platelets. Inhibition of platelet aggregation is completely consistent with the anticoagulant strategies of viperid and crotalid venoms [28].
2.3.13. Spermine Inhibits Ca2+-ATPase
Spermine inhibits Ca2+-ATPase of skeletal muscle sarcoplasmic reticulum, in part by competing with Mg2+ necessary for ATPase activity [133]. Palacios et al. [134] later reported that pig brain Ca2+-ATPase was likewise inhibited by spermine with IC50s ranging from 2.5 to 27 mM, depending upon the phospholipid with which it was reconstituted. They found, as Hughes et al. also reported, that spermine does not interfere with the enzyme’s affinity for Ca2+ or ATP, but suggested that spermine may bind to negative charges on the enzyme. This suggests a mechanism similar to Mg2+ competition proposed by Hughes et al. [133], but it may also reflect an effect on lipid binding [126]. Because Ca2+-ATPase acts as a pump to remove Ca2+ from the cell, its inhibition would raise intracellular Ca2+ concentrations, resulting in skeletal muscle (including diaphragm) paralysis and death of all cell types [135,136]. However, it is questionable whether physiological spermine concentrations could ever approach the upper end of the sensitivity range reported by Palacios et al.
2.3.14. Spermine Promotes Breakdown of the Blood–Brain Barrier
Polyamines disrupt the blood–brain barrier (BBB) under various pathological states [125,137,138,139,140]. Glantz et al. [138] reported that putrescine and spermidine, when injected into the carotid artery, caused a rapid increase in BBB permeability 1 min after injection, with a slight decline at 15 min. A slower effect was noticed after spermine administration which became significant only at 15 min. Poduslo and Curran [141] have suggested the possibility of covalently modifying proteins with polyamines in order to deliver drugs to brain areas since protein permeability increases nearly 350-fold in some cases. The relevance of this to envenomation is that exogenous spermine may permit proteinaceous venom constituents to access brain areas from which they would otherwise be excluded. However, none of the above studies measured circulating polyamine levels, so at present it is not possible to determine whether levels of polyamines induced by envenomation reach the permeability threshold of the blood–brain barrier, a threshold that may vary across species.
2.3.15. Pharmacology of Spermidine, Putrescine, and Cadaverine that Is Potentially Pertinent to Envenomation
Short-chain diamines, such as putrescine and cadaverine, strongly affect the biological activity of histamine [142]. Mongar [143] found that short-chain diamines are both substrates for and inhibitors of histaminase (=diamine oxidase) and that they can potentiate the effects of histamine. Lyons et al. [144] reported that cadaverine potentiated histamine uptake in rat small intestine segments, an effect that they attributed to inhibition of histamine catabolism. This conclusion was based on the earlier work of Taylor and Lieber [145], who found that cadaverine inhibited histamine-N-methyltransferase and diamine oxidase, the two principal histamine catabolic enzymes in the intestine. Other workers [13,146,147,148] have also implicated putrescine in histamine potentiation. Moreover, Paik Jung and Bjeldanes [142] found that the potentiation of histamine toxicity by cadaverine is greatest when administered in conjunction with putrescine. Both histamine-N-methyltransferase and diamine oxidase are widely distributed among mammalian tissues [149,150]; thus it is possible that these two diamines could potentiate histamine release in envenomation, although the cadaverine titer in most venoms is probably too small to be of consequence. In support of this possibility, Marmo et al. have reported that the hypotensive effect of i.v. injections of spermine and spermidine are due to histamine release [42].
Putrescine and spermidine, as well as spermine, have been shown to potentiate transient relaxation of isolated guinea pig trachealis (smooth muscle) [43]. De Meis and De Paula specifically attributed this relaxation to inhibition of actomyosin ATPase [151].
3. Conclusions
Snake venoms apparently all contain polyamines, although polyamine titers in elapid venoms are manifestly minor. Cadaverine, which is produced from lysine in an anabolic pathway separate from the pathway that produces the other three polyamines, is normally the least abundant. Spermidine was absent from all six elapid venoms examined and from venom of the viperid, Pseudocerastes fieldi. With this exception, all other venoms contained all polyamines, although titers of individual polyamines varied by several orders of magnitude. All venoms manifested either a spermine-dominant or a putrescine dominant pattern. If venom polyamines are not sufficiently abundant to provoke systemic effects in the prey, it seems virtually certain that in the venoms of many viperids and crotalids, there are high enough polyamine concentrations to induce local effects. Endogenous polyamine stores released from prey tissues may well unleash systemic symptoms. Spermine reduces the force of cardiac contractions, and blocks vasoconstriction via blood vessel L-type Ca2+ channels. In addition, it blocks ryanodine receptors in skeletal muscle, triggers excitotoxic Ca2+ influx at extrasynaptic NMDA receptors, causes the death of motor neurons that bear Ca2+-sensitive Type 2 AMPA receptors, inhibits platelet aggregation, and breaks down the blood-brain barrier, and other effects. Putrescine, in combination with cadaverine, inhibits histamine catabolism, thereby enhancing inflammatory responses and promoting hypotension. Thus polyamine pharmacology is consistent with snake envenomation strategies to limit prey flight via hypotension and circulatory shock as well as by direct paralysis [35].
4. Materials and Methods
4.1. Venom Samples
Lyophilized crude venoms of 31 venomous snake species were examined in this study (Table B1). Venoms were dissolved in water at a concentration of 125 mg/mL. Samples of Naja sputatrix, Bitis gabonica, Bitis nasicornis, Ovophis okinavensis, and all three Protobothrops species were from individual snakes, as were all rattlesnake venoms supplied by the first author. All others were pooled samples from multiple individuals. Ten-microliter samples (1.25 mg) were supplied for mass spectrometry. Venoms of five prairie rattlesnakes (Crotalus viridis viridis) from each of two populations were examined in order to assess the amount of individual variation in polyamine levels.
4.2. Polyamine Derivatization
All solvents and reagents used were analytical grade and were purchased from Sigma (St. Louis, MO, USA) and ThermoFisher Scientific (Waltham, MA, USA). Freshly prepared reagents were used for polyamine derivatization. The reagents were as follows: 10% perchloric acid in water, 4% benzoyl chloride in acetonitrile, 2N sodium hydroxide in water, and saturated sodium chloride in water. Reagents were prepared in glass vials (10 mL), and all reactions were also carried out in glass vials (2 mL).
Acyl- and arylpolyamine conjugates, such as those commonly known from spider venoms, were not investigated because at present there are no published reports suggesting that snakes make them. In addition, the derivatization technique employed here cannot detect conjugated amines, if those are present. Lastly, the liquid chromatography/mass spectrometry parameters employed here were optimized only for primary polyamines. However, the presence of various other unidentified chromatographic peaks of significant intensity in each species invites further analysis.
Polyamine standards and venom polyamines were derivatized using an adaptation of the procedure of Liu et al. [9]. Stock solutions (10 mg/mL) of each polyamine standard (spermine, spermidine, putrescine, and cadaverine) were prepared in water. Stock solutions of the four individual polyamines were mixed and diluted to prepare working standard mixtures at 10, 5, and 1 mg/mL. Calibration standards (100, 50, 25, 12.5, 6.2, and 3.1 ng/150 mL) were prepared by diluting the standard mixtures with water.
To 150 mL of the calibration standard solution, 125 mL of 10% perchloric acid were added, mixed, and agitated on a shaker for 3 min. After agitation, the mixture was alkalinized with 200 mL of 2N NaOH, followed by the addition of 150 mL of 4% benzoyl chloride. This reaction mixture was mixed and sonicated for 30 min at 30–35 °C. After sonication, 500 mL of saturated NaCl solution were added and mixed well so as to react with excess benzoyl chloride. Finally, the solution was extracted twice with diethyl ether (1 mL × 2). The pooled diethyl ether extracts were dried over anhydrous MgSO4 (20–30 mg), filtered through a glass pipette using cotton plug, collected in a new vial, and evaporated in a Savant SpeedVac vacuum concentrator (ThermoFisher, Waltham, MA, USA). Dried derivatives were preserved at 4 °C before MS analysis.
The above mentioned standard polyamine derivatization method was used for snake venom polyamine derivatizations. Each snake venom (5 µL; concentration = 125 µg/µL) was suspended in 20 µL water (MilliQ), vortexed (30 s), and centrifuged (9000 rpm, 10 min, room temperature). An aliquot (10 µL, equivalent to 2 µL crude snake venom solution) was transferred in a glass vial (2 mL), and 140 µL of water were added, followed by the addition of 125 µL of 10% perchloric solution. The mixture was treated in a similar fashion to that used for polyamine standards and the products were extracted with ether. Dried snake venom polyamine derivatives and the polyamine standard derivatives were dissolved in 100 µL aqueous-methanol (1% water-0.01% formic acid) and analyzed by LC-MS.
4.3. Liquid Chromatography/Mass Spectrometry of Polyamine Standards
A Waters high-definition mass spectrometer (HDMS SYNAPT G2-S, Waters, Milford, MA, USA) was used for mass spectrometry data collection. The mass spectrometer was equipped with an ESI ion source (ZSpray) and a Waters ultra-high-pressure liquid chromatograph (ACQUITY I Class UPLC) with a Binary Solvent Manager, a Sample Manager, and a PDA eλ detector.
TOF MS spectra were generated for polyamine derivatives in positive ion mode with normal resolution at mass range m/z 100–700 Da. MS conditions were set as follows: capillary 3.0 kV, sample cone 30v, source temperature at 150 °C, desolvation temperature at 550 °C, cone gas 150 L/h, desolvation gas 900 L/h, nebulizer 6 bar, scan time 0.2 s, mass window ±0.5 Da, source offset 40. Leucine-enkephalin (m/z 120.0813 and 556.2771 with collision trap energy 18) was used as a lock spray (at 30 s intervals, 3 scans merged).
Polyamine derivatives were separated on an Acquity BEH C18 column (150 × 2.1 mm, 1.7 µm, Waters). A 15-min step-gradient was used for polyamine separation (10% B for 0.0–2.0 min, 10% to 90% B for 2.0–8.0 min, hold 90% B for 8.01–10.0 min, equilibration 10% B for 10.1–15.0 min; where solvent A was water, and solvent B was acetonitrile, both solvents containing 0.1% formic acid; 300 µL/min flow rate and a column temperature of 40 °C). Samples of 3 µL were injected using the auto-sampler, and all samples were run in triplicate (Figure B1).
4.4. Data Processing
Corresponding masses for polyamine derivatives were spermine (m/z 619.3284, C38H43N4O4 MH+), spermidine (m/z 458.2440, C28H32N3O3 MH+), putrescine (m/z 297.1603, C18H21N2O2 MH+), and cadaverine (m/z 311.1760, C19H23N2O2 MH+). Peak areas for each polyamine were obtained by generating extracted ion chromatograms (XIC) using MassLynx version 4.1 (Waters, Milford, MA, USA). Polyamine concentrations in snake venom were calculated based on polyamine standard calibration curves (Figure B2).
Acknowledgments
We thank Louis Porras for allowing Steve Aird to extract venoms from some of his specimens (Zooherp), and we thank Nelson Jorge da Silva, Jr. for generous donations of various venoms (CEPB). We gratefully acknowledge research support from the Okinawa Institute of Science and Technology Graduate University to the Evolution and Ecology Unit. We thank Tomoyuki Takahashi and Gordon Arbuthnott for their expert critiques of the manuscript.
Appendix A
Table A1.
Polyamine measurements in venoms of 10 Crotalus viridis viridis from two populations in western Colorado (nmol polyamine/g venom). Two replicates were run for each specimen and each polyamine.
| Specimen | Spermine [nmol/g] | Spermidine [nmol/g] | Putrescine [nmol/g] | Cadaverine [nmol/g] | Total | ||||
|---|---|---|---|---|---|---|---|---|---|
| C. v. viridis 1501 | 24.640 | 24.576 | 11.280 | 11.324 | 28.896 | 29.695 | 0.251 | 0.251 | 65.456 |
| C. v. viridis 1502 | 17.776 | 17.586 | 9.561 | 9.385 | 9.511 | 9.366 | 0.188 | 0.188 | 36.781 |
| C. v. viridis 1505 | 17.555 | 16.985 | 10.355 | 10.134 | 29.913 | 30.130 | 0.376 | 0.376 | 57.912 |
| C. v. viridis 1506 | 8.761 | 8.445 | 6.565 | 6.565 | 18.296 | 18.223 | 0.188 | 0.251 | 33.648 |
| C. v. viridis 1507 | 15.277 | 17.302 | 11.015 | 11.720 | 12.996 | 12.923 | 0.188 | 0.188 | 40.805 |
| C. v. viridis 301 | 14.993 | 15.562 | 7.182 | 7.667 | 15.174 | 15.392 | 0.188 | 0.188 | 38.173 |
| C. v. viridis 304 | 12.873 | 13.000 | 9.694 | 9.253 | 10.745 | 11.326 | 0.188 | 0.188 | 33.634 |
| C. v. viridis 306 | 9.932 | 9.647 | 7.050 | 6.962 | 13.359 | 12.778 | 0.188 | 0.188 | 30.052 |
| C. v. viridis 308 | 16.036 | 16.131 | 6.213 | 6.169 | 3.412 | 3.340 | 0.188 | 0.188 | 25.838 |
| C. v. viridis 311 | 8.287 | 8.382 | 6.257 | 6.125 | 6.680 | 6.680 | 0.188 | 0.188 | 21.393 |
| Mean | 14.613 | 14.762 | 8.517 | 8.530 | 14.898 | 14.985 | 0.213 | 0.219 | 38.369 |
| Std. Deviation | 4.955 | 5.034 | 2.052 | 2.114 | 8.722 | 8.914 | 0.061 | 0.061 | 13.676 |
Table A2.
Polyamine abundances (nmol polyamine/g venom) in relation to preferred prey of adult snakes.
| Taxon | Polyamines (nmol/g venom) | Prey Species | References | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spermine | Spermidine | Putrescine | Cadaverine | Arthropods | Fish | Birds | Lizards | Anurans | Snakes | Mammals | ||
| Elapidae | ||||||||||||
| B. multicinctus | 0.032 | 0.000 | 0.944 | 0.376 | - | ❋ | - | - | - | ❋ | - | Mao, 1970 [152] |
| D. polylepis | 0.032 | 0.000 | 0.726 | 0.125 | - | - | ❋ | - | - | - | ❋ | Branch et al., 1995 [153] |
| M. surinamensis | 0.032 | 0.000 | 0.726 | 0.125 | - | ❋ | - | - | - | - | - | Olamendi-Portugal et al., 2008 [154]; Morais et al., 2011 [155] |
| N. kaouthia | 0.032 | 0.000 | 0.726 | 0.125 | - | - | - | - | - | ❋ | ❋ | Chanhome et al., 2001 [156]; Modal et al., 2016 [157] |
| N. sputatrix | 0.032 | 0.000 | 0.726 | 0.125 | - | - | - | - | - | ❋ | ❋ | Inferred |
| O. hannah | 0.063 | 0.000 | 0.871 | 0.188 | - | - | - | ❋ | - | ❋ | ❋ | Chanhome et al., 2001 [156] |
| Mean | 0.037 | 0.000 | 0.787 | 0.177 | ||||||||
| Std. Deviation | 0.013 | 0.000 | 0.097 | 0.100 | ||||||||
| Viperidae | ||||||||||||
| B. gabonica | 5.820 | 1.146 | 34.995 | 1.628 | - | - | ❋ | ❋ | ❋ | - | ❋ | Luiselli & Akani, 2003 [158] |
| B. nasicornis | 401.099 | 21.370 | 145.933 | 15.345 | - | - | - | - | ❋ | - | ❋ | Luiselli & Akani, 2003 [158] |
| C. cerastes | 1218.132 | 68.649 | 431.120 | 28.373 | - | - | - | ❋ | - | - | - | Bazaa et al., 2005 [159] |
| D. palestinae | 14.012 | 1.102 | 71.006 | 14.719 | - | - | - | - | - | - | ❋ | Inferred |
| D.siamensis | 251.142 | 14.320 | 406.870 | 15.220 | - | - | - | ❋ | ❋ | ❋ | ❋ | Chanhome et al., 2001 [156]; Gennaro et al., 2007 [160] |
| P. fieldi | 0.095 | 0.000 | 1.162 | 0.125 | - | - | ❋ | ❋ | - | - | ❋ | Ali et al., 2015 [161] |
| Mean | 315.050 | 17.764 | 181.848 | 12.569 | ||||||||
| Std. Deviation | 471.697 | 26.395 | 190.032 | 10.428 | ||||||||
| Crotalidae | ||||||||||||
| A. c. contortrix | 1087.374 | 3.084 | 72.386 | 14.907 | ❋ | - | ❋ | ❋ | ❋ | ❋ | ❋ | Trauth & McAllister, 1995 [162]; Heatwole et al., 1999 [163] |
| A. p. leucostoma | 398.474 | 4.318 | 14.521 | 3.006 | - | ❋ | - | - | ❋ | ❋ | ❋ | Himes, 2003 [164]; Vincent et al., 2004 [165] |
| A. nummifer | 0.253 | 0.044 | 0.871 | 0.125 | - | - | - | - | - | - | ❋ | Martins et al., 2002 [166] |
| B. schlegelii | 0.285 | 0.044 | 0.726 | 0.125 | - | - | ❋ | ❋ | ❋ | - | ❋ | Greene, 1988 [167]; Sorrel, 2007 [168] |
| B. erythromelas | 3.005 | 0.573 | 5.590 | 1.002 | ❋ | - | ❋ | - | ❋ | - | ❋ | Martins et al, 2002 [166] |
| B. moojeni | 516.295 | 10.090 | 72.313 | 14.719 | ❋ | - | ❋ | ❋ | ❋ | ❋ | ❋ | Nogueira et al., 2003 [169] |
| C. rhodostoma | 335.878 | 1.057 | 70.643 | 14.656 | ❋ | ❋ | ❋ | ❋ | ❋ | ❋ | ❋ | Daltry et al., 1998 [156]; Chanhome et al., 2001 [170] |
| C. sasai | 0.791 | 0.044 | 0.871 | 0.125 | ❋ | - | - | ❋ | ❋ | - | ❋ | Luiselli, 2006 [166]; Martins et al., 2002 [171] |
| C. adamanteus | 53.486 | 18.506 | 132.864 | 15.345 | - | - | - | - | - | - | ❋ | Margres et al., 2015 [172] |
| C. cerastes | 19.199 | 14.761 | 90.464 | 14.782 | - | - | - | ❋ | - | - | ❋ | Secure & Nagy, 1994 [173,174] |
| C. d. terrificus | 151.223 | 88.741 | 269.141 | 16.348 | - | - | - | - | - | - | ❋ | Salomao et al, 1995 [160]; Sant’anna & Abe, 2007 [175]; Gennaro et al., 2007 [176] |
| C. m. pyrrhus | 0.569 | 0.088 | 1.379 | 0.125 | - | - | - | - | - | - | ❋ | Meik et al., 2012 [177] |
| C.v.concolor | 9.805 | 7.314 | 13.795 | 0.188 | - | - | - | ❋ | - | - | ❋ | Mackessy et al., 2003 [178] |
| C. v. viridis | 14.687 | 8.524 | 14.942 | 0.216 | - | - | ❋ | ❋ | - | - | ❋ | Hayes, 1992 [179] |
| L. stenophrys | 129.683 | 6.213 | 22.943 | 2.944 | - | - | - | - | - | - | ❋ | Voss, 2013 [180] |
| O. okinavensis | 0.443 | 0.044 | 0.799 | 0.188 | - | - | ❋ | ❋ | ❋ | - | ❋ | Mori & Toda, 2011 [181] |
| P. elegans | 2320.150 | 1.234 | 70.498 | 14.719 | - | - | - | ❋ | - | - | ❋ | SDA, pers. observations |
| P. flavoviridis | 85.875 | 1.190 | 70.425 | 14.656 | - | - | ❋ | - | ❋ | - | ❋ | Gennaro et al., 2007 [160]; Chijiwo et al., 2003 [182] |
| P. mucrosquamatus | 7918.426 | 77.902 | 700.479 | 146.440 | - | - | ❋ | - | ❋ | ❋ | ❋ | Mao, 1970 [152] |
| Mean | 686.626 | 12.830 | 85.561 | 14.454 | ||||||||
| Std. Deviation | 1938.355 | 25.468 | 162.691 | 32.745 | ||||||||
Appendix B
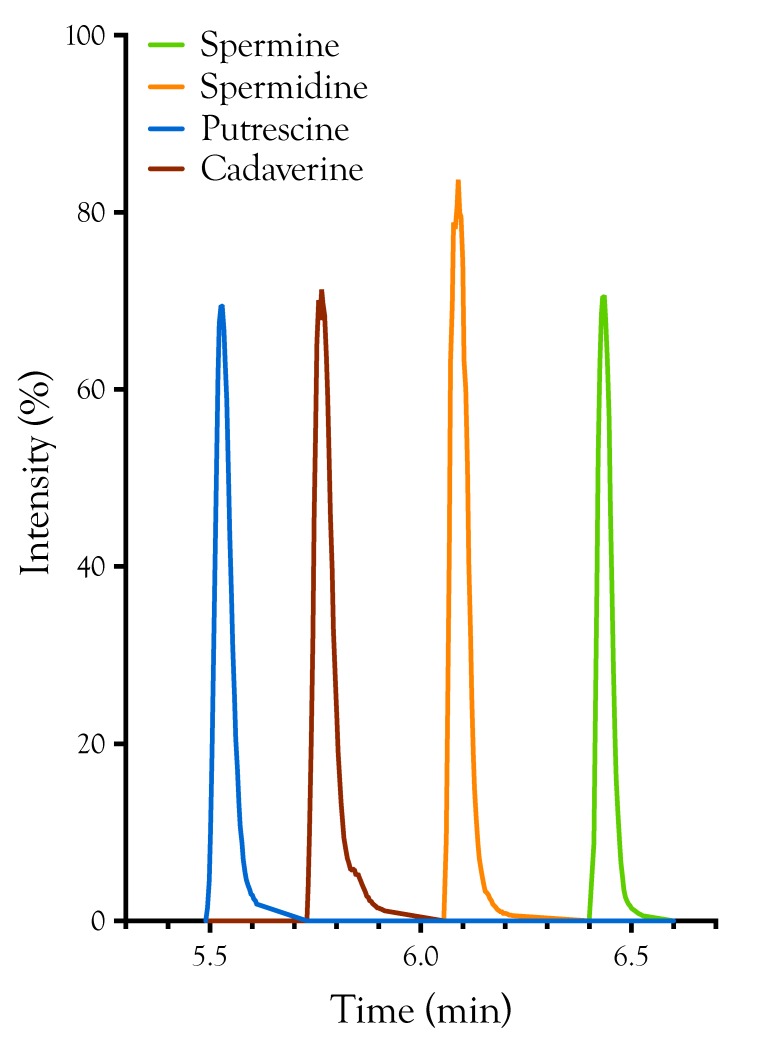
Figure B1.
Extracted ion chromatogram (XIC) showing the elution times of the four polyamines quantified in this study.
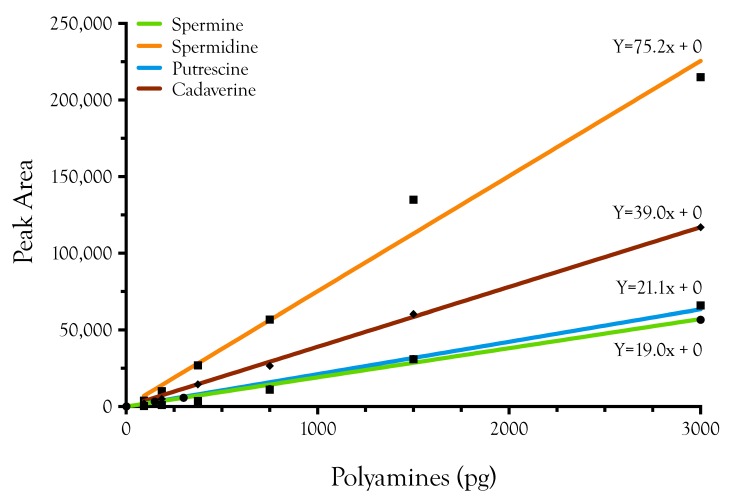
Figure B2.
Standard curves for the four polyamines. Only two samples out of the 208 analyzed by LC-MS exceeded 3000 pg/injection. For all curves, p < 0.0001.
Table B1.
Venomous snake taxa examined in this study and geographic origins of samples. Samples from Zooherp and SDA were obtained from single snakes. All other samples were pooled from multiple individuals. Sources: Biotoxins, St. Cloud, FL, USA; Clodomiro Picado, San Juan, Costa Rica; CEPB, Centro de Estudos e Pesquisas Biológicas, Pontifícia Universidade Católica de Goiás, Goiânia, Goiás, Brasil; KRZ, Kentucky Reptile Zoo, Slade, KY, USA; SVRI, Snake Venom Research Institute, Nanning, China; Zooherp, Salt Lake City, UT, USA.
| Family | Taxon | Origin | Source |
|---|---|---|---|
| Elapidae | Bungarus multicinctus | China | SVRI |
| Dendroaspis polylepis | Kenya | Biotoxins | |
| Micrurus surinamensis | Letícia, Colombia | CEPB | |
| Naja kaouthia | Unknown | KRZ | |
| Naja sputatrix | Java | Zooherp | |
| Ophiophagus hannah | Unknown | KRZ | |
| Viperidae | Bitis gabonica | Burundi | Zooherp |
| Bitis nasicornis | Unknown | Zooherp | |
| Cerastes cerastes | Unknown | KRZ | |
| Daboia palestinae | Unknown | KRZ | |
| Daboia russellii siamensis | Thailand | SVRI | |
| Pseudocerastes fieldi | Unknown | KRZ | |
| Crotalidae | Agkistrodon contortrix contortrix | South Carolina | Biotoxins |
| Agkistrodon piscivorus leucostoma | Missouri | KRZ | |
| Atropoides nummifer | SE Mexico | KRZ | |
| Bothriechis schlegelii | Costa Rica | Clodomiro Picado | |
| Bothrops moojeni | Goiás, Brasil | CEPB | |
| Bothrops erythromelas | NE Brazil | KRZ | |
| Calloselasma rhodostoma | Thailand | KRZ | |
| Cerrophidion sasai | Costa Rica | Clodomiro Picado | |
| Crotalus adamanteus | Florida | Biotoxins | |
| Crotalus durissus terrificus | Brazil | CEPB | |
| Crotalus cerastes | Yuma, Arizona | SDA | |
| Crotalus mitchellii pyrrhus | Yavapai, Arizona | SDA | |
| Crotalus viridis concolor | Rio Blanco, Colorado | SDA | |
| Crotalus viridis viridis | Rio Blanco, Colorado | SDA | |
| Lachesis stenophrys | Costa Rica | Clodomiro Picado | |
| Ovophis okinavensis | Okinawa | SDA | |
| Protobothrops elegans | Itoman, Okinawa | SDA | |
| Protobothrops flavoviridis | Itoman, Okinawa | SDA | |
| Protobothrops mucrosquamatus | Nago, Okinawa | SDA |
Author Contributions
M.C.R. developed the derivatization methodology based on published methods, performed the derivatizations and the mass spectrometry. A.V.B. performed the data analysis and wrote the LC-MS methods. A.S.M. performed statistical analyses to assess ecological and phylogenetic correlates and wrote the statistical methods. S.D.A. created the figures and wrote the remainder of the manuscript. All authors edited and approved the manuscript. We also wish to thank an anonymous reviewer of this manuscript who brought the early paper of Tabor and Rosenthal [19] to our attention. This greatly strengthened the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Sasaki T. Chemical studies on the venom of Formosan habu (Trimeresurus mucrosquamatus Cantor). III. On the dialyzable substances in the venom. J. Pharmacol. Soc. Jpn. 1960;80:844. [Google Scholar]
- 2.Shipolini R., Ivanov C.P., Dimitrov G., Alexiev B.V. Composition of the low molecular fraction of the Bulgarian viper venom. Biochim. Biophys. Acta (BBA) Gen. Subj. 1965;104:292–295. doi: 10.1016/0304-4165(65)90250-3. [DOI] [PubMed] [Google Scholar]
- 3.Merkel P., Beck A., Muhammad K., Ali S.A., Schönfeld C., Voelter W., Duszenko M. Spermine isolated and identified as the major trypanocidal compound from the snake venom of Eristocophis macmahoni causes autophagy in Trypanosoma brucei. Toxicon. 2007;50:457–469. doi: 10.1016/j.toxicon.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 4.Grishin E.V., Volkova T.M., Arsen’ev A.S., Reshetova O.S., Onoprienko V.V. Structural-functional characteristics of argiopine—The ion channel blockers from the spider Argiope lobata venom. Bioorg. Khim. 1986;12:1121–1124. [PubMed] [Google Scholar]
- 5.Adams M.E., Carney R.L., Enderlin F.E., Fu E.T., Jarema M.A., Li J.P., Miller C.A., Schooley D.A., Shapiro M.J., Venema V.J. Structures and biological activities of three synaptic antagonists from orb weaver spider venom. Biochem. Biophys. Res. Commun. 1987;148:678–683. doi: 10.1016/0006-291X(87)90930-2. [DOI] [PubMed] [Google Scholar]
- 6.Budd T., Clinton P., Dell A., Duce I.R., Johnson S.J., Quicke D.L., Taylor G.W., Usherwood P.N., Usoh G. Isolation and characterisation of glutamate receptor antagonists from venoms of orb-web spiders. Brain Res. 1988;448:30–39. doi: 10.1016/0006-8993(88)91098-0. [DOI] [PubMed] [Google Scholar]
- 7.Adams M.E., Herold E.E., Venema V.J. Two classes of channel-specific toxins from funnel web spider venom. J. Comp. Physiol. A. 1989;164:333–342. doi: 10.1007/BF00612993. [DOI] [PubMed] [Google Scholar]
- 8.Pan-Hou H., Hidemitsu P.-H., Yasuo S., Masao S., Masanori Y., Nobufumi K. A spider toxin (JSTX) inhibits l-glutamate uptake by rat brain synaptosomes. Brain Res. 1989;476:354–357. doi: 10.1016/0006-8993(89)91258-4. [DOI] [PubMed] [Google Scholar]
- 9.Ippolito J.E., Xu J., Jain S., Moulder K., Mennerick S., Crowley J.R., Townsend R.R., Gordon J.I. An integrated functional genomics and metabolomics approach for defining poor prognosis in human neuroendocrine cancers. Proc. Natl. Acad. Sci. USA. 2005;102:9901–9906. doi: 10.1073/pnas.0500756102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Häkkinen M.R., Keinänen T.A., Jouko V., Khomutov A.R., Leena A., Juhani J., Seppo A. Analysis of underivatized polyamines by reversed phase liquid chromatography with electrospray tandem mass spectrometry. J. Pharm. Biomed. Anal. 2007;45:625–634. doi: 10.1016/j.jpba.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Liu R., Ran L., Kaishun B., Ying J., Qian W., Ran Y., Qing L. Determination of polyamines in human plasma by high-performance liquid chromatography coupled with Q-TOF mass spectrometry. J. Mass Spectrom. 2012;47:1341–1346. doi: 10.1002/jms.3084. [DOI] [PubMed] [Google Scholar]
- 12.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2015. [Google Scholar]
- 13.Aird S.D., Aggarwal S., Villar-Briones A., Tin M.M.-Y., Terada K., Mikheyev A.S. Snake venoms are integrated systems, but abundant venom proteins evolve more rapidly. BMC Genom. 2015;16:279. doi: 10.1186/s12864-015-1832-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayes W.K. Factors associated with the mass of venom expended by prairie rattlesnakes (Crotalus v. viridis) feeding on mice. Toxicon. 1992;30:449–460. doi: 10.1016/0041-0101(92)90541-C. [DOI] [PubMed] [Google Scholar]
- 15.Morrison J.J., Pearn J.H., Coulter A.R. The mass of venom injected by two elapidae: The taipan (Oxyuranus scutellatus) and the Australian tiger snake (Notechis scutatus) Toxicon. 1982;20:739–745. doi: 10.1016/0041-0101(82)90121-0. [DOI] [PubMed] [Google Scholar]
- 16.Morrison J.J., Pearn J.H., Charles N.T., Coulter A.R. Further studies on the mass of venom injected by Elapid snakes. Toxicon. 1983;21:279–284. doi: 10.1016/0041-0101(83)90012-0. [DOI] [PubMed] [Google Scholar]
- 17.Cooper K.D., Shukla J.B., Rennert O.M. Polyamine distribution in cellular compartments of blood and in aging erythrocytes. Clin. Chim. Acta. 1976;73:71–88. doi: 10.1016/0009-8981(76)90307-7. [DOI] [PubMed] [Google Scholar]
- 18.Uehara N., Shirakawa S., Uchino H., Saeki Y. Elevated contents of spermidine and spermine in the erythrocytes of cancer patients. Cancer. 1980;45:108–111. doi: 10.1002/1097-0142(19800101)45:1<108::AID-CNCR2820450120>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 19.Tabor C.W., Rosenthal S.M. Pharmacology of spermine and spermidine; some effects on animals and bacteria. J. Pharmacol. Exp. Ther. 1956;116:139–155. [PubMed] [Google Scholar]
- 20.Tabor H., Tabor C.W., Rosenthal S.M. The Biochemistry of the Polyamines: Spermidine and Spermine. Annu. Rev. Biochem. 1961;30:579–604. doi: 10.1146/annurev.bi.30.070161.003051. [DOI] [Google Scholar]
- 21.Bachrach U. Oxidized polyamines. Ann. N. Y. Acad. Sci. 1970;171:939–956. doi: 10.1111/j.1749-6632.1970.tb39400.x. [DOI] [Google Scholar]
- 22.Pegg A.E. Toxicity of polyamines and their metabolic products. Chem. Res. Toxicol. 2013;26:1782–1800. doi: 10.1021/tx400316s. [DOI] [PubMed] [Google Scholar]
- 23.Aird S.D., Watanabe Y., Villar-Briones A., Roy M.C., Terada K., Mikheyev A.S. Quantitative high-throughput profiling of snake venom gland transcriptomes and proteomes (Ovophis okinavensis and Protobothrops flavoviridis) BMC Genom. 2013;14:279. doi: 10.1186/1471-2164-14-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DiScenna P.G., Ferchmin P.A., Eterovic V.A., Teyler T.J. Spermine depresses NMDA, K/AMPA and GABAA-mediated synaptic transmission in the rat hippocampal slice preparation. Brain Res. 1994;647:353–356. doi: 10.1016/0006-8993(94)91335-8. [DOI] [PubMed] [Google Scholar]
- 25.Rosenthal S.M., Tabor C.W. The pharmacology of spermine and spermidine; distribution and excretion. J. Pharmacol. Exp. Ther. 1956;116:131–138. [PubMed] [Google Scholar]
- 26.Michaelson I.A., Coffman P.Z. Histamine and spermidine in tissues of the guinea pig. Biochem. Pharmacol. 1967;16:1636–1641. doi: 10.1016/0006-2952(67)90144-X. [DOI] [PubMed] [Google Scholar]
- 27.Shimizu H., Kakimoto Y., Sano I. The determination and distribution of polyamines in mammalian nervous system. J. Pharmacol. Exp. Ther. 1964;143:199–204. [PubMed] [Google Scholar]
- 28.Perry T.L., Hansen S., Foulks J.G., Ling G.M. Aliphatic and aromatic amines of cat brain. J. Neurochem. 1965;12:397–405. doi: 10.1111/j.1471-4159.1965.tb04240.x. [DOI] [PubMed] [Google Scholar]
- 29.Paschen W. Polyamine metabolism in different pathological states of the brain. Mol. Chem. Neuropathol. 1992;16:241–271. doi: 10.1007/BF03159973. [DOI] [PubMed] [Google Scholar]
- 30.Zappia V., Porta R., Cartenì-Farina M., De Rosa M., Gambacorta A. Polyamine distribution in eukaryotes: Occurrence of sym-nor-spermidine and sym-nor-spermine in arthropods. FEBS Lett. 1978;94:161–165. doi: 10.1016/0014-5793(78)80928-4. [DOI] [PubMed] [Google Scholar]
- 31.Polticelli F., Salvi D., Mariottini P., Amendola R., Cervelli M. Molecular evolution of the polyamine oxidase gene family in Metazoa. BMC Evol. Biol. 2012;12:279. doi: 10.1186/1471-2148-12-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaisiri P., Harper M.E., Griffiths K. Plasma spermine concentrations of patients with benign and malignant tumours of the breast or prostate. Clin. Chim. Acta. 1979;92:273–282. doi: 10.1016/0009-8981(79)90123-2. [DOI] [PubMed] [Google Scholar]
- 33.Masuko T., Kusama-Eguchi K., Sakata K., Kusama T., Chaki S., Okuyama S., Williams K., Kashiwagi K., Igarashi K. Polyamine transport, accumulation, and release in brain. J. Neurochem. 2003;84:610–617. doi: 10.1046/j.1471-4159.2003.01558.x. [DOI] [PubMed] [Google Scholar]
- 34.Cino I., Formenti A. Spermine biphasically affects N-type calcium channel currents in adult dorsal root ganglion neurons of the rat. Biochim. Biophys. Acta. 2008;1778:2437–2443. doi: 10.1016/j.bbamem.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 35.Aird S.D. Ophidian envenomation strategies and the role of purines. Toxicon. 2002;40:335–393. doi: 10.1016/S0041-0101(01)00232-X. [DOI] [PubMed] [Google Scholar]
- 36.Aird S.D. Taxonomic distribution and quantitative analysis of free purine and pyrimidine nucleosides in snake venoms. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2005;140:109–126. doi: 10.1016/j.cbpc.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 37.Pellegrini-Giampietro D.E. An activity-dependent spermine-mediated mechanism that modulates glutamate transmission. Trends Neurosci. 2003;26:9–11. doi: 10.1016/S0166-2236(02)00004-8. [DOI] [PubMed] [Google Scholar]
- 38.Yoshida T., Nagai R., Hashimoto T. Lack of species specificity of a skin-reactive factor released from sensitized guinea pig spleen cells. Lab. Investig. 1973;29:329–335. [PubMed] [Google Scholar]
- 39.Hashimoto H., Unemoto T., Hayashi M. Inhibitory action of spermine on the contractions of rat uterus. Am. J. Physiol. 1973;225:743–746. doi: 10.1152/ajplegacy.1973.225.3.743. [DOI] [PubMed] [Google Scholar]
- 40.Nicchitta C.V., Williamson J.R. Spermine. A regulator of mitochondrial calcium cycling. J. Biol. Chem. 1984;259:12978–12983. [PubMed] [Google Scholar]
- 41.De Meis L. Relaxing effect of spermine and spermidine on intact and glycerol-treated muscle. Am. J. Physiol. 1967;212:92–96. doi: 10.1152/ajplegacy.1967.212.1.92. [DOI] [PubMed] [Google Scholar]
- 42.Marmo E., Berrino L., Cazzola M., Filippelli A., Cafaggi G., Persico N., Spadaro R., Nisticò G. Cardiovascular and respiratory effects of spermidine and spermine: An experimental study. Biomed. Biochim. Acta. 1984;43:509–515. [PubMed] [Google Scholar]
- 43.Chideckel E.W., Fedan J.S., Mike P. Polyamines and putreanine relax respiratory tract smooth muscle in the guinea-pig. Eur. J. Pharmacol. 1985;116:187–190. doi: 10.1016/0014-2999(85)90203-1. [DOI] [PubMed] [Google Scholar]
- 44.Chideckel E.W., Dedhia H.H., Fedan J.S., Teba L., Jain A. Spermine decreases peripheral vascular resistance in the dog and relaxes the isolated aorta of the guinea pig. Cardiovasc. Res. 1986;20:931–934. doi: 10.1093/cvr/20.12.931. [DOI] [PubMed] [Google Scholar]
- 45.Fernández A.I., Cantabrana B., Sánchez M., Hidalgo A. Extracellular and intracellular effects of polyamines on smooth muscle contractions. Life Sci. 1995;57:855–861. doi: 10.1016/0024-3205(95)02018-E. [DOI] [PubMed] [Google Scholar]
- 46.Nilsson B.O., Gomez M., Santiago Carrilho R., Nordström I., Hellstrand P. Differential actions of exogenous and intracellular spermine on contractile activity in smooth muscle of rat portal vein. Acta Physiol. Scand. 1995;154:355–365. doi: 10.1111/j.1748-1716.1995.tb09919.x. [DOI] [PubMed] [Google Scholar]
- 47.Myung S.C., Oh S.Y., Kim K.D., Kim S.C., Lee M.Y. Effects of spermine on the relaxation response of rat detrusor smooth muscles. Eur. J. Pharmacol. 2007;573:196–200. doi: 10.1016/j.ejphar.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 48.Revuelta M.P., Cantabrana B., Sánchez M., Hidalgo A. Effect of spermine and alpha-difluoromethylornithine on KCl- and CaCl2-induced contraction in rat uterine smooth muscle. J. Auton. Pharmacol. 1998;18:223–230. doi: 10.1046/j.1365-2680.1998.18489.x. [DOI] [PubMed] [Google Scholar]
- 49.Gomez M., Hellstrand P. Effects of polyamines on voltage-activated calcium channels in guinea-pig intestinal smooth muscle. Pflugers Arch. 1995;430:501–507. doi: 10.1007/BF00373886. [DOI] [PubMed] [Google Scholar]
- 50.Weston A.H., Absi M., Ward D.T., Ohanian J., Dodd R.H., Dauban P., Petrel C., Ruat M., Edwards G. Evidence in favor of a calcium-sensing receptor in arterial endothelial cells: Studies with calindol and Calhex 231. Circ. Res. 2005;97:391–398. doi: 10.1161/01.RES.0000178787.59594.a0. [DOI] [PubMed] [Google Scholar]
- 51.Quinn S.J., Ye C.P., Diaz R., Kifor O., Bai M., Vassilev P., Brown E. The Ca2+-sensing receptor: A target for polyamines. Am. J. Physiol. 1997;273:C1315–C1323. doi: 10.1152/ajpcell.1997.273.4.C1315. [DOI] [PubMed] [Google Scholar]
- 52.Sham J.S., Cleemann L., Morad M. Functional coupling of Ca2+ channels and ryanodine receptors in cardiac myocytes. Proc. Natl. Acad. Sci. USA. 1995;92:121–125. doi: 10.1073/pnas.92.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ziegelstein R.C., Xiong Y., He C., Hu Q. Expression of a functional extracellular calcium-sensing receptor in human aortic endothelial cells. Biochem. Biophys. Res. Commun. 2006;342:153–163. doi: 10.1016/j.bbrc.2006.01.135. [DOI] [PubMed] [Google Scholar]
- 54.Ventura C., Ferroni C., Flamigni F., Stefanelli C., Capogrossi M.C. Polyamine effects on [Ca2+]i homeostasis and contractility in isolated rat ventricular cardiomyocytes. Am. J. Physiol. 1994;267:H587–H592. doi: 10.1152/ajpheart.1994.267.2.H587. [DOI] [PubMed] [Google Scholar]
- 55.Guevara-Balcázar G., Querejeta-Villagómez E., Nuevo-Adalla O., Orozco-Guillen A., Rubio-Gayosso I., Hernández-Castillo J.R., Zamora-Garza M., Ceballos-Reyes G. Spermine-induced negative inotropic effect in isolated rat heart, is mediated through the release of ATP. Biochem. Pharmacol. 2003;66:157–161. doi: 10.1016/S0006-2952(03)00238-7. [DOI] [PubMed] [Google Scholar]
- 56.Reid C.A., Bekkers J.M., Clements J.D. Presynaptic Ca2+ channels: A functional patchwork. Trends Neurosci. 2003;26:683–687. doi: 10.1016/j.tins.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 57.Catterall W.A., Perez-Reyes E., Snutch T.P., Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol. Rev. 2005;57:411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- 58.Clapham D.E. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 59.Bonci A., Grillner P., Mercuri N.B., Bernardi G. L-Type calcium channels mediate a slow excitatory synaptic transmission in rat midbrain dopaminergic neurons. J. Neurosci. 1998;18:6693–6703. doi: 10.1523/JNEUROSCI.18-17-06693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Herman M.D., Reuveny E., Narahashi T. The effect of polyamines on voltage-activated calcium channels in mouse neuroblastoma cells. J. Physiol. 1993;462:645–660. doi: 10.1113/jphysiol.1993.sp019574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eterović V.A., Torres E., Ferchmin P.A. Spermine does not compete with omega-conotoxin GVIA in the striatum radiatum of the hippocampal slice. Brain Res. 1997;772:191–202. doi: 10.1016/S0006-8993(97)00814-7. [DOI] [PubMed] [Google Scholar]
- 62.Pullan L.M., Keith R.A., LaMonte D., Stumpo R.J., Salama A.I. The polyamine spermine affects omega-conotoxin binding and function at N-type voltage-sensitive calcium channels. J. Auton. Pharmacol. 1990;10:213–219. doi: 10.1111/j.1474-8673.1990.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 63.Schoemaker H. Polyamines allosterically modulate [3H]nitrendipine binding to the voltage-sensitive calcium channel in rat brain. Eur. J. Pharmacol. 1992;225:167–169. doi: 10.1016/0922-4106(92)90097-F. [DOI] [PubMed] [Google Scholar]
- 64.Joshi D.C., Singh M., Krishnamurthy K., Joshi P.G., Joshi N.B. AMPA induced Ca2+ influx in motor neurons occurs through voltage gated Ca2+ channel and Ca2+ permeable AMPA receptor. Neurochem. Int. 2011;59:913–921. doi: 10.1016/j.neuint.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 65.Cayzac S.H., Rocher A., Obeso A., Gonzalez C., Riccardi D., Kemp P.J. Spermine attenuates carotid body glomus cell oxygen sensing by inhibiting L-type Ca2(+) channels. Respir. Physiol. Neurobiol. 2011;175:80–89. doi: 10.1016/j.resp.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 66.Zucchi R., Ronca-Testoni S. The sarcoplasmic reticulum Ca2+ channel/ryanodine receptor: Modulation by endogenous effectors, drugs and disease states. Pharmacol. Rev. 1997;49:1–51. [PubMed] [Google Scholar]
- 67.Uehara A., Fill M., Vélez P., Yasukochi M., Imanaga I. Rectification of rabbit cardiac ryanodine receptor current by endogenous polyamines. Biophys. J. 1996;71:769–777. doi: 10.1016/S0006-3495(96)79276-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zarka A., Shoshan-Barmatz V. The interaction of spermine with the ryanodine receptor from skeletal muscle. Biochim. Biophys. Acta. 1992;1108:13–20. doi: 10.1016/0005-2736(92)90109-Y. [DOI] [PubMed] [Google Scholar]
- 69.MacDermott A.B., Mayer M.L., Westbrook G.L., Smith S.J., Barker J.L. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. Nature. 1986;321:519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- 70.Choi D.W. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- 71.Brackley P., Goodnow R., Jr., Nakanishi K., Sudan H.L., Usherwood P.N. Spermine and philanthotoxin potentiate excitatory amino acid responses of Xenopus oocytes injected with rat and chick brain RNA. Neurosci. Lett. 1990;114:51–56. doi: 10.1016/0304-3940(90)90427-B. [DOI] [PubMed] [Google Scholar]
- 72.McGurk J.F., Bennett M.V., Zukin R.S. Polyamines potentiate responses of N-methyl-d-aspartate receptors expressed in xenopus oocytes. Proc. Natl. Acad. Sci. USA. 1990;87:9971–9974. doi: 10.1073/pnas.87.24.9971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jin L., Miyazaki M., Mizuno S., Takigawa M., Hirose T., Nishimura K., Toida T., Williams K., Kashiwagi K., Igarashi K. The pore region of N-methyl-d-aspartate receptors differentially influences stimulation and block by spermine. J. Pharmacol. Exp. Ther. 2008;327:68–77. doi: 10.1124/jpet.108.140459. [DOI] [PubMed] [Google Scholar]
- 74.Choi D.W. Excitotoxic cell death. J. Neurobiol. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- 75.Zito K., Scheuss V. Encyclopedia of Neuroscience. Academic Press; Cambridge, MA, USA: 2009. NMDA Receptor Function and Physiological Modulation; pp. 1157–1164. [Google Scholar]
- 76.Chitravanshi V.C., Sapru H.N. NMDA as well as non-NMDA receptors mediate the neurotransmission of inspiratory drive to phrenic motoneurons in the adult rat. Brain Res. 1996;715:104–112. doi: 10.1016/0006-8993(95)01565-5. [DOI] [PubMed] [Google Scholar]
- 77.Steenland H.W., Liu H., Horner R.L. Endogenous glutamatergic control of rhythmically active mammalian respiratory motoneurons in vivo. J. Neurosci. 2008;28:6826–6835. doi: 10.1523/JNEUROSCI.1019-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Berger A.J. Development of synaptic transmission to respiratory motoneurons. Respir. Physiol. Neurobiol. 2011;179:34–42. doi: 10.1016/j.resp.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grillner S., Deliagina T., Ekeberg O., el Manira A., Hill R.H., Lansner A., Orlovsky G.N., Wallén P. Neural networks that co-ordinate locomotion and body orientation in lamprey. Trends Neurosci. 1995;18:270–279. doi: 10.1016/0166-2236(95)80008-P. [DOI] [PubMed] [Google Scholar]
- 80.Grillner S., Wallén P., Hill R., Cangiano L., El Manira A. Ion channels of importance for the locomotor pattern generation in the lamprey brainstem-spinal cord. J. Physiol. 2001;533:23–30. doi: 10.1111/j.1469-7793.2001.0023b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Williams K., Romano C., Dichter M.A., Molinoff P.B. Modulation of the NMDA receptor by polyamines. Life Sci. 1991;48:469–498. doi: 10.1016/0024-3205(91)90463-L. [DOI] [PubMed] [Google Scholar]
- 82.Marvizón J.-C., Juan-Carlos M., Michel B. NMDA receptor activation by spermine requires glutamate but not glycine. Eur. J. Pharmacol. Mol. Pharmacol. 1993;244:103–104. doi: 10.1016/0922-4106(93)90065-H. [DOI] [PubMed] [Google Scholar]
- 83.Pullan L.M., Powel R.J. Spermine reciprocally changes the affinity of NMDA receptor agonists and antagonists. Eur. J. Pharmacol. 1991;207:173–174. doi: 10.1016/0922-4106(91)90094-X. [DOI] [PubMed] [Google Scholar]
- 84.Benveniste M., Mayer M.L. Multiple effects of spermine on N-methyl-d-aspartic acid receptor responses of rat cultured hippocampal neurones. J. Physiol. 1993;464:131–163. doi: 10.1113/jphysiol.1993.sp019627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Robichaud L.J., Boxer P.A. Polyamine modulation of excitatory amino acid responses in the rat cortical wedge. Neuropharmacology. 1993;32:1025–1035. doi: 10.1016/0028-3908(93)90068-E. [DOI] [PubMed] [Google Scholar]
- 86.Araneda R.C., Zukin R.S., Bennett M.V. Effects of polyamines on NMDA-induced currents in rat hippocampal neurons: A whole-cell and single-channel study. Neurosci. Lett. 1993;152:107–112. doi: 10.1016/0304-3940(93)90495-7. [DOI] [PubMed] [Google Scholar]
- 87.Ferchmin P.A., Pérez D., Biello M. Spermine is neuroprotective against anoxia and N-methyl-d-aspartate in hippocampal slices. Brain Res. 2000;859:273–279. doi: 10.1016/S0006-8993(00)01973-9. [DOI] [PubMed] [Google Scholar]
- 88.Segal J.A., Skolnick P. Spermine-induced toxicity in cerebellar granule neurons is independent of its actions at NMDA receptors. J. Neurochem. 2000;74:60–69. doi: 10.1046/j.1471-4159.2000.0740060.x. [DOI] [PubMed] [Google Scholar]
- 89.Ran I., Israeli R., Miura R.M., Ernest P. Spermine modulates neuronal excitability and NMDA receptors in juvenile gerbil auditory thalamus. Hear. Res. 2003;176:65–79. doi: 10.1016/S0378-5955(02)00746-3. [DOI] [PubMed] [Google Scholar]
- 90.Turecek R., Vlcek K., Petrovic M., Horak M., Vlachova V., Vyklicky L., Jr. Intracellular spermine decreases open probability of N-methyl-d-aspartate receptor channels. Neuroscience. 2004;125:879–887. doi: 10.1016/j.neuroscience.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 91.Calderón F., López-Colomé A.M. Spermine inhibits [3H]glycine binding at the NMDA receptors from plexiform layers of chick retina. Neurochem. Res. 1998;23:1363–1369. doi: 10.1023/A:1020794421783. [DOI] [PubMed] [Google Scholar]
- 92.Mortensen M., Matsumoto I., Niwa S., Dodd P.R. The modulatory effect of spermine on the glutamate-NMDA receptor is regionally variable in normal human adult cerebral cortex. Pharmacol. Toxicol. 1999;84:135–142. doi: 10.1111/j.1600-0773.1999.tb00889.x. [DOI] [PubMed] [Google Scholar]
- 93.Ragnarsson L., Mortensen M., Dodd P.R., Lewis R.J. Spermine modulation of the glutamate(NMDA) receptor is differentially responsive to conantokins in normal and Alzheimer’s disease human cerebral cortex. J. Neurochem. 2002;81:765–779. doi: 10.1046/j.1471-4159.2002.00872.x. [DOI] [PubMed] [Google Scholar]
- 94.Doyle K.M., Shaw G.G. Investigation of the involvement of the N-methyl-d-aspartate receptor macrocomplex in the development of spermine-induced CNS excitation in vivo. Br. J. Pharmacol. 1996;117:1803–1808. doi: 10.1111/j.1476-5381.1996.tb15358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kirby B.P., Shaw G.G. Effect of spermine and N1-dansyl-spermine on epileptiform activity in mouse cortical slices. Eur. J. Pharmacol. 2005;524:53–59. doi: 10.1016/j.ejphar.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 96.Adriani W., Felici A., Sargolini F., Roullet P., Usiello A., Oliverio A., Mele A. N-methyl-d-aspartate and dopamine receptor involvement in the modulation of locomotor activity and memory processes. Exp. Brain Res. 1998;123:52–59. doi: 10.1007/s002210050544. [DOI] [PubMed] [Google Scholar]
- 97.Barraco R.A., Coffin V.L., Altman H.J., Phillis J.W. Central effects of adenosine analogs on locomotor activity in mice and antagonism of caffeine. Brain Res. 1983;272:392–395. doi: 10.1016/0006-8993(83)90591-7. [DOI] [PubMed] [Google Scholar]
- 98.Karlsten R., Rolf K., Torsten G., Per H., Claes P. Effects of Intrathecal Injection of the Adenosine Receptor Agonists R-Phenylisopropyl-Adenosine and N-Ethylcarboxamide-Adenosine on Nociception and Motor Function in the Rat. Anesth. Analg. 1990;71:60–64. doi: 10.1213/00000539-199007000-00010. [DOI] [PubMed] [Google Scholar]
- 99.Nikodijević O., Sarges R., Daly J.W., Jacobson K.A. Behavioral effects of A1- and A2-selective adenosine agonists and antagonists: Evidence for synergism and antagonism. J. Pharmacol. Exp. Ther. 1991;259:286–294. [PMC free article] [PubMed] [Google Scholar]
- 100.Jain N., Kemp N., Adeyemo O., Buchanan P., Stone T.W. Anxiolytic activity of adenosine receptor activation in mice. Br. J. Pharmacol. 1995;116:2127–2133. doi: 10.1111/j.1476-5381.1995.tb16421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hardingham G.E., Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: Implications for neurodegenerative disorders. Nat. Rev. Neurosci. 2010;11:682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Choi D.W. Ionic dependence of glutamate neurotoxicity. J. Neurosci. 1987;7:369–379. doi: 10.1523/JNEUROSCI.07-02-00369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tymianski M., Charlton M.P., Carlen P.L., Tator C.H. Source specificity of early calcium neurotoxicity in cultured embryonic spinal neurons. J. Neurosci. 1993;13:2085–2104. doi: 10.1523/JNEUROSCI.13-05-02085.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rao T.S., Cler J.A., Oei E.J., Emmett M.R., Mick S.J., Iyengar S., Wood P.L. The polyamines, spermine and spermidine, negatively modulate N-methyl-d-aspartate (NMDA) and quisqualate receptor mediated responses in vivo: Cerebellar cyclic GMP measurements. Neurochem. Int. 1990;16:199–206. doi: 10.1016/0197-0186(90)90088-B. [DOI] [PubMed] [Google Scholar]
- 105.Brundell P., Goodnow R., Jr., Kerry C.J., Nakanishi K., Sudan H.L., Usherwood P.N. Quisqualate-sensitive glutamate receptors of the locust Schistocerca gregaria are antagonised by intracellularly applied philanthotoxin and spermine. Neurosci. Lett. 1991;131:196–200. doi: 10.1016/0304-3940(91)90612-W. [DOI] [PubMed] [Google Scholar]
- 106.Otsuki M., Davidson M., Goodenough S., Wilce P.A., Tase C., Matsumoto I. In vivo pharmacological study of spermine-induced neurotoxicity. Neurosci. Lett. 1995;196:81–84. doi: 10.1016/0304-3940(95)11852-N. [DOI] [PubMed] [Google Scholar]
- 107.Isa T., Iino M., Ozawa S. Spermine blocks synaptic transmission mediated by Ca(2+)-permeable AMPA receptors. Neuroreport. 1996;7:689–692. doi: 10.1097/00001756-199602290-00002. [DOI] [PubMed] [Google Scholar]
- 108.Washburn M.S., Dingledine R. Block of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors by polyamines and polyamine toxins. J. Pharmacol. Exp. Ther. 1996;278:669–678. [PubMed] [Google Scholar]
- 109.Noh K.-M., Yokota H., Mashiko T., Castillo P.E., Zukin R.S., Bennett M.V.L. Blockade of calcium-permeable AMPA receptors protects hippocampal neurons against global ischemia-induced death. Proc. Natl. Acad. Sci. USA. 2005;102:12230–12235. doi: 10.1073/pnas.0505408102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Van Den Bosch L., Vandenberghe W., Klaassen H., Van Houtte E., Robberecht W. Ca(2+)-permeable AMPA receptors and selective vulnerability of motor neurons. J. Neurol. Sci. 2000;180:29–34. doi: 10.1016/S0022-510X(00)00414-7. [DOI] [PubMed] [Google Scholar]
- 111.Van Damme P., Van Den Bosch L., Van Houtte E., Callewaert G., Robberecht W. GluR2-dependent properties of AMPA receptors determine the selective vulnerability of motor neurons to excitotoxicity. J. Neurophysiol. 2002;88:1279–1287. doi: 10.1152/jn.2002.88.3.1279. [DOI] [PubMed] [Google Scholar]
- 112.Corona J.C., Tapia R. Ca2+-permeable AMPA receptors and intracellular Ca2+ determine motoneuron vulnerability in rat spinal cord in vivo. Neuropharmacology. 2007;52:1219–1228. doi: 10.1016/j.neuropharm.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 113.Perrais D., Veran J., Mulle C. Gating and permeation of kainate receptors: Differences unveiled. Trends Pharmacol. Sci. 2010;31:516–522. doi: 10.1016/j.tips.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 114.Pegg A.E. The function of spermine. IUBMB Life. 2014;66:8–18. doi: 10.1002/iub.1237. [DOI] [PubMed] [Google Scholar]
- 115.Anis N., Sherby S., Goodnow R., Jr., Niwa M., Konno K., Kallimopoulos T., Bukownik R., Nakanishi K., Usherwood P., Eldefrawi A. Structure-activity relationships of philanthotoxin analogs and polyamines on N-methyl-d-aspartate and nicotinic acetylcholine receptors. J. Pharmacol. Exp. Ther. 1990;254:764–773. [PubMed] [Google Scholar]
- 116.Szczawinska K., Ferchmin P.A., Hann R.M., Eterović V.A. Electric organ polyamines and their effects on the acetylcholine receptor. Cell. Mol. Neurobiol. 1992;12:95–106. doi: 10.1007/BF00713364. [DOI] [PubMed] [Google Scholar]
- 117.Hsu K.S. Modulation of the nicotinic acetylcholine receptor channels by spermine in Xenopus muscle cell culture. Neurosci. Lett. 1994;182:99–103. doi: 10.1016/0304-3940(94)90216-X. [DOI] [PubMed] [Google Scholar]
- 118.Haghighi A.P., Cooper E. Neuronal nicotinic acetylcholine receptors are blocked by intracellular spermine in a voltage-dependent manner. J. Neurosci. 1998;18:4050–4062. doi: 10.1523/JNEUROSCI.18-11-04050.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Law C.L., Wong P.C., Fong W.F. Effects of polyamines on the uptake of neurotransmitters by rat brain synaptosomes. J. Neurochem. 1984;42:870–872. doi: 10.1111/j.1471-4159.1984.tb02761.x. [DOI] [PubMed] [Google Scholar]
- 120.Tsvilovskyy V.V., Zholos A.V., Bolton T.B. Effects of polyamines on the muscarinic receptor-operated cation current in guinea-pig ileal smooth muscle myocytes. Br. J. Pharmacol. 2004;143:968–975. doi: 10.1038/sj.bjp.0706010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Di Giorgio R.M., De Luca G., Nisticò G., Ientile R. gamma-Aminobutyric acid metabolism and behavioral effects after intraventricular injection of spermine in chicks. J. Neurochem. 1985;45:739–743. doi: 10.1111/j.1471-4159.1985.tb04054.x. [DOI] [PubMed] [Google Scholar]
- 122.Gilad G.M., Gilad V.H., Wyatt R.J. Polyamines modulate the binding of GABAA-benzodiazepine receptor ligands in membranes from the rat forebrain. Neuropharmacology. 1992;31:895–898. doi: 10.1016/0028-3908(92)90127-B. [DOI] [PubMed] [Google Scholar]
- 123.Sábato U.C., Aguilar J.S., De Robertis E. Benzodiazepine receptors in rat brain: Action of Triton X-100 and localization in relation to the synaptic region. J. Recept. Res. 1981;2:119–133. doi: 10.3109/10799898109039257. [DOI] [PubMed] [Google Scholar]
- 124.Martijena I.D., Salvatierra N.A., Arce A. Benzodiazepine receptor recruitment after acute stress in synaptosomal membranes from forebrain of young chicks: Action of Triton X-100. J. Neural Transm. Gen. Sect. 1992;87:97–104. doi: 10.1007/BF01245011. [DOI] [PubMed] [Google Scholar]
- 125.Yermolaieva O., Leonard A.S., Schnizler M.K., Abboud F.M., Welsh M.J. Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proc. Natl. Acad. Sci. USA. 2004;101:6752–6757. doi: 10.1073/pnas.0308636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Duan B., Wang Y.-Z., Yang T., Chu X.-P., Yu Y., Huang Y., Cao H., Hansen J., Simon R.P., Zhu M.X., et al. Extracellular spermine exacerbates ischemic neuronal injury through sensitization of ASIC1a channels to extracellular acidosis. J. Neurosci. 2011;31:2101–2112. doi: 10.1523/JNEUROSCI.4351-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ahern G.P., Wang X., Miyares R.L. Polyamines are potent ligands for the capsaicin receptor TRPV1. J. Biol. Chem. 2006;281:8991–8995. doi: 10.1074/jbc.M513429200. [DOI] [PubMed] [Google Scholar]
- 128.Gewehr C., da Silva M.A., dos Santos G.T., Rossato M.F., de Oliveira S.M., Drewes C.C., Pazini A.M., Guerra G.P., Rubin M.A., Ferreira J. Contribution of peripheral vanilloid receptor to the nociception induced by injection of spermine in mice. Pharmacol. Biochem. Behav. 2011;99:775–781. doi: 10.1016/j.pbb.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 129.Israels S.J., Gerrard J.M., Robinson P. Differential effects of spermine on aggregation, inositol phosphate formation and protein phosphorylation in human platelets in response to thrombin, arachidonic acid and lysophosphatidic acid. Biochim. Biophys. Acta. 1986;883:247–252. doi: 10.1016/0304-4165(86)90315-6. [DOI] [PubMed] [Google Scholar]
- 130.Joseph S., Krishnamurthi S., Kakkar V.V. Effect of the polyamine-spermine on agonist-induced human platelet activation--specific inhibition of “aggregation-independent” events induced by thrombin, but not by collagen, thromboxane mimetic, phorbol ester or calcium ionophore. Thromb. Haemost. 1987;57:191–195. [PubMed] [Google Scholar]
- 131.Via L.D., Mariangela F., Mario M., Paola P., De Marco L., Vecchia F.D., Nicoletta R., Renzo D. On the Mechanism of the Spermine-Exerted Inhibition on α-Thrombin-Induced Platelet Activation*. Thromb. Res. 2000;98:59–71. doi: 10.1016/S0049-3848(99)00212-1. [DOI] [PubMed] [Google Scholar]
- 132.De la Peña N.C., Sosa-Melgarejo J.A., Ramos R.R., Méndez J.D. Inhibition of platelet aggregation by putrescine, spermidine, and spermine in hypercholesterolemic rabbits. Arch. Med. Res. 2000;31:546–550. doi: 10.1016/S0188-4409(00)00238-1. [DOI] [PubMed] [Google Scholar]
- 133.Hughes G., Starling A.P., East J.M., Lee A.G. Mechanism of inhibition of the Ca(2+)-ATPase by spermine and other polycationic compounds. Biochemistry. 1994;33:4745–4754. doi: 10.1021/bi00182a001. [DOI] [PubMed] [Google Scholar]
- 134.Palacios J., Sepúlveda M.R., Salvador J.M., Mata A.M. Effect of spermine on the activity of synaptosomal plasma membrane Ca(2+)-ATPase reconstituted in neutral or acidic phospholipids. Biochim. Biophys. Acta. 2003;1611:197–203. doi: 10.1016/S0005-2736(03)00057-9. [DOI] [PubMed] [Google Scholar]
- 135.Carafoli E. The calcium pumping ATPase of the plasma membrane. Annu. Rev. Physiol. 1991;53:531–547. doi: 10.1146/annurev.ph.53.030191.002531. [DOI] [PubMed] [Google Scholar]
- 136.Kucherenko Y.V., Lang F. Inhibition of cation channels in human erythrocytes by spermine. J. Membr. Biol. 2010;237:93–106. doi: 10.1007/s00232-010-9310-1. [DOI] [PubMed] [Google Scholar]
- 137.Trout J.J., Koenig H., Goldstone A.D., Lu C.Y. Blood-brain barrier breakdown by cold injury. Polyamine signals mediate acute stimulation of endocytosis, vesicular transport, and microvillus formation in rat cerebral capillaries. Lab. Investig. 1986;55:622–631. [PubMed] [Google Scholar]
- 138.Glantz L., Nates J.L., Trembovler V., Bass R., Shohami E. Polyamines induce blood-brain barrier disruption and edema formation in the rat. J. Basic Clin. Physiol. Pharmacol. 1996;7:1–10. doi: 10.1515/JBCPP.1996.7.1.1. [DOI] [PubMed] [Google Scholar]
- 139.Koenig H., Harold K., Goldstone A.D., Lu C.Y. Polyamines mediate the reversible opening of the blood-brain barrier by the intracarotid infusion of hyperosmolal mannitol. Brain Res. 1989;483:110–116. doi: 10.1016/0006-8993(89)90040-1. [DOI] [PubMed] [Google Scholar]
- 140.Poduslo J.F., Curran G.L. Polyamine modification increases the permeability of proteins at the blood-nerve and blood-brain barriers. J. Neurochem. 1996;66:1599–1609. doi: 10.1046/j.1471-4159.1996.66041599.x. [DOI] [PubMed] [Google Scholar]
- 141.Poduslo J.F., Curran G.L. Increased permeability of superoxide dismutase at the blood-nerve and blood-brain barriers with retained enzymatic activity after covalent modification with the naturally occurring polyamine, putrescine. J. Neurochem. 1996;67:734–741. doi: 10.1046/j.1471-4159.1996.67020734.x. [DOI] [PubMed] [Google Scholar]
- 142.Paik Jung H.Y., Bjeldanes L.F. Effects of cadaverine on histamine transport and metabolism in isolated gut sections of the guinea-pig. Food Cosmet. Toxicol. 1979;17:629–632. doi: 10.1016/0015-6264(79)90123-8. [DOI] [PubMed] [Google Scholar]
- 143.Mongar J.L. Effect of chain length of aliphatic amines on histamine potentiation and release. Br. J. Pharmacol. Chemother. 1957;12:140–148. doi: 10.1111/j.1476-5381.1957.tb00112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lyons D.E., Beery J.T., Lyons S.A., Taylor S.L. Cadaverine and aminoguanidine potentiate the uptake of histamine in vitro in perfused intestinal segments of rats. Toxicol. Appl. Pharmacol. 1983;70:445–458. doi: 10.1016/0041-008X(83)90162-X. [DOI] [PubMed] [Google Scholar]
- 145.Taylor S.L., Lieber E.R. In vitro inhibition of rat intestinal histamine-metabolizing enzymes. Food Cosmet. Toxicol. 1979;17:237–240. doi: 10.1016/0015-6264(79)90287-6. [DOI] [PubMed] [Google Scholar]
- 146.Maintz L., Novak N. Histamine and histamine intolerance. Am. J. Clin. Nutr. 2007;85:1185–1196. doi: 10.1093/ajcn/85.5.1185. [DOI] [PubMed] [Google Scholar]
- 147.Hui J.Y., Taylor S.L. Inhibition of in vivo histamine metabolism in rats by foodborne and pharmacologic inhibitors of diamine oxidase, histamine N-methyltransferase, and monoamine oxidase. Toxicol. Appl. Pharmacol. 1985;81:241–249. doi: 10.1016/0041-008X(85)90160-7. [DOI] [PubMed] [Google Scholar]
- 148.Rossi F., Nisticò G., De Marco G., Berrino L., Matera C., Bile G., Marmo E. Cardiovascular effects of putrescine in dogs after systemic, intra-arterial vertebral and intraventricular injection. Res. Commun. Chem. Pathol. Pharmacol. 1984;46:43–52. [PubMed] [Google Scholar]
- 149.Bowsher R.R., Verburg K.M., Henry D.P. Rat histamine N-methyltransferase. Quantification, tissue distribution, purification, and immunologic properties. J. Biol. Chem. 1983;258:12215–12220. [PubMed] [Google Scholar]
- 150.Schwelberger H.G., Hittmair A., Kohlwein S.D. Analysis of tissue and subcellular localization of mammalian diamine oxidase by confocal laser scanning fluorescence microscopy. Inflamm. Res. 1998;47:S60–S61. doi: 10.1007/s000110050273. [DOI] [PubMed] [Google Scholar]
- 151.De Meis L., De Paula H.J. Polyamines and muscle relaxation. Inhibition of actomyosin ATPase activity by spermine and spermidine. Arch. Biochem. Biophys. 1967;119:16–21. doi: 10.1016/0003-9861(67)90422-5. [DOI] [PubMed] [Google Scholar]
- 152.Shou-hsian Mao Food of the Common Venomous Snakes of Taiwan. Herpetologica. 1970;26:45–48. [Google Scholar]
- 153.Branch W.R., Haagner G.V., Shine R. Is there an ontogenetic shift in mamba diet? Taxonomic confusion and dietary records for black and green mambas (Dendroaspis: Elapidae) Herpetol. Nat. Hist. 1995 [Google Scholar]
- 154.Olamendi-Portugal T., Batista C.V., Restano-Cassulini R., Pando V., Villa-Hernandez O., Zavaleta-Martinez-Vargas A., Salas-Arruz M.C., Rodriguez de la Vega R.C., Becerril B., Possani L.D. Proteomic analysis of the venom from the fish eating coral snake Micrurus surinamensis: Novel toxins, their function and phylogeny. Proteomics. 2008;8:1919–1932. doi: 10.1002/pmic.200700668. [DOI] [PubMed] [Google Scholar]
- 155.Morais D.H., Ávila R.W., Kawashita-Ribeiro R.A. Squamata, Elapidae, Micrurus surinamensis (Cuvier, 1817): New records and distribution map in the state of Mato Grosso, Brazil, with notes on diet and activity period. Check List. 2011;7:350–351. [Google Scholar]
- 156.Chanhome L., Lawan C., Piboon J., Henry W., Cox M.J. Venomous snake husbandry in Thailand. Wilderness Environ. Med. 2001;12:17–23. doi: 10.1580/1080-6032(2001)012[0017:VSHIT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 157.Modahl C.M., Mukherjee A.K., Mackessy S.P. An analysis of venom ontogeny and prey-specific toxicity in the Monocled Cobra (Naja kaouthia) Toxicon. 2016;119:8–20. doi: 10.1016/j.toxicon.2016.04.049. [DOI] [PubMed] [Google Scholar]
- 158.Luiselli L., Luca L., Akani G.C. Diet of sympatric Gaboon Vipers (Bitis gabonica) and Nose-horned Vipers (Bitis nasicornis) in southern Nigeria. Afr. J. Herpetol. 2003;52:101–106. doi: 10.1080/21564574.2003.9635485. [DOI] [Google Scholar]
- 159.Bazaa A., Marrakchi N., El Ayeb M., Sanz L., Calvete J.J. Snake venomics: Comparative analysis of the venom proteomes of the Tunisian snakes Cerastes cerastes, Cerastes vipera and Macrovipera lebetina. Proteomics. 2005;5:4223–4235. doi: 10.1002/pmic.200402024. [DOI] [PubMed] [Google Scholar]
- 160.Gennaro J.F., Hall H.P., Casey E.R., Hayes W.K. Neurotropic effects of venoms and other factors that promote prey acquisition. J. Exp. Zool. A Ecol. Genet. Physiol. 2007;307:488–499. doi: 10.1002/jez.405. [DOI] [PubMed] [Google Scholar]
- 161.Ali S.A., Jackson T.N.W., Casewell N.R., Low D.H.W., Rossi S., Baumann K., Fathinia B., Visser J., Nouwens A., Hendrikx I., et al. Extreme venom variation in Middle Eastern vipers: A proteomics comparison of Eristicophis macmahonii, Pseudocerastes fieldi and Pseudocerastes persicus. J. Proteomics. 2015;116:106–113. doi: 10.1016/j.jprot.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 162.Trauth S.E. Vertebrate Prey of Selected Arkansas Snakes. Proc. Ark. Acad. Sci. 1995;46:188–192. [Google Scholar]
- 163.Heatwole H., Harold H., Naomie P., Peter K. Ontogenetic Changes in the Resistance of Bullfrogs (Rana catesbeiana) to the Venom of Copperheads (Agkistrodon contortrix contortrix) and Cottonmouths (Agkistrodon piscivorus piscivorus) Copeia. 1999;1999:808–814. doi: 10.2307/1447620. [DOI] [Google Scholar]
- 164.Himes J., John H. Diet composition of Nerodia sipedon (Serpentes: Colubridae) and its dietary overlap with, and chemical recognition of Agkistrodon piscivorus (Serpentes: Viperidae) Amphib-reptil. 2003;24:181–188. doi: 10.1163/156853803322390426. [DOI] [Google Scholar]
- 165.Vincent S.E., Anthony H., Irschick D.J. Ontogeny of intersexual head shape and prey selection in the pitviper Agkistrodon piscivorus. Biol. J. Linn. Soc. Lond. 2004;81:151–159. doi: 10.1111/j.1095-8312.2004.00282.x. [DOI] [Google Scholar]
- 166.Martins M., Marques O.A.V., Sazima I. Ecological and phylogenetic correlates of feeding habits in Neotropical pitvipers of the genus Bothrops. In: Schuett G.W., Hoggren M., Douglas M.E., editors. Biology of the Vipers. Eagle Mountain Publishing; Eagle Mountain, UT, USA: 2002. pp. 1–22. [Google Scholar]
- 167.Greene H.W. Species richness in tropical predators. In: Almeda F., Pringle C.M., editors. Tropical Rainforests: Diversity and Conservation. Memoirs California Academy of Science; San Francisco, CA, USA: 1988. pp. 259–279. [Google Scholar]
- 168.Sorrell G. Master’s Theise. Auburn University; Auburn, AL, USA: 2007. Natural History and Conservation of the Eyelash Palmpitviper (Bothriechis schlegelii) in Western Panamá. [Google Scholar]
- 169.Nogueira C., Cristiano N., Sawaya R.J., Marcio M. Ecology of the Pitviper, Bothrops moojeni, in the Brazilian Cerrado. J. Herpetol. 2003;37:653–659. doi: 10.1670/120-02A. [DOI] [Google Scholar]
- 170.Daltry J.C., Wolfgang W., Thorpe R.S. Intraspecific Variation in the Feeding Ecology of the Crotaline Snake Calloselasma rhodostoma in Southeast Asia. J. Herpetol. 1998;32:198–205. doi: 10.2307/1565297. [DOI] [Google Scholar]
- 171.Luiselli L. Broad geographic, taxonomic and ecological patterns of interpopulation variation in the dietary habits of snakes. Web Ecol. 2006;6:2–16. doi: 10.5194/we-6-2-2006. [DOI] [Google Scholar]
- 172.Margres M.J., McGivern J.J., Seavy M., Wray K.P., Facente J., Rokyta D.R. Contrasting modes and tempos of venom expression evolution in two snake species. Genetics. 2015;199:165–176. doi: 10.1534/genetics.114.172437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Secor S.M., Nagy K.A. Bioenergetic Correlates of Foraging Mode for the Snakes Crotalus cerastes and Masticophis flagellum. Ecology. 1994;75:1600–1614. doi: 10.2307/1939621. [DOI] [Google Scholar]
- 174.Secor S.M. Ecological Aspects of Foraging Mode for the Snakes Crotalus cerastes and Masticophis flagellum. Herpetol. Monogr. 1995;9:169–186. doi: 10.2307/1467004. [DOI] [Google Scholar]
- 175.Salomão M.D., Santos S.M.A., Guiseppe P. Activity pattern of Crotalus durissus (Viperidae, Crotalinae): Feeding, reproduction and snakebite. Stud. Neotrop. Fauna Environ. 1995;30:101–106. doi: 10.1080/01650529509360946. [DOI] [Google Scholar]
- 176.Sant’Anna S.S., Abe A.S. Diet of the rattlesnake Crotalus durissus in southeastern Brazil (Serpentes, Viperidae) Stud. Neotrop. Fauna Environ. 2007;42:169–174. doi: 10.1080/01650520601148313. [DOI] [Google Scholar]
- 177.Meik J.M., Schaack S., Ingrasci M.J., Lawing A.M. Notes on activity, body size variation, and diet in insular speckled rattlesnakes from the western Sea of Cortés, Mexico. Herpetol. Rev. 2012;43:556–560. [Google Scholar]
- 178.Mackessy S.P., Kwame W., Ashton K.G. Ontogenetic Variation in Venom Composition and Diet of Crotalus oreganus concolor: A Case of Venom Paedomorphosis? Copeia. 2003;2003:769–782. doi: 10.1643/HA03-037.1. [DOI] [Google Scholar]
- 179.Hayes W.K. Prey-Handling and Envenomation Strategies of Prairie Rattlesnakes (Crotalus v. viridis) Feeding on Mice and Sparrows. J. Herpetol. 1992;26:496–499. doi: 10.2307/1565129. [DOI] [Google Scholar]
- 180.Voss R.S. Opossums (Mammalia: Didelphidae) in the diets of Neotropical pitvipers (Serpentes: Crotalinae): Evidence for alternative coevolutionary outcomes? Toxicon. 2013;66:1–6. doi: 10.1016/j.toxicon.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 181.Mori A., Akira M., Mamoru T. Feeding Characteristics of a Japanese Pitviper, Ovophis okinavensis, on Okinawa Island: Seasonally Biased but Ontogenetically Stable Exploitation on Small Frogs. Curr. Herpetol. 2011;30:41–52. doi: 10.5358/hsj.30.41. [DOI] [Google Scholar]
- 182.Chijiwa T., Yamaguchi Y., Ogawa T., Deshimaru M., Nobuhisa I., Nakashima K., Oda-Ueda N., Fukumaki Y., Hattori S., Ohno M. Interisland evolution of Trimeresurus flavoviridis venom phospholipase A(2) isozymes. J. Mol. Evol. 2003;56:286–293. doi: 10.1007/s00239-002-2400-7. [DOI] [PubMed] [Google Scholar]